Abstract
Purpose
The neurokinin B (NKB)/NK3 receptor (NK3R) and kisspeptin (KISS1)/kisspeptin receptor (KISS1R), two systems essential for reproduction, are present in human granulosa cells (GCs) of healthy women and contribute to the control of fertility, at least partially, by acting on the gonads. However, little is known about the expression of these systems in GCs of women with polycystic ovarian syndrome (PCOS). The aim of this study was to analyze the expression of NKB/NK3R and KISS1/KISS1R in mural granulosa (MGCs) and cumulus cells (CCs) of PCOS women.
Methods
A cross-sectional study was performed in 46 healthy women and 43 PCOS women undergoing controlled ovarian stimulation. MGCs and CCs were collected from pre-ovulatory follicles after transvaginal ultrasound-guided oocyte retrieval and the expression of the genes encoding NKB (TAC3), NK3R (TACR3), KISS1, and its receptor (KISS1R) was analyzed using real-time quantitative RT-PCR.
Results
TAC3, TACR3, and KISS1 mRNA levels were decreased in MGCs and CCs of PCOS women. TAC3 positively correlated with KISS1 in MGCs of healthy women and TACR3 was positively associated with KISS1R in CCs from healthy women. These associations were not observed in PCOS women.
Conclusion
The NKB/NK3R and KISS1/KISS1R systems are dysregulated in MGCs and CCs of PCOS women. The lower expression of these systems in GCs could contribute to the abnormal follicle development and defective ovulation that characterize the pathogenesis of PCOS.
Electronic supplementary material
The online version of this article (10.1007/s10815-018-1338-7) contains supplementary material, which is available to authorized users.
Keywords: Polycystic ovarian syndrome, Neurokinin B, Kisspeptin, Granulosa cells, Cumulus cells
Introduction
Polycystic ovarian syndrome (PCOS) is a complex and highly heterogeneous endocrine disorder that affects approximately 5–15% of reproductive-aged women and is one of the most common causes of female subfertility [1–3]. PCOS is typically characterized by ovarian dysfunction, with elevated pre-antral follicle number, arrested follicular maturation, defective ovulation, and menstrual disorders, in conjunction with metabolic abnormalities such as hyperandrogenemia, insulin resistance, and abdominal obesity [1–6]. However, the symptoms of PCOS vary greatly between patients and its underlying etiology remains unclear [1–7].
The pathogenesis of PCOS seems to be strongly associated with dysfunction of granulosa cells (GCs), the specialized cells that are in direct contact with the oocyte forming the inside of the ovarian follicles [1–3, 7, 8]. At the antral stage of follicular development, two functionally different populations of GCs become recognizable: the cells that surround and remain associated with the oocyte, known as cumulus oophorus cells (CCs), and the cells that line the outer limits of the follicle, known as mural granulosa cells (MGCs) [9]. The study of MGCs and CCs may thus provide essential data for the advancement of our knowledge on the etiopathogenesis of PCOS.
Kisspeptins and tachykinins, particularly neurokinin B (NKB), are essential regulators of reproductive function [10–13] and play a key role in the modulation of GnRH secretion and gonadotropin release [4, 14–18]. In humans, kisspeptin is encoded by the KISS1 gene and their effects are mediated by the KISS1 receptor (KISS1R), which is encoded by the KISS1R gene [14–17]. NKB and its preferred tachykinin receptor, NK3 receptor (NK3R), are encoded by the genes TAC3 and TACR3, respectively [12, 16, 19–22]. Besides their expression at the central nervous system, KISS1/KISS1R and NKB/NK3R are locally synthesized in MGCs and CCs of healthy, oocyte-donor women [23–26] and their effects on ovarian function are partially mediated by a direct action at the level of the gonads [19–21, 23, 25–29]. Recent reports have shown that kisspeptin induces ovulation and triggers egg maturation in different mammalian species including women [17, 28, 30]. Moreover, follicular development is arrested at the antral stage in Kiss1 or Kiss1R null mice [31] suggesting a role in the regulation of follicular development [27–32], an event that is greatly altered in PCOS patients. With respect to NKB, a recent study has shown that this peptide regulates gonadotropin secretion, follicular development, and the timing of ovulation in healthy women [33]. Despite these evidences, little is known about the presence and eventual dysregulation of the KISS1 and NKB systems in the ovary of PCOS patients.
In this work, we analyzed the presence of KISS1, KISS1R, TAC3, and TACR3 in human MGCs and CCs from control, healthy women and PCOS patients, to investigate the possible existence of defects in the expression pattern of these systems in association with PCOS.
Materials and methods
Study population
Approval for this work was obtained from the institutional Ethics Committees of CSIC and Hospital Virgen Macarena (Sevilla, Spain) and all patients gave informed written consent. Human MGCs and CCs were collected from the pre-ovulatory follicles of 89 women, aged 19–38 years, undergoing oocyte retrieval after controlled ovarian stimulation treatment at the IVI Sevilla Centre (IVI-RMA Global) for Reproductive Care. Forty-six were healthy oocyte donors and 43 were patients with PCOS.
PCOS was diagnosed according to the 2003 Rotterdam Criteria [34, 35] including any two of the following three clinical features: (i) menstrual dysfunction (oligo/ anovulation), (ii) clinical and/or biochemical hyperandrogenism, and (iii) polycystic ovaries on ultrasound. Women with other related disorders such as adrenal congenital hyperplasia, thyroid disease, Cushing’s syndrome, or androgen-secreting tumors were excluded.
A general clinical examination of all patients was performed during the first visit to the IVI Centre. Blood samples were obtained during the early follicular phase of their menstrual cycle (day 3) and following administration of the ovulation inductor and serum hormone levels assayed enzymatically using an automated biochemistry analyzer (cobas e 411, Roche Diagnostics GmbH, Mannheim, Germany).
Stimulation protocol
All women were treated with a GnRH antagonist protocol using a combination of recombinant FSH + HMG in a dose of 150–225 IU of rFSH (Gonal, Merck Serono, Geneva, Switzerland) plus 37.5–75 IU of HMG (Menopur, Ferring Pharmaceuticals, Saint-Prex, Switzerland). A GnRH antagonist (Orgalutran, MSD, Hertfordshire, UK) was added the day that the leading follicle reached 14-mm average diameter followed by ovulation induction with 6500 UI recombinant choriogonadotropin alpha (rhCG) (Merck Serono) or 0.2 mg triptorelin (Ipsen Pharmabiotech, Signes, France) when two follicles reached 17-mm average diameter. Doses were adjusted according to patient characteristics and ovarian response, as judged by ultrasound and serum estradiol concentrations.
Collection of human MGCs and CCs
MGCs were collected from the follicular fluids (FF) obtained via ultrasound-guided transvaginal oocyte retrieval, which was performed 36 h after ovulation induction. After removal of oocyte-cumulus complexes, the remaining follicular aspirates from each patient were pooled and MGCs collected by using the Dynabeads methodology, as described previously [25].
Human CCs were obtained from the same patients than MGCs and collected following previously described procedures [25]. After follicular aspiration, the CCs surrounding the oocyte were removed by using cutting needles, by subsequent treatment of cumulus-oocyte complexes (COCs) with Sydney IVF Hyaluronidase (80 IU/ml, K-SIHY Cook Medical, Brisbane, Australia), and by carefully removing the CCs of the corona radiata with very thin glass pipettes (Swemed denudation pipette, 0.134–0.145 mm, Vitrolife, Goteborg, Sweden).
RNA extraction and real-time qPCR
Total RNA was extracted from fresh MGCs and CCs using the RNA/Protein purification kit (Norgen Biotek Corp., Ontario, Canada) and residual genomic DNA was removed with RNase-free DNase I and RNasin (Promega, Madison, WI). Complementary DNAs (cDNAs) were synthesized using the Transcriptor First Strand cDNA Synthesis kit (Roche, Mannheim, Germany). Samples were then pre-amplificated using the SsoAdvanced PreAmp supermix (Bio-Rad Laboratories, Hercules, CA) following manufacturer’s protocol.
Real-time qPCR was used to quantify the expression of KISS1, KISS1R, TAC3, and TACR3 in CCs and MGCs using the 2-∆∆CT method, as reported previously [25, 36]. qPCR was performed on a Bio-Rad iCycler iQ real-time detection apparatus using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad). The parameters of PCR amplification were 10 s at 94 °C, 20 s at 60 °C, and 30 s at 72 °C, for 50 cycles. The sequences of the specific primer pairs designed to amplify each target gene are shown in supplemental Table S1. Table S1 also shows the primers used to amplify β-actin (ACTB), hypoxanthine phosphoribosyltransferase 1 (HPRT1), cyclophilin A (PPIA), and succinate dehydrogenase complex, subunit A (SDHA) which were chosen as housekeeping genes on the basis of previous studies from other laboratories and ours [8, 25]. The specificity of PCR reactions was confirmed by melting curve analysis of the products and by size verification of the amplicon in a conventional agarose gel. A human universal reference total RNA (BD Biosciences Clontech, Palo Alto, CA) was used as a positive control of amplification and three negative controls were run for each assay: no template, no reverse transcriptase, and no RNA in the reverse transcriptase reaction.
The fold change of each target gene expression was expressed relative to the geometric mean mRNA expression of the reference genes in each sample [25, 36].
Statistical analysis
Results are expressed as mean ± standard deviation and n represents the number of experiments in MGCs or CCs from n different women. Data distribution and homogeneity of variances were analyzed with the Kolmogorov-Smirnov test and Levene test. For gene expression data, a logarithmic transformation was adapted in order to meet the normality assumptions and the statistical differences between these log-transformed values were assessed using the Student t test. Pearson correlation coefficients were calculated to analyze the association between gene expression levels in healthy and PCOS women. General linear models were performed to control for confounding variables and all models were adjusted by BMI and age. p < 0.05 values were considered significant. All the statistical analyses were performed using IBM SPSS Statistics software version 24.0.
Results
Anthropometric and biochemical parameters of healthy donors and PCOS patients are shown in Table 1. PCOS women were significantly older and had higher body mass index (BMI). The serum concentrations of follicle-stimulating hormone (FSH), luteinizing hormone (LH), testosterone (T), and 17β-estradiol (E2) fell within the reference range values in the early follicular phase of the menstrual cycle in both healthy and PCOS women (Table 1). There were no significant differences in the serum concentrations of day 3 FSH or E2 between both groups. Day 3 T, LH serum levels, and the LH/FSH ratio were higher in PCOS (Table 1). The serum concentrations of E2 and P measured on the day of administration of the ovulation inductor were similar in controls and PCOS (Table 1).
Table 1.
Anthropometrical and biochemical data of PCOS patients and healthy donors
| Healthy donors (n = 46) | PCOS (n = 43) | P value | |
|---|---|---|---|
| Age (years) | 24.67 ± 3.52 | 33.26 ± 2.83 | < 0.0001* |
| BMI (kg/m2) | 22.41 ± 2.70 | 26.26 ± 6.44 | 0.005* |
| Day 3 serum E2 (pg/ml) | 57.34 ± 37.33 | 53.12 ± 38.22 | 0.471 |
| Day 3 serum T (ng/dl) | 25.25 ± 8.15 | 38.34 ± 12.36 | < 0.001* |
| Day 3 serum FSH (mIU/ml) | 6.16 ± 1.43 | 6.00 ± 1.65 | 0.751 |
| Day 3 serum LH (mIU/ml) | 5.41 ± 1.95 | 8.70 ± 4.41 | < 0.001* |
| Day 3 serum LH/FSH | 0.86 ± 0.31 | 1.63 ± 1.27 | < 0.001* |
| E2 after ovulation induction (pg/ml) | 3214 ± 1554 | 2880 ± 1621 | 0.294 |
| P after ovulation induction (pg/ml) | 1.00 ± 0.48 | 0.84 ± 0.66 | 0.182 |
Data are means ± standard deviation. BMI body mass index, E2 estrogen, P progesterone, T total testosterone, FSH follicle-stimulating hormone, LH luteinizing hormone
Significant differences between groups (*P < 0.05) were assessed using the Student t test and are shown in italics
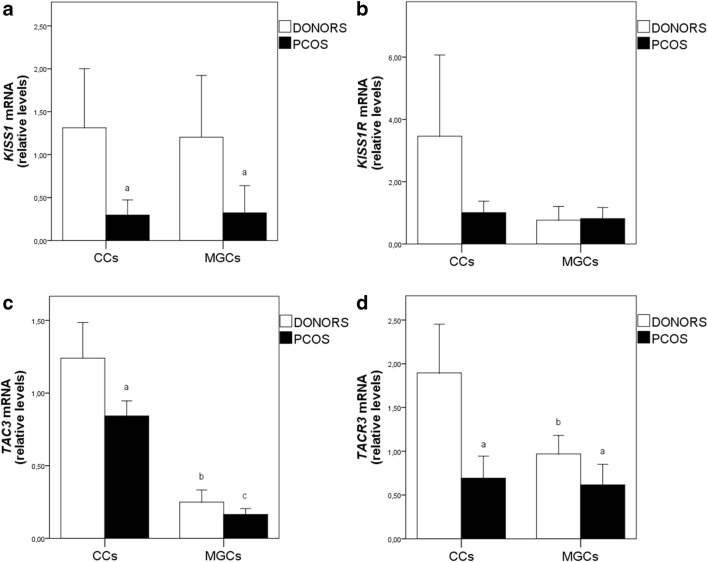
The expression levels of all the genes examined were not influenced by the use of rhCG or triptorelin for ovulation induction. The expression of KISS1 was down-regulated in MGCs (β = 0.048, p = 0.048) and CCs (β = − 0.585, p = 0.03) from PCOS patients, in comparison with mRNA levels in control healthy women (Fig. 1a) and the differences remained significant after adjusting for age and BMI. The expression levels of KISS1R showed great variations between samples, particularly in CCs from healthy women, and, as a consequence, we did not found significant differences between PCOS and control patients (Fig. 1b). The 2-∆∆CT values ranged from 0 to 43 in CCs from control women, while values in PCOS were much more regular and ranged from 0 to 4.1. There were also appreciable differences between controls and patients in the number of samples that expressed KISS1 and KISS1R. Thus, KISS1R was expressed in 50% of MGC samples from healthy women (11/22) and in 81% of PCOS samples (18/22). With respect to CCs, KISS1R was detected in 52% of controls (24/46) and in 74.4% of PCOS (32/43). Opposite to KISS1R, KISS1 was expressed in 72.7% of MGC samples from healthy women (16/22) and in 31.8% of PCOs samples (7/22). With respect to CCs, KISS1 was detected in 65.2% of controls (30/46) and in 35% of PCOS (15/43).
Fig. 1.
Expression of KISS1 (a), KISS1R (b), TAC3 (c), and TACR3 (d) in ovarian cumulus and granulosa cells of healthy women and PCOS patients. CCs, cumulus cells; MGCs, mural granulosa cells. Results are presented as means ± standard deviation. Statistically significant differences at p < 0.05 are represented as (a) between donors and PCOS, (b) between cumulus and granulosa cells in donors, and (c) between cumulus and granulosa cells in PCOS
TAC3 was present in all MGC and CC samples from healthy and PCOS women and its expression was higher in CCs than in MGCs in all women (Fig. 1c). TAC3 mRNA levels were extremely regular, particularly in CCs, where the 2-∆∆CT values ranged between 0.4 and 4 in healthy women (n = 46) and between 0.3 and 1.6 in PCOS patients (n = 43). TAC3 mRNA was down-regulated in CCs from PCOS (Fig. 1c). A multiple linear regression analysis shows that PCOS was associated with a lower expression of TAC3 in CCs which remained significant after adjusting for age and BMI (β = − 0.461, p 0.022).
TACR3 was also detected in more than 90% samples of both controls and PCOS women and its expression was significantly higher in MGCs (β = 0.721, p = 0.004) and CCs (β = 0.454, p = 0.022) of healthy women even after correcting for confounding variables (age and BMI). An increase in TACR3 mRNA levels was observed in CCs of healthy women, relative to MGCs from the same women. This increase did not occur in PCOS women (Fig. 1d).
The correlation coefficients between variables were also different in PCOS and healthy women. TACR3 was positively associated with KISSR in CCs from healthy women, and TAC3 was positively associated with KISS1 in MGCs of healthy women (Table 2). These associations remained after controlling for BMI and age (Fig. 2). However, the associations were not observed in PCOS women.
Table 2.
Partial correlations between KISS1, KISS1R, TAC3, and TACR3 in cumulus and granulosa cells of healthy women and PCOS patients
| Healthy Women | |||
| CCs | TACR3 mRNA | KISS1 mRNA | KISS1R mRNA |
| TAC3 mRNA | r = − 0.080 (p = 0.610) | r = − 0.068 (p = 0.651) | r = 0.058 (p = 0.707) |
| TACR3 mRNA | r = − 0.202 (p = 0.194) | r = 0.347 (p = 0.024) | |
| KISS1 mRNA | r = 0.043 (p = 0.777) | ||
| MGCs | TACR3 mRNA | KISS1 mRNA | KISS1R mRNA |
| TAC3 mRNA | r = − 0.075 (p = 0.741) | r = 0.652 (p = 0.006) | r = − 0.052 (p = 0.818) |
| KISS1 mRNA | r = 0.214 (p = 0.425) | r = − 0.132 (p = 0.528) | |
| KISS1R mRNA | r = 0.385 (p = 0.141) | ||
| PCOS | |||
| CCs | TACR3 mRNA | KISS1 mRNA | KISS1R mRNA |
| TAC3 mRNA | r = 0.296 (p = 0.060) | r = − 0.113 (p = 0.471) | r = 0.146 (p = 0.377) |
| TACR3 mRNA | r = 0.155 (p = 0.333) | r = 0.121 (p = 0.477) | |
| KISS1 mRNA | r = 0.238 (p = 0.144) | ||
| MGCs | TACR3 mRNA | KISS1 mRNA | KISS1R mRNA |
| TAC3 mRNA | r = 0.053 (p = 0.846) | r = 0.580 (p = 0.132) | r = 0.280 (p = 0.207) |
| TACR3 mRNA | r = − 0.411 (p = 0.312) | r = 0.172 (p = 0.400) | |
| KISS1 mRNA | r = 0.216 (p = 0.608) | ||
Significant associations (p < 0.05) are shown in italics. CCs cumulus cells, MGCs mural granulosa cells
Fig. 2.
Scatterplots showing the relationship between TAC3 and KISS1 in mural granulosa cells (a) and TACR3 and KISS1R in cumulus cells (b). A log transformation was given to the relative gene expression values. Models are controlled by age and body mass index (BMI). Correlation coefficient and p value refer to the association of gene expression in both, donors and PCOS. Significant associations (p < 0.05) are shown in bold
Discussion
The NKB/NK3R and kisspeptin/KISS1R systems are locally synthesized in MGCs and CCs of healthy women and regulate ovarian function by exerting a direct effect at the level of the gonads [25–28]. The present data show that kisspeptin, NKB, and NK3R mRNA levels are reduced in MGCs and CCs from PCOS patients, suggesting that NKB and kisspeptin could play also a role in the pathophysiology of PCOS.
The clinical manifestations of PCOS differ widely from a patient to another, suggesting a multi-factorial origin, which remains still unknown [1–6]. The peptides kisspeptin and NKB play an essential role in the neuroendocrine control of reproductive function acting coordinately at the hypothalamic-pituitary-gonadal (HPG) axis [4, 10–18, 37]. With respect to kisspeptin, several evidences argue for a potential role of this peptide in the pathophysiology of PCOS. First, PCOS is associated with several perturbations of HPG function, including a higher ratio of LH/FSH serum levels (see Table 1) [1–3, 7, 8] and kisspeptin is a key regulator of the HPG axis and, particularly, of gonadotropin secretion [4, 10, 11, 13–18, 37]. Second, PCOS is associated with metabolic symptoms, which include obesity and insulin resistance [1–5], and kisspeptin plays an important role in the metabolic regulation of fertility [4, 37–40]. Thus, fasting, which inhibits the activity of the reproductive axis, is associated with diminished expression of hypothalamic Kiss1 mRNA in rats and central administration of kisspeptin reverses the fasting-induced inhibition of gonadotrophin secretion [37]. These and other evidences, such as the deregulation of hypothalamic Kiss expression in conditions of obesity [39, 40], strongly suggest that kisspeptin neurons in the arcuate nucleus (ARC) serve as a link between metabolism and reproduction [4, 37–40]. Third, and in reference to the ovarian symptoms, different experimental data have shown that kisspeptin may participate in ovarian follicular development, can trigger egg maturation, and could contribute to the regulation of ovulation in different mammalian species including humans [17, 23, 27, 28, 30, 31]. In face of our present experimental evidence for altered KISS1 expression in GCs, these data are suggestive of a participation of kisspeptin in the pathogenesis of PCOS.
The main symptoms of PCOS are produced at the level of the ovary and are largely dependent on GC dysfunction [1–3, 5–8]. Different reports have shown that kisspeptin and kisspeptin receptor mRNAs and/or proteins are expressed in GCs of chickens [41], rats [23, 42, 43], dogs [44], and humans [23–25]. We found previously that KISS1 and KISS1R are locally synthesized in MGCs and CCs of healthy women and act coordinately with NKB and NK3R to regulate follicular function [25]. The present data show that the KISS1/KISS1R system is dysregulated in MGCs and CCs from PCOS patients with an overt suppression of KISS1 expression. Moreover, there were appreciable differences between controls and patients in the number of samples that express KISS1 and KISS1R. We also found that the expression of KISS1R showed a great dispersion between samples, particularly in CCs from healthy women, with samples which express KISS1R in high levels but approximately 50% of samples where KISS1R was not detected. This suggests that, in healthy donors, the expression of KISS1R in CCs is strictly regulated during a short period of time around ovulation. A similar event has been observed in rats where the expression of Kiss1 is dramatically increased preceding ovulation [42, 43]. This strict regulation appears to be lost in PCOS, where the expression of KISS1R was detected in a 75% of patients and was much more homogeneous.
In comparison with kisspeptin, less is known about the role of NKB and NK3R in the regulation of human ovarian physiology. However, a recent report involving treatment of 13 healthy women with the NK3R antagonist MLE4901 has shown that NKB regulates gonadotropin secretion, ovarian follicular growth, and the timing of ovulation in healthy women [33]. Our previous [25] and present data show that the TAC3/TACR3 system is widely expressed in MGC and CC samples from healthy and PCOS women and its expression is extremely regular, particularly in CCs. In healthy women, the transition between MGCs and CCs is accompanied by an increase in the TAC3/TACR3 system which does not occur in PCOS (see Fig. 1d). In addition, the present study shows that TAC3 expression was down-regulated in CCs of PCOS, and TACR3 was down-regulated in MGCs and CCs of PCOS women, in comparison with healthy controls. In the same line, a recent study by Qi et al. [26] failed to detect any expression of TACR3 in MGCs from six PCOS women. Of note, a recent clinical study showed that the NK3R antagonist MLE4901 is a useful drug for PCOS treatment and can be beneficial to reduce T and LH serum levels, which are elevated in PCOS patients (see Table 1) [45]. NKB and kisspeptin signaling has been involved in estrogen negative feedback regulation, which is altered in PCOS. While the potential contribution of kisspeptin and NKB to this phenomenon is assumed to be mainly central, our current data open up the intriguing possibility that local deregulated expression of KISS1 and NKB systems in GCs might contribute also to the miscommunication between gonadal and central levels of the HPG axis that is characteristic of PCOS.
We previously found that the NKB/NK3R and KISS1/KISS1R systems act in a coordinated manner to regulate GC function in healthy donors [25]. The present results reinforce this observation showing the existence of a positive association between TAC3 and KISS1 in MGCs and between TACR3 and KISS1R in CCs of healthy women. These associations were, however, lost in PCOS, suggesting that the coordinated action of NKB and KISS1 could be compromised in these patients. Further, studies will help to determine the precise significance of this finding in the pathogenesis of PCOS.
In conclusion, the present data show that the kisspeptin/KISS1R and NKB/NK3R systems are down-regulated in MGCs and CCs of PCOS patients. The lower levels of kisspeptin and NKB in the ovary of PCOS women could contribute to the abnormal follicular development and defective ovulation which is observed in these patients.
Electronic supplementary material
(DOCX 28 kb)
Funding information
This work was supported by a grant from the Ministerio de Economía y Competitividad (RTC-2014-1431-1), Spain, with joint financing by FEDER funds from the European Union.
Compliance with ethical standards
Approval for this work was obtained from the institutional Ethics Committees of CSIC and Hospital Virgen Macarena (Sevilla, Spain) and all patients gave informed written consent.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Norman RJ, Dewailly D, Legro RS, Hickey TE. Polycystic ovary syndrome. Lancet. 2007;370:685–697. doi: 10.1016/S0140-6736(07)61345-2. [DOI] [PubMed] [Google Scholar]
- 2.Livadas S, Diamanti-Kandarakis E. Polycystic ovary syndrome: definitions, phenotypes and diagnostic approach. Front Horm Res. 2013;40:1–21. doi: 10.1159/000341673. [DOI] [PubMed] [Google Scholar]
- 3.El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Polycystic ovarian syndrome: an updated overview. Front Physiol. 2016;7:124. [DOI] [PMC free article] [PubMed]
- 4.Navarro VM, Tena-Sempere M. Neuroendocrine control by kisspeptins: role in metabolic regulation of fertility. Nat Rev Endocrinol. 2011;8:40–53. doi: 10.1038/nrendo.2011.147. [DOI] [PubMed] [Google Scholar]
- 5.Jayasena CN, Franks S. The management of patients with polycystic ovary syndrome. Nat Rev Endocrinol. 2014;10:624–636. doi: 10.1038/nrendo.2014.102. [DOI] [PubMed] [Google Scholar]
- 6.Ramezanali F, Ashrafi M, Hemat M, Arabipoor A, Jalali S, Moini A. Assisted reproductive outcomes in women with different polycystic ovary syndrome phenotypes: the predictive value of anti-Müllerian hormone. Reprod BioMed Online. 2016;32:503–512. doi: 10.1016/j.rbmo.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Dewailly D, Robin G, Peigne M, Decanter C, Pigny P, Catteau-Jonard S. Interactions between androgens, FSH, anti-Müllerian hormone and estradiol during folliculogenesis in the human normal and polycystic ovary. Hum Reprod Update. 2016;22:709–724. doi: 10.1093/humupd/dmw027. [DOI] [PubMed] [Google Scholar]
- 8.Lv Y, Zhao SG, Lu G, Leung CK, Xiong ZQ, Su XW, Ma JL, Chan WY, Liu HB. Identification of reference genes for qRT-PCR in granulosa cells of healthy women and polycystic ovarian syndrome patients. Sci Rep. 2017;7:6961. doi: 10.1038/s41598-017-07346-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li R, Albertini DF. The road to maturation: somatic cell interaction and self-organization of the mammalian oocyte. Nat Rev Mol Cell Biol. 2013;14:141–152. doi: 10.1038/nrm3531. [DOI] [PubMed] [Google Scholar]
- 10.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KISS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100:10972–6. [DOI] [PMC free article] [PubMed]
- 11.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF, Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 12.Pintado CO, Pinto FM, Pennefather JN, Hidalgo A, Baamonde A, Sanchez T, Candenas ML. A role for tachykinins in female mouse and rat reproductive function. Biol Reprod. 2003;69:940–946. doi: 10.1095/biolreprod.103.017111. [DOI] [PubMed] [Google Scholar]
- 13.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 15.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu G, Lin C, He M, Wong AO. Neurokinin B and reproductive functions: “KNDy neuron” model in mammals and the emerging story in fish. Gen Comp Endocrinol. 2014;208:94–108. doi: 10.1016/j.ygcen.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 17.Clarke H, Dhillo WS, Jayasena CN. Comprehensive review on kisspeptin and its role in reproductive disorders. Endocrinol Metab. 2015;30:124–141. doi: 10.3803/EnM.2015.30.2.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skorupskaite K, George JT, Veldhuis JD, Millar RP, Anderson RA. Interactions between neurokinin B and kisspeptin in mediating estrogen feedback in healthy women. J Clin Endocrinol Metab. 2016;101:4628–4636. doi: 10.1210/jc.2016-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Candenas L, Lecci A, Pinto FM, Patak E, Maggi CA, Pennefather JN. Tachykinins and tachykinin receptors: effects in the genitourinary tract. Life Sci. 2005;76:835–862. doi: 10.1016/j.lfs.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Page NM. Neurokinin B and pre-eclampsia: a decade of discovery. Reprod Biol Endocrinol. 2010;8:4. doi: 10.1186/1477-7827-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lasaga M, Debeljuk L. Tachykinins and the hypothalamo-pituitary-gonadal axis: an update. Peptides. 2011;32:1972–1978. doi: 10.1016/j.peptides.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Satake H, Kawada T. Overview of the primary structure, tissue distribution, and functions of tachykinins and their receptors. Curr Drug Targets. 2006;7:963–974. doi: 10.2174/138945006778019273. [DOI] [PubMed] [Google Scholar]
- 23.Gaytán F, Gaytán M, Castellano JM, Romero M, Roa J, Aparicio B, Garrido N, Sánchez-Criado JE, Millar RP, Pellicer A, Fraser HM, Tena-Sempere M. KiSS-1 in the mammalian ovary: distribution of kisspeptin in human and marmoset and alterations in KiSS-1 mRNA levels in a rat model of ovulatory dysfunction. Am J Physiol Endocrinol Metab. 2009;296:E520–E531. doi: 10.1152/ajpendo.90895.2008. [DOI] [PubMed] [Google Scholar]
- 24.Cejudo Roman A, Pinto FM, Dorta I, Almeida TA, Hernández M, Illanes M, Tena-Sempere M, Candenas L. Analysis of the expression of neurokinin B, kisspeptin and their cognate receptors, NK3R and KISS1R in the human female genital tract. Fertil Steril. 2012;97:1213–1219. doi: 10.1016/j.fertnstert.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 25.García-Ortega J, Pinto FM, Fernández-Sánchez M, Prados N, Cejudo-Román A, Almeida TA, Hernández M, Romero M, Tena-Sempere M, Candenas L. Expression of neurokinin B/NK3 receptor and kisspeptin/KISS1 receptor in human granulosa cells. Hum Reprod. 2014;29:2736–2746. doi: 10.1093/humrep/deu247. [DOI] [PubMed] [Google Scholar]
- 26.Qi X, Salem M, Zhou W, Sato-Shimizu M, Ye G, Smitz J, Peng C. Neurokinin B exerts direct effects on the ovary to stimulate estradiol production. Endocrinology. 2016;157:3355–3365. doi: 10.1210/en.2016-1354. [DOI] [PubMed] [Google Scholar]
- 27.Gaytan F, Garcia-Galiano D, Dorfman MD, Manfredi-Lozano M, Castellano JM, Dissen GA, Ojeda SR, Tena-Sempere M. Kisspeptin receptor haplo-insufficiency causes premature ovarian failure despite preserved gonadotropin secretion. Endocrinology. 2014;155:3088–3097. doi: 10.1210/en.2014-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattacharya M, Babwah AV. Kisspeptin: beyond the brain. Endocrinology. 2015;156:1218–1227. doi: 10.1210/en.2014-1915. [DOI] [PubMed] [Google Scholar]
- 29.León S, Barroso A, Vázquez MJ, García-Galiano D, Manfredi-Lozano M, Ruiz-Pino F, Heras V, Romero-Ruiz A, Roa J, Schutz G, Kirilov M, Gaytan F, Pinilla L, Tena-Sempere M. Direct actions of kisspeptins on GnRH neurons permit attainment of fertility but are insufficient to fully preserve gonadotropic axis activity. Sci Rep. 2016;6:19206. doi: 10.1038/srep19206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jayasena CN, Abbara A, Comninos AN, Nijher GM, Christopoulos G, Narayanaswamy S, Izzi-Engbeaya C, Sridharan M, Mason AJ, Warwick J, Ashby D, Ghatei MA, Bloom SR, Carby A, Trew GH, Dhillo WS. Kisspeptin-54 triggers egg maturation in women undergoing in vitro fertilization. J Clin Invest. 2014;124:3667–3677. doi: 10.1172/JCI75730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calder M, Chan YM, Raj R, Pampillo M, Elbert A, Noonan M, Gillio-Meina C, Caligioni C, Bérubé NG, Bhattacharya M, Watson AJ, Seminara SB, Babwah AV. Implantation failure in female Kiss1-/- mice is independent of their hypogonadic state and can be partially rescued by leukemia inhibitory factor. Endocrinology. 2014;155:3065–3078. doi: 10.1210/en.2013-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandois D, Na E, Cuevas F, Cruz G, Lara HE, Paredes AH. Kisspeptin is involved in ovarian follicular development during aging in rats. J Endocrinol. 2016;228:161–170. doi: 10.1530/JOE-15-0429. [DOI] [PubMed] [Google Scholar]
- 33.Skorupskaite K, George JT, Veldhuis JD, Anderson RA. Neurokinin B regulates gonadotropin secretion, ovarian follicle growth, and the timing of ovulation in healthy women. J Clin Endocrinol Metab. 2018;103:95–104. doi: 10.1210/jc.2017-01306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 36.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–11. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Castellano JM, Navarro VM, Fernández-Fernández R, Nogueiras R, Tovar S, Roa J, Vazquez MJ, Vigo E, Casanueva FF, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Changes in hypothalamic KiSS-1 system and restoration of pubertal activation of the reproductive axis by kisspeptin in undernutrition. Endocrinology. 2005;146:3917–3925. doi: 10.1210/en.2005-0337. [DOI] [PubMed] [Google Scholar]
- 38.Dudek M, Ziarniak K, Sliwowska JH. Kisspeptin and metabolism: the brain and beyond. Front Endocrinol. 2018;9:145. doi: 10.3389/fendo.2018.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sánchez-Garrido MA, Ruiz-Pino F, Manfredi-Lozano M, Leon S, Garcia-Galiano D, Castaño JP, Luque RM, Romero-Ruiz A, Castellano JM, Diéguez C, Pinilla L, Tena-Sempere M. Obesity-induced hypogonadism in the male: premature reproductive neuroendocrine senescence and contribution of Kiss1-mediated mechanisms. Endocrinology. 2014;155:1067–1079. doi: 10.1210/en.2013-1584. [DOI] [PubMed] [Google Scholar]
- 40.Sánchez-Garrido MA, Ruiz-Pino F, Manfredi-Lozano M, Leon S, Heras V, Castellano JM, Castaño JP, Luque RM, Vázquez MJ, Roa J, Romero-Ruiz A, Diéguez C, Pinilla L, Tena-Sempere M. Metabolic and gonadotropic impact of sequential obesogenic insults in the female: influence of the loss of ovarian secretion. Endocrinology. 2015;156:2984–2998. doi: 10.1210/en.2014-1951. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Y, Ni Y, Huang Y, Wu J, Grossmann R, Zhao R. Effects of kisspeptin-10 on progesterone secretion in cultured chicken ovarian granulosa cells from preovulatory (F1-F3) follicles. Peptides. 2011;32:2091–2097. doi: 10.1016/j.peptides.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Laoharatchatathanin T, Terashima R, Yonezawa T, Kurusu S, Kawaminami M. Augmentation of metastin/kisspeptin mRNA expression by the proestrous luteinizing hormone surge in granulosa cells of rats: implications for luteinization. Biol Reprod. 2015;93:15. doi: 10.1095/biolreprod.115.127902. [DOI] [PubMed] [Google Scholar]
- 43.Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, Dieguez C, Aguilar E, Sánchez-Criado JE, Pellicer A, Pinilla L, Gaytan F, Tena-Sempere M. Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology. 2006;147:4852–4862. doi: 10.1210/en.2006-0117. [DOI] [PubMed] [Google Scholar]
- 44.Cielesh ME, McGrath BM, Scott CJ, Norman ST, Stephen CP. The localization of kisspeptin and kisspeptin receptor in the canine ovary during different stages of the reproductive cycle. Reprod Domest Anim. 2017;52:24–28. doi: 10.1111/rda.12841. [DOI] [PubMed] [Google Scholar]
- 45.George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, Veldhuis J, Skorupskaite K, Anderson RA, McIntosh S, Webber L. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101:4313–4321. doi: 10.1210/jc.2016-1202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 28 kb)