Abstract
Purpose
To evaluate the risk of prematurity and infant mortality by maternal fertility status, and for in vitro fertilization (IVF) pregnancies, by oocyte source and embryo state combinations.
Methods
Women in 14 States who had IVF-conceived live births during 2004–13 were linked to their infant’s birth and death certificates; a 10:1 sample of non-IVF births was selected for comparison; those with an indication of infertility treatment on the birth certificate were categorized as subfertile, all others were categorized as fertile. Risks were modeled separately for the fertile/subfertile/IVF (autologous-fresh only) group and for the IVF group by oocyte source-embryo state combinations, using logistic regression, and reported as adjusted odds ratios (AORs) and 95% confidence intervals (CI).
Results
The study population included 2,474,195 pregnancies. Placental complications (placenta previa, abruptio placenta, and other excessive bleeding) and prematurity were both increased with pregestational and gestational diabetes and hypertension, among subfertile and IVF groups, and in IVF pregnancies using donor oocytes. Both subfertile and IVF pregnancies were at risk for prematurity and NICU admission; IVF infants were also at risk for small-for-gestation birthweight, and subfertile infants had greater risks for neonatal and infant death. Within the IVF group, pregnancies with donor oocytes and/or thawed embryos were at greater risk of large-for-gestation birthweight, and pregnancies with thawed embryos were at greater risk of neonatal and infant death.
Conclusions
Prematurity was associated with placental complications, diabetes and hypertension, subfertility and IVF groups, and in IVF pregnancies, donor oocytes and/or thawed embryos.
Keywords: Embryo state, Fertility status, Infant morbidity, Infant mortality, Oocyte source, Placental complications, Prematurity
Introduction
In 2015 in the USA, there were nearly 73,000 babies born from in vitro fertilization (IVF), accounting for 1.8% of all births, a proportion which has doubled since 2000 [1–4]. It is well-established that both assisted reproductive technology (ART) and subfertility, independent of treatment, are associated with compromised maternal and infant perinatal outcomes [5–12]. A persistent and unresolved issue is how much of this excess risk is due to the biology of the subfertile couple versus the ART treatments used to achieve a live birth [13–17]. The purpose of this analysis is to evaluate the risk of prematurity and infant morbidity and mortality for singletons and twins by maternal fertility status, and for IVF pregnancies, by oocyte source and embryo state combinations.
Materials and methods
This study involved linking data from the national IVF database, the Society for Assisted Reproductive Technology Clinic Outcome Reporting System (SART CORS), to birth certificates as part of a larger study in 14 States on assisted reproductive technology (ART) and risk of childhood cancer (NIH grant R01 CA151973). The data for this analysis was limited to live births (≥ 22 weeks’ gestation and ≥ 300 g birthweight). Two comparison groups were identified. First, women classified as fertile, subfertile, and IVF-treated (limited to autologous oocytes-fresh embryos [autologous-fresh]) were compared, with fertile women as the reference group (fertile and subfertile are defined in the birth certificate section below). Second, within the IVF-treated population, women were categorized by oocyte source-embryo state combinations (autologous-fresh, autologous-thawed, donor-fresh, and donor-thawed) used in their IVF cycle, with women using autologous-fresh cycles as the reference group.
SART CORS data
The Society for Assisted Reproductive Technology (SART) maintains Health Insurance Portability and Accountability Act of 1996 (HIPAA)-compliant Business Associate Agreements with its 375 reporting clinics. In 2004, following a contract change with the Centers for Disease Control and Prevention, SART leveraged the SART CORS data system for the purposes of conducting research. The database includes information on demographic factors, IVF diagnoses and treatment parameters, and pregnancy outcomes. The data in the SART CORS are validated annually with some clinics having on-site visits for chart review. During each visit, data reported by the clinic are compared with information recorded in the medical record; most data fields have discrepancy rates less than 2%, with diagnosis fields ranging from 2 to 5% [4].
Birth and death certificate data
Births during the study time period (2004–13) included both the 1989 and 2003 revisions of the US Certificate of Live Birth. Of the 14 States in this study, the 2003 revision was implemented in 2003 (Pennsylvania), 2004 (Florida and New York State), 2005 (Texas), 2006 (California and Ohio), 2007 (Colorado and Michigan), 2008 (New York City), 2010 (Illinois and North Carolina), 2011 (Massachusetts), and 2012 (Virginia); New Jersey and Connecticut implemented the 2003 revision after the study time period (2015 and 2016, respectively). Therefore, data from both the 1989 and 2003 revisions of the birth certificate are included for births in this study. Data from the 1989 revision of the birth certificate included the following placental complications: abruptio placenta: premature separation of a normally implanted placenta from the uterus; placenta previa: implantation of the placenta over or near the internal opening of the cervix; and other excessive bleeding: the loss of a significant amount of blood from conditions other than abruptio placenta or placenta previa. In the 2003 revision of the birth certificate, three checkboxes were added to indicate that (1) the pregnancy resulted from infertility treatment, (worded as: if yes, check all that apply): (2) Fertility-enhancing drugs, artificial insemination, or intrauterine insemination; (3) Assisted reproductive technology (e.g., IVF [in vitro fertilization], GIFT [gamete intrafallopian transfer]). Pregnancies which linked to the SART CORS cycles were categorized as IVF; pregnancies with an indication that it resulted from infertility treatment (via the infertility checkbox) but did not link to an IVF cycle were categorized as subfertile; the remaining pregnancies were categorized as fertile. Known limitations of birth certificate data include the unreliability of selected items (such as maternal weight gain), the high rate of missing values for other items (such as father’s age and race/ethnicity, maternal height and prepregnancy weight) [1]. The validity of the 1989 and 2003 revisions of the birth certificate data using the medical record as the gold standard has been assessed, with most items reported accurately, with high specificity and wide variance in sensitivity, reflecting that if a rare condition was present, it often was not documented, but if the condition was documented, it was likely that it was present [18–27]. All States routinely link infant death certificates to their corresponding birth certificates for legal and statistical purposes. When the birth and death of an infant occur in different States, copies of the records are exchanged by the State of death and State of birth in order to affect a link. In addition, if a third State is identified as the State of residence at the time of birth or death that State is also sent a copy of the appropriate certificate by the State where the birth or death occurred. Infant deaths were classified by age at occurrence as neonatal (birth to 27 days), postneonatal (28–364 days), and infant (birth to 364 days). Cause of death, based on International Classification of Diseases, Tenth Revision (ICD-10) was summarized, and the leading ten causes by plurality and age at death (neonatal, postneonatal, and infant mortality) compared with national statistics for the United States [28].
Linkage procedure
In the course of conducting a study on childhood cancer following IVF, we linked the SART CORS data and State Vital Records. Each State received a file of cycles of women who were residents of that State. To begin the linkage process, a limited data file was generated by Redshift Technologies, Inc., the organization which maintains the SART CORS on behalf of SART, containing only the following factors: study-specific patient ID and cycle ID, woman’s first, middle name or initial, and last names, social security number, date of birth, zip code of residence, date of cycle outcome (live birth), plurality of the live birth, gender(s) and birthweight(s) of the infant(s). The State then performed a linkage to identify the IVF births; 91% of IVF-conceived births in the SART CORS were linked to their respective birth certificates. For each delivery identified as having been conceived by IVF, we requested that the subsequent 10 deliveries (all liveborn infants from a pregnancy) be selected as the non-IVF comparison group, although not all States implemented this request, providing the next 10 births (individual children) instead, and often only one infant from a twin or triplet + birth. The files of the study children were then linked to each State’s vital records (death certificates). Once all data was linked and complete, the files were stripped of all identifying elements (such as names, dates, social security numbers, and any other information that could identify an individual), but retaining the patient ID and cycle ID for the IVF group. The de-identified files were then transmitted to the investigators using secure file transfer methods. For the investigators, Redshift created a de-identified data file with the study-specific patient ID and cycle ID, and the IVF treatment parameters, and sent the file by secure file transfer methods. We then merged the two de-identified data files using the patient ID and cycle ID. This study was approved by the Institutional Review Boards at Michigan State University, the University of Michigan, the University of Minnesota, and each of the State Departments of Health.
The data files received from the States were indexed by infant. However, in this study, the analysis was by mother. Although the family structure (identification of siblings) could be reliably determined for the IVF infants, this was not true for the controls, as discussed above. Therefore, each record of a multiple birth was weighted by 1/plurality; i.e., if the birth was recorded as a twin, each record would receive the weight of ½. Summing the two records in the same family using this weight would then estimate the mother’s outcome correctly. Since some States enumerated all infants from the same birth in the comparison group, while other States sampled births rather than deliveries, the weighting would underestimate the number of mothers of multiples (i.e., will be conservative with respect to any statistical test). The standard deviations reported were not weighted, since weighting reduces the estimate of the standard deviation.
Variables
Independent variables included oocyte source-embryo state combinations (autologous-fresh, autologous-thawed, donor-fresh, donor-thawed), maternal age at delivery (continuous and as 18–29, 30–34, 35–37, 38–40, and ≥ 41 years), race (white, black, Asian, other) and Hispanic ethnicity, education (less than 8th grade, some high school, high school graduate or GED, some college or associate degree, bachelor’s degree, or post-graduate education), maternal prepregnancy medical conditions (hypertension and diabetes mellitus), and parity (nulliparous, 1, or ≥ 2). IVF treatment parameters included the number of prior IVF cycles, infertility diagnoses (male factor, endometriosis, ovulation disorders, diminished ovarian reserve, tubal factors, uterine factors, other factors, and unexplained), number of embryos transferred (1, 2, > 2), and number of fetal heartbeats at 6 weeks’ gestation (1, 2, or > 2). The pregnancy, birth, and infant outcomes included pregnancy complications (gestational diabetes and pregnancy hypertension), placental complications (other excessive bleeding, placenta previa, and abruptio placenta), mode of delivery (vaginal, cesarean, and repeat cesarean), and infant sex; State and year of birth were also included in the models. Dependent variables included preterm birth (very preterm, 22–27 weeks; early preterm, 22–32 weeks; and preterm, 22–36 weeks), very low birthweight (< 1500 g), low birthweight (< 2500 g), small-for-gestation birthweight (SGA, birthweight z-score ≤ −1.28), large-for-gestation birthweight (LGA, birthweight z-score ≥ 1.28), neonatal intensive care unit (NICU) admission, neonatal death (days 0–27 of life), postneonatal death (28–364 days), and infant death (days 0–364), as well as the placental complications. Birthweight z-scores were calculated using gender-specific national standards [29] as recommended by Land [30], with z-scores ≤ − 1.28 reflecting birthweight below the 10th percentile for gestation, and z-scores ≥ 1.28 indicating birthweight above the 90th percentile for gestation.
Statistical methods
We modeled the risk of placental complications (placenta previa, abruptio placenta, and other excessive bleeding) by diabetes and hypertension (pregestational and gestational), and fertility group and IVF group, separately by plurality. We modeled the risks of very early preterm birth, early preterm birth, preterm birth, SGA, LGA, NICU admission, neonatal death and infant death by fertility group and IVF group, by diabetes and hypertension, and placental complications, separately by plurality. The fertility group included the fertile/subfertile/IVF [autologous-fresh only] study population (pregnancies to fertile women were the reference group) (model 1), and the within IVF study population (autologous-fresh/autologous-thawed/donor-fresh/donor-thawed; pregnancies to women with autologous-fresh cycles were the reference group) (model 2) using logistic regression as adjusted odds ratios (AOR) and 95% confidence intervals. Models were adjusted for maternal fertility status, age, race and ethnicity, parity, pre-existing conditions (diabetes mellitus and chronic hypertension), pregnancy complications (gestational diabetes and pregnancy hypertension), placental complications (abruptio placenta, placenta previa, and other excessive bleeding), plurality at birth (singleton or twin), mode of delivery, State of residence, year of birth, and infant sex. Models of placental complications by plurality were limited to the preterm birth outcomes, since the other outcomes (SGA, LGA, NICU admission, and neonatal and infant death) were highly correlated with prematurity. We tested interactions and they did not significantly reduce the lack of fit of the models and therefore were not retained. Only models with sufficient sample size are presented in the tables. All analyses were performed using the SAS software, version 9.4 (SAS Institute).
Results
The study population included 2,478,459 pregnancies [2,258,460 fertile, 12,184 subfertile and 140,686 autologous-fresh, 36,509 autologous-thawed, 22,754 donor-fresh and 7866 donor-thawed]; 2,379,210 singleton pregnancies (2,379,210 infants and 9531 deaths), and 94,985 twin pregnancies (189,971 infants and 2903 deaths). A description of maternal characteristics by fertility group and plurality is shown in Table 1. Women in the fertile group were more likely to be younger, Hispanic, multiparous, and less likely to be college graduates compared to the subfertile and IVF groups, which for most characteristics tended to be similar. Within the IVF group, women using their own oocytes (autologous) averaged about 7 years younger than women using donor oocytes. Women with IVF-fresh embryo cycles were most likely to be nulliparous (47.6 to 67.7% of singletons and 24.5 to 34.1% of twins).
Table 1.
Maternal characteristics by fertility group and plurality at birth
| Factor | Categories | Singleton births | Twin births | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fertility | Fertile | Subfertile | IVF | Fertile | Subfertile | IVF | |||||||
| Group | A-fresh | A-thawed | D-fresh | D-thawed | A-fresh | A-thawed | D-fresh | D-thawed | |||||
| N, pregnancies | 2,223,647 | 9941 | 97,852 | 27,930 | 13,875 | 5965 | 34,033 | 2032 | 40,406 | 8127 | 8586 | 1801 | |
| Age (years) | Mean (SD) | 28.8 (5.9) | 33.8 (5.2) | 35.0 (4.2) | 35.0 (4.2) | 42.0 (4.7) | 42.5 (5.1) | 30.2 (5.9) | 34.4 (5.4) | 33.8 (4.0) | 34.2 (4.0) | 41.6 (4.8) | 42.2 (5.1) |
| (%) 18–29 | 54.1 | 20.8 | 10.4 | 9.6 | 1.3 | 1.5 | 44.8 | 16.7 | 14.0 | 12.2 | 1.6 | 1.7 | |
| 30–34 | 27.6 | 35.6 | 34.8 | 35.6 | 6.7 | 6.6 | 31.3 | 37.2 | 41.7 | 41.5 | 7.2 | 6.6 | |
| 35–37 | 10.7 | 19.8 | 25.3 | 27.0 | 8.3 | 8.0 | 13.6 | 21.3 | 25.2 | 25.5 | 10.0 | 9.9 | |
| 38–40 | 5.4 | 13.9 | 19.7 | 18.5 | 15.9 | 13.3 | 7.0 | 12.4 | 14.9 | 14.8 | 16.4 | 15.2 | |
| ≥ 41 | 2.2 | 9.8 | 9.8 | 9.3 | 67.9 | 70.7 | 3.3 | 12.4 | 4.1 | 6.0 | 64.8 | 66.6 | |
| Ethnicity (%) | Hispanic | 24.1 | 8.3 | 8.2 | 8.0 | 7.3 | 7.4 | 17.6 | 7.1 | 8.8 | 8.7 | 8.0 | 8.1 |
| Race (%) | White | 76.2 | 86.8 | 82.8 | 79.8 | 85.9 | 85.4 | 75.6 | 85.9 | 84.7 | 79.9 | 86.1 | 85.4 |
| Black | 14.5 | 4.0 | 5.0 | 5.7 | 4.4 | 5.0 | 17.4 | 4.0 | 4.6 | 6.6 | 4.7 | 5.3 | |
| Asian | 8.8 | 8.9 | 11.9 | 14.3 | 9.6 | 9.4 | 6.5 | 9.8 | 10.5 | 13.4 | 8.9 | 9.2 | |
| Other | 0.5 | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.5 | 0.2 | 0.3 | 0.2 | 0.2 | 0.1 | |
| Parity (%) | Nulliparous (0) | 38.6 | 56.2 | 67.7 | 49.0 | 67.5 | 47.6 | 18.1 | 29.5 | 34.1 | 26.8 | 33.5 | 24.5 |
| 1 | 33.0 | 30.4 | 23.4 | 34.6 | 22.3 | 35.9 | 32.1 | 42.1 | 40.1 | 38.6 | 39.5 | 36.4 | |
| ≥ 2 | 28.4 | 13.4 | 8.9 | 16.4 | 10.2 | 16.5 | 49.8 | 28.4 | 25.8 | 34.6 | 27.0 | 39.1 | |
Means are weighted, SDs are not weighted
The infertility diagnoses and IVF treatment parameters are shown in Table 2. Women with autologous-fresh cycles had fewer prior IVF cycles compared to women with donor or thawed cycles. Male factor infertility was the most common diagnosis in cycles using autologous oocytes (ranging from 39 to 44.1% by plurality), and diminished ovarian reserve was most common in cycles using donor oocytes (ranging from 75.3 to 77.9% by plurality). Only 11.8 to 23.7% of singleton IVF births had a single embryo transferred, 61.1 to 82.8% of twin births had two embryos transferred, indicating probable evidence of fetal loss and embryo splitting.
Table 2.
Infertility diagnoses and treatment parameters for women in the IVF group by oocyte source and embryo state
| Singleton | Twin | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A-fresh | A-thawed | D-fresh | D-thawed | A-fresh | A-thawed | D-fresh | D-thawed | ||
| N, pregnancies | 97,852 | 27,930 | 13,875 | 5965 | 40,406 | 8127 | 8586 | 1801 | |
| Prior IVF | Number of prior cycles (%) | 54.5 | 91.2 | 66.3 | 88.7 | 52.6 | 90.6 | 67.1 | 87.5 |
| Mean number of cycles (SD) | 1.6 (2.2) | 2.6 (2.5) | 2.4 (2.8) | 3.6 (3.4) | 1.5 (2.1) | 2.4 (2.2) | 2.5 (2.8) | 3.4 (3.1) | |
| Infertility | Male factor | 39.5 | 39.0 | 19.0 | 18.7 | 41.1 | 39.0 | 19.6 | 19.5 |
| Diagnosis (%) | Endometriosis | 11.4 | 10.8 | 6.3 | 6.9 | 12.1 | 11.2 | 6.4 | 7.5 |
| Ovulation disorders | 16.8 | 21.2 | 5.1 | 6.7 | 19.3 | 23.1 | 5.6 | 5.7 | |
| Diminished ovarian reserve | 14.9 | 10.2 | 77.5 | 76.0 | 10.5 | 8.6 | 77.9 | 75.3 | |
| Tubal factors | 15.9 | 16.1 | 6.9 | 7.6 | 16.1 | 16.6 | 7.1 | 8.2 | |
| Uterine factors | 4.2 | 4.3 | 4.6 | 5.4 | 3.7 | 3.6 | 4.6 | 5.2 | |
| Other | 12.6 | 13.9 | 17.8 | 18.3 | 11.5 | 13.3 | 16.2 | 17.7 | |
| Unexplained | 14.5 | 13.5 | 3.4 | 3.1 | 14.5 | 13.4 | 3.6 | 3.6 | |
| Embryos | 1 | 11.8 | 23.7 | 13.3 | 19.9 | 0.6 | 1.5 | 0.3 | 1.0 |
| Transferred (%) | 2 | 53.5 | 51.1 | 71.3 | 53.0 | 64.9 | 63.8 | 82.8 | 61.1 |
| > 2 | 34.7 | 25.2 | 15.4 | 27.2 | 34.5 | 34.7 | 16.9 | 37.9 | |
| Fetal heartbeats | 1 | 91.7 | 93.8 | 88.8 | 93.7 | 0.8 | 1.0 | 0.6 | 1.1 |
| At 6 weeks (%) | 2 | 7.4 | 5.6 | 9.9 | 5.6 | 93.4 | 93.5 | 95.3 | 93.8 |
| > 2 | 0.9 | 0.6 | 1.3 | 0.7 | 5.8 | 5.4 | 4.1 | 5.0 | |
The pregnancy, birth, and infant outcomes by fertility group and plurality are shown in Table 3. Diabetes and hypertension were more frequent in the subfertile and the donor-fresh and donor-thawed IVF groups, and placental complications generally more common in the IVF donor groups. Prematurity (and associated reduced birthweight) was more likely in the subfertile and IVF donor groups. SGA was lowest and LGA highest in the IVF thawed embryo groups (in both autologous and donor oocytes groups). Neonatal and infant mortality was highest in the subfertile group, followed by the IVF donor-thawed group.
Table 3.
Pregnancy, birth, and infant outcomes by maternal fertility group and plurality at birth
| Singleton births | Twin births | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fertility group | Fertile | Subfertile | IVF | Fertile | Subfertile | IVF | |||||||
| A-fresh | A-thawed | D-fresh | D-thawed | A-fresh | A-thawed | D-fresh | D-thawed | ||||||
| N, pregnancies | 2,223,647 | 9941 | 97,852 | 27,930 | 13,875 | 5965 | 34,033 | 2032 | 40,406 | 8127 | 8586 | 1801 | |
| Factor | Categories | ||||||||||||
| Diabetes | Pregestational (%) | 0.6 | 0.9 | 0.7 | 0.7 | 0.7 | 0.9 | 0.8 | 1.0 | 0.6 | 0.6 | 0.7 | 1.0 |
| Gestational (%) | 6.0 | 9.0 | 8.7 | 8.7 | 10.2 | 11.0 | 8.4 | 9.9 | 10.6 | 11.5 | 12.4 | 13.0 | |
| Hypertension | Pregestational (%) | 1.2 | 2.1 | 1.6 | 2.0 | 3.1 | 3.3 | 2.1 | 2.4 | 1.7 | 2.7 | 3.3 | 4.6 |
| Gestational (%) | 3.5 | 6.8 | 4.4 | 5.1 | 9.1 | 7.9 | 7.8 | 12.1 | 9.1 | 12.1 | 18.6 | 15.8 | |
| Placental | Other excessive bleeding (%) | 0.8 | 1.0 | 1.5 | 2.4 | 2.2 | 1.3 | 2.7 | 3.1 | 3.1 | 4.5 | 4.9 | 5.0 |
| Complications | Placenta previa (%) | 0.4 | 0.9 | 2.2 | 1.4 | 2.2 | 2.2 | 0.5 | 0.9 | 1.4 | 1.0 | 1.6 | 2.1 |
| Abruptio placenta (%) | 0.5 | 0.7 | 0.9 | 0.6 | 0.8 | 0.7 | 0.8 | 0.9 | 1.2 | 0.8 | 1.1 | 0.7 | |
| Mode of delivery | Vaginal (%) | 67.9 | 56.3 | 55.1 | 46.5 | 33.4 | 31.8 | 26.4 | 21.7 | 19.5 | 17.7 | 11.9 | 12.8 |
| Cesarean (%) | 32.1 | 43.7 | 44.9 | 53.5 | 66.6 | 68.2 | 73.6 | 78.3 | 80.5 | 82.3 | 88.1 | 87.2 | |
| Repeat cesarean (% of cesarean) | 42.7 | 30.2 | 22.3 | 35.2 | 17.3 | 37.7 | 22.0 | 17.6 | 12.7 | 20.5 | 13.1 | 26.7 | |
| Length of | Mean weeks (SD) | 38.7 (2.0) | 38.4 (2.4) | 38.4 (2.2) | 38.4 (2.2) | 38.2 (2.4) | 38 (2.5) | 35.3 (3.2) | 34.9 (3.6) | 35.3 (3.0) | 35.3 (3.0) | 35.2 (2.9) | 35.1 (2.9) |
| Gestation | < 28 weeks, % | 0.5 | 1.1 | 0.7 | 0.8 | 0.8 | 1.1 | 3.9 | 5.7 | 3.3 | 3.1 | 2.5 | 3.0 |
| < 32 weeks, % | 1.1 | 1.7 | 1.8 | 1.6 | 2.5 | 2.8 | 10.5 | 12.2 | 10.5 | 10.9 | 11.8 | 11.6 | |
| < 36 weeks, % | 6.5 | 9.0 | 9.2 | 8.7 | 12.1 | 13.3 | 43.6 | 41.6 | 44.7 | 45.3 | 48.2 | 49.7 | |
| ≥ 37 weeks, % | 91.9 | 88.3 | 88.3 | 88.9 | 84.6 | 82.8 | 42.0 | 40.5 | 41.4 | 40.6 | 37.4 | 35.7 | |
| N, infants (live births) | 2,223,647 | 9941 | 97,857 | 27,933 | 13,876 | 5966 | 68,065 | 4063 | 80,813 | 16,253 | 17,175 | 3602 | |
| N, infant deaths | 8931 | 64 | 343 | 112 | 49 | 32 | 1329 | 123 | 1054 | 181 | 180 | 36 | |
| Birthweight | Mean grams (SD) | 3316 (555) | 3268 (632) | 3237 (602) | 3377 (615) | 3243 (643) | 3235 (666) | 2361 (621) | 2302 (671) | 2354 (424) | 2439 (430) | 2356 (411) | 2360 (423) |
| VLBW, < 1500 g, % | 1.0 | 2.2 | 1.7 | 1.5 | 2.1 | 2.3 | 9.5 | 13.0 | 9.1 | 8.0 | 8.0 | 8.8 | |
| LBW, < 2500 g, % | 6.0 | 8.9 | 9.0 | 6.8 | 10.7 | 11.2 | 54.5 | 56.2 | 55.9 | 49.0 | 56.8 | 55.7 | |
| SGA, Z-score ≤ − 1.28, % | 8.4 | 8.6 | 9.4 | 5.3 | 8.2 | 7.1 | 21.7 | 20.8 | 22.2 | 15.0 | 20.2 | 18.9 | |
| LGA, Z-score ≥ 1.28, % | 9.6 | 9.7 | 8.7 | 14.5 | 11.1 | 12.8 | 1.9 | 2.0 | 1.5 | 2.6 | 1.8 | 2.0 | |
| Gender | Male (%) | 51.2 | 51.3 | 50.6 | 51.8 | 51.2 | 50.5 | 50.2 | 51.7 | 50.7 | 50.9 | 51.3 | 51.0 |
| NICU | NICU admission (%) | 6.0 | 9.6 | 7.9 | 8.3 | 10.5 | 10.4 | 31.2 | 36.5 | 32.5 | 32.3 | 36.1 | 36.0 |
| Infant | Infant (0–364 days, %) | 0.40 | 0.64 | 0.35 | 0.40 | 0.35 | 0.54 | 1.95 | 3.03 | 1.30 | 1.11 | 1.05 | 1.00 |
| Deaths | Neonatal (0–27 days, %) | 0.24 | 0.54 | 0.25 | 0.30 | 0.25 | 0.35 | 1.48 | 2.66 | 1.02 | 0.87 | 0.75 | 0.83 |
| Neonatal (% of all deaths) | 59.2 | 84.4 | 71.7 | 75.0 | 69.4 | 65.6 | 76.0 | 87.8 | 77.9 | 78.5 | 71.7 | 83.3 | |
The results of the placental complications models are shown in Table 4. Both pregestational and gestational diabetes were associated with increased risks for placental complications, regardless of plurality (model 1). Within the IVF population (model 2), gestational diabetes was associated with increased risk of abruptio placenta in singletons and placenta previa and other excessive bleeding in twins. Hypertension (both pregestational and gestational) was associated with increased risks of abruptio placenta and other excessive bleeding, regardless of plurality (model 1); the pattern was similar within the IVF population (model 2). Compared to fertile women, both subfertile and IVF-treated women had increased risks for placental complications, highest for placenta previa in the latter group (AOR 3.79, 95% CI 3.48, 4.13 for singletons, and AOR 2.19, 95% CI 2.19, 95% CI 1.63, 2.94 for twins) (model 1). Within the IVF population, the risks for other excessive bleeding were generally increased for pregnancies with donor oocytes and/or thawed embryos, regardless of plurality. Placental complications were, in turn, strongly associated with prematurity (Table 5). The risks with abruptio placenta was increased 10-fold (AOR 9.52, 95% CI 9.07, 9.98) and 7-fold (AOR 7.04, 95% CI 6.09, 8.14), respectively, in models 1 and 2. The pattern for placenta previa (AOR 6.94, 95% CI 6.52, 7.40, and AOR 6.52, 95% CI 5.79, 7.39, respectively, in models 1 and 2) and other excessive bleeding (AOR 1.46, 95% 1.34, 1.58, and AOR 1.35, 95% CI 1.10, 1.65, respectively, in models 1 and 2), showed a similar pattern.
Table 4.
Risk of placental complications by maternal co-morbidities, fertility group, and plurality
| Plurality | Singletons | Twins | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | Model | Outcome | Other | Placenta | Abruptio | Other | Placenta | Abruptio | ||||||
| Excessive bleeding | Previa | Placenta | Excessive bleeding | Previa | Placenta | |||||||||
| Model 1 | N | 651,746 | 854,999 | 1,524,996 | 22,352 | 29,375 | 49,915 | |||||||
| N, % | 5536 | 0.8% | 4218 | 0.5% | 7314 | 0.5% | 655 | 2.9% | 295 | 1.0% | 509 | 1.0% | ||
| Model 2 | N | 43,160 | 55,723 | 99,401 | 17,343 | 22,907 | 39,014 | |||||||
| N, % | 741 | 1.7% | 1164 | 2.1% | 799 | 0.8% | 615 | 3.5% | 323 | 1.4% | 426 | 1.1% | ||
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |||
| Diabetes | Model 1 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Pregestational | 1.53 | 0.95, 2.48 | 0.80 | 0.48, 1.33 | 1.02 | 0.76, 1.37 | 1.03 | 0.14, 7.51 | – | – | 0.38 | 0.05, 2.72 | ||
| Gestational | 1.28 | 1.04, 1.58 | 1.03 | 0.86, 1.24 | 0.93 | 0.83, 1.05 | 1.23 | 0.63, 2.37 | 1.43 | 0.82, 2.52 | 0.79 | 0.53, 1.19 | ||
| Model 2 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| Pregestational | 0.46 | 0.06, 3.27 | 1.01 | 0.44, 2.32 | 1.69 | 0.83, 3.44 | – | – | 0.85 | 0.12, 6.19 | 0.26 | 0.02, 4.25 | ||
| Gestational | 1.01 | 0.60, 1.68 | 0.79 | 0.55, 1.13 | 1.41 | 1.08, 1.84 | 1.26 | 0.65, 2.45 | 1.10 | 0.61, 1.99 | 0.83 | 0.55, 1.25 | ||
| Hypertension | Model 1 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Pregestational | 1.37 | 1.12, 1.67 | 0.49 | 0.36, 0.68 | 1.77 | 1.52, 2.07 | 1.06 | 0.55, 2.02 | 1.00 | 0.41, 2.44 | 1.40 | 0.75, 2.61 | ||
| Gestational | 1.52 | 1.34, 1.72 | 0.54 | 0.44, 0.65 | 1.96 | 1.79, 2.14 | 1.31 | 0.98, 1.74 | 0.50 | 0.30, 0.83 | 0.82 | 0.59, 1.15 | ||
| Model 2 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| Pregestational | 1.39 | 0.88, 2.20 | 0.61 | 0.37, 1.01 | 1.79 | 1.21, 2.65 | 1.05 | 0.58, 1.89 | 0.45 | 0.14, 1.41 | 0.83 | 0.37, 1.88 | ||
| Gestational | 1.02 | 0.73, 1.42 | 0.55 | 0.40, 0.74 | 1.16 | 0.87, 1.56 | 1.27 | 0.98, 1.66 | 0.58 | 0.38, 0.87 | 0.77 | 0.54, 1.08 | ||
| Fertility | Model 1 | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Group | Subfertile | 1.49 | 0.77, 2.89 | 1.30 | 0.73, 2.32 | 1.52 | 1.11, 2.06 | 2.09 | 0.64, 6.81 | 1.14 | 0.22, 5.97 | 1.16 | 0.62, 2.17 | |
| IVF | 1.53 | 1.38, 1.70 | 3.79 | 3.48, 4.13 | 1.68 | 1.52, 1.85 | 1.16 | 0.97, 1.37 | 2.19 | 1.63, 2.94 | 1.23 | 0.99, 1.52 | ||
| Model 2 | A-fresh | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| A-thawed | 1.49 | 1.24, 1.80 | 0.54 | 0.45, 0.65 | 0.63 | 0.51, 0.78 | 1.26 | 0.99, 1.61 | 0.66 | 0.44, 0.99 | 0.75 | 0.54, 1.04 | ||
| D-fresh | 1.52 | 1.16, 2.00 | 0.74 | 0.59, 0.93 | 0.87 | 0.66, 1.14 | 1.76 | 1.31, 2.34 | 0.90 | 0.61, 1.32 | 0.93 | 0.66, 1.31 | ||
| D-thawed | 0.83 | 0.52, 1.34 | 0.68 | 0.50, 0.94 | 0.73 | 0.49, 1.08 | 1.58 | 0.98, 2.54 | 1.15 | 0.63, 2.10 | 0.57 | 0.27, 1.19 | ||
Models adjusted for maternal age, parity, hypertension and diabetes (pregestational and gestational), placental complications (placenta previa, abruptio placenta, and excessive bleeding), mode of delivery, infant sex, length of gestation, small-for-gestation birthweight, State of residence, year of birth, and fertility status. For model 1, the study population was categorized by fertility status as fertile (reference), subfertile, and IVF (limited to autologous-fresh cycles only); for model 2, the study population was limited to IVF-conceived pregnancies as autologous-fresh (reference), autologous-thawed, donor-fresh, donor-thawed
Italicized AORs and 95% CIs are increased; those that are additionally emphasized in bold are significantly increased
Table 5.
Risks of adverse outcomes, infant morbidity, and infant mortality by maternal fertility group: singletons
| Very early preterm | Early preterm | Preterm | SGA | LGA | NICU | Neonatal | Infant | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model #* | 22–27 weeks | 22–32 weeks | 22–36 weeks | (≤ − 1.28) | (≥ 1.28) | Admission | Death | Death | ||||||||||
| Infants | Model 1 | N | 2,308,528 | 2,308,528 | 2,308,528 | 2,303,434 | 2,303,434 | 1,279,495 | 2,331,440 | 2,331,440 | ||||||||
| Outcome | N, % | 11,543 | 0.5% | 37,751 | 1.6% | 191,214 | 8.3% | 194,057 | 8.4% | 221,179 | 9.6% | 78,387 | 6.1% | 5583 | 0.24% | 9338 | 0.40% | |
| Model 2 | N | 143,644 | 143,644 | 143,644 | 143,341 | 143,341 | 79,622 | 145,622 | 145,622 | |||||||||
| N, % | 1104 | 0.77% | 3767 | 2.62% | 17,446 | 12.15% | 12,044 | 8.4% | 14,625 | 10.2% | 6628 | 8.3% | 385 | 0.26% | 536 | 0.37% | ||
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |||
| Fertility | Model 1 | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Group | Subfertile | 2.12 | 1.74, 2.57 | 1.53 | 1.35, 1.74 | 1.41 | 1.33, 1.51 | 1.01 | 0.94, 1.09 | 0.93 | 0.87, 0.99 | 1.19 | 1.09, 1.29 | 1.44 | 1.06, 1.96 | 1.16 | 0.88, 1.53 | |
| IVF | 1.48 | 1.37, 1.61 | 1.52 | 1.45, 1.59 | 1.48 | 1.45, 1.51 | 1.13 | 1.10, 1.15 | 0.86 | 0.84, 0.88 | 1.07 | 1.02, 1.12 | 0.75 | 0.64, 0.86 | 0.73 | 0.65, 0.83 | ||
| IVF | Model 2 | Autologous, fresh | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Group | Autologous, thawed | 1.14 | 0.97, 1.33 | 0.93 | 0.85, 1.02 | 0.92 | 0.88, 0.96 | 0.58 | 0.54, 0.61 | 1.58 | 1.52, 1.65 | 1.10 | 1.02, 1.19 | 1.36 | 1.03, 1.79 | 1.28 | 1.01, 1.63 | |
| Donor, fresh | 1.02 | 0.81, 1.29 | 1.17 | 1.04, 1.32 | 1.22 | 1.15, 1.30 | 0.82 | 0.76, 0.89 | 1.18 | 1.10, 1.26 | 1.14 | 1.03, 1.27 | 1.01 | 0.65, 1.57 | 0.91 | 0.63, 1.32 | ||
| Donor, thawed | 1.58 | 1.19, 2.11 | 1.50 | 1.29, 1.76 | 1.45 | 1.33, 1.56 | 0.76 | 0.69, 0.85 | 1.26 | 1.15, 1.37 | 1.11 | 0.96, 1.28 | 1.23 | 0.72, 2.10 | 1.18 | 0.76, 1.84 | ||
| Co-morbidities | Model 1 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Hypertension | Pregestational | 3.12 | 2.83, 3.43 | 3.51 | 3.33, 3.71 | 2.81 | 2.73, 2.89 | 1.58 | 1.52, 1.64 | 0.90 | 0.87, 0.94 | 1.52 | 1.44, 1.61 | 0.60 | 0.50, 0.73 | 0.71 | 0.61, 0.83 | |
| Gestational | 2.07 | 1.93, 2.22 | 3.36 | 3.25, 3.48 | 3.23 | 3.18, 3.29 | 1.66 | 1.62, 1.70 | 0.98 | 0.96, 1.01 | 1.67 | 1.61, 1.72 | 0.49 | 0.43, 0.56 | 0.60 | 0.54, 0.66 | ||
| Model 2 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| Pregestational | 2.04 | 1.67, 2.49 | 2.89 | 2.61, 3.19 | 3.17 | 3.00, 3.35 | 1.58 | 1.47, 1.71 | 0.90 | 0.83, 0.97 | 1.53 | 1.37, 1.70 | 0.45 | 0.28, 0.70 | 1.18 | 0.32, 0.69 | ||
| Gestational | 2.14 | 1.55, 2.95 | 2.50 | 2.12, 2.95 | 2.35 | 2.14, 2.58 | 1.51 | 1.33, 1.71 | 0.89 | 0.79, 1.01 | 1.28 | 1.06, 1.55 | 0.26 | 0.10, 0.67 | 0.58 | 0.32, 1.06 | ||
| Co-morbidities | Model 1 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Diabetes | Pregestational | 1.43 | 1.19, 1.72 | 1.55 | 1.40, 1.70 | 2.34 | 2.23, 2.45 | 0.74 | 0.68, 0.80 | 3.06 | 2.92, 3.19 | 2.87 | 2.68, 3.06 | 1.17 | 0.88, 1.56 | 1.28 | 1.02, 1.60 | |
| Gestational | 0.58 | 0.52, 0.65 | 0.93 | 0.88, 0.98 | 1.29 | 1.27, 1.32 | 0.83 | 0.81, 0.85 | 1.81 | 1.78, 1.84 | 1.71 | 1.65, 1.76 | 0.85 | 0.72, 0.99 | 0.86 | 0.77, 0.97 | ||
| Model 2 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| Pregestational | 0.42 | 0.29, 0.61 | 2.89 | 2.61, 3.19 | 3.17 | 3.00, 3.35 | 0.98 | 0.90, 1.06 | 1.36 | 1.27, 1.46 | 1.59 | 1.44, 1.77 | 0.62 | 0.32, 1.19 | 0.69 | 0.42, 1.14 | ||
| Gestational | 1.24 | 0.63, 2.42 | 2.50 | 2.12, 2.95 | 2.35 | 2.14, 2.58 | 0.79 | 0.60, 1.04 | 2.31 | 1.92, 2.77 | 2.22 | 1.71, 2.89 | 0.66 | 0.15, 2.96 | 0.42 | 0.09, 1.87 | ||
| Length of | Model 1 | Term (≥ 37 weeks) | – | – | – | – | – | – | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Gestation | Preterm (< 37 weeks) | – | – | – | – | – | – | 0.95 | 0.93, 0.97 | 1.49 | 1.46, 1.51 | 11.11 | 10.90, 11.32 | 7.24 | 6.61, 7.93 | 4.59 | 4.30, 4.91 | |
| Early Preterm (< 32 weeks) | – | – | – | – | – | – | 1.22 | 1.17, 1.27 | 1.37 | 1.33, 1.43 | 68.39 | 65.75, 71.15 | 43.05 | 39.15, 47.33 | 22.21 | 20.63, 23.92 | ||
| Very early preterm (< 28 weeks) | – | – | – | – | – | – | 1.36 | 1.28, 1.44 | 1.18 | 1.12, 1.26 | 73.43 | 69.15, 77.97 | 496.87 | 462.44, 533.86 | 234.11 | 221.55, 247.37 | ||
| Model 2 | Term (≥ 37 weeks) | – | – | – | – | – | – | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| Preterm (<37 weeks) | – | – | – | – | – | – | 0.99 | 0.93, 1.05 | 1.01 | 0.95, 1.07 | 11.79 | 11.05, 12.58 | 6.84 | 4.52, 10.36 | 5.54 | 4.02, 7.62 | ||
| Early Preterm (< 32 weeks) | – | – | – | – | – | – | 1.27 | 1.12, 1.44 | 0.66 | 0.57, 0.76 | 75.42 | 66.05, 86.13 | 56.81 | 39.01, 82.71 | 40.99 | 30.46, 55.10 | ||
| Very early preterm (< 28 weeks) | – | – | – | – | – | – | 1.71 | 1.44, 2.04 | 0.67 | 0.53, 0.84 | 77.40 | 62.67, 95.60 | 631.31 | 462.02, 862.64 | 407.65 | 318.99, 520.95 | ||
| Placental | Model 1 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | – | – | – | – | – | – | – | – | – | – |
| Complications | Other excessive bleeding | 2.39 | 1.93, 2.96 | 1.95 | 1.70, 2.24 | 1.46 | 1.34, 1.58 | – | – | – | – | – | – | – | – | – | – | |
| Placenta previa | 2.43 | 1.96, 3.00 | 3.67 | 3.29, 4.10 | 6.94 | 6.52, 7.40 | – | – | – | – | – | – | – | – | – | – | ||
| Abruptio placenta | 13.90 | 12.69, 15.22 | 13.72 | 12.93, 14.55 | 9.52 | 9.07, 9.98 | – | – | – | – | – | – | – | – | – | – | ||
| Model 2 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | – | – | – | – | – | – | – | – | – | – | |
| Other excessive bleeding | 2.95 | 1.82, 4.79 | 2.23 | 1.64, 3.04 | 1.35 | 1.10, 1.65 | – | – | – | – | – | – | – | – | – | – | ||
| Placenta previa | 1.64 | 1.02, 2.63 | 2.65 | 2.12, 3.31 | 6.54 | 5.79, 7.39 | – | – | – | – | – | – | – | – | – | – | ||
| Abruptio placenta | 9.53 | 7.12, 12.77 | 9.77 | 8.17, 11.69 | 7.04 | 6.09, 8.14 | – | – | – | – | – | – | – | – | – | – | ||
Models adjusted for maternal age, parity, hypertension and diabetes (pregestational and gestational), placental complications (placenta previa, abruptio placenta, and excessive bleeding), mode of delivery, infant sex, length of gestation, small-for-gestation birthweight, State of residence, year of birth, and fertility status. For model 1, the study population was categorized by fertility status as fertile (reference), subfertile, and IVF (limited to autologous-fresh cycles only); for model 2, the study population was limited to IVF-conceived pregnancies as autologous-fresh (reference), autologous-thawed, donor-fresh, donor-thawed. Italicized AORs and 95% CIs are increased; those that are additionally emphasized in bold are significantly increased
Prematurity was associated with deviations from normal growth (both SGA and LGA birthweights), and greater risks of NICU admission, and death (Tables 5 for singletons and Table 6 for twins). Premature singletons had a 7-fold risk of neonatal death (AOR 7.24, 95% CI 6.61, 7.93 [model 1] and AOR 6.84, 95% CI 4.52, 10.36 [model 2]) and twins had more than a twofold risk (AOR 2.64, 95% CI 1.98, 3.53 [model 1] and AOR 2.79, 95% CI 1.93, 4.05 [model 2]). Hypertension was associated with a two- to three-fold risk of prematurity, as well as greater risks of SGA birthweight, and NICU admission. Diabetes was also associated with two- to three-fold risk of prematurity, as well as increased risk of LGA birthweight, and NICU admission. The risks of prematurity and NICU admission were significantly increased for subfertile and IVF-treated women (model 1); SGA birthweight was elevated for infants of IVF women, whereas the risks of neonatal and infant death were increased for infants of subfertile women. Within the IVF group (model 2), prematurity, LGA birthweight, and NICU admission were increased for pregnancies using donor oocytes and/or thawed embryos, and the risks for neonatal or infant death were increased for infants born from thawed embryos.
Table 6.
Risks of adverse outcomes, infant morbidity, and infant mortality by maternal fertility group and plurality at birth: twins
| Very early preterm | Early preterm | Preterm | SGA | LGA | NICU | Neonatal | Infant | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model #* | 22–27 weeks | 22–32 weeks | 22–36 weeks | (≤ − 1.28) | (≥ 1.28) | Admission | Death | Death | ||||||||||
| Infants | Model 1 | N | 75,565 | 75,565 | 75,565 | 150,628 | 150,628 | 79,104 | 152,939 | 152,939 | ||||||||
| Outcome | N, % | 2760 | 3.7% | 10,740 | 14.2% | 44,090 | 58.3% | 33,021 | 21.9% | 2585 | 1.7% | 25,421 | 32.1% | 1939 | 1.27% | 2506 | 1.64% | |
| Model 2 | N | 58,110 | 58,110 | 58,110 | 115,847 | 115,847 | 61,505 | 117,837 | 117,837 | |||||||||
| N, % | 1831 | 3.15% | 8105 | 14.0% | 34,530 | 59.4% | 24,086 | 20.8% | 2001 | 1.7% | 20,371 | 33.1% | 1122 | 0.95% | 1451 | 1.23% | ||
| AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | AOR | 95% CI | |||
| Fertility | Model 1 | Fertile | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Group | Subfertile | 1.77 | 1.44, 2.18 | 1.42 | 1.25, 1.61 | 1.14 | 1.04, 1.26 | 0.93 | 0.86, 1.01 | 0.94 | 0.74, 1.19 | 1.15 | 1.05, 1.26 | 1.40 | 1.08, 1.82 | 1.24 | 0.97, 1.57 | |
| IVF | 0.88 | 0.81, 0.97 | 0.97 | 0.92, 1.02 | 1.07 | 1.03, 1.11 | 1.02 | 0.99, 1.05 | 0.83 | 0.76, 0.91 | 1.13 | 1.08, 1.19 | 0.69 | 0.61, 0.78 | 0.67 | 0.61, 0.75 | ||
| IVF | Model 2 | Autologous, fresh | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Group | Autologous, thawed | 0.97 | 0.85, 1.12 | 1.01 | 0.94, 1.08 | 0.97 | 0.92, 1.02 | 0.60 | 0.57, 0.63 | 1.70 | 1.51, 1.91 | 0.96 | 0.90, 1.02 | 1.01 | 0.82, 1.24 | 0.97 | 0.81, 1.17 | |
| Donor, fresh | 0.89 | 0.74, 1.07 | 1.15 | 1.05, 1.26 | 1.20 | 1.12, 1.28 | 0.83 | 0.78, 0.87 | 1.25 | 1.06, 1.47 | 1.15 | 1.07, 1.24 | 0.93 | 0.71, 1.21 | 0.97 | 0.77, 1.22 | ||
| Donor, thawed | 1.12 | 0.83, 1.52 | 1.23 | 1.06, 1.42 | 1.35 | 1.21, 1.51 | 0.77 | 0.70, 0.85 | 1.32 | 1.02, 1.72 | 1.09 | 0.96, 1.23 | 0.87 | 0.56, 1.35 | 0.78 | 0.52, 1.16 | ||
| Co-morbidities | Model 1 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Hypertension | Pregestational | 1.29 | 1.00, 1.67 | 1.26 | 1.10, 1.46 | 2.29 | 2.02, 2.59 | 1.18 | 1.08, 1.30 | 0.93 | 0.70, 1.23 | 1.39 | 1.20, 1.61 | 0.78 | 0.53, 1.13 | 0.83 | 0.60, 1.16 | |
| Gestational | 0.45 | 0.37, 0.54 | 0.88 | 0.82, 0.95 | 2.29 | 2.15, 2.43 | 1.12 | 1.07, 1.17 | 1.05 | 0.91, 1.20 | 1.48 | 1.39, 1.57 | 0.56 | 0.43, 0.74 | 0.63 | 0.50, 0.79 | ||
| Model 2 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| Pregestational | 0.51 | 0.41, 0.62 | 1.01 | 0.94, 1.09 | 2.62 | 2.46, 2.79 | 1.16 | 1.10, 1.22 | 0.86 | 0.74, 1.00 | 1.62 | 1.52, 1.72 | 0.53 | 0.39, 0.73 | 0.64 | 0.50, 0.83 | ||
| Gestational | 1.40 | 1.05, 1.87 | 1.37 | 1.17, 1.59 | 2.53 | 2.21, 2.90 | 1.25 | 1.12, 1.38 | 0.84 | 0.61, 1.14 | 1.46 | 1.25, 1.71 | 0.78 | 0.51, 1.19 | 0.83 | 0.57, 1.22 | ||
| Co-morbidities | Model 1 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Diabetes | Pregestational | 1.12 | 0.67, 1.87 | 1.58 | 1.23, 2.03 | 1.43 | 1.14, 1.79 | 0.96 | 0.80, 1.15 | 2.12 | 1.45, 3.11 | 1.58 | 1.28, 1.96 | 0.78 | 0.37, 1.66 | 1.05 | 0.58, 1.92 | |
| Gestational | 0.64 | 0.52, 0.79 | 1.04 | 0.95, 1.14 | 1.08 | 1.01, 1.16 | 0.92 | 0.87, 0.97 | 1.39 | 1.20, 1.61 | 1.22 | 1.14, 1.31 | 0.89 | 0.68, 1.16 | 0.86 | 0.69, 1.09 | ||
| Model 2 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| Pregestational | 0.70 | 0.56, 0.88 | 1.05 | 0.96, 1.16 | 1.09 | 1.01, 1.17 | 0.93 | 0.88, 0.99 | 1.29 | 1.09, 1.52 | 1.28 | 1.19, 1.37 | 0.81 | 0.59, 1.10 | 0.80 | 0.61, 1.05 | ||
| Gestational | 1.06 | 0.53, 2.11 | 1.34 | 0.97, 1.84 | 1.15 | 0.89, 1.50 | 1.08 | 0.88, 1.34 | 1.76 | 1.06, 2.92 | 1.58 | 1.22, 2.05 | 2.22 | 1.03, 4.78 | 2.16 | 1.09, 4.28 | ||
| Length of | Model 1 | Term (≥37 weeks) | – | – | – | – | – | – | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference |
| Gestation | Preterm (<37 weeks) | – | – | – | – | – | – | 0.44 | 0.43, 0.46 | 1.89 | 1.70, 2.10 | 10.66 | 10.15, 11.19 | 2.64 | 1.98, 3.53 | 2.27 | 1.85, 2.79 | |
| Early Preterm (<32 weeks) | – | – | – | – | – | – | 0.22 | 0.21, 0.23 | 4.58 | 4.07, 5.16 | 74.42 | 69.06, 80.20 | 17.85 | 13.45, 23.68 | 13.68 | 11.14, 16.81 | ||
| Very early preterm (<28 weeks) | – | – | – | – | – | – | 0.21 | 0.19, 0.23 | 8.84 | 7.71, 10.14 | 51.95 | 46.91, 57.53 | 499.48 | 386.61, 645.30 | 299.74 | 248.67, 361.30 | ||
| Model 2 | Term (≥37 weeks) | – | – | – | – | – | – | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |
| Preterm (<37 weeks) | – | – | – | – | – | – | 0.44 | 0.42, 0.45 | 1.86 | 1.65, 2.09 | 10.81 | 10.23, 11.42 | 2.79 | 1.93, 4.05 | 2.39 | 1.80, 3.17 | ||
| Early Preterm (<32 weeks) | – | – | – | – | – | – | 0.22 | 0.21, 0.24 | 3.96 | 3.45, 4.53 | 73.27 | 67.36, 79.70 | 22.27 | 15.57, 31.85 | 17.80 | 13.51, 23.45 | ||
| Very early preterm (<28 weeks) | – | – | – | – | – | – | 0.19 | 0.17, 0.21 | 8.64 | 7.37, 10.14 | 65.40 | 57.42, 74.48 | 540.5 | 387.13, 754.63 | 380.01 | 293.77, 491.55 | ||
| Placental | Model 1 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | – | – | – | – | – | – | – | – | – | – |
| Complications | Other excessive bleeding | 1.17 | 0.80, 1.70 | 1.20 | 0.97, 1.49 | 1.08 | 0.91, 1.28 | – | – | – | – | – | – | – | – | – | – | |
| Placenta previa | 0.75 | 0.36, 1.55 | 1.79 | 1.36, 2.37 | 3.48 | 2.56, 4.71 | – | – | – | – | – | – | – | – | – | – | ||
| Abruptio placenta | 5.79 | 4.52, 7.41 | 5.13 | 4.28, 6.16 | 3.61 | 2.85, 4.57 | – | – | – | – | – | – | – | – | – | – | ||
| Model 2 | None | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | – | – | – | – | – | – | – | – | – | – | |
| Other excessive bleeding | 0.71 | 0.43, 1.19 | 1.02 | 0.81, 1.29 | 0.95 | 0.79, 1.13 | – | – | – | – | – | – | – | – | – | – | ||
| Placenta previa | 0.94 | 0.49, 1.81 | 2.00 | 1.54, 2.60 | 3.63 | 2.69, 4.90 | – | – | – | – | – | – | – | – | – | – | ||
| Abruptio placenta | 6.84 | 5.21, 8.99 | 5.23 | 4.28, 6.38 | 3.21 | 2.50, 4.13 | – | – | – | – | – | – | – | – | – | – | ||
The N for the very early preterm, early preterm, and preterm outcomes is based on pregnancies, not infants. Models adjusted for maternal age, parity, hypertension and diabetes (pregestational and gestational), placental complications (placenta previa, abruptio placenta, and excessive bleeding), mode of delivery, infant sex, length of gestation, small-for-gestation birthweight, State of residence, year of birth, and fertility status. For model 1, the study population was categorized by fertility status as fertile (reference), subfertile, and IVF (limited to autologous-fresh cycles only); for model 2, the study population was limited to IVF-conceived pregnancies as autologous-fresh (reference), autologous-thawed, donor-fresh, donor-thawed. Italicized AORs and 95% CIs are increased; those that are additionally emphasized in bold are significantly increased
The ten leading causes of neonatal, postneonatal, and infant mortality by plurality are shown in Table 7; for comparison, national data for 2016 is included [28]. The leading causes of infant mortality among study singletons reflected national rankings, with the number 1 cause being congenital malformations and chromosomal abnormalities (ICD-10 codes Q00-Q99) accounting for 25.4% and 20.8% of deaths, respectively, and the number 2 cause being prematurity and low birthweight (ICD-10 code P07) (10.2% of deaths for study singletons and 17.0% of deaths nationally). This order was reversed for study twins, with prematurity being number 1 cause (19.1% of deaths) and congenital malformations the number 2 cause (13.2% of deaths). Newborns affected by maternal complications of pregnancy (ICD-10 code P01) was the 4th leading cause of death nationally (6.1% of deaths) and for study singletons (3.1% of deaths), and the 3rd leading cause among study twins (9.7% of deaths). Newborns affected by complications of placenta, cord, and membranes (ICD-10 code P02) was the 6th leading cause of death nationally (3.6% of deaths) and for study singletons (2.8% of deaths), and the 7th leading cause among study twins (4.2% of deaths).
Table 7.
Distribution of infant, neonatal, and postneonatal mortality by plurality: leading causes of infant death
| All US births* | Study singletons | Study twins | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICD-10 | Rank | Number | % of total | Rank | Number | % of total | Rank | Number | % of total | |
| Codes | Deaths | Deaths | Deaths | |||||||
| Infant mortality (birth to 364 days) | ||||||||||
| All causes | – | 23,161 | – | – | 8657 | – | – | 2404 | – | |
| Congenital malformations, deformations, and chromosomal abnormalities | Q00-Q99 | 1 | 4816 | 20.8 | 1 | 2200 | 25.4 | 2 | 317 | 13.2 |
| Disorders related to short gestation and low birthweight | P07 | 2 | 3927 | 17.0 | 2 | 884 | 10.2 | 1 | 459 | 19.1 |
| Sudden infant death syndrome | R95 | 3 | 1500 | 6.5 | 3 | 679 | 7.8 | 8 | 68 | 2.8 |
| Newborn affected by maternal complications of pregnancy | P01 | 4 | 1402 | 6.1 | 4 | 268 | 3.1 | 3 | 232 | 9.7 |
| Accidents (unintentional injuries) | V01-X59 | 5 | 1219 | 5.3 | 5 | 421 | 4.9 | 9 | 30 | 1.2 |
| Newborn affected by complications of placenta, cord, and membranes | P02 | 6 | 841 | 3.6 | 6 | 242 | 2.8 | 7 | 101 | 4.2 |
| Bacterial sepsis of newborn | P36 | 7 | 583 | 2.5 | 7 | 246 | 2.8 | 6 | 121 | 5.0 |
| Respiratory distress of newborn | P22 | 8 | 488 | 2.1 | 8 | 245 | 2.8 | 4 | 157 | 6.5 |
| Diseases of the circulatory system | I00-I99 | 9 | 460 | 2.0 | 9 | 211 | 2.4 | 9 | 44 | 1.8 |
| Neonatal hemorrhage | P50-P52, P54 | 10 | 398 | 1.7 | 10 | 199 | 2.3 | 5 | 119 | 5.0 |
| Neonatal mortality (birth to 27 days) | ||||||||||
| All causes | – | 15,282 | – | – | 5202 | – | 1855 | |||
| Disorders related to short gestation and low birthweight | P07 | 1 | 3855 | 25.2 | 2 | 819 | 15.7 | 1 | 424 | 22.9 |
| Congenital malformations, deformations, and chromosomal abnormalities | Q00-Q99 | 2 | 3394 | 22.2 | 1 | 1482 | 28.5 | 2 | 251 | 13.5 |
| Newborn affected by maternal complications of pregnancy | P01 | 3 | 1389 | 9.1 | 3 | 256 | 4.9 | 3 | 227 | 12.2 |
| Newborn affected by complications of placenta, cord, and membranes | P02 | 4 | 829 | 5.4 | 5 | 228 | 4.4 | 7 | 99 | 5.3 |
| Bacterial sepsis of newborn | P36 | 5 | 555 | 3.6 | 6 | 212 | 4.1 | 6 | 105 | 5.7 |
| Respiratory distress of newborn | P22 | 6 | 479 | 3.1 | 4 | 229 | 4.4 | 4 | 150 | 8.1 |
| Neonatal hemorrhage | P50-P52, P54 | 7 | 391 | 2.6 | 7 | 187 | 3.6 | 5 | 110 | 5.9 |
| Intrauterine hypoxia and birth asphyxia | P20-P21 | 8 | 331 | 2.2 | 8 | 134 | 2.6 | 10 | 19 | 1.0 |
| Necrotizing enterocolitis of newborn | P77 | 9 | 303 | 2.0 | 9 | 118 | 2.3 | 8 | 54 | 2.9 |
| Atelectasis | P28.0-P28.1 | 10 | 261 | 1.7 | 10 | 77 | 1.5 | 9 | 34 | 1.8 |
| Postneonatal mortality (28–364 days) | ||||||||||
| All causes | – | 7879 | – | – | 3449 | – | – | 544 | – | |
| Congenital malformations, deformations, and chromosomal abnormalities | Q00-Q99 | 1 | 1422 | 18.0 | 1 | 718 | 20.8 | 1 | 65 | 11.9 |
| Sudden infant death syndrome | R95 | 2 | 1380 | 17.5 | 2 | 605 | 17.5 | 2 | 62 | 11.4 |
| Accidents (unintentional injuries) | V01-X59 | 3 | 1084 | 13.8 | 3 | 339 | 9.8 | 6 | 25 | 4.6 |
| Diseases of the circulatory system | I00-I99 | 4 | 361 | 4.6 | 4 | 154 | 4.5 | 5 | 26 | 4.8 |
| Assault (homicide) | X85-Y09 | 5 | 253 | 3.2 | 5 | 96 | 2.8 | 10 | 7 | 1.3 |
| Diarrhea and gastroenteritis of infectious origin | A09 | 6 | 205 | 2.6 | 7 | 41 | 1.2 | 8 | 22 | 4.0 |
| Septicemia | A40-A41 | 7 | 190 | 2.4 | 6 | 83 | 2.4 | 7 | 23 | 4.2 |
| Influenza and pneumonia | J09-J18 | 8 | 164 | 2.1 | 8 | 70 | 2.0 | 9 | 14 | 2.6 |
| Chronic respiratory disease originating in the perinatal period | P27 | 9 | 89 | 1.1 | 10 | 59 | 1.7 | 4 | 30 | 5.5 |
| Disorders related to short gestation and low birthweight | P07 | 10 | 72 | 0.9 | 9 | 65 | 1.9 | 3 | 32 | 5.9 |
*2016 deaths, from Heron M. Deaths: Leading Causes for 2016. National Vital Statistics Reports, July 26, 2018, vol. 67, no. 6, pp. 1–77
Prematurity was the leading cause of neonatal death nationally (25.2% of deaths) and for study twins (22.9% of deaths), and the 2nd leading cause for study singletons (15.7% of deaths). Newborns affected by maternal complications of pregnancy was the 3rd leading cause of neonatal mortality nationally (9.1% of deaths), among study singletons (4.9% of deaths), and study twins (12.2% of deaths). Newborns affected by complications of placenta, cord, and membranes was the 4th, 5th, and 7th leading cause of neonatal mortality nationally (5.4% of deaths), among study singletons (4.4% of deaths) and study twins (5.3% of deaths), respectively.
Congenital malformations and chromosomal abnormalities was the leading cause of postneonatal mortality, accounting for 18.0% of national deaths, 20.8% of deaths among study singletons, and 11.9% of deaths among study twins. Two of the top 10 leading causes of postneonatal mortality reflect the residual adverse effect of prematurity on survival during infancy: chronic respiratory disease originating in the perinatal period (ICD-10 code P27) and diseases related to short gestation and low birthweight (ICD-10 code P07). These two causes ranked 9 and 10 nationally (2.0% of deaths), ranked 10 and 9 among study singletons (3.6% of deaths), and ranked 4 and 3 among study twins (11.4% of deaths).
Discussion
This analysis of nationally-representative data provides a contemporary picture of infant morbidity and mortality by maternal fertility status in the USA (Figure 1). While prematurity was the major factor associated with infant morbidity and mortality, these analyses demonstrate the substantial role of placental complications, and the contribution of pregestational and gestational hypertension and diabetes to both adverse outcomes. These analyses demonstrate the significant risks associated with subfertile and IVF births, and in IVF pregnancies from cycles using donor oocytes and/or thawed embryos. In addition to being older and of lower parity, subfertile and IVF-treated women begin pregnancy with a higher incidence of chronic disease (hypertension and diabetes) compared to their fertile counterparts, and are more likely to develop gestational hypertension and diabetes, as well as placental complications. These findings confirm and expand our prior population-based studies in Massachusetts of greater risks of bleeding and placental complications among IVF-treated women [31, 32].
Fig. 1.
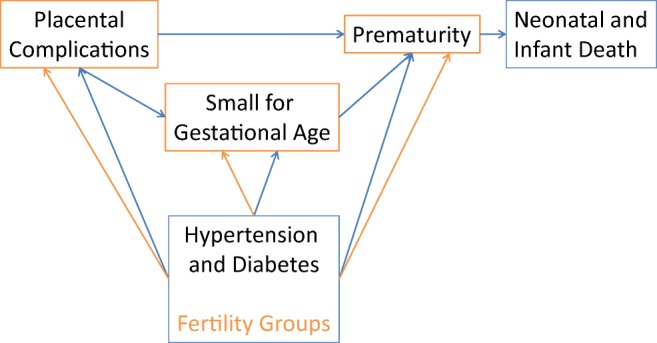
A contemporary picture of infant morbidity and mortality by maternal fertility status in the USA
Both pregestational and gestational diabetes and hypertension, particularly the latter, were associated with greater risks of placental complications. In line with other rising trends, diabetes and hypertension, both pregestational and gestational, have increased among women of childbearing ages. In their analysis of the Nationwide Inpatient Sample from 1994 to 2004, Albrecht et al. [33] reported that over this decade period the rates for all types of diabetes increased, including > 50% increase for type 1 and gestational diabetes, and greater than fourfold increase for type 2. Older maternal age was an independent predictor of any diabetes among delivery hospitalizations. Hypertensive disorders in pregnancy are also rising in the US, with older maternal age and obesity being major contributing factors; these disorders are associated with substantial risks for adverse outcomes and severe morbidity [34–36]. An evaluation of the women ages 20–44 in the 1999–2008 NHANES reported a prevalence of hypertension to be 8% (with 4.2% on anti-hypertensive medication), with significant independent risk factors of older age, non-Hispanic black race, diabetes mellitus, chronic kidney disease, and higher BMI; obesity was associated with more than a fourfold increased risk of hypertension [34]. National studies have documented the increasing contribution of pre-existing chronic disease to the rise in maternal morbidity and pregnancy-related mortality [37–40].
A consistent finding in IVF and ART pregnancies is an increased risk for uterine bleeding and placental complications, regardless of plurality [41–47]. IVF placentas have been documented to have altered morphology and gene expression, which may result in compromised development [48–51]. In their analysis of all births in Norway in 1999–2009, Ebbing et al. [46] reported increased risks for velamentous and marginal cord insertions with ART (twofold for singletons, and fourfold for twins), and a 20–80% risk of recurrence. Abnormal cord insertions were associated with a constellation of factors present in greater frequency among women who conceived after ART treatment (pre-existing medical conditions, older maternal age, pregnancy complications), as well as being associated with adverse pregnancy outcomes more common among the IVF and non-IVF ART populations (vaginal bleeding, placental complications, non-vertex presentation, prematurity, and birth defects). The authors suggest that these placental conditions share etiologic factors, and support the assumption that velamentous and marginal cord insertions represent a continuum of conditions that occur as a consequence of altered placental development. Other studies have shown that maternal pre-existing factors may lead to placental insufficiency and account for as much as one-third of spontaneous preterm births [52–54].
The use of frozen embryo transfer has increased by more than 80% since 2006 due to better cryopreservation techniques, improved live birth rates, lower risk of ectopic pregnancies, and more physiologically normal hormonal and endometrial environments [55–60]. Results indicate that singletons born after frozen embryo transfer have comparable or lower risks for low birthweight, small-for-gestational age birthweight, and preterm birth compared to singletons born after fresh IVF and ICSI, but worse outcomes compared to singletons born after spontaneous conception, including an excess of large-for-gestational age birthweights, pregnancy-induced hypertension, and placenta accreta [61–68].
Strengths and limitations
A major strength of this study is that the SART CORS data was collected prior to and separately from the vital statistics data, so we expect no differential misclassification of infant morbidity and mortality with respect to IVF. These findings are subject to several limitations. We were unable to adjust for underreported factors, including obesity, smoking and alcohol use, and inadequate or excessive gestational weight gain, which are each known to be associated with both prematurity and infant mortality. Although the models were adjusted for maternal age, there may be other important age-related factors influencing outcomes that were not available, such as central obesity. In addition, we have previously shown that infertility treatment is underreported on the birth certificate, only accurately identifying about one-third of IVF-conceived infants [69]. The subfertile group is likely to also be underreported.
Acknowledgements
The authors wish to thank SART and all of its members for providing clinical information to the SART CORS database for use by patients and researchers. Without the efforts of their members, this research would not have been possible.
The authors also gratefully acknowledge the following State agencies for their assistance in conducting this study:
California Department of Public Health, Office of Health Information and Research
Colorado Department of Public Health and Environment
Connecticut Department of Public Health
Florida Department of Health
Illinois Department of Public Health
Massachusetts Department of Public Health
Michigan Department of Health and Human Services, Division for Vital Records and Health Statistics
New Jersey Department of Health
New York City Department of Health and Mental Hygiene, Bureau of Vital Statistics
New York State Department of Health, Bureau of Health Informatics, Vital Statistics Unit
North Carolina Department of Health
Ohio Department of Health, Bureau of Vital Statistics
Pennsylvania Department of Health, Bureau of Health Statistics and Research
Texas Department of State Health Services, Center for Health Statistics
Virginia Department of Health.
Source of funding
The project described was supported by grant R01CA151973 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health, nor any of the State Departments of Health which contributed data.
Compliance with ethical standards
Conflict of interest
Barbara Luke is a research consultant to the Society for Assisted Reproductive Technology; all other authors report no conflict of interest.
References
- 1.Martin JA, Hamilton BE, Ventura SJ, Menacker F, Park MM. Births: final data for 2000. Natl Vital Stat Rep. 2002;50(5):1–102. [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Mathews TJ. Births: final data for 2015. Natl Vital Stat Rep. 2017;66(1):1–70. [PubMed] [Google Scholar]
- 3.Toner J, Coddington CC, Doody K, van Voorhis B, Seifer D, Ball GD, Luke B, Wantman E. SART & ART in the US: a 2016 update. Fertil Steril 2016 Sep 1; 106 (3):541–546. [DOI] [PubMed]
- 4.Center for Disease Control and Prevention, American Society for Reproductive Medicine, and Society for Assisted Reproductive Technology . 2015 Assisted reproductive technology success rates: national summary report. Atlanta (GA): US Dept. of Health and Human Services; 2017. [Google Scholar]
- 5.Basso O, Olsen J. Subfecundity and neonatal mortality: longitudinal study within the Danish National Birth Cohort. BMJ. 2005;330:393–394. doi: 10.1136/bmj.38336.616806.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helmerhorst FM, Perquin DAM, Donker D, Keirse JNC. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ. 2004;328:261–266. doi: 10.1136/bmj.37957.560278.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–563. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- 8.McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A, on behalf of the Knowledge Synthesis Group Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146:138–148. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Källén B, Finnström O, Lindam A, Nilsson E, Nygren K-G, Olausson PO. Trends in delivery and neonatal outcome after in vitro fertilization in Sweden: data for 25 years. Hum Reprod. 2010;25:1026–1034. doi: 10.1093/humrep/deq003. [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Li Y, Li C, Zhang W. Current overview of pregnancy complications and live-birth outcome of assisted reproductive technology in mainland China. Fertil Steril. 2014;101:385–391. doi: 10.1016/j.fertnstert.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 11.Zhu JL, Obel C, Bech BH, Olsen J, Basso O. Infertility, infertility treatment, and fetal growth restriction. Obstet Gynecol. 2007;110:1326–1334. doi: 10.1097/01.AOG.0000290330.80256.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raatikainen K, Kuivasaari-Pirinen P, Hippeläinen M, Heinonen S. Comparison of the pregnancy outcomes of subfertile women after infertility treatment and in naturally conceived pregnancies. Hum Reprod. 2012;27:1162–1169. doi: 10.1093/humrep/des015. [DOI] [PubMed] [Google Scholar]
- 13.Edwards RG, Ludwig M. Are major defects in children conceived in vitro due to innate problems in patients or to induced genetic damage? Reprod BioMed Online. 2003;7:131–138. doi: 10.1016/S1472-6483(10)61742-7. [DOI] [PubMed] [Google Scholar]
- 14.Buck Louis GM, Schisterman EF, Dukic VM, Schieve LA. Research hurdles complicating the analysis of infertility treatment and child health. Hum Reprod. 2005;20:12–18. doi: 10.1093/humrep/deh542. [DOI] [PubMed] [Google Scholar]
- 15.Buckett WM, Tan SL. Congenital abnormalities in children born after assisted reproductive techniques: how much is associated with the presence of infertility and how much with its treatment? Fertil Steril. 2005;84:1318–1319. doi: 10.1016/j.fertnstert.2005.04.065. [DOI] [PubMed] [Google Scholar]
- 16.Sutcliffe AG, Ludwig M. Outcome of assisted reproduction. Lancet. 2007;370:351–359. doi: 10.1016/S0140-6736(07)60456-5. [DOI] [PubMed] [Google Scholar]
- 17.Kondapalli LA, Perales-Puchalt A. Low birth weight: is it related to assisted reproductive technology or underlying infertility? Fertil Steril. 2013;99:303–310. doi: 10.1016/j.fertnstert.2012.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parrish KM, Holt VL, Connell FA, Williams B, LoGerfo JP. Variations in the accuracy of obstetric procedures and diagnoses on birth records in Washington State, 1989. Am J Epidemiol. 1993;138:119–127. doi: 10.1093/oxfordjournals.aje.a116834. [DOI] [PubMed] [Google Scholar]
- 19.Buescher PA, Taylor KP, Davis MH, Bowling JM. The quality of the new birth certificate data: a validation study in North Carolina. Am J Public Health. 1993;83:1163–1165. doi: 10.2105/AJPH.83.8.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piper JM, Mitchel EF, Snowden M, Hall C, Adams M, Taylor P. Validation of 1989 Tennessee birth certificate using maternal and newborn hospital records. Am J Epidemiol. 1993;137:758–768. doi: 10.1093/oxfordjournals.aje.a116736. [DOI] [PubMed] [Google Scholar]
- 21.Woolbright LA, Harshberger DS. The revised standard certificate of live birth: analysis of medical risk factor data from birth certificates in Alabama, 1988-92. Public Health Rep. 1995;110:59–63. [PMC free article] [PubMed] [Google Scholar]
- 22.Clark K, Chun-Mei F, Burnett C. Accuracy of birth certificate data regarding the amount, timing, and adequacy of prenatal care using prenatal medical records as referents. Am J Epidemiol. 1997;145:68–71. doi: 10.1093/oxfordjournals.aje.a009033. [DOI] [PubMed] [Google Scholar]
- 23.Dobie SA, Baldwin L-M, Rosenblatt RA, Fordyce MA, Andrilla CH, Hart LG. How well do birth certificates describe the pregnancies they report? The Washington State experience with low-risk pregnancies. Matern Child Health J. 1998;2:145–154. doi: 10.1023/A:1021875026135. [DOI] [PubMed] [Google Scholar]
- 24.Reichman NE, Hade EM. Validation of birth certificate data: a study of women in New Jersey’s healthy start program. Ann Epidemiol. 2001;11:186–193. doi: 10.1016/S1047-2797(00)00209-X. [DOI] [PubMed] [Google Scholar]
- 25.Roohan PJ, Josberger RE, Acar J, Dabir P, Feder HM, Gagliano PJ. Validation of birth certificate data in New York State. J Community Health. 2003;28:335–346. doi: 10.1023/A:1025492512915. [DOI] [PubMed] [Google Scholar]
- 26.Dietz P, Bombard J, Mulready-Ward C, Gauthier J, Sackoff J, Brozicevic P, Gambatese M, Nyland-Funke M, England L, Harrison L, Farr S. Validation of selected items on the 2003 US Standard Certificate of Live Birth: New York City and Vermont. Public Health Rep. 2015;130:60–70. doi: 10.1177/003335491513000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin JA, Wilson EC, Osterman MJK, Saadi EW, Sutton SR, Hamilton BE. Assessing the quality of medical and health data from the 2003 birth certificate revision: results from two states. Natl Vital Stat Rep. 2013;62(2):1–20. [PubMed] [Google Scholar]
- 28.Heron M. Deaths: leading causes for 2016. Natl Vital Stat Rep. 2018;67(6):1–77. [PubMed] [Google Scholar]
- 29.Talge NM, Mudd LM, Sikorskii A, Basso O. United States birth weight reference corrected for implausible gestational age estimates. Pediatrics. 2014;133:844–853. doi: 10.1542/peds.2013-3285. [DOI] [PubMed] [Google Scholar]
- 30.Land JA. How should we report on perinatal outcome? Hum Reprod. 2006;21:2638–2639. doi: 10.1093/humrep/del246. [DOI] [PubMed] [Google Scholar]
- 31.Luke B, Gopal D, Cabral H, Stern JE, Diop H. Pregnancy, birth, and infant outcomes by maternal fertility status: the Massachusetts Outcomes Study of Assisted Reproductive Technology. Am J Obstet Gynecol. 2017;217:327.e1–327.14. doi: 10.1016/j.ajog.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luke B, Gopal D, Cabral H, Stern JE, Diop H. Adverse pregnancy, birth, and infant outcomes in twins: effects of maternal fertility status and infant gender combinations; the Massachusetts Outcomes Study of Assisted Reproductive Technology. Am J Obstet Gynecol. 2017;217:330.e1–330.15. doi: 10.1016/j.ajog.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, Posner SF, Callaghan WM. Diabetes trends among delivery hospitalizations in the US, 1994-2004. Diabetes Care. 2010;33:768–773. doi: 10.2337/dc09-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bateman BT, Shaw KM, Kuklina EV, Callaghan WM, Seely EW. Hypertension in women of reproductive age in the United States: NHANES 1999-2008. PLoS One. 2012;7:e36171. doi: 10.1371/journal.pone.0036171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113:1299–1306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 36.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206:134.e1–134.e8. doi: 10.1016/j.ajog.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berg CJ, MacKay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States, 1993–1997 and 2001–2005. Obstet Gynecol 2009; 113:1075–1081. [DOI] [PubMed]
- 38.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116:1302–1309. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 39.Kuklina EV, Callaghan WM. Chronic heart disease and severe maternal morbidity among hospitalizations for pregnancy in the USA: 1995-2006. Br J Obstet Gynecol. 2011;118:345–352. doi: 10.1111/j.1471-0528.2010.02743.x. [DOI] [PubMed] [Google Scholar]
- 40.Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006-2010. Obstet Gynecol. 2015;125:5–12. doi: 10.1097/AOG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 41.Romundstad LB, Romundstad PR, Sunde A, von Düring V, Skjærven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod 2006; 21:2353–2358. [DOI] [PubMed]
- 42.Luke B, Stern JE, Kotelchuck M, Declercq E, Cohen B, Diop H. Birth outcomes by infertility diagnosis: analyses of the Massachusetts Outcomes Study of Assisted Reproductive Technologies (MOSART) J Reprod Med. 2015;60:480–490. [PMC free article] [PubMed] [Google Scholar]
- 43.Luke B, Stern JE, Hornstein MD, Kotelchuck M, Diop H, Cabral H, Declercq ER. Is the wrong question being asked in infertility research? J Assist Reprod Genet. 2016;33(1):3–8. doi: 10.1007/s10815-015-0610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schachter M, Tovbin Y, Arieli S, Friedler S, Ron-El R, Sherman D. In vitro fertilization is a risk factor for vasa previa. Fertil Steril. 2002;78:642–643. doi: 10.1016/S0015-0282(02)03253-3. [DOI] [PubMed] [Google Scholar]
- 45.Sheiner E, Shoham-Vardi I, Hallak M, Hershkowitz R, Katz M, Mazor M. Placenta previa: obstetric risk factors and pregnancy outcome. J Matern Fetal Med. 2001;10:414–419. doi: 10.1080/jmf.10.6.414.419. [DOI] [PubMed] [Google Scholar]
- 46.Ebbing C, Kiserud T, Johnsen SL, Albrechtsen S, Rasmussen S. Prevalence, risk factors and outcomes of velamentous and marginal cord insertions: a population-based study of 634,741 pregnancies. PLoS One. 2013;8:e70380. doi: 10.1371/journal.pone.0070380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg T, Pariente G, Serienko R, Wiznitzer A, Sheiner E. Critical analysis of risk factors and outcome of placenta previa. Arch Gynecol Obstet. 2011;284:47–51. doi: 10.1007/s00404-010-1598-7. [DOI] [PubMed] [Google Scholar]
- 48.Joy J, Gannon C, McClure N, Cooke I. Is assisted reproduction associated with abnormal placentation? Pediatr Dev Pathol. 2012;15:306–314. doi: 10.2350/11-11-1115-OA.1. [DOI] [PubMed] [Google Scholar]
- 49.Nelissen ECM, Dumoulin JCM, Daunay A, Evers JLH, Tost J, van Montfort APA. Placentas from pregnancies conceived by IVF/ICSI have a reduced DNA methylation level at the H19 and MEST differentially methylated regions. Hum Reprod. 2013;28:1117–1126. doi: 10.1093/humrep/des459. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura Y, Yaguchi C, Itoh H, Sakamoto R, Kimura T, Furuta N, Uchida T, Tamura N, Suzuki K, Sumimoto K, Matsuda Y, Matsuura T, Nishimura M, Kanayama N. Morphologic characteristics of the placental basal plate in in vitro fertilization pregnancies: a possible association with the amount of bleeding in delivery. Hum Pathol. 2015;46:1171–1179. doi: 10.1016/j.humpath.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Sakian S, Louie K, Wong EC, Havelock J, Kashyap S, Rowe T, Taylor B, Ma S. Altered gene expression of H19 and IGF2 in placentas from ART pregnancies. Placenta. 2015;36:1100–1105. doi: 10.1016/j.placenta.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 52.Catov JM, Wu CS, Olsen J, Sutton-Tyrrell K, Li J, Nohr EA. Early or recurrent preterm birth and maternal cardiovascular disease risk. Ann Epidemiol. 2010;20:604–609. doi: 10.1016/j.annepidem.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arias F, Rodriguez L, Raayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 54.Germain A, Carvajal J, Sanchez M, Valenzuela G, Tsunekawa H, Chuaqui B. Preterm labor: placental pathology and clinical correlation. Obstet Gynecol. 1999;94:284–289. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 55.Kato O, Kawasaki N, Bodri D, Kuroda T, Kawachiya S, Kato K, Takehara Y. Neonatal outcome and birth defects in 6,623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. Eur J Obstet Gyencol Reprod Biol. 2012;161:46–50. doi: 10.1016/j.ejogrb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 56.Weinerman R, Mainigi M. Why we should transfer frozen instead of fresh embryos: the translational rationale. Fertil Steril. 2014;102:10–18. doi: 10.1016/j.fertnstert.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barnhart KT. Introduction: are we ready to eliminate the transfer of fresh embryos in in vitro fertilization? Fertil Steril. 2014;102:1–2. doi: 10.1016/j.fertnstert.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Clinical rationale for cryopreservation of entire embryo cohorts in lieu of fresh transfer. Fertil Steril. 2014;102:3–9. doi: 10.1016/j.fertnstert.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 59.Shapiro BS, Daneshmand ST, De Leon L, Garner FC, Aguirre M, Hudson C. Frozen-thawed embryo transfer is associated with a significantly reduced incidence of ectopic pregnancy. Fertil Steril. 2012;98:1490–1494. doi: 10.1016/j.fertnstert.2012.07.1136. [DOI] [PubMed] [Google Scholar]
- 60.Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- 61.Pinborg A, Loft A, Henningsen A-KA, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995-2006. Fertil Steril. 2010;94:1320–1327. doi: 10.1016/j.fertnstert.2009.05.091. [DOI] [PubMed] [Google Scholar]
- 62.Ishihara O, Araki R, Kuwahara A, Itakura A, Saito H, Adamson GD. Impact of frozen-thawed single-blastocyst transfer on maternal and neonatal outcome: an analysis of 277,042 single-embryo transfer cycles from 2008 to 2010 in Japan. Fertil Steril. 2014;101:128–133. doi: 10.1016/j.fertnstert.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 63.Sutcliffe AG, D’Souza SW, Cadman J, Richards B, McKinlay IA, Lieberman B. Minor congenital anomalies, major congenital malformations and development in children conceived from cryopreserved embryos. Hum Reprod. 1995;10:3332–3337. doi: 10.1093/oxfordjournals.humrep.a135915. [DOI] [PubMed] [Google Scholar]
- 64.Belva F, Henriet S, Van den Abbeel E, Camus M, Devroey P, Van der Elst J, Liebaers I, Haentjens P, Bonduelle M. Neonatal outcome of 937 children born after transfer of cryopreserved embryos obtained by ICSI and IVF and comparison with outcome data of fresh ICSI and IVF cycles. Hum Reprod. 2008;23:2227–2238. doi: 10.1093/humrep/den254. [DOI] [PubMed] [Google Scholar]
- 65.Shih W, Rushford DD, Bourne H, Garrett C, McBain JC, Healy DL, Baker HWG. Factors affecting low birthweight after assisted reproduction technology: difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Hum Reprod. 2008;23:1644–1653. doi: 10.1093/humrep/den150. [DOI] [PubMed] [Google Scholar]
- 66.Murakami M, Egashira A, Murakami K, Araki Y, Kuramoto T. Perinatal outcome of twice-frozen-thawed embryo transfers: a clinical follow-up study. Fertil Steril. 2011;95:2648–2650. doi: 10.1016/j.fertnstert.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 67.Wennerholm U-B, Henningsen A-KA, Romundstad LB, Bergh C, Pinborg A, Skjaerven R, Forman J, Gissler M, Nygren KG, Tiitinen A. Perinatal outcomes of children born after frozen-thawed embryo transfer: a Nordic cohort study from the CoNARTaS group. Hum Reprod. 2013;28:2545–2553. doi: 10.1093/humrep/det272. [DOI] [PubMed] [Google Scholar]
- 68.Luke B, Brown MB, Wantman E, Stern JE, Toner J, Coddington CC. Increased risk of large-for-gestational age birthweight in singleton siblings conceived with in vitro fertilization in frozen versus fresh cycles. J Assist Reprod Genet. 2017;34:191–200. doi: 10.1007/s10815-016-0850-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Luke B, Brown MB, Spector LG. Validation of infertility treatment and assisted reproductive technology use on the birth certificate in eight States. (Research Letter) Am J Obstet Gynecol 2016; 215:126–7. PMID:26945609, 127. [DOI] [PMC free article] [PubMed]


