Abstract
Background
To examine the contribution of vigorous physical activity to subsequent cognitive functioning, taking into account the effect of social network.
Methods
The sample included respondents aged 65 years and older who participated in both the fourth and sixth waves of SHARE (n = 17,104). Cognitive functioning in Wave 6, measured as the average of standardized scores for recall, fluency and numeracy, was regressed on the extent of vigorous physical activity, social network size and several confounders in Wave 4, (including the corresponding cognition score at baseline). Interaction terms for physical activity and network size were also considered.
Results
Moderate and high levels of vigorous physical activity, as well as social network size, were related to the cognition outcome after controlling for the confounders. Introduction of the interaction terms showed a direct and positive association of both moderate and high physical activity with the cognition outcome scores as social network size increased. However, among respondents in small (0-1 members) and moderate sized networks (2-3 members), greater physical activity was unrelated to the cognition score at follow-up. Only the interaction of high social connectedness (4-7 network members) and vigorous physical activity was significant.
Conclusions
Vigorous physical activity is, indeed, related to subsequent cognitive functioning. However, the relationship is tempered by social network size. Therefore, interventions that increase both social connectedness and physical activity, especially among older people who are isolated and sedentary, are warranted.
Keywords: Vigorous physical effort, network size, recall, fluency, numeracy, SHARE, 65+
Introduction
Cognitive functioning is a key component of successful aging (Rowe and Kahn, 1987; 1997) while cognitive decline is a possible precursor of dementia (Whitley et al., 2016). Maintaining an active and socially integrated lifestyle into old age promotes cognition and protects against dementia (Dodge et al., 2014; Fratiglioni et al., 2004). Two specific aspects of lifestyle with special relevance for late life cognition are leisure activities (Wang et al., 2013), particularly physical activity (Depp et al., 2010) and social networks (Crooks et al., 2008; Holtzman et al., 2004). However, it is difficult to determine the unique contribution of physical activity vis a vis cognition insofar as most such activity takes place in the company of others and, therefore, reflects some degree of social as well as physical stimulation (Fratiglioni and Wang, 2007). Consequently, the current study seeks to consider the complex association between physical activity and social network in relation to late-life cognition by looking at longitudinal data from a large cross-national sample of older European adults. Toward this end, we examine the respective effects of vigorous physical activity and social network, and their interaction, on cognitive change after a four-year follow-up.
Physical activity has been linked to successful cognitive and emotional aging (Jeste et al., 2010; Plassman et al., 2010). Bodily exercise may impact cognition through the alleviation of dysphoric mood (depression reduction) or through stimulation of the nervous system by means of socially oriented action (Vance et al., 2005). A random clinical trial confirmed that physical exercise helps to maintain cognitive function (Plassman et al., 2010), and another study found exercise to be negatively related to cognitive impairment risk at a 10-year follow-up (but not at the 5-year follow-up) (Jedrziewski et al., 2014). The lack of more widespread conclusive evidence concerning the role of physical activity may be partly related to the issue of endogeneity because the domain of physical activity overlaps the realm of social engagement.
Social networks are the constellations of interpersonal ties that individuals variously maintain. Such groupings frequently provide nurturance and support to their focal members but may also provoke or otherwise challenge them. Personal social networks can impact cognition through a number of mechanisms, including stress reduction and enhancement of cognitive reserve (Kawachi and Berkman, 2001; Sorman et al., 2017). Several studies link social networks with better cognitive functioning (Holtzman et al., 2004; Kuiper et al., 2017; Santini et al., 2017). The variable of network size is frequently cited in this regard as a key predictor (Belley et al., 2013). (For an exception see Krueger et al., 2009).
Social network characteristics may, themselves, enhance or restrain engagement in physical activity. For example, network size, frequency of contact and extent of network homogeneity have been found to be positively related to both energy expenditure and exercise adherence (McNeill et al., 2006). An Australian study of persons aged 60 and older found that regular participation of friends and family was associated with being physically active (Booth et al., 2000), and a study in Portland, Oregon, revealed that neighborhood social cohesion was related to greater levels of physical activity (Fisher et al., 2004). This line of inquiry underscores the dilemma and the difficulty of disentangling the effect of physical activity from its social components.
Using data from the National Social life, Health and Aging Project in the United States, one previous study attempted to clarify the role of physical activity in relation to the mental health of older adults independent of the effect of social network. The findings showed that physical activity inversely correlated with late life depressive symptoms. However, when the interaction of physical activity with two particularly vulnerable social network types was also considered (family-limited and restricted networks), the effect of these two at-risk network constellations increased, while the effect of the physical activity indicator became non-significant (Litwin, 2012). That study did not consider the same interaction in relation to cognitive function, however.
Cognitive functioning is related to sociodemographic background and health characteristics, as are physical activity and social networks. Consequently, any analysis in this domain should control for them. They include: age (Brewster et al., 2014; Kareholt, 2012), gender (Lee et al., 2014; Stephens et al., 2015), ethnicity (Sloan and Wang, 2005), marital status (Karlamangla et al., 2009), education (Kareholt, 2012; Maurer, 2011), income (Nguyen et al., 2008), chronic diseases (Kilander et al., 2000; Schneider, 2004), limitations in basic activities of daily living (ADL) and instrumental activities of daily living (IADL) (Rajan et al., 2013) and self-rated health (Jelicic and Kempen, 1999).
Based upon this review, the current inquiry examines two hypotheses:
H1 - Vigorous physical activity is positively related to subsequent cognitive functioning.
H2 - The effect of vigorous physical activity on subsequent cognitive functioning is moderated by social network size.
Methods
The analysis used data from the fourth (2011) and sixth waves (2015) of the Survey of Health, Ageing and Retirement in Europe (SHARE), focusing on respondents aged 65 or older in Wave 4 who also participated in Wave 6, some four years later (n =17,383). Respondents came from 14 countries: Austria, Belgium, Czech Republic, Denmark, Estonia, France, Germany, Italy, Poland, Portugal, Slovenia, Spain, Sweden and Switzerland. Each country sample was drawn using probability sampling of households with persons aged 50 and older. Data were gathered through Computer Assisted Personal Interviews that took place in the respondents’ homes. Proxy responses were not permitted for the subjective survey items, for example, the questions related to personal social ties. The present analysis excluded respondents who had been diagnosed with dementia (n =237; 1.4%) and those who did not answer the probe about dementia diagnosis (n =42; 0.2%). The resultant analytical sample numbered 17,104 respondents .
Dependent variable
Cognitive functioning was measured by a combined indicator calculated from three separate tests: recall (immediate and delayed), verbal fluency and numeracy. This indicator has been employed elsewhere for the measurement of cognitive function and cognitive change (Litwin et al., 2017). In the immediate recall task, the interviewer read aloud a list of ten words and requested the respondent to recall as many as possible. In the delayed recall assessment 5-10 minutes later, the respondent was asked to mention once again the words remembered from the list. The scores for each probe ranged from 0-10 and the total recall score ranged from 0-20. Verbal fluency was measured by asking respondents to name as many animals as possible in one minute. Numeracy was examined by means of the Serials-7 test in which the respondent subtracts 7 from 100 and then proceeds to subtract 7 four more times. The score on this last measure ranged from 0-5. All correct subtractions were credited even if they followed an incorrect one. For the present analysis, a combined indicator of cognitive functioning was calculated by averaging the standardized scores of each of these three indicators.
A cognitive function score was calculated for each respondent at baseline and at followup, four years later. The dependent variable in the current study is the cognitive function indicator at follow-up. The analysis regressed the dependent variable on the study variables controlling for the measure of cognitive function at baseline as well. Thus, the outcome measure is essentially a measure of cognitive change after four years.
Independent variables
Physical activity was measured by the question “How often do you engage in vigorous physical activity, such as sports, heavy housework, or a job that involves physical labor.” This question is differentiated from a similar probe in the questionnaire about moderate physical activity (e.g. cleaning the car or doing a walk), which we purposely did not utilize in the current analysis. As such, we can confirm that the respondents understood the vigorous physical activity question to refer to action that involves considerable physical effort. The values of the vigorous physical activity measure In the present analysis were: 1 (hardly ever, or never), 2 (weekly or less often) and 3 (more than once a week).
The social network data were obtained from a name generating social network inventory that elicits the names of close persons. The questionnaire asks respondents to identify the persons with whom they spoke about things that were important to them in the previous 12 months (range=0-7), and then requests additional information about each such person cited. The social network module in SHARE is administered at the beginning of the interview in order to minimize the risk of interview fatigue that may occur, for example, when a name generator is applied much later in the interview. In the current analysis, we employed the variable of social network size as the network indicator of interest. It was operationalized as the count of persons mentioned in the name generator, from zero to seven. In part of the analysis we divided the continuous network size variable into three categories: low (0-1), moderate (2-3) and high (4-7).
The analysis also controlled for sociodemographic attributes. Gender was indicated by a dichotomous variable (males = 0; females = 1). Marital status was also dichotomous (not married or partnered = 0; married or partnered = 1). Income was considered by perceived financial capacity, measured by the question “Thinking of your household’s total monthly income, would you say that your household is able to make ends meet” [range = 1 (with great difficulty)–4 (easily)]. Education was classified into three levels, based on the International Standard Classification of Education (ISCED-1997) classification: 1) elementary education or less; 2) high school education (secondary education); and 3) further education beyond high school (post – secondary education). Finally, ethnicity was addressed by controlling for country of residence using dummy variables for each country, with Austria as the reference category.
Health variables were also controlled. The number of chronic diseases was measured by the question “has a doctor ever told you that you had any of the conditions on this card”? The conditions included, for example, diabetes, chronic lung disease and cataracts (range 0-11). Limitations in basic activities of daily living (ADL) reflected difficulties in dressing, walking across a room, bathing, eating, getting in and out of bed and using the toilet (range = 0-6, higher score means less independence). Limitations in instrumental activities of daily living (IADL) referred to difficulties performing complex practical tasks, e.g. preparing meals, doing housework and handling personal finances (range = 0-7, higher score means less independence). Finally, a 5-point subjective measure of self-perceived health ranged from 1 (poor) to 5 (excellent).
Statistical analysis
We executed univariate, bivariate and multivariate analyses. The bivariate examinations considered the unadjusted associations between the study variables and cognitive function at follow-up. In the final stage of the inquiry we regressed the cognitive function score at follow-up on the study variables using ordinary least squares regressions in several models. Model 1A regressed cognition at follow-up on physical activity only, and Model 1B did the same vis a vis social network size. Model 2 regressed cognitive function on both physical activity and social network size. Model 3 added the control variables, including the baseline cognition score. Lastly, Model 4 considered the interactions of physical activity social and network size. This analysis was repeated twice–once with the physical activity measure divided into three levels (Model 4A), and once with the social network size variable divided into three categories (Model4B).
Results
Table 1 summarizes the distributions of the study variables. The sample had a majority of women. Average age was 73 (sd=6.3; range=65-102). Slightly less than two thirds were married. A bit less than a quarter had higher education while more than a quarter completed secondary schooling. The rest, almost half the sample, had fewer years of education. About 28 percent of the respondents reported some difficulty making ends meet, while another nine percent reported great difficulty. More than a third managed fairly easily and more than a quarter managed easily.
Table 1.
Univariate description of the study variables: Background, health, social network, physical activity and mean standardized cognition
| Variables | N | (%) | mean | (std) | range |
|---|---|---|---|---|---|
| Background | |||||
| Gender | |||||
| Male | 7326 | (42.8) | |||
| Female | 9778 | (57.2) | |||
| Age | 17104 | 73.3 | (6.3) | 65 - 102 | |
| Marital status | |||||
| Not married or partnered | 5842 | (34.6) | |||
| Married or partnered | 11041 | (65.4) | |||
| Education | |||||
| Elementary or less | 8163 | (48.7) | |||
| Secondary | 4657 | (27.8) | |||
| Post-secondary | 3940 | (23.5) | |||
| Financial capacity | |||||
| With great difficulty | 1538 | (9.1) | |||
| With some difficulty | 4749 | (28.2) | |||
| Fairly easily | 5841 | (34.6) | |||
| Easily | 4739 | (28.1) | |||
| Country | |||||
| Austria | 1504 | (8.8) | |||
| Germany | 526 | (3.1) | |||
| Sweden | 925 | (5.4) | |||
| Spain | 1443 | (8.4) | |||
| Italy | 1272 | (7.4) | |||
| France | 1506 | (8.8) | |||
| Denmark | 732 | (4.3) | |||
| Switzerland | 1256 | (7.3) | |||
| Belgium | 1539 | (9.0) | |||
| Czech Republic | 1745 | (10.2) | |||
| Poland | 667 | (3.9) | |||
| Portugal | 650 | (3.8) | |||
| Slovenia | 866 | (5.1) | |||
| Estonia | 2473 | (14.5) | |||
| Health | |||||
| # chronic diseases | 17104 | 2.1 | (1.6) | 0 – 10 | |
| ADL | 17094 | 0.2 | (0.8) | 0 – 6 | |
| IADL limitations | 17094 | 0.4 | (1.0) | 0 – 7 | |
| Perceived health | 17092 | 2.6 | (1.0) | 1–5 | |
| Mean standardized cognition | |||||
| at baseline | 17104 | 10.1 | (3.7) | 0 – 36.3 | |
| at follow-up | 17104 | 9.8 | (4.1) | 0 – 44.5 | |
| Social network size (SN)a | 17104 | 2.5 | (1.6) | 0 – 7 | |
| Low SNb | 5629 | (32.9) | |||
| Moderate SNc | 7575 | (44.3) | |||
| High SNd | 3900 | (22.8) | |||
| Physical activity (PA)e | 16986 | 1.8 | (0.8) | 1–3 | |
| Lowf | 8646 | (50.1) | |||
| Moderateg | 3780 | (22.3) | |||
| Highh | 4560 | (26.9) |
Range = 0-7
SN = 0-1
SN = 2-3
SN = 4-7
Range = 1-3
Hardly ever or never
Up to once a week
More than once a week
In terms of health, respondents reported having been diagnosed with two chronic diseases, on average (sd=1.6). The mean number of ADL and IADL limitations was quite low (mean=0.2; sd=0.8 and 0.4; sd=1.0 respectively). Average self rated health was about midway on a scale of 1-5 (mean=2.6; sd=1.0). In terms of cognitive function, participants showed a mild decrease after four years. The average standardized score at baseline was 10.1 (sd=3.7). At follow-up, it dropped to 9.8 (sd=4.1).
Mean social network size was 2.5 (sd=1.6). When collapsed into three categories, more than a fifth of the respondents fell into the highest grouping, a third were in the lowest grouping, and the remainder—close to 45 percent—were in the middle grouping. As for physical activity, some 27 percent fell into the highest category and about 22 percent were in the middle category. Half of the sample hardly or never engaged in vigorous physical activity. The overall mean on the continuous version of this variable was 1.8 (sd=0.8).
Table 2 shows the unadjusted bivariate correlations with cognitive functioning at follow-up. A strong correlation between cognition at baseline and the same at follow-up was observed. Vigorous physical activity and social network size were both associated positively with the cognition outcome measure. In addition, all the control variables except gender showed significant correlations. Positive associations were found for marital status, education, and financial capacity. Negative associations emerged among all the risk-related health variables (illness and limitations) while better self-rated health was positively correlated.
Table 2.
The unadjusted associations of background, health, social network and physical activity with mean standardized cognition at follow-up: Pearson correlations
| Variables | Cognition at follow-up |
|---|---|
| R | |
| Background | |
| Femalea | −0.01 |
| Age | −0.35*** |
| Married or partneredb | 0.06*** |
| Secondary educationc | 0.14*** |
| Post-secondary educationc | 0.25*** |
| Financial capacity | |
| With some difficultyd | −0.11*** |
| Fairly easilyd | 0.02* |
| Easilyd | 0.17*** |
| Health | |
| # chronic diseases | −0.10*** |
| ADL | −0.20*** |
| IADL limitations | −0.30*** |
| Perceived health | 0.27*** |
| Mean standardized cognition at baseline | 0.61*** |
| Social network size (SN) | 0.15*** |
| Physical activity (PA) | |
| Moderatee | 0.09*** |
| Highe | 0.17*** |
p < .05;
p < .01;
p < .001
Reference categories
male
not married or partnered
primary education or less
With great difficulty
low (hardly ever or never).
The results of the linear regression analysis are presented in Table 3. Models 1A and 1B replicate the bivariate results. We ran these initial regressions to clarify the variance explained by the two independent variables— physical activity (5.4%) and 2 social network (2.2%). When cognition was regressed on both variables simultaneously (Model 2), the explained variance rose to 7.3 percent while the strength of the associations of the respective variables remained the same.
Table 3.
The association of physical activity and social network in relation to cognitive change at follow-up
| Model | Model | Model | Model | Model | Model | |
|---|---|---|---|---|---|---|
| 1A | 1B | 2 | 3e | 4Ae | 4Be | |
| VARIABLES | ||||||
| Baseline measures | ||||||
| Cognition | 0.44*** | 0.44*** | 0.44*** | |||
| Moderate PAa | 0.17*** | 0.16*** | 0.02* | −0.01 | ||
| High PAa | 0.22*** | 0.22*** | 0.03*** | −0.00 | ||
| SNb | 0.15*** | 0.14*** | 0.03*** | 0.01 | ||
| Moderate PA × SNb | 0.03* | |||||
| High PA × SNb | 0.03** | |||||
| PAc | 0.01 | |||||
| Moderate SNd | 0.02 | |||||
| High SNd | −0.00 | |||||
| Moderate SNd × PA | 0.01 | |||||
| High SNd × PA | 0.05** | |||||
| Observations | 16,986 | 17,104 | 16,986 | 16,496 | 16,496 | 16,496 |
| R – squared | 0.054 | 0.022 | 0.073 | 0.443 | 0.444 | 0.444 |
p<0.001,
p<0.01,
p<0.05
Physical activity (reference group=hardly ever or never)
Social network (range=0-7)
Physical activity (range=1-3)
Social network (reference group=none)
Adjusted for age, gender, marital status, education level, financial adequacy, chronic diseases, limitations in basic activities of daily living (ADL), limitations in instrumental activities of daily living (IADL), self-rated health and country
The addition of the control variables in Model 3 modified the previously observed associations and raised the amount of explained variance considerably (R2 = 0.44). Not surprisingly, the association of baseline cognition with cognition at follow-up was strong and positive. As for the independent variables, moderate and high vigorous physical activity, as well as social network size, all retained their positive associations, albeit to a lesser degree. They also changed somewhat in the relative strength of the respective associations, with social network now showing similar strength to that of physical activity. For the respective effects of the remaining control variables, see Supplementary Table S1 attached to the electronic version of this paper at http://journals.cambridge.org/ipg.
Model 4A shows the first of two analyses that examine the interaction of physical activity and social network size. This analysis considers three levels of vigorous physical activity with social network size calculated as a continuous measure. Low physical activity (hardly or never) served as the reference category. When the interaction terms were entered into the regression, the main effects of both physical activity and social network size lost their prior significance. Moreover, compared to low physical activity and social network size, the interactions of moderate and high physical activity were significant. Figure 1 shows a clear positive association of both moderate and high physical activity with the cognition outcome scores as social network size is greater.
Figure 1.
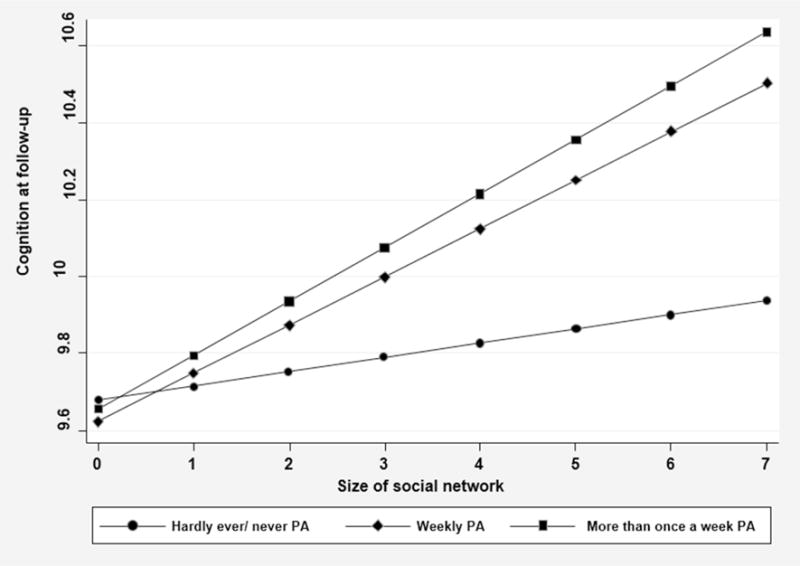
The interaction of three levels of vigorous physical activity and social network size in relation to cognitive function at follow-up
Model 4B reverses the measurement levels of the physical activity and social network size variables. In this case, the analysis considers three categories of social network size with vigorous physical activity calculated as a continuous (ordinal) measure. Low social network size served as the reference category. This model reveals that only the interaction of high social connectedness (i.e. the group with the greatest number of social network members, relatively) and vigorous physical activity was significant. Figure 2 shows that among the respondents in the smallest social networks (0-1 members), physical activity was unrelated to the degree of cognition at follow-up. Among respondents in the moderate sized social networks (2-3 members), the cognitive score at follow-up also hardly increased as the extent of vigorous physical activity increased. It was only among respondents having the largest social networks (4-7 members) that we discern a rise in cognition scores at followup, as baseline physical activity levels increase.
Figure 2.
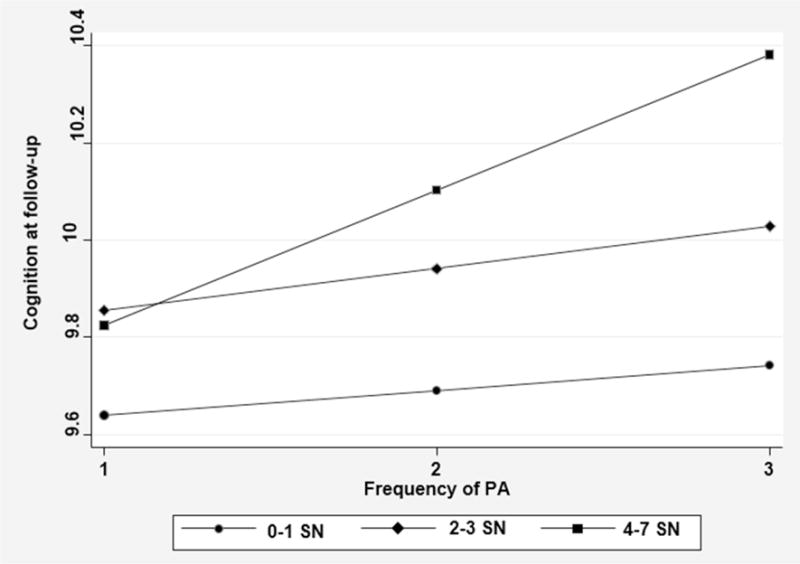
The interaction of three categories of social network size and vigorous physical activity in relation to cognitive function at follow-up
Discussion
It is difficult to distinguish the contribution of physical activity to the maintenance of cognitive function in late life insofar as physical activity is a variable with several facets, including bodily effort and social engagement. We focused, in this study, on vigorous physical activity (as opposed to moderate physical activity), in order to optimize the conceptualization of this variable. We also considered the effect of the social network, a key indicator of social engagement. In addition, we examined cognition in a longitudinal sense, looking at the change from baseline to follow-up, four years hence. The study sample numbered over 17 thousand persons aged 65 and over, drawn from two non-consecutive waves of the SHARE Survey.
In general, we found that about half of the European elders hardly or never engage in vigorous physical activity. We also found that about a third of them are embedded in personal social networks of very limited size, that is, they have only one meaningful social tie or less. As for cognitive function, the scores showed a slight decrease overall over a period of four years.
The first study hypothesis posited that physical activity is positively related to subsequent cognitive functioning. This hypothesis was fully supported by the data. The analysis revealed that moderate and high vigorous physical activity (compared to hardly ever engaging in such activity or never engaging at all) were related to the cognitive outcome variable even after network size and the control variables were considered. Stated differently, the results showed that vigorous physical activity correlated with improved cognition. We note also that social network size retained its independent association with cognitive function at follow-up, irrespective of physical activity. Thus, the number of persons with whom respondents spoke at baseline about matters that were important to them was a significant predictor of better cognitive functioning at follow-up. It seems that social ties make a meaningful contribution to better cognitive health in late life in their own right.
The second hypothesis queried whether the effect of physical activity on subsequent cognitive functioning is moderated by social network size. This was considered by entering the interaction of these two variables into the regression. The results revealed that the second study hypothesis was also confirmed. Two observations stand behind this conclusion. First, the effect of physical activity on cognition was shown to be related to the size of the social network. Second, it was mainly among respondents who were the most socially endowed, that is, those with the largest social networks, where engagement in more frequent vigorous physical activity correlated with the cognition measure at follow-up. Among those with moderate sized social networks, in comparison, greater physical activity was unrelated to cognitive function when the interactions were considered.
The key finding of this research, therefore, is that vigorous physical activity in late life is, indeed, related to subsequent cognitive functioning. However, this relationship is tempered by other variables and particularly by the interpersonal environment in which older people are embedded. The social networks of older people not only affect their cognitive mental health independently, they also moderate the contribution of vigorous physical activity to this same outcome.
A few limitations of the current study should be mentioned. Although SHARE provides a rich collection of social network data, we cannot determine whether respondents explicitly used their named social ties to enhance vigorous physical activity. We can mainly underscore that people with a greater number of meaningful social ties also engaged more frequently in energetic actions, and that those who were more endowed in both domains had better scores on cognitive functioning over time. We should also note that the current analysis considered a single network indicator. Although, as documented earlier, network size is a robust measure, further inquiry should explore the role of other social network facets in relation to the physical activity – cognitive function nexus. Similarly, our measure of physical activity is based on a self-reported measure. Future studies are encouraged to consider objective measures of one’s engagement in vigorous physical effort. Finally, further longitudinal investigation is warranted in order to verify that declines in both social network size and physical activity are not the consequence of previous cognitive decline.
Despite these limitations, the advantages of the current analysis outweigh its shortcomings. The availability of detailed information on the lives, social relations and physical activities of a very large cross-national sample of older European adults offers an unparalleled opportunity to examine a variety of factors in relation to cognitive health. Moreover, the longitudinal nature of the SHARE survey provides an excellent means by which to consider the physical activity – cognitive function nexus in late life.
The policy and practice implication of the current study findings is that it is, indeed, warranted to encourage older people to engage in sports and other energetic physical pursuits. In addition, the results of the analysis suggest that social ties are facilitative for maintaining vigorous physical activity. Nonetheless, vigorous exercise does not equally benefit all older people. As the present study demonstrates, physical activity among those who have no social ties does not seem to help their cognitive functioning, and even among those with a medium degree of social connectedness, the contribution of physical activity is limited. Thus, in order to optimize the potential benefit of vigorous physical activity, society should guarantee that its eldest members do not experience minimal social connectedness. Strengthening meaningful social ties in late life can provide a double reward, because it is both related to better cognition and it enhances the role of physical activity in preserving cognitive functioning as well.
In conclusion, this study shows that vigorous physical activity can potentially benefit the cognitive health of older persons, but it also demonstrates that the value of physical activity for cognitive functioning differs across individuals. The benefit of sports and other energetic actions is most apparent among those who are embedded in larger constellations of meaningful others. Mental health practitioners should be aware of these dynamics and consider their possible effects. This implies the necessity to consider interventions that increase both social connectedness and physical activity, especially among those older people who are isolated and sedentary.
Supplementary Material
Acknowledgments
We gratefully acknowledge funding from the European Commission (Horizon 2020), the US National Institute on Aging, and national sources, especially the German Federal Ministry of Education and Research.
Footnotes
Conflict of interest
None
Description of authors’ roles:
A. Shaul was responsible for the statistical design of the study and for carrying out the statistical analysis. H. Litwin supervised the statistical analysis and wrote the paper.
References
- Belley AM, et al. Ecological perspective on the determinants of cognitive health of seniors. Canadian Journal on Aging-Revue Canadienne Du Vieillissement. 2013;32:240–249. doi: 10.1017/S0714980813000299. [DOI] [PubMed] [Google Scholar]
- Booth ML, Owen N, Bauman A, Clavisi O, Leslie E. Social-cognitive and perceived environment influences associated with physical activity in older Australians. Preventive Medicine. 2000;31:15–22. doi: 10.1006/pmed.2000.0661. [DOI] [PubMed] [Google Scholar]
- Brewster PWH, et al. Life experience and demographic influences on cognitive function in older adults. Neuropsychology. 2014;28:846–858. doi: 10.1037/neu0000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks VC, Lubben J, Petitti DB, Little D, Chiu V. Social network, cognitive function, and dementia incidence among elderly women. American Journal of Public Health. 2008;98:1221–1227. doi: 10.2105/AJPH.2007.115923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depp C, Vahia IV, Jeste D. Successful aging: Focus on cognitive and emotional health. In: NolenHoeksema S, Cannon TD, Widiger T, editors. Annual Review of Clinical Psychology. Vol. 6. 2010. pp. 527–550. [DOI] [PubMed] [Google Scholar]
- Dodge HH, Ybarra O, Kaye JA. Tools for advancing research into social networks and cognitive function in older adults. International Psychogeriatrics. 2014;26:533–539. doi: 10.1017/S1041610213001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher KJ, Li FZ, Michael Y, Cleveland M. Neighborhood-level influences on physical activity among older adults: A multilevel analysis. Journal of Aging and Physical Activity. 2004;12:45–63. doi: 10.1123/japa.12.1.45. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. Lancet Neurology. 2004;3:343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. Journal of Alzheimers Disease. 2007;12:11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- Holtzman RE, Rebok GW, Saczynski JS, Kouzis AC, Doyle KW, Eaton WW. Social network characteristics and cognition in middle-aged and older adults. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2004;59:P278–P284. doi: 10.1093/geronb/59.6.p278. [DOI] [PubMed] [Google Scholar]
- Jedrziewski MK, Ewbank DC, Wang HD, Trojanowski JQ. The impact of exercise, cognitive activities, and socialization on cognitive function: results from the National Long-Term Care Survey. American Journal of Alzheimers Disease and Other Dementias. 2014;29:372–378. doi: 10.1177/1533317513518646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelicic M, Kempen G. Effect of self-rated health on cognitive performance in community dwelling elderly. Educational Gerontology. 1999;25:13–17. [Google Scholar]
- Jeste DV, Depp CA, Vahia IV. Successful cognitive and emotional aging. World Psychiatry. 2010;9:78–84. doi: 10.1002/j.2051-5545.2010.tb00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kareholt I. Age and sex differences in the relation between education and physical and cognitive functioning among men and women aged 76 years and older. International Journal of Behavioral Medicine. 2012;19:S224–S224. [Google Scholar]
- Karlamangla AS, Miller-Martinez D, Aneshensel CS, Seeman TE, Wight RG, Chodosh J. Trajectories of cognitive function in late life in the United States: Demographic and socioeconomic predictors. American Journal of Epidemiology. 2009;170:331–342. doi: 10.1093/aje/kwp154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, Berkman LF. Social ties and mental health. Journal of Urban Health-Bulletin of the New York Academy of Medicine. 2001;78:458–467. doi: 10.1093/jurban/78.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilander L, Nyman H, Boberg M, Lithell H. The association between low diastolic blood pressure in middle age and cognitive function in old age. A population-based study. Age and Ageing. 2000;29:243–248. doi: 10.1093/ageing/29.3.243. [DOI] [PubMed] [Google Scholar]
- Krueger KR, Wilson RS, Kamenetsky JM, Barnes LL, Bienias JL, Bennett DA. Social engagement and cognitive function in old age. Experimental Aging Research. 2009;35:45–60. doi: 10.1080/03610730802545028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper JS, Voshaar RCO, Zuidema SU, Stolk RP, Zuidersma M, Smidt N. The relationship between social functioning and subjective memory complaints in older persons: a population-based longitudinal cohort study. International Journal of Geriatric Psychiatry. 2017;32:1059–1071. doi: 10.1002/gps.4567. [DOI] [PubMed] [Google Scholar]
- Lee J, Shih R, Feeney K, Langa KM. Gender disparity in late-life cognitive functioning in india: Findings from the Longitudinal Aging Study in India. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2014;69:603–611. doi: 10.1093/geronb/gbu017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin H. Physical activity, social network type, and depressive symptoms in late life: An analysis of data from the National Social Life, Health and Aging Project. Aging & Mental Health. 2012;16:608–616. doi: 10.1080/13607863.2011.644264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin H, Schwartz E, Damri N. Cognitively stimulating leisure activity and subsequent cognitive function: A SHARE-based analysis. The Gerontologist. 2017;57(5):940–948. doi: 10.1093/geront/gnw084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer J. Education and male-female differences in later-life cognition: International evidence from Latin America and the Caribbean. Demography. 2011;48:915–930. doi: 10.1007/s13524-011-0048-x. [DOI] [PubMed] [Google Scholar]
- McNeill LH, Kreuter MW, Subramanian SV. Social environment and physical activity: A review of concepts and evidence. Social Science & Medicine. 2006;63:1011–1022. doi: 10.1016/j.socscimed.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Nguyen CT, Couture MC, Alvarado BE, Zunzunegui MV. Life course socioeconomic disadvantage and cognitive function among the elderly population of seven capitals in Latin America and the Caribbean. Journal of Aging and Health. 2008;20:347–362. doi: 10.1177/0898264308315430. [DOI] [PubMed] [Google Scholar]
- Plassman BL, Williams JW, Burke JR, Holsinger T, Benjamin S. Systematic review: Factors associated with risk for and possible prevention of cognitive decline in later life. Annals of Internal Medicine. 2010;153:182–U188. doi: 10.7326/0003-4819-153-3-201008030-00258. [DOI] [PubMed] [Google Scholar]
- Rajan KB, Hebert LE, Scherr PA, de Leon CFM, Evans DA. Disability in basic and instrumental activities of daily living is associated with faster rate of decline in cognitive function of older adults. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2013;68:624–630. doi: 10.1093/gerona/gls208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Human aging - usual and successful. Science. 1987;237:143–149. doi: 10.1126/science.3299702. [DOI] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. Successful aging. Gerontologist. 1997;37:433–440. doi: 10.1093/geront/37.4.433. [DOI] [PubMed] [Google Scholar]
- Santini ZI, et al. The protective properties of Act-Belong-Commit indicators against incident depression, anxiety, and cognitive impairment among older Irish adults: Findings from a prospective community-based study. Experimental Gerontology. 2017;91:79–87. doi: 10.1016/j.exger.2017.02.074. [DOI] [PubMed] [Google Scholar]
- Schneider MG. The intersection of mental and physical health in older Mexican Americans. Hispanic Journal of Behavioral Sciences. 2004;26:333–355. [Google Scholar]
- Sloan FA, Wang JS. Disparities among older adults in measures of cognitive function by race or ethnicity. Journals of Gerontology Series B-Psychological Sciences and Social Sciences. 2005;60:P242–P250. doi: 10.1093/geronb/60.5.p242. [DOI] [PubMed] [Google Scholar]
- Sorman DE, Ronnlund M, Sundstrom A, Norberg M, Nilsson LG. Social network size and cognitive functioning in middle-aged adults: Cross-sectional and longitudinal associations. Journal of Adult Development. 2017;24:77–88. doi: 10.1007/s10804-016-9248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens C, Spicer J, Budge C, Stevenson B, Alpass F. Accounting for differences in cognitive health between older adults in New Zealand and the USA. International Psychogeriatrics. 2015;27:591–600. doi: 10.1017/S1041610214002579. [DOI] [PubMed] [Google Scholar]
- Vance DE, Wadley VG, Ball KK, Roenker DL, Rizzo M. The effects of physical activity and sedentary behavior on cognitive health in older adults. Journal of Aging and Physical Activity. 2005;13:294–313. doi: 10.1123/japa.13.3.294. [DOI] [PubMed] [Google Scholar]
- Wang HX, et al. Late life leisure activities and risk of cognitive decline. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2013;68:205–213. doi: 10.1093/gerona/gls153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley E, Deary IJ, Ritchie SJ, Batty GD, Kumari M, Benzeval M. Variations in cognitive abilities across the life course: Cross-sectional evidence from Understanding Society: The UK Household Longitudinal Study. Intelligence. 2016;59:39–50. doi: 10.1016/j.intell.2016.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


