Abstract
The dietary R-3-hydroxybutyrate-R-1,3-butanediol monoester increases resting energy expenditure (REE) and markers of brown and white adipose thermogenesis in lean mice. The purpose of this investigation was to determine whether the ketone ester, R,S-1,3-butanediol diacetoacetate (BD-AcAc2), increases energy expenditure and markers of adipose tissue thermogenesis in the context of high-fat diet (HFD)–induced obesity. Thirty-five-week–old male C57BL/6J mice were placed on an ad libitum HFD (45% kcal) for 10 wk. The mice were then randomized to 1 of 3 groups (n = 10 per group) for an additional 12 wk: 1) control (Con), continuous HFD, 2) pair-fed (PF) to ketone ester (KE); and 3) KE: HFD+30% energy from BD-AcAc2. Mean energy intake throughout the study was ∼26% lower in the KE compared to the Con group (8.2 ± 0.5 vs. 11.2 ± 0.7 kcal/d; P < 0.05). Final body weight (26.8 ± 3.6 vs. 34.9 ± 4.8 g; P < 0.001) and fat mass (5.2 ± 1.2 vs. 11.3 ± 4.5 g; P < 0.001) of the KE group was significantly lower than PF, despite being matched for energy provisions. Differences in body weight and adiposity were accompanied by higher REE and total energy expenditure in the KE group compared to PF after adjustment for lean body mass and fat-mass (P = 0.001 and 0.007, respectively). Coupled or uncoupled mitochondrial respiratory rates in skeletal muscle were not different among groups, but markers of mitochondrial uncoupling and thermogenesis (uncoupling protein-1, deiodinase-2, and peroxisome proliferator-activated receptor γ coactivator-1α) were higher in interscapular brown adipose tissue (BAT) of mice receiving the KE diet. The absence of mitochondrial uncoupling in skeletal muscle and increased markers of mitochondrial uncoupling in BAT suggest that BD-AcAc2 initiates a transcriptional signature consistent with BAT thermogenesis in the context of HFD-induced obesity.—Davis, R. A. H., Deemer, S. E., Bergeron, J. M., Little, J. T., Warren, J. L., Fisher, G., Smith, D. L., Jr., Fontaine, K. R., Dickinson, S. L., Allison, D. B., Plaisance, E. P. Dietary R,S-1,3-butanediol diacetoacetate reduces body weight and adiposity in obese mice fed a high-fat diet.
Keywords: ketones, ketogenic diet, energy expenditure, adipose tissue thermogenesis, weight loss
During starvation, the transition from hepatic fatty acid oxidation to ketogenesis attenuates glucose demand, reducing rates of gluconeogenesis and protein degradation (1). Ketones are transported from liver to extrahepatic tissues where they undergo conversion to acetyl-CoA and generate reducing equivalents for ATP synthesis. The capacity to efficiently store excess energy as fatty acids in adipose tissue and the ability to produce ketones in the liver has increased long-term viability of humans in the presence of ongoing starvation. It can also be argued in an environment of unwavering availability that the capacity to efficiently store energy threatens the viability of humans, as evidenced by the contemporary incidence of obesity and poor success of weight-loss strategies such as calorie restriction (CR) (2). The long-term lack of success with CR is often attributed to poor compliance and reductions in each component of daily total energy expenditure (TEE) resulting from lower metabolizable mass, the thermic effects of food, and nonexercise activity thermogenesis (2, 3).
High-fat, low-carbohydrate ketogenic diets (KDs) have been shown to decrease body weight more rapidly and often to a greater extent than low-calorie diets (4–6). The mechanisms are poorly understood, but studies in rodents and humans have shown that the reduction in body weight is produced by an increase in EE (5, 7, 8). Others have shown that KDs increase mitochondrial proteins involved in oxidative phosphorylation and uncoupling in brown adipose tissue (BAT), suggesting that the increase in EE is related to enhanced mitochondrial respiratory and thermogenic capacity (9). The growing body of evidence from KD on body composition has prompted interest in the ability to increase circulating ketones in the absence of total energy or carbohydrate restriction.
Although ketones are water soluble, their existence as strong acids cause peripheral vein irritation with enteral administration (10, 11). In response, Brunengraber et al. (11) esterified the molecule R,S-1,3-butanediol with diacetoacetate (BD-AcAc2) to overcome this problem and to serve as an alternative to lipid emulsions in parenteral nutrition. Subsequent studies in rodents show that dietary BD-AcAc2 produces modest increases in circulating ketone concentrations ranging from 0.5 to 1.0 mM and reductions in body weight compared to control animals (12–14). Despite evidence of reductions in body weight, little is known regarding the effects of BD-AcAc2 on energy intake and the components of EE. Veech et al. (15, 16) showed that the ketone ester (KE) R-3-hydroxybutyrate-R-1,3-butanediol (3HB-BD) elevates circulating βHB concentrations (0.7–7.0 mM) and lowered energy intake in pelleted diets fed to rodents and in a concentration-dependent manner in humans (17). Additional findings from the same group suggest that dietary 3HB-BD promotes signaling events that activate BAT thermogenesis in mice (18). Indeed, lean mice consuming 3HB-BD showed increased markers of mitochondrial biogenesis and thermogenesis in BAT and increased resting energy expenditure (REE) compared to mice consuming an isocaloric control diet. Despite the increase in REE, there were no differences in TEE or body weight between pair-fed (PF) controls and 3HB-BD–fed mice.
Given the availability and consumption of energy-dense foods and the obesity epidemic in many developed nations, our purpose was to determine whether dietary BD-AcAc2 increases EE and adipose tissue thermogenesis in the context of a high-fat, low-carbohydrate diet (HFD) and ensuing HFD–induced obesity. Our findings demonstrate that an HFD formulated with BD-AcAc2 decreases energy intake and increases REE and TEE after adjustment for fat mass (FM) and lean body mass (LBM). The increase in EE is consistent with a transcriptional signature of interscapular BAT activation.
MATERIALS AND METHODS
Animals and diets
Male C57BL/6J mice (n = 30) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) at 5 wk of age and allowed to acclimate for 7 d upon arrival at the University of Alabama at Birmingham (UAB) animal facility. Throughout the study, the mice were single-housed in filter-top shoebox cages with wood-chip bedding and shredded-paper nesting in a ventilated cage rack system. The animals were maintained in a standard 12:12 light–dark cycle (lights on, 6 am) in a temperature-controlled room at 22–23°C. At 6 wk of age, the mice began consuming an ad libitum HFD containing 45% kcal from fat (104212; Dyets, Bethlehem, PA, USA) for 10 wk (Table 1). Mice were then randomly assigned to 1 of 3 groups using a random-number generator in Excel (Microsoft, Redmond, WA, USA) for an additional 12 wk (n = 10 per group): 1) Control group (Con, remained on HFD), 2) PF (HFD with food intake matched to the mean intake of the KE group from the previous day and provided in a single allotment at ∼10 AM each morning), and 3) KE (HFD+30% carbohydrate energy replaced by KE). BD-AcAc2 was purchased from SavInd (Urbana, IL, USA) and shipped to Dyets for production. Bomb calorimetry was performed by the manufacturer on the BD-AcAc2 and was found to be 4.7 kcal/g for the lot produced for this experiment. During the first week of the experimental phase, the KE group was acclimated with a mixture of the powdered KE and Con diets (50:50). All analyses described in Results were performed with 10 animals per group except for the PF group which had 9 after removal of 1 animal that, for reasons unknown, was not eating during wk 7 of the experimental phase of the study. We also randomly selected a cohort of 6 animals per group to perform the skeletal muscle mitochondrial respiration studies. The UAB Institutional Animal Care and Use Committee (IACUC) approved the investigation, and all conditions conformed to the care procedures used by the UAB Animal Resources Program.
TABLE 1.
High-fat control and KE diets
| Ingredient | Amount (kcal/g) | Con (g/kg) | KE (g/kg) | Con (kcal/kg) | KE (kcal/kg) |
|---|---|---|---|---|---|
| Casein | 3.6 | 200.0 | 200.0 | 716.00 | 716.00 |
| Cornstarch | 3.6 | 72.8 | 0.0 | 262.08 | 0.00 |
| Dyetrose | 3.8 | 100.0 | 0.0 | 380.00 | 0.00 |
| Sucrose | 4.0 | 162.8 | 27.5 | 651.20 | 110.00 |
| Cellulose | 0.0 | 50.0 | 50.0 | 0.00 | 0.00 |
| Soybean oil | 9.0 | 25.0 | 25.0 | 225.00 | 225.00 |
| t-Butylhydroquinone | 0.0 | 0.005 | 0.005 | 0.00 | 0.00 |
| Lard | 9.0 | 177.5 | 177.5 | 1597.50 | 1597.50 |
| Salt mix 21008 | 1.6 | 10.0 | 10.0 | 16.00 | 16.00 |
| Dicalcium phosphate | 0.0 | 13.0 | 13.0 | 0.00 | 0.00 |
| Calcium carbonate | 0.0 | 5.5 | 5.5 | 0.00 | 0.00 |
| Potassium citrate H2O | 0.0 | 16.5 | 16.5 | 0.00 | 0.00 |
| Vitamin mix 300050 | 3.9 | 10.0 | 10.0 | 39.20 | 39.20 |
| l-cystine | 4.0 | 3.0 | 3.0 | 12.00 | 12.00 |
| Choline bitrate | 0.0 | 2.0 | 2.0 | 0.00 | 0.00 |
| Peanut flavor | 4.0 | 10.0 | 10.0 | 40.00 | 40.00 |
| Ketone ester | 4.7 | 0.0 | 252.0 | 0.00 | 1184.45 |
| Total | 858.11 | 802.02 | 3938.98 | 3940.15 |
Diets are from Dyets.
Body composition and indirect calorimetry
Body composition was measured in the UAB Small Animal Phenotyping Core by quantitative magnetic resonance (EchoMRI 3-in-1 version 2013; Echo Medical Systems, Houston, TX, USA) after 10 wk of HFD and at the end of the 12-wk experimental phase. Energy intake and expenditure and locomotor activity [horizontal (XT) and vertical (YT)] were evaluated during wk 12 of the experimental phase, with a PhenoMaster indirect calorimetry system (TSE Instruments, Chesterfield, MO, USA). Mice were acclimated to the metabolic cages for 48 h with measurements obtained for ∼24 h the following day. Oxygen consumption and carbon dioxide production were measured for 1 min in each of the 8 measurement cages and then in the reference cage. Therefore, EE was calculated every 9 min for each animal. TEE was determined by calculating the mean hourly EE over the measurement period and then multiplying by 24. REE was calculated by averaging the 3 lowest 18-min periods of EE, with at least 1 h between each period.
Glucose and insulin tolerance testing
Glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were conducted at wk 10 and 11, respectively, of the experimental phase. GTTs were performed 6 h after food was withheld, starting at 11 am. After a baseline blood glucose measurement (time 0), glucose was administered at 1.5 g/kg body weight (i.p.), followed by additional measurements at 15, 30, 60, 90, and 120 min. ITTs were performed 4 h after food was withheld with 0.75 mU/g body weight (i.p.) Humalog (Lilly, Indianapolis, IN, USA) starting at 11 am. Blood samples from the tail vein were obtained at 0, 15, 30, 45, and 60 min. A glucometer (One Touch Ultra; Lifespan, New Brunswick, NJ, USA) was used for all blood glucose measurements. The incremental area under the curve (AUCI) was calculated from the GTTs and ITTs, as described by Matthews et al. (19).
Tissue collection and handling
Mice were euthanized after they were unfed for 6 h. Tissues were obtained immediately after decapitation, snap frozen in liquid N2, and stored at −80°C until analysis. Whole blood was obtained from the trunk and collected into 1.5-ml centrifuge tubes. Blood was immediately placed on ice for at least 30 min to allow time for clotting followed by centrifugation at 1500 g at 4°C for 10 min. Serum was isolated and stored at −80°C until analysis.
Adipose tissue morphology
Aliquots of each adipose tissue depot [epididymal, retroperitoneal, and inguinal white adipose tissue (WAT) and interscapular BAT] were placed in histology cassettes and fixed in neutral buffered formalin overnight. After fixation, tissue cassettes were transferred to 70% ethanol followed by submission to the UAB Comparative Pathology Core, where they were embedded in paraffin blocks, sectioned at 5 µm, and autoprocessed for hematoxylin and eosin staining. Tissue sections were viewed with a DMRB inverted light microscope (Leica, Wetzlar, Germany) at ×20 magnification, with images captured by a MicroPublisher 3.3 RTV camera (Q Imaging, Surry BC, Canada). WAT area was determined by ImageJ (National Institutes of Health, Bethesda, MD, USA), as described by Parlee et al. (20). Because of the characteristic presence of multilocular adipocytes in BAT, we developed a procedure in ImageJ with the Histogram feature to count enhanced pixels in the red mode of red-green-blue (RGB). Lipid droplets are not included within red pixilation, and thus, greater amounts of pixilation represent lower lipid content.
Quantitative real-time PCR
Total RNA was isolated from tissues with an Rneasy Mini Kit (Qiagen, Frederick, MD, USA), according to the manufacturer’s instructions. One microgram RNA was reverse transcribed to produce cDNA using I Script Reverse Transcription Supermix for quantitative (q)RT-PCR with oligo(dT) and random primers (Bio-Rad, Hercules, CA, USA). cDNA was amplified by real-time PCR in a total reaction volume of 20 μl with Sybr Green Real-time PCR Master Mix. Real-time PCR reactions were cycled in a Bio-Rad real-time PCR system with the following cycles: 95°C for 10 min, 40 cycles of 95°C for 15 s, 60°C for 30 s, and 72°C for 30 s. Cycle threshold values for each gene of interest were normalized to cyclophilin by using the ΔΔCt method. Primer sequences used were uncoupled protein (Ucp)-1: Fwd 5′-GAGTCAGGGGCCTGTGGAAA-3′, Rev 5′-GCGTTCATGTATCGGGTCTT-3′; peroxisome proliferator-activated receptor γ coactivator (Pgc)-1α: Fwd 5′-ATGTGCGCCTTCTTGCTCT-3′, Rev 5′-CACGACCTGTGTCGAGAAAA-3′; deiodinase (Dio)-2: Fwd 5′-GTCCGCAAATGACCCCTTT-3′, Rev 5′-CCCACCCACTCTCTGACTTTC-3′; fibroblast growth factor (Fgf)-21: Fwd 5′-CTGGGGGTCTACCAAGCATA-3′, Rev 5′-CACCCAGGATTTGAATGACC-3′; PR domain containing (Prdm)-16: Fwd 5′-CAGCACGGTGAAGCCATTC-3′, Rev 5′-GCGTGCATCCGCTTGTG-3′; citrate synthase (Cs): Fwd 5′-CGAGACTACATCTGGAACACAC-3′, Rev 5′-CAAACTCTCGCTGACAGGAATA-3′; complex I: Fwd 5′-CTCAGAACTCAGGATGAACTGG-3′, Rev 5′-CACAAAGCCGAAGAGATGTTTAC-3′; complex II: Fwd 5′-GGTGGGCAGAATGTCTTCTAA-3′, Rev 5′-TCACGAATGGTCGAACCTAAC-3′; complex III: Fwd 5′-CAGTCGAAGTGTCCCAGTTAAG-3′, Rev 5′-CACAACCAAGATGAGTACAGACA-3′; and complex IV: Fwd 5′-TGAGGTGTACATCCTGATCCT-3′, Rev 5′-ATGCCCATGTAGCCGAAAG-3′.
Western blot analysis
Tissues were homogenized in RIPA buffer with protease and phosphatase inhibitors (Thermo Fisher Scientific, Waltham, MA, USA). Homogenates were centrifuged for 10 min at 10,000 g (4°C) and stored at −80°C until ready for use. Protein content of the supernatant was quantified by protein assay (Dc Protein Assay; Bio-Rad) using fatty acid free bovine serum albumin standards. Proteins were separated by SDS-PAGE on 10% polyacrylamide gels and transferred to PVDF membranes (Bio-Rad) overnight. Membranes were then incubated with primary antibody for 2–3 h or overnight, followed by washing and subsequent incubation with a horseradish peroxidase–linked secondary antibody, according to the manufacturer’s instructions. The UCP-1 antibody was purchased from Thermo Fisher Scientific (PIPA529575), PGC-1α (AB3242) from MilliporeSigma (Burlington, MA, USA), and β-actin (ab8227) from Abcam (Cambridge, United Kingdom). Proteins were visualized by chemiluminescence, and the density of individual bands was quantified by ImageJ with normalization to β-actin.
Biochemical assays
Serum glucose and triglyceride (TG) concentrations were determined by enzymatic colorimetric assays, according to the manufacturer’s instructions (Cayman Chemicals, Ann Arbor, MI, USA). Serum insulin was measured with an ELISA kit from Alpco (Salem, NH, USA). Hepatic TG levels were determined by enzymatic colorimetric assay after tissue homogenization, according to the manufacturer’s instructions (Cayman Chemicals).
Skeletal muscle mitochondrial respiration
Skeletal muscle from the vastus lateralis was transferred to the laboratory on ice in buffer X containing 50 mM N-morpholino ethanesulfonic acid, 7.23 mM K2EGTA, 2.77 mM CaK2EGTA, 20 mM imidazole, 0.5 mM DTT, 20 mM taurine, 5.7 mM ATP, 14.3 mM phosphocreatine (PCr), and 6.56 mM MgCl2-6 H2O (pH 7.1, 290 mOsM) as previously described, with adaptations (21–23). The tissue specimens were cleared of adipose and connective tissue and separated into several smaller muscle bundles (∼3–5 mg wet weight). Each bundle was gently dissected along the longitudinal axis in ice-cold buffer X with a pair of antimagnetic, needle-tipped forceps under magnification. Permeabilization was achieved by incubating the tissue in buffer X containing 40 µg/ml saponin on a rotator for 30 min at 4°C. Tissue bundles were washed of saponin for 15 min in buffer Z containing 105 mM K- N-morpholino ethanesulfonic acid, 30 mM KCl, 1 mM EGTA, 10 mM K2HPO4, and 5 mM MgCl2-6 H2O, 5 µM glutamate, 2 µM malate, and 5.0 mg/ml bovine serum albumin (pH 7.4; 290 mOsM). The samples were transferred to buffer Z containing 20 mM creatine hydrate and 20 µM blebbistatin for 10 min before each experiment was begun.
High-resolution respirometry was performed with an Oroboros Oxygraph O2K (Oroboros Instruments, Innsbruck, Austria) containing 2 ml buffer Z with the addition of creatine and blebbistatin. The medium was hyperoxygenated, constantly stirred, and maintained at 37°C. Malate (2 mM) and pyruvate (10 mM) were used to drive electron input to complex I and to assess mitochondrial O2 consumption. Maximum coupled respiration (state 3) was measured after the addition of ADP (5 mM). Succinate (3 mM) was then added to drive complex II electron entry (state 3S). Uncoupled respiration (state 4) was induced by oligomycin (10 μg/ml). Cytochrome c (10 μM) was added to assess mitochondrial membrane integrity after dissection of the myofibers. The respiratory control ratio was calculated as state 3/state 4. Oxygen flux was normalized to the wet weight of each fiber bundle taken before each experiment.
Blood glucose and β-hydroxybutyrate measurements
Blood glucose and β-hydroxybutyrate concentrations were measured with a Nova Vet ketone/glucose meter from Nova Biomedical (Waltham, MA, USA). Before dark cycle measurements, animals had food withheld for 4 h and refed for 4 h before testing during wk 6 of the experimental phase of the study. AcAc concentrations are not measurable by the ketone meter and are not available.
Statistical analysis
Differences among groups (Con, PF, and KE) were analyzed by 1-wayANOVA. Body weight responses were analyzed using a 3 (group) × 12 (time) ANOVA with repeated measures on time. Blood glucose responses during the GTT were analyzed using a 3 (group) × 6 (time) ANOVA with repeated measures of time and a 3 (group) × 5 (time) ANOVA with repeated measures of time was used for the ITT. Group differences in EE (kilojoules per mouse per day) were compared by analysis of covariance (ANCOVA), calculating least-squares means that account for variation in EE attributable to differences in LBM and FM among groups (24). The covariate for FM in the model was transformed with natural log to satisfy the assumptions of homogenous slopes in ANCOVA. Fisher’s least-significant difference post hoc testing was used to explore significant differences between groups (25). Significance was set a priori at P < 0.05 (2-tailed). Data are expressed as means ± sd. All statistical analyses were conducted with IBM SPSS (v.25.0; IBM, Armonk, NY, USA).
RESULTS
Dietary BD-AcAc2 decreases body weight and adiposity in the presence of an HFD
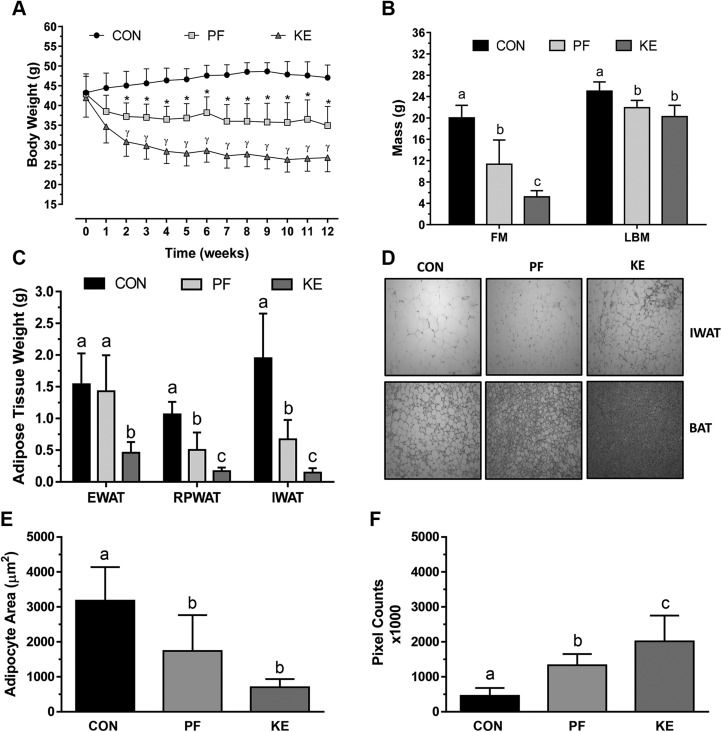
Circulating d-β-hydroxybutyric acid (d-βHB) concentrations were increased in the KE group (0.51 ± 0.12 mM) compared to the Con (0.32 ± 0.06) and PF (0.32 ± 0.10 mM) groups. Ten weeks of the HFD increased body weights of the entire cohort from 20.8 ± 0.2 g to 42.9 ± 0.8 g. Body weight continued to increase in the Con group after randomization, whereas the KE group experienced a rapid decrease in body weight that plateaued at a level that was 42% lower than in the Con group at the completion of the study (Fig. 1A). Despite the matched energy provision, the final body weight of the PF group was 23% greater than the KE group. LBM was similar between KE and PF groups, but both were lower than Con (Fig. 1B). FM was 74% lower in the KE vs. Con group and 54% lower than the PF group. WAT fat pad (epididymal, retroperitoneal, and IWAT) weights were lower in the KE group vs. the Con or PF group (Fig. 1C). The adipocyte area was smaller in the PF and KE groups than in the Con group (Fig. 1D, E), with the appearance of multilocular brown-like adipocytes observed in the IWAT of the KE group (Fig. 1D). Representative images suggest lower lipid content in the KE group compared to the Con or PF group. Color saturation enhancement and RGB pixel count revealed more extensive concentration of nonlipid structures in BAT of the KE group vs. the PF and Con groups, indicating lower lipid content (Fig. 1F).
Figure 1 .
Differences in body weight and body composition. A) Body weight responses to 12 wk of Con, PF, or KE diet. *P < 0.001, Con vs. PF beginning at wk 2; γP < 0.001, all group comparisons beginning at wk 2. B) Final FM and LBM among groups. P < 0.001. C) Final adipose tissue weights for epididymal (EWAT), retroperitoneal (RPWAT), and IWAT. Data are presented as means ± sd. Different letters indicate significant differences between groups. P < 0.05. D) Representative hematoxylin and eosin images of interscapular BAT sections. Original magnification, ×20. E) WAT area was determined by ImageJ. F) Because of the characteristic presence of multilocular adipocytes in BAT, we developed a procedure in ImageJ with the Histogram feature to count enhanced pixels in the red mode of RGB. Lipid droplets are not included within red pixilation, and thus greater amounts of pixilation represent lower lipid content.
Reductions in body weight and adiposity are attributed to lower food intake and higher EE
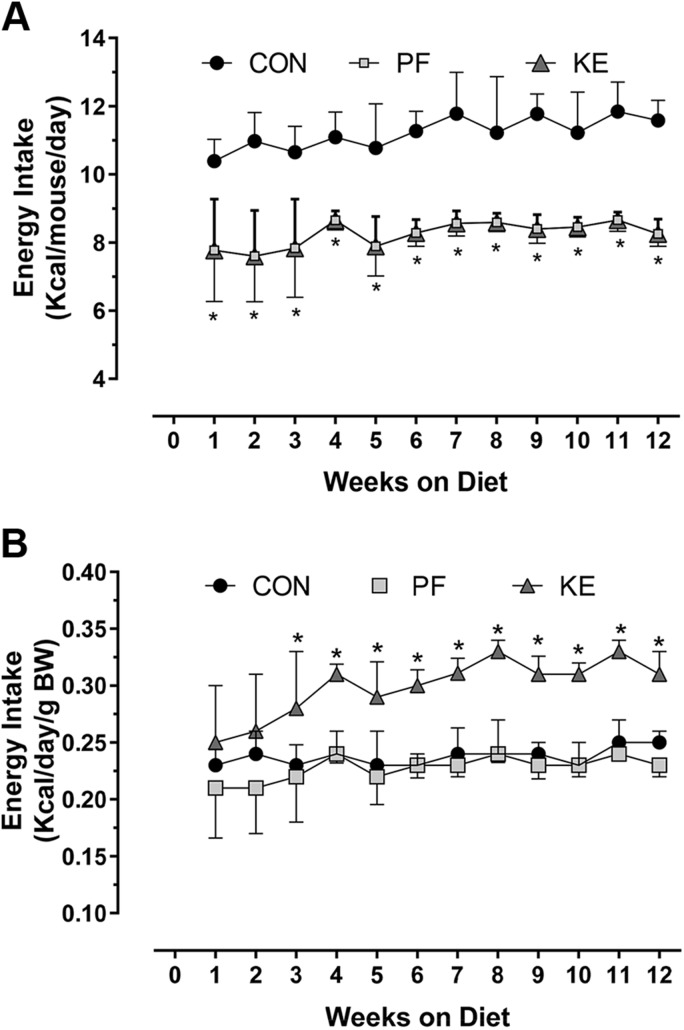
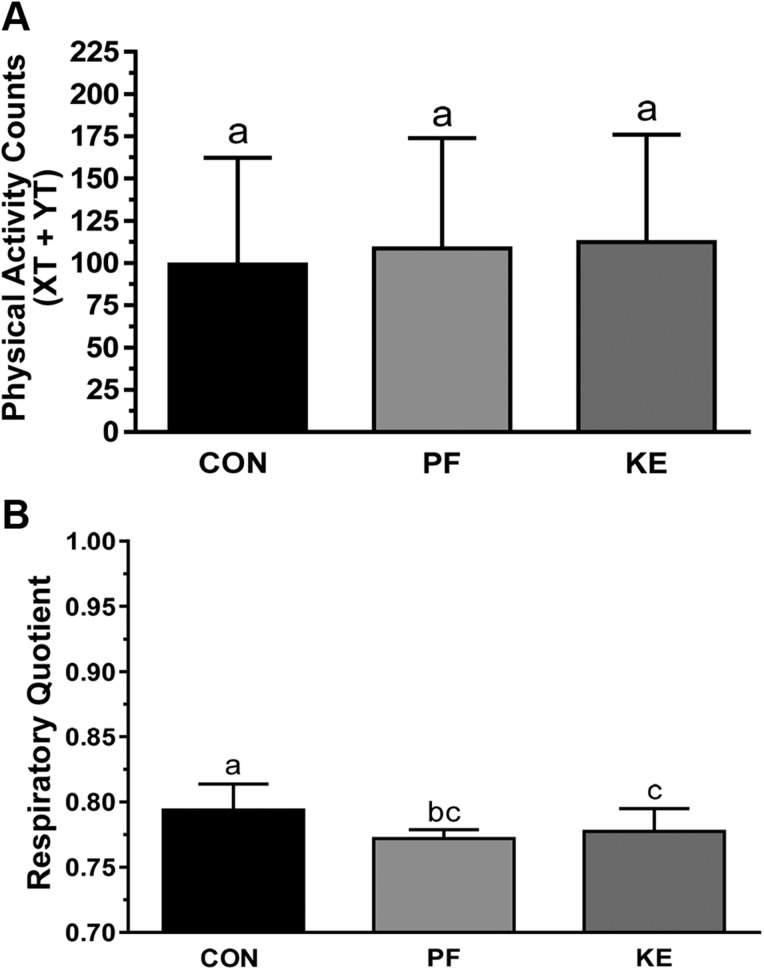
The energy intake was a mean of 11.2 ± 0.7 kcal/mouse per day in the Con group and 8.2 ± 0.5 kcals/mouse per day in the KE group (Fig. 2A). Similar energy intake per unit body weight in the PF and Con groups indicated that the loss in body weight and FM in the PF animals was accounted for by reductions in energy intake, whereas higher energy intake in the KE group indicated that mice lost more body weight because of higher EE (Fig. 2B). Although REE and TEE were not significantly different between groups before adjustment for LBM and FM, adjusted REE (P = 0.001) and TEE (P = 0.007) were higher in the KE group than in the PF group (Table 2). REE was not significantly different between KE and Con animals. More extensive evaluation of the 12-h dark cycle revealed that adjusted REE and TEE were significantly higher in the KE group than in the Con group or PF with a slight reduction in TEE for the PF and KE groups compared to the Con group during the light cycle. Ambulatory activity (XT+YT) was not different among groups during indirect calorimetry, suggesting that differences in EE are unlikely to be attributable to differences in physical activity (Fig. 3A). The respiratory quotient was lower in the KE and PF groups compared to the Con, but no differences were observed among KE and PF groups (Fig. 3B).
Figure 2 .
Assessment of effects on energy intake among the Con, PF, and KE groups. A) Energy intake in kilocalories per mouse per day. PF mice were matched for energy provisions with the KE group. B) Energy intake per unit body weight (BW). See Table 1 for energy content of the diets. Data are means ± sd. *P < 0.05, differences between all groups.
TABLE 2.
REE and TEE
| Group | Daily (kcal) | Dark cycle (kcal/h) | Light cycle (kcal/h) | |||
|---|---|---|---|---|---|---|
| REE | TEE | REE | TEE | REE | TEE | |
| Control | ||||||
| Unadjusted | 10.6 ± 0.8a | 14.1 ± 0.7a | 0.48 ± 0.04a | 0.63 ± 0.03a | 0.42 ± 0.03a | 0.54 ± 0.03a |
| Adjusted | 8.3 ± 0.4a | 12.0 ± 0.3a | 0.35 ± 0.07a | 0.50 ± 0.06a | 0.34 ± 0.06a | 0.49 ± 0.02a |
| PF | ||||||
| Unadjusted | 7.0 ± 1.9b | 10.7 ± 1.5b | 0.28 ± 0.09b | 0.44 ± 0.10b | 0.29 ± 0.05b | 0.45 ± 0.02b |
| Adjusted | 7.1 ± 0.3a,b | 10.8 ± 0.2b | 0.29 ± 0.02b | 0.45 ± 0.02b | 0.30 ± 0.02a,b | 0.46 ± 0.01b |
| KE | ||||||
| Unadjusted | 7.2 ± 1.5b | 10.4 ± 1.3b | 0.33 ± 0.08b | 0.47 ± 0.07b | 0.29 ± 0.07b | 0.39 ± 0.05c |
| Adjusted | 9.5 ± 0.4a,c | 12.4 ± 0.3a | 0.45 ± 0.07c | 0.59 ± 0.06c | 0.36 ± 0.06a,c | 0.43 ± 0.02b |
Adjusted values are adjusted for FM and LBM by using ANCOVA, where FM is natural log–transformed to maintain homogenous slopes. Values are represented as means ± sd from 24- or 12-h (dark and light cycle) continuous indirect calorimetry performed after the 12-wk intervention. Mice were acclimated for 48 h before gases were measured for 24 h. a,b,cValues with different letters are significantly different. P < 0.05.
Figure 3 .
Ambulatory activity counts and substrate utilization. A) Ambulatory activity was assessed as XT and YT activity during the final week of the study. B) Respiratory quotient was determined as Vco2/Vo2. Data are means ± sd. P < 0.05, different letters indicate statistically significant differences.
Brown adipose thermogenic and biogenic markers are increased by BD-AcAc2
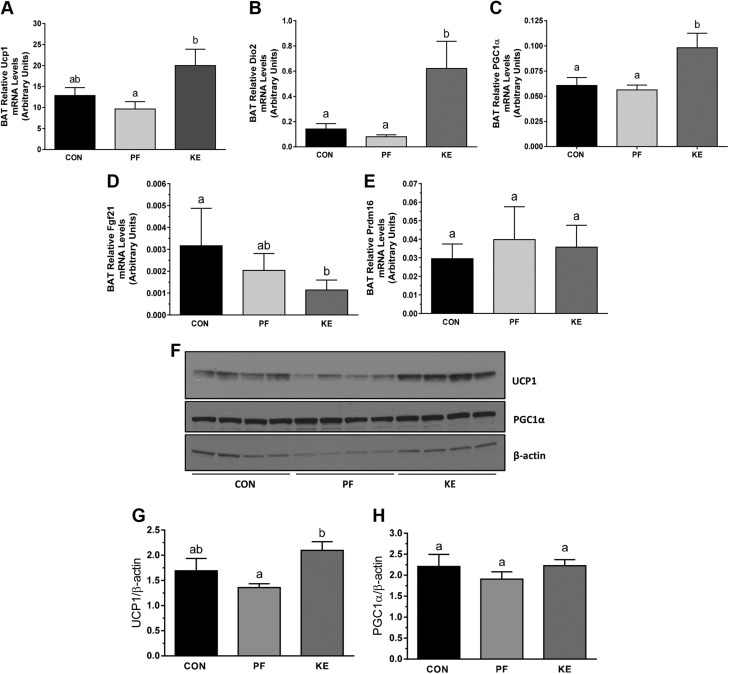
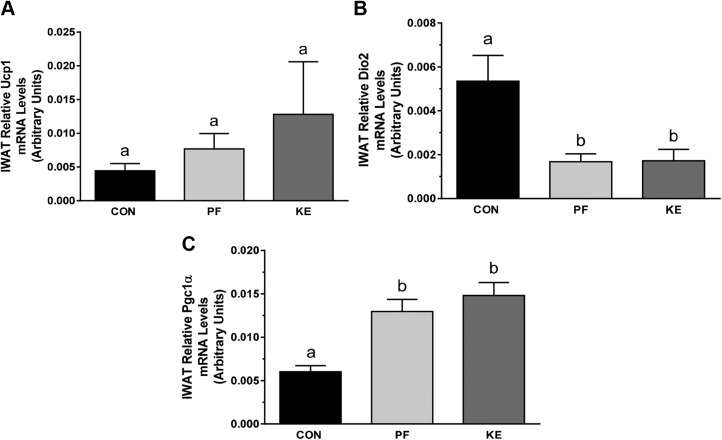
BD-AcAc2 increased Ucp1 mRNA (Fig. 4A) and protein expression (Fig. 4F) in BAT compared to PF animals. Dio2 and Pgc1a mRNA expression was also increased in the KE group vs. PF (Fig. 4B, C), but protein levels of PGC-1α were not different among groups (Fig. 4F). mRNA expression of Fgf-21 in the KE group was similar to that in the PF group, whereas no differences were observed for Prdm16. mRNA expression was also examined in IWAT, producing a transcriptional signature inconsistent with increased EE (Fig. 5A–C). In addition, Ucp1 mRNA expression was unchanged in epididymal WAT (data not shown).
Figure 4 .
Mitochondrial thermogenic and biogenic expression in BAT. A–E) Relative Ucp1 (A), Dio2 (B), Pgc1a (C), Fgf21 (D), and Prdm16 (E) gene expression in interscapular BAT. F) Representative images of protein expression for UCP-1 and PGC-1α with quantification. Data are means ± sd. P < 0.05, different letters indicate statistically significant differences.
Figure 5 .
Mitochondrial thermogenic and biogenic expression in IWAT. Relative Ucp1 (A), Dio2 (B), and Pgc1a (C) gene expression in IWAT. Data are means ± sd. P < 0.05, different letters indicate statistically significant differences.
Skeletal muscle does not appear to contribute to the increase in EE produced by BD-AcAc2
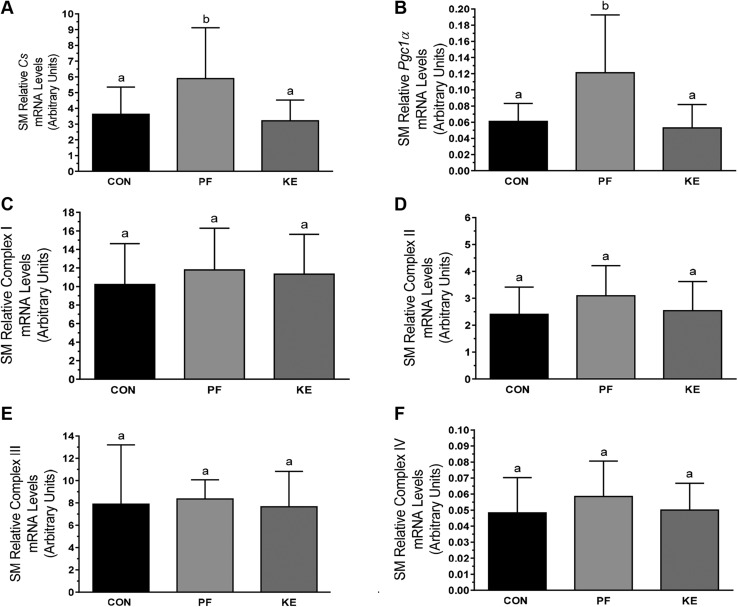
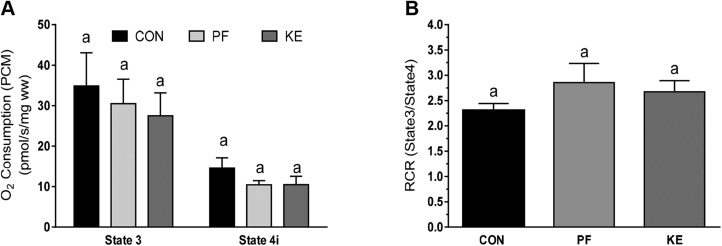
Skeletal muscle (vastus lateralis) mRNA expression of citrate synthase (Cs) and Pgc1a was higher in PF animals than in Con and KE animals (Fig. 6A, B). Furthermore, there were no differences among groups in mRNA expression of mitochondrial complexes I–IV (Fig. 6C–F). Direct examination of skeletal muscle mitochondrial respiration revealed no differences between groups in maximum oxygen uptake coupled to ATP production in the presence of malate and pyruvate (Fig. 7A, state 3). Furthermore, oligomycin-induced mitochondrial uncoupling (state 4i) and the respiratory control ratio (index of mitochondrial coupling; Fig. 7B) were also similar, demonstrating no differences in skeletal muscle mitochondrial-oxidative capacity or -coupling between groups.
Figure 6 .
Mitochondrial biogenic and respiratory expression in skeletal muscle (vastus lateralis). Relative Cs (A), Pgc1a (B), and complex I (C), II (D), III (E), and IV (F). Data are means ± sd. P < 0.05, different letters indicate statistically significant differences.
Figure 7 .
Skeletal muscle mitochondrial respiration. A) State 3 and state 4 respiration. B) Respiratory control ratio (state 3:state 4). Values are means ± sd. Values with the same letter are not statistically significant.
Effects of BD-AcAc2 on glucose and lipid metabolism
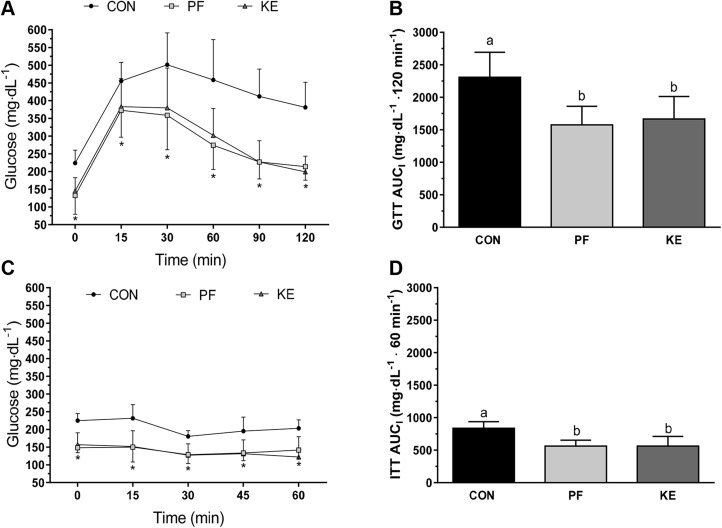
Fasting serum insulin concentrations were lower in the KE and PF groups than in the Con group, but no differences were observed between KE and PF groups (Table 3). Blood glucose was significantly lower in the PF group compared to the Con group, but no statistically significant differences were observed in the KE group compared to the Con or PF groups. Neither serum nor hepatic TG concentrations were significantly different among the PF and KE groups, but hepatic TG levels were 1.7-fold lower in the KE vs. the Con group (Table 3). Glucose concentrations were lowered to a similar extent in the KE and PF groups compared to the Con group during the GTT on an absolute basis (Fig. 8A) and when presented as the AUCI (Fig. 8B). Glucose concentrations during the ITT were also similar between PF and KE groups, and both were significantly lower than Con on an absolute basis (Fig. 8C) and when expressed as AUCI (Fig. 8D).
TABLE 3.
Biochemical parameters
| Parameter | Con | PF | KE |
|---|---|---|---|
| Insulin (ng/ml) | 5.55 ± 3.38a | 0.80 ± 0.25b | 0.61 ± 0.55b |
| Glucose (mg/dl) | 208 ± 31a | 163 ± 34b | 175 ± 39a,b |
| Serum TG (mg/dl) | 72 ± 7a | 86 ± 18a | 68 ± 21a |
| Hepatic TG (mg/dl/g) | 6712 ± 2271a | 4956 ± 1159b | 3938 ± 748b |
| Serum βHB (mM) | 0.32 ± 0.06a | 0.32 ± 0.10a | 0.51 ± 0.12b |
Mice were unfed for 6 h before blood and tissue collection with the exception of serum βHB. Serum βHB concentrations were measured in whole blood from a tail snip following a 4-h food withholding/4-h refeeding period at the start of the dark cycle (6 pm). Values are means ± sd. a,bValues with different letters are significantly different (P < 0.05).
Figure 8 .
Intraperitoneal GTTs and ITTs. A) Absolute blood glucose responses to GTTs performed at a dose of 1.5 mg/kg. *P < 0.05, significant differences for PF and KE vs. Con. B) AUCI by group for GTT. C) Absolute blood glucose responses to ITT performed at 0.75 mg/kg. *P < 0.05, significant differences for PF and KE vs. Con. D) AUCI by group for ITT. Values are means ± sd. Different letters indicate significant differences between groups.
DISCUSSION
A diet consisting of 28.6% energy from the KE 3HB-BD produces a transcriptional and metabolic signature consistent with increased interscapular BAT thermogenesis in lean male C57BL/6J mice (18). An increase in REE was consistent with the transcriptional response in BAT, but the ester did not increase TEE or decrease body weight compared to PF controls. The purpose of this investigation was to examine the feasibility and efficacy of the KE BD-AcAc2 (30% by kilocalories) on components of energy balance, weight loss and adiposity in obese mice receiving a high-fat, low-carbohydrate diet (45 and 5% by kilocalories, respectively). According to our results, replacing carbohydrate energy with BD-AcAc2 in the presence of an HFD reduced mean weekly energy intake by 26% compared with the control. Mice in the KE group lost 23% more body weight than PF mice that received matched-energy provisions, suggesting that the additional weight loss came from greater EE, altered nutrient partitioning or perhaps energy loss. These conclusions are consistent with our observation that the KE group had higher REE and TEE (after adjustment for FM and LBM) than the PF group and that dietary KE produces transcriptional changes consistent with interscapular BAT activation.
High-fat, low-carbohydrate KDs have been shown to increase EE, leading to the hypothesis that circulating ketones and the metabolic state of ketosis are key factors that affect energy balance and metabolism (5, 8). The potential for enhanced energy metabolism through nutrient-sensing pathways has led to an extensive search for dietary strategies that produce ketosis without the requirements of a KD. Desrochers and colleagues (10, 11) first reported on the esterification of BD and AcAc2 in 1995 when they showed that the ester provided a convenient way to administer ketones (without sodium load and with the capacity to trap reducing equivalents produced by hepatic BD oxidation) and increase circulating concentrations of AcAc and βHB. BD-AcAc2 has subsequently been shown to produce beneficial outcomes in rodent models of cognitive dysfunction and neurologic disease (13, 14, 26). Although the primary outcomes have not focused on energy balance and body weight, multiple studies conducted in mice and rats show that BD-AcAc2 induces body weight loss with only modest increases in circulating ketones (0.5–1.0 mM) (12–14). 3HB-BD has been shown to produce significant increases in circulating ketone concentrations (3.5–7.0 mM) (16, 18), but did not produce weight loss, despite higher REE compared to mice receiving matched energy provisions (18). The increase in REE was associated with increased circulating leptin concentrations, intracellular markers of sympathetic nervous system activation 9cAMP and cAMP response-element binding protein), and markers of thermogenesis and biogenesis in mice (18).
Early investigations in rodents show that circulating βHB concentrations increase and food intake decreases with subcutaneous delivery of βHB (27–29). The hypophagic effect is abrogated by vagotomy of the common hepatic branch (29) suggesting that vagal afferents arising from the small intestine may provide signals to the brain that affect feeding behavior (30). Multiple studies have subsequently explored central effects on eating behavior using intracerebroventricular administration of βHB into the third ventricle of the hypothalamus (31–34). Hypothalamic administration of βHB consistently decreased body weight and either decreased (33, 34) or had no effect (31, 32, 35) on food intake suggesting that increased EE contributed to the reduction in body weight. Recent unpublished findings from our laboratory and others (18) show that BD-AcAc2 also decreases food intake by ∼7–10% when incorporated as part of a low-fat, high-carbohydrate diet in a concentration-dependent manner. In the current investigation, we chose a nutritional model using obesity and a high-fat/low-carbohydrate diet, with and without KE, to study the effects of metabolic ketosis on energy intake and expenditure. The mean weekly energy intake of the KE group was 26% lower than that of the Con group, suggesting that the nutritional model either may have produced a greater level of ketosis or conferred gustatory or olfactory aversion that is not present with a higher dietary carbohydrate base. The concept of a threshold- or concentration-dependent response is in line with a study in pygmy goats where parenteral administration of βHB alone produced a plasma concentration of 0.56 mM that did not alter feeding behavior, whereas combined administration of 1,3-butanediol and βHB increased plasma concentrations to 0.75 mM and decreased food intake by decreasing meal frequency (36).
Little is known about the specific mechanisms by which ketones regulate feeding behavior, but it has been proposed that the hypophagic effects of KE in mice and rats are related, at least in part, to increases in brain malonyl-CoA levels (15, 18). This notion is based on findings that intracerebroventricular administration of C75, a potent fatty acid synthase inhibitor, increases malonyl-CoA levels leading to reductions in food intake (37). Despite the fact that serum leptin concentrations were not increased in rats treated with dietary KE (15), the researchers proposed that the increase in serum leptin of mice treated with KE could be responsible for the increase in brain malonyl-CoA and decrease in food intake. More recently, Stubbs and colleagues (38) showed in humans that a single dose of 3HB-BD decreases ghrelin, reported hunger, and desire to eat for up to 1.5 h. Circulating ghrelin concentrations increase in the presence of energy restriction–mediated weight loss and this response has been implicated in overeating and weight regain (39, 40). Taken together, these findings highlight consistent effects of ketones on feeding behavior and add further support to the notion that additional studies are needed to more thoroughly understand the mechanisms responsible for the hypophagic effect of ketones.
Despite similar energy provisions, body weight and adiposity were significantly lower in the KE vs. the PF group, suggesting that EE or energy loss was higher in the KE group. Indeed, REE and TEE in the KE group were greater than in the PF animals, particularly during the dark cycle when mice typically eat. Srivastava and colleagues (18) showed in lean mice that 3HB-BD produced greater circulating βHB concentrations than BD-AcAc2 used in the current investigation. However, TEE and body weight were not different between PF controls and the KE group. In contrast, in our study, BD-AcAc2 reduced body weight by 23% more than PF in previously obese mice in the context of a high-fat, low-carbohydrate diet.
To determine the mechanisms responsible for increased EE, we first explored skeletal muscle, a tissue well-known to use ketones as a mediator of increased fatty acid oxidation and intracellular glycogen sparing during prolonged fasting or starvation (41). In line with previous studies (42, 43), we found that energy-restricted PF mice had increased mRNA expression of Cs and Pgc1a compared to the control. However, we found no evidence that BD-AcAc2 produced transcriptional changes consistent with mitochondrial biogenesis or respiration. Using high-resolution respirometry, we found that BD-AcAc2 had no effect on mitochondria-coupled (state 3) or -uncoupled (state 4i) respiration, suggesting that skeletal muscle mitochondrial respiration was not a primary contributor to the increased EE observed. Nonetheless, we acknowledge that the skeletal muscle analysis is preliminary, and there remains a possibility that skeletal muscle contributes to the phenotype.
Because a previous study by Srivastava et al. (18) in mice showed an increase in EE associated with interscapular BAT and epididymal WAT markers of mitochondrial biogenesis and thermogenesis with dietary administration of 3HB-BD, we also explored adipose tissue. The results show that compared to PF animals, KE treatment increased expression of multiple genes associated with BAT mitochondrial biogenesis (Pgc1a) and thermogenesis (Ucp1 and Dio2). In contrast, we did not observe an increase in the expression of these genes in epididymal WAT, as Srivastava et al. (18) reported, or in IWAT where browning phenotypes and transcriptional responses have been noted with pharmacological agonists of the β3-receptor or cold exposure (44). Although the contribution of UCP-1 (45) and alternative thermogenic pathways to energy homeostasis and metabolism continue to be debated, it will be important in future studies to flesh out the role of WAT and BAT on the metabolic phenotype produced by the KE-supplemented diet. Studies are underway in our laboratory to explore the precise role of adipose tissue mitochondria on EE and to evaluate whether KE directly stimulates BAT or central activation of the sympathetic nervous system mediates the response. As previously reported, human newborns have high levels of BAT and receive high-fat, lactose-deficient colostrum during the first days after birth, suggesting that ketones could be used as a direct fuel source for thermoregulation (18, 41).
Similar improvements in insulin and glucose tolerance between the PF and KE groups suggest that the reduction in energy intake produced by the KE diet contributes to improvements in glucose metabolism. Restriction of energy produces well-known effects on insulin action and glucose regulation (46). Thus, it seems plausible that at least part of the beneficial changes in glucose metabolism of the current study are attributed to the reduction in energy intake. However, recent findings in BAT suggest that cold exposure activates the transcription factor carbohydrate–responsive element-binding protein, leading to increased de novo lipogenesis (47). It seems likely that dietary ketones could lead to an increase in glucose disposal within BAT to fuel de novo lipogenesis leading to improvements in glucose metabolism. This interpretation would also explain extensive reductions in lipid content in both BAT and liver. Future studies using hyperinsulinemic clamps with metabolite tracers to understand where insulin action is enhanced and glucose disposal is occurring are needed.
In previous studies, reduction of carbohydrates in the diet was used to account for energy content of the KE, with the intent of producing an isocaloric diet. Recognizing the experimental necessity of producing an isocaloric diet, we also removed energy from carbohydrates (matched for protein concentration). Although the PF group received energy provisions equivalent to those of the KE group, it is important to note that the consequence of producing an isocaloric diet meant that we were unable to reduce the carbohydrate content to the same level as in the KE group, which could influence the results. Despite extensive reductions in energy intake, we have no reason to suspect that the animals were in poor health, based on physical appearance and the fact that body weights eventually stabilized to indicate achievement of energy balance.
Hepatic alcohol and aldehyde dehydrogenases oxidize R,S-1,3-butanediol to R,S-β-hydroxybutyrate (RS-βHB). R(d)-βHB is the physiologic form of the ketone body and is oxidized to acetoacetate and eventually acetyl-CoA in extrahepatic tissues. In contrast, the metabolic fate of the nonphysiologic S(l)-βHB enantiomer is much different, initially forming S-β-hydroxybutyl-CoA, one of the final intermediates in the β-oxidation of fatty acids (48). In perfused rat liver, S-βHB produces R-βHB+AcAc but generates a lower amount of reducing equivalents than ketogenesis from long-chain fatty acids (48). In fact, the free NADH:free NAD+ ratio, as reflected by the R-βHB:AcAc ratio, is not increased by S-βHB metabolism, suggesting that oxidation of endogenous fatty acids is not inhibited (48). These findings support work by Webber and Edmond (49) which showed lower oxidation of S-βHB than the R isomer and superior substrate utilization for sterol and fatty acid synthesis in brain, spinal cord, and kidney. A more recent study by Stubbs et al. (50), in which they used using racemic mixtures of R- and S-βHB, showed that the S enantiomer is oxidized to a lesser extent than the R enantiomer and is not a major oxidative fuel in humans at rest (50). In contrast, we showed that the major fate of exogenous R-βHB was oxidation in peripheral tissues. Because ∼25% of BD-AcAc2 is metabolized to S-βHB, it is possible that the difference in body weight observed between the KE and PF groups was at least in part attributable to lower use as an energy substrate and to greater fatty acid oxidation as expected with provision of S-βHB alone.
Another plausible rationale for the significant weight loss caused by BD-AcAc2 could be related to the glucose-lowering effects of S-βHB and possible suppression of insulin, which could contribute to greater adipose tissue lipolysis and oxidation of fatty acids. Based on previous observations that R- and S-1,3-butanediol contribute only 6–8% of lipogenic carbon (51), it is possible that glucose is a lipogenic source and contributes to the glucose lowering effects of S-βHB. Our findings that blood glucose was similarly reduced in PF and KE mice compared to the Con mice provide further support for future studies designed to examine the specific effects of the S-βHB enantiomer on weight loss and blood glucose.
Although we observed lower βHB concentrations with BD-AcAc2 than previously reported for 3HB-BD (15, 16, 18), it is important to note that we were not able to measure circulating AcAc concentrations, which we predict would be higher than those observed using other form of KEs. Finally, it is important to note that REE and TEE were increased only in the KE group when adjusted for FM and LBM, suggesting that reduced uptake from the gut and energy loss from excretion also contributes to the phenotype observed. Studies are underway in our laboratory to explore this possibility.
In summary, dietary KE provided as part of a high-fat, low-carbohydrate diet after the establishment of diet-induced obesity resulted in reductions in energy intake, as previously reported. However, KE reduced body weight and adiposity to a greater extent than in energy-matched PF animals, which emphasizes findings of greater REE and TEE. Finally, the increase in EE does not appear to be related to skeletal muscle thermogenic activity, but evidence of interscapular BAT activation supports a role for adipose tissue as a potential mediator of the energetic effects of KE even after established diet-induced obesity.
ACKNOWLEDGMENTS
The authors thank Dr. Maria S. Johnson (University of Alabama at Birmingham, Department of Nutrition Sciences) for performing indirect calorimetry and condensing the results for analysis; Dr. Tim R. Nagy (University of Alabama at Birmingham, Department of Nutrition Sciences) for his expertise with interpretation of the indirect calorimetry results; and Michayla B. Brown (University of Alabama at Birmingham, Department of Human Studies) for development and assistance with measurement of BAT lipid content. This work was supported by Pilot and Feasibility Grant NORC, U.S. National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Grant P30DK056336 (to E.P.P.) from the UAB Nutrition Obesity Research Center; the NIH NIDDK Grant P30DK079626 (to the UAB Diabetes Research Center); the NIH National Institute on Aging Grants P30AG050886 and U24AG056053 (to the Nathan Shock Center Excellence in the Basic Biology of Aging); a T32 Predoctoral Award from the UAB Pre-Doctoral Training Program in Obesity-Related Research, NIH, National Heart, Lung and Blood institute Grant T32HL105349 (to R.A.H.D.); a T32 Postdoctoral Award from the UAB Pre-Doctoral Training Program in Obesity-Related Research, NIH NIDDK Grant T32DK062710 (to S.E.D.); and a Named New Investigator Award from the UAB NORC, NIH NIDDK Grant P30DK056336 (to E.P.P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or NIH. The authors declare no conflicts of interest.
Glossary
- 3HB-BD
R-3-hydroxybutyrate-R-1,3-butanediol
- ANCOVA
analysis of covariance
- AUCI
incremental area under the curve
- BAT
brown adipose tissue
- BD-AcAc2
R,S-1,3-butanediol diacetoacetate
- CR
calorie restriction
- Cs
citrate synthase
- DIO
deiodinase
- EE
energy expenditure
- FM
fat mass
- GTT
glucose tolerance test
- HFD
high-fat diet
- ITT
insulin tolerance test
- IWAT
inguinal white adipose tissue
- KD
ketogenic diet
- KE
ketone ester
- LBM
lean body mass
- PF
pair fed
- PGC
peroxisome proliferator-activated receptor γ coactivator
- Prdm16
PR domain containing 16
- REE
resting energy expenditure
- RGB
red-green-blue
- TEE
total energy expenditure
- TG
triglyceride
- UCP1
uncoupling protein 1
- WAT
white adipose tissue
- XT
horizontal locomotion
- YT
vertical locomotion
AUTHOR CONTRIBUTIONS
R. A. H. Davis designed the experiments, collected and analyzed the data, and prepared the manuscript; S. E. Deemer and G. Fisher collected and analyzed data and prepared the manuscript; J. M. Bergeron, J. T. Little, and J. L. Warren collected and analyzed the data; D. L. Smith, K. R. Fontaine, and D. B. Allison designed the experiments and prepared the manuscript; S. L. Dickinson analyzed the data and was responsible for statistical oversight; and E. P. Plaisance designed the experiments and oversaw their performance, collected and analyzed the data, and prepared the manuscript.
REFERENCES
- 1.Hawkins, R. A., Biebuyck, J. F. (1979) Ketone bodies are selectively used by individual brain regions. Science 205, 325–327 10.1126/science.451608 [DOI] [PubMed] [Google Scholar]
- 2.Heymsfield, S. B., Harp, J. B., Reitman, M. L., Beetsch, J. W., Schoeller, D. A., Erondu, N., Pietrobelli, A. (2007) Why do obese patients not lose more weight when treated with low-calorie diets? A mechanistic perspective. Am. J. Clin. Nutr. 85, 346–354 10.1093/ajcn/85.2.346 [DOI] [PubMed] [Google Scholar]
- 3.Redman, L. M., Heilbronn, L. K., Martin, C. K., de Jonge, L., Williamson, D. A., Delany, J. P., Ravussin, E.; Pennington CALERIE Team . (2009) Metabolic and behavioral compensations in response to caloric restriction: implications for the maintenance of weight loss. PLoS One 4, e4377 10.1371/journal.pone.0004377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreno, B., Bellido, D., Sajoux, I., Goday, A., Saavedra, D., Crujeiras, A. B., Casanueva, F. F. (2014) Comparison of a very low-calorie-ketogenic diet with a standard low-calorie diet in the treatment of obesity. Endocrine 47, 793–805 10.1007/s12020-014-0192-3 [DOI] [PubMed] [Google Scholar]
- 5.Kennedy, A. R., Pissios, P., Otu, H., Roberson, R., Xue, B., Asakura, K., Furukawa, N., Marino, F. E., Liu, F. F., Kahn, B. B., Libermann, T. A., Maratos-Flier, E. (2007) A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab. 292, E1724–E1739; errata: 293, E1846; 296, E1179 [DOI] [PubMed] [Google Scholar]
- 6.Yancy, W. S., Jr., Olsen, M. K., Guyton, J. R., Bakst, R. P., Westman, E. C. (2004) A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann. Intern. Med. 140, 769–777 10.7326/0003-4819-140-10-200405180-00006 [DOI] [PubMed] [Google Scholar]
- 7.Hall, K. D., Chen, K. Y., Guo, J., Lam, Y. Y., Leibel, R. L., Mayer, L. E., Reitman, M. L., Rosenbaum, M., Smith, S. R., Walsh, B. T., Ravussin, E. (2016) EE and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am. J. Clin. Nutr. 104, 324–333 10.3945/ajcn.116.133561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jornayvaz, F. R., Jurczak, M. J., Lee, H. Y., Birkenfeld, A. L., Frederick, D. W., Zhang, D., Zhang, X. M., Samuel, V. T., Shulman, G. I. (2010) A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing EE and preventing weight gain. Am. J. Physiol. Endocrinol. Metab. 299, E808–E815 10.1152/ajpendo.00361.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Srivastava, S., Baxa, U., Niu, G., Chen, X., Veech, R. L. (2013) A ketogenic diet increases brown adipose tissue mitochondrial proteins and UCP1 levels in mice. IUBMB Life 65, 58–66; erratum: 66, 519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desrochers, S., Dubreuil, P., Brunet, J., Jetté, M., David, F., Landau, B. R., Brunengraber, H. (1995) Metabolism of (R,S)-1,3-butanediol acetoacetate esters, potential parenteral and enteral nutrients in conscious pigs. Am. J. Physiol. 268, E660–E667 [DOI] [PubMed] [Google Scholar]
- 11.Desrochers, S., Quinze, K., Dugas, H., Dubreuil, P., Bomont, C., David, F., Agarwal, K. C., Kumar, A., Soloviev, M. V., Powers, L., Landau, B. R., Brunengraber, H. (1995) R,S-1,3-butanediol acetoacetate esters, potential alternates to lipid emulsions for total parenteral nutrition. J. Nutr. Biochem. 6, 111–118 10.1016/0955-2863(94)00011-A [DOI] [Google Scholar]
- 12.Poff, A. M., Ari, C., Arnold, P., Seyfried, T. N., D’Agostino, D. P. (2014) Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer. Int. J. Cancer 135, 1711–1720 10.1002/ijc.28809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kesl, S. L., Poff, A. M., Ward, N. P., Fiorelli, T. N., Ari, C., Van Putten, A. J., Sherwood, J. W., Arnold, P., D’Agostino, D. P. (2016) Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague-Dawley rats. Nutr. Metab. (Lond.) 13, 9 10.1186/s12986-016-0069-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ari, C., Kovács, Z., Juhasz, G., Murdun, C., Goldhagen, C. R., Koutnik, A. P., Poff, A. M., Kesl, S. L., D’Agostino, D. P. (2016) Exogenous ketone supplements reduce anxiety-related behavior in Sprague-Dawley and Wistar Albino Glaxo/Rijswijk rats. Front. Mol. Neurosci. 9, 137; erratum: February 13, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashiwaya, Y., Pawlosky, R., Markis, W., King, M. T., Bergman, C., Srivastava, S., Murray, A., Clarke, K., Veech, R. L. (2010) A ketone ester diet increases brain malonyl-CoA and uncoupling proteins 4 and 5 while decreasing food intake in the normal Wistar rat. J. Biol. Chem. 285, 25950–25956 10.1074/jbc.M110.138198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashiwaya, Y., Bergman, C., Lee, J. H., Wan, R., King, M. T., Mughal, M. R., Okun, E., Clarke, K., Mattson, M. P., Veech, R. L. (2013) A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 34, 1530–1539 10.1016/j.neurobiolaging.2012.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke, K., Tchabanenko, K., Pawlosky, R., Carter, E., Todd King, M., Musa-Veloso, K., Ho, M., Roberts, A., Robertson, J., Vanitallie, T. B., Veech, R. L. (2012) Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul. Toxicol. Pharmacol. 63, 401–408 10.1016/j.yrtph.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Srivastava, S., Kashiwaya, Y., King, M. T., Baxa, U., Tam, J., Niu, G., Chen, X., Clarke, K., Veech, R. L. (2012) Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 26, 2351–2362 10.1096/fj.11-200410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews, J. N., Altman, D. G., Campbell, M. J., Royston, P. (1990) Analysis of serial measurements in medical research. BMJ 300, 230–235 10.1136/bmj.300.6719.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parlee, S. D., Lentz, S. I., Mori, H., MacDougald, O. A. (2014) Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 537, 93–122 10.1016/B978-0-12-411619-1.00006-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuznetsov, A. V., Veksler, V., Gellerich, F. N., Saks, V., Margreiter, R., Kunz, W. S. (2008) Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 3, 965–976 10.1038/nprot.2008.61 [DOI] [PubMed] [Google Scholar]
- 22.Perry, C. G., Kane, D. A., Lin, C. T., Kozy, R., Cathey, B. L., Lark, D. S., Kane, C. L., Brophy, P. M., Gavin, T. P., Anderson, E. J., Neufer, P. D. (2011) Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem. J. 437, 215–222 10.1042/BJ20110366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry, R. J., Kim, T., Zhang, X. M., Lee, H. Y., Pesta, D., Popov, V. B., Zhang, D., Rahimi, Y., Jurczak, M. J., Cline, G. W., Spiegel, D. A., Shulman, G. I. (2013) Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 18, 740–748 10.1016/j.cmet.2013.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tschöp, M. H., Speakman, J. R., Arch, J. R., Auwerx, J., Brüning, J. C., Chan, L., Eckel, R. H., Farese, R. V., Jr., Galgani, J. E., Hambly, C., Herman, M. A., Horvath, T. L., Kahn, B. B., Kozma, S. C., Maratos-Flier, E., Müller, T. D., Münzberg, H., Pfluger, P. T., Plum, L., Reitman, M. L., Rahmouni, K., Shulman, G. I., Thomas, G., Kahn, C. R., Ravussin, E. (2011) A guide to analysis of mouse energy metabolism. Nat. Methods 9, 57–63 10.1038/nmeth.1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayter, A. J. (1986) The maximum familywise error rate of Fisher’s least significant difference test. J. Am. Stat. Assoc. 81, 1000–1004 10.1080/01621459.1986.10478364 [DOI] [Google Scholar]
- 26.D’Agostino, D. P., Pilla, R., Held, H. E., Landon, C. S., Puchowicz, M., Brunengraber, H., Ari, C., Arnold, P., Dean, J. B. (2013) Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R829–R836 10.1152/ajpregu.00506.2012 [DOI] [PubMed] [Google Scholar]
- 27.Langhans, W., Wiesenreiter, F., Scharrer, E. (1983) Different effects of subcutaneous D,L-3-hydroxybutyrate and acetoacetate injections on food intake in rats. Physiol. Behav. 31, 483–486 10.1016/0031-9384(83)90070-7 [DOI] [PubMed] [Google Scholar]
- 28.Langhans, W., Pantel, K., Scharrer, E. (1985) Ketone kinetics and D-(−)-3-hydroxybutyrate-induced inhibition of feeding in rats. Physiol. Behav. 34, 579–582 10.1016/0031-9384(85)90052-6 [DOI] [PubMed] [Google Scholar]
- 29.Langhans, W., Egli, G., Scharrer, E. (1985) Selective hepatic vagotomy eliminates the hypophagic effect of different metabolites. J. Auton. Nerv. Syst. 13, 255–262 10.1016/0165-1838(85)90014-1 [DOI] [PubMed] [Google Scholar]
- 30.Laeger, T., Metges, C. C., Kuhla, B. (2010) Role of beta-hydroxybutyric acid in the central regulation of energy balance. Appetite 54, 450–455 10.1016/j.appet.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 31.Sun, M., Martin, R. J., Edwards, G. L. (1997) ICV beta-hydroxybutyrate: effects on food intake, body composition, and body weight in rats. Physiol. Behav. 61, 433–436 10.1016/S0031-9384(9X)00455-8 [DOI] [PubMed] [Google Scholar]
- 32.Park, S., Kim, D. S., Daily, J. W. (2011) Central infusion of ketone bodies modulates body weight and hepatic insulin sensitivity by modifying hypothalamic leptin and insulin signaling pathways in type 2 diabetic rats. Brain Res. 1401, 95–103 10.1016/j.brainres.2011.05.040 [DOI] [PubMed] [Google Scholar]
- 33.Arase, K., Fisler, J. S., Shargill, N. S., York, D. A., Bray, G. A. (1988) Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am. J. Physiol. 255, R974–R981 [DOI] [PubMed] [Google Scholar]
- 34.Davis, J. D., Wirtshafter, D., Asin, K. E., Brief, D. (1981) Sustained intracerebroventricular infusion of brain fuels reduces body weight and food intake in rats. Science 212, 81–83 10.1126/science.7193909 [DOI] [PubMed] [Google Scholar]
- 35.Carneiro, L., Geller, S., Fioramonti, X., Hébert, A., Repond, C., Leloup, C., Pellerin, L. (2016) Evidence for hypothalamic ketone body sensing: impact on food intake and peripheral metabolic responses in mice. Am. J. Physiol. Endocrinol. Metab. 310, E103–E115 10.1152/ajpendo.00282.2015 [DOI] [PubMed] [Google Scholar]
- 36.Rossi, R., Dörig, S., Del Prete, E., Scharrer, E. (2000) Suppression of feed intake after parenteral administration of D-beta-hydroxybutyrate in pygmy goats. J. Vet. Med. A Physiol. Pathol. Clin. Med. 47, 9–16 10.1046/j.1439-0442.2000.00255.x [DOI] [PubMed] [Google Scholar]
- 37.Hu, Z., Cha, S. H., Chohnan, S., Lane, M. D. (2003) Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proc. Natl. Acad. Sci. USA 100, 12624–12629 10.1073/pnas.1834402100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stubbs, B. J., Cox, P. J., Evans, R. D., Cyranka, M., Clarke, K., de Wet, H. (2018) A ketone ester drink lowers human ghrelin and appetite. Obesity (Silver Spring) 26, 269–273 10.1002/oby.22051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Roux, C. W., Neary, N. M., Halsey, T. J., Small, C. J., Martinez-Isla, A. M., Ghatei, M. A., Theodorou, N. A., Bloom, S. R. (2005) Ghrelin does not stimulate food intake in patients with surgical procedures involving vagotomy. J. Clin. Endocrinol. Metab. 90, 4521–4524 10.1210/jc.2004-2537 [DOI] [PubMed] [Google Scholar]
- 40.Müller, T. D., Nogueiras, R., Andermann, M. L., Andrews, Z. B., Anker, S. D., Argente, J., Batterham, R. L., Benoit, S. C., Bowers, C. Y., Broglio, F., Casanueva, F. F., D’Alessio, D., Depoortere, I., Geliebter, A., Ghigo, E., Cole, P. A., Cowley, M., Cummings, D. E., Dagher, A., Diano, S., Dickson, S. L., Diéguez, C., Granata, R., Grill, H. J., Grove, K., Habegger, K. M., Heppner, K., Heiman, M. L., Holsen, L., Holst, B., Inui, A., Jansson, J. O., Kirchner, H., Korbonits, M., Laferrère, B., LeRoux, C. W., Lopez, M., Morin, S., Nakazato, M., Nass, R., Perez-Tilve, D., Pfluger, P. T., Schwartz, T. W., Seeley, R. J., Sleeman, M., Sun, Y., Sussel, L., Tong, J., Thorner, M. O., van der Lely, A. J., van der Ploeg, L. H., Zigman, J. M., Kojima, M., Kangawa, K., Smith, R. G., Horvath, T., Tschöp, M. H. (2015) Ghrelin. Mol. Metab. 4, 437–460 10.1016/j.molmet.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cahill, G. F., Jr. (2006) Fuel metabolism in starvation. Annu. Rev. Nutr. 26, 1–22 10.1146/annurev.nutr.26.061505.111258 [DOI] [PubMed] [Google Scholar]
- 42.Finley, L. W., Lee, J., Souza, A., Desquiret-Dumas, V., Bullock, K., Rowe, G. C., Procaccio, V., Clish, C. B., Arany, Z., Haigis, M. C. (2012) Skeletal muscle transcriptional coactivator PGC-1α mediates mitochondrial, but not metabolic, changes during calorie restriction. Proc. Natl. Acad. Sci. USA 109, 2931–2936 10.1073/pnas.1115813109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin-Montalvo, A., de Cabo, R. (2013) Mitochondrial metabolic reprogramming induced by calorie restriction. Antioxid. Redox Signal. 19, 310–320 10.1089/ars.2012.4866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plaisance, E. P., Henagan, T. M., Echlin, H., Boudreau, A., Hill, K. L., Lenard, N. R., Hasek, B. E., Orentreich, N., Gettys, T. W. (2010) Role of beta-adrenergic receptors in the hyperphagic and hypermetabolic responses to dietary methionine restriction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R740–R750 10.1152/ajpregu.00838.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keipert, S., Kutschke, M., Ost, M., Schwarzmayr, T., van Schothorst, E. M., Lamp, D., Brachthäuser, L., Hamp, I., Mazibuko, S. E., Hartwig, S., Lehr, S., Graf, E., Plettenburg, O., Neff, F., Tschöp, M. H., Jastroch, M. (2017) Long-term cold adaptation does not require FGF21 or UCP1. Cell Metab. 26, 437–446.e5 10.1016/j.cmet.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 46.McCurdy, C. E., Cartee, G. D. (2005) Akt2 is essential for the full effect of calorie restriction on insulin-stimulated glucose uptake in skeletal muscle. Diabetes 54, 1349–1356 10.2337/diabetes.54.5.1349 [DOI] [PubMed] [Google Scholar]
- 47.Sanchez-Gurmaches, J., Tang, Y., Jespersen, N. Z., Wallace, M., Martinez Calejman, C., Gujja, S., Li, H., Edwards, Y. J. K., Wolfrum, C., Metallo, C. M., Nielsen, S., Scheele, C., Guertin, D. A. (2018) Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metab. 27, 195–209.e6 10.1016/j.cmet.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lincoln, B. C., Des Rosiers, C., Brunengraber, H. (1987) Metabolism of S-3-hydroxybutyrate in the perfused rat liver. Arch. Biochem. Biophys. 259, 149–156 10.1016/0003-9861(87)90480-2 [DOI] [PubMed] [Google Scholar]
- 49.Webber, R. J., Edmond, J. (1977) Utilization of L(+)-3-hydroxybutyrate, D(-)-3-hydroxybutyrate, acetoacetate, and glucose for respiration and lipid synthesis in the 18-day-old rat. J. Biol. Chem. 252, 5222–5226 [PubMed] [Google Scholar]
- 50.Stubbs, B. J., Cox, P. J., Evans, R. D., Santer, P., Miller, J. J., Faull, O. K., Magor-Elliott, S., Hiyama, S., Stirling, M., Clarke, K. (2017) On the metabolism of exogenous ketones in humans. Front. Physiol. 8, 848 10.3389/fphys.2017.00848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desrochers, S., David, F., Garneau, M., Jetté, M., Brunengraber, H. (1992) Metabolism of R- and S-1,3-butanediol in perfused livers from meal-fed and starved rats. Biochem. J. 285, 647–653 10.1042/bj2850647 [DOI] [PMC free article] [PubMed] [Google Scholar]