Abstract
High mobility group box 1 (HMGB1) is a prototypical danger-associated molecular pattern molecule that is considered a late mediator of neuro-inflammation after traumatic brain injury (TBI). Prior studies have suggested that targeting HMGB1 may lead to neuroprotective effects, but none of these studies have reported cognitive outcomes. We hypothesized that loss of HMGB1 before and after TBI would markedly attenuate post-traumatic brain edema, blood–brain barrier (BBB) permeability, improve functional deficits and long-term neuropathology versus control mice. Using the controlled cortical impact model and conditional global HMGB1 knockout (HMGB1 KO) mice, we demonstrate that there was a neuroprotective effect seen in the HMGB1 KO versus wild-type control evidenced by a significant reduction in contusion volume. However, two surprising findings were 1) the lack of benefit on either post-traumatic brain edema or BBB permeability, and 2) that spatial memory performance was impaired in HMGB1 KO naïve mice such that the behavioral effects of HMGB1 deletion in uninjured naïve mice were similar to those observed after TBI. Our data suggest the possibility that the role of HMGB1 in TBI is a “double-edged sword”; that is, despite the benefits on selected aspects of secondary injury, the sustained absence of HMGB1 may impair cognitive function, even in naïve mice. Given the pleiotropic actions of extracellular and intracellular HMGB1, when evaluating the potential use of therapies targeting HMGB1, effects on long-term cognitive outcome should be carefully evaluated. It also may be prudent in future studies to examine cell-specific effects of manipulating the HMGB1 pathway.
Keywords: BBB permeability, cognitive impairment, HMGB1, traumatic brain injury
Introduction
Current estimates from the World Health Organization suggest that traumatic brain injury (TBI) will be the third leading cause of death and disability by the year 2020.1 In the last 2 decades, significant attention has been directed towards the innate immune response after TBI.2 It is now recognized that tissue damage can trigger the innate immune response via the release of danger-associated molecular pattern (DAMPs) molecules in TBI.3,4 Wang and colleagues5 first demonstrated the cytokine-like properties of high mobility group box 1 (HMGB1) and established HMGB1 as a prototype for endogenous danger signals or the so-called “Alarmins.”3,5,6 Alarmins were defined as endogenous intracellular molecules that 1) are released into the extracellular milieu during cell death or secreted by viable immune cells, 2) recruit and activate other immune cells, and 3) maintain homeostasis.3,7 In contrast to the early pro-inflammatory mediators, active secretion of HMGB1 from monocytes/macrophages begins 6–12 h after exposure to the inflammatory stimulus.8 Extracellular HMGB1 binds to the pattern recognition receptors such as the Toll-like receptor (TLR) 2, TLR4, and receptor for advanced glycation end products (RAGE),9,10 leading to the subsequent production of pro-inflammatory cytokines and chemokines.11 Both adult and pediatric patients demonstrate elevated levels of HMGB1 in cerebrospinal fluid (CSF) after severe TBI, supporting its role in inflammation after injury.12,13
Further corroboration was obtained from animal studies that revealed a significant reduction in cellular HMGB1 in contused brain tissue with increased expression of RAGE receptor 6 h after injury.14 A possible role of extracellular HMGB1 also was suggested by the findings of Okuma and colleagues, who noted a reduction in cerebral edema, reduced lesion volume, and improved motor function in brain-injured rats that were administered monoclonal antibodies targeting HMGB1.15 Similarly, administration of HMGB1 inhibitors led to a reduction in cerebral edema and cortical apoptotic cell death in rats subjected to TBI.16,17 Therefore, while the precise role of extracellular HMGB1 remains to be fully defined, it may represent a sub-acute or delayed mediator of neuroinflammation after TBI. To further elucidate the role of HMGB1 in TBI, we used inducible HMGB1 knockout (HMGB1 KO) mice where the HMGB1 gene was globally deleted after tamoxifen treatment. Given that prior studies using monoclonal antibodies or drugs to target HMGB1 suggested neuroprotective effects on both brain edema and neuronal death, we hypothesized that complete loss of HMGB1 before and after TBI would markedly attenuate early post-traumatic brain edema, blood–brain barrier (BBB) permeability, improve functional deficits and long-term neuropathology versus wild-type (WT) littermate control mice.
Methods
The Institutional Animal Care and Use Committee of the University of Pittsburgh School of Medicine approved the experiments used in this study. Inducible HMGB1 KO mice (n = 45) and age-matched WT littermate mice (n = 45) 16–20 weeks of age were allowed ad libitum food and water and were housed in controlled environmental conditions until the study began. Mice were apportioned to the two study protocols as described below (i.e., Study 1: assessment of markers of brain edema at 24 h after injury, or Study 2: assessment of long-term behavioral and neuropathological outcomes over 21 days).
Generation of inducible global HMGB1 KO mice
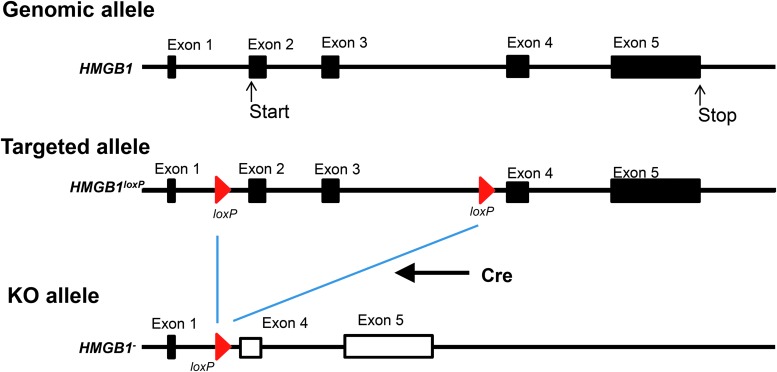
Inducible global HMGB1 KO mice were generated because embryonic deletion was shown to be lethal shortly after birth,18 preventing the in vivo functional study of the HMGB1 gene beyond this early age. The floxed HMGB1 mouse was generated as previously described.19 The targeted allele was designed to have two Lox/P elements inserted into introns 1 and 3. The flanking region of the two Lox/P elements includes exon 2, comprising the transcription initiation codon that covers most of the HMGB1 coding region. Before generating the chimeric mouse with the correct ES cell clones, the selection marker, Neo gene cassette, was removed by in vitro FRP recombination. Therefore, only two short Lox/P elements remained in the mouse genome. We confirmed that this modification of the genome did not affect HMGB1 gene expression.
Figure 1 illustrates the HMGB1 allele in wild type, floxed, and KO cells and shows the analysis of the floxed and KO mice. The strain used in this study was generated by introducing the inducible Cre transgene into HMGB1 floxed mice. The transgene is driven by a ubiquitously active CAG promoter and expresses a Cre and estrogen receptor fusion protein. This fusion protein changes its configuration upon binding to estrogen or an estrogen analog such as tamoxifen (TM) and translocates into the cell nucleus to excise the floxed gene. We confirmed that HMGB1 expression was intact in the inducible global HMGB1 KO mouse until TM administration. TM was administered to the mice by intraperitoneal injection and complete absence of HMGB1 protein expression in murine brain extracts was confirmed 6–7 weeks after TM administration. An identical approach was taken for the naïve mice assessed in the behavioral studies (see below). Although HMGB1 deletion in young mice (less than 3 weeks old) leads to death, deletion in young adult mice (6 weeks of age or older) was well tolerated.
FIG. 1.
The mouse high mobility group box 1 (HMGB1) gene loci for wild type, floxed, and knockout (KO) mice are shown. The HMGB1 protein is encoded by four exons and starts from exon 2. Lox/P sequences were placed in introns 1 and 3; therefore, Cre-mediated recombination deleted exons 2 and 3, including the initiation codon (ATG). The floxed HMGB1 mouse strain was established to produce a global, inducible HMGB1 KO mouse.
Controlled cortical impact model of TBI
All experiments were performed in accordance with guidelines in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh. TBI was performed using the controlled cortical impact (CCI) model in mice as previously described20–22 with minor modifications. Briefly, male mice (12 weeks of age) were anesthetized with 4% isoflurane (in 2:1 O2/N2O) for induction and then with 1–2% isoflurane throughout the surgical procedure via a nose cone. Animals were positioned in a stereotaxic frame, and a needle electrode (Physitemp Instruments [MT-29/1HT], Clifton, NJ) was inserted into the temporalis muscle ipsilateral to the injury to monitor brain temperature.23 A 5-mm craniotomy was performed over the left parietal cortex, the bone flap removed, and brain temperature was maintained at 37 ± 0.5°C for 5 min to confirm normothermia at the time of TBI. Body temperature also was monitored by a rectal probe (Physitemp Instruments, Clifton, NJ). Mice were then subjected to vertically directed CCI using a pneumatic cylinder with a 3-mm tip impounder at a velocity of 5 m/sec and a depth of 1.2 mm producing a moderate-severe level of injury with appreciable loss of CA1 hippocampal neurons and no mortality. This model was utilized for studies of brain edema and BBB permeability, and a severe CCI model (velocity 6 m/sec and a depth of 1.8 mm) was used for long-term outcome studies. Immediately after the injury, the bone flap was replaced, sealed with dental cement (Vernon Benshoff, Albany, NY), and the wound sutured closed. Sensorcaine was (0.25%) was applied topically to each wound site. Isoflurane was discontinued, and the mice were placed in an oxygen hood for 30 min, after which they were returned to their cages.
Study 1: Assessment of brain edema and BBB permeability over the initial 24 h after CCI
Assessment of brain edema
A total of 10 mice (n = 5 per genotype) were recovered after CCI, returned to their cages and then sacrificed at 24 h euthanized with isoflurane followed by decapitation and their brains were immediately removed and bisected into left and right hemispheres. Each hemisphere was immediately weighed (wet weight). Hemispheres were then placed in an oven, dehydrated at 110°C for 72 h, and re-weighed (dry weight). To quantify brain edema after TBI, percent brain water (%BW) content of injured and contralateral hemispheres was determined by the wet–dry weight method.
Assessment of BBB permeability
In separate mice, at 4 h after CCI (n = 10 per genotype) mice were re-anesthetized with isoflurane in oxygen, a femoral venous catheter was surgically placed, and Evans Blue (a 2% solution, 2.5 mL/kg, intravenous) was injected. After allowing the mice to recover for an additional 20 h after injection, they were anesthetized with isoflurane in oxygen followed by perfusion with ice cold heparinized normal saline. The brains were immediately removed and bisected into left and right hemispheres. Each hemisphere was immediately weighed. Subsequently, the hemispheres were extracted with 3 mL of N,N-dimethyl formamide at room temperature for 3 days to allow spectrophotometric quantification (at 620 nm) of the amount of Evans Blue in the tissue which reflects the extent of BBB permeability to albumin as previously described.24 BBB permeability was quantified in injured (left) and non-injured (right) brain hemispheres as micrograms of Evans Blue per gram of brain.
Study 2: Assessment of long-term behavioral and neuropathological outcomes over 21 days
A total of 40 mice were studied in this protocol. To determine if motor or cognitive performance was altered in HMGB1 KO mice versus WT, we evaluated the performance of both naïve and injured mice on these behavioral tasks. Mice were thus randomly divided into four groups WT (naïve), HMGB1 KO (naïve), WT (CCI) and HMGB1 KO (CCI), n = 10 per group, respectively.
Assessment of motor function and Morris water maze (MWM) performance
Analysis of motor function and MWM spatial memory acquisition was performed as previously described.21,24 For mice subjected to CCI, WT and inducible global HMGB1 KO mice underwent surgery at 6–7 weeks after administration of TM, following the CCI injury protocol exactly as described above (n = 10 in each group). For naïve animals, testing of WT and KO in the behavioral protocols (as described below) temporally matched the approach taken for the CCI groups relative to TM dosing. Motor function was assessed at Days 1–5 after injury using a beam balance task, with mice trained on Day 0 (pre-injury). Spatial memory acquisition was assessed using the MWM hidden platform task on Days 14–18 followed by probe trial. Performance in the MWM was quantified by measuring latency in finding the platform and the probe trial was quantified as percent time in the target quadrant. Visible platform testing was then performed on Days 19–20 after TBI to confirm that latencies were not confounded by visual impairments. Mice whose 20-day visible platform scores were greater than two standard deviations greater than the sham group (2-day average) were deemed to exhibit visual impairment and were removed from the study.
Assessment of lesion volume and hemispheric tissue loss
To assess lesion volume and hemispheric tissue loss, mice were deeply anesthetized with isoflurane and brains perfused with 50 mL of 0.9% NaCl, followed by 50 mL of 10% buffered formalin solution via the left ventricle 21 days after TBI (n = 10 per group). Brains were removed and coronal brain sections (10 μm) were cut on a cryostat at 0.5-mm intervals and mounted on Superfrost (Fisher Scientific) slides. Sections were stained with hematoxylin and eosin (H&E). Contralateral and remaining ipsilateral hemispheric areas were measured using an image analysis system (MCID Imaging Research, St. Catharines, ON). Volumes were calculated by multiplying slice area × slice interval thickness and adding together all slices. Lesion volume was then quantified both in mm3 and as a percent of the contralateral hemisphere as previously described.
Assessment of hippocampal neuronal survival
To assess the role of HMGB1 in neuronal death in the selectively vulnerable dorsal hippocampus ipsilateral to CCI, separate mice (n = 10 per genotype) were subjected to CCI (5 m/sec velocity, 1.2 mm depth) and recovered exactly as previously described. At 24 h after injury, mice were deeply anesthetized with isoflurane, and brains perfused with 50 mL of 0.9% NaCl, followed by 50 mL of 10% buffered formalin solution via the left ventricle. Brains were blocked through the hippocampus at the center of the lesion and embedded in paraffin. Section measuring 5 μM were cut in the center of the contusion on a microtome and stained with H&E. One section was analyzed per animal and the number of surviving hippocampal neurons in CA1 and CA3 regions were counted in the ipsilateral and contralateral hemispheres as previously described.20 Results are reported as surviving neurons/0.1 mm. Only neurons with visible nuclei were counted; shrunken or pyknotic cells were excluded from cell counts.
Western blot analysis
Brain tissue was homogenized using 500 μL of lysis buffer (0.1M sodium chloride, 0.01M Tris-Cl pH 7.6, 0.01M EDTA, protease inhibitors, and PMSF). Protein concentrations were analyzed using the BCA assay. Samples were prepared in Laemmli loading buffer and were loaded onto SDS-PAGE gels. Proteins were subsequently transferred to polyvinylidene difluoride (PVDF) membranes (GE Healthcare, Buckinghamshire, UK), and blocked with 5% non-fat dried milk in PBS for 1 h. Primary polyclonal mouse HMGB1 antibody (1:500; R&D Systems, Minneapolis, MN) and β-actin (1:500, Sigma-Aldrich) were used for Western blot. After washing twice with phosphate-buffered saline (PBS) containing 0.5% Tween 20 (PBST), secondary antibody (horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G; Sigma-Aldrich) was applied at 1:10,000 dilution for 1 h. Blots were washed in PBST thrice for 10 min, incubated in Enhanced Chemiluminescence Reagent (GE Healthcare, Buckinghamshire, UK), and used to expose x-ray film (GE Healthcare).
Statistical analysis
All data are expressed as mean ± standard error of the mean. One-way analysis of variance followed by the post hoc Student-Newman-Keuls test was used to determine the differences among multiple groups. The Mann–Whitney U test was applied on small-size comparisons with non-normal distributions between groups. The t test was applied only on experiments with normal distributions between the comparison groups. A p value <0.05 was considered statistically significant.
Results
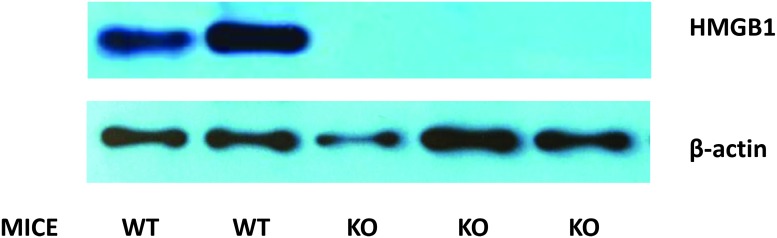
As embryonic deletion of HMGB1 is lethal, we generated an inducible HMGB1−/− mouse strain where the HMGB1 gene was globally deleted after tamoxifen treatment (Fig. 1 and Fig. 2). As shown in Figure 2, tamoxifen treatment lead to a near complete and sustained loss of HMGB1 expression in the murine brain.
FIG. 2.
Representative Western blot radiographs of whole murine brain extracts of high mobility group box 1 (HMGB1) knockout (KO) and wild-type (WT) mice after controlled cortical impact (CCI), with β-actin as a loading control.
Study 1: Assessment of brain edema and BBB permeability over the initial 24 h after CCI
Brain edema
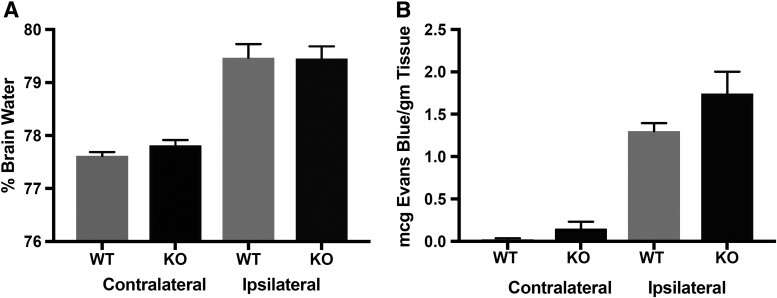
Measurement of %BW is a gold standard approach to quantifying brain edema after TBI in pre-clinical studies. Although a highly significant increase in %BW was seen in the injured hemisphere at 24 h after CCI, as previously reported in this model, there was no difference noted in the %BW in the ipsilateral (injured) hemisphere of the WT versus the HMGB1 KO (79.46 ± 0.58% vs. 79.45 ± 0.51%, respectively; p = 0.86). Similarly, no difference was noted in %BW in the contralateral hemisphere of WT versus HMGB1 KO mice (77.61 ± 0.16% vs. 77.81 ± 0.0.22%, respectively; p = 0.15; Fig. 3A). Of note, in numerous reports, %BW levels are not elevated in the hemisphere contralateral to injury in the CCI model, and the levels reported in these mice are again consistent with that finding in both WT and KO.25,26
FIG. 3.
Bar graphs demonstrating comparisons of (A) percent brain water (%BW) as a measure of brain edema (n = 5 per genotype), and (B) Evans blue dye test to investigate blood–brain barrier (BBB) permeability in a controlled cortical impact (CCI) model in wild-type (WT) and high mobility group box 1 (HMGB1) knockout (KO) mice (n = 10 per genotype). Overall, no significant difference was noted in %BW and Evans blue dye leakage in both the ipsilateral and contralateral hemisphere in the HMGB1 KO mice, compared with the WT mice (p > 0.05).
BBB permeability
Evans Blue dye has been used to investigate BBB permeability following TBI, and as applied in this model, serves as a surrogate for assessment of BBB permeability to albumin.24 Using this method, we assessed BBB permeability over a 20 h epoch, namely, between 4 and 24 h after CCI. Despite obvious BBB extravasation into the injured hemisphere, once again, we noted that there was no significant difference in BBB permeability on the ipsilateral side in the injured WT versus HMGB1 KO mice (1.30 ± 0.09 vs. 1.74 ± 0.26 mcg Evans Blue/gm tissue p = 0.27; Fig. 3B). Similarly, there was no significant difference in BBB permeability on the contralateral side in the WT versus HMGB1 KO mice (0.015 ± 0.02 vs. 0.15 ± 0.08 mcg Evans Blue/gm tissue p = 0.18). Together, these results suggest that absence of HMGB1 did not lead to a significant reduction in either brain edema or BBB permeability at 24 h after CCI.
Study 2: Assessment of behavioral and neuropathological outcomes over 21 days
Behavioral outcomes
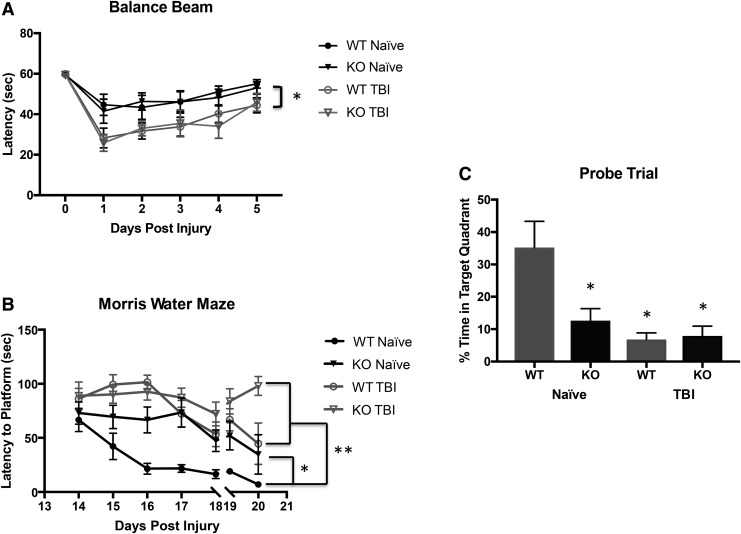
Beam balance performance was assessed in naïve WT and HMGB1 KO mice, and in WT and HMGB1 KO mice on Days 1–5 after CCI. Both WT and HMGB1 KO mice exhibited decreased latencies on the beam balance task on Day 1 post-injury versus pre-injury baseline or naïve. While latencies gradually improved for both groups on Days 2–5 after CCI, there was no significant difference in the overall performance between the injured WT and HMGB1 KO mice (Fig. 4A).
FIG. 4.
Behavioral outcomes after controlled cortical impact (CCI), in wild-type (WT) and high mobility group box 1 (HMGB1) knockout (KO) mice (n = 10 per genotype) are demonstrated by (A) beam balance test (B) spatial memory performance in the Morris water maze (MWM) acquisition paradigm, and (C) probe trail. (A) Both WT and HMGB1 KO mice exhibited decreased latencies on the beam balance task on Day 1 post-injury and improved on Days 2–5 after CCI (*p = 0.047 compared with the TBI group). (B) Naïve HMGB1 KO mice demonstrated significant impairment in learning on the MWM 14–18 days post-injury, which was comparable to the prolonged latencies noted in both the WT and HMGB1 KO groups after TBI (*p = 0.02 compared with WT naïve; **p = 0.000 compared with the TBI group). A visible platform task was performed on Days 19–20 post-injury. (C) Probe test was performed on Days 19–20 post-injury and % time in target quadrant was significantly prolonged in WT naïve group compared with HMGB1 KO naïve and post-injury WT and HMGB1 KO mice (*p < 0.05).
Spatial memory acquisition was tested using the MWM on Days 14–18 after CCI and on the identical days after TM dosing in naïve animals. Figure 4B reveals that there were significant increases noted in the submerged platform latencies for naïve HMGB1 KO versus naive WT mice on Days 15–18 of testing—implying impaired learning on the MWM in naïve HMGB1 KO mice. After CCI, MWM latencies also were increased on Days 15–18 in both WT and HMGB1 KO mice. However, there was no difference between WT and HMGB1 KO groups after injury. Surprisingly, the prolonged latency in the naïve HMGB1 KO mice was comparable to the latencies observed in both the WT and KO groups after TBI.
To discriminate between spatial and non-spatial strategies, mice were given a probe test (Fig. 4C). The % time in target quadrant was significantly different between the HMGB1 naïve and WT naïve groups (12.60 ± 3.7% vs. 35.20 ± 8.09; P = 0.02, respectively) consistent with the impairment seen in the KO in the hidden platform testing. Similarly, the % time in the target quadrant in WT and HMGB1 KO mice (6.8 ± 2.0% vs. 7.8 ± 3.0%, respectively) were markedly reduced versus naïve WT (p < 0.05) but did not differ between genotypes after TBI—and once again, the level of impairment after TBI was similar to that seen in the naïve HMGB1 KO.
Swim speeds were equal between groups, indicating that there was no motor impairment during spatial memory testing (data not shown). Visible platform latencies for both naïve mice and mice after CCI are shown in Figure 4B. An increase in latency was seen on Day 20 after CCI in the HMGB1 KO (p < 0.05 vs. all other groups).
Taken together, these data illustrate that motor function is preserved in naïve HMGB1 KO mice and is impaired after TBI to a similar level in both genotypes. In contrast, deletion of HMGB1 impaired performance on the MWM in naïve mice, although the impairments in performance after TBI on both the hidden platform task and probe trial were similar between genotypes after injury.
Lesion volume and hemispheric tissue loss
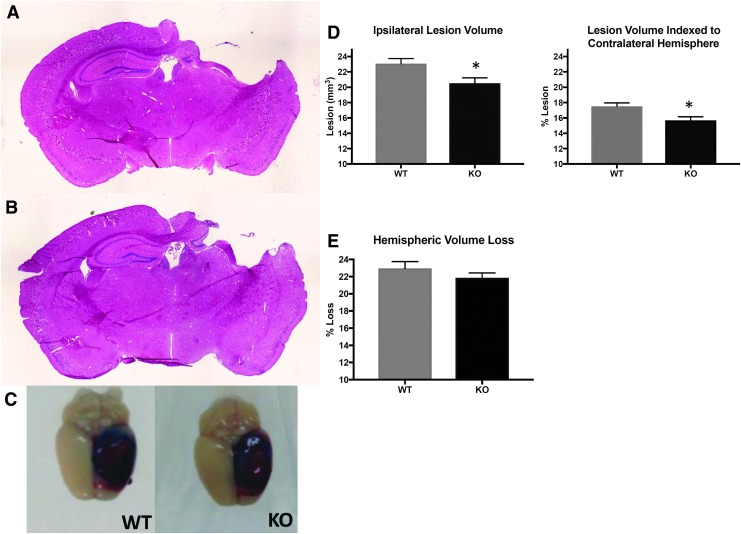
To examine whether absence of HMGB1 could attenuate tissue injury induced by TBI, lesion volumes were measured in WT and HMGB1 KO mice at 21 days after TBI (Fig. 5A, 5B). The mean lesion volume in traumatized HMGB1 KO mice was significantly less than WT mice (20.53 ± 2.17 vs. 23.06 ± 2.10 mm3; p = 0.039). Similarly, when indexed to the contralateral hemisphere, the lesion volume was significantly smaller in HMGB1 KO mice as compared to WT mice (15.69 ± 1.45 vs. 17.51 ± 1.44 %).
FIG. 5.
Lesion and hemispheric volume after controlled cortical impact (CCI; n = 10 per genotype). Twenty-one days after CCI, brains were removed, sectioned and stained with hematoxylin and eosin (H&E; A and B) and analyzed for lesion size (C-E). (A) and (B) demonstrate histological appearance of hippocampal injury in wild-type (WT) and high mobility group box 1 (HMGB1) knockout (KO) mice, respectively. (C) Representative photographs of murine brains showing Evan's blue extravasation at 24 h. (D) and (E) Lesion volume for HMGB1 KO mice was significantly smaller than WT mice even when indexed to the contralateral hemisphere. Hemispheric volume loss in the HMGB1 KO mice did not differ significantly vs. WT (p = 0.34).
Hemispheric tissue loss was also assessed in both WT and HMGB1 KO mice at 21 days after TBI (Fig. 5E). There was no significant difference in hemispheric tissue loss between HMGB1 KO versus WT (21.87 ± 0.557 vs. 22.95 ± 0.78 mm3; p = 0.34).
Hippocampal neuronal survival
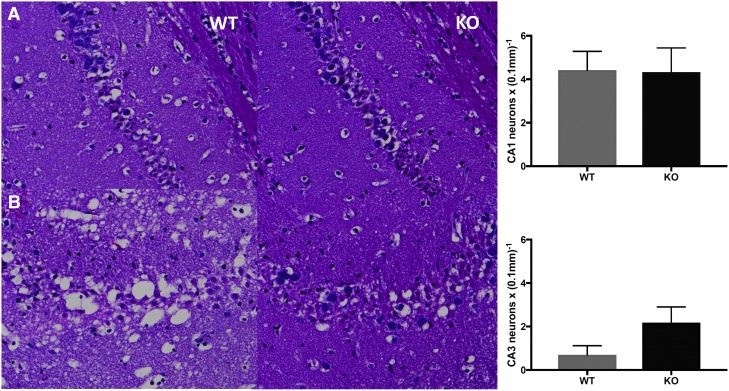
To examine if the absence of HMGB1 could protect hippocampal neurons following TBI, mice were sacrificed at 24 h post-TBI, and the number of surviving neurons in the hippocampus were counted in the CA1 and CA3 regions of the contralateral and ipsilateral hemispheres. There was no difference in the number of surviving CA1 and CA3 hippocampal neurons in the contralateral hemisphere in the HMGB1 KO versus WT mice (CA1: 24.3 ± 0.42 vs. 24.4 ± 0.95 cells/0.1 mm; p = 0.9; CA3: 19.2 ± 0.55 vs. 16.9 ± 0.69 cells/0.1 mm; p = 0.09, respectively). As anticipated, the number of surviving CA1 hippocampal neurons were reduced in the ipsilateral hemisphere after CCI, but the number was comparable in the HMGB1 KO and WT mice (4.3 ± 1.1 vs. 4.4 ± 0.8 cells/0.1 mm; p = 0.93, respectively). Once again, as anticipated, the number of surviving CA3 hippocampal neurons were reduced in the ipsilateral hemisphere after CCI, but the number did not significantly differ between the HMGB1 KO and WT mice (2.1 ± 0.72 vs. 0.69 ± 0.42 cells/0.1 mm; p = 0.07, respectively; Fig. 6). Together, these data suggest that absence of HMGB1 results in a significant reduction in contusion volume assessed at 21 days after injury without a significant improvement in hippocampal neuronal survival in either CA1 or CA3.
FIG. 6.
Representative microphotographs stained with hematoxylin and eosin (H&E), depicting the CA1 (A) and CA3 (B) hippocampal subfield in wild-type (WT) and high mobility group box 1 (HMGB1) knockout (KO) mice at 21 days post–controlled cortical impact (CCI; n = 10 per genotype). No statistically significant difference was noted in neuronal survival between WT and HMGB1 KO mice in the CA1 or CA3 hippocampus (p = 0.07).
Discussion
Understanding and selectively targeting the neuroinflammatory response that is initiated within the first few hours after TBI has the potential to make a significant impact in limiting secondary tissue damage and promoting recovery.27 Since the seminal work of Matzinger,28 HMGB1 biology has emerged as a rapidly expanding field of basic and clinical research. Since constitutive HMGB1-/- mice are non-viable, we used inducible HMGB1 KO mice to characterize the role of HMGB1 in TBI. There were three significant findings in our study. First, consistent with prior reports, an absence of HMGB1 is neuroprotective and results in significant decrease in lesion volume. Second, remarkably, naïve global HMGB1 KO mice demonstrated impairments in spatial memory acquisition and probe trial performance on the MWM versus WT to a level similar as that seen after a relatively severe CCI in WT. However, after CCI, MWM performance was impaired to a similar level in WT and HMGB1 KO mice. Third, despite prior publications in pre-clinical models of TBI strongly supporting a role for HMGB1 inhibition in contributing to brain edema and BBB permeability after injury, we saw no difference in either brain edema or BBB permeability in HMGB1 KO versus WT mice at 24 h after injury. Each of these observations merits discussion.
To the best of our knowledge, this is the first report that used inducible global HMGB1 KO mice to help characterize the role of HMGB1 in the inflammatory response after TBI. Previous studies in this field have demonstrated that neuronal HMGB1 is released into extracellular space after trauma, diffuses into the surrounding areas including the blood stream and CSF, and that inhibition of HMGB1 with either monoclonal antibodies or drugs led to improvement in brain edema and outcomes.15–17 These prior studies treated the injured animals early after injury with various anti-HMGB1 therapeutic agents; however, it is notable that no cognitive testing was assessed in those studies. Our findings thus appear to address a vital gap in the literature regarding the effects of HMGB1 in the central nervous system (CNS), using a selective approach to target HMGB1 along with classical tools to assess outcome in pre-clinical models of TBI (%BW, MWM testing, and volumetric analyses), and suggest some degree of neuroprotection (tissue sparing), but a surprising lack of benefit on brain edema and BBB permeability, and unanticipated behavioral deficits even before injury.
Global inducible HMGB1 KO mice are neuroprotected versus WT
The pro-inflammatory response that is initiated after TBI includes the release of HMGB1 that acts as a ligand for TLR2, TLR4, and RAGE. Further downstream, there is activation of several MAPKs and NF-κB, and the subsequent release of the classic pro-inflammatory cytokines to activate resident glial cells (microglia and astrocytes).29 Numerous studies have demonstrated that genetic or pharmacological inhibition of TLR4 in an animal model attenuates secondary damage after TBI with abrogation of the pro-inflammatory response and subsequent improvement in recovery.12,30,31 Corroborating this postulate, TLR4-deficient mice have smaller lesions and less inflammatory response after TBI.32 Our results agree with this hypothesis and demonstrate that HMGB1, a TLR4 ligand, is a detrimental neuroinflammatory participant in the secondary injury response since the loss of HMGB1 led to a significant reduction in the lesion volume. Our study was not powered adequately to test the hypothesis as to whether CA3 hippocampal neurons were spared after injury in our model. Based on the trend that we observed, additional studies targeting that endpoint are warranted, particularly given the fact that hippocampal injury is well known to contribute to deficits in cognitive outcome.
Failure of HMGB1 KO to alter post-traumatic brain edema or BBB permeability
We also did not observe any difference in brain edema or BBB permeability in HMGB1 KO mice versus WT after CCI. Brain edema is complex after TBI and can result from vasogenic, cytotoxic, and contusional (osmolar) mechanisms33 and a variety of water channels including both aquaporin-4 (AQP4) and transient receptor potential cation channel subfamily M member 4. HMGB1 has been suggested to play a key role via TLR4 in upregulating AQP4, since TLR4 antagonism blunted both AQP4 expression and brain edema after CCI in mice as described by Laird and colleagues.12 However, in that report, direct effects HMGB1 antagonism were not tested, and it is possible that other DAMPs could have been responsible for upregulating TLR4. Thus, although the loss of HMGB1 can blunt some aspects of the inflammatory response and secondary injury, the loss may not be sufficient to eliminate BBB permeability, AQP4 up-regulation, and brain edema. However, in recent studies, we did not observe a reduction in brain edema in rats treated with a novel AQP4 antagonist after CCI in rats.34 Other pathways for edema formation may be operating after CCI. Similarly, issues such as cell type, timing, and model differences may be operating. In any case, taken together, our work and the work of Laird and colleagues12 suggest that there may be a more robust role for TLR4 than HMGB1 in the development of brain edema and BBB damage in the specific setting of cerebral contusion, as modeled by CCI. Further study is needed of DAMPS and TLR4 signaling after TBI in this regard.
Cognitive impairment in global inducible HMGB1 KO mice
In contrast to the beneficial effects of loss of HMGB1 on tissue sparing after TBI, naïve HMGB1 KO mice exhibited impairment of MWM performance on both the hidden platform paradigm and the probe trial. The effect on spatial learning in naïve mice was similar to the behavioral deficits produced by a severe level of CCI itself. Curiously, cognitive outcomes have not been reported by other authors that used pharmacological blockade of HMGB1 in the animals that were subjected to TBI.15–17 We believe that these findings could be important.
HMGB1 is highly expressed in the CNS and plays an important role in both neurogenesis and gliogenesis in brain development.35 Also, our findings of impaired spatial learning with HMGB1 mirror those in previous studies on TLR4-/- mice.36 Hence, it is plausible that deletion of HMGB1, a TLR4 ligand, might lead to changes in TLR4 axis signaling that subsequently results in altered neurogenesis and acquired learning defects. Future studies in site-specific HMGB1 KO mice across various cell types in the CNS including neurons, astrocytes, and/or microglia could help better characterize this phenomenon, and contributed to more targeted therapies addressing the HMGB1–TLR4 axis.
It also was interesting in our results that TBI in the HMGB1 KO did not significantly impair MWM performance in either acquisition or probe performance versus that seen in naïve HMGB1 KO mice, and that MWM performance was not different in WT and HMGB1 KO mice after CCI. One might argue that—given the substantial decrement of MWM performance at baseline in HMGB1 KO mice—that the deficit after TBI might have been expected to be even more marked. However, we specifically chose a severe CCI injury level for these studies anticipating improved function after injury—and thus there was in fact a rather limited chance to demonstrated further increases in latency, which were already near maximal in the naïve HMGB1 KO.
We also observed that 20 days after CCI, the performance of HMGB1 KO mice on the visible platform task was impaired. This finding is similar to the impairment in MWM and visible platform performance seen in the inducible nitric oxide synthase (iNOS) mouse that we reported in 1999.21 It may be more than coincidence that subsequent work by other laboratories demonstrated that iNOS, another participant in the inflammatory response to TBI, is essential to neurogenesis after acute brain injury,37 and confers other endogenous neuroprotectant effects.38 The impairment in visible platform testing was not seen in naïve HMGB1 KO mice, supporting the notion that our findings relevant to MWM performance in naïve mice were not confounded by visual deficits. In any case, our data strongly suggest that inducible HMGB1 KO mice merit evaluation across a broad battery of behavioral tasks to more fully characterize any deficits that are present, and an assessment of the impact of the loss of HMGB1 on neurogenesis is needed. Future directions include interrogation of the loss of HMGB1 in a cell-specific manner since the impact of HMGB1 KO on neurons, glia, or the cerebral microcirculation could confer differential effects that might have important mechanistic and therapeutic relevance.
Unlike the effects seen on cognitive function, we did not observe a decrement in motor testing. We also, however, did not detect any benefit on motor testing after TBI in the HMGB1 KO versus WT, which would have been anticipated based on the reports testing antibodies or drugs targeting HMGB1. It is unclear why benefit after TBI in the HMGB1 KO was restricted to tissue sparing. Species differences could possibly play a role given that the both the work of Okuma and colleagues15 and Gu and colleagues17 studied rats rather than mice. Model differences, (CCI vs. fluid percussion vs. weight drop) and differences in injury severity also could play a role. We also did not administer control antibodies and our KO targeted HMG1 in all cell types—while the specific cell types targeted and/or accessible to monoclonal antibodies or drugs could differ. Finally, we recognize that HMGB1-Cre mice that did not receive TM would represent an additional control group for this study and further help in delineating the role of HMGB1 in TBI, and should be pursued in future studies.
Potential implications of this work for TBI and to the broader context of HMGB1 targeting therapeutics
It is thus tempting now to speculate on the role of HMGB1 in TBI as a “double-edged sword,” such that despite the benefits on various aspects of secondary injury, the sustained absence of HMGB1 can profoundly impair cognitive function, even in naïve mice. HMGB1 is a multi-functional protein, and its biological activity depends on the location, context and post-translational modification.39 For example, rats that survived cecal ligation and puncture to induce sepsis demonstrated increased circulating serum HMGB1 levels that were associated with selective memory impairment. Further, administration of HMGB1 monoclonal antibody in the peri-operative period alleviated the memory deficit.40 However, cognitive testing in the setting of extracerebral derangements during sepsis likely differ importantly from those produced during testing in naïve mice or after TBI. Thus, although the neuroinflammation is reduced by treatment with anti-HMGB1 monoclonal antibodies, the degree, location, and duration of HMGB1 blockade required to confer benefit on behavioral outcomes is complex and may vary across insult etiologies.
In contrast, the vital role of intracellular HMGB1 is evident in HMGB1 KO mice that present with glucose dysregulation, multi-organ failure and a diminished life-span.41 Further, HMGB1 is highly expressed in early embryonic brain and is critical for forebrain development as it regulates the proliferation of neural progenitors.42 Even though the mice used in our study were fully developed (16–20 weeks), it is recognized that HMGB1 is required for the important intracellular functions in neural progenitor regulation, a possibility that warrants further studies. Our findings, thus, have potential relevance beyond TBI, specifically, to any condition in which HMGB1 is being therapeutically targeted—particularly with sustained therapy. Insights gleaned from this study raise an interesting question as to whether the deployment of anti-HMGB1 strategies for prolonged periods of time are associated with an adverse risk-to-benefit ratio. More studies need to be conducted to define and limit the therapeutic window during which the use of such strategies is beneficial and aids in the amelioration of the acute disease process.
The discovery of HMGB1 as a late mediator sparked the quest for therapeutic agents that target the late inflammatory response to inflammation. To date, remarkably few therapeutic inhibitors of HMGB1 release have been discovered.43,44 With such limited number of clinically efficacious inhibitors available, a renewed focus is required on discovery of new therapeutic agents and strategies to pinpoint the therapeutic window to target inflammation. Based on our findings, there are important questions that still need to be addressed about the role of HMGB1 signaling in the innate immune response after TBI and other diseases. How does the absence of HMGB1 lead to spatial learning impairment? Are the effects of HMGB1 deletion on spatial learning long lasting or transient? Which cell types in the CNS are involved? Finally, how long should anti-HMGB1 therapies be deployed in TBI or other conditions for a favorable risk-benefit ratio?
Conclusions
Here, we show an unexpected outcome of spatial learning impairment in naïve global inducible HMGB1 KO mice, such that developmental behavioral effects of HMGB1 deletion in naïve mice were similar to those seen after TBI. Mirroring prior reports using HMGB1 antagonists, we noted that HMGB1 deletion is protective in terms of lesion volume after TBI. However, we did not observe a significant benefit on post-traumatic brain edema and BBB permeability, contrasting prior reports. Our data suggest that HMGB1 may be a dual-edged sword after acute brain injury and that the duration of anti-HMGB1 therapies should be carefully considered if these treatments are to be included in the armamentarium for therapeutic purposes in TBI and other conditions.
Acknowledgment
We thank the NIH T32 HD040686 (AMA), RO1 GM098474 (RKA), the US Department of Defense WH81XWH-14-2-0018 (PMK), and the Ake N. Grenvik Professorship (PMK) for support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. (2012). The changing landscape of traumatic brain injury research. Lancet Neurol. 11, 651. [DOI] [PubMed] [Google Scholar]
- 2. Corps K.N., Roth T.L., and McGavern D.B. (2015). Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 72, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bianchi M.E. (2007). DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 [DOI] [PubMed] [Google Scholar]
- 4. Manson J., Thiemermann C., and Brohi K. (2012). Trauma alarmins as activators of damage-induced inflammation. Br. J. Surg. 99 Suppl 1, 12–20 [DOI] [PubMed] [Google Scholar]
- 5. Wang H., Yang H., Czura C.J., Sama A.E., and Tracey K.J. (2001). HMGB1 as a late mediator of lethal systemic inflammation. Am. J. Respir. Crit. Care Med. 164, 1768–1773 [DOI] [PubMed] [Google Scholar]
- 6. Castiglioni A., Canti V., Rovere-Querini P., and Manfredi A.A. High-mobility group box 1 (HMGB1) as a master regulator of innate immunity. Cell. Tissue Res. 343,189–199 [DOI] [PubMed] [Google Scholar]
- 7. Gallucci S. and Matzinger P. (2001). Danger signals: SOS to the immune system. Curr. Opin. Immunol. 13, 114–119 [DOI] [PubMed] [Google Scholar]
- 8. Peltz E.D., Moore E.E., Eckels P.C., Damle S.S., Tsuruta Y., Johnson J.L., Sauaia A., Silliman C.C., Banerjee A., and Abraham E. (2009). HMGB1 is markedly elevated within 6 hours of mechanical trauma in humans. Shock 32, 17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park J.S., Svetkauskaite D., He Q., Kim J.Y., Strassheim D., Ishizaka A., and Abraham E. (2004). Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J. Biol. Chem. 279, 7370–7377 [DOI] [PubMed] [Google Scholar]
- 10. Yu M., Wang H., Ding A., Golenbock D.T., Latz E., Czura C.J., Fenton M.J., Tracey K.J., and Yang H. (2006). HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26, 174–179 [DOI] [PubMed] [Google Scholar]
- 11. Pedrazzi M., Patrone M., Passalacqua M., Ranzato E., Colamassaro D., Sparatore B., Pontremoli S., and Melloni E. (2007). Selective proinflammatory activation of astrocytes by high-mobility group box 1 protein signaling. J. Immunol. 179, 8525–8532 [DOI] [PubMed] [Google Scholar]
- 12. Laird M.D., Shields J.S., Sukumari-Ramesh S., Kimbler D.E., Fessler R.D., Shakir B., Youssef P., Yanasak N., Vender J.R., and Dhandapani K.M. (2014). High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia 62, 26–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Au A.K., Aneja R.K., Bell M.J., Bayir H., Feldman K., Adelson P.D., Fink E.L., Kochanek P.M., and Clark R.S. (2012). Cerebrospinal fluid levels of high-mobility group box 1 and cytochrome C predict outcome after pediatric traumatic brain injury. J. Neurotrauma 29, 2013–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gao T.L., Yuan X.T., Yang D., Dai H.L., Wang W.J., Peng X., Shao H.J., Jin Z.F., and Fu Z.J. (2012). Expression of HMGB1 and RAGE in rat and human brains after traumatic brain injury. J. Trauma Acute Care Surg. 72, 643–649 [DOI] [PubMed] [Google Scholar]
- 15. Okuma Y., Liu K., Wake H., Zhang J., Maruo T., Date I., Yoshino T., Ohtsuka A., Otani N., Tomura S., Shima K., Yamamoto Y., Yamamoto H., Takahashi H.K., Mori S., and Nishibori M. (2012). Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann. Neurol. 72, 373–384 [DOI] [PubMed] [Google Scholar]
- 16. Su X., Wang H., Zhao J., Pan H., and Mao L. (2011). Beneficial effects of ethyl pyruvate through inhibiting high-mobility group box 1 expression and TLR4/NF-kappaB pathway after traumatic brain injury in the rat. Mediators Inflamm. 2011, 807142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gu X.J., Xu J., Ma B.Y., Chen G., Gu P.Y., Wei D., and Hu W.X. (2014). Effect of glycyrrhizin on traumatic brain injury in rats and its mechanism. Chin. J. Traumatol. 17, 1–7 [PubMed] [Google Scholar]
- 18. Tang D., Kang R., Zeh H.J., 3rd, and Lotze M.T. (2011). High-mobility group box 1, oxidative stress, and disease. Antioxid. Redox. Signal 14, 1315–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zheng Y., Gardner S.E., and Clarke M.C. (2011). Cell death, damage-associated molecular patterns, and sterile inflammation in cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 31, 2781–2786 [DOI] [PubMed] [Google Scholar]
- 20. Tehranian R., Rose M.E., Vagni V., Griffith R.P., Wu S., Maits S., Zhang X., Clark R.S., Dixon C.E., Kochanek P.M., Bernard O., and Graham S.H. (2006). Transgenic mice that overexpress the anti-apoptotic Bcl-2 protein have improved histological outcome but unchanged behavioral outcome after traumatic brain injury. Brain Res. 1101, 126–135 [DOI] [PubMed] [Google Scholar]
- 21. Sinz E.H., Kochanek P.M., Dixon C.E., Clark R.S., Carcillo J.A., Schiding J.K., Chen M., Wisniewski S.R., Carlos T.M., Williams D., DeKosky S.T., Watkins S.C., Marion D.W., and Billiar T.R. (1999). Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice. J. Clin. Invest. 104, 647–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Statler K.D., Alexander H., Vagni V., Holubkov R., Dixon C.E., Clark R.S., Jenkins L., and Kochanek P.M. (2006). Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Res. 1076, 216–224 [DOI] [PubMed] [Google Scholar]
- 23. Busto R., Dietrich W.D., Globus M.Y., Valdes I., Scheinberg P., and Ginsberg M.D. (1987). Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. J. Cereb. Blood Flow Metab. 7, 729–738 [DOI] [PubMed] [Google Scholar]
- 24. Whalen M.J., Clark R.S., Dixon C.E., Robichaud P., Marion D.W., Vagni V., Graham S.H., Virag L., Hasko G., Stachlewitz R., Szabo C., and Kochanek P.M. (1999). Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J. Cereb. Blood Flow Metab. 19, 835–842 [DOI] [PubMed] [Google Scholar]
- 25. Thomale U.W., Griebenow M., Kroppenstedt S.N., Unterberg A.W., and Stover J.F. (2006). The effect of N-acetylcysteine on posttraumatic changes after controlled cortical impact in rats. Intensive Care Med. 32, 149–155 [DOI] [PubMed] [Google Scholar]
- 26. Kochanek P.M., Marion D.W., Zhang W., Schiding J.K., White M., Palmer A.M., Clark R.S., O'Malley M.E., Styren S.D., Ho C. and DeKosky S.T. (1995). Severe controlled cortical impact in rats: assessment of cerebral edema, blood flow, and contusion volume. J. Neurotrauma 12, 1015–1025 [DOI] [PubMed] [Google Scholar]
- 27. Gyoneva S. and Ransohoff R.M. (2015). Inflammatory reaction after traumatic brain injury: therapeutic potential of targeting cell-cell communication by chemokines. Trends Pharmacol. Sci. 36, 471–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Matzinger P. (2002). The danger model: a renewed sense of self. Science 296, 301–305 [DOI] [PubMed] [Google Scholar]
- 29. Park J.S., Arcaroli J., Yum H.K., Yang H., Wang H., Yang K.Y., Choe K.H., Strassheim D., Pitts T.M., Tracey K.J., and Abraham E. (2003). Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am. J. Physiol. Cell. Physiol. 284, C870–C879 [DOI] [PubMed] [Google Scholar]
- 30. Chen G., Zhang S., Shi J., Ai J., Qi M., and Hang C. (2009). Simvastatin reduces secondary brain injury caused by cortical contusion in rats: possible involvement of TLR4/NF-kappaB pathway. Exp. Neurol. 216, 398–406 [DOI] [PubMed] [Google Scholar]
- 31. Chen C.C., Hung T.H., Wang Y.H., Lin C.W., Wang P.Y., Lee C.Y., and Chen S.F. (2012). Wogonin improves histological and functional outcomes, and reduces activation of TLR4/NF-kappaB signaling after experimental traumatic brain injury. PLoS One 7, e30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ahmad A., Crupi R., Campolo M., Genovese T., Esposito E. and Cuzzocrea S. (2013). Absence of TLR4 reduces neurovascular unit and secondary inflammatory process after traumatic brain injury in mice. PLoS One 8, e57208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kawamata T., Mori T., Sato S., and Katayama Y. (2007). Tissue hyperosmolality and brain edema in cerebral contusion. Neurosurg. Focus 22, E5. [DOI] [PubMed] [Google Scholar]
- 34. Wallisch J., Jha R., Farr G., McGuirk P., Pelletier M., Jackson T., Kochanek P., and Manole M. (2016). Aquaporin-4 inhibitor AER-271 blocks early cerebral edema in pediatric rat asphyxial cardiac arrest. Crit. Care Med. 44, 255 [Google Scholar]
- 35. Guazzi S., Strangio A., Franzi A.T., and Bianchi M.E. (2003). HMGB1, an architectural chromatin protein and extracellular signalling factor, has a spatially and temporally restricted expression pattern in mouse brain. Gene Expr. Patterns 3, 29–33 [DOI] [PubMed] [Google Scholar]
- 36. Okun E., Barak B., Saada-Madar R., Rothman S.M., Griffioen K.J., Roberts N., Castro K., Mughal M.R., Pita M.A., Stranahan A.M., Arumugam T.V., and Mattson M.P. (2012). Evidence for a developmental role for TLR4 in learning and memory. PLoS One 7, e47522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhu D.Y., Liu S.H., Sun H.S., and Lu Y.M. (2003). Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J. Neurosci. 23, 223–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bayir H., Kagan V.E., Borisenko G.G., Tyurina Y.Y., Janesko K.L., Vagni V.A., Billiar T.R., Williams D.L., and Kochanek P.M. (2005). Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J. Cereb. Blood Flow Metab. 25, 673–684 [DOI] [PubMed] [Google Scholar]
- 39. Harris H.E., Andersson U., and Pisetsky D.S. (2012). HMGB1: a multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 8, 195–202 [DOI] [PubMed] [Google Scholar]
- 40. Chavan S.S., Huerta P.T., Robbiati S., Valdes-Ferrer S.I., Ochani M., Dancho M., Frankfurt M., Volpe B.T., Tracey K.J., and Diamond B. (2012). HMGB1 mediates cognitive impairment in sepsis survivors. Mol. Med. 18, 930–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Calogero S., Grassi F., Aguzzi A., Voigtlander T., Ferrier P., Ferrari S., and Bianchi M.E. (1999). The lack of chromosomal protein Hmg1 does not disrupt cell growth but causes lethal hypoglycaemia in newborn mice. Nat. Genet. 22, 276–280 [DOI] [PubMed] [Google Scholar]
- 42. Zhao X., Kuja-Panula J., Rouhiainen A., Chen Y.C., Panula P., and Rauvala H. (2011). High mobility group box-1 (HMGB1; amphoterin) is required for zebrafish brain development. J. Biol. Chem. 286, 23200–23213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ulloa L., Ochani M., Yang H., Tanovic M., Halperin D., Yang R., Czura C.J., Fink M.P., and Tracey K.J. (2002). Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc. Natl. Acad. Sci. U. S. A. 99, 12351–12356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang H., Ochani M., Li J., Qiang X., Tanovic M., Harris H.E., Susarla S.M., Ulloa L., Wang H., DiRaimo R., Czura C.J., Wang H., Roth J., Warren H.S., Fink M.P., Fenton M.J., Andersson U. and Tracey K.J. (2004). Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc. Natl. Acad. Sci. U. S. A. 101, 296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]