Abstract
Children under 4 years of age have the highest incidence of traumatic brain injury (TBI) among the non-elderly and may be at high risk of poor developmental outcomes. We prospectively enrolled a cohort of children injured before 31 months old with TBI or orthopedic injury (OI), from 2013 to 2015 at two pediatric level 1 trauma centers to study very young children's developmental outcomes after injury. We used Ages & Stages-3 and Ages & Stages: Social-Emotional screening tools to measure children's development at pre-injury and 3 and 12 months post-injury. The cohort included 123 children with TBI categorized as mild (n = 48), complicated-mild or moderate (n = 54), and severe (n = 21) and 45 children with OI. Generalized linear models examined effects of injury severity and age at injury controlling for pre-injury ratings. Children with mild or complicated-mild/moderate TBI generally remained on developmental track. Compared to OI, children with severe TBI tended to have a negative developmental trajectory with decrements in communication (−7.07; 95% confidence interval [CI], −13.7, −0.48), gross motor (−15.2; 95% CI, −21.1, −9.19), problem solving (−11.6; 95% CI, −17.9, −5.29), personal-social (−16.8; 95% CI, −22.8, −10.8), and social-emotional (21.0; 95% CI, 7.32, 34.7) domains 12 months post-injury. Developmental effects from TBI differed by age at injury: Infants had more difficulties than older children in communication and problem-solving domains. Despite low developmental scores in 28% of the cohort, only 5% were receiving Early Childhood Intervention (ECI) services 12 months after injury. Early age at injury is a vulnerability factor after TBI. Young age and severe injury should prompt evaluation for ECI.
Keywords: infant, neurodevelopment, pediatric, traumatic brain injury
Introduction
Children 0–4 years of age have the highest incidence of traumatic brain injury (TBI) among the non-elderly with over 300,000 emergency department (ED) visits, 9250 hospitalizations, and 760 deaths, annually.1 Infants and toddlers may be particularly vulnerable to the consequence of TBI because the infant brain is in a phase of rapid development during which it may be particularly susceptible to disruption by injury.2 Although term infants are born with the main white matter connections formed, the infant brain is actively undergoing intense fiber myelination, synapse formation, and pruning, which slows as infants become toddlers.3 Myelination and refinement of white matter is linked to gains in motor, cognitive, and socioemotional functioning.4 Poor outcomes of TBI sustained in early childhood may be explained, in part, by the timing of injury in a period of rapid brain and behavioral development.5–7 Identification of periods of vulnerability to the effects of TBI is crucial to promote awareness of appropriate referral for rehabilitation and school-based services.
Developmental consequences of TBI are shaped by injury and child characteristics and family social ecology.8 Injury severity, age at injury, time post-injury, and pre-existing reserve from factors such as pre-maturity may affect the trajectory of recovery and eventual level of skill acquisition.5,9,10 Despite the high incidence of TBI in infants and toddlers, very few studies have assessed risks and tracked developmental trajectories. The few studies including infants and toddlers have been limited by small sample sizes and/or lack of an injury comparison group.7,11,12 Despite these limitations, findings suggest that infants and pre-schoolers with moderate-severe TBI have long-term decrements in intellectual, academic, adaptive behavior, social, and some areas of attention compared to typically developing children.7,11–15 Approximately half of severe TBI in infants is attributed to abusive injury, which may have worse outcomes than unintentional injury.15–17 Very young children with mild TBI have outcomes similar to typically developing comparison groups, although subtle changes in verbal IQ, theory of mind, and post-concussive symptoms have been reported.18–20 The social environment, including socioeconomic status, family functioning, and parenting styles, is known to impact recovery in older children, with more favorable outcomes associated with positive home environments.21,22 However, the influence of these factors on outcomes of infants and pre-schoolers is largely unknown.
Our aim was to examine the impact of injury severity and age at injury on the development of infants and pre-schoolers in the first year after TBI. Secondarily, we explored differences in the relationships between time post-injury and outcomes among these groups. We hypothesized that higher injury severity, younger age at injury, and adverse family environment would negatively impact children's recovery and ongoing acquisition of developmental skills.
Methods
Patient population
Patients were children from birth to <31 months of age recruited from two level 1 pediatric trauma centers: Primary Children's Hospital (PCH) in Salt Lake City, Utah, and the University of Texas Health Science Center at Houston (UTHealth)/Children's Memorial Hermann Hospital. These children are a subset of a larger cohort of children recruited up to age 15 years.23 This young age group is reported separately to allow similar developmental outcome measures and represent children eligible for early childhood intervention (ECI) services. Children were recruited from January 2013 through September 2015 if they had sustained either a TBI or an orthopedic injury (OI). Abusive head trauma (AHT) is included in the TBI group. English- and Spanish-speaking children and families were recruited and asked for consent in person while in the ED or hospital or were contacted by telephone after review of ED logs (UTHealth) and returned consent forms by mail. Children with severe developmental delay, psychiatric diagnoses, or very pre-term birth (<32 weeks) that would preclude understanding whether changes in development could be attributed to the injury were excluded. Institutional review board approval was obtained from both the University of Utah and UTHealth.
Definitions
TBI group
TBI was defined according to the Centers for Disease Control and Prevention (CDC) 2002 report as an injury to the head with observed or reported decreased level of consciousness, amnesia, and/or neuropsychological abnormality or diagnosed intracranial lesion.24 For children <2 years of age, neuropsychological abnormality included irritability, vomiting, or lethargy. TBI severity was measured using the lowest ED pediatric Glasgow Coma Scale (GCS)25 and was divided into mild, moderate, and severe categories. Mild TBI was defined according to CDC and World Health Organization definitions as a GCS ≥13 upon presentation to healthcare with a GCS of 15 at discharge or after 24 h if hospitalized, and one or more focal signs including a period of transient confusion, loss of consciousness for 30 min or less, and/or transient neurological abnormalities.26,27 Mild TBI was subclassified as complicated mild based on the presence of an intracranial hemorrhage diagnosed on computed tomography (CT) scan.28 Children with skull fracture and no underlying parenchymal hemorrhage were classified as mild TBI. Moderate TBI was categorized as a GCS of 9–12. Severe TBI was categorized as a GCS of 3–8. For children who had received heavy sedation and/or muscle relaxants precluding evaluation, a score of 3T was assigned. Abusive injury was categorized by each institution's child abuse team. Child abuse team consultation notes were reviewed both at the time of injury and after the medical workup was completed. Injury mechanism was adjudicated by the investigators if no conclusion was reported in the medical record.
Comparison group
Children with an upper or lower-extremity long-bone fracture and no evidence of TBI were recruited contemporaneously with the TBI group. OI comparisons isolate the effect of TBI from the effect of being injured and account for unmeasured pre-injury differences between injured versus uninjured children. Injury severity was measured with the Abbreviated Injury Scale (AIS).29 To insure even distribution of children with OI and different levels of TBI severity, families were recruited sequentially until the target number was attained for each subgroup.
Data sources
Parents retrospectively completed surveys of family demographics, family functioning and social support, and child outcomes as soon as possible after injury. Demographic information included family composition, self-identified race and ethnicity, income category, parental education, employment, and health insurance status. Parents were asked to recall their child's pre-injury functioning right before injury. Follow-up assessments were collected at 3 and 12 months. English-speaking families completed assessments in person, online, or by telephone. Bilingual study coordinators interviewed Spanish-speaking families in person or by telephone.
Medical records were abstracted for clinical and injury mechanism data by trained study coordinators using standardized data abstraction forms. A hierarchy of sources was established before data abstraction for cases in which multiple people recorded information in the medical record. The hierarchy was as follows: trauma surgeon, ED attending, trauma fellow, ED fellow, nurse, and resident. Trauma registrars assigned AIS scores. CT scans were performed at the treating clinician's discretion and were read by pediatric neuroradiologists at each site.
Measures
Outcomes
Ages & Stages Questionnaire-3 (ASQ-3) assesses communication, gross motor, fine motor, problem-solving, and personal-social skills in children ages 1–60 months. Higher scores indicate more-advanced development. Children are categorized as appropriate, need to monitor (≥1 standard deviation [SD] and <2 SDs below the mean), and need to assess (≥2 SDs below the mean corresponding to ≤2nd percentile).30 Ages & Stages Questionnaire: Social-Emotional (ASQ:SE) measures seven developmental and behavioral characteristics, including self-regulation, compliance, communication, adaptive functioning, autonomy, affect, and interaction with others in ages 3–66 months.31 ASQ:SE is scored against an age-normed risk threshold. Higher scores indicate more problems. Sensitivity and specificity are favorable for identifying children who need evaluation for the ASQ (0.86 and 0.85) and ASQ:SE (0.81 and 0.83). ASQ and ASQ:SE are parent-reported measures available in both English and Spanish.32 Specifically in the 2- to 12-month age range, ASQ is 91.3% accurate at correctly identifying children not at risk for developmental delay and 84.6% accurate at correctly identifying children at risk for developmental delay when compared to the Bayley Scales of Infant Development. Test-test reliability is good for parent report (0.91) as is inter-rater reliability (0.92).30 The Spanish language version of ASQ-3 is validated.33
Family environment covariates
Pre-injury family function was assessed over the past 6 months using the McMaster Family Assessment Device (FAD)–General Functioning Scale.34 FAD includes 12 items scored 1–4, with higher scores representing worse functioning. The Social Capital Index provides a total score measuring a person's connection to their community, including perceptions of personal, family, neighborhood, and spiritual community support, with higher scores representing more support.35 Family income relative to federal poverty level was calculated using self-reported income category and family size. Families reported receipt of ECI at 3 and 12 months.
Statistical analysis
All children with evaluable outcomes pre-injury and either the 3- or 12-month time point were included in the analysis. Because of small numbers of children with moderate TBI, the moderate and complicated-mild groups were combined for modeling based on previous studies showing similar outcomes.28 Generalized linear mixed models, with a subject-level random effect, and allowing for different residual variances for each time point, were fit for each outcome using R (R Foundation for Statistical Computing) and the gls function from the nlme package.36,37 Our strategy was to develop an explanatory multi-variate model for each outcome that addressed the hypotheses that earlier age at injury and higher injury severity would lead to worse outcomes. Additionally, we wished to identify other variables potentially important to very young children's outcomes that had been identified in studies of older children.
We used the following approach to construct multi-variable models for each outcome. First, we created a reference model that included a priori identified covariates to address our hypotheses. Theses covariates included the following: injury group (OI reference), time post-injury (centered at 12 months), and age at injury (continuous, centered at mean); clinical covariates known to affect outcome in very young children (pre-maturity and abusive injury); and covariates needed because of the study structure (study site and pre-injury scores). All two- and three-way interactions among injury group, time post-injury, and age at injury with likelihood ratio p value <0.15 were also included in the reference model for that outcome. Because sample size was not adequate to include all potentially relevant covariates, candidate covariates selected a priori that have been important to studies of older children were individually screened for inclusion using the reference model. These covariates included child demographics: sex, race/ethnicity, and previous diagnosis of health or behavioral problem; family demographics: income relative to poverty level, parent education, parent employment, parent marital status, and parent preferred language; and family functioning and Social Capital Index. Candidate covariates were retained if p < 0.15. Finally, in order to achieve a parsimonious model, a “full model” including all reference model variables and candidate covariates from the screening step was iteratively reduced by removal of covariates with p > 0.1. Reference model variables were retained in the final model regardless of significance. Statistical significance was evaluated at the 0.05 level. All tests were two-tailed. Finally, we created figures displaying interaction terms using categorized age and time for ease of interpretation.
Results
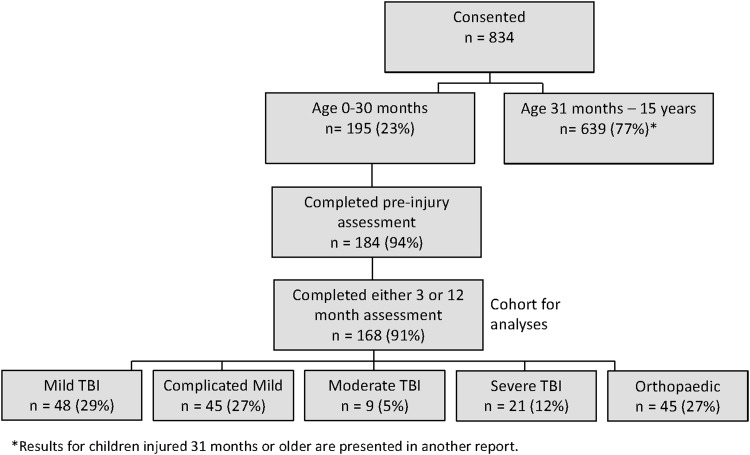
Consent was provided for 195 children injured before 31 months of age. Of those, 184 (94%) completed the pre-injury assessment; 168 (91%) with a pre-injury assessment completed either a 3- or 12-month assessment; the latter group comprises the cohort. Of the 168 children, 152 (90%) completed all follow-up assessments, whereas 9 (5%) completed only the 3-month and 7 (4%) completed only the 12-month assessment (Fig. 1). Parents completed the pre-injury interview a median of 7 days (interquartile range [IQR], 3, 13) after injury.
FIG. 1.
Flow diagram of cohort recruitment. TBI traumatic brain injury.
The study cohort consists of 48 (29%) children with mild TBI, 45 (27%) with complicated mild TBI, 9 (5%) with moderate TBI, and 21 (12%) with severe TBI. There are 45 (27%) children with OI. Overall, the cohort was composed of poor (37% under the poverty level), working (85% employed), and two-parent (66% married) families. The sample was diverse, with 51% non-Hispanic Caucasian and 31% Hispanic. Most families completed assessments by the Web (65%) and in the English language (86%; Table 1).
Table 1.
Child and Family Characteristics by Injury Severity and Type
| Mild TBI (N = 48) | Complicated mild TBI (N = 45) | Moderate TBI (N = 9) | Severe TBI (N = 21) | TBI Overall (N = 123) | Orthopedic (N = 45) | |
|---|---|---|---|---|---|---|
| Characteristic | n % | n % | n % | n % | n % | n % |
| Enrollment site | ||||||
| Utah | 19 (40) | 23 (51) | 6 (67) | 16 (76) | 64 (52) | 25 (56) |
| Texas | 29 (60) | 22 (49) | 3 (33) | 5 (24) | 59 (48) | 20 (44) |
| Response by web | 30 (63) | 31 (69) | 5 (56) | 15 (71) | 81 (66) | 29 (64) |
| Preferred language | ||||||
| English | 40 (83) | 40 (89) | 7 (78) | 20 (95) | 107 (87) | 37 (82) |
| Spanish | 8 (17) | 5 (11) | 2 (22) | 1 (5) | 16 (13) | 8 (18) |
| Age at injury (months)a: mean ± SD | 13.0 ± 9.0 | 8.9 ±(8.3) | 17.0 ± 7.1 | 12.0 ± 10.2 | 11.6 ± 9.0 | 20.2 ± 7.5 |
| Child sex: boy | 26 (54) | 21 (47) | 7 (78) | 14 (67) | 68 (55) | 27 (60) |
| Prematurea (<37 weeks) | 4 (8) | 6 (13) | 1 (11) | 1 (5) | 12 (10) | 4 (9) |
| Gestational age in weeks: range | 34, 36 | 34, 36 | 36, 36 | 36, 36 | 34, 36 | 32, 36 |
| Child race: | ||||||
| American Indian or Alaska Native | 0 (0) | 2 (4) | 0 (0) | 0 (0) | 2 (2) | 0 (0) |
| Asian | 0 (0) | 1 (2) | 1 (11) | 0 (0) | 2 (2) | 2 (4) |
| Black of African American | 9 (19) | 3 (7) | 1 (11) | 1 (5) | 14 (11) | 3 (7) |
| Native Hawaiian or other Pacific Islander | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 0 (0) |
| Caucasian | 36 (75) | 34 (76) | 5 (56) | 13 (65) | 88 (72) | 38 (84) |
| Mixed | 1 (2) | 5 (11) | 2 (22) | 6 (30) | 14 (11) | 2 (4) |
| Child ethnicity: Hispanic/Latino | 16 (33) | 12 (27) | 2 (25) | 6 (32) | 36 (30) | 14 (32) |
| Child with diagnosed health or behavioral problem (school or MD) | 2 (4) | 0 (0) | 1 (11) | 1 (5) | 4 (3) | 3 (7) |
| Respondent sex: female | 40 (83) | 36 (80) | 8 (89) | 16 (76) | 100 (81) | 38 (84) |
| Married: yes | 26 (55) | 29 (69) | 6 (67) | 14 (67) | 75 (63) | 33 (73) |
| Either caregiver employed | 40 (85) | 38 (86) | 8 (89) | 17 (81) | 103 (85) | 38 (84) |
| Respondent education | ||||||
| Less than high school | 8 (17) | 5 (11) | 2 (22) | 2 (10) | 17 (14) | 9 (20) |
| High school | 11 (23) | 18 (40) | 4 (44) | 4 (19) | 37 (30) | 8 (18) |
| Vocational/some college | 20 (42) | 11 (24) | 1 (11) | 10 (48) | 42 (34) | 13 (29) |
| Bachelor's degree or higher | 9 (19) | 11 (24) | 2 (22) | 5 (24) | 27 (22) | 15 (33) |
| Income at or below poverty level | 18 (41) | 13 (32) | 5 (62) | 3 (16) | 39 (35) | 18 (42) |
| Insurance type | ||||||
| None | 4 (8) | 3 (7) | 0 (0) | 1 (5) | 8 (7) | 0 (0) |
| Medicaid/CHIP | 24 (50) | 26 (58) | 5 (56) | 11 (52) | 66 (54) | 23 (51) |
| Commercial/private/military | 20 (42) | 16 (36) | 4 (44) | 9 (43) | 49 (40) | 22 (49) |
| McMaster FAD: mean ± SD | 1.4 ± 0.4 | 1.5 ± 0.5 | 1.5 ± 0.4 | 1.4 ± 0.5 | 1.4 ± 0.4 | 1.4 ± 0.5 |
| Social Capital Index: mean ± SD | 3.6 ± 1.1 | 3.4 ± 1.3 | 3.0 ± 1.3 | 3.9 ± 1.0 | 3.5 ± 1.2 | 3.5 ± 1.2 |
SD, standard deviation; MD, medical doctor; CHIP, Children's Health Insurance Program; FAD, Family Assessment Device; TBI, traumatic brain injury.
Enrollment was similar by site; however, more children with severe TBI were enrolled in Utah because of differences in Child and Family Services' decisions regarding enrollment of abused children. Injury groups had similar proportions of child sex, pre-maturity (32–36 weeks gestational age), race/ethnicity, preferred language, parent employment, and respondent education. Of the 16 pre-mature children, 9 (56%) were born at 36 weeks. Pre-injury ratings of family environment covariates were similar between injury groups. Demographic and family environment variables did not differ in severely injured children with AHT and other injury mechanisms.
Falls were the most frequent injury mechanism (n = 124; 74%) and the primary injury mechanism for the OI (87%), mild TBI (83%), and complicated mild TBI (84%) groups. AHT was the most frequent injury mechanism for children with severe TBI (52%). Most children with TBI had an isolated TBI (Table 2).
Table 2.
Injury Information by Injury Severity and Type
| Mild TBI (N = 48) | Complicated mild TBI (N = 45) | Moderate TBI (N = 9) | Severe TBI (N = 21) | All TBI (N = 123) | Orthopedic injury (N = 45) | |
|---|---|---|---|---|---|---|
| Injury severity/type | n % | n % | n % | n % | n % | n % |
| Admission type | ||||||
| ED/OBS only | 34 (71) | 7 (16) | 1 (11) | 0 (0) | 42 (34) | 31 (69) |
| Hospital, not PICU | 13 (27) | 23 (51) | 0 (0) | 0 (0) | 36 (29) | 14 (31) |
| PICU | 1 (2) | 15 (33) | 8 (89) | 21 (100) | 45 (37) | 0 (0) |
| Transport mode | ||||||
| Ambulance | 25 (52) | 24 (53) | 5 (56) | 0 (0) | 54 (44) | 18 (40) |
| Air transport | 6 (12) | 11 (24) | 4 (44) | 21 (100) | 42 (34) | 0 (0) |
| Private vehicle | 17 (35) | 10 (22) | 0 (0) | 0 (0) | 27 (22) | 27 (60) |
| Injury mechanism | ||||||
| Assault/child abuse | 2 (4) | 5 (11) | 3 (33) | 11 (52) | 21 (17) | 1 (2) |
| Fall | 40 (83) | 38 (84) | 4 (44) | 3 (14) | 85 (69) | 39 (87) |
| Motorized vehicle | 3 (6) | 1 (2) | 0 (0) | 4 (19) | 8 (7) | 0 (0) |
| Pedestrian/bicycle | 0 (0) | 0 (0) | 2 (22) | 1 (5) | 3 (2) | 2 (4) |
| Struck by or against | 2 (4) | 0 (0) | 0 (0) | 0 (0) | 2 (2) | 2 (4) |
| Other | 1 (2) | 1 (2) | 0 (0) | 2 (10) | 4 (3) | 1 (2) |
| Which extremity was fractured? | ||||||
| Arm | — | — | — | — | — | 22 (49) |
| Leg | — | — | — | — | — | 22 (49) |
| Both | — | — | — | — | — | 1 (2) |
| Head imaging in ED | 46 (96) | 45 (100) | 9 (100) | 21 (100) | 121 (98) | — |
| Median (IQR) | ||||||
|---|---|---|---|---|---|---|
| Glasgow Coma Scale | ||||||
| ED lowest post-resuscitation | 15 (15, 15) | 15 (15, 15) | 11 (10, 11) | 3 (3, 6) | — | — |
| ED motor score | 6 (6, 6) | 6 (6, 6) | 5 (5, 5) | 1 (1, 4) | — | — |
| Injury severity score | 4 (1, 6) | 9 (8, 10) | 16 (10, 24) | 20 (16, 25) | 9 (4, 16) | 5 (4, 9) |
| Abbreviated injury score | ||||||
| Head and neck | 2 (1, 2) | 3 (2, 3) | 3 (2, 4) | 4 (3, 4) | 3 (2, 3) | 0 (0, 0) |
| Maximum excluding head | 0 (0, 1) | 0 (0, 1) | 1 (1, 2) | 1 (0, 2) | 0 (0, 1) | 2 (2, 3) |
ED, emergency department; OBS, observation unit; PICU, pediatric intensive care unit; TBI, traumatic brain injury; IQR, interquartile range.
Pre-injury, 47 (28%) children were in the assess range on one or more ASQ-3 domains. Pre-injury ratings on the ASQ-3 and ASQ:SE did not differ between children with OI and TBI. Comparing only children with severe TBI to children with OI showed that more children with severe TBI had pre-injury ASQ-3 communication (24% vs. 2%; p = 0.01) and problem-solving (33% vs. 7%; p = 0.009) scores in the assess range. Both pre-maturity (odds ratio [OR], 3.2; 95% confidence interval [CI], 1.1, 10.2) and child abuse (OR, 2.7; 95% CI, 0.98, 7.5) were associated with at least one score in the assess range on pre-injury ASQ after adjustment for child sex, poverty, and Social Capital Index.
Injury severity and time post-injury
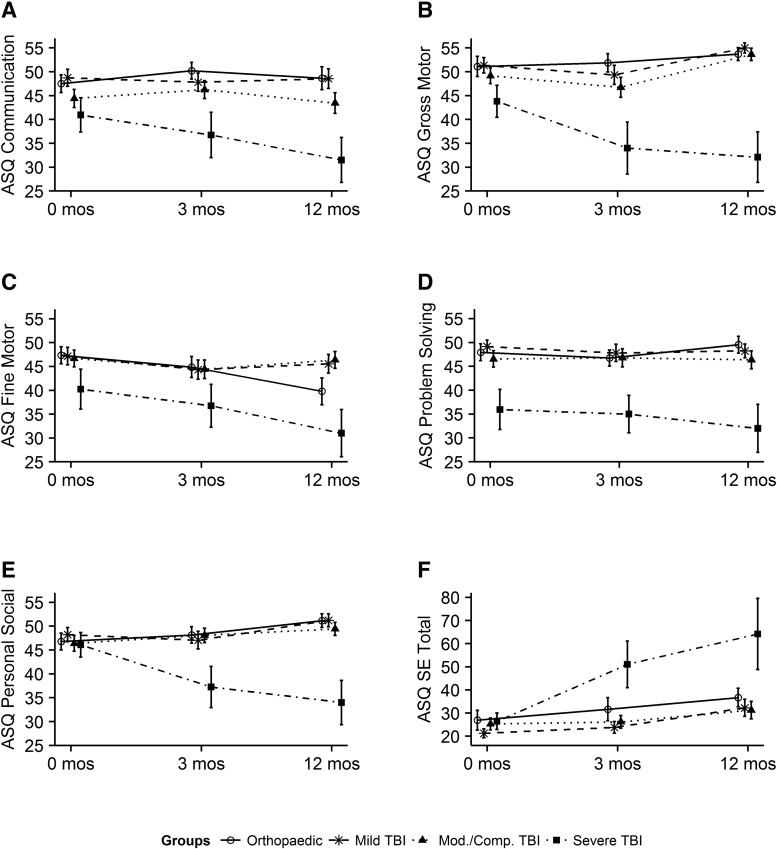
Children with mild and complicated mild/moderate TBI remained largely on their developmental track on the ASQ-3 and ASQ:SE. Figure 2 displays unadjusted mean scores of the ASQ-3 and ASQ:SE at pre-injury and 3 and 12 months by injury type and severity. Supplementary Figure 1 (see online supplementary material at http://www.liebertpub.com) displays the ASQ-3 and ASQ:SE scores at each time point excluding children with an abuse mechanism of injury. Patterns of scores are similar with and without those children injured by abuse. The pattern shown by the unadjusted scores remains similar after scores are adjusted for pre-injury ratings, AHT, and other model covariates seen in Table 3. AHT was included to ensure that associations of outcomes with age were not confounded by abuse as a mechanism of injury.
FIG. 2.
Raw mean scores of ASQ:3 domains and ASQ:SE by injury severity over time with standard errors. ASQ-3, Ages & Stages-3; ASQ:SE, Ages & Stages: Social-Emotional.
Table 3.
Ages & Stages-3 and Ages & Stages: Socioemotional Multi-Variate Model Results
| Communication | Gross motor | Fine motor | Problem solving | Personal social | Social emotional total | |
|---|---|---|---|---|---|---|
| Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | Estimate (95% CI) | |
| Pre-injury and demographic | ||||||
| (Intercept)a | 47.8 (43.7, 52.0) | 53.0 (49.4, 56.6)e | 41.3 (36.2, 46.4) | 47.7 (43.9, 51.4) | 51.7 (48.2, 55.3) | 40.5 (29.7, 51.3) |
| Site (Texas) | −0.37 (−3.82, 3.08) | 1.70 (−1.32, 4.71) | −0.73 (−4.34, 2.87) | 0.51 (−2.83, 3.86) | 0.02 (−3.01, 3.04) | 2.06 (−5.39, 9.52) |
| Pre-injury ratingb | 0.47 (0.34, 0.60) | 0.43 (0.30, 0.57) | 0.38 (0.25, 0.52) | 0.31 (0.17, 0.44) | 0.40 (0.27, 0.53) | 0.63 (0.44, 0.81) |
| Premature | −3.26 (−9.02, 2.49) | −1.90 (−7.19, 3.40) | −8.68 (−14.7, −2.69) | −10.2 (−15.6, −4.79) | −6.15 (−11.0, −1.31) | −9.49 (−21.7, 2.69) |
| Abusive injury | −7.53 (−13.5, −1.56) | −5.54 (−10.8, −0.30) | −3.01 (−9.25, 3.22) | −2.84 (−8.41, 2.72) | −6.11 (−11.1, −1.16) | 6.6 (−6.18, 19.4) |
| Injury severity and time | ||||||
| TBI severity | ||||||
| Mild | −1.70 (−6.41, 3.00) | 0.07 (−4.34, 4.47) | 4.56 (−1.92, 11.0) | −0.03 (−4.46, 4.41) | −0.34 (−4.8, 4.11) | −3.35 (−13.3, 6.60) |
| Cmild/moderate | −0.60 (−5.35, 4.15) | 0.89 (−3.55, 5.33) | 6.40 (−0.05, 12.8) | 0.87 (−3.64, 5.38) | −0.37 (−4.84, 4.11) | −1.38 (−11.4, 8.66) |
| Severe | −7.07 (−13.7, −0.48) | −15.2 (−21.1, −9.19)e | −6.72 (−15.4, 1.91) | −11.6 (−17.9, −5.29) | −16.8 (−22.8, −10.8) | 21.0 (7.32, 34.7) |
| Time (mos)c | −0.19 (−0.43, 0.06) | 0.52 (0.06, 0.97) | −0.59 (−1.13, −0.05) | 0.05 (−0.18, 0.28) | 0.31 (−0.06, 0.68) | 0.77 (0.36, 1.19) |
| Injury age (mos)b | 0.32 (0.06, 0.57) | −0.06 (−0.25, 0.14) | −0.16 (−0.38, 0.06) | 0.25 (0.02, 0.48) | 0.07 (−0.11, 0.25) | 0.46 (0.02, 0.89) |
| Interactions | ||||||
| Time × injury age | 0.04 (0.01, 0.06)d | −0.04 (−0.06, −0.01) | — | 0.03 (0.00, 0.05) | — | — |
| TBI severity × time | ||||||
| Mild | — | 0.05 (−0.56, 0.66) | 0.66 (−0.09, 1.40) | — | 0.16 (−0.35, 0.68) | — |
| Cmild/mod | — | 0.06 (−0.56, 0.67) | 0.79 (0.06, 1.52) | — | −0.14 (−0.64, 0.37) | — |
| Severe | — | −0.77 (−1.54, −0.01)e | −0.16 (−1.09, 0.77) | — | −0.72 (−1.37, −0.07) | — |
| Family environment | ||||||
| Family functionb | — | −2.71 (−5.87, 0.45) | — | — | — | — |
| Poverty levela | — | — | — | 1.70 (0.62, 2.77) | 1.31 (0.36, 2.26) | — |
| Married | — | — | — | — | — | −8.67 (−16.6, −0.79) |
Cmild/moderate = complicated mild and moderate; mos. = months.
Reference levels of model variables: Utah site, child demographics (not premature, non-abusive injury, orthopedic injury), parents not married.
The intercept represents the average score of a child with orthopedic injury at 12 months after injury and other variables at their reference levels.
Centered at the mean for analysis.
Time from injury at assessment was centered at 1 year from injury.
For each 1-month increase in age at injury, the slope between 3 and 12 months post-injury increases, on average, by 0.04 (95% CI, 0.01, 0.06) points per month, adjusting for other covariates. Because the slope for Time is negative, −0.19 (95% CI, −0.43, 0.06), children at younger ages have negative slopes, and children at older ages have positive slopes. The slope is 0 (no change over time) at an age at injury of 18.65 months, because 0.04*(18.65-average age:13.9)-0.19 = 0.
At the final 12-month time point, the orthopedic group has an average Gross Motor score of 56.88 (95% CI, 51.32, 62.44, with other variables at their reference levels), and the severe group is 15.16 (95% CI, −21.13, −9.19; p < 0.001) points lower, adjusting for pre-injury Gross Motor and other covariates. The slope between 3 and 12 months is, on average, 0.77 (95% CI, −1.54, −0.01) points/month lower in the severe group in comparison to the orthopedic group (p = 0.049).
TBI, traumatic brain injury; CI, confidence interval.
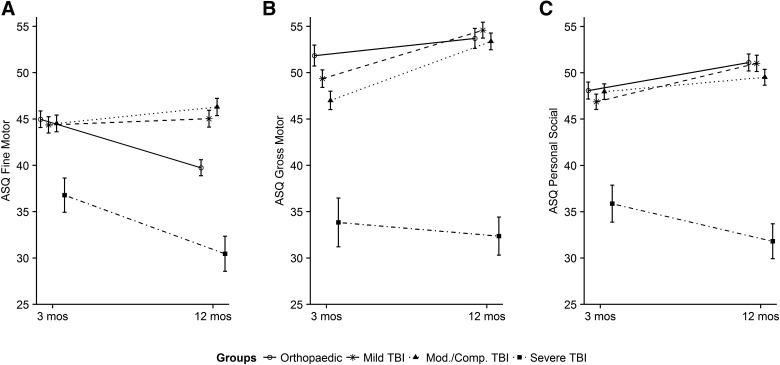
Sixty percent of children with severe TBI were in the assess range of at least one domain of the ASQ. Children with severe TBI had significant decrements in ASQ-3 communication, gross motor, problem-solving, personal-social, and social-emotional domains. For example, in the gross motor domain, the OI group had an average gross motor score of 53.0 at 12 months post-injury (intercept). The severe TBI group was 15.2 points (95% CI, −21.1, −9.9) lower than the OI group at 12 months post-injury after adjusting for pre-injury score and all other covariates. An interaction between TBI severity and time post-injury showed that the severe TBI group worsened by 0.77 points per month (95% CI, −1.54, −0.01) between the 3- and 12-month time points compared to the OI group, as shown in Figure 3. On the ASQ:SE, the severe group, on average, scored 21.0 points higher (worse) after adjustment for model covariates.
FIG. 3.
Interaction of injury severity with time since injury for ASQ:3 fine motor, gross motor, and problem-solving domains with standard errors. ASQ-3, Ages & Stages-3; TBI traumatic brain injury.
Age at injury
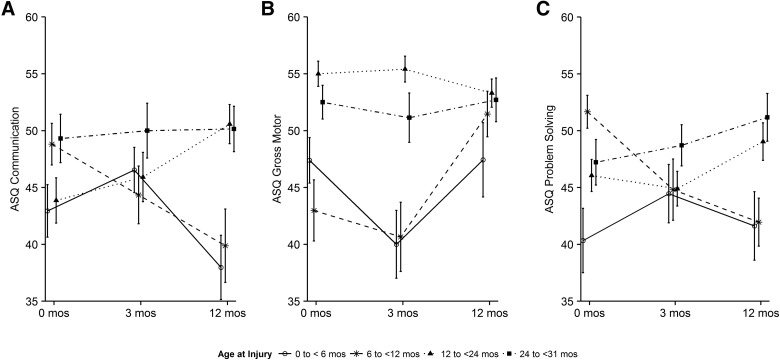
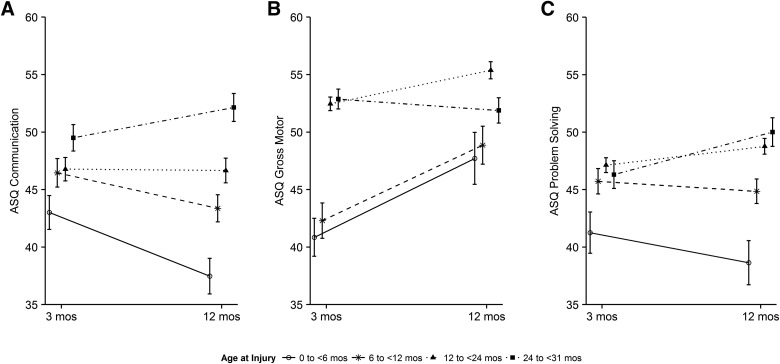
Age at injury interacted with time post-injury for ASQ communication, gross motor, and problem-solving domains. Figure 4 displays raw mean scores of age groups over time for these outcomes. Table 3 shows that ASQ communication scores were increased at the 1-year follow-up by 0.32 points for every 1-month increase in age at injury. The time by injury age interaction term shows that over time, on average, a 1-month increase in age was associated with a 0.04-point (95% CI, 0.01, 0.06) increase in score; however, because the slope for time is negative (−0.19; 95% CI, −0.43, 0.06), the average slope was negative for younger children, indicating worsening outcomes over time and positive for older children indicating improving outcomes over time. Figure 5 helps to visualize the interaction of age at injury (categorized) with time post-injury in the modeled results, which take the children's pre-injury scores into account. Consistent with the model results, Figure 5 shows that children in the youngest three age groups (0 to <6, 6 to <12 months, and 12 to <24) have decreasing scores in communication from 3 to 12 months, whereas children in the 24- to 31-month age group improved. Results for problem solving were similar: The youngest two age groups had decreasing scores, and the two older groups improved. Gross motor scores were significantly reduced in the two youngest groups at 3 months, but improved between 3 and 12 months. Age at injury was significantly associated with the ASQ:SE, with worse scores in older children. Age effects were consistent in strength and direction when premature children were excluded from the models (data not shown).
FIG. 4.
Raw mean scores of ASQ:3 communication, gross motor and problem-solving by age group with standard errors. ASQ-3, Ages & Stages-3.
FIG. 5.
Interaction of age at injury with time post-injury for ASQ:3 communication, gross motor, and problem-solving with standard errors. ASQ-3, Ages & Stages-3.
Injury mechanism and family covariates
Children with AHT as the injury mechanism had worse scores in ASQ-3 communication, gross motor, and personal-social skills domains in the adjusted analysis. Poverty was associated with both ASQ-3 problem-solving and personal-social scales. Relative to a child whose family was at the federal poverty level, a child from a family at 5 times above the poverty level had a 6.8-point higher score on problem-solving and 5.2-point higher personal-social score. Children living with married parents scored 8.7 points lower (better) on the ASQ:SE compared to children with single or unmarried parents.
Early childhood intervention services
At 12 months post-injury, 25% of children were in the monitor and 28% in the assess range on one or more ASQ-3 scales. Few families reported ECI services at 3 (n = 9; 6%) or 12 months (n = 8; 5%) post-injury. Most children (n = 7) receiving services at 12 months were in the moderate or severe TBI groups.
Discussion
Our data demonstrate that key risk factors for adverse outcomes 1 year after injury include injury sustained in infancy and severe TBI. Understanding which young children with TBI are at risk for developmental deficits is important given that ECI services are known to be helpful, but are not always considered or mandated for children with TBI.38
After controlling for the effects of pre-injury functioning, pre-term birth, AHT, and other covariates, younger age at injury was associated with impairments in core outcomes. On the ASQ-3 communication and problem-solving outcomes, children <6 months of age failed to make gains at a developmentally appropriate rate, resulting in a progressively larger deficit between their scores and those of their peers over the first year post-injury. Children in the 6- to <12-month age group also failed to make gains in communication. In gross motor skills, these two youngest groups of children scored lower than their older peers at the 3-month follow-up time point, but had made gains at the 12-month follow-up. These findings extend those of pre-school– and school-aged children, where younger age at injury negatively affected the trajectory of children's development and subsequent cognitive and academic performance.6,7,39
The overall good outcomes of infants and toddlers with mild, complicated mild/moderate TBI are reassuring. These children, on average, remained developmentally on track, comparable to children with OI. This finding is consistent with an earlier study of children with mild TBI preceding 30 months of age in whom IQ and behavior 2 years after injury were similar to typically developing children.11 Bellerose and colleagues found that whereas children with mild TBI injured between 18 and 60 months of age scored similarly to children with OI and uninjured children on tests of intelligence and adaptive behavior, they scored more poorly when asked to think about another person's desires (theory of mind).18 This suggests that children with mild TBI may have subtle pre-injury differences or post-injury changes not reflected on the ASQ:SE or ASQ:3 personal-social skills.
Children with severe TBI have a global injury reflected in decrements across ASQ-3 and ASQ:SE scales, including increasing relative deficits in gross motor skills over time. Children with AHT represented approximately half of those with severe TBI consistent with previous studies.16 This group had increased difficulties in communication, gross motor, and personal-social outcomes compared to children injured by other mechanisms, even after adjusting for family environment. This result is similar to other reports of children's development after abusive TBI.15,17,40 In spite of their low level of functioning and poor prognosis, less than one third of children in the severe injury group received ECI services. These rates are low, given that 60% of children with severe TBI scored in the assess range, with developmental scores below the third percentile, at the 12-month follow-up. Rehabilitative services improve outcomes of young children with abusive and non-abusive severe TBI.41 Physicians may not consider ECI for TBI, although it is routine for infants with congenital disorders and pre-maturity. Additionally, funding challenges for ECI have limited its availability for some children, resulting in substantial unmet service needs.42 Failure to receive ECI likely reflects a gap in the continuum of care from acute hospitalization to outpatient community-based rehabilitation services. Additionally, failure to receive ECI may contribute to gaps in subsequent educational service delivery given that ECI is a pipeline for referring pre-school–aged children to public school intervention programs.
Lower levels of pre-injury functioning contributed to poor outcomes. A high percentage of children with TBI scored below-expected levels in one or more ASQ-3 domains pre-injury. Our findings are similar to the lower scores noted on several pre-injury ASQ:SE scales in infants and toddlers with mild TBI.43 Ascertainment of pre-injury levels of functioning is important to allow discrimination of post-injury changes and identify children at high risk for post-injury developmental difficulties.
Poverty, social capital, and family function were important for specific ASQ domains. However, family environment was not as important to outcomes as observed in an older cohort where social capital and family income were important across cognitive and behavioral domains.23 It is possible that the effects of family environment are more pronounced in older children or will emerge in this very young cohort over time. Very young children also have a very broad range of normal development; therefore, it is possible that we were unable to detect the effects of family environment on outcome with precision.
Results of this study should be viewed in light of its limitations. ASQ-3 and ASQ:SE are screening tools that use parent report. These tools are used widely by general pediatricians, useful to follow children longitudinally, accurate from parent report, correlate highly with results of individualized assessments, and cover the main developmental tasks required by infants and children. However, they may not detect subtle problems that children may have after a mild TBI. Thus, whereas it is reassuring that children with mild and complicated mild/moderate TBI remain developmentally on track, we cannot say that these children will have no consequence of their injury. Imaging was not used to further classify injury severity beyond differentiating mild and complicated mild TBI; therefore, we were unable to assess extent of parenchymal injury among moderate and severe injury types. It is possible for children within GCS levels and age groups to have differing severity of physical injury to the brain, which may have affected outcomes.
The number of children with moderate TBI was very small. We chose to group them with complicated mild TBI for analysis as in previous studies.28 However, grouping moderate and complicated mild TBI may obscure outcome differences.
Strengths of the study include the sample size, documentation of pre-injury development, injury comparison group, consideration of demographic and family environment factors that impact outcomes, and a longitudinal design allowing evaluation of age, injury characteristics, and time on the trajectory of developmental outcomes.
Conclusion
Severe TBI sustained during the most rapid phase of brain development has serious adverse consequences for children's developmental outcomes and places them at high risk for later problems. Clinical implications of this work include that a wait-and-see approach for consideration of ECI for infants with TBI and infants and toddlers who sustain a severe TBI should not be taken. These groups should be followed closely for their subsequent development and referred for ECI promptly if problems are detected.
Supplementary Material
Acknowledgments
This study was supported by the CDC under Cooperative Agreement U01/CE002188. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC. This study was supported also by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; K24HD072984). Neither the NICHD nor the CDC played a role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Taylor C.A., Bell J.M., Breiding M.J., and Xu L. (2017). Traumatic brain injury-related emergency department visits, hospitalizations, and deaths—United States, 2007 and 2013. MMWR Surveill. Summ. 66, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hebb D.O. (1942). The effects of early and late brain injury upon test scores, and the nature of normal adult intelligence. Proc. Am. Phil. Soc. 85, 275–292 [Google Scholar]
- 3. Dubois J., Dehaene-Lambertz G., Kulikova S., Poupon C., Huppi P.S., and Hertz-Pannier L. (2014). The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 276, 48–71 [DOI] [PubMed] [Google Scholar]
- 4. Rogers C.E., Smyser T., Smyser C.D., Shimony J., Inder T.E., and Neil J.J. (2016). Regional white matter development in very preterm infants: perinatal predictors and early developmental outcomes. Pediatr. Res. 79, 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ewing-Cobbs L., Barnes M.A., and Fletcher J.M. (2003). Early brain injury in children: development and reorganization of cognitive function. Dev. Neuropsychol. 24, 669–704 [DOI] [PubMed] [Google Scholar]
- 6. Ewing-Cobbs L., Barnes M., Fletcher J.M., Levin H.S., Swank P.R., and Song J. (2004). Modeling of longitudinal academic achievement scores after pediatric traumatic brain injury. Dev. Neuropsychol. 25, 107–133 [DOI] [PubMed] [Google Scholar]
- 7. Anderson V., Catroppa C., Morse S., Haritou F., and Rosenfeld J. (2005). Functional plasticity or vulnerability after early brain injury? Pediatrics 116, 1374–1382 [DOI] [PubMed] [Google Scholar]
- 8. Bronfenbrenner U. (1977). Toward an experimental model of human development. Am. Psychol. 32, 515–531 [Google Scholar]
- 9. Cheong J.L., Doyle L.W., Burnett A.C., Lee K.J., Walsh J.M., Potter C.R., Treyvaud K., Thompson D.K., Olsen J.E., Anderson P.J., and Spittle A.J. (2017). Association between moderate and late preterm birth and neurodevelopment and social-emotional development at age 2 Years. JAMA Pediatr. 171, e164805. [DOI] [PubMed] [Google Scholar]
- 10. Dennis M., Spiegler B.J., Simic N., Sinopoli K.J., Wilkinson A., Yeates K.O., Taylor H.G., Bigler E.D., and Fletcher J.M. (2014). Functional plasticity in childhood brain disorders: when, what, how, and whom to assess. Neuropsychol. Rev. 24, 389–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crowe L.M., Catroppa C., Babl F.E., and Anderson V. (2012). Intellectual, behavioral, and social outcomes of accidental traumatic brain injury in early childhood. Pediatrics 129, e262–e268 [DOI] [PubMed] [Google Scholar]
- 12. Ewing-Cobbs L., Prasad M.R., Kramer L., Cox C.S., Jr., Baumgartner J., Fletcher S., Mendez D., Barnes M., Zhang X., and Swank P. (2006). Late intellectual and academic outcomes following traumatic brain injury sustained during early childhood. J. Neurosurg. 105, 287–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crowe L.M., Catroppa C., Babl F.E., and Anderson V. (2013). Executive function outcomes of children with traumatic brain injury sustained before three years. Child Neuropsychol. 19, 113–126 [DOI] [PubMed] [Google Scholar]
- 14. Ewing-Cobbs L., Prasad M.R., Mendez D., Barnes M.A., and Swank P. (2013). Social interaction in young children with inflicted and accidental traumatic brain injury: relations with family resources and social outcomes. J. Int. Neuropsychol. Soc. 19, 497–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Keenan H.T., Hooper S.R., Wetherington C.E., Nocera M., and Runyan D.K. (2007). Neurodevelopmental consequences of early traumatic brain injury in 3-year-old children. Pediatrics 119, e616–e623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keenan H.T., Runyan D.K., Marshall S.W., Nocera M.A., Merten D.F., and Sinal S.H. (2003). A population-based study of inflicted traumatic brain injury in young children. JAMA 290, 621-626 [DOI] [PubMed] [Google Scholar]
- 17. Ewing-Cobbs L., Kramer L., Prasad M., Canales D.N., Louis P.T., Fletcher J.M., Vollero H., Landry S.H., and Cheung K. (1998). Neuroimaging, physical, and developmental findings after inflicted and noninflicted traumatic brain injury in young children. Pediatrics 102, 300–307 [DOI] [PubMed] [Google Scholar]
- 18. Bellerose J., Bernier A., Beaudoin C., Gravel J., and Beauchamp M.H. (2017). Long-term brain-injury-specific effects following preschool mild TBI: a study of theory of mind. Neuropsychology 31, 229–241 [DOI] [PubMed] [Google Scholar]
- 19. Ewing-Cobbs L., Fletcher J.M., Levin H.S., Francis D.J., Davidson K., and Miner M.E. (1997). Longitudinal neuropsychological outcome in infants and preschoolers with traumatic brain injury. J. Int. Neuropsychol. Soc. 3, 581–591 [PubMed] [Google Scholar]
- 20. Bernard C.O., Ponsford J.A., McKinlay A., McKenzie D., and Krieser D. (2016). Predictors of post-concussive symptoms in young children: injury versus non-injury related factors. J. Int. Neuropsychol. Soc. 22, 793–803 [DOI] [PubMed] [Google Scholar]
- 21. Wade S.L., Zhang N., Yeates K.O., Stancin T., and Taylor H.G. (2016). Social environmental moderators of long-term functional outcomes of early childhood brain injury. JAMA Pediatr. 170, 343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yeates K.O., Bigler E.D., Dennis M., Gerhardt C.A., Rubin K.H., Stancin T., Taylor H.G., and Vannatta K. (2007). Social outcomes in childhood brain disorder: a heuristic integration of social neuroscience and developmental psychology. Psychol. Bull. 133, 535–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keenan H.T., Clark A.E., Holubkov R., Cox C.S., and Ewing-Cobbs L. (2017). Psychosocial and executive function recovery trajectories one year after pediatric traumatic brain injury: the influence of age and injury severity. J. Neurotrauma 15, 286–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Marr A.L., and Coronado V.G. (2004). Central Nervous System Injury Surveillance Data Submission Standards: 2002. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 25. Reilly P.L., Simpson D.A., Sprod R., and Thomas L. (1988). Assessing the conscious level in infants and young children: a paediatric version of the Glasgow Coma Scale. Childs Nerv. Syst. 4, 30–33 [DOI] [PubMed] [Google Scholar]
- 26. National Center for Injury Prevention and Control. (2003). Report to Congress on Mild Traumatic Brain Injury in the US: Steps to Prevent a Serious Public Health Probelm. Centers for Disease Control and Prevention: Atlanta, GA [Google Scholar]
- 27. Carroll L.J., Cassidy J.D., Holm L., Kraus J., and Coronado V.G.; WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. (2004). Methodological issues and research recommendations for mild traumatic brain injury: the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 36, Suppl. 43, 113–125 [DOI] [PubMed] [Google Scholar]
- 28. Levin H.S., Hanten G., Roberson G., Li X., Ewing-Cobbs L., Dennis M., Chapman S., Max J.E., Hunter J., Schachar R., Luerssen T.G., and Swank P. (2008). Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. J. Neurosurg. Pediatr. 1, 461–470 [DOI] [PubMed] [Google Scholar]
- 29. Gennarelli T.A., and Wodzin E. (2008). Abbreviated Injury Scale 2005. Association for the Advancement of Automotive Medicine: Des Plaines, IL [Google Scholar]
- 30. Squires J., Potter L., and Bricker D. (2009). The ASQ Users Guide. Brookes: Baltimore, MD [Google Scholar]
- 31. Squires J., Bricker D., and Twombly E. (2002). Ages & Stages Questionnaire: Social-Emotional (ASQ:SE): A Parent-Completed, Child Monitoring System for Social-Emotional Behavior. Brookes: Baltimore, MD [Google Scholar]
- 32. Squires J., and Bricker D. (2009). Ages & Stages Questionnaires in Spanish. Brookes: Baltimore, MD [Google Scholar]
- 33. Schonhaut L., Armijo I., Schonstedt M., Alvarez J., and Cordero M. (2013). Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics 131, e1468–e1474 [DOI] [PubMed] [Google Scholar]
- 34. Miller I.W., Bishop D.S., Epstein N.B., and Kietner G.I. (1985). The McMaster family assessment device: Reliability and validity. J. Marital Fam. Ther. 11, 345–356 [Google Scholar]
- 35. Runyan D.K., Hunter W.M., Socolar R.R., Amaya-Jackson L., English D., Landsverk J., Dubowitz H., Browne D.H., Bangdiwala S.I., and Mathew R.M. (1998). Children who prosper in unfavorable environments: the relationship to social capital. Pediatrics 101, 12–18 [DOI] [PubMed] [Google Scholar]
- 36. R Core Team. (2016). R: a language and environment for statistical computing. R Foundation for Statistical Computing: Vienna, Austria [Google Scholar]
- 37. Pinheiro J., Bates D., DebRoy S., Sarkar D., and R Core Team. (2016). _nlme: Linear and Nonlinear Mixed Effects Models_. T package version 3.1-128. https://cran.r-project.org/web/packages/nlme/index.html (last accessed August27, 2018)
- 38. Prasad M.R,. and Ewing-Cobbs L. (2014). Pediatric traumatic brain injury: outcome, assessment, and intervention, in: Handbook on the Neuropsychology of Traumatic Brain Injury. Sherer M., and Sander A.M. (eds). Springer: New York, NY [Google Scholar]
- 39. Prasad M.R., Swank P.R., and Ewing-Cobbs L. (2017). Long-term school outcomes of children and adolescents with traumatic brain injury. J. Head Trauma Rehabil. 32, E24–E32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barlow K.M., Thomson E., Johnson D., and Minns R.A. (2005). Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics 116, e174–e185 [DOI] [PubMed] [Google Scholar]
- 41. Risen S.R., Suskauer S.J., Dematt E.J., Slomine B.S., and Salorio C.F. (2014). Functional outcomes in children with abusive head trauma receiving inpatient rehabilitation compared with children with nonabusive head trauma. J. Pediatr. 164, 613–619.e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Texans Care for Children and Methodist Healthcare Ministries of South Texas, Inc. (2016). Left out: the impact of state cuts to early childhood intervention (ECI) for young Texas kids with disabilities. http://www.tcdd.texas.gov/wp-content/uploads/2016/10/Handout-Left-Out-ECI-Report-1031.pdf (last accessed June12, 2018)
- 43. Kaldoja M.L., and Kolk A. (2015). Does gender matter? Differences in social-emotional behavior among infants and toddlers before and after mild traumatic brain injury: a preliminary study. J. Child Neurol. 30, 860–867 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.