Abstract
To determine whether the effects of symptom duration on fibromyalgia physical impairment are moderated by symptom self-efficacy, data from 572 female participants, who were members of a large health maintenance organization and had a diagnosis of fibromyalgia syndrome (FMS) were assessed. Age, symptom duration, history of physical, sexual, and emotional abuse, fibromyalgia-specific self-efficacy (Arthritis Self-Efficacy Scale adapted for FMS [ASES]), depression (Centers for Epidemiological Studies Depression Scale [CES-D]), fibromyalgia physical impairment (Fibromyalgia Impact Questionnaire [FIQ]), and pain (McGill Present Pain Index [PPI]) were measured five times across 18 months. Linear regressions were performed to predict baseline FIQ and PPI cross-sectionally. Of primary interest was a hypothesized interaction between ASES and symptom duration, which was significant in relation to FIQ but not PPI. Multilevel mixed models were performed to determine whether the same pattern existed longitudinally controlling for baseline symptom duration as an effect of time and ASES. The interaction was significant in the models for both FIQ and PPI. These results suggest that the effects of age and symptom duration on FMS are unique, and that self-efficacy plays a crucial role in moderating disease course (measured by symptom duration or time) in FMS.
Keywords: Fibromyalgia, self-efficacy, symptom duration, moderation, age
Fibromyalgia syndrome [FMS] is a chronic, painful, debilitating condition of unknown etiology. It is frequently treatment-resistant, though some pharmacological and behavioral interventions may help (Bradley, 2009; Goldenberg, Burckhardt, & Crofford, 2004; Hassett, Cone, Patella, & Sigal, 2004; Recla, 2010). Approximately five million people in the United States, predominantly women, are affected by FMS (Silveman, Sadosky, Evans, Yeh, Alvir, & Zlateva, 2010).
Diagnostic criteria for FMS are largely subjective, including a patient-reported history of chronic, widespread musculoskeletal pain and painful sensitivity (Wolfe et al., 1990). Fatigue, sleep disturbances, cognitive problems, and psychiatric disorders are also common (Giesecke et al., 2003; Wolfe et al., 2010). Pain in FMS is difficult to treat. Intragroup differences among FMS sufferers increase the challenge of developing effective interventions (Malt, Olafsson, Lund, & Ursin, 2002; Turk, Okifuji, Sinclair, & Starz, 1996; Turk, Okifuji, Sinclair, & Starz, 1998). Intragroup classifications that consider psychological factors (e.g., Giesecke et al. 2003) have received increasing interest (Alegre de Miquel et al., 2010). The American College of Rheumatology [ACR] now acknowledges the importance of non-myalgic factors in FMS (Wolfe et al., 2010).
Previously, researchers have suggested that FMS has less impact as duration and age increase (Cronan, Serber, Walen, & Jaffe, 2002; Wigers, 1996; Kennedy & Felson, 1996). This is reasonable given the consensus that FMS is not a progressive disorder (Kennedy & Felson, 1996). However, these effects could be mediated or moderated by various factors. For example, greater age and symptom duration may produce more effective strategies for coping with complications that arise from FMS. Or, continued exposure might result in habituation after prolonged pain (cf. Smith et al., 2008). In either case, social-cognitive processes, such as self-efficacy, may moderate the effect of symptom duration on pain experiences (Smith et al., 2008; Edwards, Bingham III, & Haythornthwaite, 2006).
Self-efficacy—one’s beliefs about his or her ability to accomplish a given objective (Bandura, 1977)— may predict adjustment to FMS and present a mechanism through which positive change could be effected. Self-efficacy is related to the primary symptoms of FMS: pain, depression, and disability (Arnstein, Caudill, Mandle, Norris, & Beasley, 1999; Denison, Åsenlöf, & Lindberg, 2004; Keefe, Lefebvre, Maixner, Salley Jr, & Caldwell, 1997; Smarr et al., 1997). A strong sense of self-efficacy increases resilience and minimizes susceptibility to stress and depression in FMS (Bandura, 2001; Sahar, Thomas, & Clarke, 2016). Self-efficacy may buffer the relationships between health and perceived stress, stress vulnerability, and negative life events in pain populations. Self-efficacy was a more important determinant of disability than pain intensity or duration among primary care patients reporting musculoskeletal pain (Asghari, Julaeiha, & Godarsi, 2008). It also has been identified as a more important predictor of physical functioning than re-injury or pain-related beliefs among chronic low back pain sufferers (Lorig, Chastain, Ung, Shoor, & Holman, 1989). Self-efficacy predicts long-term disability and pain behavior over and above pain, distress, and personality factors (Asghari & Nicholas, 2001), and it was a significant determinant of depression and disability, even after controlling for pain intensity and demographic variables, among chronic pain patients (Asghari et al., 2008).
The present study aimed to examine the extent to which self-efficacy affects FMS impact over time. We hypothesized that the effects of symptom duration on physical impairment and pain in FMS would be moderated by self-efficacy, such that higher self-efficacy would predict improved outcomes controlling for depression symptomology (Asghari & Nicholas, 2001; Asghari et al., 2008), exercise habit (Fink & Lewis, 2017), history of abuse (the only traumas measured in the study; Smith et al., 2010), and age (Carstensen, Pasupathi, Mayr, & Nesselroade, 2000; Shallcross et al., 2013; Yezierski, 2012) given their established relationships with the outcomes.
Method
Participants
The original randomized clinical trial included 572 female members of a health maintenance organization [HMO]. For participant demographics, see Table 1. All participants reviewed and signed an informed consent and were treated in accordance with American Psychology Association (2010) ethical guidelines. The original study was approved by the University and HMO Institutional Review Boards (IRBs).
Table 1.
Participant Demographics
| Item | Valid % | N |
|---|---|---|
| Ethnicity | ||
| White | 85.11 | 486 |
| Non-White | 14.89 | 85 |
| Age | ||
| 18 to 30 years | 2.44 | 14 |
| 31 to 50 years | 37.17 | 213 |
| 51 to 65 years | 44.33 | 254 |
| 66 years or older | 16.06 | 92 |
| Education | ||
| High School Graduate or Less | 19.06 | 109 |
| Associate’s Degree/Trade School | 50.70 | 290 |
| Bachelor’s Degree | 15.21 | 87 |
| Graduate Level Degree | 15.03 | 86 |
| Income | ||
| Less than $10,000 | 5.06 | 29 |
| $10,001-$20,000 | 10.82 | 62 |
| $20,001-$30,000 | 15.53 | 89 |
| $30,001-$40,000 | 21.47 | 123 |
| $40,001-$50,000 | 15.18 | 87 |
| $50,001-$60,000 | 9.60 | 55 |
| $60,001-$70,000 | 6.63 | 38 |
| $70,001 or more | 12.57 | 72 |
| Decline to state | 3.14 | 18 |
| Employment Status | ||
| Full-Time | 32.64 | 91 |
| Part-Time | 15.88 | 187 |
| Unemployed | 7.85 | 45 |
| Retired | 22.86 | 131 |
| Disabled | 11.34 | 65 |
| Homemaker | 9.08 | 52 |
| Student | 0.35 | 2 |
| Relationship Status | ||
| Single | 10.30 | 59 |
| Married/Remarried | 63.87 | 366 |
| Widow | 4.89 | 28 |
| Separated | 1.4 | 8 |
| Divorced | 19.55 | 112 |
Measures
Self-Efficacy.
Perceived self-efficacy was measured using the Arthritis Self-Efficacy Scale (ASES; Lorig et al., 1989) with the term “arthritis” changed to “fibromyalgia.” This 20-item, self-administered scale measures participants’ confidence in their ability to perform specific tasks, such as decreasing their pain or walking 100 feet on flat ground in 20 seconds. It uses a scale ranging from 0 (very uncertain) to 100 (very certain) and yields three subscale scores: self-efficacy for pain, function, and other symptoms. Subscale scores were summed to create a total self-efficacy score. In the present study, the scale showed high internal consistency (α = .92, n = 571), and test-retest reliability (ICC = .608).
Depression.
The Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977) is a 20-item, self-administered assessment of depression symptomatology. It uses a 4-point Likert-type scale ranging from 0 (rarely or none of the time) to 3 (most or all of the time). Scores can range from 0 to 60, with ≥ 19 indicating depressed mood for chronic pain populations (Turk & Okifuji, 1994). In the present study, the CES-D was moderately consistent (.625 ≤ α ≤ .719, 464 ≤ n ≤ 572) and stable over time, ICC = .490. Depression and ASES were significantly correlated at all time points, −.5501 ≤ r ≤ −.4912, ps < .001.
Symptom Duration.
Symptom duration was measured through a medical history in which participants were asked in which year they first started experiencing FMS symptoms. Participants reported a mean of 13.91 years of symptoms (SD = 13.20, Min = 0, Max = 66). Participants’ ages were significantly correlated with symptom durations, r = .315, p < .001.
History of Abuse.
Participants reported whether they had experienced physical, emotional, or sexual abuse (1 = No, 2 = Yes) at baseline. In our sample, 17.28% (n = 99) reported a history of physical abuse, 16.93% (n = 97) reported a history of sexual abuse, and 46.6% (n = 267) reported a history of emotional abuse. The correlations among these were statistically significant, ps < .001: physical and emotional, r = .47; physical and sexual, r = .51; emotional and sexual, r = .41.
Exercise.
Participants reported whether they had exercised in the past two weeks (1 = No, 2 = Yes). The following prevalence of exercise was observed across the respective time points: 78.5% (n = 449), 73.85% (n = 353), 75.96% (n = 357), and 73.71% (n= 342), ICC = .400.
Fibromyalgia Impact.
Fibromyalgia impact was measured using the Fibromyalgia Impact Questionnaire (FIQ). The FIQ is a self-administered questionnaire that includes 19 items that assess the impact of FMS on physical functioning, psychological symptoms, social activities, and global wellbeing in people (Burckhardt, Clark, & Bennett, 1991). For the present study, only the physical function subscale was used to remove any undue overlap that may exist between the other subscales and the predictors in the model (e.g., CES-D and psychological symptoms). It consists of 10 items that measure functioning or physical impairment in everyday tasks during the past week (e.g., preparing meals and doing laundry; Bennett, 2005). The FIQ has demonstrated reliability (.56 ≤ r ≤ .95 for function) and validity for people with FMS (Burkhardt et al., 1991). The physical function subscale had excellent internal consistency (α = .91) and good re-test reliability, ICC = .578.
Pain.
The Present Pain Intensity (PPI) index from the McGill Pain Questionnaire (MPQ; Melzack, 1975) was used to assess pain. Participants were asked to rate their pain using a 6-point scale, where 0 = no pain, 1 = mild, 2 = discomforting, 3 = distracting, 4 = horrible, and 5 = excruciating. The short-form MPQ (0–5 vs. 0–10) PPI scale was designed to accelerate administration while maintaining accuracy (Dworkin et al., 2009, Melzack, 1987). Test–retest reliability for the PPI item was fair, ICC = .325, and reasonable given it measures present pain. The FIQ Physical Subscale and MPQ PPI were significantly correlated at all time points, .4522 ≤ r ≤ .5027, ps < .001.
Procedure
Participants from the HMO were recruited using a variety of methods including newspaper advertisements, mass mailings, flyers in physicians’ offices, and physician referrals. To be eligible for the study, participants had to be diagnosed by a physician and meet the ACR’s 1990 diagnostic criteria for FMS (Wolfe et al., 1990, cf. Wolfe et al., 2010). At an initial interview, informed consent was obtained, and a trained examiner performed a manual tender point exam to confirm the diagnosis. At the initial assessment, participants completed questionnaires. Then participants were randomly assigned. The intervention arms included control (no treatment), social support (group meetings), and social support plus education (self-management strategies). No intervention effects were found in the original study (Oliver, Cronan, Walen & Tomita, 2001).
Analytic Strategy
First, linear regressions were performed to assess the effects of age, symptom duration, ASES, CES-D, history of abuse, and exercise on FIQ and MPQ PPI scores at baseline (pre-intervention). Symptom duration and ASES were centered to create an interaction term. Finally, an interaction of ASES by age was entered the same way.
To explore these processes using a longitudinal approach, linear growth models were performed in Stata 12.1 using data from baseline, 6 months, 12 months, and 18 months. Random intercepts and slopes and an unstructured covariance matrix were specified. The first models explored the fixed effects of time, ASES, CES-D, exercise in the past two weeks, age at baseline, baseline symptom duration, presence of abuse reported at baseline, and intervention arm. The second models included an interaction of time by ASES on FIQ and MPQ PPI scores.
Results
The linear regressions performed to assess the effects of age, symptom duration, ASES, CES-D, history of abuse, and exercise on FIQ scores at baseline were statistically significant (see Table 2). The main effects of age and ASES demonstrated that, holding other predictors constant, as individuals became older and had higher ASES scores, they were predicted to have less FMS physical impairment. The main effect of depression revealed that as depression increased, FMS physical impairment increased. The interaction of symptom duration by ASES showed that, controlling for other variables, a longer duration of symptoms predicted lower FIQ scores only for those who had ASES scores above the mean (see Figure 1). The effect of symptom duration on FIQ was nearly zero at the mean of ASES, and the effect of symptom duration on FIQ became positive when ASES was below the mean. Thus, greater symptom duration predicted less FMS physical impairment only for those with above-average efficacy; for those with below-average efficacy greater FMS physical impairments were predicted. For the distribution of ASES scores in the present sample, see Figure 2.
Table 2.
Baseline Regression Models for Physical Functioning (FIQ Physical Function Subscale)
| Main Effects Model | Interaction 1 | Interaction 2 | ||||
|---|---|---|---|---|---|---|
| F(8, 561) = 101.11, p < .001, R2 = .59 | F(9, 560) = 91.57, p < .001, R2 = .60 | F(10, 559) = 83.20, p < .001, R2 = .60 | ||||
| Effect | B | p | B | p | B | p |
| Age | −.1426 | .001* | −.1423 | .001* | .1054 | .433 |
| ASES | −.2912 | < .001* | −.2216 | < .001* | .0137 | .914 |
| CES-D | .7534 | < .001* | .7542 | < .001* | .7631 | < .001* |
| Symptom Duration | .0082 | .816 | .2740 | .011* | .2297 | .036* |
| Physical Abuse | 1.2060 | .403 | 1.1832 | .409 | 1.0979 | .446 |
| Sexual Abuse | −.5077 | .716 | −.4302 | .757 | −.4139 | .765 |
| Emotional Abuse | −.8308 | .426 | −.8132 | .434 | −.7904 | .446 |
| Fortnightly Exercise | 1.2121 | .263 | 1.1003 | .308 | .9649 | .371 |
| Symptom Duration × ASES | −.0047 | .009* | −.0039 | .035* | ||
| Age × ASES | −.0045 | .053 | ||||
Note: FIQ = Fibromyalgia Impact Questionnaire; ASES = Arthritis Self-Efficacy Scale; CES-D = Center for Epidemiological Study Depression Scale.
p ≤ .05
Figure 1.
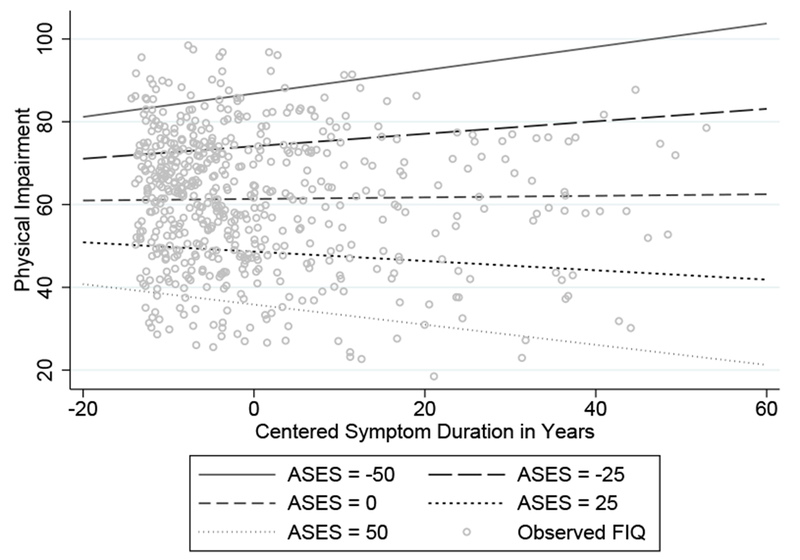
The effects of symptom duration by self-efficacy on Fibromyalgia Impact questionnaire.
Figure 2.
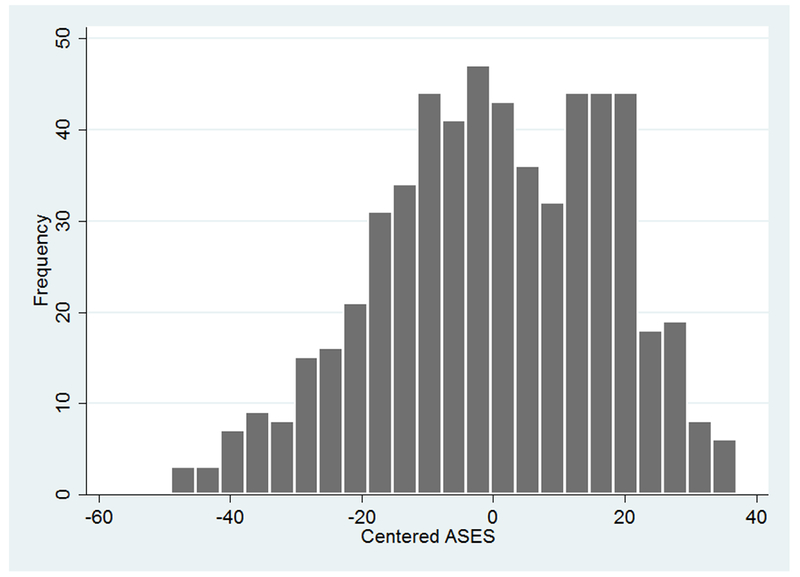
Distribution of Arthritis-Specific Self-Efficacy Scores.
The linear regressions performed to assess the effects of age, symptom duration, ASES, CES-D, history of abuse, and exercise on MPQ PPI scores at baseline were statistically significant (see Table 3). The main effect of ASES demonstrated that, holding other predictors constant, as individuals had higher ASES scores, they were predicted to have less pain intensity. The effects of symptom duration and the interaction were not significant. Among covariates, only CES-D was a significant and positive predictor of pain intensity.
Table 3.
Baseline Regression Models for Pain (McGill PPI)
| Main Effects Model | Interaction 1 | Interaction 2 | ||||
|---|---|---|---|---|---|---|
| F(8, 561) = 11.57, p < .001, R2 = .14 | F(9, 560) = 10.36, p < .001, R2 = .14 | F(10, 559) = 9.46, p < .001, R2 = .14 | ||||
| Effect | B | p | B | p | B | p |
| Age | −.0009 | .780 | −.0009 | .782 | .0108 | .309 |
| ASES | −.0077 | .001* | −.0060 | .054 | .0052 | .610 |
| CES-D | .0195 | < .001* | .0195 | < .001* | .0199 | < .001* |
| Symptom Duration | .0014 | .612 | .0081 | .343 | .0059 | .493 |
| Physical Abuse | .2035 | .073 | .2030 | .074 | .1987 | .080 |
| Sexual Abuse | −.0366 | .739 | −.0346 | .752 | −.0339 | .758 |
| Emotional Abuse | −.0545 | .506 | −.0541 | .510 | −.0530 | .518 |
| Fortnightly Exercise | .0193 | .820 | .0165 | .846 | .0101 | .906 |
| Symptom Duration × ASES | −.0001 | .408 | −.0001 | .586 | ||
| Age × ASES | −.0002 | .247 | ||||
Note: PPI = Present Pain Intensity; ASES = Arthritis Self-Efficacy Scale; CES-D = Center for Epidemiological Study Depression Scale.
p ≤ .05
For the longitudinal analyses, the overall models predicting FIQ scores were statistically significant (see Table 4). The main effect of ASES demonstrated that as self-efficacy went up FIQ scores decreased. As CES-D scores increased, so did FIQ scores. There was no main effect of age on FIQ in the presence of the other variables; however, there was a main effect of exercise, such that across all assessments endorsement of fortnightly exercise predicted significantly lower mean levels of physical impairment. There was a main effect of time; but the marginal effect was not significant. However, the moderating effect of self-efficacy demonstrated that increases in impairment occurred for those low in self-efficacy; however, at the highest levels of self-efficacy, impairment scores decreased significantly over time (see Figure 3).
Table 4.
Longitudinal Multilevel Models for Physical Function (FIQ Physical Function Subscale)
| Main Effects Model | Interaction Model | |||||
|---|---|---|---|---|---|---|
| Wald χ2(11) = 1678.48, p < .001 | Wald χ2(12) = 1691.58, p < .001 | |||||
| Fixed Effect | B | p | B | p | Marginal B | p |
| Intervention Arm | ||||||
| Control v SS | −1.1936 | .176 | −1.2176 | .167 | - | - |
| Control v Combo | −1.2852 | .143 | −1.2779 | .144 | - | - |
| Age | −.1530 | < .001* | −.1550 | < .001* | - | - |
| ASES | −.2757 | < .001* | −.2440 | < .001* | −.2842 | < .001 |
| CES-D | .7121 | < .001* | .7158 | < .001* | - | - |
| Symptom Duration | .0353 | .216 | .0348 | .222 | - | - |
| Time | .1025 | .630 | 1.8200 | .014* | .1195 | .571 |
| Physical Abuse | 1.8392 | 1.1645 | 1.8767 | .106 | - | - |
| Sexual Abuse | .3240 | 1.1323 | .3228 | .775 | - | - |
| Emotional Abuse | −.0754 | .928 | −.0571 | .946 | - | - |
| Fortnightly Exercise | 2.3253 | < .001* | 2.2767 | < .001* | - | - |
| Time × ASES | −.0284 | .015* | - | - | ||
Note: FIQ = Fibromyalgia Impact Questionnaire; SS = Social Support Group; Combo = Social Support plus Education Group; ASES = Arthritis Self-Efficacy Scale; CES-D = Center for Epidemiological Study Depression Scale.
p ≤ .05
Figure 3.
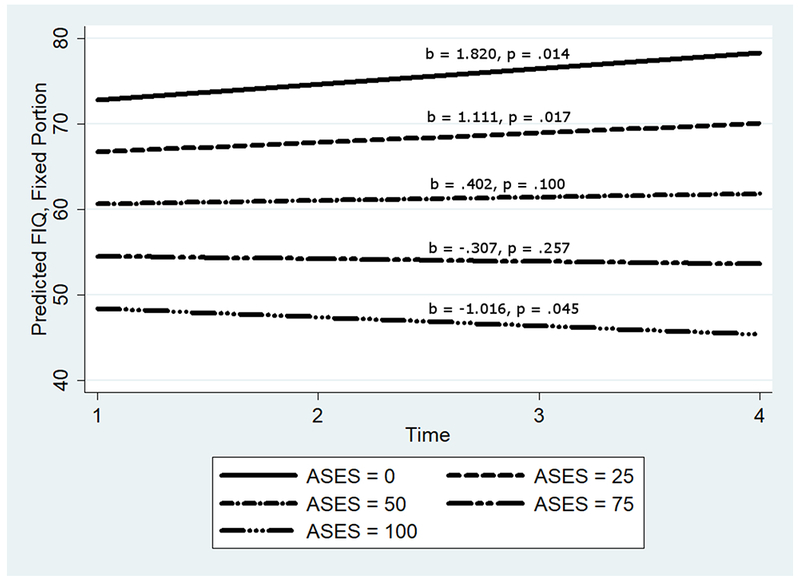
The longitudinal effects of time and self-efficacy on Fibromyalgia Impact Questionnaire.
The models for MPQ PPI scores were statistically significant (see Table 5). The main effect of ASES demonstrated that as efficacy increased McGill PPI scores decreased. As CES-D scores increased, so did PPI scores. There was no effect of age or exercise on PPI in the presence of the other variables; however, there was an effect of physical abuse history, such that endorsement physical abuse at baseline predicted significantly higher PPI scores. There was no significant main effect of time on pain experience, but the marginal effect showed significant decreases in pain over time. However, the interaction of efficacy and time demonstrated that although those with high self-efficacy experienced significant reductions in pain, those with low self-efficacy experienced non-significant increases in pain (see Figure 4).
Table 5.
Longitudinal Multilevel Models for Pain (McGill PPI)
| Main Effects Model | Interaction Model | |||||
|---|---|---|---|---|---|---|
| Wald χ2(11) = 279.28, p < .001 | Wald χ2(12) = 287.19, p < .001 | |||||
| Fixed Effect | B | p | B | p | Marginal B | p |
| Intervention Arm | ||||||
| Control v SS | .0283 | .641 | .0277 | .648 | - | - |
| Control v Combo | .0271 | .653 | .0276 | .646 | - | - |
| Age | −.0008 | .721 | −.0010 | .669 | - | - |
| ASES | −.0126 | < .001* | −.0097 | < .001* | −.0129 | < .001 |
| CES-D | .0130 | < .001* | .0132 | < .001* | - | - |
| Symptom Duration | .0008 | .684 | .0007 | .717 | - | - |
| Time | −.0406 | .010* | .0929 | .093 | −.0402 | .010 |
| Physical Abuse | .2226 | .006* | .2225 | .005* | - | - |
| Sexual Abuse | .0267 | .733 | .0259 | .739 | - | - |
| Emotional Abuse | −.0282 | .626 | −.0281 | .626 | - | - |
| Fortnightly Exercise | −.0013 | .978 | −.0056 | .902 | - | - |
| Time × ASES | −.0022 | .012* | - | - | ||
Note: PPI = Present Pain Intensity; SS = Social Support Group; Combo = Social Support plus Education Group; ASES = Arthritis Self-Efficacy Scale; CES-D = Center for Epidemiological Study Depression Scale.
p ≤ .05
Figure 4.
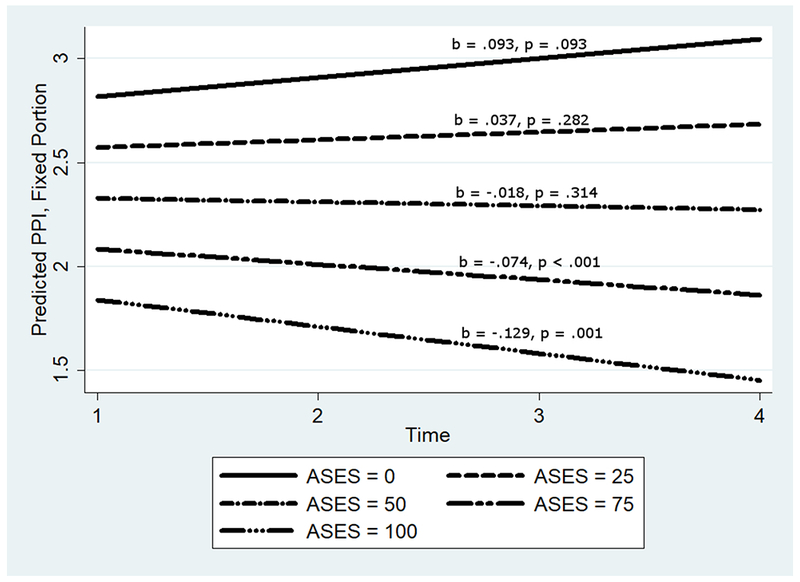
The longitudinal effects of time and self-efficacy on McGill present pain intensity.
Discussion
The results from this study provide further evidence of the relationship between age, symptom duration, psychosocial processes, and fibromyalgia physical impairment (Cronan et al., 2002; Kennedy & Felson, 1996, Wigers, 1996). This study corroborates research in FMS indicating that understanding the multifactorial nature of the disorder is crucial (Alegre de Miquel et al., 2010; Giesecke et al., 2003; Malt et al., 2002; Turk et al., 1996; Turk et al., 1998). Consistent with the ACR’s provisional diagnostic criteria (Wolfe et al., 2010), the analysis supports the importance personal and illness histories and psychological characteristics in FMS.
The prominence of self-efficacy in determining positive health outcomes has been documented for FMS. Symptom self-efficacy has been shown to significantly affect the relationships between self-reported pain and physical functionality, anxiety, and depression (Miró, Martínez, Sánchez, Prados, & Medina, 2011; Van Liew, Brown, Cronan, Bigatti, & Kothari, 2013). Puente et al. (2015) found that self-efficacy affected emotional outcomes and self-report measures of pain but noted that pressure induced pain measures were not affected. This is indicative of the complex role that metacognitions play in determining pain and function in FMS (Kollmann, Gollwitzer, Spada, & Fernie, 2016). Our study extends this knowledge by demonstrating that self-efficacy predicted—both cross-sectionally and longitudinally—wholly unique functional trajectories by moderating symptom duration (or time, longitudinally) on the impact of FMS. For those with above-average self-efficacy, improvements were expected with increased symptom duration or over time; however, for those below average in self-efficacy, increased symptom duration predicted worsening self-reported pain and functioning. Interestingly, the effect of time and the interaction of time by self-efficacy was significant longitudinally, but not cross-sectionally, for pain. The marginal slope showed that on average participants experienced decreases in pain throughout the course of the study. Although it is possible that this difference reflects a placebo (attentional) effect or regression to the mean, the moderation by self-efficacy provides valuable insight even if these are the reasons for the improvement.
Additionally, although symptom duration and age were correlated, their effects on FMS physical impairment and pain appear to be unique. The effect of age on physical impairment, but not pain, was negative and statistically significant beyond the effects of symptom duration. Unlike symptom duration, the effect of age was not moderated by self-efficacy. Longitudinally, the moderation of the effect of time cannot be distinguished as “symptom duration” or “age” per se; however, the baseline models suggest that the moderation effect is stronger for symptom duration. This is reasonable as the cumulative strain on coping resources would be amassed more directly as time with symptoms increased, not age.
Although it may be tempting to consider differences in functioning measured by the FIQ as being attributed to a lack of FMS specificity, greater age predicted less functional issues suggesting that general changes in functioning because of age are not a plausible explanation. It is possible that symptom duration reflected some form of disease course in FMS, but this seems unlikely given other studies have not revealed significant changes in pain over time in FMS (Felson & Goldenberg, 1986; Haviland, Banta, & Przekop, 2011; Kennedy & Felson, 1996; Noller & Sprott, 2003). The effect of age on physical impairment could reflect differences in neural pain signaling across age groups (Yezierski, 2012), the psychological impacts of negative stimuli or negative emotional experience across the lifespan (Carstensen et al., 2000; Noller & Sprott, 2003; Shallcross et al., 2013), or, possibly, disease progression as a function of age of onset. However, if differences in pain processing were the mechanism, it seems unlikely that the relationship with pain would not be significant. Researchers have shown that aging predicts decreased negative affect (Kratz et al., 2007), greater levels of acceptance (Shallcross et al., 2013), and acceptance has been found to buffer the effects of pain on negative affect in FMS (Kratz, Davis, & Zautra, 2007). In FMS, quality of life and health status satisfaction have been found to improve over time despite unchanging pain (Noller & Sprott, 2003).
Lastly, exercise predicted greater improvements in function longitudinally, but not cross-sectionally, and baseline reports of physical abuse predicted higher pain longitudinally, but not cross-sectionally. The effect of physical abuse was marginally significant in the baseline model, so it seems reasonable that this difference is a result of increased power in the longitudinal model (Hedeker & Gibbons, 2006). With respect to exercise, power alone does not seem to explain the difference. Research in FMS has revealed significant effects of exercise on functioning longitudinally (Da Costa et al., 2005; Sañudo, Carrasco, de Hoyo, & McVeigh, 2012), but it is interesting that these differences emerged as significant in our sample only when differences were considered over time.
Researchers should continue to explore the intricacies of age, psychosocial (e.g., affect and acceptance) and behavioral (e.g., exercise) factors, symptom duration, and self-efficacy simultaneously to further explicate these processes. The PPI index from the MPQ was administered in this FMS-specific study, but it is possible that participants reported pain other than FMS-specific pain. Future studies should investigate the relationship between self-efficacy and objective pain, which seems to behave distinctly in these processes (Puente et al., 2015). Researchers could also attempt an ecological momentary assessment of pain approach to exploring these relationships. Finally, although the sample reflects typical FMS sufferers, future studies could examine these relationships within different demographics, as our sample was mostly White and middle-aged and exclusively female.
Acknowledgments
The original study was funded by NIH grant AR-40423
Glossary
- FMS
Fibromyalgia Syndrome
- ACR
American College of Rheumatology
- HMO
Health Maintenance Organization
- IRB
Institutional Review Boards
- ASES
Arthritis Self-Efficacy Scale
- CES-D
Center for Epidemiologic Studies Depression Scale
- FIQ
Fibromyalgia Impact Questionnaire
- PPI
Present Pain Intensity
- MPQ
McGill Pain Questionnaire
Footnotes
There are no conflicts of interest to report.
References
- Alegre de Miquel C, García Campayo J, Tomás Flórez M, Gómez Arguelles JM, Blanco Tarrio E, Gobbo Montoya M, Gómez de la Cámara A (2010). Interdisciplinary consensus document for the treatment of fibromyalgia. Actas Españolas de Psiquiatria, 38(10), 108–120. [PubMed] [Google Scholar]
- Arnstein P, Caudill M, Mandle CL, Norris A, & Beasley R (1999). Self-efficacy as a mediator of the relationship between pain intensity, disability and depression in chronic pain patients. Pain, 80(4), 83–91. [DOI] [PubMed] [Google Scholar]
- Asghari A, & Nicholas MK (2001). Pain self-efficacy beliefs and pain behaviour. A prospective study. Pain, 94(1), 85–100. doi: 10.1016/S0304-3959(01)00344-X [DOI] [PubMed] [Google Scholar]
- Asghari A, Julaeiha S, & Godarsi M (2008). Disability and depression patients with chronic pain: pain or pain-related beliefs?. Archives of Iranian Medicine, 11(3), 263–269. doi:08113/AIM.006 [PubMed] [Google Scholar]
- Bandura A (1977). Self-efficacy: Toward a unifying theory of behavioral change. Psychological Review, 84(2), 191–215. [DOI] [PubMed] [Google Scholar]
- Bandura A (2001). Social cognitive theory: An agentic perspective. Annual Review of Psychology, 52, 1–26. doi: 10.1146/annurev.psych.52.1.1 [DOI] [PubMed] [Google Scholar]
- Beal C, Stuifbergen A & Brown A (2009). Predictors of a health promoting lifestyle in women with Fibromyalgia syndrome. Psychology, Health, & Medicine, 14(3), 343–353. doi: 10.1080/13548500902730093 [DOI] [PubMed] [Google Scholar]
- Bennett R (2005). The Fibromyalgia Impact Questionnaire (FIQ): A review of its development, current version, operating characteristics and uses. Clinical and Experimental Rheumatology, 23, 154–62. [PubMed] [Google Scholar]
- Bennett RM, Jones J, Turk DC, Russell IJ, & Matallana L (2007). An internet survey of 2,596 people with fibromyalgia. BMC Musculoskeletal Disorders, 8(1), 27–37. doi: 10.1186/1471-2474-8-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley LA (2009). Pathophysiology of fibromyalgia. American Journal of Medicine, 122(Suppl 12), 22–34. doi: 10.1016/j.amjmed.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt CS, Clark SR, & Bennett RM (1991). The fibromyalgia impact questionnaire: Development and validation. Journal of Rheumatology, 18(5), 728–33. [PubMed] [Google Scholar]
- Byrne M, Troy A, Bradley LA, Marchisello PJ, Geisinger KF, Van der Heide LH, & Prieto EJ (1982). Cross-validation of the factor structure of the McGill Pain Questionnaire. Pain, 13(2), 193–201. [DOI] [PubMed] [Google Scholar]
- Carstensen LL, Pasupathi M, Mayr U & Nesselroade JR (2000). Emotional experience in everyday life across the adult life span. Journal of Personality and Social Psychology, 79(4), 644–655. [PubMed] [Google Scholar]
- Cronan TA, Serber ER, Walen HR, & Jaffe M (2002). The influence of age on fibromyalgia symptoms. Journal of Aging and Health, 14(3), 370–384. doi: 10.1177/08964302014003004 [DOI] [PubMed] [Google Scholar]
- Da Costa D, Abrahamowicz M, Lowensteyn I, Bernatsky S, Dritsa M, Fitzcharles MA, & Dobkin PL (2005). A randomized clinical trial of an individualized home-based exercise programme for women with fibromyalgia. Rheumatology, 44(11), 1422–1427. [DOI] [PubMed] [Google Scholar]
- Denison E, Åsenlöf P, & Lindberg P (2004). Self-efficacy, fear avoidance, and pain intensity as predictors of disability in subacute and chronic musculoskeletal pain patients in primary health care. Pain, 111(3), 245–252. doi: 10.1016/j.pain.2004.07.001 [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Revicki DA, Harding G, Coyne KS, Peirce-Sandner S, … Melzack R (2009). Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain, 144(1-2), 35–42. doi: 10.1016/j.pain.2009.02.007 [DOI] [PubMed] [Google Scholar]
- Edwards RR, Bingham III JB, & Haythornthwaite JA (2006). Catastrophizing and pain in arthritis, fibromyalgia, and other rheumatic diseases. Arthritis & Rheumatology, 55(3), 325–332. doi: 10.1002/art.21865 [DOI] [PubMed] [Google Scholar]
- Fink L, & Lewis D (2017). Exercise as a Treatment for Fibromyalgia: A Scoping Review. Journal for Nurse Practitioners, 13(8), 546–551. doi: 10.1016/j.nurpra.2017.06.018 [DOI] [Google Scholar]
- Felson DT & Goldenberg DL (1986). The natural history of fibromyalgia. Arthritis and Rheumatology, 29(12), 1522–1526. [DOI] [PubMed] [Google Scholar]
- Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, Clauw DJ (2003). Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis & Rheumatology, 48(10), 2916–2922. doi: 10.1002/art.11272 [DOI] [PubMed] [Google Scholar]
- Goldenberg DL, Burckhardt C, & Crofford L (2004). Management of fibromyalgia syndrome. Journal of the American Medical Association, 292(19), 2388–2395. doi: 10.1001/jama.292.19.2388 [DOI] [PubMed] [Google Scholar]
- Harrison AL (2004). The influence of pathology, pain, balance, and self-efficacy on function in women with osteoarthritis of the knee. Physical Therapy, 84(8), 822–831. doi: 10.1093/ptj/84.9.822 [DOI] [PubMed] [Google Scholar]
- Hassett AI, Cone JD, Patella SJ, & Sigal LH (2000). The role of catastrophizing in the pain and depression of women with fibromyalgia syndrome. Arthritis & Rheumatology, 43(11), 2493–2500. doi: [DOI] [PubMed] [Google Scholar]
- Häuser W, Kosseva M, Üceyler N, Klose P, & Sommer C (2011). Emotional, physical, and sexual abuse in fibromyalgia syndrome: A systematic review with meta analysis. Arthritis Care & Research, 63(6), 808–820. [DOI] [PubMed] [Google Scholar]
- Haviland MG, Banta JE & Przekop P (2011). Fibromyalgia: prevalence, course, and co-morbities in hospitalized patients in the United States, 1999-2007. Clinical and Experimental Rheumatology, 29, S79–87. [PubMed] [Google Scholar]
- Hedeker D, & Gibbons RD (2006). Longitudinal data analysis (Vol. 451). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Keefe FJ, Lefebvre JC, Maixner W, Salley AN Jr, & Caldwell DS (1997). Self-efficacy for arthritis pain: Relationship to perception of thermal laboratory pain stimuli. Arthritis Care and Research, 10(3), 177–184. [DOI] [PubMed] [Google Scholar]
- Kennedy M, & Felson DT (1996). A prospective long-term study of fibromyalgia syndrome. Arthritis & Rheumatology, 39(4), 682–685. [DOI] [PubMed] [Google Scholar]
- Kollmann J, Gollwitzer M, Spada M & Fernie B (2016). The association between metacognitions and the impact of Fibromyalgia in a German sample. Journal of Psychosomatic Research, 83, 1–9. doi: 10.1016/j.jpsychores.2016.02.002 [DOI] [PubMed] [Google Scholar]
- Kratz AL, Davis MC, & Zautra AJ (2007). Pain acceptance moderates the relation between pain and negative affect in osteoarthritis and fibromyalgia patients. Annals of Behavioral Medicine, 33(3), 291–301. doi: 10.1080/08836610701359860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JM, Carosella AM, & Feuerstein M (1996). Pain expectancies, pain, and functional self-efficacy expectancies as determinants of disability in patients with chronic low back disorders. Journal of Consulting and Clinical Psychology, 64(1), 212–220. [DOI] [PubMed] [Google Scholar]
- Lorig K, Chastain RL, Ung E, Shoor S & Holman HR (1989). Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis & Rheumatology, 31(1), 37–44. [DOI] [PubMed] [Google Scholar]
- Malt EA, Olafsson S, Lund A, & Ursin H (2002). Factors explaining variance in perceived pain in women with fibromyalgia. BMC Musculoskeletal Disorders, 3, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R (1975). The McGill Pain Questionnaire: Major properties and scoring methods. Pain, 1(3), 277–99. [DOI] [PubMed] [Google Scholar]
- Melzack R (1987). The short-form McGill Pain Questionnaire. Pain, 30(2), 191–197. [DOI] [PubMed] [Google Scholar]
- Miró E, Martínez M, Sánchez A, Prados G & Medina A (2011). When is pain related to emotional distress and daily functioning in fibromyalgia syndrome? The mediating roles of self-efficacy and sleep quality. British Journal of Health Psychology, 16(4), 799–814. doi: 10.1111/j.2044-8287.2011.02016.x [DOI] [PubMed] [Google Scholar]
- Noller V & Sprott H (2003). Prospective epidemiological observations on the course of the disease in fibromyalgia patients. Journal of Negative Results in BioMedicine, 2(4), 1–6. doi: 10.1186/1477-5751-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary A (1992). Self-efficacy and health: Behavioral and stress-physiological mediation. Cognitive Therapy and Research, 16(2), 229–245. [Google Scholar]
- Oliver K, Cronan TA, Walen HR, & Tomita M (2001). Effects of social support and education on health care costs for patients with fibromyalgia. Journal of Rheumatology, 28(12), 2711–2719. [PubMed] [Google Scholar]
- Puente CP, Furlong LV, Gallardo CE, Méndez MC, Cruz DB & Fernández-de-las-Peñas C (2015). Self-efficacy and affect as mediators between pain dimensions and emotional symptoms and functional limitation in women with fibromyalgia. Pain Management Nursing, 16(1), 60–68. doi: 10.1016/j.pmn.2014.04.005 [DOI] [PubMed] [Google Scholar]
- Radloff L (1977). The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- Recla JM (2010). New and emerging therapeutic agents for the treatment of fibromyalgia: An update. Journal of Pain Research, 3, 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar K, Thomas SA, & Clarke SP (2016). Adjustment to fibromyalgia: The role of domain-specific self-efficacy and acceptance. Australian Journal of Psychology, 68(1), 29–37. doi: 10.1111/ajpy.12089 [DOI] [Google Scholar]
- Sañudo B, Carrasco L, de Hoyo M, & McVeigh JG (2012). Effects of exercise training and detraining in patients with fibromyalgia syndrome: a 3-yr longitudinal study. American Journal of Physical Medicine & Rehabilitation, 91(7), 561–573. [DOI] [PubMed] [Google Scholar]
- Shallcross AJ, Ford BQ, Floerke VA, & Mauss IB (2013). Getting better with age: The relationship between age, acceptance, and negative affect. Journal of Personality and Social Psychology, 104(4), 734–749. doi: 10.1037/a0031180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S, Sadosky A, Evans C, Yeh Y, Alvir JMJ, & Zlateva G (2010). Toward characterization and definition of fibromyalgia severity. BMC Musculoskeletal Disorders, 11, 66–74. doi: 10.1186/1471-2474-11-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr KL, Parker JC, Wright GE, Stucky-Ropp RC, Buckelew SP, Hoffman RW, Hewett JE (1997). The importance of enhancing self-efficacy in rheumatoid arthritis. Arthritis Care and Research, 10(1), 18–26. [DOI] [PubMed] [Google Scholar]
- Smith B, Papp Z, Tooley E, Montague E, Robinson A, & Cosper C (2010). Traumatic events, perceived stress and health in women with fibromyalgia and healthy controls. Stress & Health: Journal of the International Society for the Investigation of Stress, 26(1), 83–93. doi: 10.1002/smi.1269 [DOI] [Google Scholar]
- Smith BW, Tooley EM, Montague EQ, Robinson AE, Cosper CJ, & Mullins PG (2008). Habituation and sensitization to heat and cold pain in women with fibromyalgia and healthy controls. Pain, 140(3), 420–428. doi: 10.1016/j.pain.2008.09.018 [DOI] [PubMed] [Google Scholar]
- Turk DC & Okifuji A (1994). Detecting depression in chronic pain patients: Adequacy of self-reports. Behavioral Research and Therapy, 32(1), 9–16. [DOI] [PubMed] [Google Scholar]
- Turk DC, Okifuji A, Sinclair JD, & Starz TW (1996). Pain, disability, and physical function in subgroups of patients with fibromyalgia. Journal of Rheumatology, 23(7), 1255–1562. [PubMed] [Google Scholar]
- Turk DC, Okifuji A, Sinclair JD, & Starz TW (1998). Differential responses by psychosocial subgroups of fibromyalgia syndrome patients to an interdisciplinary treatment. Arthritis Care and Research, 11(5), 397–404. [DOI] [PubMed] [Google Scholar]
- Van Liew C, Brown KC, Cronan TA, Bigatti SM & Kothari DJ (2013). Predictors of pain and functioning over time in Fibromyalgia syndrome: An autoregressive path analysis. Arthritis Care Research, 65(2), 251–256. doi: 10.1002/acr.21792. [DOI] [PubMed] [Google Scholar]
- Wigers SH (1996). Fibromyalgia outcome: the predictive values of symptom duration, physical activity, disability pension, and critical life events—a 4.5 year prospective study. Journal of Psychosomatic Research, 41(3), 235–243. [DOI] [PubMed] [Google Scholar]
- Wolfe F, Clauw DJ, Fitzcharles M, Goldenberg DL, Katz RS, Mease P, Yunus MB (2010). The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care & Research, 62(5), 600–610. doi: 10.1002/acr.20140 [DOI] [PubMed] [Google Scholar]
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Sheon RP (1990). The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: Report of the multicenter criteria committee. Arthritis & Rheumatology, 33(2), 160–172. [DOI] [PubMed] [Google Scholar]
- Yezierski RP (2012). The effects of age on pain sensitivity: Pre-clinical studies. Pain Medicine, 13(2), S27–36. doi: 10.1111/j.1526-4637.2011.01311.x [DOI] [PMC free article] [PubMed] [Google Scholar]


