Abstract
KCNE5 is an X-linked gene encoding KCNE5, an ancillary subunit to voltage-gated potassium (KV) channels. Human KCNE5 mutations are associated with atrial fibrillation (AF)– and Brugada syndrome (BrS)–induced cardiac arrhythmias that can arise from increased potassium current in cardiomyocytes. Seeking to establish underlying molecular mechanisms, we created and studied Kcne5 knockout (Kcne5−/0) mice. Intracardiac ECG revealed that Kcne5 deletion caused ventricular premature beats, increased susceptibility to induction of polymorphic ventricular tachycardia (60 vs. 24% in Kcne5+/0 mice), and 10% shorter ventricular refractory period. Kcne5 deletion increased mean ventricular myocyte KV current density in the apex and also in the subpopulation of septal myocytes that lack fast transient outward current (Ito,f). The current increases arose from an apex-specific increase in slow transient outward current-1 (IKslow,1) (conducted by KV1.5) and Ito,f (conducted by KV4) and an increase in IKslow,2 (conducted by KV2.1) in both apex and septum. Kcne5 protein localized to the intercalated discs in ventricular myocytes, where KV2.1 was also detected in both Kcne5−/0 and Kcne5+/0 mice. In HL-1 cardiac cells and human embryonic kidney cells, KCNE5 and KV2.1 colocalized at the cell surface, but predominantly in intracellular vesicles, suggesting that Kcne5 deletion increases IK,slow2 by reducing KV2.1 intracellular sequestration. The human AF-associated mutation KCNE5-L65F negative shifted the voltage dependence of KV2.1-KCNE5 channels, increasing their maximum current density >2-fold, whereas BrS-associated KCNE5 mutations produced more subtle negative shifts in KV2.1 voltage dependence. The findings represent the first reported native role for Kcne5 and the first demonstrated Kcne regulation of KV2.1 in mouse heart. Increased KV current is a manifestation of KCNE5 disruption that is most likely common to both mouse and human hearts, providing a plausible mechanistic basis for human KCNE5-linked AF and BrS.—David, J.-P., Lisewski, U., Crump, S. M., Jepps, T. A., Bocksteins, E., Wilck, N., Lossie, J., Roepke, T. K., Schmitt, N., Abbott, G. W. Deletion in mice of X-linked, Brugada syndrome– and atrial fibrillation–associated Kcne5 augments ventricular KV currents and predisposes to ventricular arrhythmia.
Keywords: MiRP4, I Kslow , I to , potassium channel
The potassium voltage-gated channel, subfamily E (KCNE) family of ion channel β subunits consists of 5 members, KCNE1–5, also called minK and minK-related peptides 1–4 respectively. They are single-pass membrane proteins with an extracellular N terminus and an intracellular C terminus and act as ancillary subunits for various voltage-gated potassium (KV) and other channels (for reviews, see refs, 1–4). Expression profiles vary, yet all KCNE genes are reportedly expressed in the human heart (5–7). Likewise, all KCNE family members have been linked to cardiac arrhythmias, including atrial fibrillation (AF), Brugada syndrome (BrS), and long QT syndrome (8). Best characterized is the role of KCNE1 in the heart, where it is considered the primary β-subunit for the KV7.1 (KCNQ1) channel in the human heart. Together, KV7.1 and KCNE1 are thought to be the predominant molecular correlate of the slow delayed rectifier potassium current (IKs) which is a major contributor to the repolarization of human ventricular myocyte action potentials (9–11).
Whereas KCNE1-4 are relatively well studied, knowledge of the X-linked KCNE5 (also termed KCNE1L) gene is limited. KCNE5 was originally identified as one of several deleted genes in patients with the rare Alport syndrome, mental retardation, midface hypoplasia, and elliptocytosis contiguous gene syndrome characterized by a large deletion of the X chromosome (Xq22.3) (12). Notable features of the disease are Alport syndrome, mental retardation, midface hypoplasia, and elliptocytosis (13, 14). In one study, affected men show a more complex phenotype including mild abnormalities of the heart: 1 patient displayed a slow right atrial rhythm and short PR interval and also had some minor cardiac morphologic abnormalities (12). Several KCNE5 variants are associated with AF or BrS, and different ion channels have been shown to be misregulated by the various KCNE5 variants, including the KV7.1 and KV4 channels (15–17). In addition, we have demonstrated that the KCNE5 protein interacts with KV2.1 in a heterologous expression system, suppressing KV2.1 current density without changing kinetic parameters (18).
Despite the association of genetic variation in KCNE5 with disease, knowledge regarding the underlying molecular mechanisms is sparse. Therefore, for the present study, we generated Kcne5 knockout (Kcne5−/0) mice to determine the implications for cardiac function. Moreover, we examined how human KCNE5 mutations that are linked to AF and BrS affect the KV2.1 current.
MATERIALS AND METHODS
Kcne5 knockout mice
Kcne5 knockout mice were generated by the commercial provider Taconic (Oxnard, CA, USA) from a cryopreserved Kcne5 knockout line (Taconic TF0342, background: 129/SvEv-C57BL/6) in their knockout repository. In these mice, the Kcne5 gene is completely deleted. Positive clones were screened by Southern blot for correct targeting events. Genotyping was performed by PCR with primers 18 (5′-TGTATGCTTCATTCAGGGCC-3′) and 27 (5′-GGGTTCAAATGATCTTCCTGCC-3′) to yield products of 313 bp for wild-type (WT) or 376 bp for mutant animals. Mice were bred and genotyped at Taconic and delivered to our university facilities at least 2 wk before the experiments. The mice were provided food and water ad libitum and housed in a room kept at 22°C with a 12-h light-dark schedule. Knockout (hemizygous, Kcne5−/0)) male mice were bred from WT (Kcne5+/0) or hemizygous males (Kcne5−/0) with heterozygous (Kcne5−/+) female mice. Kcne5−/0 mice are viable and fertile. We also generated a second Kcne5−/0 mouse line, on a C57BL/6NCrl background, in collaboration with Dr. Boris Jerchow [Transgenic Core Facility, Max Delbrück Center for Molecular Medicine (MDC), Berlin, Germany], using a zinc finger approach (Supplemental Fig. S1). To verify the successful homology-directed repair in founder animals, we used PCR and enzyme digestion, as well as sequencing. Specific primers (Supplemental Fig. S1A, yellow and blue) were used for PCR analysis of genomic DNA. Positive founder animals, the DNA of which contained the new restriction site, displayed 2 fragments instead of 1 (Supplemental Fig. S1B). In addition, the positive founder animals were examined by sequencing (Supplemental Fig. S1C, primers yellow and gray). Sequence analysis showed correct homology-directed repair with the 3 stop codons (Supplemental Fig. S1C, underlined red) and the new TfiI restriction site (underlined green) in the investigated animal. The breeding strategy for the generation and maintenance of Kcne5−/0 mice was as follows: y/+ (male) x−/+ (female) to receive male y/− (Kcne5−/0 mice) and y/+ (Kcne5 WT littermates). Genotyping was performed by PCR (Supplemental Fig. S1D). Neither Kcne5−/0 mouse line exhibited cardiac hypertrophy, other overt structural defects, or KVα subunit transcript remodeling; each exhibited similar ventricular myocyte KV current upregulation. We conclude that the functional effects we observed in this study are specific to Kcne5 deletion, as they arose in 2 different strains generated by different methodologies.
All animal work conformed to national guidelines for the protection of vertebrate animals used for experimental and other scientific purposes. All mice were housed in pathogen-free facilities, and experiments were approved by the Institutional Animal Care and Use Committees at University of California, Irvine, and at MDC-Berlin. Studies were performed during the light cycle, in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (NIH), Bethesda, MD, USA. For all experiments requiring postmortem harvest of Kcne5−/0 mouse tissue, each mouse was euthanized by CO2 inhalation before cervical dislocation.
Heart size analysis
To compare the size of the hearts of adult (8-mo-old) male Kcne5−/0 mice with age-matched control (WT), the mice were euthanized with CO2 before cervical dislocation. After opening of the chest cavities, their hearts were transected below the major vessels and rinsed free of blood in normal saline. The saline was removed from the hearts with a napkin before being weighed. Furthermore, we isolated the right tibia, which was rinsed to remove skin and muscle tissue and measured from the crest to the medial malleolus. The size of the hearts was normalized to the length of the tibia and reported as the ratio of heart size (mg)/tibia length (mm). The unpaired Student’s t test with Welch’s correction was performed with Prism (GraphPad Software, La Jolla, CA, USA) for statistical analysis. Values of P < 0.05 were considered statistically significant. Data are expressed as means ± sem.
Body surface electrocardiograph recordings
Mice were anesthetized with 2% isoflurane in oxygen and were spontaneously breathing. They were placed on a heating pad to avoid a dramatic drop in body temperature, while the subcutaneous needle electrodes on shield cables were placed on the left side near the middle part of the rib cage (plus electrode), in the right foreleg pit (minus electrode), and in the right hind leg (reference electrode), to record a single-lead surface ECG. Before signals were recorded, the isoflurane in the oxygen was lowered to 1.75%, and the heating pad was disconnected because of an increase in electrical noise. Signals were recorded for 5 min with Powerlab 8/35 (ADInstruments, Dunedin, New Zealand) at 2 kHz and filtered with a high-pass setting of 0.3 Hz and a low-pass setting of 1 kHz. Beats within min 1 and 4 of the recording were used to generate a mean-signal ECG and were analyzed manually with LabChart 7 (ADinstruments). The isoelectric level was calculated by the program from the pre-P wave segment or from the interval between the end of the P-wave and the beginning of the Q-wave, if possible. Because of electrical noise, the end of the T-wave was difficult to determine on the mean signal. Hence, the QT interval could not be determined accurately. Therefore, for each ECG recording, the mean QT interval was manually determined from 5 single heart beats showing a minimum of electrical noise. Unpaired Student’s t test with Welch’s correction was performed with Prism 5.01 for statistical analysis and values of P < 0.05 were considered statistically significant. Pooled data are expressed as means ± sem.
Invasive electrophysiology studies
For invasive electrophysiology studies, we used a digital electrophysiology lab (EP Tracer; CardioTek, Maastricht, The Netherlands) and octapolar 2-French electrode catheters (CIBermouse Cath; Numed, Cross Roads, TX, USA), to determine standard parameters of electrical conduction and refractoriness and to test for inducibility of arrhythmias. Mice were lightly anesthetized with isoflurane (1.6 vol. % isoflurane/air) at a constant body temperature. The catheter was placed via the right jugular vein into the right atrium and ventricle. We performed programmed electrical stimulation with a standardized protocol with documentation of occurrence and duration of induced arrhythmias (19).
RNA extraction and reverse transcription
For expression analysis, mouse atria and the walls of each ventricle were isolated without excising the whole heart, frozen in liquid nitrogen, and kept at −80°C until further use. RNA was extracted from the atria and ventricles of male Kcne5−/0 and control (Kcne5+/0) mice by using the RNeasy RNA Purification Kit (Qiagen, Germantown, MD, USA), according to the manufacturer’s protocol. The RNA was reverse-transcribed with the QuantiTect Reverse Transcription Kit (Qiagen) according to the manufacturer’s instructions.
Human cardiac tissue samples from donor hearts that were unsuitable for transplantation were kindly provided by Dr. András Varró (University of Szeged, Szeged, Hungary). The procedure was approved by the national ethics committee, ethics approval number 4991-0/2010-1018EKU (339/PI/010), and informed consent was given to use the tissue for research purposes. The investigation conformed to the principles outlined in the Declaration of Helsinki. All donors received 25,000 Na-heparin, flushing solution (Custodiol; Dr. Franz Köhler Chemie, Bensheim, Germany), methylprednisolone sodium succinate (Solu-Medrol; Pfizer, New York, NY, USA), norephnephrine (Arterenol; MilliporeSigma, Burlington, MA, USA), and hydroxyethyl starch (Voluven; Pfizer) before heart explantation. The hearts were placed in ice-cold cardioplegic solution, cut into pieces, frozen, and stored in liquid nitrogen until further use. RNA extraction and cDNA generation from human heart tissues were performed as described by Soltysinska et al. (20).
Quantitative PCR
Quantitative analyses of the KCNE5/Kcne5 gene in the cDNA samples from human and mouse hearts were performed in duplicate reactions of 20 μl volumes containing 10 μl PrimerDesign 2X Precision Mastermix (Primerdesign, Southampton, United Kingdom) with the 7300 qPCR System (Thermo Fisher Scientific, Waltham, MA, USA) and the CFX96 Real-Time PCR System (Bio-Rad, Hercules, CA, USA), respectively. Data were obtained with SDS1.2 software and CFX96 software, respectively. Double-dye assays were designed and synthesized by Primerdesign (primer sequences for mouse, sense: 5′-GCACGAAGAGACCTCAGACAT-3′, antisense: 5′-GGACAGGAAAACAAGAACACCAT-3′; and for human, sense: 5′-TACTTCCAAATGCCTCTCCTCTAT-3′, antisense: 5′-AACAACCTTTATTACCTGCCTCTG-3′). The amplification protocol used for each reaction was as follows: a 10 min initiation step at 95°C before 40 cycles of 15 s at 95°C and 1 min at 60°C. For optimal normalization analysis of the cDNA samples, we used the geNorm Reference Gene Selection Kit (Primerdesign) (21). β-Actin (ACTB) and topoisomerase-1 (TOP1) were chosen as optimal reference genes for the human cDNA samples, and for mouse cDNA samples, glyceraldehyde phosphate dehydrogenase, and ActB were used (Primerdesign). The 2−ΔCt method (22) was used to determine the relative expression of KCNE5/Kcne5 in both human and mouse cDNA samples. A 2-way ANOVA with Bonferroni posthoc and an unpaired Student’s t test with Welch’s correction were performed with using Prism 5.01 (GraphPad) for statistical analysis. Values of P < 0.05 were considered statistically significant. Data are expressed as means ± sem.
Plasmids
Human KV2.1 (GenBank Accession number NM_004975) in the eGFP-N1 vector (Takara Bio USA, Mountain View, CA, USA) and human KCNE5 (hKCNE5, NM_012282) in pXOOM are described in refs. 18 and 20. Mutant constructs P33S (c.97C > T), L65F (c.193C > T), R85H (c.254G > A), Y81H (c.241T > C) or D92E/E93X (c.276-277CG > AT) were generated by mutated oligo extension PCR with hKCNE5 in pXOOM as template using PfuUltraII HS Fusion Polymerase (Agilent Technologies, Santa Clara, CA, USA) and subsequent restriction with DpnI (Thermo Fisher Scientific). Reaction samples were transformed into Escherichia coli XL1 Blue cells applying a standard heat-shock procedure. After plasmid DNA preparation, constructs were verified by complete DNA sequencing of the cDNA insert (Macrogen, Seoul, South Korea).
Ventricular tissue immunofluorescence
Cryosections (8 mm) from snap-frozen ventricular tissue were fixed with ice-cold acetone for 10 min at 4°C, blocked, and permeabilized in 10% goat serum, 0.3% Triton X-100 and 0.2% bovine serum albumin for 30 min. The following primary antibodies were used and incubated overnight at 4°C: rabbit anti-Kcne5 (1:20; MilliporeSigma, Burlington, MA, USA), mouse anti-β-catenin (1:20; MilliporeSigma), rabbit anti-KV2.1 (1:20; Thermo Fisher Scientific), and mouse anti-Cx43 (1:20; Thermo Fisher Scientific). As secondary antibodies we used AlexaFluor 555 goat anti-rabbit and AlexaFluor 647 goat anti-mouse (1:1000; Thermo Fisher Scientific), incubated for 60 min at room temperature. Kcne5 staining was amplified with biotin-conjugated anti-rabbit (1:500; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) for 30 min followed by incubation with streptavidin-conjugated AlexaFluor 555 (1:1000; Thermo Fisher Scientific) for 60 min at room temperature. Stained cryosections were mounted with fluorescence mounting medium (Agilent Technologies) and imaged on a confocal laser scanning microscope (TSC SP5 with software, ver. LAS AF 2.632; Leica Microsystems, Wetzlar, Germany). Images were assembled in Photoshop CS4 (Adobe, San Diego, CA, USA) and CorelDraw 34 (Corel, Ottawa, ON, Canada).
Electrophysiological analysis of adult mouse cardiomyocytes
Ventricular cardiomyocytes were isolated from 7-mo-old male mice, as described in detail by Hu et al. (23). Only rod-shaped, quiescent cardiomyocytes with clear striations were used for recording. Whole-cell patch-clamp recordings from dispersed ventricular myocytes (derived from 2 to 8 mice of each genotype) were obtained at room temperature with an IX50 inverted microscope (Olympus, Center Valley, PA, USA) equipped with an FHD chamber from IonOptix (Westwood MA, USA) and a Multiclamp 700A Amplifier, a Digidata 1300 Analog/Digital converter, and a PC with pClamp9.2 software (all from Molecular Devices, Sunnyvale, CA, USA). For whole-cell patch-clamp recordings, the bath solution contained (in mM) 117 NaCl, 4 KCl, 1.7 MgCl2, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1 KH2PO4, 4 NaHCO3, 3 CoCl2, and 10 d-glucose (pH 7.4 with NaOH). Bath solutions were treated with either no drug, 50 μM 4-aminopyridine (4-AP; ICN Biomedicals, Irvine, CA, USA), 25 mM tetraethylammonium (TEA; MilliporeSigma), or 500 nM heteropodatoxin (HpTx2) for KV current inhibition. Pipettes were of 2.0–3.1 MΩ resistance when filled with intracellular solution containing (in millimolars) 130 KCl, 2 MgCl2, 20 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 11 EGTA, 5 Na2ATP, 0.4 Na2GTP, and 5 Na2CP (pH 7.4 with KOH). Outward K+ currents were evoked during 4-s voltage steps to test potentials between −60 and +60 mV in 20 mV increments from a holding potential of −70 mV after a 100-ms prepulse to −40 mV. Uncorrected leak currents were always <100 pA. Data were analyzed with pClamp9.2 software (Molecular Devices), and statistical analysis (ANOVA) was performed with Origin 6.1 (Microcal, Northampton, MA, USA).
Human embryonic kidney cell culture, transient transfection, and patch-clamp electrophysiology
Human embryonic kidney (HEK)293 cells were cultured as previously described (18). HEK293 cell cultures (70–80% confluence) were cotransfected with 250 ng KV2.1 and 1 µg WT or mutant hKCNE5 with Lipofectamine 2000 reagent (Thermo Fisher Scientific). Green fluorescent protein cDNA (0.5 µg) was cotransfected as a transfection marker. Transfected cells were dissociated with trypsin and used for electrophysiological analysis 16–24 h after transfection. Patch-clamp recordings from HEK293 cells were performed as previously described (18). Series resistance was compensated (80%) for, and cells with a voltage error exceeding 5 mV after compensation were excluded from analysis. The Boltzmann equation, y = 1/{1+exp[−(V−V1/2)/k]}, where V is the voltage applied, V1/2 is the voltage at which 50% of the channels are (in)activated, and k is the slope factor, was used to fit the voltage-dependence of activation and inactivation. Single and double exponential functions were used to fit the activation and deactivation kinetics, respectively. Student’s t test or Mann-Whitney rank sum test was used for statistical analysis, and values of P < 0.05 were considered statistically significant.
HL-1 cell culture, transient transfection, and imaging
HL-1 cells were cultured and transfected in gelatin/fibronectin-coated T25 flasks (Nunc, Roskilde, Denmark) with Claycomb medium (MilliporeSigma), supplemented with 10% fetal bovine serum (Batch 11A568; MilliporeSigma), 100 U/ml penicillin (Thermo Fisher Scientific), 100 mg/ml streptomycin (Thermo Fisher Scientific), 0.1 mM norepinephrine (MilliporeSigma), and 2 mM l-glutamine (MilliporeSigma) at 37°C in 5% CO2. At 90–100% confluence, the cells were trypsinized and resuspended in 5 ml medium, and 1.25 ml cell solution was transfected with 2 μg DNA in total, with 3.5 μl Silentfect Lipid Reagent (Bio-Rad) according to the manufacturer’s protocol. Transfected cells were plated into a 35-mm tissue culture dish (Greiner Bio-One, Frickenhausen, Germany) containing 4 glass cover slips (diameter, 12 mm; Thermo Fisher Scientific) and coated with gelatin/fibronectin. The cells were incubated at 37°C in 5% CO2 for 16–24 h before they were fixed by adding 4% paraformaldehyde in PBS for 10 min at room temperature.
Immunofluorescence staining and imaging with the LSM710 or LSM780 laser scanning confocal microscope (Zeiss, Jena, Germany) were performed with published methods (18, 24). Primary antibodies were: rabbit polyclonal anti-KCNE5 (1:100, in-house) (18), mouse monoclonal anti-KV2.1 (1:100, clone K39/25; NeuroMab, University of California, Davis/NIH NeuroMab Facility, Davis, CA, USA), and rat monoclonal anti-hemagglutinin (HA) (1:50, clone 3F10; Roche Diagnostics, Indianapolis, IN, USA). Alexa-Fluor 647–conjugated and rhodamine-conjugated phalloidin (1.5 U/ml; Thermo Fisher Scientific) were used as plasma membrane markers in HL-1 cells. Rat monoclonal anti-lysosomal-associated membrane protein (LAMP)-2 (1:100, clone GL2A7; Abcam, Cambridge, United Kingdom) was used as a lysosomal marker. Depending on the antibodies used, the plasma membrane was visualized with either rhodamine-conjugated phalloidin or Alexa Fluor 647 phalloidin to identify the submembranous F-actin in HL-1 cell secondary antibodies: AlexaFluor 488 donkey anti-rabbit IgG [heavy (H)+light (L) chain] (1:200), AlexaFluor 488 donkey anti-mouse IgG (H+L) (1:200), AlexaFluor 488 donkey anti-rat (H+L) (1:200), AlexaFluor 568 donkey anti-rabbit IgG (H+L) (1:500), and AlexaFluor 555 donkey anti-mouse IgG (H+L) (1:500). All secondary antibodies were purchased from Thermo Fisher Scientific.
RESULTS
The KCNE5 gene is expressed in both mouse and human hearts
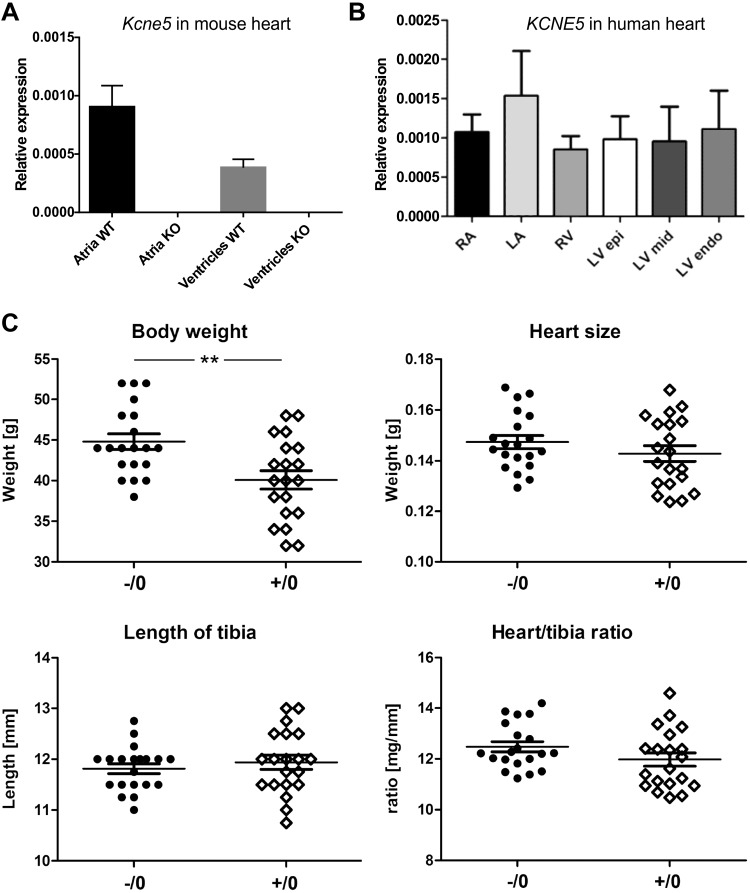
In WT (Kcne5+/0) male mice, we detected Kcne5 transcript in both the atria and ventricles (Fig. 1A). Because the level of KCNE5 expression in the human heart is still a matter of debate (5–7), we also analyzed the relative expression in cardiac tissue samples from 6 male donor hearts, and found that the KCNE5 gene was expressed in all human heart regions analyzed (Fig. 1B).
Figure 1 .
Kcne5 is expressed in mouse and human hearts. A) Quantification of Kcne5 transcript expression in atria and ventricles (n = 5 and n = 6, respectively) for male Kcne5+/0 (WT) and Kcne5−/0 (knockout) mice by real-time qPCR, normalized to the reference genes ActB and glyceraldehyde phosphate dehydrogenase. Data are means ± sem. B) Relative KCNE5 regional expression in male human hearts (n = 6): left (LA) and right (RA) atria and the left ventricular epi-, mid- and endomyocardial wall. No significant differences in expression levels between the regions were found. Quantification was by real-time qPCR with ACTB and TOP1 as reference genes. C) Body weight, heart size, and tibia length of ∼8-mo-old male mice (n = 20 in each group; means ± sem shown). Kcne5−/0 mice showed a significant increase in body weight (44.8 ± 1.0 g) compared to control mice (40.1 ± 1.1 g). P = 0.0030. Heart size (0.147 ± 0.003 vs. 0.143 ± 0.003 g, respectively) and the length of tibia (11.8 ± 0.1 vs. 11.9 ± 0.1 mm, respectively) did not show any differences. Likewise, there was no difference in heart size when normalized to tibia length [(mg/mm) 12.5 ± 0.2 vs. 12.0 ± 0.3, respectively]. **P < 0.01.
Kcne5 deletion predisposes to ventricular arrhythmia
Because we found that Kcne2−/− pups from homozygous crosses show cardiac hypertrophy as early as 3 wk (25), we investigated whether deletion of Kcne5 had a similar effect (Fig. 1C). Kcne5 deletion did not alter heart weight, normalized to either body weight or tibia length, in either of the strains we assessed (Fig. 1C and Supplemental Table S1). In addition, echocardiographic analysis uncovered no evidence of cardiac hypertrophy (Supplemental Table S2).
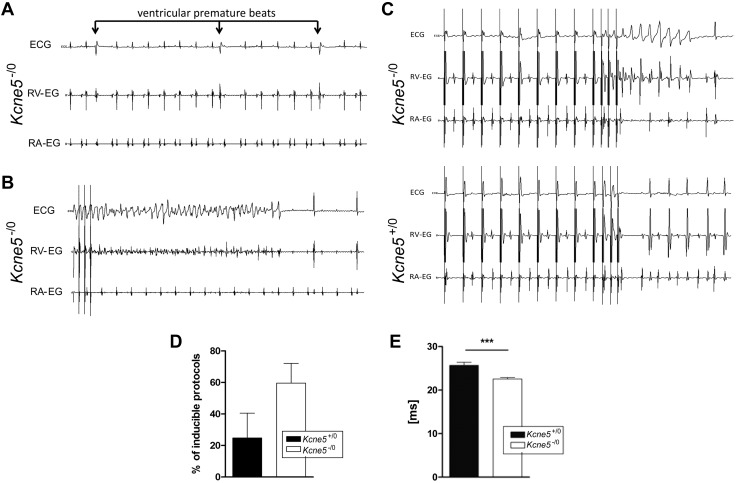
Noninvasive body surface ECG analysis in anesthetized male mice (Supplemental Fig. S2), aged ∼5 mo (n = 9–10 in each group) (Supplemental Fig. S2A) and 8 mo (n = 16–20 in each group) (Supplemental Fig. S2B), did not reveal any genotype-dependent changes. In contrast, invasive ECG uncovered ventricular premature beats (VPBs), which were followed by postextrasystolic compensatory pauses, in male Kcne5–/0 mice (Fig. 2A). In 1 Kcne5–/0 mouse, we observed a spontaneously occurring episode of polymorphic ventricular tachycardia VT. The episode could not be terminated by burst pacing, but eventually converted into an atrial arrhythmia with a 2:1 and 3:1 atrioventricular conduction, (Fig. 2B). The Kcne5–/0 mice also had increased susceptibility to polymorphic VT induction with a defined pacing protocol followed by 3 extra stimuli (Fig. 2C, D). Furthermore, Kcne5 deletion shortened the ventricular effective refractory period (Fig. 2E).
Figure 2 .
Kcne5 deletion causes ventricular arrhythmias. A) Spontaneously occurring monomorphic VPBs were detectable during continuous ECG monitoring in sedated live Kcne5−/0 mice. No such VPBs were found in Kcne5+/0 mice. Upper ECG curves represent body surface ECGs, middle ECG curves represent intracardiac ECG from the ventricular catheter, and lower ECG curves represent intracardiac ECG registration from the atrial catheter. Note postextrasystolic compensatory pauses after VPBs are pathognomonic for ventricular extrasystolic beats. B) A spontaneously occurring episode of polymorphic VT was noted in 1 Kcne5−/0 mouse. The episode could not be terminated by burst pacing (pacing spikes on the left). The VT finally converted into an atrial arrhythmia with a 2:1 and 3:1 atrioventricular conduction. C) Polymorphic VTs were inducible during continuous ECG monitoring of sedated live Kcne5−/0 mice. After a defined pacing protocol followed by 3 extrastimuli polymorphic VTs were highly inducible in Kcne5−/0 mice as compared to WT controls. Data for ECG curves were obtained as described in A. D) Percentage of inducible protocols was increased in Kcne5−/0 mice vs. WT controls. E) The ventricular effective refractory period was reduced in Kcne5−/0 mice vs. Kcne5+/0 controls. ***P < 0.001.
Kcne5 gene deletion augments specific ventricular KV currents
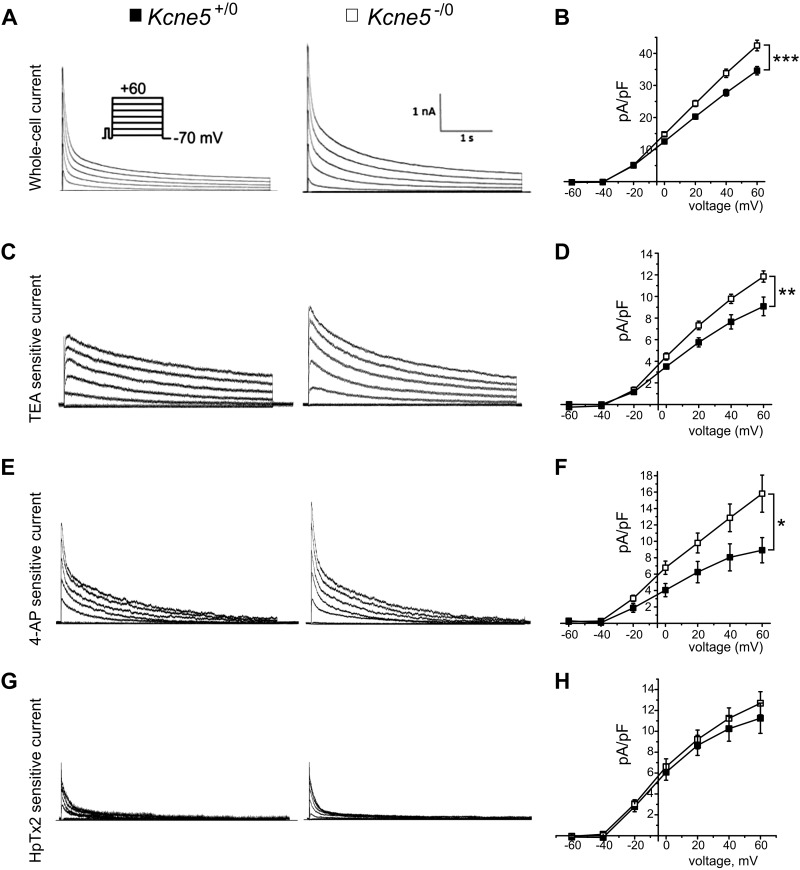
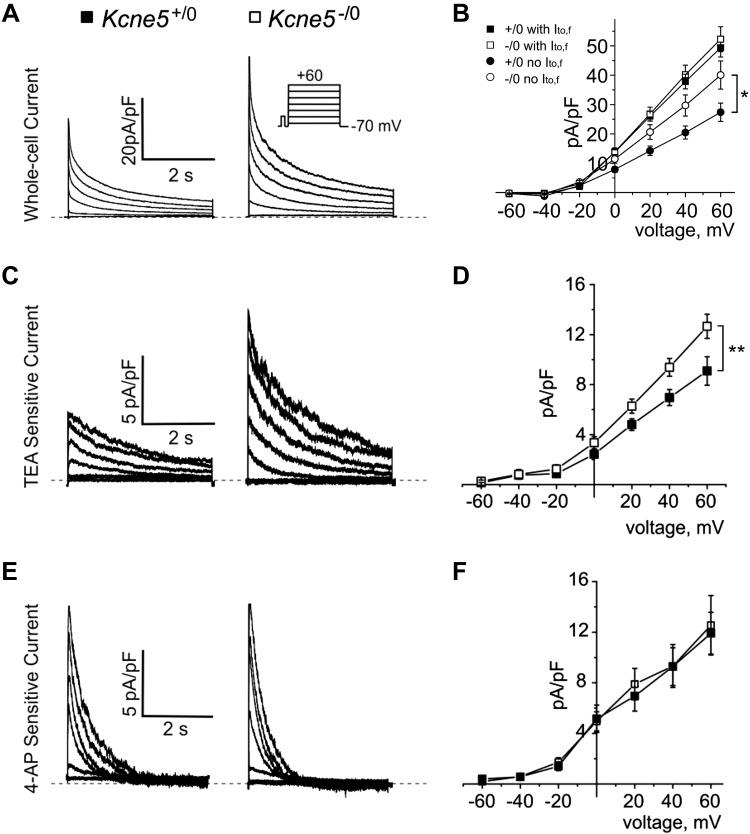
We next used whole-cell patch-clamp electrophysiology to quantify KV currents in ventricular myocytes from adult male mice, to examine possible mechanisms for arrhythmogenesis in Kcne5–/0 mice. In previous studies of adult mice, it was discovered that their ventricular myocyte KV current is composed primarily of the fast outward transient current (Ito,f), which is generated by KV4.2, KV4.3, or both channels and is sensitive to heteropodotoxin (HpTx)-2; the slow transient outward current (Ito,s), probably generated by KV1.4 (IK,slow); and a steady-state component (Iss) (26). IK,slow comprises the 50 μM 4-AP-sensitive KV1.5 current (IK,slow1) (27, 28) and the 25 mM TEA-sensitive KV2.1 current (IK,slow2) (27, 29). We studied ventricular myocytes isolated from the apex (Fig. 3) and the septum (Fig. 4). Similar to our prior work with the Kcne2−/− mouse line (30), we found that apical myocytes isolated from either Kcne5–/0 or Kcne5+/0 mice lacked Ito,s, whereas septal myocytes were divided roughly equally into those with Ito,f and those without (as assessed by curve fitting the current decay at +40 mV) (Table 1).
Figure 3 .
Kcne5 gene deletion augments ventricular apical myocyte KV currents. A) Representative native whole-cell KV currents in Kcne5+/0 (left) and Kcne5−/0 (right) ventricular apex cardiomyocytes (voltage protocol inset) (n = 27–31). B) Mean current density vs. voltage characteristics (I-V curve) from recordings as in A for Kcne5+/0 (filled squares) and Kcne5−/0 (open squares) apical myocytes (n = 27–31). ***P < 0.001 at +60 mV. C) Typical digitally subtracted 25 mM TEA-sensitive current (IK,slow2) for Kcne5+/0 (left) and Kcne5−/0 (right) ventricular myocytes. D) Mean I-V relationships for TEA-sensitive currents as in C (n = 9–10 cells/genotype, derived from 3 mice/genotype). **P = 0.01 at +60 mV. E) Typical digitally subtracted current traces for 50 μM 4-AP-sensitive current (IK,slow1) for Kcne5+/0 (left) and Kcne5−/0 (right) ventricular myocytes. F) Mean I-V relationships for myocytes as in E (n = 9–10 cells/genotype, derived from 5 mice/genotype). P < 0.05 at +60 mV. G) Typical digitally subtracted 500 nM HpTx-sensitive current (IK,slow2) for Kcne5+/0 (left) and Kcne5−/0 (right) ventricular myocytes. H) Mean I-V relationships for HpTx-sensitive currents as in G (n = 6 cells/genotype, derived from 4 to 5 mice per genotype). P = 0.45 at +60 mV.
Figure 4 .
Kcne5 gene deletion augments IK,slow2 in ventricular septum myocytes lacking Ito,f. A) Typical current traces from Kcne5+/0 and Kcne5−/0 adult ventricular septum myocytes (voltage protocol inset). B) Mean I-V relationships for Kcne5+/0 (solid) and Kcne5−/0 (open) septal myocytes with (squares) or without (circles) Ito,f (n = 15–19 cells per group). *P < 0.05 at +60 mV. C) Typical digitally subtracted 25 mM TEA-sensitive KV2.1 current (IK,slow2) for Kcne5+/0 (left) and Kcne5−/0 (right) ventricular septum myocytes. D) Mean I-V relationships for TEA-sensitive currents as in C (n = 6 cells/genotype, derived from 2 mice/genotype). **P = 0.009 at +60 mV. E) Typical digitally subtracted current traces for 50 μM 4-AP-sensitive KV1.5 current (IK,slow1) for Kcne5+/0 (left) and Kcne5−/0 (right) ventricular myocytes. Dashed lines: 0 current level. F) Mean I/V relationships for myocytes as in E (n = 8 cells/genotype, derived from 2 mice/genotype). P = 0.984 at +60 mV. Dashed lines: 0 current level.
TABLE 1.
Fitted KV current densities and inactivation kinetics in ventricular apex and septum myocytes from Kcne5+/0 and Kcne5−/0 mice at +40 mV
| Genotype and myocyte type | I to,f | I to,s | I K,slow | n |
|---|---|---|---|---|
| Kcne5+/0 apex | 68.6 ± 2.5 | NA | 1351 ± 59.5 | 27 |
| 14.7 ± 0.9 | NA | 8.5 ± 0.6 | 27 | |
| Kcne5+/0 septum, with Ito,f | 52.1 ± 3.3 | 526.3 ± 35 | 3869 ± 943 | 19 |
| 14.3 ± 1.6 | 9.2 ± 0.5 | 11.2 ± 1.0 | 19 | |
| Kcne5+/0 septum, no Ito,f | NA | 165.7 ± 11.6 | 1601.8 ± 76 | 16 |
| NA | 5.8 ± 0.8 | 9.5 ± 1.1 | 16 | |
| Kcne5−/0 apex | 66.7 ± 2.6 | NA | 1134 ± 31.4*** | 31 |
| 17.5 ± 0.9 * | NA | 11.6 ± 0.7*** | 31 | |
| Kcne5−/0 septum, with Ito,f | 40.8 ± 1.8** | 383.9 ± 22.8*** | 1857 ± 135 | 15 |
| 16.3 ± 1.4 | 8.6 ± 1.1 | 11.2 ± 1.1 | 15 | |
| Kcne5−/0 septum, no Ito,f | NA | 159.2 ± 15 | 1250 ± 88*** | 16 |
| NA | 8.5 ± 1.4 | 12.9 ± 1.7 * | 16 |
Values are means ± se. Current properties: τdecay (ms); I (pA/pF). I, current density; τdecay, inactivation time constant; n, number of cells; NA, not applicable (current not present). Statistically significant changes in current density are underlined.
P < 0.05, **P < 0.01, ***P < 0.005 vs. equivalent criteria in WT mice.
In apical myocytes, Kcne5 deletion increased peak total KV current density by 22% (Fig. 3A, B). As assessed by curve fitting, the primary effect in apical myocytes of Kcne5 deletion was a 36% increase in IK,slow density. Kcne5 deletion also increased apical myocyte Ito,f density, by 20% (Table 1). We next used pharmacological analysis as an additional methodology for quantifying effects on myocyte KV currents. In apical myocytes, Kcne5 deletion increased the density at +60 mV of both components of IK,slow: the 50 μM 4-AP-sensitive KV1.5 current (IK,slow1) by 78% and the 25 mM TEA-sensitive KV2.1 current (IK,slow2) by 30%. The apical myocyte HpTx2-sensitive KV4 current (Ito,f) was increased by 13% by Kcne5 deletion, although the increase did not reach statistical significance (Fig. 3C–H). Thus, the results of curve fitting and pharmacological analyses were essentially congruent.
In the septum, Kcne5 deletion increased the myocyte peak total KV current density at +40 mV by 32% in myocytes lacking Ito,f, but left the peak total KV current unchanged in septal myocytes with Ito,f (Fig. 4A, B). As quantified by curve fitting, the sole effect of Kcne5 deletion on specific currents in the septum was an increase in IK,slow density by 36% in septal myocytes lacking Ito,f. In contrast, Kcne5 deletion left IK,slow, Ito,s, and Ito,f density unchanged in septal myocytes with Ito,f (Table 1). Pharmacological analysis in a population of septal myocytes including both those with Ito,f and those without (as assessed by curve fitting) showed that mean Kcne5–/0 septum myocyte KV2.1 (25 mM TEA-sensitive) current (IK,slow2) amplitude was 37% greater at +40 mV than that of Kcne5+/0 myocytes (Fig. 4C, D). In contrast, KV1.5 (50 µM 4-AP-sensitive) current (IK,slow1) amplitude at +40 mV was unchanged by Kcne5 deletion (Fig. 4E, F). HpTx2 sensitivity was not quantified in septal myocytes because of the challenges of pharmacologically assessing Ito,f with a toxin in this mixed cell population. As described above, curve-fitting data indicated that Kcne5 deletion did not alter Ito,f in the septum (Table 1). Finally, Kcne5 deletion did not alter ventricular KVα subunit transcript expression (Supplemental Fig. S3), suggesting that the effects on currents were primarily from loss of Kcne5 interaction rather than from transcript remodeling.
Kcne5 and KV2.1 colocalize at ventricular myocyte intercalated discs
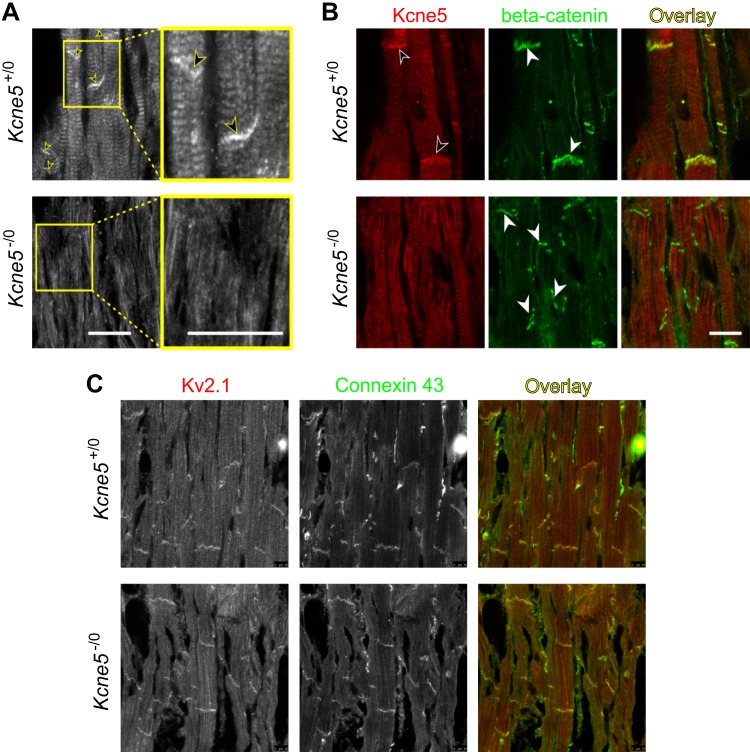
We analyzed the KV2.1-Kcne5 interaction in more detail, because it occurred in both apical and septal myocytes. Using immunofluorescence, we found that Kcne5 localizes strongly to the intercalated discs (ICDs) in ventricular myocytes, with Kcne5–/0 tissue serving as a negative control for antibody specificity (Fig. 5A) and β-catenin as a marker for the ICDs (Fig. 5B). Similarly, we detected KV2.1 strongly localized to the ICDs, using Connexin 43 as an ICD marker in these experiments (Fig. 5C). There was no evidence that Kcne5 deletion altered the ability of KV2.1 to specifically target the ICDs and no evidence of increased KV2.1 protein expression overall.
Figure 5 .
Kcne5 and KV2.1 colocalize in the ICD of mouse ventricular cardiomyocytes. A) Immunofluorescence staining of ventricular heart sections of Kcne5+/+ and Kcne5−/− mice with an anti-Kcne5 antibody. Kcne5 is detectable in ICDs of cardiomyocytes (arrows) whereas Kcne5 signal is absent in Kcne5−/− hearts. Yellow framed areas are shown in higher magnification as indicated. B) Coimmunofluorescence staining with antibodies against Kcne5 (red) and β-Catenin (green) for ICD visualization. No Kcne5 signal was detectable in Kcne5−/− hearts. C) Coimmunofluorescence staining with antibodies against KV2.1 (red) and Connexin-43 (green) as ICD marker proteins. Scale bars, 20 µm.
KV2.1 and KCNE5 colocalize at the plasma membrane and in intracellular vesicles in HL-1 cells
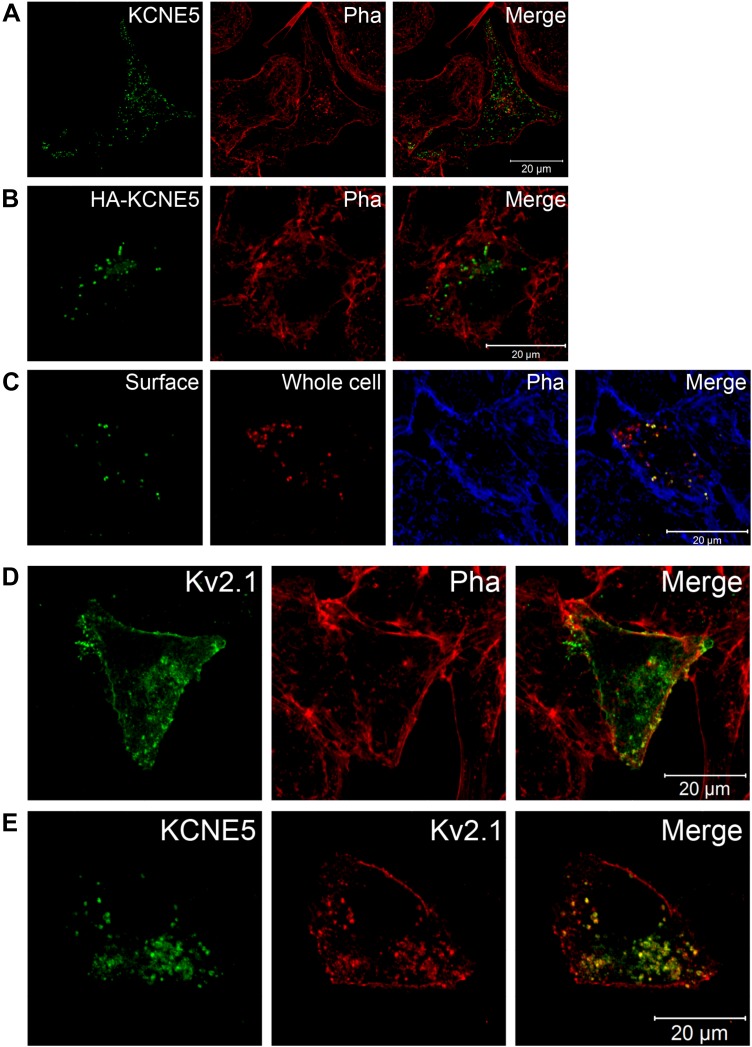
To facilitate more detailed analysis of KCNE5-KV2.1 localization and examination of both mouse and human subunits, we used transient expression of epitope-tagged KCNE5 and KV2.1 in the mouse atrial cardiomyocyte tumor lineage HL-1. When individually expressed, detection with an in-house KCNE5-specific antibody revealed that human KCNE5 was located both in small, dense clusters, and in some vesicle-like structures (Fig. 6A). The ratio between these 2 different entities varied. To address possible species differences, we also transfected HL-1 cells with mouse Kcne5. The mouse protein localized similar to the human protein in these cells (Supplemental Fig. S4). To deduce whether these KCNE5+ clusters were at the membrane or inside the cell, we generated an N-terminal, HA-tagged KCNE5 construct. The tag did not disturb the trafficking of the subunit, as the HA-KCNE5 localized similarly to the untagged KCNE5 (Fig. 6B), nor did the tag affect biophysical properties when coexpressed with KV2.1 (Supplemental Fig. S5). When a specific HA-tag antibody was applied without permeabilizing the cell (Fig. 6C, surface), the same dense clusters appeared, indicating plasma membrane expression. Permeabilization of the same cells and exposure to the anti-KCNE5 antibody revealed additional KCNE5+ intracellular vesicles (whole cell).
Figure 6 .
KCNE5 and KV2.1 colocalize in HL-1 cells. A, B) Confocal images of HL-1 cells heterologously expressing WT KCNE5 (A) or HA-tagged WT KCNE5 (B). Phalloidin (Pha) labeled the submembranous actin cytoskeleton and was used to visualize the cell membrane. C) Nonpermeabilized HA-KCNE5–expressing HL-1 cells stained with a specific HA-tagged antibody (surface staining, green), permeabilized, and then stained with a KCNE5-specific antibody (red) and Phalloidin (blue). D) Colabeling of single transfected KV2.1 (green) and endogenous Phalloidin (red) in HL-1 cells. E) Colabeling of cotransfected KCNE5 (green) and KV2.1 (red) in HL-1 cells.
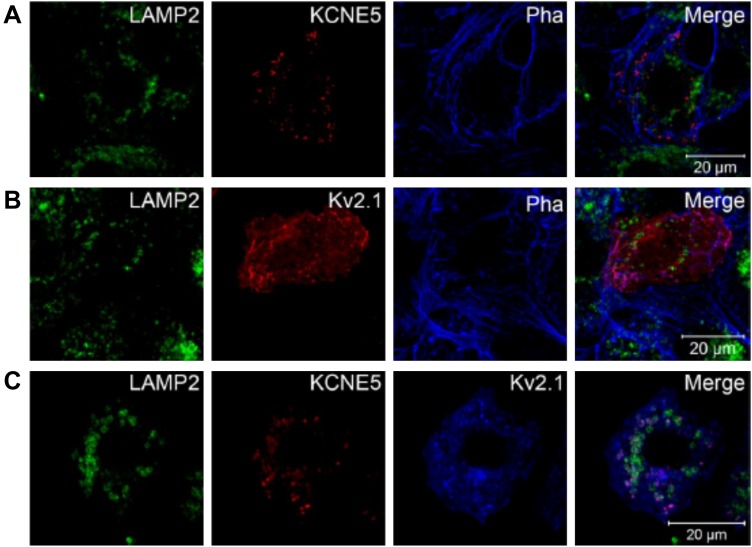
Next, we evaluated colocalization of KCNE5 with KV2.1 in mouse atrial cardiomyocyte tumor lineage HL-1 cells. When expressed alone, KV2.1 was predominantly located at the cell surface, overlapping phalloidin, which labels the actin cytoskeleton just beneath the plasma membrane. In most cells KV2.1 could also be found in dense clusters and vesicles, similar to KCNE5 and consistent with results from studies of HEK293 cells (20, 30–33) (Fig. 6D). Upon coexpression of KCNE5 with KV2.1, the channel subunits colocalized in the same clusters and vesicles in most of the cells (Fig. 6E), also in line with our recent results in HEK293 cells (18). Upon costaining with LAMP-2, we observed that KCNE5 and KV2.1 localized to intracellular compartments that were in proximity to the lysosomes, indicative of late endosome compartments (Fig. 7A–C).
Figure 7 .
KCNE5 and KV2.1 colocalize in intracellular compartments in proximity to the lysosomes. A, B) Laser confocal microscopy was used to acquire images of KCNE5 and KV2.1 subunits singly transfected into HL-1 cells, respectively. Both were found to be located in dense clusters and vesicles for which some appear very close to the lysosomal marker LAMP2. Phalloidin (Pha) was used to identify the cell membrane. C) When KV2.1 and KCNE5 were coexpressed in the same HL-1 cells, they colocalized in vesicles and dense clusters, similar to the above-mentioned structures, which again were located in proximity to the lysosome maker, LAMP2—possibly indicative of late endosome compartments.
Arrhythmia-associated KCNE5 mutations confer functional changes on the KV2.1-KCNE5 channel complex
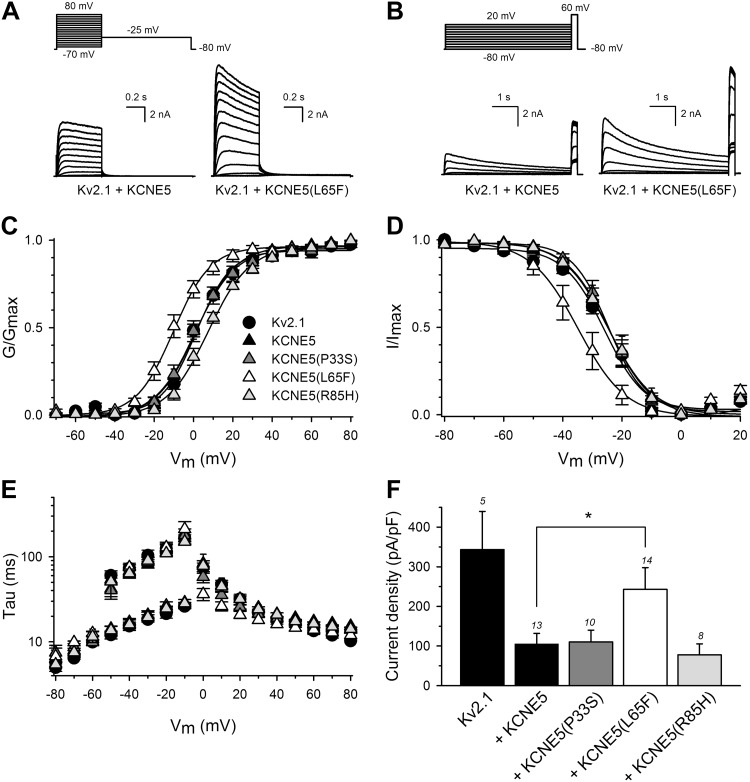
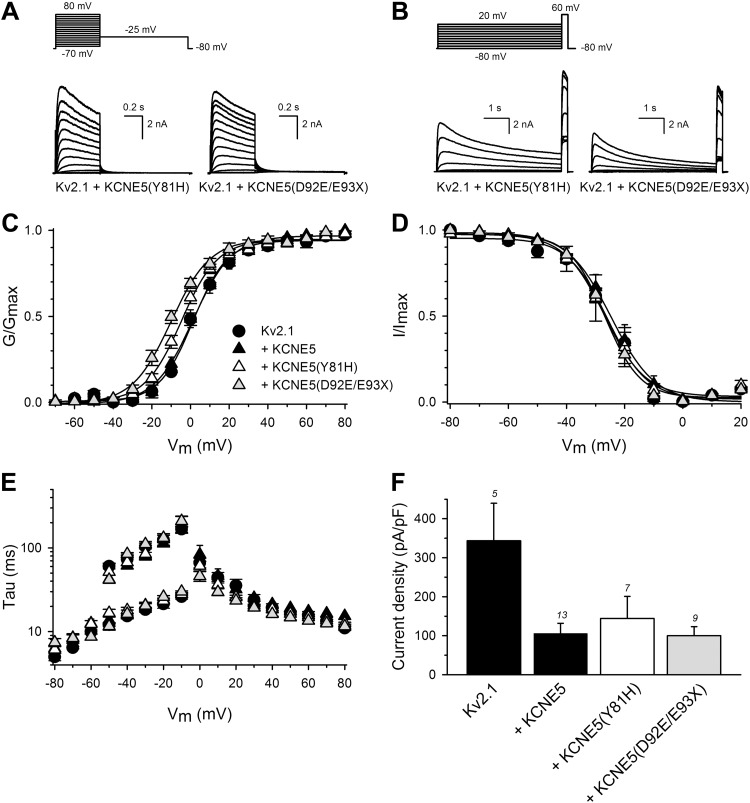
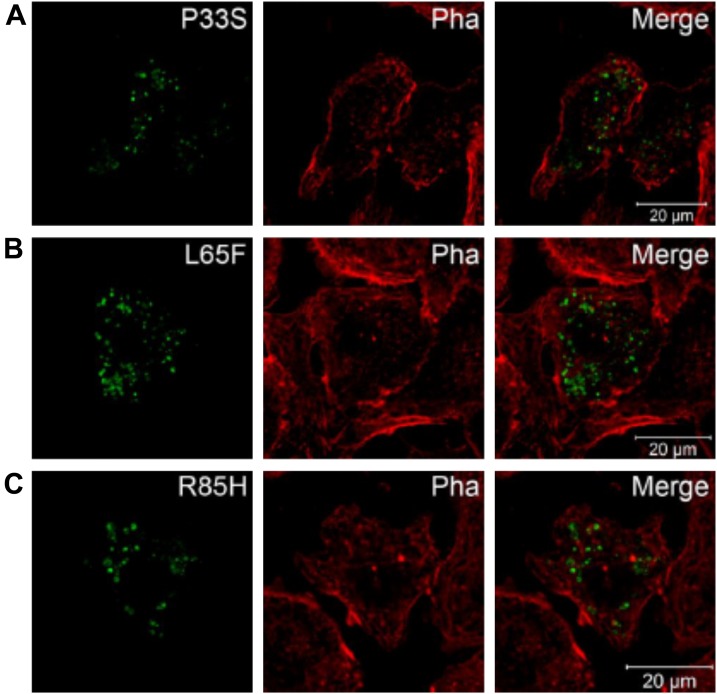
Although KV2.1 is primarily characterized in rodent cardiac electrophysiology (31, 32), both KV2.1 mRNA (33) and protein (34) are reportedly expressed in human atria and ventricles. With further evidence of an interaction between KCNE5 and KV2.1 from our previous work (18) and the current study, we next investigated the effect of human KCNE5 genetic variants that had been linked to AF and BrS (15–17, 35). We coexpressed the KCNE5 AF variants P33S, L65F, or R85H (Fig. 8) and the KCNE5 BrS variants Y81H or D92E/E93X (Fig. 9) with KV2.1 in HEK293 cells. Currents generated by KV2.1 with KCNE5-P33S and Y81H were similar to those generated by KV2.1 and WT KCNE5. In contrast, KCNE5-L65F shifted both the KV2.1 voltage dependence of activation (Fig. 8A–C) and the voltage-dependence of inactivation (Fig. 8D) ∼10 mV toward negative potentials. KCNE5-L65F also modified the KV2.1 activation kinetics significantly (with a ∼2-fold acceleration at 0 mV) without affecting the deactivation kinetics (Fig. 8E). In addition, KCNE5-L65F increased the KV2.1 current density (∼2-fold) compared with KCNE5-WT (243 ± 55 and 100 ± 25 pA/pF, respectively) (Fig. 8F). Coexpression of KV2.1 with KCNE5-R85H changed neither the voltage-dependence of inactivation (Fig. 8D) nor the activation or deactivation kinetics (Fig. 8E). Yet, KCNE5-R85H tended to shift the voltage dependence of activation toward positive potentials (Fig. 8C) and to further reduce KV2.1 current density (Fig. 8F), albeit not reaching statistical significance. KCNE5-D92E/E93X shifted the KV2.1 voltage-dependence of activation (Fig. 9A–C) ∼6 mV toward more negative potentials. Data for channels containing mutant KCNE5 are summarized in Table 2. The mutant forms of KCNE5 did not perturb KCNE5 trafficking in mouse atrial cardiomyocyte tumor lineage HL-1 cells (36) (Fig. 10A–C). Thus, the KCNE5-L65F and D92E/E93X mutations exerted their effects by their impact on the electrical properties of KV2.1.
Figure 8 .
AF-associated KCNE5-L65F causes a gain-of-function of KV2.1-KCNE5 channels. A–D) Biophysical properties of KV2.1 coexpressed with WT or mutant human KCNE5. All recordings were performed on cells from 3 to 5 repeated transfections of 2–5 independent cell cultures. Representative current recordings determined the activation (A) and inactivation (B) properties of KV2.1+KCNE5 and KV2.1+KCNE5-L65F. The applied pulse protocols are given on top. Voltage-dependence of activation (C) and of inactivation (D) of KV2.1+KCNE5, KV2.1+KCNE5-P33S, KV2.1+KCNE5-L65F, and KV2.1+KCNE5-R85H. Activation and inactivation curves were obtained by plotting the normalized current amplitudes at −25 and +60 mV as a function of the 500-ms and 5-s prepulse potentials, respectively. Solid lines: Boltzmann fits. Coexpression of KCNE5-L65F shifted both the voltage dependence of activation and of inactivation. P < 0.05. E) Time constants of activation and deactivation of KV2.1 coexpressed with KCNE5 variants as indicated; symbols as in C. Activation and deactivation constants were derived from single and double exponential fits of the raw current recordings, respectively. F) Current densities at 0 mV of KV2.1 coexpressed with KCNE5 variants, as indicated. The numbers above each bar represent the number of cells analyzed. The L65F mutation increased current density compared with WT KV2.1-KCNE5 channels. *P < 0.05.
Figure 9 .
BrS-associated KCNE5 mutations cause a negative shift in the voltage dependence of KV2.1-KCNE5 channels. All recordings were performed on cells from 3 to 5 repeated transfections of 2–5 independent cell cultures A–D) Biophysical properties of KV2.1 coexpressed with WT or mutant human KCNE5. Representative current recordings determined the activation (A) and inactivation (B) properties of KV2.1+KCNE5-Y81H and KV2.1+KCNE5- D92E/E93X. The applied pulse protocols are given on top. Voltage-dependence of activation (C) and of inactivation (D) of KV2.1+KCNE5, KV2.1+KCNE5-Y81H, and KV2.1+KCNE5- D92E/E93X. Activation and inactivation curves were obtained as in Fig. 4. Solid lines: the Boltzmann fits. Coexpression of KCNE5-D92E/E93X shifted the voltage dependence of activation. P < 0.05. E) Time constants of activation and deactivation of KV2.1 coexpressed with KCNE5 variants as indicated; symbols as in C. Activation and deactivation constants as in Fig. 4E. F) Current densities at 0 mV of KV2.1 coexpressed with KCNE5 variants as indicated. The numbers above each bar represent the number of analyzed cells.
TABLE 2.
Biophysical properties of human KV2.1 alone or coexpressed with WT or mutant human KCNE5
| Subunits | Activation |
Inactivation |
Deactivation |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| V 1/2 | k | τ (at +60 mV) | n | V 1/2 | k | n | τ1 (at −40 mV) | τ2 (at −40 mV) | n | |
| KV2.1 | 0.0 ± 1.9 | 9.4 ± 0.5 | 15.7 ± 1.6 | 6 | −26.5 ± 1.6 | 5.2 ± 0.5 | 5 | 15.3 ± 1.1 | 73.1 ± 11.1 | 4 |
| KV2.1+ | ||||||||||
| WT | 1.3 ± 2.2 | 9.7 ± 0.7 | 17.4 ± 0.9 | 7 | −24.3 ± 2.8 | 5.7 ± 0.2 | 8 | 15.6 ± 1.0 | 61.4 ± 5.1 | 8 |
| P33S | −0.2 ± 2.5 | 10.0 ± 1.1 | 16.0 ± 1.2 | 6 | −23.9 ± 2.1 | 5.6 ± 0.4 | 6 | 16.2 ± 2.6 | 68.0 ± 7.6 | 5 |
| L65F | −10.4 ± 2.4 | 9.1 ± 1.3 | 13.9 ± 1.1 | 7 | −34.8 ± 3.3 | 5.6 ± 0.2 | 7 | 17.0 ± 1.1 | 75.3 ± 5.2 | 4 |
| R85H | 7.0 ± 1.8 | 10.4 ± 1.0 | 16.4 ± 0.7 | 6 | −24.1 ± 2.4 | 6.4 ± 0.3 | 6 | 16.0 ± 1.1 | 63.9 ± 7.7 | 5 |
| Y81H | −2.4 ± 2.1 | 9.6 ± 0.6 | 14.8 ± 0.3 | 6 | −26.9 ± 3.9 | 5.4 ± 0.3 | 5 | 18.0 ± 1.5 | 67.2 ± 10.7 | 6 |
| D92E/E93X | −5.2 ± 1.4 | 8.8 ± 0.5 | 13.4 ± 0.9 | 7 | −26.4 ± 1.3 | 5.8 ± 0.3 | 5 | 16.3 ± 1.8 | 84.4 ± 3.5 | 5 |
Values are means ± sem. V1/2, midpoint (mV) of activation or inactivation; k, slope factor; τ, time constant (ms); n, number of cells. Values underlined showed a statistically significant difference compared to KV2.1+KCNE5 values. P < 0.05.
Figure 10 .
AF-associated KCNE5 mutants locate similarly to WT KCNE5 in HL-1 cells. KCNE5-P33S (A), KCNE5-L65F (B), and KCNE5-R85H (C) expressed in HL-1 cells alone were found in compartments similar to WT KCNE5 (compare to Fig. 6) visualized with confocal microcopy. Thus, the mutations did not seem to confer a trafficking deficiency. Phalloidin (Pha) was used to mark the cell membrane.
DISCUSSION
Human KCNE5 sequence variants are associated with both AF (16, 17) and BrS (15), conditions that increase the risk of mortality (35, 36). The functional mechanisms linking KCNE5 to these cardiac arrhythmia syndromes have been suggested to be dysregulation of IKslow (16) or Ito (15), respectively, but little is known of the roles of KCNE5 in vivo. To aid in this endeavor, we studied the effects of germline deletion of Kcne5 in mice. Transgenic mouse models have been instrumental in detecting and characterizing novel native functions of KCNE subunits (23, 25, 30, 37–45). By phenotypically studying Kcne5–/0 mice, we discovered that Kcne5 regulates KV2.1 current (IK,slow,2) (27, 29) in mouse ventricles. Furthermore, we demonstrated region- and myocyte-subtype–specific differences in the ventricular role of Kcne5, observing that Kcne5 deletion augments IK,slow,1 (generated by KV1.5) (in addition to IKslow,2) in the apex but not the septum. Previously, we found that Kcne2 deletion downregulates ventricular IKslow,2 by hampering KV1.5 trafficking to the ICDs (30). We also found that, despite not being expressed in mouse heart, Kcne3 deletion predisposes to ventricular arrhythmias and AF via an autoimmune attack on the adrenal glands that increases serum aldosterone (19, 23). In the atria, this tendency was demonstrated to occur via increased KV1.5 recycling, leading again to diminished IKslow,2 (19). Future studies will focus on elucidating the mechanism for KCNE5 modulation of KV1.5 current density.
In the present study, Kcne5 deletion increased Ito,f density, but the change was less dramatic than we observed for IK,slow and was also restricted to the apical myocytes, despite the known capacity for KCNE5 to robustly regulate KV4 channels in vitro (15, 46, 47). This finding, together with the apical-specific IK,slow,1 regulation by KCNE5 suggests that myocyte subtype-specific mechanisms are in place to limit KVα subunit regulation by KCNE5, despite its capacity to interact in other cell subtypes or contexts.
Our finding that Kcne5 is expressed in both atria and ventricles in mice is congruent with our expression data for human cardiac tissue, where we identified KCNE5 throughout the human heart, in line with prior studies (5–7), with slightly raised expression in the atria. We also detected Kcne5 protein expression specifically at the ventricular ICDs. We observed neither cardiac hypertrophy nor KVα subunit transcript remodeling in Kcne5–/0 mice, suggesting that the arrhythmias we detected by intracardiac ECG arose primarily from a purely electrical defect.
Our findings are the first, to our knowledge, to demonstrate β-subunit regulation of KV2.1 in mice, and the augmentation of IK,slow arising from Kcne5 deletion is consistent with current knowledge of the mechanistic bases for human AF and BrS (i.e., pathologic increase in specific KV currents; the more common basis for BrS is loss of function of voltage-gated sodium channels, but gain in KV current has a similar effect). The relatively subtle and apically restricted effect of Kcne5 deletion on Ito,f in mouse ventricles is of interest, given that Ohno and colleagues (15) reported that BrS-linked KCNE5 mutants increase the current conducted by human KV4 channels. KV4 channels may be regulated differently with respect to KCNEs in mouse vs. human hearts, with more extensive modulation in the latter. Alternatively, the capacity of KCNE5 mutants to alter KV4-KCNE5 channel function in vitro may not be representative of the primary molecular mechanism underlying human BrS disease pathogenesis. In the mouse model presented herein, Kcne5 deletion gave rise to specific KV current augmentation, and macroscopically caused VPBs, which were followed by postextrasystolic compensatory pauses and in one case, a spontaneous polymorphic VT. Thus, as is thought to occur in human BrS, augmentation of specific KV currents in mice via Kcne5 disruption caused ventricular arrhythmia.
Our data present strong evidence of a novel KCNE5-KV2.1 interaction in mouse cardiac myocytes; however, little is known about how these subunits interact. Expression of the subunits in HL-1 cells revealed that neither subunit affected the trafficking or localization of the other; however, our electrophysiology data suggest that KCNE5 directly regulates KV2.1 channel attributes at the plasma membrane, in line with our previous work in HEK293 cells (20). We detected most of both human and mouse KCNE5 in dense clusters at the membrane and in some intracellular clusters and vesicles located in proximity to the lysosomal marker LAMP2. KV2.1 was mainly located at the border of the cell, overlapping the cell membrane marker phalloidin, but could also be found in vesicular structures similar to KCNE5. We speculate that some of the intracellular compartments positive for KCNE5 subunits are compartments of the late endosomal pathway and could serve either as a recycling reserve or as part of an endocytic mechanism regulating the number of KV2.1 channels at the membrane. These observations are in line with results obtained in HEK293 cells (18). In this model, Kcne5 deletion would prevent this recycling, thereby contributing to augmentation of KV2.1 current density. A similar model could apply to other channels inhibited by KCNE5.
Finally, in our study, KCNE5-L65F lacked the inhibitory effect on KV2.1 exhibited by WT KCNE5 and shifted the voltage dependence of activation and inactivation to more negative potentials. To date, no native current within the human heart has been assigned to KV2.1, yet both KV2.1 mRNA and protein have been detected in human ventricles and atria (33, 34). Ravn et al. (16) speculated that the L65F mutation in KCNE5 is associated with nonfamilial or acquired forms of AF, given that it causes a gain-of-function of IKs. Our findings, at the very least, suggest mechanistic commonalties between KCNE5 regulation of KV7.1 and KV2.1 and the possibility that human KCNE5-linked AF is more complex than first thought and perhaps involves KV2.1, a channel of which little is known in terms of its importance in the human heart.
CONCLUSIONS
We provide the first description of the physiologic roles of KCNE5 in vivo. Mouse Kcne5 functions as a ventricular myocyte subtype-specific inhibitor of IKslow,1 and IKslow2, which are conducted by KV1.5 and KV2.1, respectively, and to a lesser extent Ito,f (KV4 α subunits). Kcne5 deletion increases ventricular arrhythmia susceptibility. These findings provide potential mechanistic clues to the molecular basis of human KCNE5-linked BrS and AF.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Morten Thomsen [University of Copenhagen (UCPH), Copenhagen, Denmark] for advice on mouse ECG analysis and the Core Facility for Integrated Microscopy at UCPH. This work was supported by a framework grant of the Danish National Research Foundation (to N.S.); a Ph.D. stipend from the Faculty of Health and Medical Sciences, UCPH (to J.-P.D.); funding from the Arvid Nilsson Foundation (to J.-P.D.); the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme Grant FP7/2007–2013 (Research Executive Agency Grant Agreement 608765) and a grant from the Lundbeck Foundation (to T.A.J.); a postdoctoral fellowship from the Research Foundation–Flanders (FWO) (to E.B.); and U.S. National Institutes of Health/National Heart, Lung and Blood Institute Grant HL079275 and Postdoctoral Diversity Supplement Grant HL079275-S1 (to G.W.A.). The authors declare no conflicts of interest.
Glossary
- 4-AP
4-aminopyridine
- ACTB
β-actin
- AF
atrial fibrillation
- BrS
Brugada syndrome
- ECG
electrocardiograph
- HA
hemagglutinin
- HEK
human embryonic kidney
- HpTx
heteropodatoxin
- ICD
intercalated disc
- I K,slow
slow transient outward current generated by KV1.4
- I K,slow1
slow transient outward current generated by KV1.5
- I K,slow2
slow transient outward current generated by KV2.1
- I ss
steady-state current
- I to,s
slow transient outward current
- Ito,f
fast transient outward current, Kcne, potassium voltage-gated channel, subfamily E
- KV
voltage-gated potassium channel
- LAMP
lysosomal-associated membrane protein
- TEA
tetraethylammonium
- TOP1
topoisomerase-1
- VPB
ventricular premature beat
- VT
ventricular tachycardia
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J.-P. David, U. Lisewski, T. K. Roepke, N. Schmitt, and G. W. Abbott designed the research; J.-P. David, U. Lisewski, S. M. Crump, T. A. Jepps, E. Bocksteins, N. Wilck, and J. Lossie performed the research; J.-P. David, U. Lisewski, S. M. Crump, T. A. Jepps, E. Bocksteins, N. Wilck, J. Lossie, and G. W. Abbott analyzed the data; and J.-P. David, S. M. Crump, T. A. Jepps, E. Bocksteins, T. K. Roepke, N. Schmitt, and G. W. Abbott wrote the manuscript.
REFERENCES
- 1.Abbott, G. W. (2015) The KCNE2 K+ channel regulatory subunit: ubiquitous influence, complex pathobiology. Gene 569, 162–172 10.1016/j.gene.2015.06.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott, G. W. (2016) KCNE1 and KCNE3: the yin and yang of voltage-gated K(+) channel regulation. Gene 576, 1–13 10.1016/j.gene.2015.09.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abbott, G. W. (2016) KCNE4 and KCNE5: K(+) channel regulation and cardiac arrhythmogenesis. Gene 593, 249–260 10.1016/j.gene.2016.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCrossan, Z. A., Abbott, G. W. (2004) The minK-related peptides. Neuropharmacology 47, 787–821 10.1016/j.neuropharm.2004.06.018 [DOI] [PubMed] [Google Scholar]
- 5.Bendahhou, S., Marionneau, C., Haurogne, K., Larroque, M. M., Derand, R., Szuts, V., Escande, D., Demolombe, S., Barhanin, J. (2005) In vitro molecular interactions and distribution of KCNE family with KCNQ1 in the human heart. Cardiovasc. Res. 67, 529–538 10.1016/j.cardiores.2005.02.014 [DOI] [PubMed] [Google Scholar]
- 6.Gaborit, N., Le Bouter, S., Szuts, V., Varro, A., Escande, D., Nattel, S., Demolombe, S. (2007) Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart. J. Physiol. 582, 675–693 10.1113/jphysiol.2006.126714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundquist, A. L., Manderfield, L. J., Vanoye, C. G., Rogers, C. S., Donahue, B. S., Chang, P. A., Drinkwater, D. C., Murray, K. T., George, A. L., Jr. (2005) Expression of multiple KCNE genes in human heart may enable variable modulation of I(Ks). J. Mol. Cell. Cardiol. 38, 277–287 10.1016/j.yjmcc.2004.11.012 [DOI] [PubMed] [Google Scholar]
- 8.Crump, S. M., Abbott, G. W. (2014) Arrhythmogenic KCNE gene variants: current knowledge and future challenges. Front. Genet. 5, 3 10.3389/fgene.2014.00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barhanin, J., Lesage, F., Guillemare, E., Fink, M., Lazdunski, M., Romey, G. (1996) K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature 384, 78–80 10.1038/384078a0 [DOI] [PubMed] [Google Scholar]
- 10.Sanguinetti, M. C., Curran, M. E., Zou, A., Shen, J., Spector, P. S., Atkinson, D. L., Keating, M. T. (1996) Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature 384, 80–83 10.1038/384080a0 [DOI] [PubMed] [Google Scholar]
- 11.Schmitt, N., Grunnet, M., Olesen, S. P. (2014) Cardiac potassium channel subtypes: new roles in repolarization and arrhythmia. Physiol. Rev. 94, 609–653 10.1152/physrev.00022.2013 [DOI] [PubMed] [Google Scholar]
- 12.Piccini, M., Vitelli, F., Seri, M., Galietta, L. J., Moran, O., Bulfone, A., Banfi, S., Pober, B., Renieri, A. (1999) KCNE1-like gene is deleted in AMME contiguous gene syndrome: identification and characterization of the human and mouse homologs. Genomics 60, 251–257 10.1006/geno.1999.5904 [DOI] [PubMed] [Google Scholar]
- 13.Jonsson, J. J., Renieri, A., Gallagher, P. G., Kashtan, C. E., Cherniske, E. M., Bruttini, M., Piccini, M., Vitelli, F., Ballabio, A., Pober, B. R. (1998) Alport syndrome, mental retardation, midface hypoplasia, and elliptocytosis: a new X linked contiguous gene deletion syndrome? J. Med. Genet. 35, 273–278 10.1136/jmg.35.4.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lane, W., Robson, M., Lowry, R. B., Leung, A. K. (1994) X-linked recessive nephritis with mental retardation, sensorineural hearing loss, and macrocephaly. Clin. Genet. 45, 314–317 [DOI] [PubMed] [Google Scholar]
- 15.Ohno, S., Zankov, D. P., Ding, W. G., Itoh, H., Makiyama, T., Doi, T., Shizuta, S., Hattori, T., Miyamoto, A., Naiki, N., Hancox, J. C., Matsuura, H., Horie, M. (2011) KCNE5 (KCNE1L) variants are novel modulators of Brugada syndrome and idiopathic ventricular fibrillation. Circ. Arrhythm Electrophysiol. 4, 352–361 10.1161/CIRCEP.110.959619 [DOI] [PubMed] [Google Scholar]
- 16.Ravn, L. S., Aizawa, Y., Pollevick, G. D., Hofman-Bang, J., Cordeiro, J. M., Dixen, U., Jensen, G., Wu, Y., Burashnikov, E., Haunso, S., Guerchicoff, A., Hu, D., Svendsen, J. H., Christiansen, M., Antzelevitch, C. (2008) Gain of function in IKs secondary to a mutation in KCNE5 associated with atrial fibrillation. Heart Rhythm 5, 427–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ravn, L. S., Hofman-Bang, J., Dixen, U., Larsen, S. O., Jensen, G., Haunsø, S., Svendsen, J. H., Christiansen, M. (2005) Relation of 97T polymorphism in KCNE5 to risk of atrial fibrillation. Am. J. Cardiol. 96, 405–407 10.1016/j.amjcard.2005.03.086 [DOI] [PubMed] [Google Scholar]
- 18.David, J. P., Stas, J. I., Schmitt, N., Bocksteins, E. (2015) Auxiliary KCNE subunits modulate both homotetrameric Kv2.1 and heterotetrameric Kv2.1/Kv6.4 channels. Sci. Rep. 5, 12813 10.1038/srep12813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisewski, U., Koehncke, C., Wilck, N., Buschmeyer, B., Pieske, B., Roepke, T. K. (2016) Increased aldosterone-dependent Kv1.5 recycling predisposes to pacing-induced atrial fibrillation in Kcne3−/− mice. FASEB J. 30, 2476–2489 10.1096/fj.201600317R [DOI] [PubMed] [Google Scholar]
- 20.Soltysinska, E., Olesen, S. P., Christ, T., Wettwer, E., Varró, A., Grunnet, M., Jespersen, T. (2009) Transmural expression of ion channels and transporters in human nondiseased and end-stage failing hearts. Pflugers Arch. 459, 11–23 10.1007/s00424-009-0718-3 [DOI] [PubMed] [Google Scholar]
- 21.Vandesompele, J., De Preter, K., Pattyn, F., Poppe, B., Van Roy, N., De Paepe, A., Speleman, F. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak, K. J., Schmittgen, T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 23.Hu, Z., Crump, S. M., Anand, M., Kant, R., Levi, R., Abbott, G. W. (2014) Kcne3 deletion initiates extracardiac arrhythmogenesis in mice. FASEB J. 28, 935–945 10.1096/fj.13-241828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.David, J. P., Andersen, M. N., Olesen, S. P., Rasmussen, H. B., Schmitt, N. (2013) Trafficking of the IKs-complex in MDCK cells: site of subunit assembly and determinants of polarized localization. Traffic 14, 399–411 10.1111/tra.12042 [DOI] [PubMed] [Google Scholar]
- 25.Roepke, T. K., King, E. C., Reyna-Neyra, A., Paroder, M., Purtell, K., Koba, W., Fine, E., Lerner, D. J., Carrasco, N., Abbott, G. W. (2009) Kcne2 deletion uncovers its crucial role in thyroid hormone biosynthesis. Nat. Med. 15, 1186–1194 10.1038/nm.2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu, H., Guo, W., Nerbonne, J. M. (1999) Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J. Gen. Physiol. 113, 661–678 10.1085/jgp.113.5.661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bou-Abboud, E., Li, H., Nerbonne, J. M. (2000) Molecular diversity of the repolarizing voltage-gated K+ currents in mouse atrial cells. J. Physiol. 529, 345–358 10.1111/j.1469-7793.2000.00345.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.London, B., Jeron, A., Zhou, J., Buckett, P., Han, X., Mitchell, G. F., Koren, G. (1998) Long QT and ventricular arrhythmias in transgenic mice expressing the N terminus and first transmembrane segment of a voltage-gated potassium channel. Proc. Natl. Acad. Sci. USA 95, 2926–2931 10.1073/pnas.95.6.2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou, J., Kodirov, S., Murata, M., Buckett, P. D., Nerbonne, J. M., Koren, G. (2003) Regional upregulation of Kv2.1-encoded current, IK,slow2, in Kv1DN mice is abolished by crossbreeding with Kv2DN mice. Am. J. Physiol. Heart Circ. Physiol. 284, H491–H500 10.1152/ajpheart.00576.2002 [DOI] [PubMed] [Google Scholar]
- 30.Roepke, T. K., Kontogeorgis, A., Ovanez, C., Xu, X., Young, J. B., Purtell, K., Goldstein, P. A., Christini, D. J., Peters, N. S., Akar, F. G., Gutstein, D. E., Lerner, D. J., Abbott, G. W. (2008) Targeted deletion of kcne2 impairs ventricular repolarization via disruption of I(K,slow1) and I(to,f). FASEB J. 22, 3648–3660 10.1096/fj.08-110171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCrossan, Z. A., Roepke, T. K., Lewis, A., Panaghie, G., Abbott, G. W. (2009) Regulation of the Kv2.1 potassium channel by minK and MiRP1. J. Membr. Biol. 228, 1–14 10.1007/s00232-009-9154-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connell, K. M., Whitesell, J. D., Tamkun, M. M. (2008) Localization and mobility of the delayed-rectifer K+ channel Kv2.1 in adult cardiomyocytes. Am. J. Physiol. Heart Circ. Physiol. 294, H229–H237 10.1152/ajpheart.01038.2007 [DOI] [PubMed] [Google Scholar]
- 33.Kääb, S., Dixon, J., Duc, J., Ashen, D., Näbauer, M., Beuckelmann, D. J., Steinbeck, G., McKinnon, D., Tomaselli, G. F. (1998) Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation 98, 1383–1393 10.1161/01.CIR.98.14.1383 [DOI] [PubMed] [Google Scholar]
- 34.Van Wagoner, D. R., Pond, A. L., McCarthy, P. M., Trimmer, J. S., Nerbonne, J. M. (1997) Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. Circ. Res. 80, 772–781 10.1161/01.RES.80.6.772 [DOI] [PubMed] [Google Scholar]
- 35.Olesen, M. S., Andreasen, L., Jabbari, J., Refsgaard, L., Haunsø, S., Olesen, S. P., Nielsen, J. B., Schmitt, N., Svendsen, J. H. (2014) Very early-onset lone atrial fibrillation patients have a high prevalence of rare variants in genes previously associated with atrial fibrillation. Heart Rhythm 11, 246–251 [DOI] [PubMed] [Google Scholar]
- 36.Claycomb, W. C., Lanson, N. A., Jr., Stallworth, B. S., Egeland, D. B., Delcarpio, J. B., Bahinski, A., Izzo, N. J., Jr. (1998) HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. USA 95, 2979–2984 10.1073/pnas.95.6.2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbott, G. W., Jepps, T. A. (2016) Kcne4 deletion sex-dependently alters vascular reactivity. J. Vasc. Res. 53, 138–148 10.1159/000449060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crump, S. M., Hu, Z., Kant, R., Levy, D. I., Goldstein, S. A., Abbott, G. W. (2016) Kcne4 deletion sex- and age-specifically impairs cardiac repolarization in mice. FASEB J. 30, 360–369 10.1096/fj.15-278754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu, Z., Kant, R., Anand, M., King, E. C., Krogh-Madsen, T., Christini, D. J., Abbott, G. W. (2014) Kcne2 deletion creates a multisystem syndrome predisposing to sudden cardiac death. Circ. Cardiovasc. Genet. 7, 33–42 10.1161/CIRCGENETICS.113.000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu, Z., Wei, W., Zhou, L., Chen, M., Abbott, G. W. (2018) Kcne4 deletion sex-specifically predisposes to cardiac arrhythmia via testosterone-dependent impairment of RISK/SAFE pathway induction in aged mice. Sci. Rep. 8, 8258 10.1038/s41598-018-26599-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee, S. M., Baik, J., Nguyen, D., Nguyen, V., Liu, S., Hu, Z., Abbott, G. W. (2017) Kcne2 deletion impairs insulin secretion and causes type 2 diabetes mellitus. FASEB J. 31, 2674–2685 10.1096/fj.201601347 [DOI] [PubMed] [Google Scholar]
- 42.Lee, S. M., Nguyen, D., Anand, M., Kant, R., Köhncke, C., Lisewski, U., Roepke, T. K., Hu, Z., Abbott, G. W. (2016) Kcne2 deletion causes early-onset nonalcoholic fatty liver disease via iron deficiency anemia. Sci. Rep. 6, 23118 10.1038/srep23118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Drici, M. D., Arrighi, I., Chouabe, C., Mann, J. R., Lazdunski, M., Romey, G., Barhanin, J. (1998) Involvement of IsK-associated K+ channel in heart rate control of repolarization in a murine engineered model of Jervell and Lange-Nielsen syndrome. Circ. Res. 83, 95–102 10.1161/01.RES.83.1.95 [DOI] [PubMed] [Google Scholar]
- 44.Vetter, D. E., Mann, J. R., Wangemann, P., Liu, J., McLaughlin, K. J., Lesage, F., Marcus, D. C., Lazdunski, M., Heinemann, S. F., Barhanin, J. (1996) Inner ear defects induced by null mutation of the isk gene. Neuron 17, 1251–1264 10.1016/S0896-6273(00)80255-X [DOI] [PubMed] [Google Scholar]
- 45.Warth, R., Barhanin, J. (2002) The multifaceted phenotype of the knockout mouse for the KCNE1 potassium channel gene. Am. J. Physiol. Regul. Integr. Comp. Physiol. 282, R639–R648 10.1152/ajpregu.00649.2001 [DOI] [PubMed] [Google Scholar]
- 46.Radicke, S., Cotella, D., Graf, E. M., Banse, U., Jost, N., Varró, A., Tseng, G. N., Ravens, U., Wettwer, E. (2006) Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc. Res. 71, 695–703 10.1016/j.cardiores.2006.06.017 [DOI] [PubMed] [Google Scholar]
- 47.Abbott, G. W. (2017) β subunits functionally differentiate human Kv4.3 potassium channel splice variants. Front. Physiol. 8, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.