Abstract
Infants of obese mothers have an increased risk of developing obesity, insulin resistance, and type 2 diabetes. The underlying mechanisms remain elusive, and no effective interventions to limit the transmission of metabolic disease from the obese mother to her infant are currently available. Obese pregnant women have decreased circulating levels of adiponectin, which is associated with increased placental nutrient transport and fetal overgrowth. We have reported that normalization of adiponectin levels during late gestation reversed placental dysfunction and fetal overgrowth in a mouse model of maternal obesity in pregnancy. In the current study, we hypothesized that adiponectin supplementation during pregnancy in obese mice attenuates the adverse metabolic outcomes in adult offspring. Adult male offspring of obese mice developed obesity, fatty liver, and insulin resistance, with adult female offspring of obese mice having a less pronounced metabolic phenotype. These metabolic abnormalities in offspring born to obese mice were largely prevented by normalization of maternal adiponectin levels in late pregnancy. We provide evidence that low circulating maternal adiponectin is a critical mechanistic link between maternal obesity and the development of metabolic disease in offspring. Strategies aimed at improving maternal adiponectin levels may prevent long-term metabolic dysfunction in offspring of obese mothers.—Paulsen, M. E., Rosario, F. J., Wesolowski, S. R., Powell, T. L., Jansson, T. Normalizing adiponectin levels in obese pregnant mice prevents adverse metabolic outcomes in offspring.
Keywords: fetal programming, insulin resistance, fatty liver
Maternal overnutrition and obesity in pregnancy affects the intrauterine environment during critical periods of fetal growth and development, resulting in an increased risk for the infant to develop disease later in life (1). This paradigm, the developmental origins of health and disease hypothesis, has broad public health consequences because almost two thirds of American women now enter pregnancy either overweight or obese (2). A series of recent reports that link pregnancy complicated by obesity, large-for-gestational-age birth weight, and later development of metabolic syndrome and cardiovascular disease are particularly alarming (3–5). Specifically, infants of obese mothers are susceptible to develop obesity, high triglycerides, low HDL cholesterol, high blood pressure, and elevated fasting glucose in childhood, followed by type 2 diabetes and cardiovascular disease in adulthood (3, 6). Fetal exposure to maternal obesity potentially creates a vicious cycle, transmitting metabolic disease across generations and thus exacerbating the epidemic of obesity (7).
There are currently no specific or effective intervention strategies available to alleviate the short- and long-term adverse outcomes after exposure to the abnormal metabolic environment associated with a pregnancy complicated by maternal obesity. A recent metaanalysis shows that dietary and lifestyle interventions in overweight and obese women have only marginal beneficial effects on birth weight and other key obstetrical outcomes (8). A large maternal prenatal weight loss, on average 37 kg, after bariatric surgery did decrease the incidence of large-for-gestational-age infants; however, this procedure also increased the risk of having an infant born small for gestational age (9). Furthermore, lifestyle interventions before pregnancy have low compliance and miss a large portion of at-risk women because of unintended pregnancy rates, which are highest in populations where obesity is most prevalent (10, 11).
The mechanisms linking the adverse metabolic environment in maternal obesity to programming of the fetus for later disease remain poorly understood, constituting a major roadblock for the development of specific intervention strategies. One key mechanism by which maternal nutrition and metabolism influence fetal development and growth is through modulating the function of the placenta, which serves as a nutrient sensor regulating fetal growth and programming the fetus for the development of chronic disease in childhood and later in life (12–14). In mothers who are overweight or obese, there is an abundance of nutrients available, which likely results in an increased flux of nutrients across the placenta (13). In addition, pregnant women with high body mass index have altered circulating levels of metabolic hormones, which in some women cause up-regulation of placental transporters for glucose, fatty acids, and amino acids, thereby promoting fetal adiposity, insulin resistance, and/or fetal overgrowth (15–17).
Serum levels of adiponectin (ADN), an adipokine secreted by adipose tissue, are inversely correlated to metabolic health with low levels of ADN associated with insulin resistance, obesity, metabolic syndrome, type 2 diabetes, cardiovascular disease, and premature death (18–20). Emerging evidence suggests that ADN constitutes an endocrine link between maternal adipose stores, placental function, and fetal growth (20–22). Circulating levels of ADN are decreased in pregnant women with obesity and/or gestational diabetes mellitus (21–25). Numerous studies demonstrate that low maternal ADN in these women is correlated to increased fetal growth (26–29). Moreover, low ADN levels early in pregnancy strongly predict the development of gestational diabetes mellitus independent of maternal adiposity (26–28, 30, 31).
We have reported that ADN, in contrast to its well-known insulin-sensitizing effects in skeletal muscle and liver, inhibits insulin, mammalian target of rapamycin (mTOR) signaling, and amino acid transport in cultured primary human trophoblast cells (32, 33) and in pregnant mice in vivo (22, 34). Indeed, chronic infusion of full-length ADN to normal-weight pregnant mice decreased fetal weight, demonstrating that maternal ADN limits fetal growth (34). This conclusion was recently confirmed in elegant studies in which knockdown of the maternal ADN gene resulted in increased fetal growth (35, 36). We have further demonstrated that ADN regulates placental function mediated by activation of trophoblast peroxisome proliferator-activated receptor α (PPAR-α) signaling, which increases ceramide synthesis resulting in inhibition of insulin receptor substrate 1 (IRS-1) (33). We have reported that normalization of maternal ADN levels in obese pregnant mice significantly increases maternal insulin sensitivity and prevents fetal overgrowth by normalizing placental function (22).
Collectively, these findings suggest that low maternal ADN is mechanistically linked to the adverse placental and fetal outcomes in maternal obesity. In this study, we tested the hypothesis that ADN supplementation during pregnancy attenuates the adverse metabolic outcomes in adult offspring of obese mice. Mouse recombinant full-length ADN was infused using miniosmotic pumps the last 4 d of pregnancy in rates that increased circulating ADN in obese mice to the levels observed in lean mice (22).
MATERIALS AND METHODS
Animals
All protocols were approved by the Institutional Animal Care and Use Committee at the University of Colorado Anschutz Medical Center. Twelve-week-old C57BL/6J female mice (The Jackson Laboratory, Bar Harbor, ME, USA) were fed either a control diet (D12489; Research Diets, New Brunswick, NJ, USA) or an obesogenic diet (Western Diet D12089B; Research Diets) consisting of pellets containing 10 and 40% calories from fat, respectively. Female mice fed the obesogenic diet had ad libitum access to 20% sucrose solution supplemented with micronutrients, vitamins (Vitamin Mix V10001; Research Diets), and minerals (Mineral Mix S10001; Research Diets) (37). Females were mated once females in the obesogenic diet group had gained 25% of their initial body weight. All males were fed the control diet. The presence of a postcopulatory plug was defined as embryonic day (E)0.5. Pregnant mice delivered spontaneously, and litters were culled to equal size (n = 6–8). Pregnant mice were maintained on their respective diets throughout gestation and lactation. Offspring, once weaned, were fed a control diet. For a particular outcome, each studied offspring originated from separate litters.
ADN infusion
At E14.5, pregnant mice were anesthetized with isoflurane and a miniosmotic pump (Alzet 1003D; Alza, Mountain View, CA, USA) was implanted subcutaneously (22, 34). Animals received a continuous infusion of either sterile PBS or mouse recombinant full-length ADN (0.62 μg/g of body weight per day; ADN, ALX-522-059; Enzo Life Sciences, Farmingdale, NY, USA) from E14.5 until delivery. Metabolic profiles of mice at gestational d 18.5 have been reported previously (22, 34). In obese pregnant mice, this infusion rate increases circulating levels of high-molecular-weight ADN to those observed in normal-weight pregnant mice (22), whereas it results in circulating ADN levels that are ∼3-fold higher than normal when infused into control pregnant mice (34). As a result of this design, 4 groups of offspring were studied: C/PBS, offspring of pregnant mice fed control diet infused with vehicle, resulting in normal maternal ADN; C/ADN, offspring of pregnant mice fed control diet infused with ADN, resulting in high maternal ADN; OB/PBS, offspring of obese pregnant mice infused with vehicle, resulting in low maternal ADN; and OB/ADN, offspring of obese pregnant mice with infusion of ADN, resulting in normal maternal ADN. Offspring were not supplemented with ADN.
Body composition
Live mice were placed into a thin-walled plastic cylinder (4.7 cm ID, 0.15 cm thick), where they were limited to 4-cm vertical movements, and then placed into the quantitative magnetic resonance imaging machine (EchoMRI; Echo Medical Systems, Houston, TX, USA) for measurement of lean and fat mass.
Intraperitoneal glucose/insulin tolerance tests
Intraperitoneal injection of glucose (2 g/kg) or insulin (0.5 U/kg) was administered in awake animals after 4 h of being denied access to food. Blood was collected by tail vein sampling, and blood glucose (mM) was measured in triplicate with a OneTouch (LifeScan, Milpitas, CA, USA) glucometer.
Tissue and blood collection and serum assays
The 4 groups of animals were humanely killed at 14 wk of age after 4 h of being denied access to food. Blood was collected by cardiac puncture. Tissues and serum were stored at −80°C until analysis. Serum insulin was determined by ELISA (Alpco, Salem, NH, USA). Serum triglyceride was determined by colorimetric assay by the Mouse Metabolic Phenotyping Center at the University of Cincinnati (Cincinnati, OH, USA).
Protein expression
Frozen tissues were transferred into 0.5 ml of buffer D (mM: 250 sucrose, 1 Tris-HEPES, 1 EDTA, pH 7.4) with standard protease and phosphatase inhibitor cocktail (MilliporeSigma, Burlington, MA, USA) and then homogenized using a Polytron homogenizer (Kinematica, Bohemia, NY, USA). Protein concentrations were determined using the bicinchoninic acid assay as per the manufacturer’s instructions using bovine serum albumin as the standard (Thermo Fisher Scientific, Waltham, MA, USA). Skeletal muscle and liver homogenates (25–50 µg) were resolved on Mini-Protean Tris Glycine any kDa polyacrylamide gels (Bio-Rad, Hercules, CA, USA). Proteins from the gel were transferred to a PVDF membrane (Thermo Fisher Scientific) by electroblotting. Antibodies, purchased from Cell Signaling Technology (Danvers, MA, USA) and diluted (1:1000) in 1% bovine serum albumin unless otherwise noted, included: Akt (1:500), phospho-Akt (Thr308; Ser473), GSK3α, phospho–glycogen synthase kinase alpha (GSKα) (Ser21, 1:2000), Akt substrate of 160 kDa (AS160), phospho-AS160 (Thr642), forkhead box protein O1 (FOX-O1), phospho-FOX-O1 (Thr21), perilipin-2 (PLIN-2), insulin receptor β (IR-β; C-19, 1:200; Santa Cruz Biotechnology, Dallas, TX, USA). Membranes were incubated in ECL Western blotting detection reagents (Pierce, Rockford, IL, USA) and exposed on G:Box ChemiXL1.4 (Syngene, Cambridge, United Kingdom). Densitometric analyses were performed by ImageJ software (Image Processing and Analysis in Java; National Institutes of Health, Bethesda, MD, USA; https://imagej.nih.gov/ij/). Membranes were also stained for total protein using Amido Black Stain (MilliporeSigma) to account for variation in loading or transfer as previously described (38). For each target protein, the mean density of the control sample bands was assigned the arbitrary value of 1.
Tissue glycogen and triglyceride assay
One hundred milligrams of hepatic tissue was pulverized and digested in 2 ml of 30% KOH at 95°C for 30 min. The homogenate was placed on No. 1 Whatman filter paper and washed in 66% ethanol with constant stirring for 30 min. The filter paper was removed, dried, and cut into small pieces. Glycogen was converted to glucose with 31.1 U amyloglucosidase (MilliporeSigma) in 0.2 M acetate buffer (pH 4.8, 0.5% glacial acetic acid, 0.12 M sodium acetate) at 37°C for 60 min. Liver triglyceride content was measured using Infinity Triglyceride Reagent following lipid extraction by the Folch method. Results are expressed as milligrams glycogen or triglyceride per gram of hepatic tissue (wet weight).
Gene expression
Total RNA was isolated using Trizol extraction from 20 mg liver tissue homogenized in buffer RLT using a Bead Beater (1.0 mm zirconium oxide beads; 8 min). cDNA was synthesized using a cDNA kit (Thermo Fisher Scientific). Gene expression was assessed using predesigned exon spanning primers utilizing the TaqMan gene expressions system (Thermo Fisher Scientific) on a Real Time PCR System (Thermo Fisher Scientific). Data were normalized to 18S rRNA using the cycle threshold (ΔΔCt) method.
Oil Red O staining
Oil Red O Kit (Abcam, Cambridge, United Kingdom) was used to stain for neutral lipids. Frozen liver parenchymal sections (3/individual liver) at room temperature were placed in 70% ethanol for 5 s, stained with Oil Red O Solution for 25 min, counterstained with Harris hematoxylin for 5 s, and placed in 1% HCl–70% ethanol for 5 s. After rinsing, sections were mounted with glycerin jelly. Three predetermined areas of each frozen liver section image were captured using a light microscope. Lipid droplets from liver sections of offspring were quantified by ImageJ software, and droplet numbers were averaged between the 3 areas.
Data presentation and statistical analyses
C/PBS animals were used as the control. Data are presented as means ± sem, and n represents the number of offspring per group. Statistical analysis and graphics were performed by GraphPad Prism 7 (GraphPad Software, La Jolla, CA, USA). One-way ANOVA followed by Tukey’s multiple comparison post hoc tests was used to analyze results. The Pearson correlation coefficient was used as a measure of linear correlation. A value of P < 0.05 was considered statistically significant.
RESULTS
Normalization of ADN levels in obese pregnant mice prevents obesity in offspring
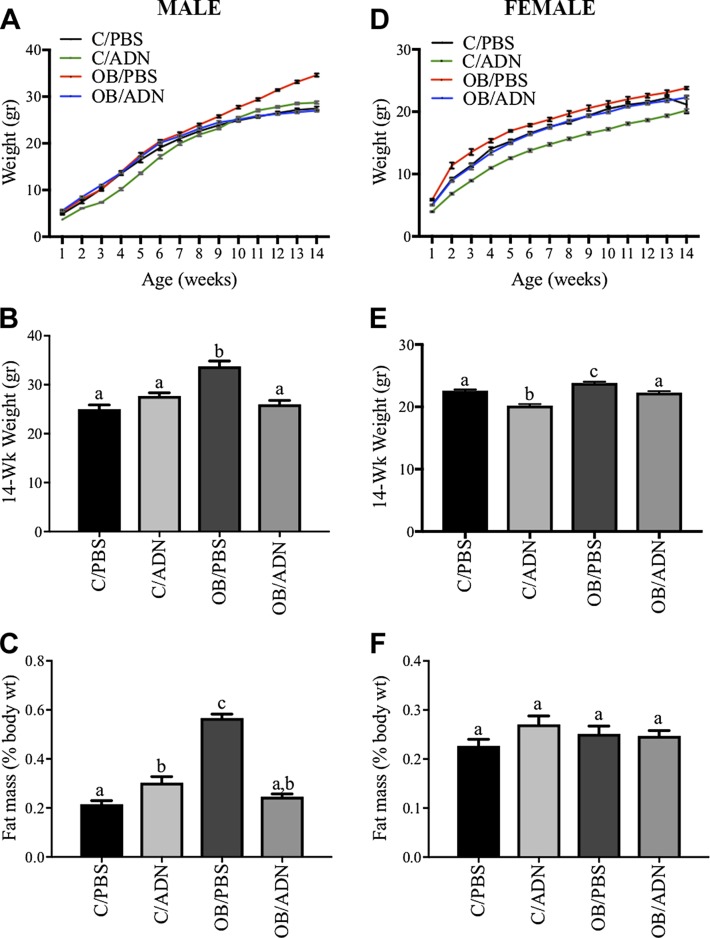
We determined the growth of offspring during the first 14 wk of life (Fig. 1). C/ADN males were 25% lighter (P = 0.03) at 1 wk but no different in weight at 14 wk (Fig. 1A, B) compared to the offspring of control mice. In females, C/ADN mice were 22 and 11% lighter at 1 and 14 wk of life, respectively (P < 0.0001, Fig. 1D, E). Male OB/PBS mice were 40% heavier than offspring of control mice (P = 0.0001) at 14 wk (Fig. 1A, B). Female OB/PBS mice were 14% heavier at 1 wk (P = 0.0002) and 5% heavier at 14 wk (P = 0.006) (Fig. 1D, E). The postnatal weights in male or female OB/ADN mice were not different compared to offspring of control mice at any time point (Fig. 1A, B, D, E).
Figure 1 .
Normalization of ADN levels in obese pregnant mice prevents obesity in offspring. Offspring weights were measured weekly from 1 to 14 wk of life (A, B, D, E). Percentage fat mass of total body weight was measured by quantitative magnetic resonance imaging at 14 wk of life (C, F). Males (A–C) and females (D–F) were studied separately. Means ± sem; n = 8–16 per group. Different letters (a–c) denote statistically significant differences. P < 0.05.
We measured body composition to determine the degree of adiposity in offspring at 3 mo of age. In males, C/ADN mice had 1.4-fold greater fat mass (P < 0.0001, Fig. 1C). In females, fat mass in C/ADN mice was not significantly different from controls (P = 0.22, Fig. 1F). Male OB/PBS mice had the expected 2.6-fold greater fat mass (P < 0.0001, Fig. 1C), whereas the fat mass in female OB/PBS mice was not significantly different from control offspring (P < 0.22, Fig. 1F). Importantly, fat mass in OB/ADN mice did not differ from controls in either sex at 3 mo of age (Fig. 1C, F). These data demonstrate that normalization of maternal ADN during pregnancy (22) prevents developmental programming of obesity in offspring of obese mice.
Normalization of ADN levels in obese pregnant mice prevents insulin resistance in offspring
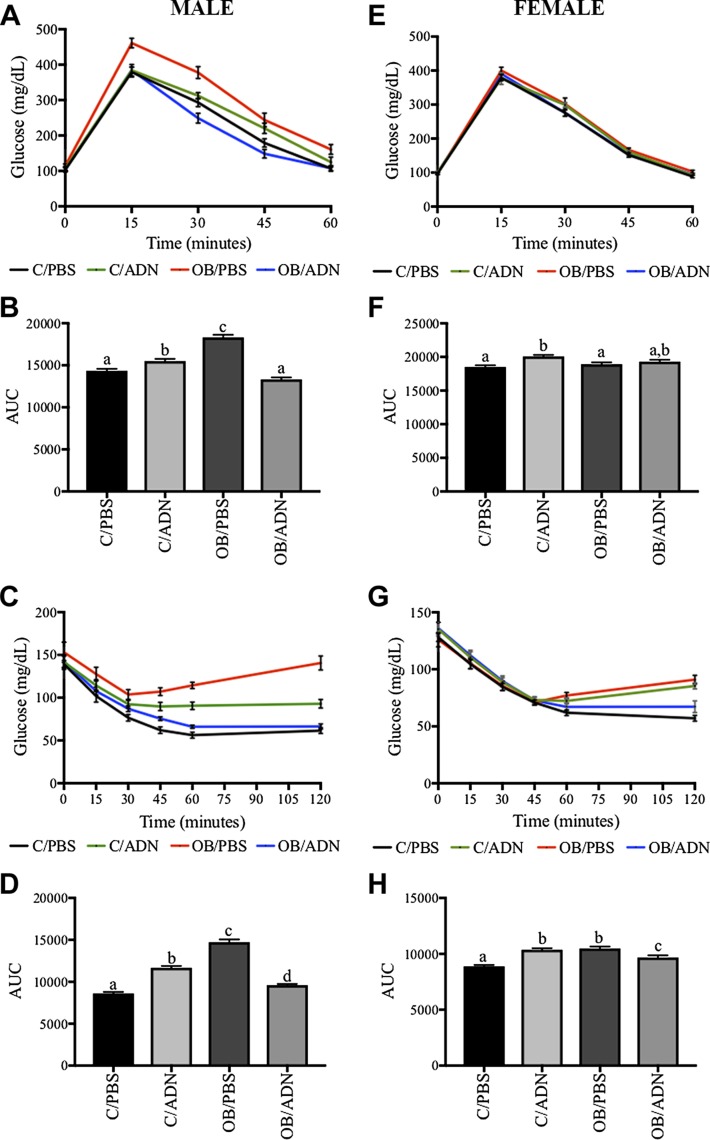
We performed a glucose tolerance test (GTT) in male and female 3-mo-old offspring (Fig. 2A, B, E, F). Results reported are compared to offspring of control mice. There was no difference in the fasting glucose levels between the different groups of offspring at baseline (P = 0.49, Fig. 2A; P = 0.76, Fig. 2E). Male and female C/ADN mice had small increases (8 and 4%, respectively) in the area under the curve (AUC) (P = 0.03, Fig. 2B; P = 0.0007, Fig. 2F). Male OB/PBS mice had elevated postprandial blood glucose levels at 15 min (P = 0.002) that did not return to baseline at 90 min (P = 0.006, Fig. 2A). As a result, OB/PBS mice had a 28% greater AUC (P < 0.0001, Fig. 2B). Female OB/PBS mice did not differ in GTT AUC (P = 0.75) from control offspring (Fig. 2F). Additionally, AUC in OB/ADN mice (male or female) was not different from offspring in the control group (P = 0.06, Fig. 2B; P = 0.18, Fig. 2F).
Figure 2 .
Normalization of ADN levels in obese pregnant mice largely prevents insulin resistance in offspring. GTT (A, B, E, F) and IRT (C, D, G, H). Blood glucose was measured over 120 min after glucose load (A, E) and insulin load (C, G) in offspring. AUC of GTT (B, F), measure of glucose tolerance, and IRT (D, H), readout of insulin resistance. Males (A–D) and females (E–H) were studied separately. Means ± sem; n = 6–10 per group. Different letters (a–c) denote statistically significant differences. P < 0.05.
Next, we performed an insulin resistance test (IRT) in male and female offspring (Fig. 2C, D, G, H). Similar to fasting glucose concentrations during the GTT, there was no significant difference in the fasting glucose levels between the 4 groups of offspring (P = 0.60, Fig. 2C; P = 0.45, Fig. 2G). Male and female C/ADN mice had a 51% increase in AUC in their respective insulin tolerance tests (P < 0.0001, Fig. 2D; P < 0.0001, Fig. 2H) compared to the offspring of controls. Male and female OB/PBS mice had 187 and 59% increased AUC, respectively (P < 0.0001, Fig. 2D; P < 0.0001, Fig. 2H), again suggesting a greater impact of maternal obesity in male OB/PBS mice compared to females. The insulin sensitivity as measured by IRT AUC in the OB/ADN mice was largely normalized compared to OB/PBS mice in both sexes (P < 0.0001, Fig. 2F; P = 0.01, Fig. 2H). While remaining significantly different from controls, in part because of the low variation, the AUC differed only by 10% (males) and 8% (females) between the 2 groups.
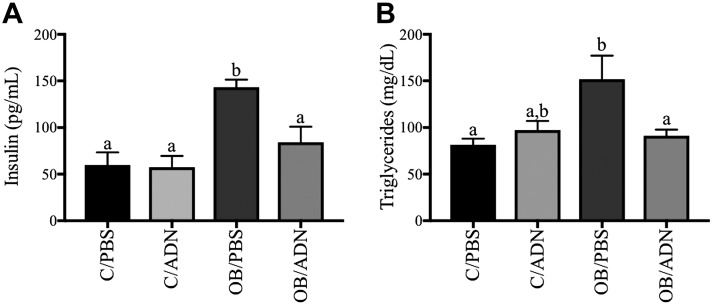
Because OB/PBS adult males, but not females, had impaired glucose tolerance and the males, but not the females, were markedly insulin resistant as assessed by the IRT, we performed detailed studies of the metabolic status of the male offspring. We found markedly higher fasting serum insulin (2.4-fold increase, P = 0.0004, Fig. 3A) and triglyceride concentrations (1.9-fold increase, P = 0.008, Fig. 3B) in the 3-mo male OB/PBS mice compared to control mice, consistent with insulin resistance and hypertriglyceridemia. Importantly, serum insulin concentrations (P > 0.99) and serum triglyceride concentrations (P = 0.86) in male OB/ADN mice did not differ significantly from the control group.
Figure 3 .
Normalization of ADN levels in obese pregnant mice prevents fasting hyperinsulinemia and hypertriglyceridemia in male offspring. Fasting serum insulin (A) and triglyceride (B) concentrations were measured in adult males at 14 wk of life. Means ± sem; n = 9 per group. Different letters (a, b) denote statistically significant differences. P < 0.05.
Normalization of ADN levels in obese pregnant mice prevents hepatic steatosis in adult male offspring
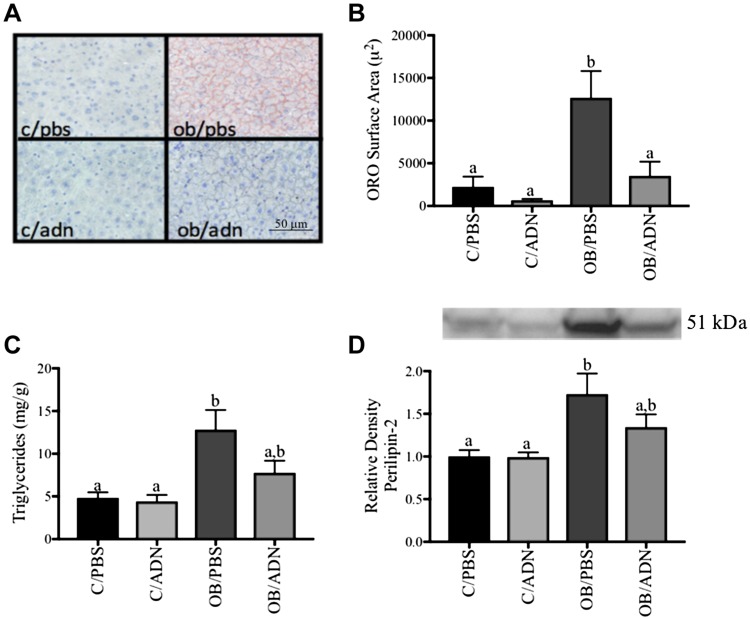
We studied liver neutral lipid staining (Fig. 4A, B), triglyceride deposition (Fig. 4C), and PLIN-2 expression (Fig. 4D) as markers of hepatic steatosis. Compared to control mice, there were no significant differences in neutral lipid staining (P = 0.931), triglyceride deposition (P = 0.97), or PLIN-2 expression (P > 0.99) in the livers of male C/ADN mice. However, in the liver of male OB/PBS mice, there was a 5.5-fold increase in neutral lipid staining (P = 0.002), a 2.7-fold increase in triglyceride content (P = 0.002), and a 1.7-fold increase in PLIN-2 expression (P = 0.009). We also observed a strong correlation between the 3 markers of hepatic steatosis (R = 0.86–0.89, P ≤ 0.0001, n = 36, data not shown), as well as a moderate correlation between insulin and neutral lipid staining (R = 0.49, P = 0.005, n = 36), PLIN-2 protein expression (R = 0.60, P = 0.0001, n = 36), and hepatic triglyceride deposition (R = 0.60, P = 0.0003, n = 36, data not shown). Importantly, these indicators of hepatic steatosis in the male OB/PBS mice were normalized by maternal ADN supplementation in pregnancy, as there were no differences in hepatic neutral lipid staining (P = 0.96), triglyceride deposition (P = 0.48), or PLIN-2 protein expression (P = 0.38) between male OB/ADN mice and male offspring of controls.
Figure 4 .
Normalization of ADN levels in obese pregnant mice prevents hepatic steatosis in male offspring. Liver tissue from unfed males was stained with Oil Red O neutral lipid stain for neutral lipid deposition (A, B), assayed for triglyceride deposition (C), and evaluated for lipid protein coat protein expression of PLIN-2 (D). Means ± sem; n = 9 per group. Different letters (a, b) denote statistically significant differences. P < 0.05.
Normalization of ADN levels in obese pregnant mice prevents peripheral insulin resistance and hepatic glycogen deposition in adult male offspring
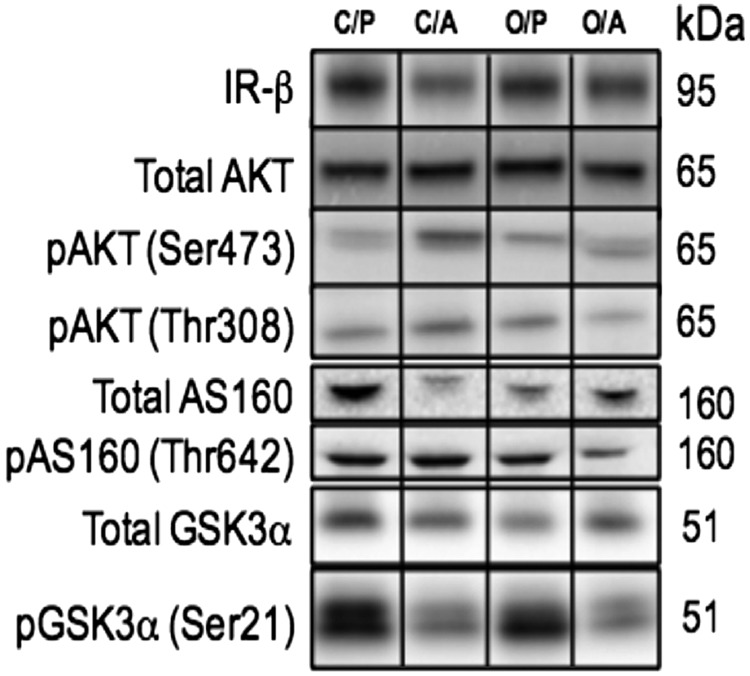
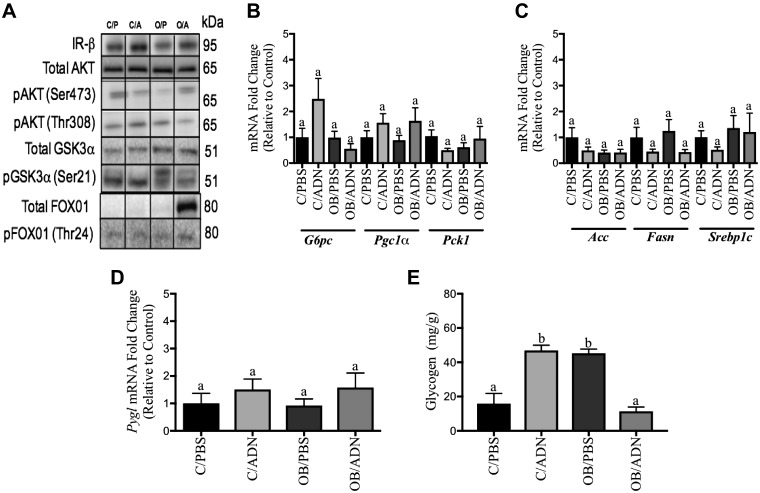
Peripheral insulin signaling was assessed by determining the total expression and phosphorylation of key components in the insulin signaling pathway in male offspring skeletal muscle and hepatic tissue. In C/ADN mice, fasting insulin levels were normal (Fig. 3A), and we observed no change in the protein expression of IR-β, AS160 (involved in insulin-mediated GLUT4 trafficking), phosphorylation of protein kinase B (AKT), or GSK3α (involved in insulin-mediated glycogen synthesis) in skeletal muscle compared to control (Fig. 5 and Supplemental Table S1). Interestingly, despite a marked increase in fasting insulin levels in male OB/PBS mice (Fig. 3A), the insulin signaling pathway in skeletal muscle of these offspring was not activated compared to offspring of control mice (Fig. 5 and Supplemental Table S1). Similar to the findings in skeletal muscle, there was no change in protein expression of hepatic IR-β or phosphorylation of AKT in either C/ADN or OB/PBS mice (Fig. 6 and Supplemental Table S2) compared to controls. Additionally, we did not detect changes in hepatic protein or mRNA expression of FOX-O1, glucose-6-phosphatase catalytic subunit (G6pc), peroxisome proliferator-activated receptor γ coactivator 1-α (Pgc1a), and phosphoenolpyruvate carboxykinase 1 (Pck1) (gluconeogenesis); acetyl-CoA carboxylase (Acc1), fatty acid synthase (Fasn), and sterol regulatory element-binding protein 1 (Srebp1c) (lipogenesis); and GSK3α οr glycogen phosphorylase (Pygl) (glycogen synthesis or breakdown) in C/ADN or OB/PBS mice compared to controls (Fig. 6). Similarly, there were no differences in the expression or phosphorylation of insulin signaling pathway components in OB/ADN mice compared to controls (Figs. 5 and 6 and Supplemental Tables S1 and S2). These findings are consistent with the normal fasting insulin levels in these animals.
Figure 5 .
Insulin signaling in skeletal muscle of unfed male adult offspring. Representative Western blots and relative protein expression of insulin targets in skeletal muscle of unfed adult male offspring. C/PBS protein expression was assigned arbitrary value of 1. Means ± sem; n = 9 per group. Different letters (a, b) denote statistically significant differences. P < 0.05. See Supplemental Table S1.
Figure 6 .
Insulin signaling activity, expression of insulin targets, and glycogen deposition in liver of unfed male adult offspring. Representative Western blots and relative protein expression of hepatic insulin targets. C/PBS protein expression was assigned arbitrary value of 1. A) Offspring mRNA expression of genes associated with insulin mediated hepatic gluconeogenesis: glucose-6-phosphatase catalytic subunit (G6pc) (P > 0.99), Pgc1α (P = 0.31), and phosphoenolpyruvate carboxykinase 1 (Pck1) (P = 0.48). B, C) Fatty acid oxidation: acetyl-CoA carboxylase (Acc1) (P > 0.99), fatty acid synthase (Fasn) (P = 0.31) (B), and sterol regulatory element-binding protein 1 (Srebp1c) (P = 0.48) (C). D)Offspring mRNA transcription of Pgyl . E) Offspring hepatic glycogen deposition. Means ± sem; n = 9 per group. Different letters (a, b) denote statistically significant differences. P < 0.05. See Supplemental Table S2.
There were no differences in glycogen phosphorylase gene expression between the different groups of offspring (P = 0.52, Fig. 6D). Interestingly, there was a 2.9-fold increase in hepatic glycogen deposition in male C/ADN mice (P < 0.0001) and 2.8-fold increase (P < 0.0001) in offspring of OB/PBS mice compared to control mice (Fig. 6E). Importantly, hepatic glycogen deposition was normalized in the 3-mo-old OB/ADN mice (P = 0.96, Fig. 6E).
DISCUSSION
This work provides, for the first time, direct evidence that low circulating maternal ADN is a critical mechanistic link between maternal obesity and the development of metabolic disease in offspring. Specifically, infusion of ADN the last 4 d of pregnancy in rates that increased circulating ADN in obese mice to the levels observed in lean mice prevented the development of obesity, glucose intolerance, insulin resistance, hypertriglyceridemia, and hepatic steatosis in the 3-mo-old male offspring. This work provides new mechanistic insight into the intrauterine transmission of metabolic disease from the obese mother to her infant and may lead to novel specific intervention strategies to alleviate the adverse effects of maternal obesity on the offspring.
We have previously reported that ADN inhibits insulin/mTOR signaling and amino acid transport in cultured primary human trophoblast cells (32, 33) and in the placenta of pregnant mice in vivo (22, 34). Low ADN levels, commonly found in both obesity and gestational diabetes, are therefore predicted to activate placental insulin signaling, which is correlated with increased fetal growth (23, 39, 40). Indeed, in our mouse model of obesity in pregnancy, characterized by maternal insulin resistance and low circulating ADN levels, activation of placental insulin, and mTOR signaling, as well as subsequent increased placental nutrient transport (37), we demonstrated that normalization of circulating levels of ADN in obese mice normalizes placental function and prevents fetal overgrowth (22). We therefore propose that normalization of maternal ADN levels prevents metabolic programming in the offspring mediated by its direct effects on the placenta. However, it is possible that increased maternal insulin sensitivity, and the resulting decrease in maternal insulin levels, contributes to the beneficial effects of normalization of maternal ADN levels in obesity on both placental function and fetal growth, and thereby on the long-term metabolic outcomes of the offspring (14, 22, 41).
We determined the expression and phosphorylation of key proteins in the insulin signaling pathway in skeletal muscle and liver to better understand potential mechanisms underpinning insulin resistance in the offspring of obese mice. Both skeletal muscle, which is responsible for 90% of insulin-mediated glucose uptake, and liver are key peripheral tissues responsive to insulin signaling (42, 43). If peripheral insulin sensitivity was maintained at normal levels in offspring of obese mice, then we would anticipate that the 2.4-fold higher insulin would cause an activation of muscle and liver insulin signaling in the male offspring of obese mice. However, muscle insulin signaling activity and insulin receptor expression were unchanged. This lack of activation suggests early signs of insulin resistance in skeletal muscle and liver in offspring of obese mice. Our measurements of whole-body insulin sensitivity, IRT, also indicated a reduced insulin responsiveness in male offspring of obese mice. These data together indicate that the male offspring of obese mice are in a prediabetic state with higher fat mass, normal fasting glucose levels, elevated postprandial glucose excursions, and insulin resistance in peripheral tissues.
Previous work has shown hepatic steatosis in fetuses of obese mice (14, 43, 44), and the current study extends these observations by demonstrating that hepatic steatosis persists in the 3 mo old offspring of obese mice, which was prevented by normalizing circulating maternal ADN levels in late pregnancy. Consistent with the findings in our mouse model, intrahepatic fat is increased in the 1- to 3-wk-old infant of obese mothers with gestational diabetes (45) and in the adolescent offspring of obese mothers (46). Other disturbances in lipid metabolism in the offspring of obese mice included increased circulating levels of triglycerides; further, similar to findings in skeletal muscle, higher insulin levels in offspring of obese mice were not associated with an activation of insulin signaling in the liver, both consistent with hepatic insulin resistance. Although there were no significant differences in the expression of regulators and mediators of liver glycogen synthesis, glycogen breakdown, fatty acid synthesis, or fatty acid oxidation, offspring of obese mice had increased hepatic glycogen and triglyceride deposition. In the absence of clear evidence of up-regulation of metabolic pathways, glycogen and triglyceride accumulation in the liver of the offspring of obese mice may be caused by increased substrate supply as a result of peripheral insulin resistance and prolonged elevation of postprandial glucose levels.
We have previously reported that infusion of ADN to normal-weight pregnant mice increased circulating ADN levels 3-fold, inhibited placental insulin and mTOR signaling, and decreased placental nutrient transport, which resulted in a modest fetal growth restriction (34). In the current study, we explored the effects of increasing maternal ADN in control mice on offspring long-term metabolic health. Offspring born to normal-weight mice supplemented with ADN had decreased body weight at 1 wk of age and showed catch-up growth by 3 mo; further, they were modestly glucose intolerant and insulin resistant, with increased hepatic glycogen at 3 mo of age. These results are consistent with a body of evidence that fetal growth restriction in both humans and in a range of animal models is associated with metabolic disease later in life (47–50). These important data suggest that maternal ADN levels are critically important during pregnancy, both in relationship to obese women (low ADN) and in lean women (high ADN) in regulating nutrient allocation between the mother and her developing fetus, resulting in programming the metabolic health of offspring on both ends of the spectrum.
Male offspring born to obese mice had a more pronounced metabolic phenotype at 3 mo of age compared to females. Adult female offspring born to obese mice were slightly heavier, with a small decrease in insulin sensitivity; these changes were not associated with an increase in fat mass, as found in males. Although the exact mechanisms underpinning sexual dimorphism in early programming of metabolic health are yet to be fully elucidated, it is generally accepted that males often have earlier and more significant metabolic phenotypes than females (51). Whether these sex-specific differences occur as a result of placental programming, or during postnatal critical periods of development, or whether they are innate to biologic differences between the sexes remains an active area for investigation (52, 53). Specific to this study, the finding that females were less affected compared to their male littermates indicates a sex difference in the intergenerational transmission of obesity. Normalization of ADN levels in late pregnancy prevented the adverse metabolic effects of maternal obesity in both male and female offspring, giving support to further investigation of ADN supplementation in preventing transmission of metabolic disease in both sexes.
Other adipokines have been implicated in the programming of metabolic disease. In particular, the effect of leptin administration in late pregnancy and throughout lactation on offspring adiposity and glucose metabolism in rats has been previously reported (54, 55). In these studies, leptin administration markedly increased endogenous leptin levels and was reported to decrease the susceptibility in male intrauterine growth restriction offspring to gain weight and develop insulin resistance in response to a high-fat diet (54) and to largely prevent diet-induced obesity and impaired glucose tolerance in offspring of normal mice (55). Although the paradigms in the previous reports on perinatal leptin administration in the rat and the scientific questions addressed in the current study are very different, they collectively demonstrate the critical importance of maternal adipokines in programming glucose metabolism and in susceptibility to develop obesity in the offspring.
Limitations of the current study include that offspring outcomes were only studied at 3 mo of age and it is possible that, for example, the metabolic phenotype in female offspring of obese mice would have been more pronounced if studied older animals. In addition, it is unknown if the prevention of adverse metabolic outcomes in offspring of obese dams by normalization of maternal ADN levels during pregnancy is permanent and can be demonstrated also at 6 or 9 mo of age. Our mouse model of maternal obesity in pregnancy shows extensive similarities with the human condition, including elevated levels of maternal leptin, glucose intolerance, activation of placental insulin and mTOR signaling, increased placental nutrient transport, and fetal overgrowth (37). However, pregnant mice and women differ in many respects, including differences in maturity at birth and in lipid metabolism, and extrapolation of our findings to women has to be done with caution, representing another limitation of our study.
In summary, our report identifies low maternal ADN in pregnancy as a mechanism programming metabolic disease in offspring of obese mice. We propose that these effects are mediated through normalizing placental function, which attenuates the excessive nutrient transfer to the fetus and prevents fetal overgrowth in maternal obesity (22). Given that the majority of Americans now enter pregnancy overweight or obese (2), with similar trends worldwide (56), intrauterine programming of metabolic disease in infants of obese mothers represents a daunting public health problem. Unfortunately, there are few interventions that are effective in preventing the transmission of metabolic risk from mother to her fetus in pregnancies complicated by obesity. Our data suggest that interventions that elevate maternal ADN levels, or possibly use of oral ADN receptor agonists (57), may serve as an effective therapeutic strategy to prevent the development of metabolic disease in infants born to mothers with obesity and/or gestational diabetes mellitus.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank A. Kramer and N. Anderson (University of Colorado) for providing technical assistance in the acquisition of data, and the Mouse Metabolic Phenotyping Center at the University of Cincinnati (Cincinnati, OH, USA) [U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases; Grant DK59630] for performing colorimetric assay of serum triglycerides. This work was supported by NIH Office of the Director Grant R24OD016724, and NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants R01HD065007 and T32HD07186. The authors declare no conflicts of interest.
Glossary
- ADN
adiponectin
- AS160
Akt substrate of 160 kDa
- AUC
area under the curve
- C/ADN
offspring of pregnant mice fed control diet infused with adiponectin
- C/PBS
offspring of pregnant mice fed control diet infused with vehicle
- FOX-O1
forkhead box protein O1
- GTT
glucose tolerance test
- IRT
insulin resistance test
- IR-β
insulin receptor β
- mTOR
mammalian target of rapamycin
- OB/ADN
offspring of obese pregnant mice infused with adiponectin
- OB/PBS
offspring of obese pregnant mice infused with vehicle
- PLIN-2
perilipin-2
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. L. Powell and T. Jansson conceived and designed the project and are the guarantors of this work, and as such had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis; M. E. Paulsen, F. J. Rosario, T. L. Powell, and T. Jansson researched data and designed the experiments; F. J. Rosario maintained the mouse colony (feeding, implantation of osmotic pump, weaning, weighing, and tissue collection) and performed body composition, GTT, and insulin tolerance test studies in offspring; S. R. Wesolowski performed hepatic glycogen and triglyceride deposition experiments; M. E. Paulsen was responsible for data analysis and presentation of data, performed experiments, and wrote the manuscript; and all authors critically revised the manuscirpt for substantive content and approved the final version.
REFERENCES
- 1.Alfaradhi, M. Z., Ozanne, S. E. (2011) Developmental programming in response to maternal overnutrition. Front. Genet. 2, 27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher, S. C., Kim, S. Y., Sharma, A. J., Rochat, R., Morrow, B. (2013) Is obesity still increasing among pregnant women? Prepregnancy obesity trends in 20 states, 2003–2009. Prev. Med. 56, 372–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evagelidou, E. N., Kiortsis, D. N., Bairaktari, E. T., Giapros, V. I., Cholevas, V. K., Tzallas, C. S., Andronikou, S. K. (2006) Lipid profile, glucose homeostasis, blood pressure, and obesity-anthropometric markers in macrosomic offspring of nondiabetic mothers. Diabetes Care 29, 1197–1201 [DOI] [PubMed] [Google Scholar]
- 4.Reynolds, R. M., Allan, K. M., Raja, E. A., Bhattacharya, S., McNeill, G., Hannaford, P. C., Sarwar, N., Lee, A. J., Bhattacharya, S., Norman, J. E. (2013) Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 347, f4539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawlor, D. A., Smith, G. D., O’Callaghan, M., Alati, R., Mamun, A. A., Williams, G. M., Najman, J. M. (2007) Epidemiologic evidence for the fetal overnutrition hypothesis: findings from the Mater-University Study of Pregnancy and Its Outcomes. Am. J. Epidemiol. 165, 418–424 [DOI] [PubMed] [Google Scholar]
- 6.Wang, X., Liang, L., Junfen, F. U., Lizhong, D. U. (2007) Metabolic syndrome in obese children born large for gestational age. Indian J. Pediatr. 74, 561–565 [DOI] [PubMed] [Google Scholar]
- 7.Catalano, P. M. (2003) Obesity and pregnancy—the propagation of a vicious cycle? J. Clin. Endocrinol. Metab. 88, 3505–3506 [DOI] [PubMed] [Google Scholar]
- 8.Thangaratinam, S., Rogozinska, E., Jolly, K., Glinkowski, S., Roseboom, T., Tomlinson, J. W., Kunz, R., Mol, B. W., Coomarasamy, A., Khan, K. S. (2012) Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta-analysis of randomised evidence. BMJ 344, e2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansson, K., Stephansson, O., Neovius, M. (2015) Outcomes of pregnancy after bariatric surgery. N. Engl. J. Med. 372, 2267 [DOI] [PubMed] [Google Scholar]
- 10.Finer, L. B., Zolna, M. R. (2016) Declines in unintended pregnancy in the United States, 2008–2011. N. Engl. J. Med. 374, 843–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogden, C. L., Carroll, M. D., Kit, B. K., Flegal, K. M. (2014) Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 311, 806–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornburg, K. L., Kolahi, K., Pierce, M., Valent, A., Drake, R., Louey, S. (2016) Biological features of placental programming. Placenta 48 (Suppl 1), S47–S53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jansson, T., Powell, T. L. (2013) Role of placental nutrient sensing in developmental programming. Clin. Obstet. Gynecol. 56, 591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wesolowski, S. R., Kasmi, K. C., Jonscher, K. R., Friedman, J. E. (2017) Developmental origins of NAFLD: a womb with a clue. Nat. Rev. Gastroenterol. Hepatol. 14, 81–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansson, N., Rosario, F. J., Gaccioli, F., Lager, S., Jones, H. N., Roos, S., Jansson, T., Powell, T. L. (2013) Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J. Clin. Endocrinol. Metab. 98, 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalano, P. M., Presley, L., Minium, J., Hauguel-de Mouzon, S. (2009) Fetuses of obese mothers develop insulin resistance in utero. Diabetes Care 32, 1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahlsson, F. S., Diderholm, B., Ewald, U., Gustafsson, J. (2007) Lipolysis and insulin sensitivity at birth in infants who are large for gestational age. Pediatrics 120, 958–965 [DOI] [PubMed] [Google Scholar]
- 18.Spranger, J., Kroke, A., Möhlig, M., Bergmann, M. M., Ristow, M., Boeing, H., Pfeiffer, A. F. (2003) Adiponectin and protection against type 2 diabetes mellitus. Lancet 361, 226–228 [DOI] [PubMed] [Google Scholar]
- 19.Berg, A. H., Combs, T. P., Scherer, P. E. (2002) ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 13, 84–89 [DOI] [PubMed] [Google Scholar]
- 20.Aye, I. L., Powell, T. L., Jansson, T. (2013) Review: adiponectin—the missing link between maternal adiposity, placental transport and fetal growth? Placenta 34 (Suppl), S40–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atègbo, J. M., Grissa, O., Yessoufou, A., Hichami, A., Dramane, K. L., Moutairou, K., Miled, A., Grissa, A., Jerbi, M., Tabka, Z., Khan, N. A. (2006) Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J. Clin. Endocrinol. Metab. 91, 4137–4143 [DOI] [PubMed] [Google Scholar]
- 22.Aye, I. L., Rosario, F. J., Powell, T. L., Jansson, T. (2015) Adiponectin supplementation in pregnant mice prevents the adverse effects of maternal obesity on placental function and fetal growth. Proc. Natl. Acad. Sci. USA 112, 12858–12863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jansson, N., Nilsfelt, A., Gellerstedt, M., Wennergren, M., Rossander-Hulthén, L., Powell, T. L., Jansson, T. (2008) Maternal hormones linking maternal body mass index and dietary intake to birth weight. Am. J. Clin. Nutr. 87, 1743–1749 [DOI] [PubMed] [Google Scholar]
- 24.Hendler, I., Blackwell, S. C., Mehta, S. H., Whitty, J. E., Russell, E., Sorokin, Y., Cotton, D. B. (2005) The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am. J. Obstet. Gynecol. 193, 979–983 [DOI] [PubMed] [Google Scholar]
- 25.Nien, J. K., Mazaki-Tovi, S., Romero, R., Erez, O., Kusanovic, J. P., Gotsch, F., Pineles, B. L., Gomez, R., Edwin, S., Mazor, M., Espinoza, J., Yoon, B. H., Hassan, S. S. (2007) Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J. Perinat. Med. 35, 522–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai, P. J., Yu, C. H., Hsu, S. P., Lee, Y. H., Huang, I. T., Ho, S. C., Chu, C. H. (2005) Maternal plasma adiponectin concentrations at 24 to 31 weeks of gestation: negative association with gestational diabetes mellitus. Nutrition 21, 1095–1099 [DOI] [PubMed] [Google Scholar]
- 27.Soheilykhah, S., Mohammadi, M., Mojibian, M., Rahimi-Saghand, S., Rashidi, M., Hadinedoushan, H., Afkhami-Ardekani, M. (2009) Maternal serum adiponectin concentration in gestational diabetes. Gynecol. Endocrinol. 25, 593–596 [DOI] [PubMed] [Google Scholar]
- 28.Bomba-Opon, D. A., Wielgos, M., Horosz, E., Bartkowiak, R., Szymusik, I., Mazanowski, N., Bochenska, K. (2009) Maternal plasma cytokines concentrations and insulin resistance in first trimester in relation to fetal growth. Neuroendocrinol. Lett. 30, 729–732 [PubMed] [Google Scholar]
- 29.Lekva, T., Roland, M. C. P., Michelsen, A. E., Friis, C. M., Aukrust, P., Bollerslev, J., Henriksen, T., Ueland, T. (2017) Large reduction in adiponectin during pregnancy is associated with large-for-gestational-age newborns. J. Clin. Endocrinol. Metab. 102, 2552–2559 [DOI] [PubMed] [Google Scholar]
- 30.Retnakaran, R., Qi, Y., Connelly, P. W., Sermer, M., Hanley, A. J., Zinman, B. (2010) Low adiponectin concentration during pregnancy predicts postpartum insulin resistance, beta cell dysfunction and fasting glycaemia. Diabetologia 53, 268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez, V. I., Miller, E., Meireles, C. L., Gelfond, J., Krummel, D. A., Powell, T. L. (2014) Adiponectin and IGFBP-1 in the development of gestational diabetes in obese mothers. BMJ Open Diabetes Res. Care 2, e000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones, H. N., Jansson, T., Powell, T. L. (2010) Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino acid transport in human primary trophoblast cells. Diabetes 59, 1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aye, I. L., Gao, X., Weintraub, S. T., Jansson, T., Powell, T. L. (2014) Adiponectin inhibits insulin function in primary trophoblasts by PPARα-mediated ceramide synthesis. Mol. Endocrinol. 28, 512–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosario, F. J., Schumacher, M. A., Jiang, J., Kanai, Y., Powell, T. L., Jansson, T. (2012) Chronic maternal infusion of full-length adiponectin in pregnant mice down-regulates placental amino acid transporter activity and expression and decreases fetal growth. J. Physiol. 590, 1495–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao, L., Wattez, J. S., Lee, S., Guo, Z., Schaack, J., Hay, W. W., Jr., Zita, M. M., Parast, M., Shao, J. (2016) Knockout maternal adiponectin increases fetal growth in mice: potential role for trophoblast IGFBP-1. Diabetologia 59, 2417–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiao, L., Wattez, J. S., Lee, S., Nguyen, A., Schaack, J., Hay, W. W., Jr., Shao, J. (2017) Adiponectin deficiency impairs maternal metabolic adaptation to pregnancy in mice. Diabetes 66, 1126–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosario, F. J., Kanai, Y., Powell, T. L., Jansson, T. (2015) Increased placental nutrient transport in a novel mouse model of maternal obesity with fetal overgrowth. Obesity (Silver Spring) 23, 1663–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lanoix, D., St-Pierre, J., Lacasse, A. A., Viau, M., Lafond, J., Vaillancourt, C. (2012) Stability of reference proteins in human placenta: general protein stains are the benchmark. Placenta 33, 151–156 [DOI] [PubMed] [Google Scholar]
- 39.Acosta, O., Ramirez, V. I., Lager, S., Gaccioli, F., Dudley, D. J., Powell, T. L., Jansson, T. (2015) Increased glucose and placental GLUT-1 in large infants of obese nondiabetic mothers. Am. J. Obstet. Gynecol. 212, 227.e1–227.e7 [DOI] [PubMed] [Google Scholar]
- 40.Yogev, Y., Chen, Y., Hod, M., Coustan, D., Oats, J.J., McIntyre, H.D., Metzger, B.E., Lowe, L.P., Dyer, A.R., Dooley, S.L., Trimble, E.R., McCance, D. R., Hadden, D. R., Persson, B., Rogers, M. S.; Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study Cooperative Research Group . (2010) Hyperglycemia and adverse pregnancy outcome (HAPO) study: preeclampsia. Am. J. Obstet. Gynecol. 202, 255.e1–255.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorn, S. R., Baquero, K. C., Newsom, S. A., El Kasmi, K. C., Bergman, B. C., Shulman, G. I., Grove, K. L., Friedman, J. E. (2014) Early life exposure to maternal insulin resistance has persistent effects on hepatic NAFLD in juvenile nonhuman primates. Diabetes 63, 2702–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leto, D., Saltiel, A. R. (2012) Regulation of glucose transport by insulin: traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 13, 383–396 [DOI] [PubMed] [Google Scholar]
- 43.Vatner, D. F., Majumdar, S. K., Kumashiro, N., Petersen, M. C., Rahimi, Y., Gattu, A. K., Bears, M., Camporez, J. P., Cline, G. W., Jurczak, M. J., Samuel, V. T., Shulman, G. I. (2015) Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc. Natl. Acad. Sci. USA 112, 1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Díaz, P., Harris, J., Rosario, F. J., Powell, T. L., Jansson, T. (2015) Increased placental fatty acid transporter 6 and binding protein 3 expression and fetal liver lipid accumulation in a mouse model of obesity in pregnancy. Am. J. Physiol. Regul. Integr. Comp. Physiol. 309, R1569–R1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brumbaugh, D. E., Tearse, P., Cree-Green, M., Fenton, L. Z., Brown, M., Scherzinger, A., Reynolds, R., Alston, M., Hoffman, C., Pan, Z., Friedman, J. E., Barbour, L. A. (2013) Intrahepatic fat is increased in the neonatal offspring of obese women with gestational diabetes. J. Pediatr. 162, 930–936.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellatorre, A., Scherzinger, A., Stamm, E., Martinez, M., Ringham, B., Dabelea, D. (2018) Fetal overnutrition and adolescent hepatic fat fraction: the Exploring Perinatal Outcomes in Children study. J. Pediatr. 192, 165–170.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gluckman, P. D., Hanson, M. A. (2004) Living with the past: evolution, development, and patterns of disease. Science 305, 1733–1736 [DOI] [PubMed] [Google Scholar]
- 48.Gluckman, P. D., Hanson, M. A., Cooper, C., Thornburg, K. L. (2008) Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 359, 61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simmons, R. A., Templeton, L. J., Gertz, S. J. (2001) Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50, 2279–2286 [DOI] [PubMed] [Google Scholar]
- 50.Stoffers, D. A., Desai, B. M., DeLeon, D. D., Simmons, R. A. (2003) Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes 52, 734–740 [DOI] [PubMed] [Google Scholar]
- 51.Kautzky-Willer, A., Harreiter, J., Pacini, G. (2016) Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 37, 278–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabory, A., Ferry, L., Fajardy, I., Jouneau, L., Gothié, J. D., Vigé, A., Fleur, C., Mayeur, S., Gallou-Kabani, C., Gross, M. S., Attig, L., Vambergue, A., Lesage, J., Reusens, B., Vieau, D., Remacle, C., Jais, J. P., Junien, C. (2012) Maternal diets trigger sex-specific divergent trajectories of gene expression and epigenetic systems in mouse placenta. PLoS One 7, e47986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dearden, L., Bouret, S. G., Ozanne, S. E. (2018) Sex and gender differences in developmental programming of metabolism. Mol. Metab. 15, 8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stocker, C., O’Dowd, J., Morton, N. M., Wargent, E., Sennitt, M. V., Hislop, D., Glund, S., Seckl, J. R., Arch, J. R., Cawthorne, M. A. (2004) Modulation of susceptibility to weight gain and insulin resistance in low birthweight rats by treatment of their mothers with leptin during pregnancy and lactation. Int. J. Obes. Relat. Metab. Disord. 28, 129–136 [DOI] [PubMed] [Google Scholar]
- 55.Stocker, C. J., Wargent, E., O’Dowd, J., Cornick, C., Speakman, J. R., Arch, J. R., Cawthorne, M. A. (2007) Prevention of diet-induced obesity and impaired glucose tolerance in rats following administration of leptin to their mothers. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1810–R1818 [DOI] [PubMed] [Google Scholar]
- 56.Branca, F., Nikogosian, H., Lobstein, T. (2007) The Challenge of Obesity in the WHO European Region and the Strategies for Response: Summary, Regional Office for Europe, Copenhagen, Denmark [Google Scholar]
- 57.Okada-Iwabu, M., Yamauchi, T., Iwabu, M., Honma, T., Hamagami, K., Matsuda, K., Yamaguchi, M., Tanabe, H., Kimura-Someya, T., Shirouzu, M., Ogata, H., Tokuyama, K., Ueki, K., Nagano, T., Tanaka, A., Yokoyama, S., Kadowaki, T. (2013) A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature 503, 493–499 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.