Abstract
Skeletal muscle mass is regulated by the coordinated activation of several anabolic and catabolic pathways. The endoplasmic reticulum (ER) is a major site of protein folding and a reservoir for calcium ions. Accretion of misfolded proteins or depletion in calcium concentration causes stress in the ER, which leads to the activation of a signaling network known as the unfolded protein response (UPR). In the present study, we investigated the role of the protein kinase R-like endoplasmic reticulum kinase (PERK) arm of the UPR in the regulation of skeletal muscle mass and function in naive conditions and in a mouse model of cancer cachexia. Our results demonstrate that the targeted inducible deletion of PERK reduces skeletal muscle mass, strength, and force production during isometric contractions. Deletion of PERK also causes a slow-to-fast fiber type transition in skeletal muscle. Furthermore, short hairpin RNA–mediated knockdown or pharmacologic inhibition of PERK leads to atrophy in cultured myotubes. While increasing the rate of protein synthesis, the targeted deletion of PERK leads to the increased expression of components of the ubiquitin–proteasome system and autophagy in skeletal muscle. Ablation of PERK also increases the activation of calpains and deregulates the gene expression of the members of the FGF19 subfamily. Furthermore, the targeted deletion of PERK increases muscle wasting in Lewis lung carcinoma tumor-bearing mice. Our findings suggest that the PERK arm of the UPR is essential for the maintenance of skeletal muscle mass and function in adult mice.—Gallot, Y. S., Bohnert, K. R., Straughn, A. R., Xiong, G., Hindi, S. M., Kumar, A. PERK regulates skeletal muscle mass and contractile function in adult mice.
Keywords: ER stress, unfolded protein response, cancer cachexia, proteostasis, lewis lung carcinoma
Skeletal muscle is a highly plastic tissue in the human body that undergoes considerable adaptations in response to both intrinsic and extrinsic stimuli. Skeletal muscle mass is regulated by the processes of protein synthesis and degradation in conjunction with systems that manage protein folding, trafficking, and clearance (1, 2). In several catabolic states (e.g., starvation, cancer, diabetes, bed rest, loss of neural input, or high dosages of corticosteroids), the rate of protein degradation exceeds the rate of protein synthesis, which eventually leads to an overall loss of skeletal muscle mass (i.e., muscle atrophy) (1, 3, 4). Although the loss of skeletal muscle mass is an independent predictor of morbidity and mortality in many disease states and conditions, there is almost a complete lack of medication to prevent muscle atrophy (1, 2). The development of therapeutic interventions for muscle atrophy requires a better understanding of the cellular and molecular mechanisms that regulate skeletal muscle growth and atrophy.
Skeletal muscle is composed of an extended network of specialized endoplasmic reticulum (ER), also known as the sarcoplasmic reticulum. The ER is responsible for the regulated release of calcium ions into the cytoplasm to initiate muscle contraction (5). The ER is also an important site for proper folding, packaging, and trafficking of freshly synthesized secretory and transmembrane proteins. However, these functions of the ER are disrupted in many conditions, such as acute exercise, hypoxia, depletion of calcium ions, redox imbalance, nutrient deprivation, and microbial infections, leading to the accumulation of misfolded and unfolded proteins in the ER lumen and associated stress (6). To alleviate the stress, the ER elicits the unfolded protein response (UPR), which is initiated through the activation of 3 ER transmembrane sensors: protein kinase R-like endoplasmic reticulum kinase (PERK), inositol-requiring protein (IRE)1α, and activating transcription factor (ATF)6. The UPR improves ER function through modulating the rate of protein synthesis and increasing the gene expression of many ER chaperones and regulatory proteins (6–9). Studies using genetic mouse models have now provided unequivocal evidence that UPR pathways play pivotal roles in the development and function of various tissues, including the liver, pancreas, bones, and antibody-secreting B cells (6, 8). The activation of the UPR is the main mechanism to ensure correct protein handling, but chronic ER stress induces numerous intracellular pathways that can lead to pathologic consequences, such as inflammation and cell death through apoptosis (8).
Accumulating evidence suggests that the abundance and activity of various components of the UPR are increased in skeletal muscle of rodents and humans in multiple conditions of muscle growth and atrophy (5, 10). For example, the expression of various ER stress and UPR markers is increased in skeletal muscle in response to both endurance and acute exercise (5, 11). It has been reported that the cleaved form of ATF6α interacts with peroxisome proliferator-activated receptor-γ coactivator-1α to induce an adaptive UPR in skeletal muscle after acute exercise (12). Moreover, markers of ER stress and the UPR have been found to be elevated in skeletal muscle undergoing atrophy (11, 13, 14). For example, levels of CCAAT/enhancer-binding protein (C/EBP) homologous protein (CHOP), a downstream target of the PERK arm of the UPR, are increased in skeletal muscle of mice upon denervation. Similarly, ER stress markers have been found to be increased in skeletal muscle of AR113Q mice, a rodent model of spinal and bulbar muscular atrophy (15). The increased expression of CHOP appears to be a protective mechanism, given that the genetic ablation of CHOP exaggerates muscle wasting in AR113Q mice and wild-type (WT) mice upon denervation (15). We have reported that the markers of all 3 arms of the UPR are increased in skeletal muscle of 2 mouse models of cancer cachexia: Lewis lung carcinoma (LLC) tumor-bearing mice and ApcMin/+ mice. Prolonged administration of 4-phenylbutyrate (4-PBA), a chemical chaperone and inhibitor of ER stress, reduces skeletal muscle mass in naive conditions and increases myofiber atrophy in LLC-bearing mice (16). However, the role of the individual arms of the UPR in the regulation of skeletal muscle mass has not yet been investigated with genetic approaches.
Upon induction of ER stress, PERK becomes activated through undergoing oligomerization and autophosphorylation. Activated PERK then phosphorylates eukaryotic translation initiation factor (eIF)2α on S51, which facilitates general translation repression while selectively increasing the translation of ATF4 and the expression of stress-responsive genes, such as CHOP and glucose-regulated protein 78 (6–9). It has been reported that the targeted constitutive activation of PERK causes a loss of skeletal muscle mass in adult mice (17, 18). Intriguingly, forced activation of PERK in skeletal muscle of mice also improves amino acid metabolism, antioxidant response, and energy expenditure, potentially through increased expression and secretion of fibroblast growth factor (FGF)21 (17). However, the physiologic role of PERK in the regulation of skeletal muscle mass has not yet been investigated.
The primary goal of this study was to investigate the role of PERK in the regulation of skeletal muscle mass and function in adult mice and during cancer cachexia. To achieve this objective, we generated mice in which PERK can be specifically deleted in skeletal muscle through Cre-mediated recombination using the injection of tamoxifen. Our results demonstrate that the targeted deletion of PERK reduces skeletal muscle contractile function and grip strength in adult mice. Inactivation of PERK also causes a slow-to-fast fiber-type transition and skeletal muscle wasting. Moreover, knockdown of PERK causes atrophy of cultured myotubes. We also found that the ablation of PERK leads to the increased expression of components of the ubiquitin-proteasome system (UPS) and autophagy and higher activation of calpains in skeletal muscle both in vivo and in vitro. Finally, we observed that the targeted deletion of PERK increases the loss of skeletal muscle mass in LLC tumor-bearing mice.
MATERIALS AND METHODS
Mice
Skeletal muscle (sm)–specific inducible PERK knockout (KO) mice (smPERK-KO) were generated by crossing human α-skeletal actin (HSA) promoter–driven Cre mice [HSA-MerCreMer; Jax Strain: transgenic (Tg) (ACTA1-cre/Esr1*)2Kesr/J] with floxed PERK (PERKf/f; Jax strain: Eif2ak3tm1.2Drc/J) mice. All mice were bred on the C57BL6/J background, and their genotype was determined by PCR from tail DNA. The mice were housed in a 12-h light–dark cycle and given water and food ad libitum. Inactivation of PERK through Cre-mediated recombination in smPERK-KO mice was achieved by injections of tamoxifen (75 mg/kg body weight, i.p.; MilliporeSigma, Burlington, MA, USA) for 4 consecutive days. After a washout period of 4 d, the mice were fed a tamoxifen-containing chow (250 mg/kg; Harlan Laboratories, Madison, WI, USA) for the duration of the experiment. For 1 experiment, 12-wk-old mice were inoculated with 2 × 106 LLC cells in 100 μl sterile PBS (16, 19). Control mice received PBS alone. The mice were euthanized on d 21 after inoculation of LLC cells. All experimental protocols with mice were approved in advance by the Institutional Biosafety Committee and the Institutional Animal Care and Use Committee at the University of Louisville (13097 and 16663).
Generation and use of adenoviral vector for PERK shRNA
We first identified a target small interfering RNA sequence for mouse PERK mRNA using Block-it RNAi Designer online software (Thermo Fisher Scientific, Waltham, MA, USA). At least 2–3 siRNA sequences were tested for efficient knockdown of PERK mRNA in cultured myoblasts. Finally, shRNA oligonucleotides were synthesized to contain the sense strand of the target sequence for mouse PERK (GCAGGTCCTTGGTAATCATCA), a short spacer (CTCGAG), and the reverse complement sequences, followed by 5 thymidines as an RNA polymerase III transcriptional stop signal. The oligonucleotides were annealed and cloned into pLKO.1-Puro plasmid with AgeI/EcoRI sites. The insertion of shRNA sequence in the plasmid was confirmed by performing DNA sequencing. Adenovirus-carrying PERK shRNA was generated with AdEasy Adenoviral Vector System (Agilent Technologies, Santa Clara, CA, USA). In brief, PERK shRNA was PCR amplified from the pLKO.1 plasmid and inserted into the pAdTrack-CMV vector at KpnI and XbaI sites. The resulted AdTrack-CMV-PERK shRNA plasmid was linearized with PmeI and transformed into Escherichia coli BJ5183 cells stably transformed with pAdEasy-1 plasmid. Clones undergoing AdTrack–AdEasy recombination were selected with kanamycin antibiotic, and positive clones were identified by digestion with restriction endonuclease. The recombinant plasmid was linearized with PacI and transfected into the 293 T cell line (American Type Culture Collection, Manassas, VA, USA) with Effectene transfection reagent (Qiagen, Germantown, MD, USA) to package into adenovirus particles. Viruses were amplified by serial passage to concentrate. The titer was monitored under a microscope by visualizing the GFP marker coexpressed with PERK shRNA in the AdTrack–AdEasy recombinants. Finally, C2C12 myotubes were transduced with adenoviral vectors at 1:50 multiplicities of infection for 24 h.
Cell culture and indirect immunofluorescence
Mouse primary myoblasts or C2C12 myoblasts were grown in Ham’s F-10 Nutrient Mix supplemented with 20% fetal bovine serum or DMEM supplemented with 10% fetal bovine serum, respectively. The cells were differentiated into myotubes by incubation in medium containing 2% horse serum for 48–144 h. To understand the role of PERK in activating the UPS and autophagy in vitro, we transduced C2C12 myotubes with Ad.Control shRNA or Ad.PERK shRNA vectors for 24 h, after which the cells were washed and incubated in differentiation medium for an additional 48 h. Control shRNA or PERK shRNA-expressing myotubes were then treated with vehicle alone, 40 µM MG132 (for 1 h), or 100 µM chloroquine (for 1 or 2 h), after which the cells were collected and processed for immunoblot analysis. For pharmacologic inhibition of PERK studies, cultured myotubes were treated with vehicle alone or 2 μM GSK2606414 (Bio Techne, Minneapolis, MN, USA) for 48 h. After treatment, the myotubes were fixed with 4% paraformaldehyde in PBS for 15 min at room temperature and then permeabilized with 0.1% Triton X-100 in PBS for 5–8 min. Cells were blocked in 2% bovine serum albumin in PBS for 60 min and incubated with a primary mouse monoclonal sarcomeric myosin heavy chain antibody (clone MF-20, 1:250 dilution; Developmental Studies Hybridoma Bank, Iowa City, IA, USA) in blocking solution overnight at 4°C. A brief PBS wash was applied before incubation with a goat anti-mouse Alexa Fluor 568–conjugated secondary antibody (1:3000; Thermo Fisher Scientific) for 60 min at room temperature. The cultures were washed 3 times for 15 min with PBS, followed by incubation with DAPI (1:5000 dilution) for 3 min and washed 2 times for 10 min with PBS. The stained myotubes were then visualized at room temperature under a fluorescence inverted microscope (Eclipse TE 2000-U; Nikon, Melville, NY, USA) equipped with a digital camera (Digital Sight DS-Fi1; Nikon). Images were captured, and the diameter of the myotubes was measured with Fiji software (National Institutes of Health, Bethesda, MD, USA). The myotube diameter was quantified as follows: 10 fields were chosen randomly, and 10 myotubes were measured per field. The mean diameter per myotube was calculated as the means of the 3 measurements taken along the length of the myotube.
Grip strength measurements
Forepaw and total (4-paw) grip strength of mice were measured using a digital grip-strength meter (Columbus Instruments, Columbus, OH, USA) and normalized by total body weight as described in one of our studies (20). In brief, mice were acclimatized for 5 min before the beginning of the test. The mouse was allowed to grasp the forepaw pull-bar assembly and, in a separate experiment, the 4-paw pull-bar assembly. Then, the mouse was gently drawn with constant force in a straight line until it could no longer grasp the bar. The force at the time of release was recorded as the peak tension. Each mouse was tested 5 times with a 20–40-s break between tests. The mean peak tension from 5 trials normalized by total body weight was defined as forepaw (2-paw) or total (4-paw) grip strength, depending on the pull-bar assembly used.
In vivo muscle functional assay
In vivo force measurements of the posterior lower leg muscles were conducted with the 1300A 3-in-1 Whole Animal System (Aurora Scientific, Aurora, ON, Canada) to evoke plantarflexion. Mice were anesthetized with 2,2,2-tribromoethanol (Avertin; MilliporeSigma) and placed on the isothermal stage. Optimal muscle length (Lo) that allows maximal isometric twitch force (Pt) was determined by a sequence of twitch contractions at 150 Hz every 1 s with small varying changes to muscle tension and current. After muscle optimization, a mean specific twitch force (sPt) was generated from 5 stimulations at 150 Hz. To obtain maximum isometric tetanic force (Po), muscles were stimulated from 25 to 300 Hz in 25 Hz intervals resulting in 12 specific tetanic forces (sPo). There was a 30-s delay between the first 2 recordings and a 1-min delay between subsequent recordings. After the 300 Hz stimulation, a baseline recording was obtained. Then, the muscles were fatigued at 150 Hz with 1 contraction per second for 180 s. Force recordings for obtained specific isometric twitch force sPt (mN/m) and tetanic force sPo (mN/m) were normalized by total mouse body weight. For all experiments, a prestimulus and poststimulus baseline of 200 ms was recorded to establish a baseline recording, and a 0.2 ms pulse width was used. Current was adjusted on an individual basis to evoke the maximum amount of force. Contractile events were recorded with the ASI611A Dynamic Muscle Control software (Aurora Scientific) at a sampling rate of 2000 Hz. Force (mN/m) and corresponding integral values for specific twitch force (mN/m/s) and tetanic force (mN/m/s) were calculated with the accompanying ASI611A Dynamic Muscle Analysis software (Aurora Scientific).
Histology and morphometric analysis
The tibialis anterior (TA) and soleus muscles of the mice were isolated, flash frozen in liquid nitrogen, mounted in embedding medium, and sectioned with a microtome cryostat. To assess tissue morphology, we stained 10 µm–thick transverse sections with hematoxylin and eosin (H&E). The sections were examined under an inverted microscope (Eclipse TE 2000-U; Nikon) at room temperature with a Plan ×10/0.25 NA Ph1 DL or a Plan Fluor ELWD ×20/0.45 NA Ph1 DM objective lens, a digital camera (Digital Sight DS-Fi1; Nikon), and NIS Elements BR 3.10 software (Nikon). Images of H&E- or laminin-stained TA or soleus muscle sections were quantified with Fiji software to measure myofiber cross-sectional area (CSA). The distribution of myofiber CSA was calculated by analyzing ∼500 myofibers per muscle.
Immunohistochemistry
Frozen TA or soleus muscle sections were fixed in acetone for 10 min at 4°C, rehydrated with PBS for 5 min, and blocked in 2% bovine serum albumin in PBS for 60 min. Sections were incubated with a primary rabbit polyclonal laminin antibody (1:500 dilution; MilliporeSigma) in blocking solution at 4°C overnight in humidified conditions. The next day, the sections were washed briefly with PBS and then incubated with a goat anti-rabbit Alexa Fluor 546–conjugated secondary antibody (1:1500 dilution; Thermo Fisher Scientific) for 60 min at room temperature. Finally, the sections were washed 3 times for 15 min with PBS. Nuclei were counterstained with DAPI (1:5000 dilution). The slides were mounted in a nonfluorescing aqueous mounting medium (Polysciences, Warrington, PA, USA) and visualized at room temperature under an Eclipse TE 2000-U microscope (Nikon). Image levels were equally adjusted with Photoshop CS6 software (Adobe, San Jose, CA, USA).
Muscle fiber-type immunostaining
The composition of slow- and fast-type myofibers in TA and soleus muscles was analyzed according to a published protocol (16, 21). We used whole TA or soleus muscle sections to quantify the percentage of type I, IIa, IIx, and IIb myosin heavy chain (MyHC) fibers after staining with mAb against type I (clone: BA-D5-c), type IIa (clone: SC-71-c), and type IIb (clone: BF-F3-c) MyHC isoforms (Developmental Studies Hybridoma Bank).
Western blot analyses
The relative amount of various proteins was determined by performing Western blot analysis (22). In brief, gastrocnemius (GA) muscles or cell pellets were homogenized in ice-cold lysis buffer [50 mM Tris (pH 8.0), 200 mM NaCl, 50 mM NaF, 0.3% Igepal, 1 mM DTT, 1 mM benzamidine, 1 mM sodium orthovanadate, and 100 μM PMSF] supplemented with protease inhibitor cocktail (Thermo Fisher Scientific). The samples were centrifuged at 14,000 g for 10 min at 4°C, and the supernatants were collected for subsequent analyses. About 100–150 µg of protein was resolved on 10–12% SDS-polyacrylamide gel, blotted onto 0.45 μm nitrocellulose membrane, and probed overnight with specific primary antibody. The following antibodies were used for Western blot analysis: anti-PERK (3192), anti-phospho-protein kinase B (Akt) (4060), anti-Akt (2211), anti-phospho-rpS6 (4060), anti-rpS6 (2217), anti-phospho-p65 (3033), anti-p65 (8242), anti-p100/p52 (4882), anti-dynamin-related protein (DRP)1 (5391), anti-Beclin-1 (3495), anti–cleaved poly (ADP-ribose) polymerase (PARP; 9548), anti-light chain (LC)-3B (2775), and anti-GAPDH (2118) (Cell Signaling Technology, Danvers, MA, USA); and anti-ubiquitin (sc-8017; Santa Cruz Biotechnology, Dallas, TX, USA). Bound antibodies were detected by secondary antibodies conjugated to horseradish peroxidase (Cell Signaling Technology). Signal detection was performed by an ECL detection reagent (Bio-Rad, Hercules, CA, USA). Approximate molecular masses were determined by comparison with the migration of prestained protein standards (Bio-Rad). Band intensities were quantified with Fiji software.
Surface sensing of translation assay
The relative rate of protein synthesis was measured with a non-isotope–labeled surface sensing of translation (Sunset) assay (10, 21). In brief, the mice were anesthetized and given an injection of puromycin (0.04 μM/g body mass, i.p.). Hind limb muscles were collected 30 min after injection and flash frozen in liquid nitrogen. For measuring protein synthesis in vitro, C2C12 myotubes were transduced with Ad.Control shRNA or Ad.PERK shRNA vectors for 24 h, after which cells were washed and incubated in differentiation medium for an additional 48 h. Before their collection, myotubes were treated with 1 μM puromycin for exactly 30 min. Finally, protein lysates were prepared from skeletal muscles or myotubes, and newly synthesized proteins were detected by performing Western blot analysis with a primary mouse monoclonal puromycin antibody (clone 12D10, 1:500 dilution; MilliporeSigma).
Casein zymography
Activation of calpains in skeletal muscle was determined according to a protocol described by Dutt et al. (23). In brief, GA muscles were lysed in ice-cold extraction buffer [20 mM Tris (pH 7.5), 5 mM EDTA, 5 mM EGTA, 0.2% Triton X-100, 1 mM DTT, 50 μM NaF, and 50 μM sodium orthovanadate] supplemented with protease inhibitor cocktail (Thermo Fisher Scientific). The samples were centrifuged at 14,000 g for 20 min at 4°C, and the supernatants were collected for subsequent analyses. An equal amount (30 μg) of protein per sample was resolved on a 12% SDS-polyacrylamide gel containing 0.17% (w/v) casein (MilliporeSigma) in nondenaturing and nonreducing conditions at 4°C. The gels were washed briefly with distilled water followed by incubation with a calpain activation buffer [20 mM Tris (pH 7.5), 10 mM DTT, and 4 mM CaCl2] at room temperature on a shaker for 60 min. Finally, the gels were incubated with fresh calpain activation buffer at room temperature on a shaker overnight. To visualize caseinolytic activity, gels were stained with Coomassie Brilliant Blue G-250 dye at room temperature followed by extensive washing in destaining buffer (40% methanol and 10% acetic acid in distilled water). To determine calpain activity, we photographed the gels and quantified them with Fiji software.
Calpain activity assay
The activity of calpains in muscle extracts was assessed with the Calpain-Glo Protease Assay Kit (Promega, Madison, WI, USA) according to a protocol suggested by the manufacturer.
RNA isolation and real-time quantitative PCR assay
Total RNA isolated from skeletal muscle tissues of mice was subjected to reverse transcription and real-time quantitative PCR (qPCR) analysis, as previously described (16, 19). The sequence of the primers is described in Supplemental Table 1.
Statistical analyses
Results are expressed as means ± sd. An unpaired, 2-tailed Student’s t test was used to compare quantitative data populations with normal distribution and equal variance. The α-level of significance was set at 0.05 for all comparison, unless otherwise specified.
RESULTS
Targeted inactivation of PERK inhibits skeletal muscle strength and contractile function in adult mice
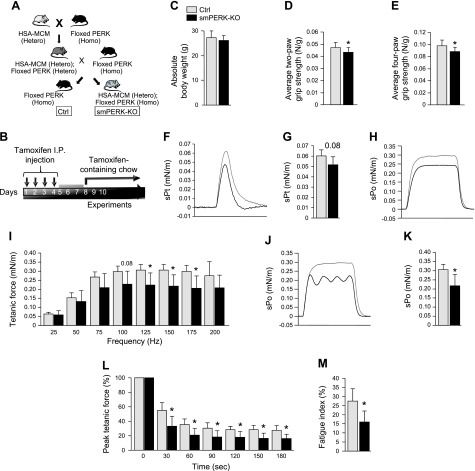
Published studies have shown that the germline deletion of PERK in mice leads to postnatal mortality, potentially because of a degeneration of β cells in the pancreas, reduced bone formation, and metabolic abnormalities (24–27). To understand the role of PERK in the regulation of skeletal muscle mass in adults, we generated skeletal muscle–specific inducible PERK- KO mice. Floxed PERK mice (24) were crossed with HSA-MerCreMer mice (28) to generate littermate PERKf/f (henceforth, control) and PERKf/f;HSA-MerCreMer (smPERK-KO) mice (Fig. 1A). To understand the role of PERK in skeletal muscle of adult mice, we gave 12-wk-old control and smPERK-KO mice daily intraperitoneal injections of tamoxifen for 4 consecutive days. The mice were then fed a diet containing tamoxifen for the duration of the experiment (Fig. 1B). Although not statistically significant, the mean body weight of smPERK-KO mice was slightly lower than that of corresponding control mice after 4 wk from the first injection of tamoxifen (Fig. 1C). We then investigated the impact of PERK deletion on skeletal muscle strength by analysis of grip strength. There was a reduction in the mean absolute forelimb (2-paw, P = 0.07) and total grip strength (4-paw, P < 0.05) in smPERK-KO mice compared with control mice (Supplemental Fig. S1A, B). Moreover, the mean forelimb and total grip strength, normalized by total body weight, were significantly reduced in smPERK-KO mice compared with control mice (Fig. 1D, E).
Figure 1 .
Ablation of PERK reduces body weight and diminishes muscle strength in adult mice. A) Schematic representation of the breeding strategy used for the generation of control (Ctrl) and smPERK-KO mice. B) Treatment protocol for tamoxifen-induced Cre-mediated recombination in smPERK-KO mice. C) Mean body weight of Ctrl and smPERK-KO mice 4 wk after the start of tamoxifen injections. D, E) Mean forelimb (D) and total 4-paw (E) grip strength per gram of body weight of Ctrl and smPERK-KO mice (n = 10–12/group). Contractile properties of the posterior compartment of the lower limb were measured in vivo. F) Representative traces of normalized specific twitch force (sPt). G) Quantification of mean specific twitch forces. H) Representative normalized traces for specific tetanic force (sPo) optimization. I) Normalized tetanic force to stimulation frequency relationship. J) Representative normalized traces for maximum tetanic force (sPo). K) Quantification of mean specific maximum tetanic forces. L) Fatigue traces over 180 s for each mouse. M) Percent fatigue index (forcet180/forcet0) for mice (n = 5/group). Error bars represent ± sd. *P < 0.05, **P < 0.01, ***P < 0.001 from corresponding littermate Ctrl mice (unpaired, 2-tailed Student’s t test).
We next evaluated skeletal muscle contractile properties in vivo by measuring the specific twitch force, force–frequency relationship, maximum isometric tetanic force, time to fatigue, and fatigue index, all normalized by body weight, of control and smPERK-KO mice (Fig. 1F–M). In addition to force values, integral values representing the amount of work between the onset and termination of muscle stimulation were calculated (Supplemental Fig. S1C–G). For these assays, we first optimized muscle length and positioning and the current, to ensure maximum force production (Fig. 1F, H). No statistically significant differences were observed in specific twitch force between smPERK-KO and control mice. However, there was a trend toward a reduction (P = 0.08) in the specific twitch force production in smPERK-KO mice compared with the control mice (Fig. 1G). There was also no significant difference in the corresponding twitch force integral values in smPERK-KO mice compared with control mice (Supplemental Fig. S1C). Upon initial stimulation of plantarflexion, we obtained normal force production waveforms in both control and smPERK-KO mice (Fig. 1H). After muscle length and position optimization, plantarflexion was stimulated at different frequencies and the specific and maximum muscle force produced in isometric contractions was measured. Tetanic force produced during low-stimulation frequency (25–75 Hz) was not significantly different between the 2 groups. Nevertheless, when generated at high-stimulation frequency (100–175 Hz), tetanic force was significantly decreased in smPERK-KO mice compared with control mice (Fig. 1I). The integral values corresponding to each frequency showed a similar trend where lower frequencies did not yield a significant difference, but the higher frequency integral values were significantly diminished in smPERK-KO mice compared with control mice (Supplemental Fig. S1D). As the force–frequency data were generated, the muscles of the smPERK-KO mice began to display an unstable tetanic force production (Fig. 1J) and a significant decrease in maximum tetanic force in smPERK-KO mice compared with control mice (Fig. 1K). Similarly, a decrease in the maximum tetanic force integral was observed in smPERK-KO mice compared with control mice (Supplemental Fig. S1E). Finally, we found that the resistance to fatigue as well as the fatigue index was significantly reduced in smPERK-KO mice compared with control mice (Fig. 1L, M). A similar trend was seen for the corresponding fatigue force integral values (Supplemental Fig. S1F, G). These results suggest that the genetic ablation of PERK reduces skeletal muscle strength and contractile properties in adult mice.
Targeted inactivation of PERK reduces skeletal muscle mass in adult mice
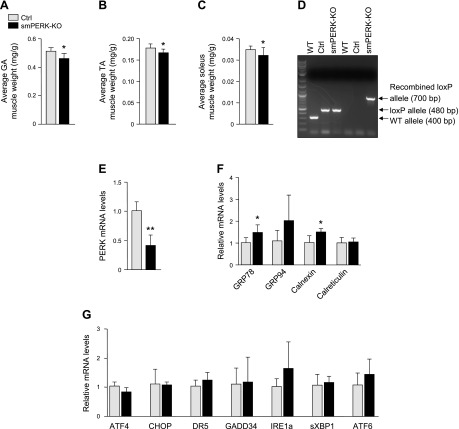
We next examined the effect of the deletion of PERK on skeletal muscle mass and gene expression of various ER stress markers. The hind limb muscles of control and smPERK-KO mice (after 4 wk of ablation of PERK) were isolated, and their wet weight was measured. We found that the mean absolute wet weights of GA and TA muscles were significantly reduced in smPERK-KO mice compared with control mice (Supplemental Fig. S2A). Wet weights of GA, TA, and soleus muscles, normalized with the total body weight, were also found to be significantly reduced in smPERK-KO mice compared with control mice (Fig. 2A–C). To evaluate the efficiency of gene disruption, PCR analysis of Cre-mediated recombination of the locus of X-over P1 (loxP) allele of PERK was performed on genomic DNA extracted from the GA muscle of WT, PERKf/f, and smPERK-KO mice. The PERK WT allele band (400 bp) was present in WT mice. The loxP-flanked allele of PERK (480 bp) was detectable in both control and smPERK-KO mice, whereas the PERK recombined loxP allele (700 bp) band appeared only in the GA muscle of smPERK-KO mice (Fig. 2D). We also observed a significant reduction in the mRNA level of PERK in GA muscle of smPERK-KO mice compared with control mice (Fig. 2E).
Figure 2 .
Deletion of PERK reduces skeletal muscle mass in adult mice. A–C) Mean wet weight of isolated GA (A), TA (B), and soleus (C) muscle of control (Ctrl) and smPERK-KO, normalized with body weight, after 4 wk of the start of tamoxifen injection (n = 9–14/group). D) PCR analysis of Cre-mediated recombination performed on genomic DNA extracted from the GA muscle of WT, Ctrl (i.e., PERKf/f), and smPERK-KO mice. An agarose gel image of PCR products is shown. Arrows: to bands corresponding to PERK WT allele (400 bp), the PERK loxP-flanked allele (480 bp) and the PERK recombined loxP allele (700 bp). E) Relative mRNA levels of PERK in GA muscle from Ctrl and smPERK-KO mice (n = 3–4/group). F) Relative mRNA levels of ER-resident chaperones, GRP-78, GRP-94, calnexin, and calreticulin in GA muscle from Ctrl and smPERK-KO mice (n = 3–5/group). G) Relative mRNA levels of select ER stress/UPR markers: ATF4, CHOP, death receptor-5, GADD34, IRE1a, spliced XBP1, and ATF6 in GA muscle of Ctrl and smPERK-KO mice (n = 3–5/group). Error bars ± sd. *P < 0.05, **P < 0.01, ***P < 0.001 vs. corresponding littermate Ctrl mice (unpaired, 2-tailed Student’s t test).
Because the PERK arm of the UPR regulates the expression of several ER-related molecular chaperones, we investigated how the expression of ER stress markers are regulated in skeletal muscle of control and smPERK-KO mice. We found that there was a small but significant increase in the mRNA levels of the peptide-binding molecular chaperone GRP78 and the lectin-like chaperone calnexin in the GA muscle of smPERK-KO mice compared with levels in the control mice. However, there was no significant difference in the mRNA levels of 2 other major ER-resident chaperones, GRP94 and calreticulin (29), in the skeletal muscle of control and smPERK-KO mice (Fig. 2F). There was also no significant difference in the mRNA levels of other markers of ER stress: ATF4, CHOP, death receptor 5, GADD34, IRE1α, spliced X-box–binding protein (XBP)1, and ATF6 in GA muscle of control and smPERK-KO mice (Fig. 2G). These results suggest that, whereas inducible deletion of PERK reduces skeletal muscle mass, it does not have any overt impact on the expression of ER stress markers or those of the UPR.
Inhibition of PERK causes skeletal muscle atrophy in vivo and in vitro
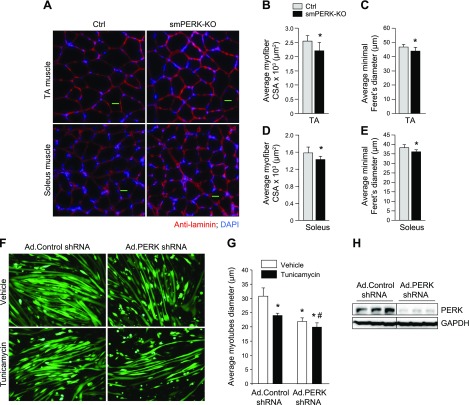
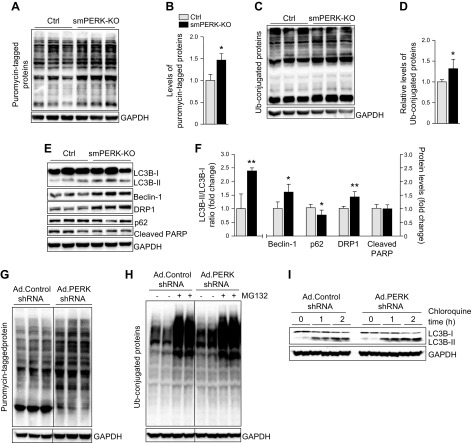
We found that wet muscle weight was significantly reduced in smPERK-KO mice, but it is not clear whether deletion of PERK results in myofiber atrophy. To investigate, we generated transverse sections of TA and soleus muscle followed by performing anti-laminin or H&E staining and morphometric analysis (Fig. 3A and Supplemental Fig. S2B). Gross examination of H&E-stained sections did not reveal any sign of myopathy, such as mononuclear infiltration or centronucleated myofibers in skeletal muscle of control or smPERK-KO mice. However, morphometric analysis showed that the mean myofiber CSA and the minimal Feret’s diameter were significantly reduced in both TA and soleus muscles of smPERK-KO mice compared with control mice (Fig. 3B–E). We also found that there was no significant difference in the number of myofibers within TA or soleus muscle of control and smPERK-KO mice (data not shown).
Figure 3 .
Ablation of PERK causes skeletal muscle atrophy. A) Representative photomicrographs of TA and soleus muscle sections from control (Ctrl) and smPERK-KO mice after staining with anti-laminin. Nuclei were counterstained with DAPI. Scale bars, 50 μm. B, C) Quantification of mean myofiber CSA (B), and mean minimal Feret’s diameter (C) in TA muscle sections (n = 4–6/group). D, E) Quantification of mean myofiber CSA (D) and mean minimal Feret’s diameter (E) in soleus muscle sections (n = 5–6/group). Error bars ± sd. *P < 0.05 vs. corresponding Ctrl mice (unpaired, 2-tailed Student’s t test). F) C2C12 myotubes were transduced with adenoviral vectors expressing control shRNA or PERK shRNA for 24 h. The cells were washed and incubated in differentiation medium for an additional 48 h, with or without 1 µg/ml tunicamycin. Representative images of the cultures are presented. Scale bars, 50 µm. G) Quantification of mean diameter of myotubes in control and PERK shRNA-expressing cultures (n = 3/group). Error bars ± sd. *P < 0.05 vs. vehicle alone-treated cultures transduced with Ad.Control shRNA, #P < 0.05 vs. vehicle alone–treated cultures transduced with Ad.PERK shRNA (unpaired, 2-tailed Student’s t test). H) Immunoblots demonstrating levels of PERK and unrelated protein GAPDH in C2C12 myotube cultures transduced with Ad.Control or Ad.PERK shRNA vectors. Horizontal solid lines on the immunoblots indicate that intervening lanes have been spliced out.
To further understand the role of PERK in the regulation of muscle mass, we next investigated the effect of PERK knockdown on the size of cultured myotubes. Because differentiated myotubes are resistant to transfection by chemical methods, we generated adenoviral vectors expressing a scrambled (control) or PERK shRNA. Fully differentiated C2C12 cultures were transduced with Ad.Control shRNA or Ad.PERK shRNA vectors for 24 h. The cultures were then incubated for an additional 48 h with vehicle alone or tunicamycin, a well-known ER stressor. We found that the mean myotube diameter was significantly reduced in vehicle-alone cultures transduced with Ad.PERK shRNA compared with those transduced with Ad.Control shRNA. Moreover, we found that the tunicamycin-induced loss of myotube diameter was further exaggerated in cultures transduced with Ad.PERK shRNA (Fig. 3F, G). Immunoblot analysis confirmed that the levels of PERK were drastically reduced in cultures transduced with Ad.PERK shRNA compared with those transduced with Ad.Control shRNA (Fig. 3H).
GSK2606414 is a pharmacologic inhibitor that also abolishes the phosphorylation of PERK in cultured myogenic cells (10, 30). Using GSK2606414, we also examined the effect of pharmacologic inhibition of PERK on the size of primary myotubes. We first isolated myoblasts from hind limb muscles of WT mice and differentiated them into myotubes. Fully differentiated myotubes were then incubated with vehicle alone or 2 µM GSK2606414 for 48 h. The cultures were then fixed and immunostained for MyHC and DAPI (Supplemental Fig. S2C). We observed a significant reduction in myotube diameter in GSK2606414-treated cultures compared with those treated with vehicle alone (Supplemental Fig. S2D). Altogether, these results suggest that PERK promotes the maintenance of skeletal muscle mass, both in vivo and in vitro.
Inhibition of PERK causes a slow-to-fast fiber-type transition in adult mice
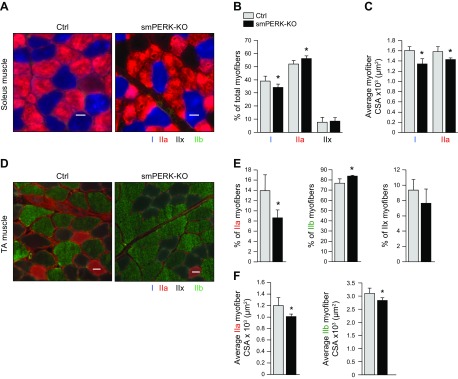
We next investigated whether the ablation of PERK affects the proportion of oxidative and glycolytic myofibers in skeletal muscle of adult mice. The soleus muscle of mice consists of mostly type I (slow, oxidative) and IIa (fast, oxidative) fibers and a limited population of type IIx (fast, oxidative/glycolytic), whereas the TA muscle is primarily composed of type IIx and IIb (fast, glycolytic) fibers and a relatively smaller proportion of IIa fibers (31, 32). Transverse muscle sections were prepared from soleus and TA muscle of control and smPERK-KO mice and immunostained with antibodies against MyHC type I, IIa, and IIb. Unstained fibers were considered type IIx. Results showed that the proportion of type I myofibers was significantly reduced, whereas the proportion of type IIa myofibers was significantly increased in soleus muscle of smPERK-KO mice compared with that of control mice (Fig. 4A–C). Similar analysis of TA muscle sections showed that the proportion of type IIa myofibers was significantly reduced, whereas the proportion of type IIb myofibers was significantly increased in smPERK-KO mice compared with control mice (Fig. 4D, E).
Figure 4 .
Inactivation of PERK causes a slow-to-fast fiber-type transition in adult mice. A) Muscle sections prepared from soleus muscle of control (Ctrl) and smPERK-KO mice were subjected to triple immunostaining against MyHCI, IIa, and IIb proteins. Representative photomicrographs of triple-stained sections of soleus muscle. Scale bar, 50 μm. B, C) Quantification of percentage of each fiber type (B) and mean myofiber CSA of type I and IIa fibers (C) in soleus muscle of Ctrl and smPERK-KO mice (n = 6/group). D) Muscle sections prepared from TA muscle of Ctrl and smPERK-KO mice were subjected to triple immunostaining against MyHCI, IIa, and IIb proteins. Representative photomicrographs of triple-stained TA muscle sections are presented here. Scale bars, 50 μm. E, F) Quantification of percentage of each fiber type (E), and mean myofiber CSA of type IIa, and IIb (F) fibers in TA muscle of Ctrl and smPERK-KO mice (n = 3–5/group). Error bars ± sd. *P < 0.05 vs. corresponding littermate Ctrl (unpaired, 2-tailed Student’s t test).
To understand whether the deletion of PERK causes atrophy in slow, fast, or both fiber-types, we quantified the mean CSA of all types of fibers in soleus and TA muscle of control and PERK-KO mice. Our analysis showed that the mean CSA of both type I and IIa fibers was significantly reduced in the soleus muscle of smPERK-KO mice compared with control mice (Fig. 4C). We also found a significant reduction in the mean CSA of type IIa and IIb fibers in the TA muscle of smPERK-KO mice compared with control mice (Fig. 4F). These results suggest that, although the inactivation of PERK promotes a slow-to-fast fiber-type transition, atrophy occurs in both slow- and fast-type myofibers.
Inactivation of PERK disrupts protein turnover and increases the expression of atrophy-related molecules
The activation of the PERK/eIF2α arm of the UPR inhibits general translation in mammalian cells (33). By performing a Sunset assay, we investigated whether the ablation of PERK affects the rate of protein synthesis in skeletal muscle of mice. We found that the rate of protein synthesis was significantly increased in skeletal muscle of smPERK-KO mice compared with control mice (Fig. 5A, B). The Akt/mammalian target of rapamycin pathway is a pivotal regulator of skeletal muscle growth in response to growth factors and exercise (1, 34). However, we found no significant difference in the levels of phosphorylated Akt or ribosomal protein S6 (rpS6) in the skeletal muscle of smPERK-KO mice compared with control mice (Supplemental Fig. S3A, B) suggesting that the ablation of PERK does not disrupt the activation of the Akt pathway in skeletal muscle, while still promoting the synthesis of new proteins.
Figure 5 .
Ablation of PERK disrupts protein turnover in skeletal muscle of adult mice. Control (Ctrl) and smPERK-KO mice were given an injection of puromycin (0.04 μM/g body weight, i.p.). The mice were euthanized 30 min later, and GA muscle was collected and processed for Western blot analysis. A) Immunoblots demonstrating levels of puromycin-tagged proteins and unrelated protein GAPDH in GA muscle of Ctrl and smPERK-KO mice. B) Densitometry quantification of relative levels of puromycin-tagged proteins in GA muscle of Ctrl and smPERK-KO mice (n = 3/group). C) Immunoblots showing relative amounts of ubiquitin-conjugated proteins and unrelated protein GAPDH in GA muscle of Ctrl and smPERK-KO mice. D) Densitometry quantification of relative levels of ubiquitin-conjugated proteins (n = 4–5/group). E) Representative immunoblots showing levels of autophagy markers: LC-3BI/II, Beclin-1, DRP1, p62, and an apoptosis marker, cleaved PARP, and unrelated protein GAPDH in GA muscle of Ctrl and smPERK-KO mice. F) Densitometry quantification of levels of LC-3BII/I, Beclin-1, DRP1, p62, and cleaved PARP (n = 3–5/group). Error bars ± sd. *P < 0.05, **P < 0.01 from corresponding Ctrl mice (unpaired, 2-tailed Student’s t test). G) Fully differentiated C2C12 myotubes were transduced with adenoviral vectors expressing control shRNA or PERK shRNA for 24 h. The cells were washed and incubated in differentiation medium for an additional 48 h. The myotubes were treated with 1 μM puromycin for exactly 30 min, followed by analysis for puromycin-tagged proteins. Immunoblot shows the levels of puromycin-tagged protein in Ad.Control shRNA and Ad.PERK shRNA cultures. H, I) In a separate experiment, control shRNA or PERK shRNA-expressing myotube cultures were treated with vehicle alone, 40 µM MG132 (for 1 h), or 100 µM chloroquine (for 1 or 2 h) followed by Western blot analysis to detect the levels of ubiquitinated protein or LC-3BI/II protein. Representative immunoblots from 2 independent experiments demonstrating levels of ubiquitin-conjugated proteins (H), and LC-3BI/II and unrelated protein GAPDH (I) in control shRNA- and PERK shRNA–expressing cultures. Black lines on the immunoblots indicate that intervening lanes have been spliced out.
The bulk of protein degradation in atrophying skeletal muscle occurs through the UPS and autophagy (2). By performing immunoblot analysis, we first determined relative amounts of ubiquitin-conjugated proteins in skeletal muscle of control and smPERK-KO mice. There was a significant increase in the levels of ubiquitin-tagged proteins in skeletal muscle of smPERK-KO mice compared with control mice (Fig. 5C, D). NF-κB is a proinflammatory transcription factor that can be activated through a canonical or noncanonical signaling pathway (4). In addition to augmenting gene expression of proinflammatory molecules, NF-κB has been found to increase the expression of a few components of the UPS in atrophying skeletal muscle (4). We found that the levels p100 and p52 proteins (markers of the noncanonical NF-κB pathway) were significantly elevated, whereas there was no significant difference in the levels of phosphorylated p65 protein (a marker of the canonical NF-κB pathway) in skeletal muscle of control and smPERK-KO mice (Supplemental Fig. S3A, B). These results suggest that PERK regulates the activation of the UPS and noncanonical NF-κB signaling in skeletal muscle.
We next investigated whether PERK regulates the activation of autophagy in skeletal muscle of adult mice. There was a significant increase in autophagy-related proteins: Beclin-1, DRP1, and the LC-3BII:LC-3BI ratio in skeletal muscle of smPERK-KO mice compared with control mice. Moreover, the levels of p62 protein, which is degraded through autophagy, were found to be significantly reduced in skeletal muscle of smPERK-KO mice compared with control mice, further suggesting that the inhibition of PERK activates autophagy in skeletal muscle (Fig. 5E, F). In the same experiment, we did not find any significant difference in the levels of cleaved PARP, a marker of apoptosis, in skeletal muscle of control and smPERK-KO mice (Fig. 5E, F).
In another experiment, we investigated the effect of shRNA-mediated knockdown of PERK on the rate of protein synthesis and activation of the UPS and autophagy in cultured myotubes. The results showed that the levels of puromycin-tagged proteins (in the Sunset assay) were increased in PERK shRNA-expressing cultures compared with control cultures (Fig. 5G). We also found that the levels of ubiquitin-tagged proteins, as well as LC-3BII, were increased in PERK-knockdown myotubes. Inhibition of the UPS or autophagy flux using MG132 or chloroquine, respectively, demonstrated that knockdown of PERK stimulates these proteolytic systems in cultured myotubes (Fig. 5H, I).
We also measured the gene expression of some other molecules that regulate skeletal muscle mass. Class IIa histone deacetylases (HDAC) isoforms, HDAC4 and 5, promote skeletal muscle atrophy through increasing the expression of myogenin, a basic helix–loop–helix transcription factor (35, 36). Our real-time qPCR analysis showed that the mRNA levels of HDAC5 and myogenin were significantly increased in the skeletal muscle of smPERK-KO mice compared with control mice (Supplemental Fig. S3C). Although not statistically significant, there was an increase in mRNA levels of HDAC4 in the smPERK-KO mice vs. control mice.
Fibroblast growth factors (FGFs) play pivotal roles in the proliferation and differentiation of various cell types. FGF19, 21, and 23 are members of the same FGF subset, called the FGF19 subfamily (37). FGF21 is expressed in skeletal muscle, and its level is increased in various catabolic conditions (38–40). In addition, it was recently reported that FGF19 drives hypertrophic effects on skeletal muscle mass (41). We found that the mRNA levels of FGF21 were significantly increased, whereas transcript levels of FGF19 were significantly decreased in the skeletal muscle of smPERK-KO mice compared with control mice (Supplemental Fig. S3D). We also found that there was an increase (P = 0.07) in the mRNA expression of their coreceptor, β-Klotho, although it was not statistically significant (Supplemental Fig. S3D). FGF23 is also expressed in skeletal muscle and known to improve exercise performance in mice (42). Our results showed that the mRNA levels of FGF23 were significantly reduced in the skeletal muscle of smPERK-KO mice compared with control mice. These results suggest that the genetic ablation of PERK causes skeletal muscle wasting, potentially through dysregulation of the UPS, autophagy, HDAC isoforms, and members of the FGF19 subfamily in adult mice.
Targeted ablation of PERK activates calpains in skeletal muscle
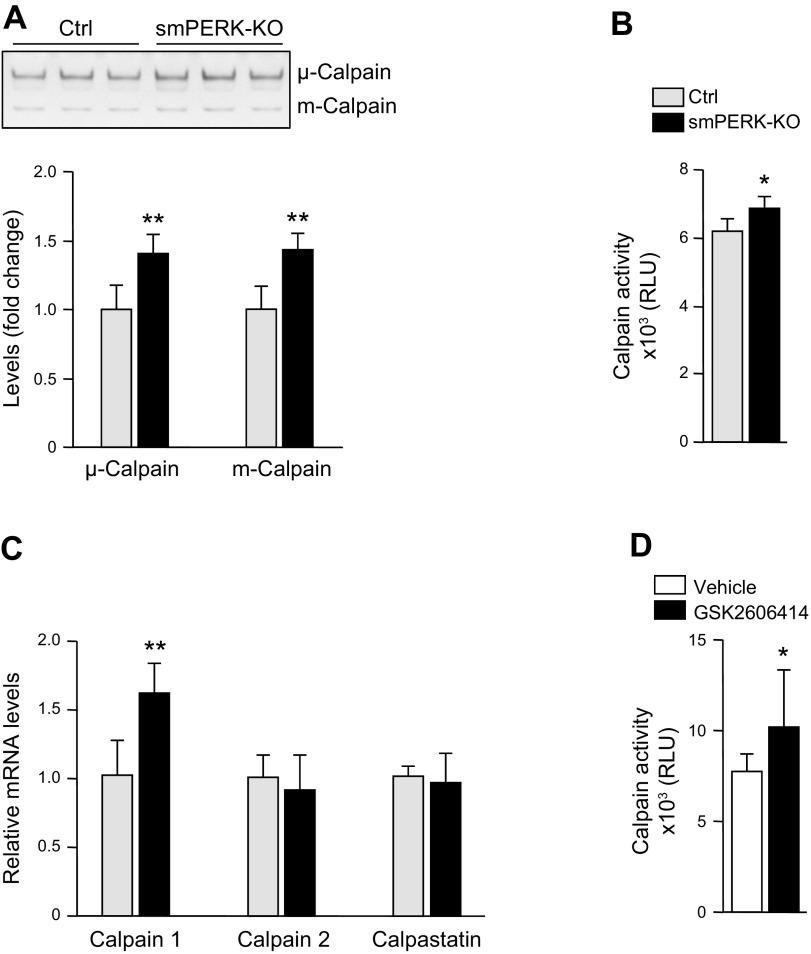
We observed that skeletal muscle of smPERK-KO mice fails to generate stable tetanic force, which could be related to aberrant calcium handling. Previous studies have shown that an acute inhibition of PERK in human and rodent β-cells causes disruption in subcellular calcium signaling because of alterations in store-operated Ca2+ entry and sarcoplasmic ER Ca2+-ATPase activity. Calpains are nonlysosomal cysteine proteases that are activated by calcium (43). The activity of calpains is also modulated by calpastatin, a specific endogenous inhibitor of calpains (44). Calpains are also known to cause muscle proteolysis in multiple catabolic conditions (45). By performing casein zymography, we first studied proteolytic activity of 2 ubiquitous calpain isoforms, μ-calpain (calpain 1) and m-calpain (calpain 2), in skeletal muscle of control and smPERK-KO mice. A significant increase in both μ- and m-calpain activity was observed in the skeletal muscle of smPERK-KO mice compared with control mice (Fig. 6A). Using a commercially available kit (Promega), we next measured the enzymatic activity of calpains in muscle extracts. Consistent with casein zymography, there was a significant increase in the enzymatic activity of calpains in the skeletal muscle of smPERK-KO mice compared with the control mice (Fig. 6B). Our real-time qPCR analysis showed that, although the mRNA levels of calpain 2 and calpastatin remained unchanged, there was a significant increase in the mRNA levels of calpain 1 in skeletal muscle of smPERK-KO mice compared with contol mice (Fig. 6C). In a separate experiment, we also studied the effect of pharmacologic inhibition of PERK on the enzymatic activity of calpains in cultured myotubes. As shown in Fig. 6D, treatment with GSK2606414 significantly increased calpain activity in cultured myotubes. Collectively, these results suggest that the molecular or pharmacologic inhibition of PERK increases the activity of calpains in skeletal muscle.
Figure 6 .
Ablation of PERK activates calpains in skeletal muscle. A) Representative photograph and quantification of bands of μ- and m-calpain in a casein zymogram performed on GA muscle extracts from control (Ctrl) and smPERK-KO mice (n = 4/group). B) Enzymatic activity of calpains in GA muscle of Ctrl and smPERK-KO mice (n = 4 per group). C) Relative mRNA levels of calpain 1, calpain 2, and calpastatin in GA muscle of Ctrl and smPERK-KO mice (n = 4–5/group). D) Differentiated myotubes were incubated with vehicle alone (DMSO) or 2 µM GSK2606414 for 48 h, and protein extracts were analyzed for calpain activity (n = 8/group). Error bars ± sd. *P < 0.05, **P < 0.01 vs. corresponding littermate Ctrl mice (unpaired, 2-tailed Student’s t test).
Role of PERK in the regulation of skeletal muscle mass during cancer cachexia
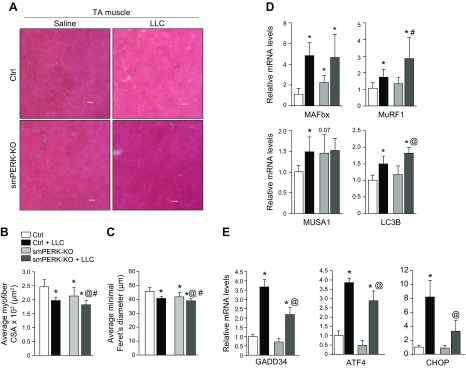
We have previously shown that the PERK arm of the UPR is activated in skeletal muscle in 2 models of cancer cachexia (16). However, the role of PERK in the regulation of skeletal muscle mass in the tumor-bearing host remains unknown. To understand the role of PERK in skeletal muscle wasting during cancer growth, control and smPERK-KO were inoculated in the left flank with 2 × 106 LLC cells. The mice were euthanized 21 d after implantation of LLC, and right hind limb muscles were isolated and analyzed by performing H&E staining (Fig. 7A). Consistent with our preceding results, the mean myofiber CSA and minimal Feret’s diameter in TA muscle were significantly reduced in smPERK-KO mice compared with control mice (Fig. 7B, C). As expected, inoculation of LLC cells in control mice resulted in a significant reduction in myofiber size. We found that the mean size of myofibers in TA muscle was further reduced in LLC-bearing smPERK-KO mice compared with control smPERK-KO mice (Fig. 7B, C). We also quantified mRNA levels of a few markers of the UPS and autophagy. A significant increase in the mRNA levels of muscle atrophy F-box/Atrogin-1, muscle ring finger (MuRF)1, muscle ubiquitin ligase of Skp, Cullin, F-box–containing complex in atrophy 1, and LC3B was observed in skeletal muscle of LLC-bearing mice compared with their corresponding controls. Although not statistically significant, there was also an increase in the mRNA levels of MuRF1 and LC3B in skeletal muscle of LLC-bearing smPERK-KO mice compared with control mice (Fig. 7D). We have shown that LLC tumor increases the gene expression of molecules regulated through the PERK arm of the UPR (16). We investigated how the gene expression of various markers of the PERK/eIF2α pathway is regulated in skeletal muscle of LLC tumor-bearing smPERK-KO mice. Results showed that LLC-induced gene expression of GADD34, ATF4, and CHOP was significantly reduced in skeletal muscle of smPERK-KO mice compared with control mice (Fig. 7E). Altogether, these results suggest that the targeted inhibition of PERK does not attenuate LLC tumor–induced muscle wasting in mice. In fact, the ablation of PERK leads to additional atrophy in skeletal muscle of LLC tumor-bearing mice.
Figure 7 .
Effect of ablation of PERK on skeletal muscle wasting during cancer-induced cachexia. Control (Ctrl) and smPERK-KO mice were inoculated with 2 × 106 LLC cells in 100 μl of sterile PBS in the left flank. After 21 d, mice were euthanized for histologic and biochemical analysis. A) Representative photomicrographs of H&E-stained TA muscle sections from Ctrl and smPERK-KO mice. Scale bars, 50 μm. B, C) Quantification of mean myofiber CSA (B) and minimal Feret’s diameter (C) of myofibers in TA muscle sections of control (Ctrl) and smPERK-KO mice (n = 7–10/group). D) Relative mRNA levels of muscle atrophy F-box/Atrogin-1, MuRF1, MUSA1, and LC3B in GA muscle of Ctrl and smPERK-KO mice (n = 3–6/group). E) Relative mRNA levels of GADD34, ATF4, and CHOP in TA muscle of Ctrl and smPERK-KO mice (n = 3/group). Error bars ± sd. *P < 0.05 vs. Ctrl mice injected with saline alone, #P < 0.05 vs. smPERK-KO mice injected with saline alone, @P < 0.05 vs. Ctrl LLC-bearing mice (unpaired, 2-tailed Student’s t test).
DISCUSSION
Accumulation of unfolded proteins leads to ER stress and the activation of the UPR (i.e., PERK, IRE1α, and ATF6 pathways) in mammalian cells. The UPR either resolves the stress through increasing the protein folding capacity in the ER or by initiating apoptosis in the cell. In addition, to detect protein folding errors, the PERK arm of the UPR is involved in the regulation of calcium levels in the ER. Moreover, once the stress has been relieved, the PERK pathway terminates the UPR through increasing the expression of GADD34, which promotes dephosphorylation of eIF2α, leading to recovery from translational inhibition (46, 47). Recent studies have provided evidence that UPR pathways, including PERK, play important roles in the regulation of myogenesis and satellite-cell–mediated myofiber regeneration in adult mice (10, 48–51). However, the role of UPR pathways in the regulation of skeletal muscle mass remain largely unknown.
Our results demonstrate that skeletal muscle of smPERK-KO mice does not generate stable force and undergoes fatigue at a faster rate compared with control mice (Fig. 1). Previous studies have shown that PERK acts as a calcium sensor in the ER and its ablation disrupts calcium homoeostasis (52). Because muscle contraction is governed by the regulated release and uptake of Ca2+ ions by sarcoplasmic/ER, a reduction in the overall force production and the inability to develop stabilized force in isometric contractions in smPERK-KO mice may be a result of aberrant calcium homeostasis and signaling. Alternatively, deletion of PERK may diminish the quantity or quality of contractile proteins, a common feature of atrophying skeletal muscle (2, 19, 53), leading to reduced force production and fatigability. Indeed, muscle mass and myofiber size are significantly reduced after 4 wk of ablation of PERK in adult mice (Figs. 2 and 3). Further, it is within the realm of plausibility that the reduction of force production and muscle mass are occurring in tandem.
We have previously reported that the pan inhibition of ER stress through prolonged administration of 4-PBA causes a loss of skeletal muscle mass and strength in adult mice (16). However, one of the drawbacks of the study was that 4-PBA inhibited ER stress, leading to the inhibition of all of the 3 arms of the UPR, not only in skeletal muscle, but also in other tissues. Moreover, being a pharmacologic compound, 4-PBA can disrupt the activity of other signaling and metabolic pathways in vivo. Our present study provides genetic evidence that the skeletal muscle–specific inhibition of PERK causes a noticeable atrophy in adult mice. We also found that the proportion of fast-type myofibers, which are known to undergo atrophy at a higher rate (54), is increased in smPERK-KO mice, further emphasizing that the deletion of PERK leads to skeletal muscle wasting in mice (Fig. 4). The loss of skeletal muscle mass and fiber-type transition observed in smPERK-KO is similar to that observed through pan inhibition of ER stress using 4-PBA in WT mice (16). Although we found a significant reduction in muscle mass and myofiber size in smPERK-KO mice within 4 wk of inactivation of PERK, the effect was not dramatic. This finding could be attributable to the difficulty in completely inactivating a gene by using tissue-specific Cre lines. Moreover, we measured progressive muscle atrophy in naive conditions, but not in response to acute stimuli, such as denervation or unloading, which cause a more rapid loss of skeletal muscle mass. Finally, it is possible that some of the effects of the ablation of PERK are compensated for through the activation of other pathways in skeletal muscle of mice in naive conditions. Indeed, we found that acute inhibition of PERK through shRNA-mediated knockdown (Fig. 3F–H) or pharmacologic inhibition (Supplemental Fig. S2C, D) leads to drastic loss in the mean diameter of cultured myotubes that is comparable to that observed in response to proinflammatory cytokines and conditioned medium of tumor cells that cause cachexia (16, 55).
Through the generation of skeletal muscle–specific Fv2E-PERK [a fusion protein of artificial dimerization domain (Fv2E) and PERK kinase domain] Tg mice, Miyake et al. (17, 18) recently investigated the effects of forced activation of PERK on the regulation of skeletal muscle mass and metabolic function. These studies showed that while improving whole body metabolism, skeletal muscle mass and strength were reduced upon inducible expression of an activated PERK isoform (17, 18). A major function of the PERK/eIF2α pathway is to attenuate global protein synthesis while augmenting synthesis of a few select proteins, such as ATF4 (5, 6, 11). ATF4 has been found to cause skeletal muscle atrophy during starvation (56). Therefore, the loss of skeletal muscle mass in Fv2E-PERK transgender mice could be a result of inhibition of protein synthesis, activation of ATF4–dependent pathways, or both.
Our results demonstrate that the inducible ablation of PERK also causes muscle atrophy in adult mice, suggesting that a certain level of PERK is essential for the maintenance of skeletal muscle mass, whereas its overexpression causes muscle atrophy, as has been found in Fv2E-PERK transgender mice (17, 18). Consistent with the inhibitory effect of PERK on translation initiation, we found that the inhibition of PERK significantly increased the rate of protein synthesis in skeletal muscle both in vivo and in vitro (Fig. 5A, B, G). This increase in protein synthesis appears to be a direct result of PERK inhibition and not the result of the disruption of other regulatory pathways, given that we found no significant difference in phosphorylation levels of the components of the Akt/mammalian target of rapamycin signaling pathway, a major regulator of protein synthesis (Supplemental Fig. S3A, B). However, why skeletal muscle of smPERK-KO mice undergoes atrophy, even though the rate of protein synthesis is increased, remains an enigma. It is possible that increases in the rate of protein synthesis results in an increase in unfolded protein load in the ER and ensuing stress that is not resolved because of the deficiency of PERK. There is also a possibility that a lack of PERK-mediated UPR leads to the activation of other intracellular pathways and proteolytic systems. In fact, our results demonstrate that levels of ubiquitin-conjugated proteins are increased in skeletal muscle of smPERK-KO mice as well as in cultured myotubes upon knockdown of PERK, suggesting an increase in the activity of the UPS (Fig. 5C, D, H).
Depending on the stimulus or underlying conditions, activation of the UPR pathways can stimulate or inhibit autophagy in mammalian cells (11, 57). We observed that levels of multiple autophagy-related proteins are increased in skeletal muscle of smPERK-KO mice (Fig. 5E, F) and in cultured myotubes after knockdown of PERK (Fig. 5I). These results are consistent with a previously published report also suggesting that the components of the PERK/eIF2α pathway inhibits autophagy in denervated skeletal muscle of mice (15). Furthermore, we observed a small but significant increase in the activation of calpains in skeletal muscle of smPERK-KO mice (Fig. 6). Because calpains are calcium-dependent enzymes, their increased activation could be related to aberrant calcium homeostasis in skeletal muscle related to a lack of PERK. Therefore, the disruption of proteostasis appears to be one of the important mechanisms for the loss of skeletal muscle mass and function in smPERK-KO mice.
Our results also demonstrate that gene expression of endocrine-acting FGF19 subfamily molecules is dysregulated in skeletal muscle of smPERK-KO mice. Consistent with the role of FGF21 in skeletal muscle atrophy (38–40), we found that the mRNA level of FGF21 is increased in skeletal muscle of smPERK-KO (Supplemental Fig. S3D). It is noteworthy that the level of FGF21 is also increased in Fv2E-PERK Tg mice (17). Because ER stress is an important stimulus of FGF21 production (40), the increased expression of FGF21 in skeletal muscle of smPERK-KO or Fv2E-PERK Tg mice could be a result of dysregulation of ER stress/UPR pathways. We also found that the mRNA levels of FGF19 and 23, which stimulate muscle growth (41, 42), are diminished in skeletal muscle of mice after ablation of PERK. More investigations are needed, but dysregulation of the FGF19 subfamily of molecules could be another mechanism for muscle atrophy in smPERK-KO mice.
Accumulating evidence suggests that the markers of ER stress and the UPR are increased in skeletal muscle in multiple physiologic and pathologic conditions (5, 11). We have previously reported that the PERK arm of the UPR is induced in skeletal muscle of mice within 24 h of withholding food (14). Several components of the PERK arm of the UPR have also been found to be increased in skeletal muscle of aged rats or mice (58, 59). The exact role of the UPR in the regulation of skeletal muscle mass during aging is unknown, but a study has suggested that ER stress is not involved in anabolic resistance in skeletal muscle of aged mice (59). By contrast, the markers of ER stress have been found to be increased in adult mice fed with a high-fat diet, as well as in a genetic model of murine obesity (ob/ob). In fact, there is evidence that ER stress plays a key role in the development of insulin resistance and inflammation in multiple organs, including the liver and adipose tissues (11, 60, 61). Although the cause-and-effect relationship has yet to be established, ER stress may also be involved in the development of insulin resistance in skeletal muscle (11, 62, 63). A recent study has also shown that a high-fat diet increases the protein levels of BiP, IRE1α, and phospho-PERK and the gene expression of XBP1, ATF4, and CHOP in skeletal muscle of mice (62). Furthermore, several ER stress markers have been found to be elevated in muscle biopsies from patients with myositis and in skeletal muscle of mouse models of inflammatory myopathy (64–66). We have reported that markers of all 3 arms of the UPR are increased in skeletal muscle during cancer cachexia (16), suggesting that heightened ER stress in skeletal muscle is a common feature of multiple conditions involving muscle wasting and metabolic dysfunction.
The present study also demonstrates that the inhibition of PERK does not attenuate muscle wasting during cancer cachexia. However, at present, we cannot rule out the possibility that PERK regulates other aspects of muscle health, including metabolic function, during cancer cachexia. Because all 3 arms of the UPR are activated during cancer cachexia (5), it is possible that the IRE1α or ATF6 arm of the UPR plays a more pronounced role in the regulation of skeletal muscle mass during cancer cachexia. It is also possible that the role of individual arms of the UPR in skeletal muscle is dependent on the underlying condition and duration of activation. As our results show, a basal level of activation of PERK is necessary for the maintenance of skeletal muscle mass and contractile function in naive conditions. By contrast, chronic heightened activation of PERK can lead to skeletal muscle wasting, which was also evidenced in Fv2E-PERK Tg mice (17, 18). In fact, studies in other tissues have provided unequivocal evidence that UPR pathways act like a double-edged sword for the cell and for normal physiology (7, 8).
In summary, our study provides initial evidence that the PERK arm of the UPR is important for the maintenance of skeletal muscle mass in adults. Because PERK regulates protein folding and calcium homeostasis, it is important to investigate how these functions of PERK are affected in skeletal muscle of smPERK-KO mice. How the activation of PERK is regulated in conditions of muscle growth and atrophy and whether modulation of its activity can improve skeletal muscle mass in various catabolic states is also an important area of future research.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Disease Grants AG029623, AR059810 and AR068313 (to A.K.) Figure 1A was produced with the assistance of Servier Medical Art (https://smart.servier.com). The authors declare no conflicts of interest.
Glossary
- 4-PBA
4-phenylbutyrate
- Akt
protein kinase B
- ATF
activating transcription factor
- CHOP
C/EBP homologous protein
- CSA
cross-sectional area
- DRP
dynamin-related protein
- eIF2α
eukaryotic translation initiation factor 2α
- ER
endoplasmic reticulum
- FGF
Fibroblast growth factor
- GA
gastrocnemius
- GADD
growth arrest and DNA damage-inducible protein
- GRP
glucose-regulated protein
- H&E
hematoxylin and eosin
- HDAC
histone deacetylase
- IRE
inositol-requiring protein
- KO
knockout
- LC
light chain
- LLC
Lewis lung carcinoma
- loxP
locus of X over P1
- MuRF
muscle ring finger
- MyHC
myosin heavy chain
- PARP
poly (ADP-ribose) polymerase
- PERK
protein kinase R–like endoplasmic reticulum kinase
- qPCR
quantitiative PCR
- shRNA
short hairpin RNA
- smPERK-KO
skeletal muscle PERK-KO
- Sunset
surface sensing of translation
- TA
tibialis anterior
- Tg
transgenic
- UPR
unfolded protein response
- UPS
ubiquitin-proteasome system
- WT
wild type
- XBP
X-box-binding protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. Kumar conceived and designed the study; Y. S. Gallot and A. Kumar wrote the original draft of the manuscript; Y. S. Gallot, K. R. Bohnert, A. R. Straughn, G. Xiong, and S. M. Hindi performed the experiments and data analysis; and all authors edited the manuscript.
REFERENCES
- 1.Egerman, M. A., Glass, D. J. (2014) Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 49, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonaldo, P., Sandri, M. (2013) Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 6, 25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kandarian, S. C., Jackman, R. W. (2006) Intracellular signaling during skeletal muscle atrophy. Muscle Nerve 33, 155–165 [DOI] [PubMed] [Google Scholar]
- 4.Li, H., Malhotra, S., Kumar, A. (2008) Nuclear factor-kappa B signaling in skeletal muscle atrophy. J. Mol. Med. (Berl.) 86, 1113–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohnert, K. R., McMillan, J. D., Kumar, A. (2018) Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease. J. Cell. Physiol. 233, 67–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hetz, C., Chevet, E., Oakes, S. A. (2015) Proteostasis control by the unfolded protein response. Nat. Cell Biol. 17, 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotamisligil, G. S. (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hetz, C. (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 [DOI] [PubMed] [Google Scholar]
- 9.Wang, M., Kaufman, R. J. (2014) The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 14, 581–597 [DOI] [PubMed] [Google Scholar]
- 10.Xiong, G., Hindi, S. M., Mann, A. K., Gallot, Y. S., Bohnert, K. R., Cavener, D. R., Whittemore, S. R., Kumar, A. (2017) The PERK arm of the unfolded protein response regulates satellite cell-mediated skeletal muscle regeneration. Elife 6, e22871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Afroze, D., Kumar, A. (2017) ER stress in skeletal muscle remodeling and myopathies. [E-pub ahead of print] FEBS J. doi:10.1111/febs.14358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu, J., Ruas, J. L., Estall, J. L., Rasbach, K. A., Choi, J. H., Ye, L., Boström, P., Tyra, H. M., Crawford, R. W., Campbell, K. P., Rutkowski, D. T., Kaufman, R. J., Spiegelman, B. M. (2011) The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab. 13, 160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma, L., Chu, W., Chai, J., Shen, C., Li, D., Wang, X. (2017) ER stress and subsequent activated calpain play a pivotal role in skeletal muscle wasting after severe burn injury. PLoS One 12, e0186128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paul, P. K., Bhatnagar, S., Mishra, V., Srivastava, S., Darnay, B. G., Choi, Y., Kumar, A. (2012) The E3 ubiquitin ligase TRAF6 intercedes in starvation-induced skeletal muscle atrophy through multiple mechanisms. Mol. Cell. Biol. 32, 1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu, Z., Wang, A. M., Adachi, H., Katsuno, M., Sobue, G., Yue, Z., Robins, D. M., Lieberman, A. P. (2011) Macroautophagy is regulated by the UPR-mediator CHOP and accentuates the phenotype of SBMA mice. PLoS Genet. 7, e1002321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohnert, K. R., Gallot, Y. S., Sato, S., Xiong, G., Hindi, S. M., Kumar, A. (2016) Inhibition of ER stress and unfolding protein response pathways causes skeletal muscle wasting during cancer cachexia. FASEB J. 30, 3053–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyake, M., Nomura, A., Ogura, A., Takehana, K., Kitahara, Y., Takahara, K., Tsugawa, K., Miyamoto, C., Miura, N., Sato, R., Kurahashi, K., Harding, H. P., Oyadomari, M., Ron, D., Oyadomari, S. (2016) Skeletal muscle-specific eukaryotic translation initiation factor 2α phosphorylation controls amino acid metabolism and fibroblast growth factor 21-mediated non-cell-autonomous energy metabolism. FASEB J. 30, 798–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyake, M., Kuroda, M., Kiyonari, H., Takehana, K., Hisanaga, S., Morimoto, M., Zhang, J., Oyadomari, M., Sakaue, H., Oyadomari, S. (2017) Ligand-induced rapid skeletal muscle atrophy in HSA-Fv2E-PERK transgenic mice. PLoS One 12, e0179955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paul, P. K., Gupta, S. K., Bhatnagar, S., Panguluri, S. K., Darnay, B. G., Choi, Y., Kumar, A. (2010) Targeted ablation of TRAF6 inhibits skeletal muscle wasting in mice. J. Cell Biol. 191, 1395–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hindi, S. M., Sato, S., Choi, Y., Kumar, A. (2014) Distinct roles of TRAF6 at early and late stages of muscle pathology in the mdx model of Duchenne muscular dystrophy. Hum. Mol. Genet. 23, 1492–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hindi, S. M., Sato, S., Xiong, G., Bohnert, K. R., Gibb, A. A., Gallot, Y. S., McMillan, J. D., Hill, B. G., Uchida, S., Kumar, A. (2018) TAK1 regulates skeletal muscle mass and mitochondrial function. JCI Insight 3, e98441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallot, Y. S., McMillan, J. D., Xiong, G., Bohnert, K. R., Straughn, A. R., Hill, B. G., Kumar, A. (2017) Distinct roles of TRAF6 and TAK1 in the regulation of adipocyte survival, thermogenesis program, and high-fat diet-induced obesity. Oncotarget 8, 112565–112583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutt, V., Saini, V., Gupta, P., Kaur, N., Bala, M., Gujar, R., Grewal, A., Gupta, S., Dua, A., Mittal, A. (2018) S-allyl cysteine inhibits TNFα-induced skeletal muscle wasting through suppressing proteolysis and expression of inflammatory molecules. Biochim. Biophys. Acta 1862, 895–906 [DOI] [PubMed] [Google Scholar]
- 24.Zhang, P., McGrath, B., Li, S., Frank, A., Zambito, F., Reinert, J., Gannon, M., Ma, K., McNaughton, K., Cavener, D. R. (2002) The PERK eukaryotic initiation factor 2 alpha kinase is required for the development of the skeletal system, postnatal growth, and the function and viability of the pancreas. Mol. Cell. Biol. 22, 3864–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang, W., Feng, D., Li, Y., Iida, K., McGrath, B., Cavener, D. R. (2006) PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab. 4, 491–497 [DOI] [PubMed] [Google Scholar]
- 26.Harding, H. P., Zeng, H., Zhang, Y., Jungries, R., Chung, P., Plesken, H., Sabatini, D. D., Ron, D. (2001) Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol. Cell 7, 1153–1163 [DOI] [PubMed] [Google Scholar]
- 27.Harding, H. P., Zhang, Y., Zeng, H., Novoa, I., Lu, P. D., Calfon, M., Sadri, N., Yun, C., Popko, B., Paules, R., Stojdl, D. F., Bell, J. C., Hettmann, T., Leiden, J. M., Ron, D. (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 [DOI] [PubMed] [Google Scholar]
- 28.McCarthy, J. J., Srikuea, R., Kirby, T. J., Peterson, C. A., Esser, K. A. (2012) Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skelet. Muscle 2, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaufman, R. J. (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 13, 1211–1233 [DOI] [PubMed] [Google Scholar]
- 30.Axten, J. M., Romeril, S. P., Shu, A., Ralph, J., Medina, J. R., Feng, Y., Li, W. H., Grant, S. W., Heerding, D. A., Minthorn, E., Mencken, T., Gaul, N., Goetz, A., Stanley, T., Hassell, A. M., Gampe, R. T., Atkins, C., Kumar, R. (2013) Discovery of GSK2656157: an optimized PERK inhibitor selected for preclinical development. ACS Med. Chem. Lett. 4, 964–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agbulut, O., Noirez, P., Beaumont, F., Butler-Browne, G. (2003) Myosin heavy chain isoforms in postnatal muscle development of mice. Biol. Cell 95, 399–406 [DOI] [PubMed] [Google Scholar]
- 32.Kammoun, M., Cassar-Malek, I., Meunier, B., Picard, B. (2014) A simplified immunohistochemical classification of skeletal muscle fibres in mouse. Eur. J. Histochem. 58, 2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harding, H. P., Zhang, Y., Ron, D. (1999) Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 [DOI] [PubMed] [Google Scholar]
- 34.Bodine, S. C., Stitt, T. N., Gonzalez, M., Kline, W. O., Stover, G. L., Bauerlein, R., Zlotchenko, E., Scrimgeour, A., Lawrence, J. C., Glass, D. J., Yancopoulos, G. D. (2001) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat. Cell Biol. 3, 1014–1019 [DOI] [PubMed] [Google Scholar]
- 35.Mielcarek, M., Toczek, M., Smeets, C. J., Franklin, S. A., Bondulich, M. K., Jolinon, N., Muller, T., Ahmed, M., Dick, J. R., Piotrowska, I., Greensmith, L., Smolenski, R. T., Bates, G. P. (2015) HDAC4-myogenin axis as an important marker of HD-related skeletal muscle atrophy. PLoS Genet. 11, e1005021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moresi, V., Williams, A. H., Meadows, E., Flynn, J. M., Potthoff, M. J., McAnally, J., Shelton, J. M., Backs, J., Klein, W. H., Richardson, J. A., Bassel-Duby, R., Olson, E. N. (2010) Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 143, 35–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ornitz, D. M., Itoh, N. (2015) The fibroblast growth factor signaling pathway. Wiley Interdiscip. Rev. Dev. Biol. 4, 215–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumiya, Y., Bina, H. A., Ouchi, N., Akasaki, Y., Kharitonenkov, A., Walsh, K. (2008) FGF21 is an Akt-regulated myokine. FEBS Lett. 582, 3805–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tezze, C., Romanello, V., Desbats, M. A., Fadini, G. P., Albiero, M., Favaro, G., Ciciliot, S., Soriano, M. E., Morbidoni, V., Cerqua, C., Loefler, S., Kern, H., Franceschi, C., Salvioli, S., Conte, M., Blaauw, B., Zampieri, S., Salviati, L., Scorrano, L., Sandri, M. (2017) Age-associated loss of OPA1 in muscle impacts muscle mass, metabolic homeostasis, systemic inflammation, and epithelial senescence. Cell Metab. 25, 1374–1389.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guridi, M., Tintignac, L. A., Lin, S., Kupr, B., Castets, P., Rüegg, M. A. (2015) Activation of mTORC1 in skeletal muscle regulates whole-body metabolism through FGF21. Sci. Signal. 8, ra113 [DOI] [PubMed] [Google Scholar]
- 41.Benoit, B., Meugnier, E., Castelli, M., Chanon, S., Vieille-Marchiset, A., Durand, C., Bendridi, N., Pesenti, S., Monternier, P. A., Durieux, A. C., Freyssenet, D., Rieusset, J., Lefai, E., Vidal, H., Ruzzin, J. (2017) Fibroblast growth factor 19 regulates skeletal muscle mass and ameliorates muscle wasting in mice. Nat. Med. 23, 990–996 [DOI] [PubMed] [Google Scholar]
- 42.Li, D. J., Fu, H., Zhao, T., Ni, M., Shen, F. M. (2016) Exercise-stimulated FGF23 promotes exercise performance via controlling the excess reactive oxygen species production and enhancing mitochondrial function in skeletal muscle. Metabolism 65, 747–756 [DOI] [PubMed] [Google Scholar]
- 43.Ohno, S., Emori, Y., Imajoh, S., Kawasaki, H., Kisaragi, M., Suzuki, K. (1984) Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature 312, 566–570 [DOI] [PubMed] [Google Scholar]
- 44.Murachi, T., Tanaka, K., Hatanaka, M., Murakami, T. (1980) Intracellular Ca2+-dependent protease (calpain) and its high-molecular-weight endogenous inhibitor (calpastatin). Adv. Enzyme Regul. 19, 407–424 [DOI] [PubMed] [Google Scholar]
- 45.Bartoli, M., Richard, I. (2005) Calpains in muscle wasting. Int. J. Biochem. Cell Biol. 37, 2115–2133 [DOI] [PubMed] [Google Scholar]
- 46.Ron, D., Walter, P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 47.Novoa, I., Zeng, H., Harding, H. P., Ron, D. (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J. Cell Biol. 153, 1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakanishi, K., Sudo, T., Morishima, N. (2005) Endoplasmic reticulum stress signaling transmitted by ATF6 mediates apoptosis during muscle development. J. Cell Biol. 169, 555–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zismanov, V., Chichkov, V., Colangelo, V., Jamet, S., Wang, S., Syme, A., Koromilas, A. E., Crist, C. (2016) Phosphorylation of eIF2α is a translational control mechanism regulating muscle stem cell quiescence and self-renewal. Cell Stem Cell 18, 79–90 [DOI] [PubMed] [Google Scholar]
- 50.Acosta-Alvear, D., Zhou, Y., Blais, A., Tsikitis, M., Lents, N. H., Arias, C., Lennon, C. J., Kluger, Y., Dynlacht, B. D. (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol. Cell 27, 53–66 [DOI] [PubMed] [Google Scholar]
- 51.Alter, J., Bengal, E. (2011) Stress-induced C/EBP homology protein (CHOP) represses MyoD transcription to delay myoblast differentiation. PLoS One 6, e29498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang, S. H., Zhang, W., McGrath, B. C., Zhang, P., Cavener, D. R. (2006) PERK (eIF2alpha kinase) is required to activate the stress-activated MAPKs and induce the expression of immediate-early genes upon disruption of ER calcium homoeostasis. Biochem. J. 393, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Acharyya, S., Ladner, K. J., Nelsen, L. L., Damrauer, J., Reiser, P. J., Swoap, S., Guttridge, D. C. (2004) Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Invest. 114, 370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciciliot, S., Rossi, A. C., Dyar, K. A., Blaauw, B., Schiaffino, S. (2013) Muscle type and fiber type specificity in muscle wasting. Int. J. Biochem. Cell Biol. 45, 2191–2199 [DOI] [PubMed] [Google Scholar]
- 55.Bhatnagar, S., Mittal, A., Gupta, S. K., Kumar, A. (2012) TWEAK causes myotube atrophy through coordinated activation of ubiquitin-proteasome system, autophagy, and caspases. J. Cell. Physiol. 227, 1042–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebert, S. M., Monteys, A. M., Fox, D. K., Bongers, K. S., Shields, B. E., Malmberg, S. E., Davidson, B. L., Suneja, M., Adams, C. M. (2010) The transcription factor ATF4 promotes skeletal myofiber atrophy during fasting. Mol. Endocrinol. 24, 790–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rashid, H. O., Yadav, R. K., Kim, H. R., Chae, H. J. (2015) ER stress: autophagy induction, inhibition and selection. Autophagy 11, 1956–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwee, D. T., Baehr, L. M., Philp, A., Baar, K., Bodine, S. C. (2014) Maintenance of muscle mass and load-induced growth in muscle RING finger 1 null mice with age. Aging Cell 13, 92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chalil, S., Pierre, N., Bakker, A. D., Manders, R. J., Pletsers, A., Francaux, M., Klein-Nulend, J., Jaspers, R. T., Deldicque, L. (2015) Aging related ER stress is not responsible for anabolic resistance in mouse skeletal muscle. Biochem. Biophys. Res. Commun. 468, 702–707 [DOI] [PubMed] [Google Scholar]
- 60.Ozcan, U., Cao, Q., Yilmaz, E., Lee, A. H., Iwakoshi, N. N., Ozdelen, E., Tuncman, G., Görgün, C., Glimcher, L. H., Hotamisligil, G. S. (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 [DOI] [PubMed] [Google Scholar]
- 61.Kawasaki, N., Asada, R., Saito, A., Kanemoto, S., Imaizumi, K. (2012) Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2, 799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deldicque, L., Cani, P. D., Philp, A., Raymackers, J. M., Meakin, P. J., Ashford, M. L., Delzenne, N. M., Francaux, M., Baar, K. (2010) The unfolded protein response is activated in skeletal muscle by high-fat feeding: potential role in the downregulation of protein synthesis. Am. J. Physiol. Endocrinol. Metab. 299, E695–E705 [DOI] [PubMed] [Google Scholar]
- 63.Rieusset, J., Chauvin, M. A., Durand, A., Bravard, A., Laugerette, F., Michalski, M. C., Vidal, H. (2012) Reduction of endoplasmic reticulum stress using chemical chaperones or Grp78 overexpression does not protect muscle cells from palmitate-induced insulin resistance. Biochem. Biophys. Res. Commun. 417, 439–445 [DOI] [PubMed] [Google Scholar]
- 64.Vitadello, M., Doria, A., Tarricone, E., Ghirardello, A., Gorza, L. (2010) Myofiber stress-response in myositis: parallel investigations on patients and experimental animal models of muscle regeneration and systemic inflammation. Arthritis Res. Ther. 12, R52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nagaraju, K., Casciola-Rosen, L., Lundberg, I., Rawat, R., Cutting, S., Thapliyal, R., Chang, J., Dwivedi, S., Mitsak, M., Chen, Y. W., Plotz, P., Rosen, A., Hoffman, E., Raben, N. (2005) Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 52, 1824–1835 [DOI] [PubMed] [Google Scholar]
- 66.Li, C. K., Knopp, P., Moncrieffe, H., Singh, B., Shah, S., Nagaraju, K., Varsani, H., Gao, B., Wedderburn, L. R. (2009) Overexpression of MHC class I heavy chain protein in young skeletal muscle leads to severe myositis: implications for juvenile myositis. Am. J. Pathol. 175, 1030–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.