Abstract
Cytochrome c (Cytc) plays a vital role in the mitochondrial electron transport chain (ETC). In addition, it is a key regulator of apoptosis. Cytc has multiple other functions including ROS production and scavenging, cardiolipin peroxidation, and mitochondrial protein import. Cytc is tightly regulated by allosteric mechanisms, tissue-specific isoforms, and post-translational modifications (PTMs). Distinct residues of Cytc are modified by PTMs, primarily phosphorylations, in a highly tissue-specific manner. These modifications downregulate mitochondrial ETC flux and adjust the mitochondrial membrane potential (ΔΨm), to minimize reactive oxygen species (ROS) production under normal conditions. In pathologic and acute stress conditions, such as ischemia–reperfusion, phosphorylations are lost, leading to maximum ETC flux, ΔΨm hyperpolarization, excessive ROS generation, and the release of Cytc. It is also the dephosphorylated form of the protein that leads to maximum caspase activation. We discuss the complex regulation of Cytc and propose that it is a central regulatory step of the mammalian ETC that can be rate limiting in normal conditions. This regulation is important because it maintains optimal intermediate ΔΨm, limiting ROS generation. We examine the role of Cytc PTMs, including phosphorylation, acetylation, methylation, nitration, nitrosylation, and sulfoxidation and consider their potential biological significance by evaluating their stoichiometry.—Kalpage, H. A., Bazylianska, V., Recanati, M. A., Fite, A., Liu, J., Wan, J., Mantena, N., Malek, M. H., Podgorski, I., Heath, E. I., Vaishnav, A., Edwards, B. F., Grossman, L. I., Sanderson, T. H., Lee, I., Hüttemann, M. Tissue-specific regulation of cytochrome c by post-translational modifications: respiration, the mitochondrial membrane potential, ROS, and apoptosis.
Keywords: electron transport chain, ischemia–reperfusion, phosphorylation, reactive oxygen species, signal transduction
Cytochrome c (Cytc) plays a pivotal role in making life-and-death decisions for the fate of the cell. It is a small, globular, highly conserved, 104 aa protein with a covalently attached heme group that performs multiple functions and catalytic activities. In the mitochondrial electron transport chain (ETC), Cytc acts as an electron carrier from complex III to complex IV, which are also known as the cytochrome bc1 complex and Cytc oxidase (COX), respectively. ETC function fully relies on the presence of Cytc, as can be seen in Cytc-knockout mice that die at midgestation (1), when fetal metabolism switches from glycolysis to oxidative phosphorylation (2) to sustain the increasing energy supply of the growing organism. It has been shown with Cytc knockout mouse fibroblasts that Cytc plays a prominent role in supercomplex formation and is essential for the stability and assembly of COX (3). Cytc also plays a key role in apoptosis by acting as a signaling molecule that is released from the mitochondria into the cytosol during cellular stress. In the cytosol, it interacts with apoptosis activating factor (Apaf)-1, leading to the formation of the heptameric apoptosome, which activates downstream caspases and initiates the cell death pathway. Furthermore, early-on during apoptosis, Cytc functions as a peroxidase of cardiolipin, a mitochondria-specific lipid that binds to Cytc. This interaction leads to conformational changes around the heme group, converting Cytc into a pentacoordinated structure from its native hexacoordinated form (4, 5). This conversion enhances cardiolipin peroxidase activity of Cytc in the presence of H2O2. This proapoptotic event promotes the dissociation of Cytc from the outer leaflet of the inner mitochondrial membrane and its release into the cytosol, where apoptosis is induced (6). There are 2 distinct binding sites for cardiolipin: the A and C sites. The A site overlaps with the ATP binding site, which is located on the left side of the molecule in the conventional view. The C site allows hydrophobic interaction between cardiolipin and Cytc (7, 8).
Cytc has been referred to as a “moonlighting” protein because of its multifunctional properties in addition to apoptosis and respiration (9, 10). It has been shown to play a role in regulated production of reactive oxygen species (ROS), mainly H2O2, by reducing p66Shc (11), a splice variant of p52Shc/p46Shc, which are adaptor proteins involved in the Ras signaling pathway (12). p66Shc is a negative regulator of life span with increased survival observed in p66Shc knockout mice and cells (13–15). These knockouts also demonstrate increased resistance to ROS and UV damage. p66Shc is regulated by phosphorylation through the PKC-β signaling pathway (16). Phosphorylation of p66Shc at Ser36 is necessary for its role in ROS production (13, 17, 18). Prevention of Cytc binding by mutation of p66Shc W134F has been shown to attenuate ROS-induced renal toxicity (19–21).
In contrast to the role of Cytc in regulated ROS formation by interaction with p66Shc, Cytc is also a well-known ROS scavenger because of its ability to take up and donate electrons quickly. Horse heart Cytc has been shown to act as an H2O2 scavenger in rat heart mitochondria linked to reverse electron transfer from succinate to NAD+ involving complex I (22, 23). In addition, Cytc plays a role in oxidation of superoxide to oxygen, where the electron removed from superoxide is channeled to COX and used for energy production (22, 24). Recently, Cytc was shown to function as an H2S oxidase and thus as a modulator of H2S signaling (25).
Cytc also has a rodent testes-specific isoform that is 86% homologous to the somatic form (26). It has been shown that testes-specific Cytc results in 3 times better H2O2 scavenging compared to somatic Cytc. However, the testes-specific Cytc also demonstrates significantly higher apoptotic activity, possibly to help maintain sperm integrity (27). In humans, this testes-specific isoform exists as a nontranscribed pseudogene (28, 29). The loss of the testes-specific isoform in humans may at first sight be surprising. However, the loss—engendered by a stop codon at position 49—took place on the primate stem ∼65 million years ago (30). The loss of the testes isoform coincided with a period of accelerated evolution of COX in anthropoid primates, such that the COX acceleration was much greater for the residues that are part of the Cytc-COX binding domain than at other COX residues (31). The result is that COX has undergone several amino acid replacements, leading to reduced electrostatic interactions between these 2 proteins in anthropoid primates (31).
In conjunction with its potential as an electron carrier, Cytc is also involved in redox-coupled import of proteins containing twin CX3C and CX9C proteins through the Mia40-Erv1 disulfide relay system. These cysteine-rich proteins are transported to the intermembrane space of the mitochondria via Mia40-catalyzed oxidation of the 4 cysteine residues of the proteins that enter the intermembrane space through the translocase of the outer membrane proteins. Mia40 is reoxidized by sulfhydryl oxidase Erv1, and Erv1 is reoxidized by transfer of electrons to Cytc, thus connecting this protein import pathway to the ETC (32, 33).
Cytc has also been reported to bind the inositol-1,4,5-triphosphate receptor in the endoplasmic reticulum during apoptosis, which leads to calcium overload in the mitochondria and cytosol, resulting in a positive-feedback mechanism that causes more Cytc release and activation of apoptosis (34–36). In addition, once released, Cytc competes with the Apaf-1 inhibitor 14-3-3ε for binding to Apaf-1, further promoting caspase activation (37). Furthermore, Cytc acts as an oxidizing agent that facilitates conversion of sulfite to sulfate by sulfite oxidase, a molybdenum-dependent mitochondrial protein (38, 39). Sulfite is a harmful metabolic byproduct of the amino acids containing sulfur, methionine and cysteine. Cytc helps reduce sulfite toxicity by interaction with sulfite oxidase in a binding domain similar to that of COX (40).
Cytc can translocate to the nucleus in response to DNA damage, where it has been shown to play a role in apoptosis by inducing chromatin condensation independent of caspase activity (41). Other studies have suggested that nuclear translocation of Cytc takes place before caspase activation. Human Cytc and plant Cytc inhibit the nucleosome assembly function of SET/template-activating factor Ib (SET/TAF-Ib) chaperones in humans and nucleosome assembly protein-1–related protein-1 (NRP-1) chaperones in plants (42, 43). Cytc is a highly positively charged protein that promotes its interaction with low-complexity acidic regions of the above histone-binding chaperones. This inhibitory interaction of Cytc has been proposed to be a cellular life-or-death mechanism, depending on the severity of cell damage (44). Finally, Cytc was recently shown to have yet another catalytic function by acting as a plasmalogenase in vitro, cleaving the vinyl–ether linkage of important phospholipids called plasmalogens, which are involved in lipid signaling (45). However, more research is needed to confirm that Cytc acts as a plasmalogenase in vivo.
POST-TRANSLATIONAL MODIFICATIONS OF CYTc AND THEIR FUNCTIONAL EFFECTS
Considering the multiple roles of Cytc in energy production, ROS balance, and apoptosis (Fig. 1), this protein should be tightly regulated. Regulation of mitochondrial proteins is also necessary because of the differences in the cellular metabolic requirements between tissues and organs. Protein regulation can be achieved by several mechanisms, starting at the gene expression level, through translational regulation, and finally at the post-translational level through post-translational modifications (PTMs) and protein turnover. In multicellular organisms, cell signaling through PTMs is one of the most crucial regulatory pathways. Despite many years of research on Cytc, the fact that it is a target of cell signaling through PTMs was revealed only recently. In this review we focus on the published literature of Cytc modifications such as phosphorylations, methylations, acetylations, nitrations, nitrosylations, and sulfoxidations (Table 1). A guiding theme of this review is that the biologic consequences of PTMs should be interpreted with respect to how much of the entire protein pool contains a particular modification. This information is critically important but is missing in most studies. Despite major methodological advances in mapping PTMs to specific residues of a protein, primarily by mass spectrometry, which has become increasingly sensitive, quantitative information is often lacking. If, for example, only 1% of a metabolic enzyme carries a PTM, it is most likely adventitious and unlikely to have a biologic effect. Even Western blot analysis with PTM-specific antibodies can only estimate a relative increase or decrease of the modification, and the same issue remains, despite the often-reported several-fold increase in signal. This point is often overlooked, not just in studies related to mitochondria, but also in publications related to cell signaling in general.
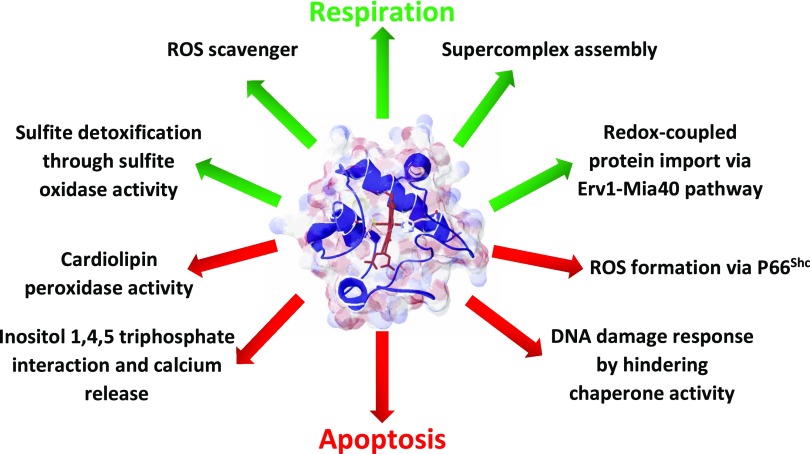
Figure 1 .
Pro-life (green arrows) and pro-death functions (red arrows) of Cytc.
TABLE 1.
Identified PTMs of Cytc
| Site | Modification | Methodology | Tissue/condition | Primary functional effect | Reference |
|---|---|---|---|---|---|
| Lys8 | Acetylation | High-throughput Nano-HPLC/MS/MS | Mouse liver mitochondria (unfed state) | Not tested | 112 |
| Thr28 | Phosphorylation | ESI-MS/MS | Bovine kidney | Lower respiration, membrane potential, and ROS | 76 |
| Human phosphomimetic | 77 | ||||
| High-throughput LC-MS/MS | Human muscle (resting state) | Not tested | 74 | ||
| Human muscle (after exercise) | Not tested | 75 | |||
| Ser47 | Phosphorylation | High-throughput LC-MS/MS | Human muscle (resting state) | Not tested | 73, 74 |
| Human muscle (after exercise) | Not tested | 75 | |||
| Human phosphomimetic | Lower caspase activity | 77 | |||
| Tyr48 | Phosphorylation | IMAC/nano-LC/ESI-MS | Bovine liver | Lower COX activity, abolished caspase-3 activity, and lower cardiolipin peroxidase activity | 62, 66 |
| Tyr67 | Nitration | ESI-MS | Horse heart (peroxynitrite treatment); osteoclasts | Reduced mitochondrial respiration | 135, 137 |
| Lys72 (yeast numbering) | Trimethylation | Radioactive labeling | Fungi/plants | Helps Cytc import into the mitochondria, prevents apoptosis, and improves protein stability against proteolytic degradation | 98, 102, 103, 152, 153 |
| Tyr74 | Nitration | MALDI-TOF-MS | Recombinant human (peroxynitrite treatment) | Increased cardiolipin peroxidase activity and suppressed caspase-9 activity | 124 |
| Met80 | Nitrosylation | EPR spectroscopy | Horse heart (reaction with NO) | Increased cardiolipin peroxidase activity | 142, 143 |
| Met80 | Sulfoxidation | MALDI-TOF-MS | Horse heart (oxidative stress) | Increased cardiolipin peroxidase activity | 118, 139 |
| Tyr97 | Phosphorylation | IMAC/nano-LC/ESI-MS | Bovine heart; porcine brain (after insulin treatment) | Lower COX activity; reduced Cytc release and apoptosis after ischemia/reperfusion when treated with insulin | 53, 59 |
ESI-MS/MS, electronspray ionization MS/MS; EPR, electron paramagnetic resonance; HPLC/MS/MS, high-performance liquid chromatography tandem mass spectrometry; MALDI-TOF-MS, matrix-assisted laser desorption ionization-time of flight-MS.
CYTc PHOSPHORYLATION
Most reports that studied functional effects of PTMs focused on Cytc phosphorylation. Protein phosphorylation and dephosphorylation are critical cell-signaling mechanisms essential for many cellular functions. In the past, mitochondria were viewed as relatively independent organelles, and not much interest was paid to their regulation by cell signaling. Despite the fact that there are a few long-known examples demonstrating that mitochondrial metabolism is decisively regulated via PTMs, such as the pyruvate dehydrogenase complex by pyruvate dehydrogenase kinases, which was discovered ∼50 yr ago (46), only recently have mitochondria been recognized as hubs for cell signaling. At the level of the ETC, >100 phosphorylation sites have been mapped, but the functions of most of them remain unknown (47). Of the few sites with a known function, most have been reported for Cytc and its partner COX. Examples for COX include subunit I phosphorylation of Tyr304 mediated by the cAMP-dependent and TNF-α pathways (48, 49), and COX subunit II phosphorylation by Src kinase and receptor tyrosine kinases EGFR and ERBB2, although the sites remain unknown (50–52). On mammalian Cytc, 4 phosphorylation sites have been mapped by mass spectrometry. These are Tyr97, Tyr48, Thr28, and Ser47, which appear to be present in a highly tissue-specific manner and are discussed below in the sequence of their identification. All 4 sites are located on the right side of the molecule in the conventional view (Fig. 2) and are conserved in mammals (Fig. 3).
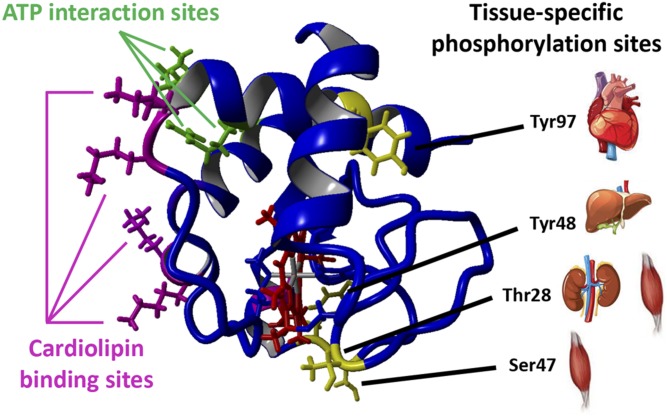
Figure 2 .
Tissue-specific phosphorylation sites are located on the right side of Cytc, whereas cardiolipin and ATP binding sites are located on the left side. Crystallographic data from oxidized rat Cytc were used (76) and analyzed with the molecular-graphics, -modeling and -simulation program YASARA (http://www.yasara.org/). Cytc is shown in the conventional view. The heme group is shown in red, and amino acids known to be phosphorylated in a tissue-specific manner are highlighted in yellow, together with the organs in which they have been identified under baseline condition. Amino acids implicated in ATP binding are Lys88, Arg91, and Glu62 (150) and are shown in green. Residues involved in cardiolipin binding are Lys87, Lys86, Lys73, Lys72 (A site), and Asn52 (C site) (5) and are shown in magenta. A third site for electrostatic interaction of Cytc with phospholipids consists of Lys22, Lys25, Lys27, His26, and His33 (151).
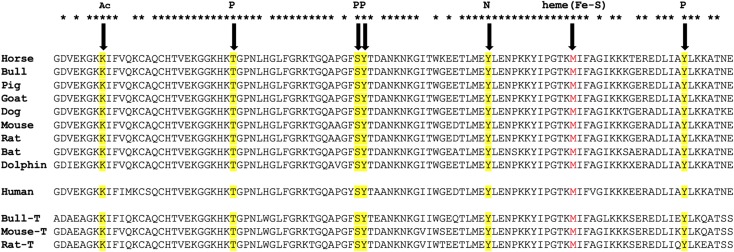
Figure 3 .
Sequence alignment of Cytc with highlighted post-translational modifications identified in mammals in vivo. Top, somatic Cytc from selected mammalian species with matching sequences of the corresponding testes-specific isoforms (T) where available (bottom). Note that humans express only a single ubiquitous Cytc (middle), which shares sequence homology with the somatic and testis isoforms. Ac, acetylation; P, phosphorylation. Met80 of the heme group shown in red is likely involved in sulfoxidation and nitrosylation as a result of nitro-oxidative stress. UniProt (https://www.uniprot.org/) Protein sequence accession numbers were F7D4V9 (horse); P62894 (bull); P62895 (pig); A0A0C4Y1X3 (goat); P00011 (dog); P62897 (mouse); P62898 (rat); S7QBN9 (bat); I6VH08 (dolphin); P99999 (human); Q3SZT9 (bull testes); P00015 (mouse testes); and P10715 (rat testes).
Tyr97 phosphorylation
In 2006, our lab reported for the first time that mammalian Cytc is phosphorylated in vivo (53). Cytc was isolated from bovine heart under conditions that preserve in vivo phosphorylation in the presence of the phosphatase inhibitor vanadate, a nonspecific tyrosine phosphatase inhibitor; fluoride, a nonspecific serine/threonine phosphatase inhibitor; and EGTA, a calcium chelator that prevents the activation of calcium-dependent protein phosphatases. Analysis with immobilized metal affinity chromatography/nanoliquid chromatography/electrospray ionization mass spectrometry (IMAC/nano-LC/ESI-MS) showed that Tyr97 is phosphorylated. Spectral analysis of the protein showed a shift of the characteristic 695 nm peak to 687 nm, which was reversed by alkaline phosphatase treatment, indicating that the shift is due to phosphorylation. The 695 nm absorption band results from the heme Fe-S (Met80) bond, and a shift suggests a conformational change in the heme environment that potentially affects its function. The absence of the 695 nm peak is pathologic because it suggests a misfolded, dysfunctional conformation of Cytc (54).
The in vivo phosphorylated Cytc demonstrated sigmoidal kinetics in the reaction with COX, as compared with the phosphatase-treated dephosphorylated Cytc, which produced a hyperbolic response, shifting the Km value of COX for Cytc from 5.5 to 2.5 μM. This finding indicates that phosphorylation leads to an inhibition of the reaction with COX when Cytc concentrations are not saturated. However, maximum turnover was similar for both the phosphorylated and nonphosphorylated forms of the protein (53). The differences in enzyme kinetics and spectral changes indicate that phosphorylation of Tyr97, located on the opposite site of the heme crevice (Fig. 2), affects the function of the enzyme.
Tyr97 is located in a relatively hydrophobic area in Cytc, called the “right channel,” which contributes to the interaction with lipids (55). The location of Tyr97 between this channel and the heme moiety could provide a pathway for electron transfer when Cytc functions as a cardiolipin peroxidase. Previously identified phosphorylation sites—Tyr304 on subunit I of COX (48, 49) and Tyr97 in cow heart Cytc—share a similar sequence motif, and this phosphoepitope is unique to Cytc and the COX subunit I in the ETC, suggesting that both sites are targeted by the same kinase/phosphatase. Cytc Lys7, a key residue involved in apoptosome formation, is located next to Tyr97 (56). The effect of Tyr97 phosphorylation on apoptosis has not yet been analyzed. However, Tyr97 phosphomimetic Cytc showed only a small reduction in caspase-9 activity in vitro (57, 58).
Another study showed that Tyr97 is phosphorylated in the brain after neuroprotective treatment with insulin in the context of brain ischemia–reperfusion injury (59). The study also showed that Cytc is not phosphorylated after global brain ischemia, but for brain Cytc, the basal phosphorylation state remains unknown. Insulin-treated rats showed an increase in neuronal survival and reduction of Cytc release from the mitochondria, suggesting that insulin-mediated Tyr97 phosphorylation is a mechanism that limits apoptotic cell death during ischemia-reperfusion (59, 60). These findings are consistent with our model (Fig. 4), proposing that Cytc is phosphorylated under normal physiological conditions. During stress, Cytc becomes dephosphorylated, which allows maximum flux in the ETC. However, in the context of ischemia, absence of oxygen leads to a cellular energy crisis, and the ETC becomes primed for hyperactivity, but because of the lack of its terminal substrate, oxygen, the ETC can resume function only when blood flow is restored to the ischemic tissue, leading to mitochondrial membrane potential (ΔΨm) hyperpolarization and excessive ROS production at complexes I and III, which initiates the apoptotic cascade.
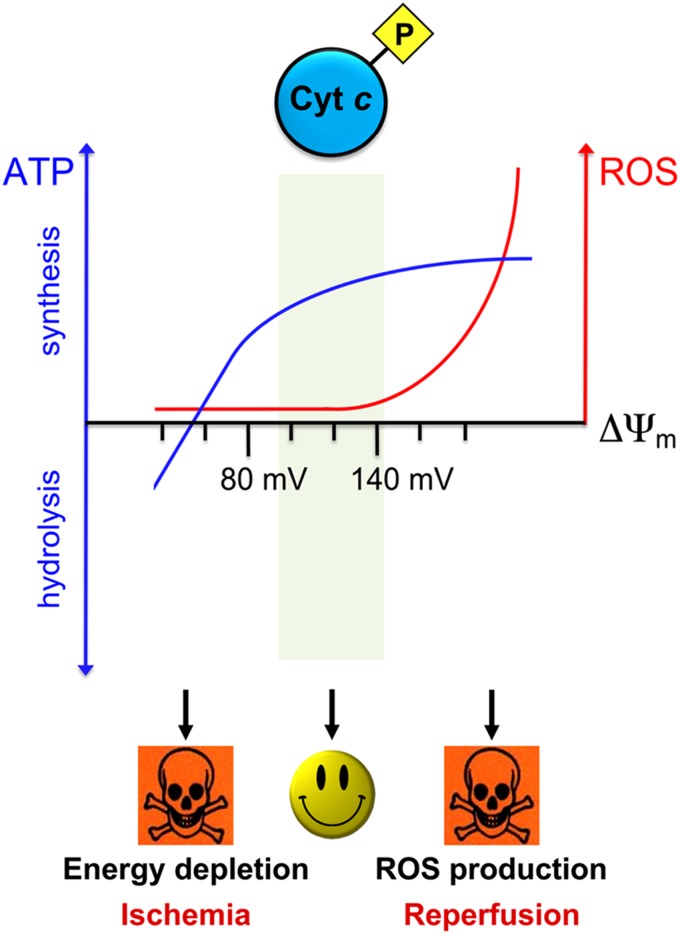
Figure 4 .
A model of the connection of the phosphorylation state of Cytc in the regulation of electron flux in the ETC, ΔΨm, and ROS production. In normal conditions (middle) Cytc is phosphorylated, which lowers electron flux in the ETC, maintaining healthy intermediate ΔΨm levels, which are sufficient for effective ATP generation, but limit the production of ROS that are generated exponentially at ΔΨm levels exceeding 140 mV. During stress conditions, such as ischemia (left), the tissue becomes energy depleted because of the lack of oxygen, leading to dephosphorylation of Cytc and other mitochondrial proteins. This occurrence renders the ETC primed for hyperactivation. When the tissue is reperfused and oxygen re-enters the cells, the ETC generates high ΔΨm levels, leading to ROS production and initialization of cell death cascades, adding to the damage already generated during the ischemic phase.
Tyr48 phosphorylation
This site was first mapped on Cytc purified from cow liver. Both Tyr48 and Tyr97 are conserved in mammals (Fig. 3) and eukaryotes in general (61), in which tyrosine phosphorylation is an important regulatory mechanism in many processes ranging from hormone responses, to cellular growth, to metabolism. Tyr48 phosphorylation resulted in a ∼50% decrease in maximum turnover when analyzed with cow liver COX (62). This result suggests that Tyr48 phosphorylation has a more profound impact on controlling COX activity of Cytc than does Tyr97. Tyr97 phosphorylation produced sigmoidal kinetics, however, maximum turnover rates were similar for the phosphorylated and nonphosphorylated forms (53, 62). In contrast, Tyr48 phosphorylated Cytc produced hyperbolic kinetics. Tyr48 is located on the lower median frontal area of the molecule in proximity to the heme crevice (Fig. 2). It is also important to note that Tyr48 is located in the 40–57 Ω loop, which requires the least free energy to be unfolded (63, 64). Both Tyr48 and Tyr97 residues showed NMR signal intensity changes after cardiolipin binding, whereas Tyr67 and Tyr74 did not, suggesting that changes in Tyr48 and Tyr97 more strongly affect the molecular dynamics of Cytc (65).
The functional effects of Tyr48 phosphorylation were further characterized by generation of a phosphomimetic Cytc mutant, Tyr48Glu, which carries a negative charge similar to that of the phosphate group, and a control mutant Tyr48Phe, which cannot be phosphorylated on this site. Similar to in vivo Tyr48 phosphorylation, the phosphomimetic Tyr48Glu mutant showed a reduced maximum turnover rate compared to the wild type (WT), suggesting that it is a useful model (66). The reduction of maximum turnover in the reaction with COX was less pronounced with heart COX than with liver COX, most likely because of the presence of 3 heart-specific COX subunit isoforms (VIa-H, VIIa-H, and VIII-H) (67). Furthermore, a higher Km was observed, suggesting a lower binding affinity toward heart COX (66). In addition to the commonly used glutamate and aspartate replacements to simulate phosphorylation, Guerra-Castellano et al. (68) recently introduced p‐carboxymethyl‐l‐phenylalanine as a replacement for tyrosine residues that can be phosphorylated. This substitution not only introduces a negative charge similar to glutamate and aspartate, it also better mimics the tyrosine side chain spatially, because of the presence of the phenyl ring.
The phosphomimetic Tyr48Glu substitution resulted in a lower redox potential by 45 mV, possibly because of the perturbation of the heme crevice and stabilization of the iron in its oxidized state. A midpoint redox potential lower than that of cytochrome c1 in the bc1 complex suggests that Tyr48 phosphorylation controls and reduces the electron transfer rate of Cytc at the bc1 complex. This reduction may cause Cytc to take part in other functions such as ROS scavenging (24). The phosphomimetic Tyr48Glu Cytc demonstrated significant effects on the apoptotic function of Cytc by decreasing cardiolipin peroxidase activity and, strikingly, by abolishing downstream caspase-3 activation (66). A study using the artificial phosphomimetic amino acid, p-carboxymethyl phenylalanine, substituted for Tyr48, showed an ∼70% reduction in caspase 3 activity, whereas cardiolipin peroxidase activity did not change when data were normalized for baseline peroxidase activity in the absence of cardiolipin (64). This study also concluded that Tyr48pCMF Cytc reduces ETC activity compared to WT protein.
Cytc mutations are very rare in humans and to date, there are only 2 known amino acids with a disease-causing mutation. The first reported mutation causes a Gly41Ser replacement (69), which renders Cytc more susceptible to Met80 oxidation by H2O2, generating a Met80 sulfoxide (70). The second reported mutation was Tyr48His in patients from an Italian family (63). Both substitutions cause the same mild disease, thrombocytopenia, which is a condition that results in lower blood platelet levels. In patients with both Gly41Ser and Tyr48His mutant Cytc, mitochondria were morphologically normal, but platelet counts were reduced by about half. The replacement of Tyr48 by the positively charged histidine caused several functional changes, including reduced oxygen consumption rate and increased apoptosis (63). A recent study on the Tyr48His variant showed that the pentacoordinated form of Cytc, which promotes cardiolipin peroxidase activity, is more prevalent in the mutant, further supporting the increased apoptotic activity (71). Molecular dynamics simulations concluded that both Gly41Ser and Tyr48His mutations result in a less stable and more open protein structure, which would promote cardiolipin peroxidase activity (72). At this point, it is unclear why these mutations cause such a cell-type–specific phenotype. For the Tyr48His mutation it is even more surprising that no liver phenotype has been reported, because this mutation would prevent liver Cytc from being Tyr48 phosphorylated and consequently make liver cells more prone to apoptosis.
Thr28 phosphorylation
Thr28 was first identified as being phosphorylated in human skeletal muscle by a high-throughput phosphoproteomics study (73). An independent high-throughput study of the same tissue detected Thr28 and Ser47 phosphorylation (74). Furthermore, in 2015 Hoffman and colleagues (75) found Thr28 and Ser47 phosphorylation in human skeletal muscle after exercise. However, no functional experiments were conducted in any of these studies.
We recently identified Thr28 phosphorylation in mammalian kidney (76). Over 80% of the Cytc pool isolated from bovine kidney carried this PTM, suggesting that it is biologically relevant. Thr28-phosphorylated and recombinant Thr28Glu phosphomimetic Cytc exhibited inhibition of COX activity, by 50 and 73%, respectively. The finding that phosphomimetic replacement leads to a more pronounced effect may be explained by the presence of some nonphosphorylated Cytc (∼20%) when isolated from kidney, whereas the recombinant phosphomimetic protein is homogeneous. Additional cell culture experiments were performed in WT and Thr28Glu Cytc transfected Cytc-knockout mouse lung fibroblasts to further validate these findings. The phosphomimetic Thr28Glu Cytc-expressing cells showed lower intact cell respiration, ΔΨm, and ROS levels. These data suggest that modification of Cytc controls ETC flux and can prevent ΔΨm hyperpolarization and ROS production. This important discovery is the first indication that a Cytc PTM can regulate the overall flux in the ETC. It fits our model where, in normal conditions, the activity of the ETC is downregulated or “controlled” to prevent ΔΨm hyperpolarization and consequent ROS production (Fig. 4). Furthermore, AMPK has been identified as the mediator of this site-specific phosphorylation by both in vitro and pharmacological approaches, making it one of the few examples where a phosphorylation site has been mapped with a larger fraction of the protein containing the PTM, together with the kinase and functional effects. In contrast to other tissues, AMPK has a higher basal activity in kidney and may account for the finding that Cytc is phosphorylated under basal conditions in this organ.
Thr28 is present in a region of Cytc called the “negative classical gamma turn” (residues 27–29; Figs. 2 and 3) located near the heme crevice and is a part of the COX binding domain of Cytc. Structural analysis of Cytc has shown that the residues present in this turn have the highest root-mean-square deviations of the whole Cytc molecule, suggesting that this is the most flexible element of the protein (76). However, Thr28 phosphorylation does not have an impact on apoptosome activity, because Thr28 is not part of the Apaf-1 binding domain of Cytc (76, 77). It would be interesting to analyze whether Cytc in skeletal muscle is targeted by AMPK (74, 75) or a different kinase. AMPK follows its more traditional role in muscle as an energy sensor and metabolic activator when energy levels drop, and it may seem counterintuitive to trigger this PTM, which partially inhibits the ETC, when increased mitochondrial activity would be needed.
Ser47 phosphorylation
Ser47 phosphorylation of Cytc was also mapped in the above phosphoproteomic studies of human skeletal muscle along with Thr28 (74, 75), but it was not identified in a third study on the same tissue (73). In vitro studies with phosphomimetic Ser47Asp Cytc suggest that it prevents caspase activity, opposite to the Thr28Asp phosphomimetic substitution, suggesting that this site is important for the regulation and execution of apoptosis (77).
Tissue-Specific Regulation Of Respiration And Apoptosis Through Reversible Phosphorylations: An Integrated Model
The fact that basal phosphorylations of Cytc are tissue specific—Tyr97 in heart, Tyr48 in liver, and Thr28 in kidney—may at first seem surprising. All 3 functionally characterized phosphorylations lead to partial inhibition of the reaction between Cytc and COX or “controlled respiration” (Fig. 4). Such a mechanism can help maintain healthy intermediate levels of the ΔΨm to prevent excessive ROS generation that takes place at high membrane potentials (ΔΨm > 140 mV). Proper regulation of the membrane potential is crucial because it is directly related to the production of ROS. It has been shown experimentally that ΔΨm levels exceeding 140 mV lead to an exponential increase in ROS production at ETC complexes I and III, whereas mitochondria of resting cells with an intermediate ΔΨm do not produce significant amounts of ROS (78–80). Studies performed in perfused rat hearts (81), lymphocytes (82), embryonic heart cells (83), intact cultured fibroblasts, and neuroblastoma cells (84) showed ΔΨm values in the range of 80–140 mV, which may resemble the healthy physiologic ΔΨm range in intact cells and organs under normal conditions. These results are favorable because maintenance of physiologically intermediate ΔΨm values prevents excessive ROS generation but provides the full capacity to produce ATP, because maximum ATP synthesis by ATP synthase takes place at a ΔΨm of 100–120 mV (85). Because the electron transfer reaction from Cytc to COX to oxygen is the proposed rate-limiting step of the ETC under physiological conditions (86–90), it is not surprising that it is highly regulated to prevent ΔΨm hyperpolarization and ROS generation under normal nonstressed conditions.
However, the situation is different with regard to apoptosis, where some PTMs seem to affect apoptosome formation and downstream caspase activation, whereas others do not. It is noteworthy that Cytc Tyr48 phosphorylation fully protects against caspase activation, as shown with phosphomimetic Cytc (64, 66), whereas Thr28 phosphorylation in kidney has no such effect. Liver cells are constantly bombarded with molecules that the organism takes up through the digestive system and even the lung (91). It may therefore seem reasonable to add another layer of protection, through Tyr48 phosphorylation, to prevent apoptosis in the relatively toxic environment to which liver cells are exposed. Other organs that are not in “direct” contact with the environment and instead face a mostly homeostatic milieu may not need an extra safeguarding mechanism.
CYTc METHYLATION
In histones, the ε-NH2 group of lysine residues can be mono-, di-, or trimethylated, and such modifications regulate many biological functions, such as gene regulation and signal transduction (92). In general, lysine, arginine, histidine, and dicarboxylic amino acids are able to accept a methyl group to their side chains. Methylation occurs in a species-specific manner. In mammals, arginine and lysine methylation are found, and in humans they occur at a ratio of ∼4:1 for Arg:Lys (93). However, of the 18 lysine and 2 arginine residues present in human Cytc (Fig. 3), none was found to be modified (93). As discussed below, Cytc methylation appears to be important in some organisms other than mammals, where it occurs on lysine residues.
Under physiological conditions the unmodified lysine side chain amino group with a side-chain pKa of 10.5 is protonated and carries a positive charge, which is essential for ionic interactions between a protein with other molecules including proteins, DNA, RNA, and metabolites. Addition of methyl groups further increases the pKa modifications of the ε-amino group of lysine residues resulting in a remarkable chemical diversity of ∼20 different modifications of this amino acid, including mono-, di-, and trimethylation, acetylation, and ubiquitination (94).
It has been shown in Saccharomyces cerevisiae and in plants that apocytochrome c expressed from the CYC1 gene is immediately trimethylated by the Cytc methyltransferase Ctm1p in the cytosol at an evolutionarily conserved site, Lys78, often referred to as Lys72, based on the corresponding homologous residue in vertebrates (95–97). Functionally, it has been shown that the import of methylated yeast Cytc into yeast mitochondria is much greater than the import of nonmethylated Cytc (98). However, this effect was not observed with rat liver mitochondria. It is possible that in yeast Cytc is imported into the mitochondria through a methylation-dependent receptor mechanism. Earlier work showed that, in the fungus class Ascomycetes, methylation of Cytc Lys72 maintains the binding of the hemoprotein to mitochondria and the researchers concluded that methylation of the protein is essential for its physiological functions, including electron transport through the ETC (99). More recently, it has been demonstrated that methylation of yeast Cytc (Cyc1p) increases its interactions with Erv1p and the Cytc heme lyase Cyc3p, further supporting the role of methylation in the import of apocytochrome c to yeast mitochondria (100). Metazoan Cytc, methylated at Lys72 after expression in yeast, completely prevented proapoptotic activity of Cytc by lowering the binding affinity toward Apaf-1 (101).
Structurally, methylation of yeast Lys-72 Cytc increases protein stability in vivo against proteolytic degradation (102, 103) and may modulate its geometry, isoelectric point, or conformation (104–106). In addition, a structural analysis demonstrated that trimethyllysine 72 is positioned near the heme crevice loop (Ω loop D, residues 70–85) and plays a crucial role in opening the crevice, suggesting a potential role in regulating peroxidase activity during apoptosis (107).
CYTc ACETYLATION
Acetylation of lysine side chains is another important PTM catalyzed by lysine acetyltransferases that use acetyl-CoA as a substrate for transfer of its acetyl moiety to the ε-group of a lysine residue. Acetylation also occurs nonenzymatically, especially in the mitochondrial matrix where high acetyl-CoA concentrations and a more basic pH favor the transfer of the acetyl group and make it the most prevalent PTM (94). Recent studies have confirmed that the mitochondrial proteome is highly acetylated, with more than 60% of proteins analyzed having 1 or more acetylation sites (108–111). Lysine acetylation leads to neutralization of the positive charge related to amide bond formation, increasing hydrophobicity, and spatial layout of the lysine side chain. In vitro acetylation of horse heart Cytc was shown to abolish the H2O2 scavenging function of Cytc (22). The only reported in vivo acetylation site of mammalian Cytc is Lys8 (Fig. 3), which was identified in a high-throughput proteomics study in mouse liver mitochondria when animals were unfed but not when they were fed (112). The extent of acetylation, the functional effect, and specificity—enzymatic vs. nonenzymatic acetylation—remain unknown.
CYTc SULFOXIDATION
H2O2 is one of the main reactive oxygen species involved in oxidative stress. One established modification of Cytc in response to increased oxidative stress is Met80 sulfoxidation (5). Cytc, when bound to cardiolipin, is more accessible to H2O2. Another characteristic change in Cytc upon cardiolipin binding is a significant negative shift in the redox potential (350–400 mV) (113). Typically, a redox potential in the range of 220–270 mV is observed for Cytc in mammals (114, 115). The shift in redox potential caused by cardiolipin binding suggests that it is thermodynamically unfeasible for cardiolipin-bound Cytc to engage as an efficient electron carrier in the ETC. Furthermore, it makes Cytc incapable of acting as a ROS scavenger (113). In Cytc–cardiolipin complexes, submillimolar H2O2 concentrations oxidize the sulfur atom of the Met80 axial ligand. Therefore, Met80 sulfoxidation of Cytc significantly enhances peroxidase activity of Cytc, promoting Cytc release and apoptosis (116, 117).
Cytc sulfoxidation is difficult to detect and study in vivo. However, Wang et al. (118) functionally studied this modification by creating a mutant that models the conformation of Cytc when bound to cardiolipin. To create this mutant, 2 residues (Val83 and Gly84) from the loop containing Met80 were deleted. Through mass spectrometry, this mutant was confirmed to be specifically autoxidized to methionine sulfoxide when purified in the presence of molecular oxygen. Functional changes, such as increased cardiolipin peroxidase activity and Cytc release with regard to Met80 modifications, were also found with the Met80Ala-Cytc mutant (119).
CYTc TYROSINE NITRATION
Tyrosine nitration is a PTM that takes place in the presence of a reaction between superoxide and NO that forms peroxynitrite, which acts as the nitration agent (120). In contrast to phosphorylation, tyrosine nitration is a stable PTM. It has been estimated that only 1–5 of 10,000 tyrosine residues are nitrated in a protein under inflammatory conditions in vivo. Given the very low frequency of this modification of <0.1%, mapping nitrated tyrosine residues has been a challenging process (121). The first protein identified as nitrated in vivo was manganese superoxide dismutase (122, 123). Human Cytc has 5 tyrosine residues at sites 46, 48, 67, 74, and 97, of which 4 are evolutionarily conserved in mammals (61, 124). Of the 5 tyrosine residues, only 3 (Tyr67, Tyr74, and Tyr97) are nitrated in vitro in the presence of peroxynitrite (125). Tyr67- and Tyr74-nitrated Cytc are less efficient in restoring respiratory capacity in Cytc-deficient mitochondria compared to Tyr97 (125, 126). Tyr48, which is highly conserved, and Tyr46, which is present only in humans and plants, were the least commonly nitrated residues (127).
Tyrosine nitration of Cytc has been reported under several conditions in vivo. In a renal ischemia–reperfusion model, Cytc was nitrated 3 h after the ischemic insult, as confirmed by nitrotyrosine immunoprecipitation (128). Furthermore, an increased release of nitrated Cytc was observed in brain tissue sections of rats exposed to ischemia-reperfusion (129). Another study suggested that tyrosine nitration of Cytc was induced by chemoradiotherapies, such as 5-fluorouracil, cis-diaminedicholorplatinum, and γ-rays (130). However, the researchers reported that nitration of Cytc alone does not suppress apoptosis in squamous cell carcinoma cells, probably because only a small fraction of Cytc carries the modification. Cytc nitration was also observed in a rat model of chronic allograft nephropathy 4 wk after transplantation, suggesting that nitration of Cytc occurs during kidney injury (131). However, no specific nitration site has been reported under physiological conditions, suggesting that most of these modifications are relatively nonspecific and driven by nitro-oxidative stress. Furthermore, some of the observations reported in vivo do not mirror the functional studies performed with nitrated Cytc in vitro because most in vitro studies conclude that nitration prevents caspase activation (124, 132, 133). This again is likely to be explained by the small portion of Cytc that is nitrated in vivo, even under severe stress conditions. Because tyrosine residues can also be phosphorylated, it should be noted that tyrosine nitration and phosphorylation of the same residue in a protein are mutually exclusive events (134).
Tyr67 nitration
Several in vitro studies have analyzed peroxynitrite-mediated nitration of Tyr67 and reported changes in redox function, cardiolipin peroxidation, and respiration (135, 136). Furthermore, spectrophotometric studies of Tyr67-nitrated Cytc have shown a loss of the 695 nm band, indicating the disruption of the axial Fe-S (Met80) bond. This finding makes sense because Tyr67 is adjacent to the heme group of Cytc and points toward the Fe-Met80 bond. A study using osteoclasts confirmed Tyr67 nitration by mass spectrometry (137). Tyr67-nitrated Cytc was then proposed to be less prone to apoptosis due to intramolecular hydrogen bonding that promotes stronger interactions with the cardiolipin phosphates, making Cytc release less favorable. Cardiolipin peroxidase activity studies have demonstrated that the Tyr67Phe mutation leads to a partial loss of peroxidase activity, suggesting that this conserved tyrosine could be the reactive tyrosyl radical involved in the oxygenase half reaction of Cytc-cardiolipin peroxidation (65). Finally, studies with human Cytc in which all tyrosines except for Tyr67 were replaced with phenylalanine showed >50% decreased caspase activation at saturating Cytc concentrations after in vitro nitration of Tyr67 (126).
Tyr74 nitration
Tyr74 is fully surface exposed and the most commonly nitrated tyrosine residue in Cytc. An in vitro study showed that Tyr74 nitration results in increased cardiolipin peroxidase activity (124). However, this mutant also prevents downstream caspase-9 activity. Peroxynitrite-mediated nitration of Tyr74 resulted in a disruption of the axial Fe-S (Met80) bond and was replaced by Lys72 as confirmed by NMR (138). Lys72 is a crucial residue for the interaction of Cytc with Apaf-1, which may explain the low caspase-9 activity (56). In vitro studies on Met80 sulfoxidation and Tyr74 nitration suggested that, in the presence of both of these modifications, the binding affinity for cardiolipin was 4 times higher than with WT Cytc (116). Furthermore, Tyr74 nitration leads to a lower midpoint redox potential, interfering with electron transfer (139).
Tyr46 and Tyr48 nitration
There is no current evidence of in vivo nitration at Cytc residues Tyr46 and Tyr48, even though they are solvent exposed and located close to the heme group that can catalyze nitration. Tyr46 is only present in human and plant Cytc, whereas Tyr48 is conserved in Cytc across all species. In vitro studies showed that Tyr46 and Tyr48 nitration promote the formation of the heme iron pentacoordinated form, which increases peroxidase activity. Nitration of these residues also affects the surrounding heme propionates. Furthermore, the use of monotyrosine mutants of both sites results in rapid degradation of Cytc in Jurkat cell extracts, suggesting that these nitrations are not physiologically favorable (127). The same group also reported that neither nitration has an effect on the rate of electron transfer to COX. However, light-scattering experiments have shown that these modifications lead to poor interactions with Apaf-1, forming a nonfunctional apoptosome and reducing caspase-9 activity (133). Even though Tyr sites 74, 46, and 48 result in a reduction in caspase activity, most in vivo studies suggest that Cytc nitration is associated with increased apoptosis and mitochondrial dysfunction (128–131). As discussed above, the low stoichiometries of Tyr nitration in in vivo conditions make their biological effect debatable. In fact, Cytc tyrosine nitration may be a good model system to study the effect of phosphorylation related to the introduction of a negative charge and spatial requirements that are similar to those of a phosphate group. Looking at tyrosine nitration from this angle, it may not be surprising that in vitro data for tyrosine phosphorylation and nitration, as well as phosphomimetic substitution, show mostly consistent results.
CYTc NITROSYLATION
Nitrosylation involves the addition of an NO group to a free cysteine or a transition metal. Therefore, it takes place in a limited number of proteins in vivo (140, 141). Because Cytc has no free cysteines, its only target of nitrosylation is the heme group. A few studies have shown that Cytc can be nitrosylated in vitro and in U-937 monocytic cells (142–144). Nitrosylated Cytc was found to be mostly present in the cytosol. However, when antiapoptotic proteins such as Bcl-2 and -XL were overexpressed, most of the nitrosylated Cytc was found in the mitochondria. The researchers concluded that nitrosylation of Cytc may play a role in apoptosis regulation (144). Endogenous heme nitrosylation is a fairly rare event that is observed in only a few other proteins, such as COX and guanylyl cyclase, the only known receptor for NO (145–148). In both COX and guanylyl cyclase, the heme iron is pentacoordinated, unlike in Cytc, where it is hexacoordinated (149). Because Cytc can undergo a conformational change into a pentacoodinated form, it may be nitrosylated in conditions when the Fe-S (Met80) bond is displaced, leading to greater cardiolipin peroxidase activity.
CONCLUSIONS
Cytc plays a significant role in balancing cellular life- and death-related functions. Both the primary functions of Cytc—respiration leading to energy production and apoptosis leading to cell death—are regulated to meet tissue- and organ-specific needs. Therefore, Cytc has tissue-specific phosphorylations that regulate respiration, apoptosis, and ROS production and scavenging. For instance, the liver-specific Tyr48 phosphorylation abolishes apoptosis, which is sensible, given that liver cells are exposed to toxic molecules primarily through food uptake. Therefore, these cells have a higher threshold for cell death commitment, supporting the concept that Tyr48 is an important regulatory site for apoptosis. In contrast, the Thr28 phosphorylation observed in kidney results in the same level of apoptotic activity as unmodified WT Cytc, suggesting that an apoptosis-suppressing mechanism is not needed in this organ. However, Thr28 phosphorylation and all other phosphorylations studied to date indirectly protect the tissues from ROS damage where they are found by reducing mitochondrial respiration and thus the ΔΨm. Maintenance of intermediate ΔΨm prevents excessive ROS generation but allows efficient energy production. However, during cellular stress such as ischemia-reperfusion, which is found under pathologic conditions in many organs including the heart, kidney, and brain, Cytc becomes dephosphorylated, resulting in ΔΨm hyperpolarization (>140 mV) during reperfusion and a consequent burst of ROS, adding to the tissue damage and death already generated during the ischemic phase (Fig. 4).
When interpreting the biological significance of PTMs identified on Cytc the stoichiometry has to be considered. Some PTMs such as nitrations of Cytc have been reported under several pathological conditions. These modifications tend to take place in a nonspecific manner and can be attributed as spontaneous chemical reactions to nitro-oxidative stress. In mammals, under normal physiological conditions only tissue-specific phosphorylation of Cytc has exceeded 50% of the Cytc pool. Methylation of Cytc, even though important in yeast and plants, has not been reported to be physiologically relevant in mammalian species. Acetylation could be an interesting regulatory modification given that the mammalian mitochondrial proteome is highly acetylated, and a specific Cytc acetylation site has been identified in mouse liver of unfed animals. In the future, an in-depth understanding of PTMs of Cytc will necessitate the identification of signaling molecules, including kinases, phosphatases, and scaffolding proteins, that mediate cell signaling in a tissue-specific manner.
ACKNOWLEDGMENTS
This work was supported by the U.S. National Institutes of Health (NIH), Institute of General Medical Sciences Grant R01 GM116807, and NIH National Institute of Neurological Disorders and Stroke Grants R01 NS091242, and R42 NS105238; the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program under Award W81XWH-16-1-0175; and the Karmanos Cancer Institute Prostate Cancer Research Team (PCRT) Postdoctoral Award Program. Opinions, interpretations, conclusions, and recommendations are those of the authors, and are not necessarily endorsed by the funding agencies including the U.S. Department of Defense or the NIH. The authors declare no conflicts of interest.
Glossary
- Apaf
apoptosis activating factor
- COX
cytochrome c oxidase
- Cytc
cytochrome c
- ΔΨm
mitochondrial membrane potential
- Erv
essential for respiration and viability
- ETC
electron transport chain
- IMAC/nano-LC/ESI-MS
immobilized metal affinity chromatography/nanoliquid chromatography/electrospray ionization mass spectrometry
- Mia
mitochondrial intermembrane space import and assembly
- PTM
post-translational modification
- ROS
reactive oxygen species
- WT
wild type
AUTHOR CONTRIBUTIONS
H. A. Kalpage, V. Bazylianska, N. Mantena, and M. Hüttemann designed the figures; and all authors wrote, edited, and approved the final version of the manuscript.
REFERENCES
- 1.Li, K., Li, Y., Shelton, J. M., Richardson, J. A., Spencer, E., Chen, Z. J., Wang, X., Williams, R. S. (2000) Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 101, 389–399 [DOI] [PubMed] [Google Scholar]
- 2.Morriss, G. M., New, D. A. (1979) Effect of oxygen concentration on morphogenesis of cranial neural folds and neural crest in cultured rat embryos. J. Embryol. Exp. Morphol. 54, 17–35 [PubMed] [Google Scholar]
- 3.Vempati, U. D., Han, X., Moraes, C. T. (2009) Lack of cytochrome c in mouse fibroblasts disrupts assembly/stability of respiratory complexes I and IV. J. Biol. Chem. 284, 4383–4391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belikova, N. A., Vladimirov, Y. A., Osipov, A. N., Kapralov, A. A., Tyurin, V. A., Potapovich, M. V., Basova, L. V., Peterson, J., Kurnikov, I. V., Kagan, V. E. (2006) Peroxidase activity and structural transitions of cytochrome c bound to cardiolipin-containing membranes. Biochemistry 45, 4998–5009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagan, V. E., Bayir, H. A., Belikova, N. A., Kapralov, O., Tyurina, Y. Y., Tyurin, V. A., Jiang, J., Stoyanovsky, D. A., Wipf, P., Kochanek, P. M., Greenberger, J. S., Pitt, B., Shvedova, A. A., Borisenko, G. (2009) Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic. Biol. Med. 46, 1439–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajagopal, B. S., Edzuma, A. N., Hough, M. A., Blundell, K. L., Kagan, V. E., Kapralov, A. A., Fraser, L. A., Butt, J. N., Silkstone, G. G., Wilson, M. T., Svistunenko, D. A., Worrall, J. A. (2013) The hydrogen-peroxide-induced radical behaviour in human cytochrome c-phospholipid complexes: implications for the enhanced pro-apoptotic activity of the G41S mutant. Biochem. J. 456, 441–452 [DOI] [PubMed] [Google Scholar]
- 7.Rytömaa, M., Kinnunen, P. K. (1994) Evidence for two distinct acidic phospholipid-binding sites in cytochrome c. J. Biol. Chem. 269, 1770–1774 [PubMed] [Google Scholar]
- 8.Hagihara, Y., Tan, Y., Goto, Y. (1994) Comparison of the conformational stability of the molten globule and native states of horse cytochrome c. Effects of acetylation, heat, urea and guanidine-hydrochloride. J. Mol. Biol. 237, 336–348 [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Paggi, D., Hannibal, L., Castro, M. A., Oviedo-Rouco, S., Demicheli, V., Tórtora, V., Tomasina, F., Radi, R., Murgida, D. H. (2017) Multifunctional cytochrome c: learning new tricks from an old dog. Chem. Rev. 117, 13382–13460 [DOI] [PubMed] [Google Scholar]
- 10.Martínez-Fábregas, J., Díaz-Moreno, I., González-Arzola, K., Díaz-Quintana, A., De la Rosa, M. A. (2014) A common signalosome for programmed cell death in humans and plants. Cell Death Dis. 5, e1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giorgio, M., Migliaccio, E., Orsini, F., Paolucci, D., Moroni, M., Contursi, C., Pelliccia, G., Luzi, L., Minucci, S., Marcaccio, M., Pinton, P., Rizzuto, R., Bernardi, P., Paolucci, F., Pelicci, P. G. (2005) Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233 [DOI] [PubMed] [Google Scholar]
- 12.Migliaccio, E., Mele, S., Salcini, A. E., Pelicci, G., Lai, K. M., Superti-Furga, G., Pawson, T., Di Fiore, P. P., Lanfrancone, L., Pelicci, P. G. (1997) Opposite effects of the p52shc/p46shc and p66shc splicing isoforms on the EGF receptor-MAP kinase-fos signalling pathway. EMBO J. 16, 706–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Migliaccio, E., Giorgio, M., Mele, S., Pelicci, G., Reboldi, P., Pandolfi, P. P., Lanfrancone, L., Pelicci, P. G. (1999) The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature 402, 309–313 [DOI] [PubMed] [Google Scholar]
- 14.Francia, P., delli Gatti, C., Bachschmid, M., Martin-Padura, I., Savoia, C., Migliaccio, E., Pelicci, P. G., Schiavoni, M., Lüscher, T. F., Volpe, M., Cosentino, F. (2004) Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 110, 2889–2895 [DOI] [PubMed] [Google Scholar]
- 15.Orsini, F., Migliaccio, E., Moroni, M., Contursi, C., Raker, V. A., Piccini, D., Martin-Padura, I., Pelliccia, G., Trinei, M., Bono, M., Puri, C., Tacchetti, C., Ferrini, M., Mannucci, R., Nicoletti, I., Lanfrancone, L., Giorgio, M., Pelicci, P. G. (2004) The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. J. Biol. Chem. 279, 25689–25695 [DOI] [PubMed] [Google Scholar]
- 16.Pinton, P., Rimessi, A., Marchi, S., Orsini, F., Migliaccio, E., Giorgio, M., Contursi, C., Minucci, S., Mantovani, F., Wieckowski, M. R., Del Sal, G., Pelicci, P. G., Rizzuto, R. (2007) Protein kinase C beta and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science 315, 659–663 [DOI] [PubMed] [Google Scholar]
- 17.Lebiedzinska, M., Duszynski, J., Rizzuto, R., Pinton, P., Wieckowski, M. R. (2009) Age-related changes in levels of p66Shc and serine 36-phosphorylated p66Shc in organs and mouse tissues. Arch. Biochem. Biophys. 486, 73–80 [DOI] [PubMed] [Google Scholar]
- 18.Sun, L., Xiao, L., Nie, J., Liu, F. Y., Ling, G. H., Zhu, X. J., Tang, W. B., Chen, W. C., Xia, Y. C., Zhan, M., Ma, M. M., Peng, Y. M., Liu, H., Liu, Y. H., Kanwar, Y. S. (2010) p66Shc mediates high-glucose and angiotensin II-induced oxidative stress renal tubular injury via mitochondrial-dependent apoptotic pathway. Am. J. Physiol. Renal Physiol. 299, F1014–F1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arany, I., Clark, J. S., Reed, D. K., Juncos, L. A., Dixit, M. (2013) Role of p66shc in renal toxicity of oleic acid. Am. J. Nephrol. 38, 226–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arany, I., Clark, J. S., Reed, D., Szabó, I., Ember, I., Juncos, L. A. (2013) The role of p66shc in taxol- and dichloroacetic acid-dependent renal toxicity. Anticancer Res. 33, 3119–3122 [PubMed] [Google Scholar]
- 21.Arany, I., Clark, J., Reed, D. K., Juncos, L. A. (2013) Chronic nicotine exposure augments renal oxidative stress and injury through transcriptional activation of p66shc. Nephrol. Dial. Transplant. 28, 1417–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korshunov, S. S., Krasnikov, B. F., Pereverzev, M. O., Skulachev, V. P. (1999) The antioxidant functions of cytochrome c. FEBS Lett. 462, 192–198 [DOI] [PubMed] [Google Scholar]
- 23.Wang, Z. B., Li, M., Zhao, Y., Xu, J. X. (2003) Cytochrome C is a hydrogen peroxide scavenger in mitochondria. Protein Pept. Lett. 10, 247–253 [DOI] [PubMed] [Google Scholar]
- 24.Pereverzev, M. O., Vygodina, T. V., Konstantinov, A. A., Skulachev, V. P. (2003) Cytochrome c, an ideal antioxidant. Biochem. Soc. Trans. 31, 1312–1315 [DOI] [PubMed] [Google Scholar]
- 25.Vitvitsky, V., Miljkovic, J. L., Bostelaar, T., Adhikari, B., Yadav, P. K., Steiger, A. K., Torregrossa, R., Pluth, M. D., Whiteman, M., Banerjee, R., Filipovic, M. R. (2018) Cytochrome c reduction by H2S potentiates sulfide signaling. ACS Chem. Biol. 13, 2300–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mills, G. C. (1991) Cytochrome c: gene structure, homology and ancestral relationships. J. Theor. Biol. 152, 177–190 [DOI] [PubMed] [Google Scholar]
- 27.Liu, Z., Lin, H., Ye, S., Liu, Q. Y., Meng, Z., Zhang, C. M., Xia, Y., Margoliash, E., Rao, Z., Liu, X. J. (2006) Remarkably high activities of testicular cytochrome c in destroying reactive oxygen species and in triggering apoptosis. Proc. Natl. Acad. Sci. USA 103, 8965–8970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hüttemann, M., Jaradat, S., Grossman, L. I. (2003) Cytochrome c oxidase of mammals contains a testes-specific isoform of subunit VIb: the counterpart to testes-specific cytochrome c? Mol. Reprod. Dev. 66, 8–16 [DOI] [PubMed] [Google Scholar]
- 29.Zhang, Z., Gerstein, M. (2003) The human genome has 49 cytochrome c pseudogenes, including a relic of a primordial gene that still functions in mouse. Gene 312, 61–72 [DOI] [PubMed] [Google Scholar]
- 30.Pierron, D., Opazo, J. C., Heiske, M., Papper, Z., Uddin, M., Chand, G., Wildman, D. E., Romero, R., Goodman, M., Grossman, L. I. (2011) Silencing, positive selection and parallel evolution: busy history of primate cytochromes C. PLoS One 6, e26269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt, T. R., Wildman, D. E., Uddin, M., Opazo, J. C., Goodman, M., Grossman, L. I. (2005) Rapid electrostatic evolution at the binding site for cytochrome c on cytochrome c oxidase in anthropoid primates. Proc. Natl. Acad. Sci. USA 102, 6379–6384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen, S., Balabanidou, V., Sideris, D. P., Lisowsky, T., Tokatlidis, K. (2005) Erv1 mediates the Mia40-dependent protein import pathway and provides a functional link to the respiratory chain by shuttling electrons to cytochrome c. J. Mol. Biol. 353, 937–944 [DOI] [PubMed] [Google Scholar]
- 33.Bihlmaier, K., Mesecke, N., Terziyska, N., Bien, M., Hell, K., Herrmann, J. M. (2007) The disulfide relay system of mitochondria is connected to the respiratory chain. J. Cell Biol. 179, 389–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boehning, D., Patterson, R. L., Sedaghat, L., Glebova, N. O., Kurosaki, T., Snyder, S. H. (2003) Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis [published correction appears in Nat. Cell Biol. (2004) 6, 77]. Nat. Cell Biol. 5, 1051–1061 [DOI] [PubMed] [Google Scholar]
- 35.Boehning, D., Patterson, R. L., Snyder, S. H. (2004) Apoptosis and calcium: new roles for cytochrome c and inositol 1,4,5-trisphosphate. Cell Cycle 3, 252–254 [PubMed] [Google Scholar]
- 36.Beresewicz, M., Kowalczyk, J. E., Zabłocka, B. (2006) Cytochrome c binds to inositol (1,4,5) trisphosphate and ryanodine receptors in vivo after transient brain ischemia in gerbils. Neurochem. Int. 48, 568–571 [DOI] [PubMed] [Google Scholar]
- 37.Elena-Real, C. A., Díaz-Quintana, A., González-Arzola, K., Velázquez-Campoy, A., Orzáez, M., López-Rivas, A., Gil-Caballero, S., De la Rosa, M. A., Díaz-Moreno, I. (2018) Cytochrome c speeds up caspase cascade activation by blocking 14-3-3ε-dependent Apaf-1 inhibition. Cell Death Dis. 9, 365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen, H. J., Betcher-Lange, S., Kessler, D. L., Rajagopalan, K. V. (1972) Hepatic sulfite oxidase: congruency in mitochondria of prosthetic groups and activity. J. Biol. Chem. 247, 7759–7766 [PubMed] [Google Scholar]
- 39.Velayutham, M., Hemann, C. F., Cardounel, A. J., Zweier, J. L. (2016) Sulfite oxidase activity of cytochrome c: role of hydrogen peroxide. Biochem. Biophys. Rep. 5, 96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Webb, M., Stonehuerner, J., Millett, F. (1980) The use of specific lysine modifications to locate the reaction site of cytochrome c with sulfite oxidase. Biochim. Biophys. Acta 593, 290–298 [DOI] [PubMed] [Google Scholar]
- 41.Nur-E-Kamal, A., Gross, S. R., Pan, Z., Balklava, Z., Ma, J., Liu, L. F. (2004) Nuclear translocation of cytochrome c during apoptosis. J. Biol. Chem. 279, 24911–24914 [DOI] [PubMed] [Google Scholar]
- 42.González-Arzola, K., Díaz-Moreno, I., Cano-González, A., Díaz-Quintana, A., Velázquez-Campoy, A., Moreno-Beltrán, B., López-Rivas, A., De la Rosa, M. A. (2015) Structural basis for inhibition of the histone chaperone activity of SET/TAF-Iβ by cytochrome c. Proc. Natl. Acad. Sci. USA 112, 9908–9913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.González-Arzola, K., Díaz-Quintana, A., Rivero-Rodríguez, F., Velázquez-Campoy, A., De la Rosa, M. A., Díaz-Moreno, I. (2017) Histone chaperone activity of Arabidopsis thaliana NRP1 is blocked by cytochrome c. Nucleic Acids Res. 45, 2150–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Díaz-Moreno, I., Velázquez-Cruz, A., Curran-French, S., Díaz-Quintana, A., De la Rosa, M. A. (2018) Nuclear cytochrome c: a mitochondrial visitor regulating damaged chromatin dynamics. FEBS Lett. 592, 172–178 [DOI] [PubMed] [Google Scholar]
- 45.Jenkins, C. M., Yang, K., Liu, G., Moon, S. H., Dilthey, B. G., Gross, R. W. (2018) Cytochrome c is an oxidative stress-activated plasmalogenase that cleaves plasmenylcholine and plasmenylethanolamine at the sn-1 vinyl ether linkage. J. Biol. Chem. 293, 8693–8709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linn, T. C., Pettit, F. H., Reed, L. J. (1969) Alpha-keto acid dehydrogenase complexes, X: Regulation of the activity of the pyruvate dehydrogenase complex from beef kidney mitochondria by phosphorylation and dephosphorylation. Proc. Natl. Acad. Sci. USA 62, 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Covian, R., Balaban, R. S. (2012) Cardiac mitochondrial matrix and respiratory complex protein phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 303, H940–H966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee, I., Salomon, A. R., Ficarro, S., Mathes, I., Lottspeich, F., Grossman, L. I., Hüttemann, M. (2005) cAMP-dependent tyrosine phosphorylation of subunit I inhibits cytochrome c oxidase activity. J. Biol. Chem. 280, 6094–6100 [DOI] [PubMed] [Google Scholar]
- 49.Samavati, L., Lee, I., Mathes, I., Lottspeich, F., Hüttemann, M. (2008) Tumor necrosis factor α inhibits oxidative phosphorylation through tyrosine phosphorylation at subunit I of cytochrome c oxidase. J. Biol. Chem. 283, 21134–21144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyazaki, T., Neff, L., Tanaka, S., Horne, W. C., Baron, R. (2003) Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J. Cell Biol. 160, 709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boerner, J. L., Demory, M. L., Silva, C., Parsons, S. J. (2004) Phosphorylation of Y845 on the epidermal growth factor receptor mediates binding to the mitochondrial protein cytochrome c oxidase subunit II. Mol. Cell. Biol. 24, 7059–7071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding, Y., Liu, Z., Desai, S., Zhao, Y., Liu, H., Pannell, L. K., Yi, H., Wright, E. R., Owen, L. B., Dean-Colomb, W., Fodstad, O., Lu, J., LeDoux, S. P., Wilson, G. L., Tan, M. (2012) Receptor tyrosine kinase ErbB2 translocates into mitochondria and regulates cellular metabolism. Nat. Commun. 3, 1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee, I., Salomon, A. R., Yu, K., Doan, J. W., Grossman, L. I., Hüttemann, M. (2006) New prospects for an old enzyme: mammalian cytochrome c is tyrosine-phosphorylated in vivo. Biochemistry 45, 9121–9128 [DOI] [PubMed] [Google Scholar]
- 54.Dickerson, R., Timkovich, R. (1975) Cytochrome c. In The Enzymes (Boyer, P., ed.), Vol. 11, pp. 397–472, Academic Press, New York [Google Scholar]
- 55.Dickerson, R. E., Takano, T., Eisenberg, D., Kallai, O. B., Samson, L., Cooper, A., Margoliash, E. (1971) Ferricytochrome c, I: General features of the horse and bonito proteins at 2.8 A resolution. J. Biol. Chem. 246, 1511–1535 [PubMed] [Google Scholar]
- 56.Yu, T., Wang, X., Purring-Koch, C., Wei, Y., McLendon, G. L. (2001) A mutational epitope for cytochrome C binding to the apoptosis protease activation factor-1. J. Biol. Chem. 276, 13034–13038 [DOI] [PubMed] [Google Scholar]
- 57.García-Heredia, J. M., Díaz-Quintana, A., Salzano, M., Orzáez, M., Pérez-Payá, E., Teixeira, M., De la Rosa, M. A., Díaz-Moreno, I. (2011) Tyrosine phosphorylation turns alkaline transition into a biologically relevant process and makes human cytochrome c behave as an anti-apoptotic switch. J. Biol. Inorg. Chem. 16, 1155–1168 [DOI] [PubMed] [Google Scholar]
- 58.Guerra-Castellano, A., Díaz-Quintana, A., Pérez-Mejías, G., Elena-Real, C. A., González-Arzola, K., García-Mauriño, S. M., De la Rosa, M. A., Díaz-Moreno, I. (2018) Oxidative stress is tightly regulated by cytochrome c phosphorylation and respirasome factors in mitochondria. Proc. Natl. Acad. Sci. USA 115, 7955–7960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanderson, T. H., Mahapatra, G., Pecina, P., Ji, Q., Yu, K., Sinkler, C., Varughese, A., Kumar, R., Bukowski, M. J., Tousignant, R. N., Salomon, A. R., Lee, I., Hüttemann, M. (2013) Cytochrome C is tyrosine 97 phosphorylated by neuroprotective insulin treatment. PLoS One 8, e78627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sanderson, T. H., Kumar, R., Sullivan, J. M., Krause, G. S. (2008) Insulin blocks cytochrome c release in the reperfused brain through PI3-K signaling and by promoting Bax/Bcl-XL binding. J. Neurochem. 106, 1248–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaidi, S., Hassan, M. I., Islam, A., Ahmad, F. (2014) The role of key residues in structure, function, and stability of cytochrome-c. Cell. Mol. Life Sci. 71, 229–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu, H., Lee, I., Salomon, A. R., Yu, K., Hüttemann, M. (2008) Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochim. Biophys. Acta 1777, 1066–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Rocco, D., Cerqua, C., Goffrini, P., Russo, G., Pastore, A., Meloni, F., Nicchia, E., Moraes, C. T., Pecci, A., Salviati, L., Savoia, A. (2014) Mutations of cytochrome c identified in patients with thrombocytopenia THC4 affect both apoptosis and cellular bioenergetics. Biochim. Biophys. Acta 1842, 269–274 [DOI] [PubMed] [Google Scholar]
- 64.Moreno-Beltrán, B., Guerra-Castellano, A., Díaz-Quintana, A., Del Conte, R., García-Mauriño, S. M., Díaz-Moreno, S., González-Arzola, K., Santos-Ocaña, C., Velázquez-Campoy, A., De la Rosa, M. A., Turano, P., Díaz-Moreno, I. (2017) Structural basis of mitochondrial dysfunction in response to cytochrome c phosphorylation at tyrosine 48. Proc. Natl. Acad. Sci. USA 114, E3041–E3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kapralov, A. A., Yanamala, N., Tyurina, Y. Y., Castro, L., Samhan-Arias, A., Vladimirov, Y. A., Maeda, A., Weitz, A. A., Peterson, J., Mylnikov, D., Demicheli, V., Tortora, V., Klein-Seetharaman, J., Radi, R., Kagan, V. E. (2011) Topography of tyrosine residues and their involvement in peroxidation of polyunsaturated cardiolipin in cytochrome c/cardiolipin peroxidase complexes. Biochim. Biophys. Acta 1808, 2147–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pecina, P., Borisenko, G. G., Belikova, N. A., Tyurina, Y. Y., Pecinova, A., Lee, I., Samhan-Arias, A. K., Przyklenk, K., Kagan, V. E., Hüttemann, M. (2010) Phosphomimetic substitution of cytochrome C tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry 49, 6705–6714 [DOI] [PubMed] [Google Scholar]
- 67.Sinkler, C. A., Kalpage, H., Shay, J., Lee, I., Malek, M. H., Grossman, L. I., Hüttemann, M. (2017) Tissue- and condition-specific isoforms of mammalian cytochrome c oxidase subunits: from function to human disease. Oxid. Med. Cell. Longev. 2017, 1534056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guerra-Castellano, A., Díaz-Quintana, A., Moreno-Beltrán, B., López-Prados, J., Nieto, P. M., Meister, W., Staffa, J., Teixeira, M., Hildebrandt, P., De la Rosa, M. A., Díaz-Moreno, I. (2015) Mimicking tyrosine phosphorylation in human cytochrome c by the evolved tRNA synthetase technique. Chemistry 21, 15004–15012 [DOI] [PubMed] [Google Scholar]
- 69.Morison, I. M., Cramer Bordé, E. M., Cheesman, E. J., Cheong, P. L., Holyoake, A. J., Fichelson, S., Weeks, R. J., Lo, A., Davies, S. M., Wilbanks, S. M., Fagerlund, R. D., Ludgate, M. W., da Silva Tatley, F. M., Coker, M. S., Bockett, N. A., Hughes, G., Pippig, D. A., Smith, M. P., Capron, C., Ledgerwood, E. C. (2008) A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nat. Genet. 40, 387–389 [DOI] [PubMed] [Google Scholar]
- 70.Parakra, R. D., Kleffmann, T., Jameson, G. N. L., Ledgerwood, E. C. (2018) The proportion of Met80-sulfoxide dictates peroxidase activity of human cytochrome c. Dalton Trans. 47, 9128–9135 [DOI] [PubMed] [Google Scholar]
- 71.Deacon, O. M., Karsisiotis, A. I., Moreno-Chicano, T., Hough, M. A., Macdonald, C., Blumenschein, T. M. A., Wilson, M. T., Moore, G. R., Worrall, J. A. R. (2017) Heightened dynamics of the oxidized Y48H variant of human cytochrome c increases its peroxidatic activity. Biochemistry 56, 6111–6124 [DOI] [PubMed] [Google Scholar]
- 72.Muneeswaran, G., Kartheeswaran, S., Pandiaraj, M., Muthukumar, K., Sankaralingam, M., Arunachalam, S. (2017) Investigation of structural dynamics of Thrombocytopenia Cargeeg mutants of human apoptotic cytochrome c: a molecular dynamics simulation approach. Biophys. Chem. 230, 117–126 [DOI] [PubMed] [Google Scholar]
- 73.Højlund, K., Bowen, B. P., Hwang, H., Flynn, C. R., Madireddy, L., Geetha, T., Langlais, P., Meyer, C., Mandarino, L. J., Yi, Z. (2009) In vivo phosphoproteome of human skeletal muscle revealed by phosphopeptide enrichment and HPLC-ESI-MS/MS. J. Proteome Res. 8, 4954–4965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhao, X., León, I. R., Bak, S., Mogensen, M., Wrzesinski, K., Højlund, K., Jensen, O. N. (2011) Phosphoproteome analysis of functional mitochondria isolated from resting human muscle reveals extensive phosphorylation of inner membrane protein complexes and enzymes. Mol. Cell. Proteomics 10, M110.000299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoffman, N. J., Parker, B. L., Chaudhuri, R., Fisher-Wellman, K. H., Kleinert, M., Humphrey, S. J., Yang, P., Holliday, M., Trefely, S., Fazakerley, D. J., Stöckli, J., Burchfield, J. G., Jensen, T. E., Jothi, R., Kiens, B., Wojtaszewski, J. F., Richter, E. A., James, D. E. (2015) Global phosphoproteomic analysis of human skeletal muscle reveals a network of exercise-regulated kinases and AMPK substrates [published correction appears in Cell Metab. (2015) 22, 948]. Cell Metab. 22, 922–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mahapatra, G., Varughese, A., Ji, Q., Lee, I., Liu, J., Vaishnav, A., Sinkler, C., Kapralov, A. A., Moraes, C. T., Sanderson, T. H., Stemmler, T. L., Grossman, L. I., Kagan, V. E., Brunzelle, J. S., Salomon, A. R., Edwards, B. F., Hüttemann, M. (2017) Phosphorylation of cytochrome c threonine 28 regulates electron transport chain activity in kidney: implications for AMP kinase. J. Biol. Chem. 292, 64–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guerra-Castellano, A., Diaz-Moreno, I., Velazquez-Campoy, A., De la Rosa, M. A., Diaz-Quintana, A. (2016) Structural and functional characterization of phosphomimetic mutants of cytochrome c at threonine 28 and serine 47. Biochim Biophys Acta 1857, 387–395 [DOI] [PubMed] [Google Scholar]
- 78.Liu, S. S. (1999) Cooperation of a “reactive oxygen cycle” with the Q cycle and the proton cycle in the respiratory chain: superoxide generating and cycling mechanisms in mitochondria. J. Bioenerg. Biomembr. 31, 367–376 [DOI] [PubMed] [Google Scholar]
- 79.Korshunov, S. S., Skulachev, V. P., Starkov, A. A. (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 416, 15–18 [DOI] [PubMed] [Google Scholar]
- 80.Rottenberg, H., Covian, R., Trumpower, B. L. (2009) Membrane potential greatly enhances superoxide generation by the cytochrome bc1 complex reconstituted into phospholipid vesicles. J. Biol. Chem. 284, 19203–19210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wan, B., Doumen, C., Duszynski, J., Salama, G., Vary, T. C., LaNoue, K. F. (1993) Effects of cardiac work on electrical potential gradient across mitochondrial membrane in perfused rat hearts. Am. J. Physiol. 265, H453–H460 [DOI] [PubMed] [Google Scholar]
- 82.Brand, M. D., Felber, S. M. (1984) Membrane potential of mitochondria in intact lymphocytes during early mitogenic stimulation. Biochem. J. 217, 453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Backus, M., Piwnica-Worms, D., Hockett, D., Kronauge, J., Lieberman, M., Ingram, P., LeFurgey, A. (1993) Microprobe analysis of Tc-MIBI in heart cells: calculation of mitochondrial membrane potential. Am. J. Physiol. 265, C178–C187 [DOI] [PubMed] [Google Scholar]
- 84.Zhang, H., Huang, H. M., Carson, R. C., Mahmood, J., Thomas, H. M., Gibson, G. E. (2001) Assessment of membrane potentials of mitochondrial populations in living cells. Anal. Biochem. 298, 170–180 [DOI] [PubMed] [Google Scholar]
- 85.Kaim, G., Dimroth, P. (1999) ATP synthesis by F-type ATP synthase is obligatorily dependent on the transmembrane voltage. EMBO J. 18, 4118–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dalmonte, M. E., Forte, E., Genova, M. L., Giuffrè, A., Sarti, P., Lenaz, G. (2009) Control of respiration by cytochrome c oxidase in intact cells: role of the membrane potential. J. Biol. Chem. 284, 32331–32335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Piccoli, C., Scrima, R., Boffoli, D., Capitanio, N. (2006) Control by cytochrome c oxidase of the cellular oxidative phosphorylation system depends on the mitochondrial energy state. Biochem. J. 396, 573–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Villani, G., Attardi, G. (1997) In vivo control of respiration by cytochrome c oxidase in wild-type and mitochondrial DNA mutation-carrying human cells. Proc. Natl. Acad. Sci. USA 94, 1166–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Villani, G., Greco, M., Papa, S., Attardi, G. (1998) Low reserve of cytochrome c oxidase capacity in vivo in the respiratory chain of a variety of human cell types. J. Biol. Chem. 273, 31829–31836 [DOI] [PubMed] [Google Scholar]
- 90.Kunz, W. S., Kudin, A., Vielhaber, S., Elger, C. E., Attardi, G., Villani, G. (2000) Flux control of cytochrome c oxidase in human skeletal muscle. J. Biol. Chem. 275, 27741–27745 [DOI] [PubMed] [Google Scholar]
- 91.El-Zayadi, A. R. (2006) Heavy smoking and liver. World J. Gastroenterol. 12, 6098–6101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paik, W. K., Paik, D. C., Kim, S. (2007) Historical review: the field of protein methylation. Trends Biochem. Sci. 32, 146–152 [DOI] [PubMed] [Google Scholar]
- 93.Bremang, M., Cuomo, A., Agresta, A. M., Stugiewicz, M., Spadotto, V., Bonaldi, T. (2013) Mass spectrometry-based identification and characterisation of lysine and arginine methylation in the human proteome. Mol. Biosyst. 9, 2231–2247 [DOI] [PubMed] [Google Scholar]
- 94.Hosp, F., Lassowskat, I., Santoro, V., De Vleesschauwer, D., Fliegner, D., Redestig, H., Mann, M., Christian, S., Hannah, M. A., Finkemeier, I. (2017) Lysine acetylation in mitochondria: from inventory to function. Mitochondrion 33, 58–71 [DOI] [PubMed] [Google Scholar]
- 95.Holzschu, D., Principio, L., Conklin, K. T., Hickey, D. R., Short, J., Rao, R., McLendon, G., Sherman, F. (1987) Replacement of the invariant lysine 77 by arginine in yeast iso-1-cytochrome c results in enhanced and normal activities in vitro and in vivo. J. Biol. Chem. 262, 7125–7131 [PubMed] [Google Scholar]
- 96.Paik, W. K., Cho, Y. B., Frost, B., Kim, S. (1989) Cytochrome c methylation. Biochem. Cell Biol. 67, 602–611 [DOI] [PubMed] [Google Scholar]
- 97.Polevoda, B., Martzen, M. R., Das, B., Phizicky, E. M., Sherman, F. (2000) Cytochrome c methyltransferase, Ctm1p, of yeast. J. Biol. Chem. 275, 20508–20513 [DOI] [PubMed] [Google Scholar]
- 98.Park, K. S., Frost, B., Tuck, M., Ho, L. L., Kim, S., Paik, W. K. (1987) Enzymatic methylation of in vitro synthesized apocytochrome c enhances its transport into mitochondria. J. Biol. Chem. 262, 14702–14708 [PubMed] [Google Scholar]
- 99.Polastro, E., Schneck, A. G., Leonis, J., Kim, S., Paik, W. K. (1978) Cytochrome c methylation. Int. J. Biochem. 9, 795–801 [DOI] [PubMed] [Google Scholar]
- 100.Winter, D. L., Abeygunawardena, D., Hart-Smith, G., Erce, M. A., Wilkins, M. R. (2015) Lysine methylation modulates the protein-protein interactions of yeast cytochrome C Cyc1p. Proteomics 15, 2166–2176 [DOI] [PubMed] [Google Scholar]
- 101.Kluck, R. M., Ellerby, L. M., Ellerby, H. M., Naiem, S., Yaffe, M. P., Margoliash, E., Bredesen, D., Mauk, A. G., Sherman, F., Newmeyer, D. D. (2000) Determinants of cytochrome c pro-apoptotic activity: the role of lysine 72 trimethylation. J. Biol. Chem. 275, 16127–16133 [DOI] [PubMed] [Google Scholar]
- 102.Farooqui, J., DiMaria, P., Kim, S., Paik, W. K. (1981) Effect of methylation on the stability of cytochrome c of Saccharomyces cerevisiae in vivo. J. Biol. Chem. 256, 5041–5045 [PubMed] [Google Scholar]
- 103.Frost, B., Park, K. S., Kim, S., Paik, W. K. (1989) Effect of enzymatic methylation of apocytochrome c on holocytochrome c formation and proteolysis. Int. J. Biochem. 21, 1407–1414 [DOI] [PubMed] [Google Scholar]
- 104.Brems, D. N., Stellwagen, E. (1981) The effect of methylation on cytochrome c fragment complementation. J. Biol. Chem. 256, 11688–11690 [PubMed] [Google Scholar]
- 105.Kim, C. S., Kueppers, F., Dimaria, P., Farooqui, J., Kim, S., Paik, W. K. (1980) Enzymatic trimethylation of residue-72 lysine in cytochrome c: effect on the total structure. Biochim. Biophys. Acta 622, 144–150 [DOI] [PubMed] [Google Scholar]
- 106.Pollock, W. B., Rosell, F. I., Twitchett, M. B., Dumont, M. E., Mauk, A. G. (1998) Bacterial expression of a mitochondrial cytochrome c: trimethylation of lys72 in yeast iso-1-cytochrome c and the alkaline conformational transition. Biochemistry 37, 6124–6131 [DOI] [PubMed] [Google Scholar]
- 107.Cherney, M. M., Junior, C. C., Bowler, B. E. (2013) Mutation of trimethyllysine 72 to alanine enhances His79-heme-mediated dynamics of iso-1-cytochrome c. Biochemistry 52, 837–846 [DOI] [PubMed] [Google Scholar]
- 108.Anderson, K. A., Hirschey, M. D. (2012) Mitochondrial protein acetylation regulates metabolism. Essays Biochem. 52, 23–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hebert, A. S., Dittenhafer-Reed, K. E., Yu, W., Bailey, D. J., Selen, E. S., Boersma, M. D., Carson, J. J., Tonelli, M., Balloon, A. J., Higbee, A. J., Westphall, M. S., Pagliarini, D. J., Prolla, T. A., Assadi-Porter, F., Roy, S., Denu, J. M., Coon, J. J. (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 49, 186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.König, A. C., Hartl, M., Boersema, P. J., Mann, M., Finkemeier, I. (2014) The mitochondrial lysine acetylome of Arabidopsis. Mitochondrion 19, 252–260 [DOI] [PubMed] [Google Scholar]
- 111.Smith-Hammond, C. L., Hoyos, E., Miernyk, J. A. (2014) The pea seedling mitochondrial Nε-lysine acetylome. Mitochondrion 19, 154–165 [DOI] [PubMed] [Google Scholar]
- 112.Kim, S. C., Sprung, R., Chen, Y., Xu, Y., Ball, H., Pei, J., Cheng, T., Kho, Y., Xiao, H., Xiao, L., Grishin, N. V., White, M., Yang, X. J., Zhao, Y. (2006) Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol. Cell 23, 607–618 [DOI] [PubMed] [Google Scholar]
- 113.Basova, L. V., Kurnikov, I. V., Wang, L., Ritov, V. B., Belikova, N. A., Vlasova, I. I., Pacheco, A. A., Winnica, D. E., Peterson, J., Bayir, H., Waldeck, D. H., Kagan, V. E. (2007) Cardiolipin switch in mitochondria: shutting off the reduction of cytochrome c and turning on the peroxidase activity. Biochemistry 46, 3423–3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cammack, R. (1995) Redox states and potentials. In Bioenergetics: A Practical Approach (Brown, G. C., Cooper, C. E., ed.), pp. 93–95, IRL Press, Oxford, United Kingdom [Google Scholar]
- 115.Nicholls, D. G., Ferguson, S. J. (1992) Bioenergetics 2, Academic Press Limited, London [Google Scholar]
- 116.Capdevila, D. A., Oviedo Rouco, S., Tomasina, F., Tortora, V., Demicheli, V., Radi, R., Murgida, D. H. (2015) Active site structure and peroxidase activity of oxidatively modified cytochrome c species in complexes with cardiolipin. Biochemistry 54, 7491–7504 [DOI] [PubMed] [Google Scholar]
- 117.Vladimirov, Y. A., Proskurnina, E. V., Izmailov, D. Y., Novikov, A. A., Brusnichkin, A. V., Osipov, A. N., Kagan, V. E. (2006) Cardiolipin activates cytochrome c peroxidase activity since it facilitates H(2)O(2) access to heme. Biochemistry (Mosc.) 71, 998–1005 [DOI] [PubMed] [Google Scholar]
- 118.Wang, Z., Ando, Y., Nugraheni, A. D., Ren, C., Nagao, S., Hirota, S. (2014) Self-oxidation of cytochrome c at methionine80 with molecular oxygen induced by cleavage of the Met-heme iron bond. Mol. Biosyst. 10, 3130–3137 [DOI] [PubMed] [Google Scholar]
- 119.Godoy, L. C., Muñoz-Pinedo, C., Castro, L., Cardaci, S., Schonhoff, C. M., King, M., Tórtora, V., Marín, M., Miao, Q., Jiang, J. F., Kapralov, A., Jemmerson, R., Silkstone, G. G., Patel, J. N., Evans, J. E., Wilson, M. T., Green, D. R., Kagan, V. E., Radi, R., Mannick, J. B. (2009) Disruption of the M80-Fe ligation stimulates the translocation of cytochrome c to the cytoplasm and nucleus in nonapoptotic cells. Proc. Natl. Acad. Sci. USA 106, 2653–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Radi, R., Cassina, A., Hodara, R., Quijano, C., Castro, L. (2002) Peroxynitrite reactions and formation in mitochondria. Free Radic. Biol. Med. 33, 1451–1464 [DOI] [PubMed] [Google Scholar]
- 121.Radi, R. (2004) Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA 101, 4003–4008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu, S., Ying, J., Jiang, B., Guo, W., Adachi, T., Sharov, V., Lazar, H., Menzoian, J., Knyushko, T. V., Bigelow, D., Schöneich, C., Cohen, R. A. (2006) Detection of sequence-specific tyrosine nitration of manganese SOD and SERCA in cardiovascular disease and aging. Am. J. Physiol. Heart Circ. Physiol. 290, H2220–H2227 [DOI] [PubMed] [Google Scholar]
- 123.MacMillan-Crow, L. A., Crow, J. P., Kerby, J. D., Beckman, J. S., Thompson, J. A. (1996) Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc. Natl. Acad. Sci. USA 93, 11853–11858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.García-Heredia, J. M., Díaz-Moreno, I., Nieto, P. M., Orzáez, M., Kocanis, S., Teixeira, M., Pérez-Payá, E., Díaz-Quintana, A., De la Rosa, M. A. (2010) Nitration of tyrosine 74 prevents human cytochrome c to play a key role in apoptosis signaling by blocking caspase-9 activation. Biochim. Biophys. Acta 1797, 981–993 [DOI] [PubMed] [Google Scholar]
- 125.Batthyány, C., Souza, J. M., Durán, R., Cassina, A., Cerveñansky, C., Radi, R. (2005) Time course and site(s) of cytochrome c tyrosine nitration by peroxynitrite. Biochemistry 44, 8038–8046 [DOI] [PubMed] [Google Scholar]
- 126.Rodríguez-Roldán, V., García-Heredia, J. M., Navarro, J. A., De la Rosa, M. A., Hervás, M. (2008) Effect of nitration on the physicochemical and kinetic features of wild-type and monotyrosine mutants of human respiratory cytochrome c. Biochemistry 47, 12371–12379 [DOI] [PubMed] [Google Scholar]
- 127.Díaz-Moreno, I., García-Heredia, J. M., Díaz-Quintana, A., Teixeira, M., De la Rosa, M. A. (2011) Nitration of tyrosines 46 and 48 induces the specific degradation of cytochrome c upon change of the heme iron state to high-spin. Biochim. Biophys. Acta 1807, 1616–1623 [DOI] [PubMed] [Google Scholar]
- 128.Cruthirds, D. L., Novak, L., Akhi, K. M., Sanders, P. W., Thompson, J. A., MacMillan-Crow, L. A. (2003) Mitochondrial targets of oxidative stress during renal ischemia/reperfusion. Arch. Biochem. Biophys. 412, 27–33 [DOI] [PubMed] [Google Scholar]
- 129.Alonso, D., Encinas, J. M., Uttenthal, L. O., Boscá, L., Serrano, J., Fernández, A. P., Castro-Blanco, S., Santacana, M., Bentura, M. L., Richart, A., Fernández-Vizarra, P., Rodrigo, J. (2002) Coexistence of translocated cytochrome c and nitrated protein in neurons of the rat cerebral cortex after oxygen and glucose deprivation. Neuroscience 111, 47–56 [DOI] [PubMed] [Google Scholar]
- 130.Ueta, E., Kamatani, T., Yamamoto, T., Osaki, T. (2003) Tyrosine-nitration of caspase 3 and cytochrome c does not suppress apoptosis induction in squamous cell carcinoma cells. Int. J. Cancer 103, 717–722 [DOI] [PubMed] [Google Scholar]
- 131.MacMillan-Crow, L. A., Cruthirds, D. L., Ahki, K. M., Sanders, P. W., Thompson, J. A. (2001) Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free Radic. Biol. Med. 31, 1603–1608 [DOI] [PubMed] [Google Scholar]
- 132.Nakagawa, H., Komai, N., Takusagawa, M., Miura, Y., Toda, T., Miyata, N., Ozawa, T., Ikota, N. (2007) Nitration of specific tyrosine residues of cytochrome C is associated with caspase-cascade inactivation. Biol. Pharm. Bull. 30, 15–20 [DOI] [PubMed] [Google Scholar]
- 133.García-Heredia, J. M., Díaz-Moreno, I., Díaz-Quintana, A., Orzáez, M., Navarro, J. A., Hervás, M., De la Rosa, M. A. (2012) Specific nitration of tyrosines 46 and 48 makes cytochrome c assemble a non-functional apoptosome. FEBS Lett. 586, 154–158 [DOI] [PubMed] [Google Scholar]
- 134.Kong, S. K., Yim, M. B., Stadtman, E. R., Chock, P. B. (1996) Peroxynitrite disables the tyrosine phosphorylation regulatory mechanism: lymphocyte-specific tyrosine kinase fails to phosphorylate nitrated cdc2(6-20)NH2 peptide. Proc. Natl. Acad. Sci. USA 93, 3377–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cassina, A. M., Hodara, R., Souza, J. M., Thomson, L., Castro, L., Ischiropoulos, H., Freeman, B. A., Radi, R. (2000) Cytochrome c nitration by peroxynitrite. J. Biol. Chem. 275, 21409–21415 [DOI] [PubMed] [Google Scholar]
- 136.Pal, P. K., Verma, B., Myer, Y. P. (1975) Conformational and functional studies of chemically modified cytochrome c: nitrated and iodinated cytochromes c. Biochemistry 14, 4325–4334 [DOI] [PubMed] [Google Scholar]
- 137.Oursler, M. J., Bradley, E. W., Elfering, S. L., Giulivi, C. (2005) Native, not nitrated, cytochrome c and mitochondria-derived hydrogen peroxide drive osteoclast apoptosis. Am. J. Physiol. Cell Physiol. 288, C156–C168 [DOI] [PubMed] [Google Scholar]
- 138.Abriata, L. A., Cassina, A., Tórtora, V., Marín, M., Souza, J. M., Castro, L., Vila, A. J., Radi, R. (2009) Nitration of solvent-exposed tyrosine 74 on cytochrome c triggers heme iron-methionine 80 bond disruption nuclear magnetic resonance and optical spectroscopy studies. J. Biol. Chem. 284, 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Capdevila, D. A., Álvarez-Paggi, D., Castro, M. A., Tórtora, V., Demicheli, V., Estrín, D. A., Radi, R., Murgida, D. H. (2014) Coupling of tyrosine deprotonation and axial ligand exchange in nitrocytochrome c. Chem. Commun. (Camb.) 50, 2592–2594 [DOI] [PubMed] [Google Scholar]
- 140.Stamler, J. S., Lamas, S., Fang, F. C. (2001) Nitrosylation. the prototypic redox-based signaling mechanism. Cell 106, 675–683 [DOI] [PubMed] [Google Scholar]
- 141.Hess, D. T., Stamler, J. S. (2012) Regulation by S-nitrosylation of protein post-translational modification. J. Biol. Chem. 287, 4411–4418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ascenzi, P., Coletta, M., Santucci, R., Polizio, F., Desideri, A. (1994) Nitric oxide binding to ferrous native horse heart cytochrome c and to its carboxymethylated derivative: a spectroscopic and thermodynamic study. J. Inorg. Biochem. 53, 273–280 [DOI] [PubMed] [Google Scholar]
- 143.Sharpe, M. A., Cooper, C. E. (1998) Reactions of nitric oxide with mitochondrial cytochrome c: a novel mechanism for the formation of nitroxyl anion and peroxynitrite. Biochem. J. 332, 9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schonhoff, C. M., Gaston, B., Mannick, J. B. (2003) Nitrosylation of cytochrome c during apoptosis. J. Biol. Chem. 278, 18265–18270 [DOI] [PubMed] [Google Scholar]
- 145.Brown, G. C., Cooper, C. E. (1994) Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 356, 295–298 [DOI] [PubMed] [Google Scholar]
- 146.Cleeter, M. W., Cooper, J. M., Darley-Usmar, V. M., Moncada, S., Schapira, A. H. (1994) Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 345, 50–54 [DOI] [PubMed] [Google Scholar]
- 147.Yoo, B. K., Lamarre, I., Martin, J. L., Rappaport, F., Negrerie, M. (2015) Motion of proximal histidine and structural allosteric transition in soluble guanylate cyclase. Proc. Natl. Acad. Sci. USA 112, E1697–E1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arnold, W. P., Mittal, C. K., Katsuki, S., Murad, F. (1977) Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. USA 74, 3203–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cooper, C. E. (1999) Nitric oxide and iron proteins. Biochim. Biophys. Acta 1411, 290–309 [DOI] [PubMed] [Google Scholar]
- 150.Patriarca, A., Eliseo, T., Sinibaldi, F., Piro, M. C., Melis, R., Paci, M., Cicero, D. O., Polticelli, F., Santucci, R., Fiorucci, L. (2009) ATP acts as a regulatory effector in modulating structural transitions of cytochrome c: implications for apoptotic activity. Biochemistry 48, 3279–3287 [DOI] [PubMed] [Google Scholar]
- 151.Kawai, C., Pessoto, F. S., Graves, C. V., Carmona-Ribeiro, A. M., Nantes, I. L. (2013) Effects of transmembrane potential and pH gradient on the cytochrome c-promoted fusion of mitochondrial mimetic membranes. J. Bioenerg. Biomembr. 45, 421–430 [DOI] [PubMed] [Google Scholar]
- 152.DeLange, R. J., Glazer, A. N., Smith, E. L. (1969) Presence and location of an unusual amino acid, epsilon-N-trimethyllysine, in cytochrome c of wheat germ and Neurospora. J. Biol. Chem. 244, 1385–1388 [PubMed] [Google Scholar]
- 153.Scott, W. A., Mitchell, H. K. (1969) Secondary modification of cytochrome c by Neurospora crassa. Biochemistry 8, 4282–4289 [DOI] [PubMed] [Google Scholar]