Abstract
Signal transducer and activator of transcription 3 (Stat3) has been shown to play a role in intestinal regeneration and colitis-associated colon carcinogenesis. However, the role of Stat3 in the Wnt-driven sporadic intestinal tumorigenesis remains poorly understood. We examined the roles of Stat3 in intestinal regeneration and tumorigenesis by organoid culture experiments using Stat3∆IEC mouse–derived intestinal epithelial cells in which Stat3 was disrupted. The regeneration of intestinal mucosa and organoid formation were significantly suppressed by Stat3 disruption, which was compensated by Wnt activation. Furthermore, once organoids were recovered, Stat3 was no longer required for organoid growth. These results indicate that Stat3 and Wnt signaling cooperatively protect epithelial cells at the early phase of intestinal regeneration. In contrast, intestinal tumorigenesis was not suppressed by Stat3 disruption in adenomatous polyposis coli (Apc)Δ716 and Apc∆716 Tgfbr2∆IEC mice, thus indicating that Stat3 is not required for Wnt activation–driven intestinal tumorigenesis. Mechanistically, Itga5 and Itga6 were down-regulated by Stat3 disruption, and focal adhesion kinase (FAK) activation was also suppressed. Notably, FAK inhibitor suppressed the organoid formation of wild-type epithelial cells. These results indicate that Stat3 is indispensable for the survival of epithelial cells through the activation of integrin signaling and the downstream FAK pathway; however, it is not required for the Wnt signaling-activated normal or tumor epithelial cells.—Oshima, H., Kok, S.-Y., Nakayama, M., Murakami, K., Voon, D. C.-C., Kimura, T., Oshima, M. Stat3 is indispensable for damage-induced crypt regeneration but not for Wnt-driven intestinal tumorigenesis.
Keywords: colon cancer, organoids, anoikis, integrin, FAK
It has been established that inflammatory responses promote cancer development and malignant progression (1, 2). In the inflammatory tumor microenvironment, macrophages and fibroblasts express cytokines and chemokines, resulting in the activation of transcription factors, such as NF-κB and signal transducer and activator of transcription 3 (Stat3). The aberrant activation of Stat3 was found in >70% of human cancers (3, 4), and Stat3 activation is correlated with the invasiveness, proliferation, and stemness of human colon cancer cells (5–7). Furthermore, studies using a colitis-associated colon cancer (CAC) mouse model have shown that the disruption of Stat3 in epithelial cells significantly suppresses CAC development through the suppression of proliferation and induction of apoptosis (8, 9). It has also been shown that IL-11 is a dominant IL-6 family cytokine in gastrointestinal tumors, and IL-11 receptor signaling promotes tumorigenesis through Stat3 activation (10). On the basis of these results, the Stat3 pathway is thought to be an important target in the development of anticancer drugs for various cancers, including colorectal cancer (CRC) (11).
In contrast to these findings, several studies have demonstrated a tumor suppressor role of Stat3 in intestinal tumorigenesis (12, 13). In those reports, the disruption of Stat3 in ApcMin mice, a model of familial adenomatous polyposis, did not suppress tumorigenesis; instead, it induced the submucosal invasion of tumor cells, suggesting that Stat3 prevents the malignant progression of intestinal tumors. This discrepancy in the role of Stat3 in intestinal tumorigenesis may be due to the different mechanisms of cancer development in these models (i.e., inflammation- and regeneration-associated tumorigenesis in the CAC model and Wnt activation–driven tumorigenesis in ApcMin mice). A comprehensive genome analysis indicated that >90% of human CRCs carry genetic alterations in APC or CTNNB1 resulting in Wnt signaling activation (14); thus, it is important to investigate the precise role of Stat3 in Wnt activation–driven CRC development.
The Stat3 function has been shown to be absolutely required for the intestinal stem-cell survival (15). Consistently, Stat3 plays a role in the regeneration of the damaged intestinal mucosa after irradiation or dextran sodium sulfate treatment (16, 17). However, the mechanism through which Stat3 signaling is involved in stem-cell survival and proliferation during regeneration has not been fully elucidated. Wnt/β-catenin signaling is a key driver of the tissue stem-cell property, making the Wnt pathway important for both regeneration and cancer development (18, 19). Furthermore, pathways through focal adhesion kinase (FAK) or lysin methyltransferase SETD7 are responsible for both mucosal regeneration and tumorigenesis in the intestine through the activation of Wnt signaling (20, 21). However, the role of Stat3 in intestinal regeneration and tumorigenesis has not been studied in the context of Wnt-driven carcinogenesis.
In the present study, we used genetic mouse models and organoid culture systems to show that Stat3 is required for the survival and proliferation of the residual stem cells in damaged mucosa or isolated crypts, leading to crypt formation in the regenerating mucosa as well as organoid formation in Matrigel. However, Stat3 is not required for crypt development when Wnt signaling is activated by exogenous ligand stimulation or when the Apc gene is lost. Consistently, Stat3 is dispensable for Wnt activation–driven tumor development and malignant progression, including submucosal invasion and liver metastasis. These results suggest that the Wnt activation status and regeneration phenotype are important factors to consider with regard to the efficacy of Stat3 inhibitors in the prevention of CRC development.
MATERIALS AND METHODS
Mouse experiments
Apc∆716, Stat3flox/flox, and villin-CreER mice have been previously described (22–24). Tgfbr2flox/flox mice were obtained from the Mouse Repository (NCI-Frederick, Frederick, MD, USA) (25). The genetic background of all strains used in this study was C57BL/6. For organoid culture experiments, villin-CreER Stat3flox/flox mice were treated with tamoxifen (Tam; 4 mg, i.p./mouse) for 3 continuous days at 4 to 8 wk of age, resulting in Stat3 disruption in the intestinal epithelial cells (hereafter Stat3∆IEC). X-ray irradiation (9 Gy) was performed to induce intestinal mucosal regeneration [n = 9 for wild-type (WT) mice and n = 12 for Stat3∆IEC mice]. For the in vivo experiments, Apc∆716 villin-CreER Stat3 flox/flox mice and Apc∆716 villin-CreER Tgfbr2flox/flox Stat3 flox/flox mice were generated by crossing and treated with Tam (4 mg, i.p./mouse) once a week from 6 to 19 wk of age. The total number of intestinal polyps and the size distribution were scored under a dissection microscope (n = 10 for Apc∆716 mice, n = 12 for Apc∆716 Stat3∆IEC mice, n = 10 for Apc∆716 Tgfbr2∆IEC mice, and n = 8 for Apc∆716 Tgfbr2∆IEC Stat3∆IEC mice). Invasion frequency was scored using hematoxylin and eosin (H&E)–stained histology sections (n = 4 each for Apc∆716 and Apc∆716 Stat3∆IEC mice).
The protocols for the animal experiments were approved by the Committee on Animal Experimentation of Kanazawa University.
Organoid culture experiments
The organoid cultures were prepared from the intestinal mucosa and tumors of the small intestine according to a previously described method (26). In brief, dissected tissues were soaked in 2 mM EDTA for 30 min, and crypts were isolated by shaking. Isolated crypts were then collected and suspended in Matrigel with Advanced DMEM/F-12 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 50 ng/ml epidermal growth factor (Thermo Fisher Scientific), 100 ng/ml Noggin (PeproTech, Rocky Hill, NJ, USA) and 10% R-Spondin1 conditioned medium prepared from human R-Spondin1–expressing 293T cells (kindly provided by N. Barker, A-Star, Singapore). For mechanical passage, 15 organoids (200 µm < ϕ < 400 µm) were collected using Cell Recovery Solution (Corning, Corning, NY, USA), and crypt structures roughly dissociated into fragments by pipetting were mixed with Matrigel and transferred to 3 wells of a 48-well plate. For enzymatic passage, the collected organoids were dissociated by treatment with 25 mM trypsin for 5 min, suspended in Matrigel, and seeded at 105 cells per well (for WT and Stat3∆IEC mice) or 106 cells per well (for Apc∆716 and Apc∆716 Stat3∆IEC mice) to each well of 48-well plate. The respective passage experiments were performed 3 times, and the mean organoid numbers were counted. For the rescue experiments, 10 µM Rho-associated protein kinase inhibitor (Y-27632; Fujifilm Wako Pure Chemical Industries, Osaka, Japan) and 5 µM glycogen synthase kinase (GSK) 3 inhibitor (CHIR-99021; Tocris Bioscience, Bristol, United Kingdom) were added, and the total numbers of organoids derived from about 300 crypts were counted under a dissection microscope. Rescue experiments for each inhibitor combination were performed 3 times, and the mean organoid numbers were counted. For the activation of Wnt signaling, conditioned medium including afamin-associated Wnt3a (AFM-Wnt3a) was obtained from T. Sato (Keio University, Tokyo, Japan) using a cell line established by J. Takagi (Osaka University, Osaka, Japan) and added to the medium (27). The organoid cell proliferation was examined using the Click-iT 5-ethynyl-2′-deoxyuridine (EdU) Imaging System (Thermo Fisher Scientific), and the EdU labeling index was calculated by counting the number of positive cells in at least 5 organoids. For FAK inhibition experiments, organoids were treated with FAK inhibitor 14 (Merck, Darmstadt, Germany) at 15 µM.
Histology and immunohistochemistry
The tissue specimens were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 4-μm thick sections. Crypts were isolated by EDTA, and organoids were fixed in 4% paraformaldehyde and embedded in iPGell (Genostaff, Tokyo, Japan). The sections were stained with H&E. For immunohistochemistry, antibodies against Ki-67 (Thermo Fisher Scientific), CD44s (Merck), phosphorylated Stat3 at Tyr705 (Cell Signaling Technology, Danvers, MA, USA), and phosphorylated FAK at Tyr397 (Cell Signaling Technology) were used as the primary antibodies. Staining signals were visualized using a Vectastain Elite Kit (Vector Laboratories, Burlingame, CA, USA). Apoptosis was examined using an Apoptag Peroxidase In Situ Apoptosis Detection Kit (Merck).
Cell culture and transplantation experiments
A mouse metastatic intestinal cancer cell line (AKTP) was established as recently described (28). AKTP cells carry Apc∆716 Kirsten rat sarcoma viral oncogene (Kras)G12D Tgfbr2−/− Trp53R270H mutations. To disrupt the Stat3 gene in AKTP cells, Stat3-specific single guide RNA (sgRNA) was cloned into the px330 expression vector (Addgene, Cambridge, MA, USA), which bicistronically expresses sgRNA and Cas9 nuclease, and transfected to AKTP cells. The sgRNA sequence was determined by the Crspr Design Tool (http://crispr.mit.edu/). Disruption of the Stat3 gene was confirmed by Western blot analysis using a Stat3 antibody (Cell Signaling Technology). Cell proliferation was examined using a Cell Titer-Glo 2.0 Assay (Promega, Madison, WI, USA).
For the metastasis analysis, 1 × 106 cells were transplanted into the spleen of immunodeficient NOD/Shi-scid Il2rg−/− mice (NSG mice; CIEA, Kawasaki, Japan) (n = 3 for each cell line), and metastatic liver lesions were examined histologically at 4 wk after transplantation. The efficiency of metastasis was scored by the measurement of the areas of metastasized foci per total liver on H&E-stained sections.
Expression analyses
Total RNA was extracted from isolated crypts using an RNeasy Plus Micro Extraction Kit (Qiagen, Hilden, Germany). An expression analysis was performed using the RT2 Profiler PCR Array (Mouse Cell Junction Pathway Finder).
RT-PCR
For the real-time RT-PCR, total RNA was extracted from isolated crypts or organoids using an RNeasy Plus Micro Extraction Kit (Qiagen), reverse transcribed using a PrimeScript RT Reagent Kit (Takara Bio, Otsu, Japan), and amplified using ExTaqII SYBR Premix (Takara Bio) on a Stratagene Mx3000P Real-Time Thermocycler (Agilent Technologies, Santa Clara, CA, USA). The purchased primer sequences were as follows: (Itga5-forward) 5′-GAAGCTCTGAAGATGCCCTACCA-3′, (Itga5-reverse) 5′-TGATGATCCACAACGGGACAC-3′; and (Itga6-forward) 5′-GTCACCGCTGCTGCTCAGAATA-3′, (Itga6-reverse) 5′-AGCATCAGAATCCCGGCAAG-3′ (Takara Bio).
Statistical analyses
The data were analyzed by an unpaired Student’s t test and are presented as means ± sd. A value of P < 0.05 was considered to indicate statistical significance.
RESULTS
Stat3 is required for regeneration of intestinal mucosa
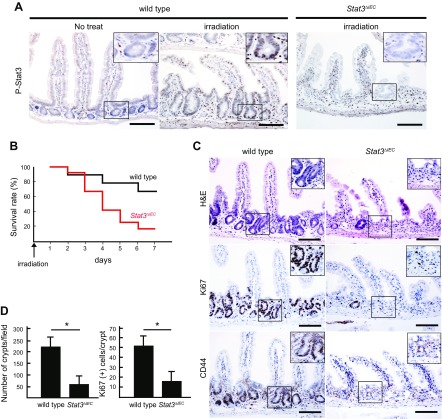
Epithelial Stat3 expression has been shown to be required for the regeneration of the damaged intestinal mucosa (17, 29, 30). We confirmed that phosphorylated Stat3 (P-Stat3) was detected in the cryptic cells of the X-ray–irradiated WT mouse intestine, while P-Stat3 in the normal intestinal epithelia was below the detection limit (Fig. 1A). These results indicate that the Stat3 activation is significantly increased in epithelial cells of regenerating mucosa. We then constructed Stat3∆IEC mice, in which Stat3 was disrupted in the intestinal epithelial cells in a cell-specific manner by the treatment of villin-CreER Stat3flox/flox mice with Tam. We confirmed that P-Stat3 was not detected in the X-ray–irradiated Stat3∆IEC mouse intestine (Fig. 1A). X-ray irradiation caused a more severe phenotype in Stat3∆IEC mice than in WT mice; the 7-d survival rates of WT and Stat3∆IEC mice were 67% and 17%, respectively (Fig. 1B). Histologically, mucosal regeneration was significantly suppressed in Stat3∆IEC mice, and the number of remaining crypts at 3 d after irradiation in Stat3∆IEC mice was significantly decreased compared to WT mice (Fig. 1C, D). The numbers of Ki-67–positive proliferating cells and CD44-positive undifferentiated cells in the residual crypts were also significantly decreased in the Stat3∆IEC intestinal mucosa. These results indicate that the activation of epithelial Stat3 is required for crypt repopulation via the survival and proliferation of residual stem cells in the damaged mucosa, which leads to regeneration.
Figure 1 .
Histologic analysis of X-ray–irradiated mouse small intestine. A) Representative sections immunohistochemically stained for P-Stat3 in small intestine of control WT and irradiated WT and irradiated Stat3ΔIEC mice. Scale bars, 100 μm. Insets indicate enlarged images of boxed areas. B) Survival rate of WT and Stat3ΔIEC mice after X-ray irradiation (n = 9 for WT and n = 12 for Stat3∆IEC mice). C) Representative histology sections from WT (left) and Stat3ΔIEC (right) mouse intestines (H&E) (top) and immunohistochemical staining for Ki-67 (middle) and CD44 (bottom). Insets show enlarged image views of boxed areas. Scale bars, 100 μm. D) Number of crypts in microscopy fields (left) and Ki-67–positive cells per crypt (right) are shown (means ± sd). *P < 0.05.
Stat3 is required for intestinal organoid formation
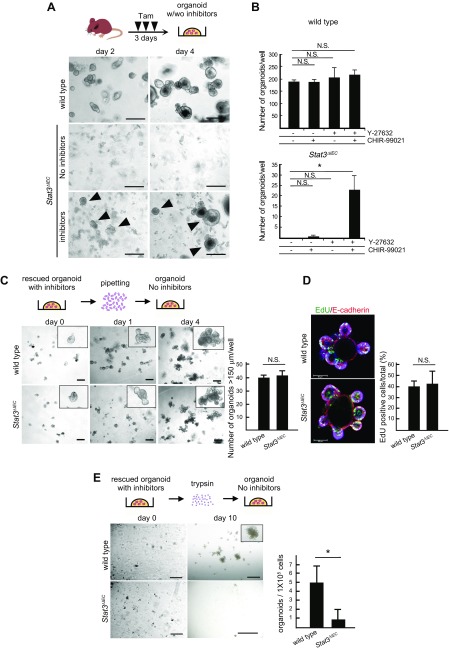
To understand the biologic mechanism of Stat3 in the regeneration of the intestinal mucosa, we next examined the in vitro organoid formation of intestinal epithelial cells, which mimics crypt regeneration in vivo. Intestinal cryptic cells isolated from WT mice formed organoids through the construction of minicrypt structures in Matrigel (Fig. 2A). Importantly, however, intestinal cryptic cells derived from Stat3∆IEC mice failed to generate organoids in Matrigel. Consistently, previous reports indicated that exogenous IL-22 signaling promotes organoid formation and mucosal regeneration through the induction of Stat3 activation (30, 31). Taken together, these results indicate that Stat3 activation is required for intestinal crypt reconstruction.
Figure 2 .
Role of Stat3 in organoid formation from intestinal crypts. A) Schematic illustration of Tam treatment and organoid formation (top). Representative microscopic photographs of organoid cultures of intestinal crypts of WT and Stat3ΔIEC mice on d 2 (left) and d 4 (right). Arrowheads indicate organoids rescued by inhibitors (Y-27632 and CHIR-99021). Scale bars, 250 μm. B) Numbers of organoids per well developed from intestinal crypts of WT (top) and Stat3ΔIEC mice (bottom) in indicated inhibitor conditions (means ± sd). *P < 0.05. Inhibitor treatment experiments were performed 3 times for each mouse genotype. C) Schematic illustration of organoid passaging by mechanical pipetting (top). Representative microscopic photographs of organoids passaged from WT and Stat3ΔIEC rescued organoids (bottom). Insets show enlarged views. Scale bars, 250 μm. Numbers of organoids >150 µm are shown in bar graph (means ± sd). Mechanical passage experiments were performed 3 times. D) Immunohistochemical staining of WT (top) and Stat3ΔIEC (bottom) rescued organoids for EdU (green) and E-cadherin (red). EdU labeling efficiency is shown in bar graph (means ± sd). Scale bars, 50 μm. E) Schematic illustration of organoid passaging by trypsin treatment (top). Representative microscopic photographs of organoids passaged from WT (top) and Stat3ΔIEC-rescued organoids (bottom). Scale bars, 200 μm (d 0) and 1 mm (d 10). Numbers of organoids are shown in bar graph (means ± sd). *P < 0.05. Enzymatic passage experiments were performed 3 times. N.S., not significant.
Next, we treated isolated cryptic cells from Stat3∆IEC mice with Rho-associated protein kinase inhibitor (Y-27632) and GSK3 inhibitor (CHIR-99021), as these inhibitors increase the efficiency of organoid formation through the suppression of anoikis and the activation of Wnt signaling, respectively (32). Notably, the addition of both inhibitors significantly increased the number of Stat3∆IEC mouse–derived organoids, while treatment with Y-27632 or CHIR-99021 alone was not sufficient for organoid recovery (Fig. 2A, B). We confirmed the disruption of Stat3 in the rescued organoids by Western blot analysis, which ruled out the possibility of contamination of Stat3 WT cells (Supplemental Fig. 1).
Intriguingly, once the organoids were generated from Stat3∆IEC intestinal crypts in the presence of inhibitors, the rescued Stat3∆IEC organoids no longer required inhibitor treatment for further growth (Fig. 2C). The organoid-forming efficiency of Stat3∆IEC epithelial cells was unaltered compared to WT after mechanical passage. Furthermore, the EdU labeling efficiency of the rescued and mechanically passaged Stat3∆IEC organoids was similar to that of WT mouse–derived organoids (Fig. 2D). In contrast, the development of Stat3∆IEC organoids was significantly suppressed when the rescued organoids were passaged after enzymatic dissociation by trypsin (Fig. 2E). These results suggest that Stat3 is required for crypt formation from the remnant stem cells in the damaged mucosa due to protection from anoikis, whereas Stat3 is no longer required for the maintenance and growth of the established crypts. It is noteworthy that the conditional Stat3 disruption in the intestinal epithelial cells of adult mice did not change the mucosal morphology, proliferation rate, or differentiation into lysozymes or Muc2-expressing cells (Supplemental Fig. 2). Furthermore, the numbers of SOX7-positive undifferentiated cells in the Stat3∆IEC mice were similar to those in WT mice. These results support the idea that Stat3 is only necessary to generate new crypts from the remnant stem cells after radiation injury.
Wnt activation compensates for Stat3 disruption to support organoid formation
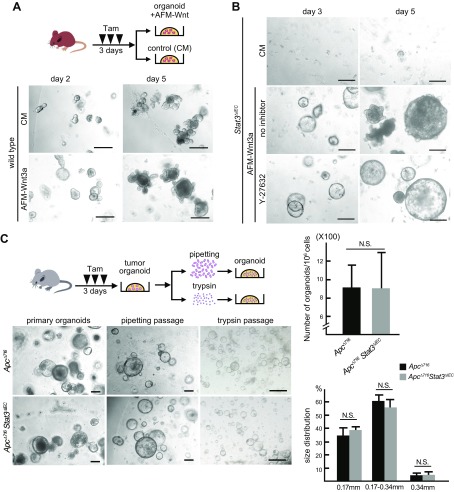
It has been shown that GSK3 inhibitor increases the colony formation efficiency of intestinal stem cells through Wnt signaling activation (32, 33). Because treatment with CHIR-99021 is required for the recovery of Stat3∆IEC organoids, it is possible that Wnt signaling activation is involved in the recovery of Stat3∆IEC organoid formation. To assess this possibility, we cultured Stat3∆IEC mouse intestinal crypts in medium supplemented with the active water-soluble form of lipidated Wnt3a, which is associated with bovine serum protein afamin (AFM-Wnt3a) (27). As expected, AFM-Wnt3a treatment increased the number and size of the organoids derived from WT mouse crypts compared to organoid cultures with control medium, which is consistent with the findings of a previous report in human intestinal organoids (27) (Fig. 3A). Importantly, AFM-Wnt3a treatment rescued the organoid formation of Stat3∆IEC mouse intestinal crypts, even in the absence of an inhibitor (Fig. 3B). These results indicate that Wnt signaling activation can compensate for Stat3 disruption to support the survival and proliferation of isolated intestinal cryptic cells.
Figure 3 .
Wnt activation compensates for Stat3 disruption to support organoid formation. A) Schematic illustration of Tam treatment and organoid formation (top). Representative microscopic photographs of organoids of intestinal crypts from WT mice cultured with control medium (top) and AFM-Wnt3a (bottom) on d 2 (left) and d 5 (right). Scale bars, 250 μm. B) Representative microscopic photographs of organoid cultures of intestinal crypts from Stat3ΔIEC mice cultured with control medium (top), AFM-Wnt3a without inhibitor (middle), and with inhibitor (bottom) on d 3 (left) and d 5 (right). Scale bars, 250 μm. C) Schematic illustration of Tam treatment, tumor-derived organoid formation, and treatment with pipetting or trypsin to passage (top). Representative photomicrographs of primary organoids (left; scale bars, 250 μm) and organoids passaged by pipetting (middle; scale bars, 250 μm), and trypsin treatment (right; scale bars, 500 μm) derived from intestinal tumors of ApcΔ716 simple mutant mice (top) and ApcΔ716 Stat3ΔIEC compound mice (bottom). Numbers of organoids (top) and size distributions (bottom) after passaging with trypsin treatment in respective genotype are shown in bar graphs (means ± sd). Experiments were performed 3 times.
We further examined whether Stat3 was required for organoid formation by Wnt signaling–activated epithelial cells using a different mouse model. Adenoma cells were isolated from the intestinal polyps of Apc∆716 mice and Apc∆716 Stat3∆IEC compound mice, and cultured in Matrigel. In these tumor cells, Wnt/β-catenin signaling is constitutively activated because of an Apc mutation (22). When tumor-cell organoids were mechanically passaged by pipetting, the organoids developed at the same level in both genotypes, similar to normal intestine–derived organoids (Fig. 3C). Notably, after the enzymatic dissociation of organoids by trypsin, Apc∆716 Stat3∆IEC tumor cells formed organoids that were similar in total number and size distribution to simple Apc∆716 tumor–derived organoids. Taken together, these results indicate that Wnt signaling activation compensates for Stat3 disruption to support the survival and proliferation of the dissociated normal and tumor epithelial cells through protection from anoikis.
Stat3 is dispensable for Wnt activation–driven intestinal tumorigenesis
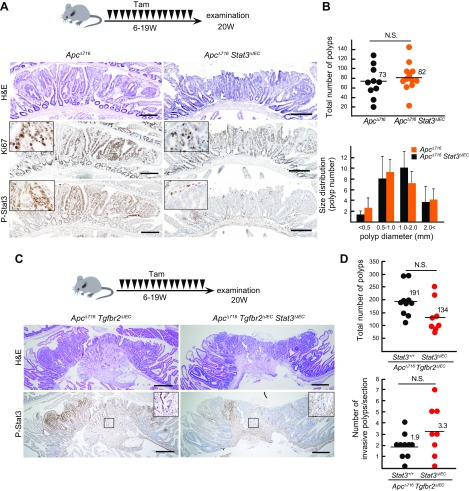
These results prompted us to examine the role of Stat3 in the Wnt activation–driven intestinal tumorigenesis by Apc mutations. We examined the phenotypes of Apc∆716 Stat3∆IEC mice and age-matched simple Apc∆716 mutant mice. P-Stat3 was detected in the nuclei of the tumor cells of Apc∆716 mouse polyps but not in Apc∆716 Stat3∆IEC mice, indicating that Stat3 is activated in the Wnt-driven sporadic tumor cells (Fig. 4A). However, there was no significant difference in the total number of tumors between Apc∆716 and Apc∆716 Stat3∆IEC mice (Fig. 4B). The size distributions of the tumors and the number of Ki-67–positive cells were also similar, and no significant difference was found in Apc∆716 Stat3∆IEC mice. These results indicate that Stat3 is dispensable for Wnt activation–driven intestinal tumorigenesis.
Figure 4 .
Dispensable role of Stat3 in Wnt-driven intestinal tumorigenesis. A) Schematic illustration of Tam treatment of ApcΔ716 mice (n = 10) and Apc∆716 villin-CreER Stat3flox/flox mice (n = 12) (top). Representative histology sections of small intestinal tumors of ApcΔ716 mice (left) and ApcΔ716 Stat3ΔIEC mice (right) for H&E (top) and immunohistochemical staining for Ki-67 (middle) and phosphorylated Stat3 (P-Stat3) (bottom). Insets show enlarged views. Scale bars, 200 μm. B) Total numbers of polyps in each mouse are shown with dots with mean values (top). Size distribution is shown in bar graph (means ± sd). C) Schematic illustration of Tam treatment of ApcΔ716 villin-CreER Tgfbr2flox/flox mice (n = 10) and ApcΔ716 villin-CreER Tgfbr2flox/flox Stat3flox/flox mice (n = 8) (top). Representative histology sections of small intestinal tumor of ApcΔ716 Tgfbr2ΔIEC mice (left) and ApcΔ716 Tgfbr2ΔIEC Stat3ΔIEC mice (right) for H&E (top) and immunohistochemical staining for P-Stat3 (bottom). Insets show enlarged views of boxed areas. Scale bars, 400 μm. D) Total numbers of polyps (top) and invasive polyps (bottom) per section are shown with mean numbers.
Submucosal invasion was found in ∼36% of intestinal polyps >1 mm in size in Apc∆716 Stat3∆IEC mice, while invasion was not found in simple Apc∆716 mice (Supplemental Fig. 3A, B), which is consistent with the previous findings in ApcMin mice (12, 13). We confirmed that the invasion frequency was significantly different between Apc∆716 mice and Apc∆716 Stat3∆IEC mice (Supplemental Fig. 3C). These results suggest a tumor suppressor role of Stat3; however, further investigations will be needed to confirm this point.
Next, we introduced the Stat3∆IEC allele into Apc∆716 Tgfbr2∆IEC compound mice, which develop invasive adenocarcinomas due to a combination of Wnt activation and TGF-β suppression (34). Notably, nuclear-accumulated P-Stat3 was detected in the tumor cells and stromal cells at the invasion front of Apc∆716 Tgfbr2∆IEC mouse tumors, whereas it was detected only in the stroma cells of Apc∆716 Tgfbr2∆IEC Stat3∆IEC tumors (Fig. 4C). However, the total number of intestinal tumors and submucosal invasion efficiency in Apc∆716 Tgfbr2∆IEC and Apc∆716 Tgfbr2∆IEC Stat3∆IEC mice did not differ to a statistically significant extent (Fig. 4D), indicating that Stat3 is not required for the submucosal invasion of intestinal tumor cells.
Stat3 is dispensable in Wnt activation–associated intestinal tumor metastasis
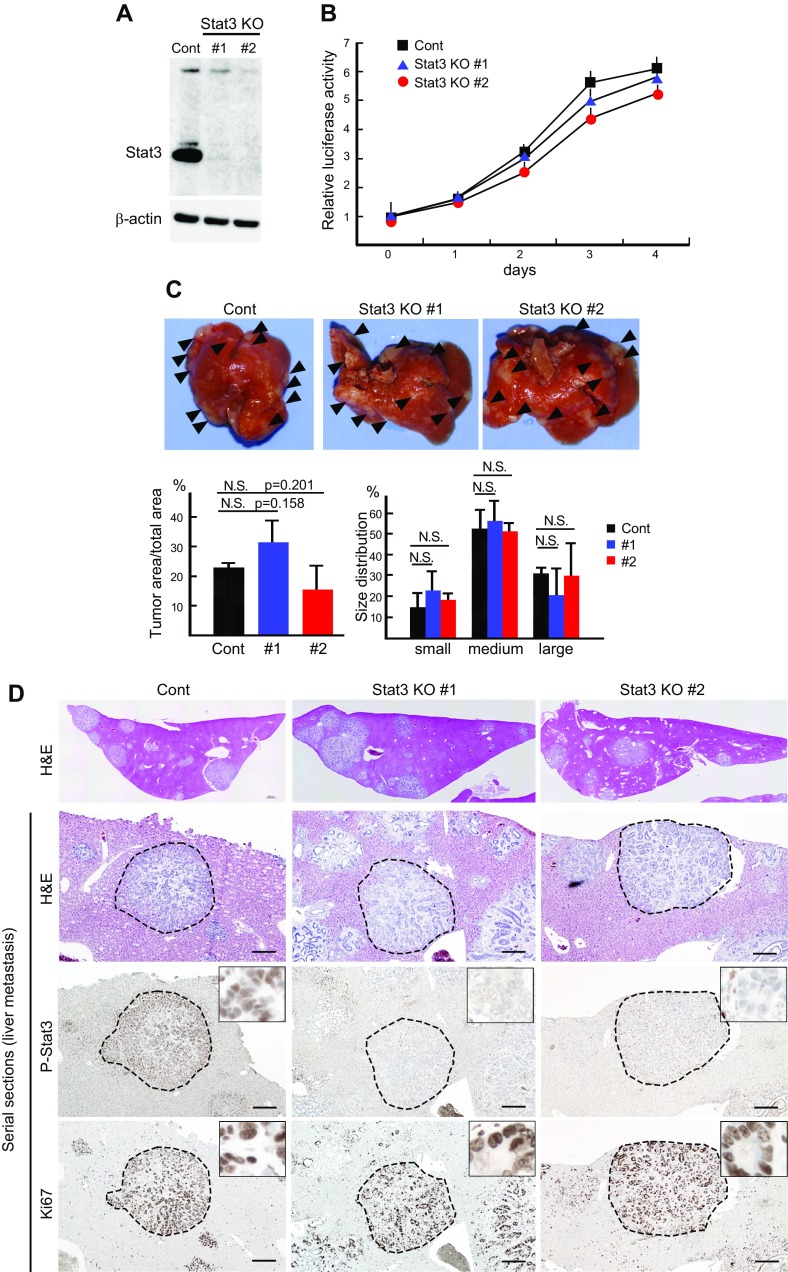
We recently established tumor-derived organoid cells (AKTP cells) from the intestinal tumors of Apc∆716 KrasG12D Tgfbr2−/− Trp53R270H quadruple compound mice (28). AKTP cells efficiently metastasized to the liver when transplanted into the mouse spleen. To examine whether Stat3 is involved in the metastatic process of Wnt-activated CRC cells, we disrupted the Stat3 gene in AKTP cells using the Crispr/Cas9 system (Fig. 5A). The disruption of Stat3 did not significantly change the cell proliferation rate of AKTP cells (Fig. 5B). Furthermore, multiple metastatic foci were developed in the livers of mice after the injection of 2 independent lines of Stat3-disrupted AKTP cells into the spleen [Stat3 knockout (KO) #1 and Stat3 KO#2], and the multiplicity did not differ from that of control AKTP cells to a statistically significant extent (P = 0.158 and 0.201 for control vs. KO#1 and KO#2, respectively) (Fig. 5C). Moreover, there was no significant difference in the size distributions of metastatic foci between control AKTP cells and Stat3 KO#1 or Stat3 KO#2 cells. Histologically, we confirmed that Stat3 is phosphorylated in metastasized AKTP tumor cells but not in Stat3 KO cells; however, the Ki-67 labeling efficiency of the Stat3-disrupted AKTP cells did not differ from that of control metastasized tumor cells (Fig. 5D). Furthermore, the expression level of total and active form β-catenin in Stat3-disrupted cells was similar to that of control AKTP cells, indicating that Wnt signaling activation is not affected by Stat3 gene disruption (Supplemental Fig. 4). These results indicate that Stat3 is not required for metastasis of Wnt activation–associated CRC cells.
Figure 5 .
Dispensable role of Stat3 in Wnt activation–associated cancer cell metastasis. A) Western blot results for Stat3 in control AKTP cells (Cont) and Stat3-targeted AKTP cell lines, Stat3 KO#1 and KO#2, by Crispr/Cas9. β-Actin was used for internal controls. B) Results of cell proliferation analysis of control and Stat3 KO#1 and KO#2 AKTP cells (means ± sd). C) Control AKTP cells (Cont) and Stat3-targeted AKTP cell lines (Stat3 KO#1 and KO#2) were transplanted to spleen of NOD/Shi-scid Il2rg−/− (NSG) mice (n = 3 for each cell line). Representative macroscopic photographs of mouse livers at 4 wk after spleen transplantation are shown (top). Arrowheads indicate metastatic foci. Mean metastasis tumor areas (bottom left) and size distribution of metastatic foci (bottom right) on histologic sections are shown in bar graphs (means ± sd). D) Representative low-power images of liver metastasis (H&E) (top) and high-power images of serial sections of metastatic foci with H&E and immunohistochemical staining for P-Stat3 and Ki-67 (from top to bottom). Dotted lines indicate metastatic foci for each genotype. Insets show enlarged views inside of metastatic foci. Scale bars, 200 μm.
Stat3-induced integrin expression is associated with FAK activation in crypts
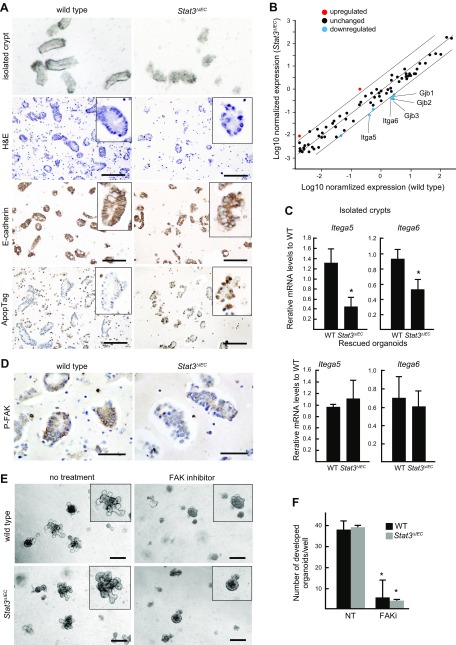
When intestinal crypts were isolated by EDTA treatment, we realized that Stat3∆IEC mouse–derived crypts showed a distinct morphology from the clear cylinder-like structure of WT crypts (Fig. 6A, top). We therefore examined the histology of isolated crypts that were collected from the intestine of WT and Stat3ΔIEC mice by EDTA treatment for 30 min. Notably, epithelial cell alignment was impaired, and apoptosis was induced in the isolated Stat3∆IEC crypts, while the alignment of columnar epithelial cells with the lateral expression of E-cadherin was confirmed in the WT crypts (Fig. 6A). These results indicate that Stat3 is important for the survival of cryptic cells after isolation from intestine following detachment from the extracellular matrix (ECM).
Figure 6 .
Stat3-dependent integrin expression and FAK activation. A) Representative photographs of isolated crypts under dissection microscope, histologic section of isolated crypts (H&E), and immunohistochemical staining for E-cadherin and ApopTag (from top to bottom) of WT (left) and Stat3ΔIEC isolated intestinal crypts (right). Insets show enlarged views. Scale bars, 100 μm. B) Results of filter array expression analysis are shown in dot graph, which displays up-regulated (>4 fold) and down-regulated (<0.25 fold) genes in Stat3ΔIEC crypts in different colors. C) Relative mRNA levels in isolated crypts (top) and rescued organoids (bottom) (means ± sd) were examined by RT-PCR for Itga5 and Itga6. *P < 0.05. D) Isolated crypts from WT (left) and Stat3∆IEC mice (right) subjected to immunohistochemical staining for P-FAK. Scale bars, 50 μm. E) Representative microscopic photographs of organoids derived from WT (top) and Stat3∆IEC mouse (bottom) crypts in absence (left) or presence (right) of FAK inhibitor in Matrigel. Insets show enlarged views. Note that crypt growth was suppressed by FAK inhibitor regardless of genotype. Scale bars, 250 μm. F) Numbers of developed organoids (budding number >5) in Matrigel with or without FAK inhibitor (FAKi and NT, respectively) (means ± sd). *P < 0.05 vs. NT.
We therefore examined the changes in the expression of cell adhesion molecules using a PCR array. Notably, the expression of Gjb1, Gjb2, and Gjb3, which encode the gap junction proteins connexin 32, 26, and 31, respectively, and Itga5 and Itga6, which encode integrins α5 and α6, respectively, was significantly down-regulated in the isolated Stat3∆IEC crypts (Fig. 6B). We confirmed that the expression of Itga5 and Itga6 was significantly decreased in the isolated Stat3∆IEC mouse intestinal crypts but not in the inhibitor-rescued Stat3∆IEC organoids by RT-PCR (Fig. 6C). Integrin α5 and α6 bind integrin β1 to form receptor complexes for fibronectin and laminin, respectively. It has been shown that the signaling of these ECM components through integrin complexes is important for the survival of epithelial cells through their activation of FAK, which protects cells from anoikis after detachment from the ECM (35). Importantly, phosphorylated FAK (P-FAK) was detected by immunostaining in epithelial cells of WT mouse–derived crypts; however, the staining intensity for P-FAK was significantly decreased in the Stat3∆IEC mouse–derived cryptic cells (Fig. 6D). To examine whether FAK signaling is indeed required for the organoid formation of the isolated crypts, we treated cultured organoids derived from WT crypts and inhibitor-rescued Stat3∆IEC organoids with FAK inhibitor. Of note, FAK inhibition significantly suppressed organoid development in both genotypes (Fig. 6E, F). Taken together, these results indicate that Stat3 prevents anoikis of the isolated crypts that are detached from the ECM by inducing the expression of integrin, which is associated with the activation of the FAK pathway.
DISCUSSION
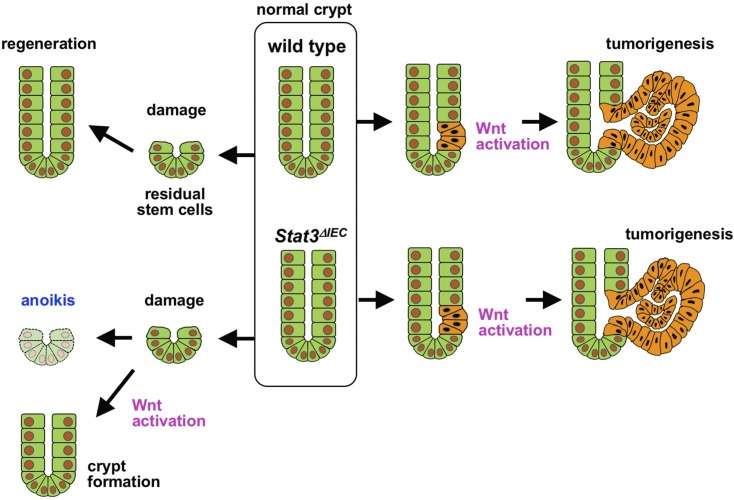
It has been shown that Stat3 is required for regeneration of the damaged intestinal mucosa (17, 23). We confirmed the role of Stat3 in intestinal regeneration as well as organoid formation in Matrigel, which mimics crypt formation in vitro. Importantly, however, we showed that Stat3 is not required for the growth of established organoids and that organoids can be maintained indefinitely by mechanical dissociation in the absence of Stat3. In contrast, Stat3-disrupted organoid cells were unable to survive when organoids were enzymatically dissociated by trypsin. These results indicate that although Stat3 is important in the protection of intestinal epithelial cells from acute injury and the subsequent induction of anoikis, it is not required for the maintenance and proliferation of steady-state cryptic cells (Fig. 7).
Figure 7 .
Schematic illustration of role of Stat3 in regeneration and tumorigenesis of intestinal crypts. WT (top) and Stat3ΔIEC crypts (bottom) are shown. Stat3 is required for regeneration from damaged mucosa, which may be compensated by Wnt activation (left). In contrast, Stat3 is dispensable for Wnt activation–driven tumorigenesis (right).
One possible mechanism underlying the impaired survival of Stat3ΔIEC epithelial cells after tissue damage or crypt isolation is the down-regulation of integrin α5 and α6, which complex with integrin β1 to form the receptors for fibronectin and laminin, respectively. It has been shown that integrin signaling from ECM activates FAK, which is important for the survival of epithelial cells through the further activation of the PI3K/Akt and Ras/MEK signaling pathways (35). It has also been shown that signaling through α5β1 integrin protects the intestinal epithelial cells from apoptosis (36). Thus, Stat3-dependent integrin signaling may contribute to the survival of epithelial cells through the activation of FAK signaling.
Importantly, we showed that Wnt signaling activation by an exogenous ligand compensated for Stat3 disruption to support organoid development. It has been shown that c-Myc–induced FAK activation is required for mucosal regeneration downstream of the Wnt signaling pathway (20). It has also been shown that treatment of ApcMin mice with FAK inhibitor significantly suppressed intestinal polyposis (37). Accordingly, it is possible that the Stat3 pathway and Wnt signaling cooperatively regulate the survival of the epithelial cells in the damaged mucosa and isolated crypts through the activation of integrin/FAK signaling. It was recently shown that subepithelial telocytes are a source of Wnt ligands for intestinal epithelial cells (38). It is therefore possible that such a stromal network lining the intestinal crypts in lamina propria is disrupted in the damaged intestinal mucosa, resulting in a decrease in Wnt signaling activity in regenerating epithelial cells, which may impair the stem-cell properties of the residual stem cells. Under such Wnt-suppressed circumstances, Stat3 is important for the survival of epithelial cells through the activation of FAK signaling. In contrast, when Wnt signaling is sufficiently activated, Stat3 is not required for the survival of epithelial cells (Fig. 7). This hypothesis may explain why Wnt activation–driven tumor cells do not require Stat3 activation.
It has been shown that Paneth cells generate a stem-cell niche through Wnt ligand secretion (39), and IL-6–induced Stat3 activation in Paneth cells increases organoid cell proliferation through Wnt activation (40). Accordingly, it is also possible that suppression of Paneth cell–dependent Wnt signaling causes impaired regeneration and crypt formation in Stat3ΔIEC mice.
With regard to the role of Stat3 in tumorigenesis, it has been shown that the blocking of IL-6 and IL-11 cytokine signaling or downstream transcription factor Stat3 causes the significant suppression of CAC development in mouse models, indicating that Stat3 is indispensable for inflammation-associated colonic tumorigenesis (8–10). However, we showed in the present study that Stat3 was not required for intestinal tumor development in ApcΔ716 mice and that submucosal invasion was not suppressed in ApcΔ716 Tgfbr2ΔIEC mice by Stat3 disruption. Furthermore, Stat3 is also dispensable for liver metastasis of ApcΔ716 KrasG12D Tgfbr2−/− Trp53R270H tumor cells. These genetic results indicate that Stat3 does not play an important role in Wnt activation–driven colonic tumorigenesis or the malignant progression of CRC (Fig. 7); thus, its role differs from that in CAC development. Accordingly, in CAC development, it is possible that Stat3 is required for the survival and proliferation of tumor cells initiated in the ulcerative colitis mucosa, similar to normal epithelial cells. In contrast, in sporadic CRC development, Wnt signaling is constitutively activated by the genetic alteration of APC or CTNNB1, which is sufficient to support the survival of tumor cells independently of the Stat3 pathway. In the present study, we showed that the epithelial Stat3 pathway is not required for Wnt-driven tumorigenesis; however, Stat3 in stromal cells is also activated in the inflammatory microenvironment of intestinal tumors. Thus, it is still possible that stromal Stat3 plays a role in sporadic tumorigenesis.
We also found that Stat3 disruption induced the submucosal invasion of intestinal tumors of ApcΔ716 mice, which is consistent with the findings of previous reports using ApcMin Stat3−/− compound mice (12, 13). It has also been shown that Stat3 suppresses KRAS-induced lung tumorigenesis and p19ARF expression–associated prostate cancer metastasis (41, 42). Thus, Stat3 may play a tumor suppressor role in the malignant progression of CRC. However, a further analysis to compare the results of mouse experiments and human CRC findings is needed to draw hard conclusions in this regard.
In summary, Stat3 is required for the survival and proliferation of the remaining epithelial cells in the damaged mucosa; thus, Stat3 is indispensable for mucosal regeneration and CAC development. In contrast, Stat3 is not required for Wnt activation–driven tumor development or the malignant progression of CRC (Fig. 7). Mechanistically, Wnt signaling and Stat3 cooperate to support the survival of cryptic epithelial cells through the activation of the integrin/FAK signaling pathway, which protects epithelial cells from anoikis. When Wnt signaling is sufficiently activated, Stat3 is no longer required for the survival or proliferation of normal and tumor epithelial cells. Thus, the degree of Wnt activation in cancer cells has an important impact on the efficacy of Stat3 inhibitor treatment in CRC patients.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank M. Watanabe, A. Tsuda, and Y. Jomen (Division of Genetics, Cancer Research Institute, Kanazawa University) for their technical assistance. This study was supported by the Japan Agency for Medical Research and Development–Core Research for Evolutional Science and Technology (AMED–CREST) (JP17gm0410014) and AMED (JP17ck0106259); and Grants-in-Aid for Scientific Research (A) (JP18H04030) and (C) (JP16K07111) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; Takeda Science Foundation; and Mitsubishi Foundation. The authors declare no conflicts of interest.
Glossary
- AFM-Wnt3a
afamin-associated Wnt3a
- Apc
adenomatous polyposis coli
- CAC
colitis-associated colon cancer
- CRC
colorectal cancer
- ECM
extracellular matrix
- EdU
5-ethynyl-2′-deoxyuridine
- FAK
focal adhesion kinase
- GSK
glycogen synthase kinase
- H&E
hematoxylin and eosin
- KO
knockout
- KRAS
Kirsten rat sarcoma viral oncogene
- P-FAK
phosphorylated focal adhesion kinase
- P-Stat3
phosphorylated signal transducer and activator of transcription 3
- sgRNA
single guide RNA
- Stat3
signal transducer and activator of transcription 3
- Tam
tamoxifen
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
H. Oshima, D. C.-C. Voon, and M. Oshima designed the study; H. Oshima and S.-Y. Kok performed the study; M. Nakayama and K. Murakami provided resources and analytic tools; T. Kimura performed pathological analysis; M. Oshima wrote the report; and all authors discussed the results and edited the final manuscript.
REFERENCES
- 1.Grivennikov, S. I., Greten, F. R., Karin, M. (2010) Immunity, inflammation, and cancer. Cell 140, 883–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He, G., Karin, M. (2011) NF-κB and STAT3—key players in liver inflammation and cancer. Cell Res. 21, 159–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank, D. A. (2007) STAT3 as a central mediator of neoplastic cellular transformation. Cancer Lett. 251, 199–210 [DOI] [PubMed] [Google Scholar]
- 4.Roeser, J. C., Leach, S. D., McAllister, F. (2015) Emerging strategies for cancer immunoprevention. Oncogene 34, 6029–6039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kusaba, T., Nakayama, T., Yamazumi, K., Yakata, Y., Yoshizaki, A., Nagayasu, T., Sekine, I. (2005) Expression of p-STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. J. Clin. Pathol. 58, 833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corvinus, F. M., Orth, C., Moriggl, R., Tsareva, S. A., Wagner, S., Pfitzner, E. B., Baus, D., Kaufmann, R., Huber, L. A., Zatloukal, K., Beug, H., Ohlschläger, P., Schütz, A., Halbhuber, K.-J., Friedrich, K. (2005) Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia 7, 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin, L., Liu, A., Peng, Z., Lin, H.-J., Li, P.-K., Li, C., Lin, J. (2011) STAT3 is necessary for proliferation and survival in colon cancer–initiating cells. Cancer Res. 71, 7226–7237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bollrath, J., Phesse, T. J., von Burstin, V. A., Putoczki, T., Bennecke, M., Bateman, T., Nebelsiek, T., Lundgren-May, T., Canli, O., Schwitalla, S., Matthews, V., Schmid, R. M., Kirchner, T., Arkan, M. C., Ernst, M., Greten, F. R. (2009) gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 15, 91–102 [DOI] [PubMed] [Google Scholar]
- 9.Grivennikov, S., Karin, E., Terzic, J., Mucida, D., Yu, G.-Y., Vallabhapurapu, S., Scheller, J., Rose-John, S., Cheroutre, H., Eckmann, L., Karin, M. (2009) IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 15, 103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Putoczki, T. L., Thiem, S., Loving, A., Busuttil, R. A., Wilson, N. J., Ziegler, P. K., Nguyen, P. M., Preaudet, A., Farid, R., Edwards, K. M., Boglev, Y., Luwor, R. B., Jarnicki, A., Horst, D., Boussioutas, A., Heath, J. K., Sieber, O. M., Pleines, I., Kile, B. T., Nash, A., Greten, F. R., McKenzie, B. S., Ernst, M. (2013) Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 24, 257–271 [DOI] [PubMed] [Google Scholar]
- 11.Johnson, D. E., O’Keefe, R. A., Grandis, J. R. (2018) Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 15, 234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musteanu, M., Blaas, L., Mair, M., Schlederer, M., Bilban, M., Tauber, S., Esterbauer, H., Mueller, M., Casanova, E., Kenner, L., Poli, V., Eferl, R. (2010) Stat3 is a negative regulator of intestinal tumor progression in ApcMin mice. Gastroenterology 138, 1003–1011.e1–5 [DOI] [PubMed] [Google Scholar]
- 13.Lee, J., Kim, J. C. K., Lee, S. E., Quinley, C., Kim, H., Herdman, S., Corr, M., Raz, E. (2012) Signal transducer and activator of transcription 3 (STAT3) protein suppresses adenoma-to-carcinoma transition in Apcmin/+ mice via regulation of Snail-1 (SNAI) protein stability. J. Biol. Chem. 287, 18182–18189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas Network . (2012) Comprehensive molecular characterization of human colon and rectal cancer. Nature 487, 330–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews, J. R., Sansom, O. J., Clarke, A. R. (2011) Absolute requirement for STAT3 function in small-intestine crypt stem cell survival. Cell Death Differ. 18, 1934–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Willson, T. A., Jurickova, I., Collins, M., Denson, L. A. (2013) Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice. Inflamm. Bowel Dis. 19, 512–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phesse, T. J., Buchert, M., Stuart, E., Flanagan, D. J., Faux, M., Afshar-Sterle, S., Walker, F., Zhang, H. H., Nowell, C. J., Jorissen, R., Tan, C. W., Hirokawa, Y., Eissmann, M. F., Poh, A. R., Malaterre, J., Pearson, H. B., Kirsch, D. G., Provero, P., Poli, V., Ramsay, R. G., Sieber, O., Burgess, A. W., Huszar, D., Vincan, E., Ernst, M. (2014) Partial inhibition of gp130-Jak-Stat3 signaling prevents Wnt–β-catenin–mediated intestinal tumor growth and regeneration. Sci. Signal. 7, ra92 [DOI] [PubMed] [Google Scholar]
- 18.Krausova, M., Korinek, V. (2014) Wnt signaling in adult intestinal stem cells and cancer. Cell. Signal. 26, 570–579 [DOI] [PubMed] [Google Scholar]
- 19.Nusse, R., Clevers, H. (2017) Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169, 985–999 [DOI] [PubMed] [Google Scholar]
- 20.Ashton, G. H., Morton, J. P., Myant, K., Phesse, T. J., Ridgway, R. A., Marsh, V., Wilkins, J. A., Athineos, D., Muncan, V., Kemp, R., Neufeld, K., Clevers, H., Brunton, V., Winton, D. J., Wang, X., Sears, R. C., Clarke, A. R., Frame, M. C., Sansom, O. J. (2010) Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling. Dev. Cell 19, 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oudhoff, M. J., Braam, M. J. S., Freeman, S. A., Wong, D., Rattray, D. G., Wang, J., Antignano, F., Snyder, K., Refaeli, I., Hughes, M. R., McNagny, K. M., Gold, M. R., Arrowsmith, C. H., Sato, T., Rossi, F. M. V., Tatlock, J. H., Owen, D. R., Brown, P. J., Zaph, C. (2016) SETD7 controls intestinal regeneration and tumorigenesis by regulating Wnt/β-catenin and Hippo/YAP signaling. Dev. Cell 37, 47–57 [DOI] [PubMed] [Google Scholar]
- 22.Oshima, M., Oshima, H., Kitagawa, K., Kobayashi, M., Itakura, C., Taketo, M. (1995) Loss of Apc heterozygosity and abnormal tissue building in nascent intestinal polyps in mice carrying a truncated Apc gene. Proc. Natl. Acad. Sci. USA 92, 4482–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda, K., Kaisho, T., Yoshida, N., Takeda, J., Kishimoto, T., Akira, S. (1998) Stat3 activation is responsible for IL-6–dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell–specific Stat3-deficient mice. J. Immunol. 161, 4652–4660 [PubMed] [Google Scholar]
- 24.El Marjou, F., Janssen, K. P., Chang, B. H., Li, M., Hindie, V., Chan, L., Louvard, D., Chambon, P., Metzger, D., Robine, S. (2004) Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 [DOI] [PubMed] [Google Scholar]
- 25.Chytil, A., Magnuson, M. A., Wright, C. V., Moses, H. L. (2002) Conditional inactivation of the TGF-β type II receptor using Cre:Lox. Genesis 32, 73–75 [DOI] [PubMed] [Google Scholar]
- 26.Sato, T., Vries, R. G., Snippert, H. J., van de Wetering, M., Barker, N., Stange, D. E., van Es, J. H., Abo, A., Kujala, P., Peters, P. J., Clevers, H. (2009) Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 [DOI] [PubMed] [Google Scholar]
- 27.Mihara, E., Hirai, H., Yamamoto, H., Tamura-Kawakami, K., Matano, M., Kikuchi, A., Sato, T., Takagi, J. (2016) Active and water-soluble form of lipidated Wnt protein is maintained by a serum glycoprotein afamin/α-albumin. eLife 5, e11621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakai, E., Nakayama, M., Oshima, H., Kouyama, Y., Niida, A., Fujii, S., Ochiai, A., Nakayama, K. I., Mimori, K., Suzuki, Y., Hong, C. P., Ock, C. Y., Kim, S. J., Oshima, M. (2018) Combined mutation of Apc, Kras, and Tgfbr2 effectively drives metastasis of intestinal cancer. Cancer Res. 78, 1334–1346 [DOI] [PubMed] [Google Scholar]
- 29.Pickert, G., Neufert, C., Leppkes, M., Zheng, Y., Wittkopf, N., Warntjen, M., Lehr, H. A., Hirth, S., Weigmann, B., Wirtz, S., Ouyang, W., Neurath, M. F., Becker, C. (2009) STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J. Exp. Med. 206, 1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindemans, C. A., Calafiore, M., Mertelsmann, A. M., O’Connor, M. H., Dudakov, J. A., Jenq, R. R., Velardi, E., Young, L. F., Smith, O. M., Lawrence, G., Ivanov, J. A., Fu, Y. Y., Takashima, S., Hua, G., Martin, M. L., O’Rourke, K. P., Lo, Y. H., Mokry, M., Romera-Hernandez, M., Cupedo, T., Dow, L., Nieuwenhuis, E. E., Shroyer, N. F., Liu, C., Kolesnick, R., van den Brink, M. R. M., Hanash, A. M. (2015) Interleukin-22 promotes intestinal-stem-cell–mediated epithelial regeneration. Nature 528, 560–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aparicio-Domingo, P., Romera-Hernandez, M., Karrich, J. J., Cornelissen, F., Papazian, N., Lindenbergh-Kortleve, D. J., Butler, J. A., Boon, L., Coles, M. C., Samsom, J. N., Cupedo, T. (2015) Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. J. Exp. Med. 212, 1783–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujii, M., Matano, M., Nanki, K., Sato, T. (2015) Efficient genetic engineering of human intestinal organoids using electroporation. Nat. Protoc. 10, 1474–1485 [DOI] [PubMed] [Google Scholar]
- 33.Wang, F., Scoville, D., He, X. C., Mahe, M. M., Box, A., Perry, J. M., Smith, N. R., Lei, N. Y., Davies, P. S., Fuller, M. K., Haug, J. S., McClain, M., Gracz, A. D., Ding, S., Stelzner, M., Dunn, J. C., Magness, S. T., Wong, M. H., Martin, M. G., Helmrath, M., Li, L. (2013) Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology 145, 383–395.e1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oshima, H., Nakayama, M., Han, T. S., Naoi, K., Ju, X., Maeda, Y., Robine, S., Tsuchiya, K., Sato, T., Sato, H., Taketo, M. M., Oshima, M. (2015) Suppressing TGFβ signaling in regenerating epithelia in an inflammatory microenvironment is sufficient to cause invasive intestinal cancer. Cancer Res. 75, 766–776 [DOI] [PubMed] [Google Scholar]
- 35.Guo, W., Giancotti, F. G. (2004) Integrin signalling during tumour progression. Nat. Rev. Mol. Cell Biol. 5, 816–826 [DOI] [PubMed] [Google Scholar]
- 36.Lee, J. W., Juliano, R. L. (2000) α5β1 integrin protects intestinal epithelial cells from apoptosis through a phosphatidylinositol 3-kinase and protein kinase B–dependent pathway. Mol. Biol. Cell 11, 1973–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao, C., Chen, G., Kuan, S. F., Zhang, D. H., Schlaepfer, D. D., Hu, J. (2015) FAK/PYK2 promotes the Wnt/β-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3β. eLife 4, e10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shoshkes-Carmel, M., Wang, Y. J., Wangensteen, K. J., Tóth, B., Kondo, A., Massasa, E. E., Itzkovitz, S., Kaestner, K. H. (2018) Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature 557, 242–246; erratum: Nature 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato, T., van Es, J. H., Snippert, H. J., Stange, D. E., Vries, R. G., van den Born, M., Barker, N., Shroyer, N. F., van de Wetering, M., Clevers, H. (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeffery, V., Goldson, A. J., Dainty, J. R., Chieppa, M., Sobolewski, A. (2017) IL-6 signaling regulates small intestinal crypt homeostasis. J. Immunol. 199, 304–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grabner, B., Schramek, D., Mueller, K. M., Moll, H. P., Svinka, J., Hoffmann, T., Bauer, E., Blaas, L., Hruschka, N., Zboray, K., Stiedl, P., Nivarthi, H., Bogner, E., Gruber, W., Mohr, T., Zwick, R. H., Kenner, L., Poli, V., Aberger, F., Stoiber, D., Egger, G., Esterbauer, H., Zuber, J., Moriggl, R., Eferl, R., Győrffy, B., Penninger, J. M., Popper, H., Casanova, E. (2015) Disruption of STAT3 signalling promotes KRAS-induced lung tumorigenesis. Nat. Commun. 6, 6285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pencik, J., Schlederer, M., Gruber, W., Unger, C., Walker, S. M., Chalaris, A., Marié, I. J., Hassler, M. R., Javaheri, T., Aksoy, O., Blayney, J. K., Prutsch, N., Skucha, A., Herac, M., Krämer, O. H., Mazal, P., Grebien, F., Egger, G., Poli, V., Mikulits, W., Eferl, R., Esterbauer, H., Kennedy, R., Fend, F., Scharpf, M., Braun, M., Perner, S., Levy, D. E., Malcolm, T., Turner, S. D., Haitel, A., Susani, M., Moazzami, A., Rose-John, S., Aberger, F., Merkel, O., Moriggl, R., Culig, Z., Dolznig, H., Kenner, L. (2015) STAT3 regulated ARF expression suppresses prostate cancer metastasis. Nat. Commun. 6, 7736; erratum Nat. Commun. 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.