Abstract
Despite effective control of HIV infection with antiretroviral drugs, individuals with HIV have high incidences of secondary diseases. These sequelae, such as cardiovascular disease (CVD), are poorly understood and represent a major health burden. To date, predictive biomarkers of HIV-associated secondary disease have been elusive, making preventative clinical management essentially impossible. Here, we applied a newly developed and easy to deploy, multitarget, and high-throughput glycomic analysis to banked HIV+ human plasma samples to determine whether the glycome may include biomarkers that predict future HIV-associated cardiovascular events or CVD diagnoses. Using 324 patient samples, we identified a glycomic fingerprint that was predictive of future CVD events but independent of CD4 counts, diabetes, age, and birth sex, suggesting that the plasma glycome may serve as a biomarker for specific HIV-associated sequelae. Our findings constitute the discovery of novel glycan biomarkers that could classify patients with HIV with elevated risk for CVD and reveal the untapped prognostic potential of the plasma glycome in human disease.—Oswald, D. M., Sim, E. S., Baker, C., Farhan, O., Debanne, S. M., Morris, N. J., Rodriguez, B. G., Jones, M. B., Cobb, B. A. Plasma glycomics predict cardiovascular disease in patients with ART-controlled HIV infections.
Keywords: biomarker, glycan, glycome, lectin, CVD
In the modern day of antiretroviral therapy (ART), the health outcomes of patients with HIV have shifted from a rapid decline into AIDS-related illnesses and mortality to more manageable, chronic morbidity (1). Patients with HIV are living decades longer, but their infection still presents a health burden to the individual, mirroring early development of late-life chronic diseases associated with immune activation, including cardiovascular disease (CVD) (2), liver or kidney failure (3), type II diabetes (4), frailty (5), and cancer (6), as has been reviewed (7). Although overall rates of HIV deaths and AIDS-related illnesses have fallen in recent years, mortality from HIV sequelae has surpassed AIDS-related illnesses as the major killer of individuals with HIV, highlighting the importance of understanding other causes of death (8).
The mechanisms that underlie the development of chronic illness in infected individuals are not understood, and it is likely that traditional risk factors and drug-related complications, in addition to HIV-induced immune responses, play a role in disease etiology. Although it is well recognized that some populations of patients with HIV have higher rates of traditional CVD risk factors, such as dyslipidemia and diabetes, it has been repeatedly demonstrated in large trials that these factors do not fully explain the increased disease risk in patients with HIV as a whole (8). There are documented effects of ART drugs on lipid levels and metabolism (9), but recent iterations of ART drugs have not shown associations with these changes in risk factors (10), presumably limiting their contribution to disease burden. Interruption of ART itself increases the risk of incurring a cardiovascular incident (11), supporting evidence of a link between viral replication and CVD. In addition, it has been demonstrated that HIV elite controllers (patients who maintain low viral load even in the absence of ART) still develop CVD in chronic HIV infection, demonstrating that there is an essential link between chronic virus infection and disease development (12). To date, inflammation in HIV is the most likely cause of CVD, and the link between these is well documented and has been reviewed extensively (2).
Biomarker discovery and application has long had an important impact in the HIV field, with CD4+ T-cell counts and HIV-1 RNA being critical in the clinic to indicate overall immune function and viral suppression, respectively (13). CVD alone has been correlated with low CD4+ T-cell counts (14), CD8+ T-cell activation (15), and multiple inflammatory markers (i.e., sCD163, sCD14, D-Dimer, hsCRP, IL-6) (15–18). The majority of these markers are also associated with CVD in HIV-uninfected populations. In addition to these studies, HIV-specific effects regarding changes in lipid metabolism (19, 20) and oxidation (21), endothelial activation (VCAM-1, ICAM-1, E-selectin, P-selectin) (22), and the development of atherosclerosis have been observed (23). Despite intense study in these areas, there has been difficulty translating HIV-specific CVD biomarkers or interventions to the clinic, highlighting the need for a novel approach to identify and analyze CVD markers.
Glycans are one unexplored avenue for analyzing the relationship between HIV and CVD. Carbohydrates covalently linked to proteins or lipids (termed “glycans”) are a critical mediator of biologic functions, constituting a large portion of all cellular surfaces and acting as a critical component of nearly every surface and secreted protein (24). Since the discovery of the original ABO blood grouping system, glycans have played an important role in the discovery and application of biomarkers. Glycan-recognizing affinity reagents, including antibodies and plant lectins, have been used as biomarkers for human stem cells and pancreatic cancer (25, 26). It has been speculated that the use of lectins as biomarkers could compound with protein and lipid analyses to yield more specific and powerful biomarkers that reflect more specific information about the metabolic and secretory state of the cell or tissue they comprise (27).
Here, we report the discovery of a novel set of biomarkers for CVD in the context of HIV infection. Although immune activation and the deposition of abnormal hyaluronan matrices has been reported in the context of diabetes or atherosclerosis (28, 29), changes in plasma glycosylation have not previously been shown to predict CVD. By analyzing banked plasma samples using a panel of lectins with broad glycan specificity in a high-throughput glycomic screening format, we were able to identify a glycomic fingerprint that was indicative of future CVD development. This proof-of-concept study demonstrates the applicability of this format in screening for new biomarker targets and provides the rationale for future large-scale prospective studies focused on the plasma glycome.
MATERIALS AND METHODS
Study definition
We performed a cross-sectional analysis using plasma samples obtained from 324 subjects who were patients at the Case Western Reserve University School of Medicine and University Hospitals of Cleveland Center for AIDS Research (CFAR) clinic. Subjects were selected for case status based on the experience of at least 1 CVD event (see Results for a comprehensive list). Case samples chosen were the most recent sample prior to CVD event. Control subjects were selected via sample matching to control for age, sex, diabetes status, and time in the CFAR clinic. Control samples chosen were the most recent sample. CD4 levels were obtained directly from the medical record. This study was approved by the institutional review boards at the University Hospitals, Cleveland Medical Center.
Sample processing
Plasma samples were pulled from a bank of frozen samples and brought to 4°C. Samples were fixed using 1 volume of 4% paraformaldehyde to a final concentration of 2% paraformaldehyde and incubated for 1 h at 4°C. To remove viral and any other large particles, the samples were spun at 16,000 g for 1 h at 4°C, and the supernatant was transferred to a new tube and spun a second time. Clarified supernatants were frozen at −80°C until use.
Protein concentration determination
Plasma protein in clarified supernatants was quantified using the Pierce BCA Kit as indicated by the manufacturer (23225; Thermo Fisher Scientific, Waltham, MA, USA).
Multiplex lectin ELISA
Samples processed for the lectin ELISA were coated to a 384-well, high-binding ELISA plate (784077; Greiner Bio-One, Monroe, NC, USA) at 0.5 µg/ml in carbonate coating buffer (0.1 M NaHCO3, 0.03 M NaCO3, pH 9.5) in a volume of 20 µl (as for all steps) overnight at 4°C. Between each step, plates were washed 3 times in PBS with Tween 20 by a plate washer (MW 96/384; Beckman-Coulter, Brea, CA, USA). The plates were then incubated with carbohydrate-free blocking solution (SP5040; Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature and incubated with divalent ion solution (1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2) for 10 min at room temperature, which was aspirated from the plate without washing. Biotinylated lectins were added at the concentrations listed in Supplemental Table 1. The lectin mixture was diluted in carbohydrate-free blocking solution and incubated for 1 h at room temperature. Europium-conjugated streptavidin (1244-360; PerkinElmer, Waltham, MA, USA) was diluted in europium dilution buffer (50 mM Tris, 150 mM NaCl, 20 μM EDTA) to 100 ng/ml and incubated in the plate for 1 h at room temperature. Enhancement solution (4001-0010; PerkinElmer, Waltham, MA, USA) was added to the plate and allowed to rock for 5 min at room temperature in the dark before being analyzed using time-resolved fluorescence on a Victor 3V 1420 multilabel counter (PerkinElmer) using time-resolved fluorescence. Assay plate layout is shown in Supplemental Fig. 1A. Optimization data (Supplemental Fig. 1B) demonstrate the detection of various concentrations of coated plasma using different concentrations of each lectin to determine the optimal concentration (highlighted in red) that ensures detection of positive or negative changes in staining. On each plate, a plasma standard from a healthy donor was included to assay variation between plates. These standards were averaged and used to normalize the data across plates.
Statistical methods
Variables were compared using nonparametric testing (Mann-Whitney). Correlations were performed using the Pearson correlation. Heatmaps were generated in R (The R Project; https://www.r-project.org/) software using the “heatmap.2” package. Samples were sorted between control subjects and patients with CVD and randomized within each group. Lectin signal was normalized to a z score. Principal component analysis (PCA) was performing using R and the “prcomp” package. Correlogram analysis was performed in R using the “corrgram” package. Receiving operator characteristic analysis was performed using SPSS (IBM, Armonk, NY, USA).
RESULTS
Study design
Samples were selected from banked HIV+ plasma from the CFAR repository. Seventy-seven case samples were selected from patients who went on to have CVD, and 247 control samples were identified to optimally reduce variability in age, sex, and diabetes, for a total of 324 samples. CVD events (and number of patients) included angina (n = 5), cerebral artery occlusion (n = 1), cerebrovascular disease (n = 5), cardiac chest pain (n = 1), cerebrovascular accident (n = 6), ischemic heart disease (n = 5), acute nitric oxide synthase myocardial infarction (n = 25), non-ST elevation myocardial infarction (n = 4), ST elevation myocardial infarction (n = 2), stroke (n = 16), and transient ischemic attack (n = 7). Time points were chosen such that cases used the most recent available precardiac event plasma, and controls were the most recent available sample. Seventy-eight percent of patients were male, and 20% had diabetes. Average age at last visit was 49.8 yr. Population demographic information is presented in Table 1.
TABLE 1.
Patient demographics
| Variable | All | CVD case | Control | P |
|---|---|---|---|---|
| Demographics | ||||
| Age at first visit (yr) | 39.00 | 39.97 | 38.70 | NS |
| Duration in study (yr) | 10.77 | 9.75 | 11.09 | NS |
| Sex (% male) | 78 | 79 | 78 | NS |
| CVD | ||||
| Case (n) | 77 | 77 | 0 | NA |
| Control (n) | 247 | 0 | 247 | NA |
| Risk factors | ||||
| Diabetes (%) | 20 | 21 | 20 | NS |
| CD4 (cells/µl) | 481.0 | 478.0 | 490.7 | NS |
NA, not available; NS, not significant (P > 0.05, Mann-Whitney).
Lectins selected for analysis were chosen for the broad specificity of targets represented among their determinants and their ability to detect changes in glycoprotein concentration in the lectin ELISA format (Supplemental Fig. 1B). Lectin specificities are given in Supplemental Table 1, including a visual representation of the target molecule and a description of which glycan classes are relevant to the lectin. The lectins can be grouped into 4 groups: 1) lectins that bind to sialic acids (or its absence), including Sambucus nigra agglutinin (binds to α2,6-linked sialic acid), Maackia amurensis lectin II (MAL-II; binds to α2,3-linked sialic acid on O-GalNAc glycans), Ricinus communis agglutinin I (RCA120; binds to asialylated β-linked terminal galactose), and wheat germ agglutinin (WGA; binds to GlcNAc or sialic acid); 2) lectins binding total glycoprotein, including concanavalin A (ConA; binds to total N-linked glycans) and jacalin (Jac; binds to total O-GalNac glycans); 3) lectins binding branching N-glycans, including Phaseolus vulgaris erythroagglutinin (PHA-E; binds to bisecting GlcNAc), Phaseolus vulgaris leucoagglutinin (PHA-L; binds to branching on α6 mannose arm), and Datura stramonium lectin (DSL; binds to branching on α3 mannose arm); and 4) lectins that bind fucose, including Lens culinaris agglutinin (binds to α1,6 core fucose) and Aleuria aurantia lectin (binds to α1,3 or α1,6 fucose) (30). Supplemental Table 2 provides links to lectin pages on the Consortium for Functional Glycomics website, where detailed information about lectin binding specificities using glycan microarrays is available.
Analysis of trends
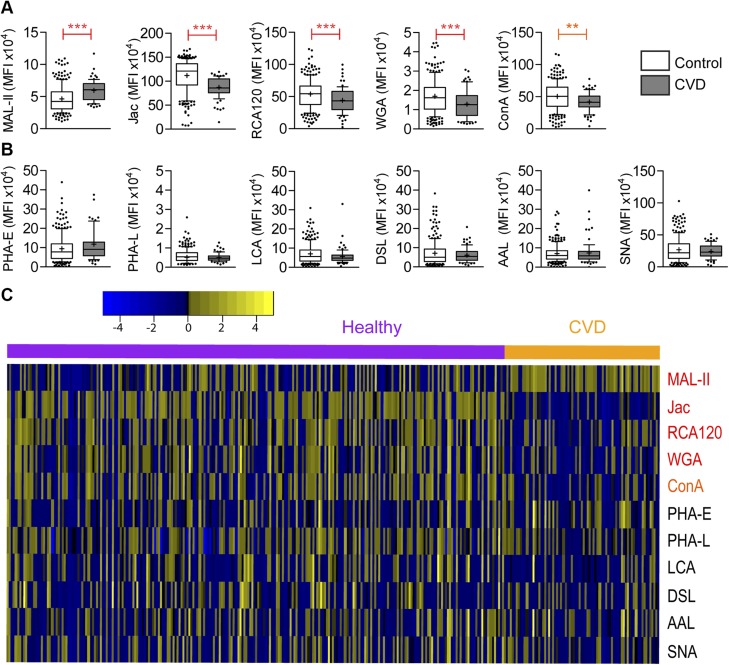
Lectin data were compiled and sorted into groups to test for differences in the means between populations in a Mann-Whitney test. Case status and traditional risk factors were used to stratify the populations and tested individually. Using control vs. CVD as a discriminator, 5 lectins stained statistically different between the populations: MAL-II, Jac, RCA120, WGA, and ConA (Fig. 1A). In this analysis, each of the significantly different lectin signals trended down with CVD status, with the exception of MAL-II, which trended up. The remaining lectins were unable to distinguish between case and control (Fig. 1B). To visualize the complete glycomic fingerprint from all lectins used, these data are presented as a heatmap (Fig. 1C).
Figure 1 .
The staining of 5 lectins is correlated with the development of CVD in patients with HIV. A) Lectins correlated with CVD (P < 0.05). **P < 0.005, ***P < 0.0005 (Mann-Whitney). B) Lectins not correlated with CVD (P > 0.05). C) Data are represented as a heatmap, with signal indicated by z score from A and B. Yellow indicates higher staining; blue indicates lower staining. Control and CVD populations are sorted separately. Lectins are presented in order of decreasing significance.
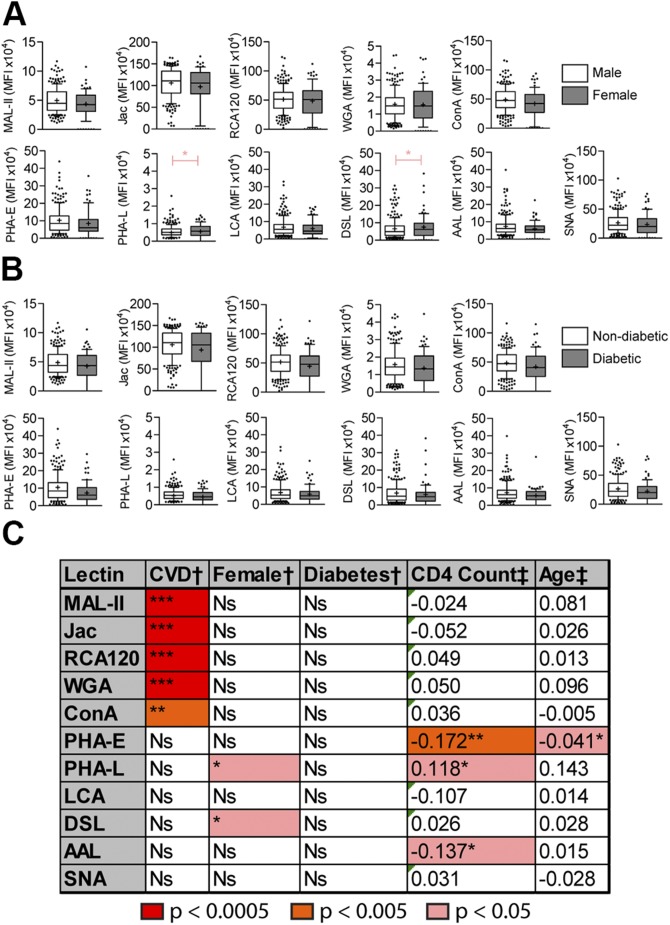
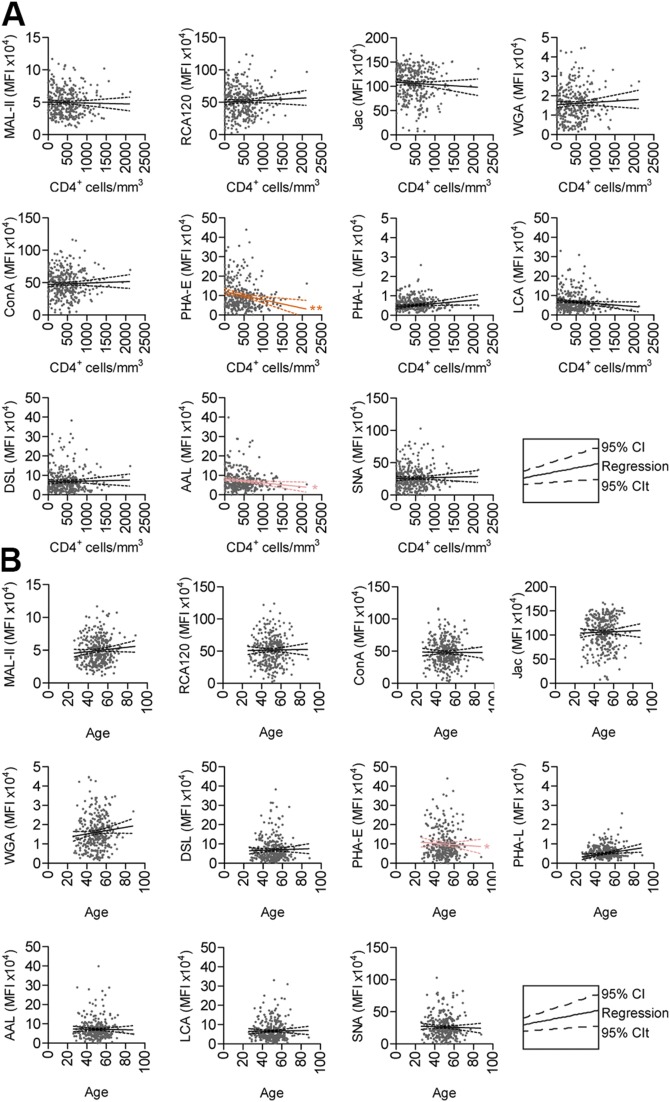
When samples were stratified by birth sex as the discriminant, DSL and PHA-L trended higher in female patients (Fig. 2A). No lectins were able to distinguish between patients with or without diabetes (Fig. 2B). For the continuous variable of age, we plotted lectin signal against the respective variable and tested Pearson linear regression correlations (Fig. 3B) and found that age correlated with PHA-E staining.
Figure 2 .
Controlled for factors analyzed for correlation with CVD. A, B) Lectin staining between male and female patients (A) and lectin staining between patients with diabetes and patients without diabetes (B). Box and whisker charts indicate median (+), mean (−), quartile (box), decile (whisker), and outliers (dots). C) Summary of the data shown from A and B. Color indicates increasing thresholds of significance. *P < 0.05, **P < 0.005, ***P < 0.0005 (Mann-Whitney test for discrete variables; Pearson correlation for continuous variables).
Figure 3 .
Age and CD4+ cell counts do not correlate with CVD. A) Lectin staining (red) as a scatterplot with CD4+ cells/µl. B) Lectin staining (red) as a scatterplot with age. *P < 0.05, **P < 0.005 (Pearson correlation).
It is well documented that CD4+ T cells play crucial roles in the health of individuals with HIV, and recent studies have linked CD4 counts <550 cells/mm3 with CVD risk in patients with HIV (14). Therefore, we looked for links between lectin staining and CD4 counts in our population. Linear regression analysis shows clear trends in a number of lectin staining and CD4 counts (Fig. 3A). Staining in PHA-E and Aleuria aurantia lectin trends negatively with CD4 count, whereas staining with PHA-L is positively correlated with CD4 count. None of the 5 lectins identified as markers of CVD was correlated with CD4 count, age, birth sex, or diabetes. The results of these statistical probes are summarized in Fig. 2C.
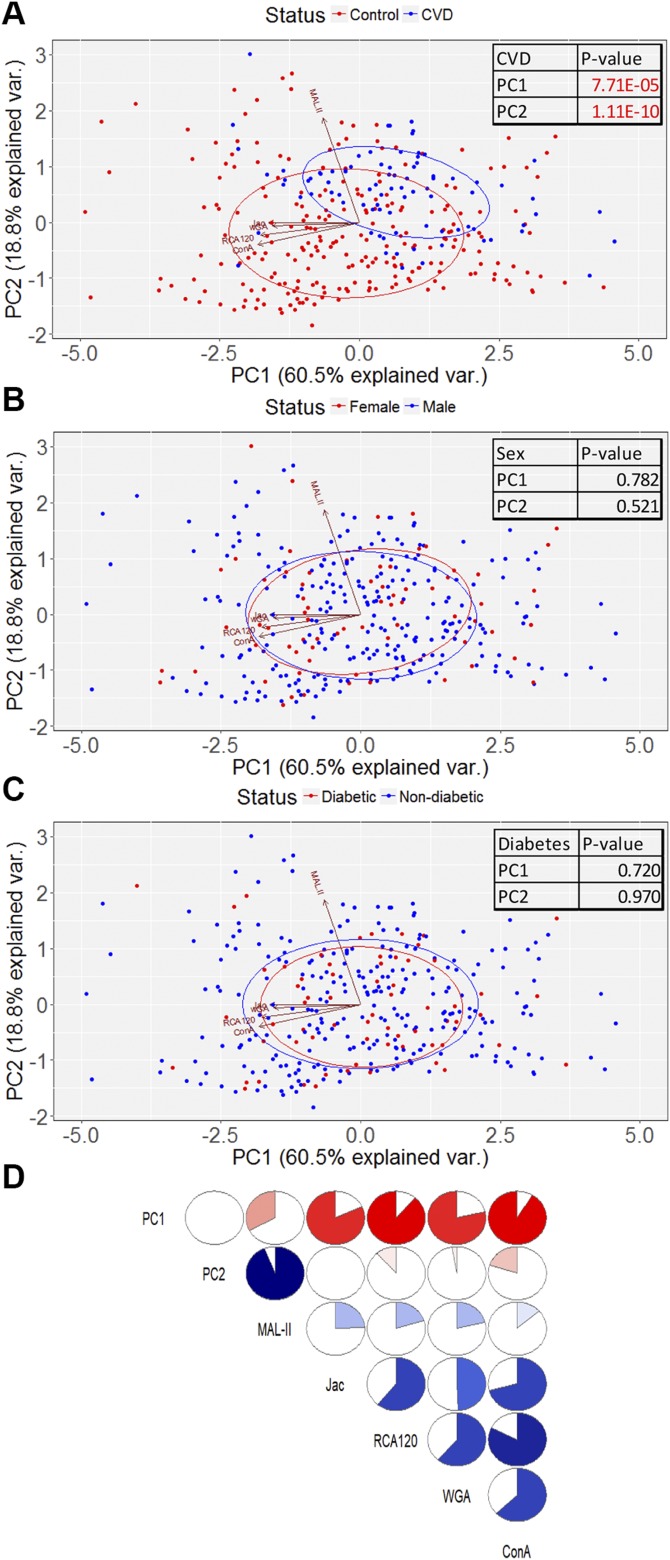
Dimensional reduction analysis
To test the dimensionality of the data, we performed PCA on the set of 5 lectins that were significantly different between patients with CVD and control subjects. This generated 2 components that were statistically different between control subjects and patients with CVD patients, PC1 and PC2, accounting for 60.5 and 18.8% of the population variance, respectively (Fig. 4A). These components were plotted to visualize the glycomic profile, which demonstrated that patients with CVD scattered differently from the controls (Fig. 4A). When discriminated by birth sex, male and female subjects were not different in either PC1 or PC2 and were visually indistinguishable (Fig. 4B). Likewise, stratifying by diabetes status yielded no differences (Fig. 4C).
Figure 4 .
Dimensionality reduction analysis. A–C) PCA discriminating CVD status (A), sex (B), and diabetes status (C). Boxed numbers indicate comparisons between patients and control subjects (Mann-Whitney). D) Correlogram indicating comparisons between lectins and principal components. Degree of correlation is indicated by fullness of circle and depth of color. Blue indicates a positive correlation; red indicates a negative correlation.
Correlations between these 5 lectin staining levels and PC1 and PC2 are presented as a correlogram (Fig. 4D). PC1 is dominated by low staining of the lectins RCA120, ConA, Jac, and WGA; each of these lectins is moderately correlated with each other. PC2 is associated with an increase in MAL-II staining. These data are also visualized by vectors on the PCA plots in Fig. 4A–C.
Logistic regression analysis
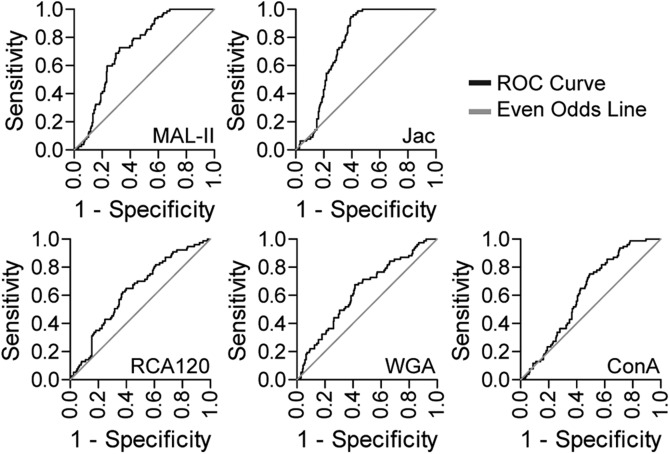
Receiving operator characteristic analyses on logistic regression models of individual lectin staining provided insight into their ability to specifically and sensitively detect CVD in our patient population. Each model was plotted individually as a curve of sensitivity vs. 1-specificity, with a diagonal line representing the even/odds ratio. The point to the highest left of the plot represents the maximization of sensitivity and specificity (Fig. 5 and Table 2), and the area under the curve is a measure of model robustness (Table 3). Jac and MAL-II showed a strong ability to predict case status. As the z statistic indicates, the multiple logistic regression model accounting for Jac and MAL-II is independent of diabetes, sex, age, and CD4+ cell count, demonstrating again that these factors have not had undue influence on our analysis (Table 2). Using this logistic regression model, we can generate odds ratios that describe the relative risk in the development of CVD when lectin staining deviates from normal. For an increase of 1 sd from mean for Jac staining, a patient’s odds ratio falls to 45.8%. For MAL-II, an increase in 1 sd from mean increases the CVD odds ratio to 192%.
Figure 5 .
Receiving operator characteristic (ROC) analyses showing prediction ability of each lectin. Black line represents pairs of sensitivity and 1-specificity indicated by the model; gray line represents even-odds ratio.
TABLE 2.
Multiple logistic regression analysis indicates Jac and MAL-II model is independent of diabetes, sex, age, and CD4 cell counts
| Model | Variable | Z statistic |
|---|---|---|
| Jac + MAL-II + diabetes | Intercept | −0.440 |
| MAL-II | 6.637*** | |
| Jacalin | −6.923*** | |
| Diabetes | 0.217 | |
| Jac + MAL-II + sex | Intercept | −0.088 |
| MAL-II | 6.648*** | |
| Jacalin | −6.917*** | |
| Birthsex | 0.541 | |
| Jac + MAL-II + age | Intercept | 0.506 |
| MAL-II | 6.655*** | |
| Jacalin | −6.925*** | |
| Age | −0.912 | |
| Jac + MAL-II + CD4 | Intercept | −0.414 |
| MAL-II | −6.917*** | |
| Jacalin | 6.642*** | |
| CD4 | 0.089 |
P < 0.05, **P < 0.005, ***P < 0.0005.
TABLE 3.
Odds ratios
| Lectin | AUC | se | Sensitivity (%) | Specificity (%) | Asymptotic significance |
|---|---|---|---|---|---|
| MAL-II | 0.722 | 0.029 | 72.7 | 67.5 | *** |
| RCA120 | 0.633 | 0.035 | 64.9 | 60.1 | *** |
| ConA | 0.618 | 0.032 | 75.3 | 50.8 | ** |
| Jac | 0.761 | 0.025 | 88.3 | 62.6 | *** |
| WGA | 0.631 | 0.036 | 67.5 | 58.5 | ** |
AUC, area under the curve. **P < 0.005, ***P < 0.0005.
DISCUSSION
In this study, we used a panel of plant lectins with defined glycan structure specificities (Supplemental Fig. 1) to demonstrate that changes in the glycome of total plasma protein are predictive of CVD among patients with HIV. These lectins represent a diverse array of glycans, including total N-linked (ConA) and total O-GalNAc (Jac) glycans as well as sialylation of O-linked glycans (MAL-II), asialylated galactose (RCA120), and sialic acid or GlcNAc (WGA). Taken together, these changes indicate that patients who developed CVD had decreased total glycosylation of their serum proteins and a proportional increase in the sialylation of the O-linked glycans. Moreover, the signal strengths of each lectin were internally consistent in that RCA120 (asialylated galactose) decreased as sialylation (MAL-II) increased. These complex relationships between the varied lectin specificities provide strength to the interpretation of the glycomic fingerprint that emerges from these data.
A number of the lectins that do not have statistical power to predict CVD development do have relatively weak association with some of the other variables (Fig. 2D). The association with DSL and PHA-L with female birth sex and the PHA-E correlation with age are significant at P < 0.05. These may indeed be biomarkers of these characteristics, and it is tempting to speculate that there may be plasma glycome differences between the sexes. However, because no such observation has been reported, it may be a biologically insignificant phenomenon and will require further testing to fully understand. In contrast, the stark lack of significance in any of the 5 CVD-predictive markers with sex, diabetes status, or patient age indicates that, within this patient cohort, the underlying glycans recognized by these lectins are independent of these risk factors and therefore potentially specific for CVD in patients with HIV.
The observation that CD4 counts also fail to trend with the 5 predictive CVD markers is interesting because CD4 counts have been reported to correlate with CVD in other cohorts of subjects with HIV (14). Although this study was not controlled appropriately to test whether CD4 counts correlated with CVD within this cohort, it is likely that plasma glycans and T cells are unrelated enough to not correlate. Rather, both may independently serve as a readout of immune health and/or immune activation. If correct, it is conceivable that a biomarker index based on the combination of CD4 counts and plasma glycomics could more robustly predict CVD risk.
Reducing the multivariate nature of our lectin data into 2 principal components helps to generate and visualize a glycomic fingerprint for CVD in patients with HIV. The visualization of this difference clearly demonstrates that a profile exists and that it can broadly be classified by either an increasing MAL-II (PC2) signal or by a decreasing signal in the other 4-lectin cluster (WGA, ConA, RCA120, Jac; PC1) (Fig. 4A). This helps to identify associations within the glycomic fingerprint so that noninformative redundancies within the fingerprint can be ignored while realistically distinguishable differences can be identified. Moreover, each of these 5 lectins was tested for its ability to act as a diagnostic and prognostic test of HIV-linked CVD. In this analysis, MAL-II and Jac emerged as the clearest and most powerful predictors and were almost entirely uncorrelated with each other (Pearson r = 0.241). Each is represented by independent components within the PCA, suggesting that the 2 lectins may be valuable to use in tandem or complementary fashions.
There is an unmet scientific and clinical need for biomarkers that predict HIV-linked CVD for use in clinical trials and risk stratification in the clinic (31). These biomarkers must be prognostic: for applications in the clinic, they must be able to predict future events with a high level of sensitivity and specificity to enable additional monitoring or intervention prior to a cardiac event. Our analyses were performed on samples taken prior to the observation of CVD; thus, both MAL-II and Jac qualify as prognostic biomarkers.
There is a long history of using plasma proteomics to predict and diagnose health and disease. Total blood protein profiling is still used in the modern clinic to identify inflammatory conditions, as with serum protein electrophoresis, and can indicate disease states from malnutrition and anemia to malignant lymphomas and myelomas to rheumatoid diseases (32) and has even been applied to HIV disease progression (33). Likewise, in biomedical research, changes in the glycome have long been associated with a host of disease processes, including inflection and cancer (34). Although the glycomic analysis described herein focused on whole plasma, the success of identifying a glycomic fingerprint capable of predicting CVD suggests that the glycome of specific plasma glycoproteins should also be determined in large scale. This applies especially to the glycome of plasma proteins already reported to be associated with CVD, such as IL-6 (15), even though it represents a relatively small percentage of the total protein in plasma. As previously suggested (27), our data strongly support the exploration of glycoform-specific glycoprotein biomarkers, which could dramatically increase the reliability of novel and established biomarkers.
The mechanistic connection, if any, between the plasma glycome and CVD in patients with HIV remains unknown. The vast majority of plasma proteins are secreted by the liver, with the notable exception of IgG. As a result, the changes measured are likely related to liver physiology, pathology, and metabolism. It has long been recognized that the liver is highly responsive to the inflammatory state, including the inducible and repressible production of essential glycan-synthesizing enzymes (35). In fact, many liver-released glycosidases and glycosyltransferases are active in circulation (36–38), including a sialyltransferase that modifies circulating IgG (36). As a result, understanding liver function, positioned at a fulcrum of glycoprotein clearance and secretion, will be essential to deciphering the underlying mechanisms of our observations.
We have discovered a glycomic fingerprint based on 2 key lectins that could serve as a new biomarker of CVD in individuals with HIV. Although future prospective cohort studies are warranted to validate these markers while exploring whether this is an HIV-dependent observation or common to all patients with CVD, our study clearly demonstrates the applicability of our system in the identification of potential glycan biomarker studies without the use of specialized equipment. Future studies could apply higher-throughput analyses for screening glycan binding proteins, such as the use of lectin microarray technology (39). Through the establishment of a link between immune health and circulatory protein glycosylation, we believe that the identified glycomic changes represent a novel target for further scientific and potential clinical application.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank the CFAR staff for assistance in designing the study and in selecting, gathering, and processing the samples; the members of the B.A.C. laboratory for valuable feedback and input; Dr. Michael Lederman (CFAR) for critical evaluation of this manuscript; and Dr. Jonathan Karn (CFAR) for the invitation and encouragement to work in the HIV field. This work was supported by U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grants GM082916 and GM115234 (to B.A.C.), an administrative supplement to B.A.C. through the CWRU CFAR grant AI036219 to Jonathan Karn, and NIH National Institute of Allergy and Infectious Diseases Training Grant T32 AI089474 (to D.M.O.). This work has been presented, in part, at the 2017 Society for Glycobiology Meeting. The authors declare no conflicts of interest.
Glossary
- ART
antiretroviral therapy
- CVD
cardiovascular disease
- CFAR
Case Western Reserve University School of Medicine and University Hospitals of Cleveland Center for AIDS Research
- ConA
concanavalin A
- DSL
Datura stramonium lectin
- Jac
jacalin
- MAL-II
Maackia amurensis lectin II
- PCA
principal component analysis
- PHA-E
Phaseolus vulgaris erythroagglutinin
- PHA-L
Phaseolus vulgaris leucoagglutinin
- RCA120
Ricinus communis agglutinin I
- WGA
wheat germ agglutinin
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. M. Oswald, N. J. Morris, B. G. Rodriguez, M. B. Jones, and B. A. Cobb designed the research; D. M. Oswald, C. Baker, O. Farhan, and S. M. Debanne analyzed the data; D. M. Oswald and E. S. Sim performed the research; B. G. Rodriguez contributed samples and tools; B. A. Cobb edited manuscript; and D. M. Oswald wrote the manuscript.
REFERENCES
- 1.Marin, B., Thiébaut, R., Bucher, H. C., Rondeau, V., Costagliola, D., Dorrucci, M., Hamouda, O., Prins, M., Walker, S., Porter, K., Sabin, C., Chêne, G. (2009) Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS 23, 1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsue, P. Y., Deeks, S. G., Hunt, P. W. (2012) Immunologic basis of cardiovascular disease in HIV-infected adults. J. Infect. Dis. 205 (Suppl 3), S375–S382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Justice, A. C., Freiberg, M. S., Tracy, R., Kuller, L., Tate, J. P., Goetz, M. B., Fiellin, D. A., Vanasse, G. J., Butt, A. A., Rodriguez-Barradas, M. C., Gibert, C., Oursler, K. A., Deeks, S. G., Bryant, K.; VACS Project Team . (2012) Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin. Infect. Dis. 54, 984–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, T. T., Tassiopoulos, K., Bosch, R. J., Shikuma, C., McComsey, G. A. (2010) Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 33, 2244–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erlandson, K. M., Ng, D. K., Jacobson, L. P., Margolick, J. B., Dobs, A. S., Palella, F. J., Jr., Lake, J. E., Bui, H., Kingsley, L., Brown, T. T. (2017) Inflammation, immune activation, immunosenescence, and hormonal biomarkers in the frailty-related phenotype of men with or at risk for HIV infection. J. Infect. Dis. 215, 228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breen, E. C., Hussain, S. K., Magpantay, L., Jacobson, L. P., Detels, R., Rabkin, C. S., Kaslow, R. A., Variakojis, D., Bream, J. H., Rinaldo, C. R., Ambinder, R. F., Martinez-Maza, O. (2011) B-cell stimulatory cytokines and markers of immune activation are elevated several years prior to the diagnosis of systemic AIDS-associated non-Hodgkin B-cell lymphoma. Cancer Epidemiol. Biomarkers Prev. 20, 1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunt, P. W. (2012) HIV and inflammation: mechanisms and consequences. Curr. HIV/AIDS Rep. 9, 139–147 [DOI] [PubMed] [Google Scholar]
- 8.Smith, C., Sabin, C. A., Lundgren, J. D., Thiebaut, R., Weber, R., Law, M., Monforte, A. d., Kirk, O., Friis-Moller, N., Phillips, A., Reiss, P., El Sadr, W., Pradier, C., Worm, S. W.; Data Collection on Adverse Events of Anti-HIV drugs (D:A:D) Study Group . (2010) Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D study. AIDS 24, 1537–1548 [DOI] [PubMed] [Google Scholar]
- 9.Grinspoon, S., Carr, A. (2005) Cardiovascular risk and body-fat abnormalities in HIV-infected adults. N. Engl. J. Med. 352, 48–62 [DOI] [PubMed] [Google Scholar]
- 10.Monforte, A., Reiss, P., Ryom, L., El-Sadr, W., Dabis, F., De Wit, S., Worm, S. W., Law, M. G., Weber, R., Kirk, O., Pradier, C., Phillips, A. N., Lundgren, J. D., Sabin, C. A. (2013) Atazanavir is not associated with an increased risk of cardio- or cerebrovascular disease events AIDS 27, 407–415 [DOI] [PubMed] [Google Scholar]
- 11.El-Sadr, W. M., Lundgren, J., Neaton, J. D., Gordin, F., Abrams, D., Arduino, R. C., Babiker, A., Burman, W., Clumeck, N., Cohen, C. J., Cohn, D., Cooper, D., Darbyshire, J., Emery, S., Fätkenheuer, G., Gazzard, B., Grund, B., Hoy, J., Klingman, K., Losso, M., Markowitz, N., Neuhaus, J., Phillips, A., Rappoport, C.; Strategies for Management of Antiretroviral Therapy (SMART) Study Group . (2006) CD4+ count-guided interruption of antiretroviral treatment. N. Engl. J. Med. 355, 2283–2296 [DOI] [PubMed] [Google Scholar]
- 12.Pereyra, F., Lo, J., Triant, V. A., Wei, J., Buzon, M. J., Fitch, K. V., Hwang, J., Campbell, J. H., Burdo, T. H., Williams, K. C., Abbara, S., Grinspoon, S. K. (2012) Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 26, 2409–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Justice, A. C., Erlandson, K. M., Hunt, P. W., Landay, A., Miotti, P., Tracy, R. P. (2018) Can biomarkers advance HIV research and care in the antiretroviral therapy era? J. Infect. Dis. 217, 521–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lichtenstein, K. A., Armon, C., Buchacz, K., Chmiel, J. S., Buckner, K., Tedaldi, E. M., Wood, K., Holmberg, S. D., Brooks, J. T.; HIV Outpatient Study (HOPS) Investigators . (2010) Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin. Infect. Dis. 51, 435–447 [DOI] [PubMed] [Google Scholar]
- 15.Longenecker, C. T., Funderburg, N. T., Jiang, Y., Debanne, S., Storer, N., Labbato, D. E., Lederman, M. M., McComsey, G. A. (2013) Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 14, 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuller, L. H., Tracy, R., Belloso, W., De Wit, S., Drummond, F., Lane, H. C., Ledergerber, B., Lundgren, J., Neuhaus, J., Nixon, D., Paton, N. I., Neaton, J. D.; INSIGHT SMART Study Group . (2008) Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 5, e203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Triant, V. A., Meigs, J. B., Grinspoon, S. K. (2009) Association of C-reactive protein and HIV infection with acute myocardial infarction. J. Acquir. Immune Defic. Syndr. 51, 268–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulware, D. R., Hullsiek, K. H., Puronen, C. E., Rupert, A., Baker, J. V., French, M. A., Bohjanen, P. R., Novak, R. M., Neaton, J. D., Sereti, I.; INSIGHT Study Group . (2011) Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J. Infect. Dis. 203, 1637–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddler, S. A., Smit, E., Cole, S. R., Li, R., Chmiel, J. S., Dobs, A., Palella, F., Visscher, B., Evans, R., Kingsley, L. A. (2003) Impact of HIV infection and HAART on serum lipids in men. JAMA 289, 2978–2982 [DOI] [PubMed] [Google Scholar]
- 20.Longenecker, C. T., Jiang, Y., Yun, C. H., Debanne, S., Funderburg, N. T., Lederman, M. M., Storer, N., Labbato, D. E., Bezerra, H. G., McComsey, G. A. (2013) Perivascular fat, inflammation, and cardiovascular risk in HIV-infected patients on antiretroviral therapy. Int. J. Cardiol. 168, 4039–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khovidhunkit, W., Kim, M.-S., Memon, R. A., Shigenaga, J. K., Moser, A. H., Feingold, K. R., Grunfeld, C. (2004) Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J. Lipid Res. 45, 1169–1196 [DOI] [PubMed] [Google Scholar]
- 22.Calza, L., Pocaterra, D., Pavoni, M., Colangeli, V., Manfredi, R., Verucchi, G., Chiodo, F., Cantù, M., Pariali, M. (2009) Plasma levels of VCAM-1, ICAM-1, E-Selectin, and P-Selectin in 99 HIV-positive patients versus 51 HIV-negative healthy controls. J. Acquir. Immune Defic. Syndr. 50, 430–432 [DOI] [PubMed] [Google Scholar]
- 23.Zanni, M. V., Grinspoon, S. K. (2012) HIV-specific immune dysregulation and atherosclerosis. Curr. HIV/AIDS Rep. 9, 200–205 [DOI] [PubMed] [Google Scholar]
- 24.Varki, A. (2017) Biological roles of glycans. Glycobiology 27, 3–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue, T., Goldstein, I. J., Hollingsworth, M. A., Kaul, K., Brand, R. E., Haab, B. B. (2009) The prevalence and nature of glycan alterations on specific proteins in pancreatic cancer patients revealed using antibody-lectin sandwich arrays. Mol. Cell. Proteomics 8, 1697–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang, Y. C., Nakagawa, M., Garitaonandia, I., Slavin, I., Altun, G., Lacharite, R. M., Nazor, K. L., Tran, H. T., Lynch, C. L., Leonardo, T. R., Liu, Y., Peterson, S. E., Laurent, L. C., Yamanaka, S., Loring, J. F. (2011) Specific lectin biomarkers for isolation of human pluripotent stem cells identified through array-based glycomic analysis. Cell Res. 21, 1551–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haab, B. B. (2012) Using lectins in biomarker research: addressing the limitations of sensitivity and availability. Proteomics Clin. Appl. 6, 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauer, M. E., Aytekin, M., Comhair, S. A., Loftis, J., Tian, L., Farver, C. F., Hascall, V. C., Dweik, R. A. (2014) Modification of hyaluronan by heavy chains of inter-α-inhibitor in idiopathic pulmonary arterial hypertension. J. Biol. Chem. 289, 6791–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, A., de la Motte, C., Lauer, M., Hascall, V. (2011) Hyaluronan matrices in pathobiological processes. FEBS J. 278, 1412–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cummings, R. D., Darvill, A. G., Etzler, M. E., Hahn, M. G. (2015) Glycan-recognizing probes as tools. In Essentials of Glycobiology (Varki, A., Cummings, R. D., Esko, J. D., Stanley, P., Har, G. W., Aebi, M., Darvil, A. G., Kinoshita, T., Packer, N. H., Prestegard, J. H., Schnaar, R. L., Seeberger, P. H., eds.), pp. 611–625, Cold Spring Harbor Laboratory Press, New York: [PubMed] [Google Scholar]
- 31.Baker, J. V., Duprez, D. (2010) Biomarkers and HIV-associated cardiovascular disease. Curr. Opin. HIV AIDS 5, 511–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connell, T. X., Horita, T. J., Kasravi, B. (2005) Understanding and interpreting serum protein electrophoresis. Am. Fam. Physician 71, 105–112 [PubMed] [Google Scholar]
- 33.Adedeji, A. L., Adenikinju, R. O., Ajele, J. O., Olawoye, T. L. (2014) Serum protein electrophoresis under effective control of HIV-1 disease progression. EXCLI J. 13, 761–771 [PMC free article] [PubMed] [Google Scholar]
- 34.Kreisman, L. S. C., Cobb, B. A. (2012) Infection, inflammation and host carbohydrates: a glyco-evasion hypothesis. Glycobiology 22, 1019–1030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan, H. A., Woloski, B. M., Hellman, M., Jamieson, J. C. (1983) Studies on the effect of inflammation on rat liver and serum sialyltransferase. Evidence that inflammation causes release of Gal beta 1 leads to 4GlcNAc alpha 2 leads to 6 sialyltransferase from liver. J. Biol. Chem. 258, 11505–11509 [PubMed] [Google Scholar]
- 36.Jones, M. B., Oswald, D. M., Joshi, S., Whiteheart, S. W., Orlando, R., Cobb, B. A. (2016) B-cell-independent sialylation of IgG. Proc. Natl. Acad. Sci. USA 113, 7207–7212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, W. H., Aziz, P. V., Heithoff, D. M., Mahan, M. J., Smith, J. W., Marth, J. D. (2015) An intrinsic mechanism of secreted protein aging and turnover. Proc. Natl. Acad. Sci. USA 112, 13657–13662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pagan, J. D., Kitaoka, M., Anthony, R. M. (2018) Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell 172, 564–577.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribeiro, J. P., Mahal, L. K. (2013) Dot by dot: analyzing the glycome using lectin microarrays. Curr. Opin. Chem. Biol. 17, 827–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.