Abstract
Intermittent administration of parathyroid hormone (PTH) stimulates bone formation in vivo and also suppresses the volume of bone marrow adipose tissue (BMAT). In contrast, a calorie-restricted (CR) diet causes bone loss and induces BMAT in both mice and humans. We used the CR model to test whether PTH would reduce BMAT in mice by both altering cell fate and inducing lipolysis of marrow adipocytes. Eight-week-old mice were placed on a control (Ctrl) diet or CR diet. At 12 wk, CR and Ctrl mice were injected daily with PTH (CR/PTH or Ctrl/PTH) or vehicle for 4 wk. Two other cohorts were CR and simultaneously injected (CR + PTH or CR + Veh) for 4 wk. CR mice had low bone mass and increased BMAT in the proximal tibias. PTH significantly increased bone mass in all cohorts despite calorie restrictions. Adipocyte density and size were markedly increased with restriction of calories. PTH reduced adipocyte numbers in CR + PTH mice, whereas adipocyte size was reduced in CR/PTH-treated mice. In contrast, osteoblast number was increased 3–8-fold with PTH treatment. In vitro, bone marrow stromal cells differentiated into adipocytes and, treated with PTH, exhibited increased production of glycerol and fatty acids. Moreover, in cocultures of bone marrow adipocyte and osteoblast progenitors, PTH stimulated the transfer of fatty acids to osteoblasts. In summary, PTH administration to CR mice increased bone mass by shifting lineage allocation toward osteogenesis and inducing lipolysis of mature marrow adipocytes. The effects of PTH on bone marrow adiposity could enhance its anabolic actions by providing both more cells and more fuel for osteoblasts during bone formation.—Maridas, D. E., Rendina-Ruedy, E., Helderman, R. C., DeMambro, V. E., Brooks, D., Guntur, A. R., Lanske, B., Bouxsein, M. L., Rosen, C. J. Progenitor recruitment and adipogenic lipolysis contribute to the anabolic actions of parathyroid hormone on the skeleton.
Keywords: PTH, bone, marrow, fat, adipocytes
Introduction
Parathyroid hormone (PTH) is a potent anabolic treatment for osteoporosis, although its mechanisms of action are complex and multifaceted, targeting several cell types. Numerous studies have shown that PTH can recruit progenitors from bone lining cells and mesenchymal stromal cells (1–3). Induction of IGF 1 and enhanced glycolysis mediate the accelerated rate of bone formation due to greater osteoblastic activity with intermittent PTH treatment (4). At least 1 study has also shown that oxidative phosphorylation is enhanced by PTH in vitro. Indirectly, PTH stimulates osteoclastogenesis and bone resorption by activation of the receptor activator of nuclear factor κB ligand (5). Moreover, PTH can modulate osteocyte activity by suppressing sclerostin and regulating salt-inducible kinases (6). Recently, we and others reported that PTH can shift marrow lineage in favor of osteoblasts at the expense of marrow adipocytes (7, 8). However, a recent in vitro report also suggested that PTH stimulates lipolysis via hormone-sensitive lipase, thereby reducing bone marrow adipose tissue (BMAT) and potentially liberating fatty acids for possible use by osteoblasts (9).
Calorie restriction in mice and young adults results in bone loss and enhanced BMAT. The most striking example is anorexia nervosa, a psychiatric disease characterized by self-induced starvation and distorted body image that affects 0.3% of young female subjects (10, 11). These individuals have poor bone quality and an extremely high risk of fracture, even during youth. Anorexia nervosa is associated with a loss of peripheral fat tissue but a paradoxical increase in BMAT (12–14). Therapies that address the skeletal fragility from anorexia nervosa have been limited to strategies to encourage weight gain or hormone replacement. However, patient compliance with these treatments is extremely low (15, 16). One promising but small trial reported an enhanced skeletal response to intermittent PTH in young anorexic subjects, despite very low body weight, poor carbohydrate intake, and high BMAT volume (17). In the current study, using calorie restriction as a model for induction of BMAT, we hypothesized that PTH would reduce marrow adiposity by both altering lineage allocation toward osteogenesis but also by stimulating lipolysis of marrow adipocytes. The latter action, we postulated, might lead to an enhanced skeletal response to anabolic treatment through utilization of free fatty acids by osteoblasts.
MATERIALS AND METHODS
Animals
Six-week-old C57BL/6J female mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Mice were placed on the AIN-93M diet (D10012M; Research Diets, Inc., New Brunswick, NJ, USA) upon arrival (Fig. 1A). The mice were singly housed in polycarbonate cages with sterilized paper bedding with 14:10-h light:dark cycles and ad libitum access to sterilized water. At 8 wk of age, mice were either left on the AIN93M diet [control (Ctrl)] or placed on a 30% calorie-restricted (CR) diet. CR mice were fed 70% of the averaged daily food weight consumed by the Ctrl cohort. The 30% CR diet was formulated as to provide all minerals and vitamins and reduce the amount of carbohydrates consumed when fed at 70% (Supplemental Table S1). Mice were kept on their respective diets and were weighed every 4 d throughout the duration of the study.
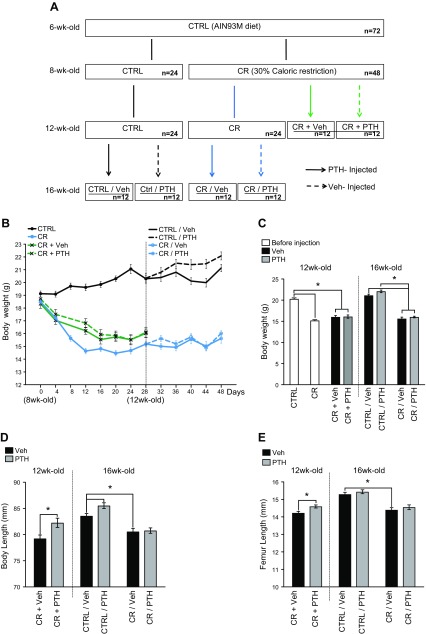
Figure 1 .
PTH partially rescues the blunting effect of calorie restriction on growth. Six-week-old female mice were placed on a Ctrl AIN93M diet for 2 wk. At 8 wk of age, mice were allocated to a Ctrl or a CR group. Ctrl mice were not injected until they reached 12 wk of age. CR mice were separated into 3 groups [not injected (CR), injected with Veh (CR + Veh), or injected with PTH (CR + PTH)] for 4 wk until 12 wk of age. After 4 wk, CR + Veh and CR + PTH mice were killed for harvest. Injections with either Veh (Ctrl/Veh, CR/Veh) or PTH (Ctrl/PTH, CR/PTH) were started on the previously uninjected Ctrl and CR mice while the diet manipulation continued for 4 more weeks. At 16 wk of age, those mice were killed for harvest (A). The weight of each mouse was measured every 4 d for the duration of the study (B). At harvest, mice were weighed (C), and body (D) and femora (E) lengths were measured to observe the impact of calorie restriction on growth. Ctrl, not injected cohort; CR, CR not injected cohort; CR + Veh, CR cohort injected with Veh at the same time calorie restriction started; CR + PTH, CR cohort injected with PTH at the same time calorie restriction started; Ctrl/Veh, Ctrl cohort injected with Veh 4 wk after diet manipulation started; Ctrl/PTH, Ctrl cohort injected with PTH 4 wk after diet manipulation started; CR/Veh, CR cohort injected with Veh 4 wk after diet manipulation started; CR/PTH, CR cohort injected with PTH 4 wk after diet manipulation started. *P < 0.05 (n = 12/group; 2-way ANOVA).
PTH (1–34 fragment; Bachem Americas, Torrance, CA, USA) was resuspended in 4 mM HCl, 0.1% bovine serum albumin to yield a 100 μM stock. The stock was divided into aliquots and stored at −80°C. Aliquots were thawed and diluted in 0.9% saline daily for the injections. The vehicle (Veh) was 4 mM HCl, 0.1% bovine serum albumin diluted at the same concentration as the PTH aliquots in 0.9% saline. Subcutaneous injections of PTH (80 μg/kg of body weight) or Veh were performed daily between 1:00 and 4:00 pm (Eastern Standard Time). Four cohorts were injected 4 wk after the diet manipulation: Ctrl/Veh, Ctrl/PTH, CR/Veh, and CR/PTH. Two cohorts were injected at the same time as the CR diet started: CR + Veh and CR + PTH. These differences in study design allowed us to determine whether PTH could reverse and/or protect against CR-induced bone loss and marrow adiposity.
Mice were intraperitoneally injected with calcein and alizarin red 5 and 2 d, respectively, before killing. The study was terminated after 4 wk of daily PTH injections to collect serum, bones, and adipose tissues for analyses. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the Maine Medical Center Research Institute.
Dual-energy X-ray absorptiometry
Dual-energy X-ray absorptiometry (DXA) of the mice was performed at 8, 12, and 16 wk of age by using a PixImus Densitometer (GE Lunar Corporation, Fairfield, CT, USA), as previously described (18). The PixImus was calibrated before each measurement by using a phantom standard provided by the manufacturer.
Microcomputed tomography
Femora and vertebrae were isolated from formalin-fixed tissues and stored in 70% ethanol before being processed for microcomputed tomography (µCT) imaging. A high-resolution desktop microtomographic imaging system (µCT40; Scanco Medical AG, Brüttisellen, Switzerland) was used to assess trabecular bone microarchitecture in the distal femoral metaphysis and L5 vertebral body and cortical bone morphology at the femoral mid-diaphysis. Scans were acquired by using a 10 µm3 isotropic voxel size, 70 kVp peak X-ray tube intensity, 114 mA X-ray tube current, and 200 ms integration time, and were subjected to Gaussian filtration and segmentation. Image acquisition and analysis protocols adhered to guidelines for the use of µCT imaging for the assessment of bone microstructure in rodents (19). In the femur, trabecular bone was evaluated in a 1500-µm-long region (150 transverse slices) beginning 200 µm above the peak of the distal growth plate and extending proximally. In the L5 vertebral body, trabecular bone was evaluated in a region beginning 100 µm inferior to the cranial growth plate and extending to 100 µm superior to the caudal growth plate. Trabecular bone was segmented from soft tissue by using thresholds of 365 and 450 mgHA/cm3 in femur and L5 vertebrae, respectively. The Scanco Evaluation Program Trabecular Morphology script was used to measure trabecular bone volume fraction (BV/TV; %), trabecular thickness (mm), trabecular number (per mm), and trabecular separation (mm). Cortical bone architecture was evaluated in a 500-µm-long region (50 transverse slices) at the femoral mid-diaphysis. Cortical bone was segmented by using a threshold of 700 mgHA/cm3 and then evaluated with the Scanco Midshaft Evaluation script to measure cortical bone area fraction (%), thickness (mm), and porosity (%).
Metabolic phenotyping
Metabolic parameters were measured by using the Promethion Metabolic Cage System (Sable Systems Intl., North Las Vegas, NV, USA) located in the Physiology Core Department of the Maine Medical Center Research Institute. Data acquisition and instrument control were analyzed as previously described (20). Indirect calorimetry measurements were performed by using the Promethion Metabolic Cage System (Sable Systems Intl.) located in the Physiology Core Department of the Maine Medical Center Research Institute. Data acquisition and instrument control were performed by using Metascreen software v.2.3.12 Sable Systems Intl., and the obtained raw data were processed by using ExpeData v.1.9.14 (Sable Systems Intl.) using an analysis script detailing all aspects of data transformation.
Mice were subjected to a standard 12-h light:dark cycle during the study, which consisted of a 12-h acclimation period followed by a 72-h sampling duration. Data shown are representative of the 24-h average of this period. Each cage in the 16-cage system consists of a cage with standard bedding, a food hopper, water bottle, and house-like enrichment tube for body mass measurements, connected to load cells for continuous monitoring, as well as an 11.5 cm running wheel connected to a magnetic reed switch to record revolutions. Ambulatory activity and position were monitored by using the XYZ beam arrays with a beam spacing of 0.25 cm. From these data, animal pedestrian locomotion and speed within the cage were calculated. Respiratory gases were measured by using the GA-3 gas analyzer (Sable Systems International) using a pull-mode, negative-pressure system. Air flow is measured and controlled by the FR-8 (Sable Systems International), with a set flow rate of 2000 ml/min. Oxygen consumption and carbon dioxide production were reported in milliliters per minute. Water vapor is continuously measured, and its dilution effect on oxygen and carbon dioxide are mathematically compensated for in the analysis stream (21). Energy expenditure (EE) was calculated by using the Weir equation:
and the respiratory quotient was calculated as the ratio of Vco2/ Vo2 (carbon dioxide elimination/oxygen consumption). Ambulatory activity and wheel running were determined simultaneously with the collection of the calorimetry data. Thus, resting EE was able to be determined and is calculated as the average EE of 30 min intervals of no activity. Sleep hours were determined as any inactivity lasting ≥40 s. Correlations were performed between EE and lean, fat, and total body mass to determine if these variables could explain changes in EE. If no significant correlations were found between EE and any of the variables, EE is reported in kilocalories per hour. However, if a significant correlation was found, EE is reported as adjusted EE to correct for the effects of these variables on EE (22–25). ANCOVA was performed between EE and lean mass to determine if this variable could explain changes in EE. No significant correlations were found in the 12 wk of age cohort; thus, these data are reported in kilocalories per hour. However, in the 16-wk-old cohort, lean mass was found to be a significant covariate, and thus EE was adjusted to lean mass and reported as such.
Histology
The hind limbs of animals were fixed for overnight in 10% neutral buffered formalin. Tissues were then stored in 70% ethanol before being processed. For each mouse, 1 tibia was used for hematoxylin and eosin staining, and the other tibia for mineralization surface assessment.
For hematoxylin and eosin staining, tibias were first decalcified by using Richard-Allan Scientific Decalcifying Solution (Thermo Fisher Scientific, Waltham, MA, USA) for 12 h. Decalcified tibias were dehydrated through a gradient of ethanol before being placed in xylene and embedded in paraffin. Paraffin blocks were cut by using a microtome to obtain 5-μm sections. Slides were then stained with hematoxylin and eosin to quantify adipocyte ghosts residing in the bone marrow and in adipose tissues. Adipocyte ghosts are present histologically after decalcification; in the current article, however, the size and number of these ghosts are referred to as being adipocytes. Adipocyte number was reported as a density per square millimeter of marrow area.
For mineralization surface, tibias were processed for plastic embedding by being submerged through a gradient of ethanol for dehydration. They were then incubated on a shaker at 4°C in 3 monomer mixes for 48 h each as follows: monomer mix I (60% methyl methacrylate, 35% butyl methacrylate, 5% methyl benzoate, and 1.2 ml polyethylene glycol), monomer mix II (monomer mix I with 0.5% w/v of benzoyl peroxide), and monomer mix III (monomer mix I with 1% w/v benzoyl peroxide). Samples were then placed in scintillation vials poured with monomer mix III supplemented with 0.4% N, N-dimethyl-p-toluidine. The vials were stored at 4°C, and the plastic was allowed to solidify for 72 h. Methyl methacrylate, butyl methacrylate, benzoyl peroxide, and N, N-dimethyl-p-toluidine were purchased from MilliporeSigma (Burlington, MA, USA); methyl benzoate and polyethylene glycol were obtained from Thermo Fisher Scientific. Plastic blocks were sectioned by using a diamond blade to obtain 5 μm sections that remained unstained to visualize calcein labeling or tetrachrome stained to quantify osteoblasts.
Parameters were quantified in a region of interest 50 μm distal from the proximal growth plate and 50 μm from the endocortical surface with a height averaging 1.5 mm. Marrow area was calculated as total area minus the bone surface. Bioquant software (v.18.2.60; Bioquant Image Analysis, Nashville, TN, USA) was used to examine adipocyte numbers and size, count osteoblast numbers, and assess mineralization surface.
Quantitative PCR
Total RNA was isolated from flash frozen femurs using Trizol (Thermo Fisher Scientific) method for tissues. Briefly, whole frozen femurs were crushed and placed in TRIzol and chloroform. Samples were centrifuged for 15 min at 10,000 g at 4°C to separate the phenol phase and the phase containing the RNA. RNA was then precipitated from the samples by using isopropanol. RNA concentrations were estimated by using Nanodrop 2000 (Thermo Fisher Scientific), and 500 ng RNA was used as a template for the reverse transcription reaction into cDNA using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific). The cDNA was diluted 1:20 in Nanopure (Thermo Fisher Scientific) water before being used for quantitative PCR. The iQ SYBR Green Supermix and iQ5 Thermal Cycler Detection System (Bio-Rad, Hercules, CA, USA) were used to quantify RNA expression. The geNorm Kit (Primer Design, Chandler’s Ford, United Kingdom) was used to find the most stable housekeeping gene for bone, which was determined as Hprt. Primers for the housekeeping gene and the genes of interest were purchased from Primerdesign (Southampton, United Kingdom) and designed to be 95–100% efficient.
Cell culture
Bone marrow stromal cells (BMSCs) were isolated and cultured as previously described (26). Briefly, 8-wk-old C57BL/6J female mice were killed to collect femora, tibias, and iliac bones. Bones were sectioned at their epiphyses to allow the bone marrow contents to be flushed out by quick centrifugation. Pelleted cells were suspended in cell culture medium composed of MEMα (Thermo Fisher Scientific), 10% fetal bovine serum (VWR International, Radnor, PA, USA), and 1% penicillin/streptomycin (Thermo Fisher Scientific). After 48 h in culture, the adherent stromal cell (BMSC) population was trypsinized and counted. Cells were plated in culture wells before adipogenesis was induced.
Adipogenesis
BMSCs were differentiated into adipocytes for 7 d by using the adipogenic media previously described (26). Briefly, the medium is composed of DMEM high glucose medium containing 10% fetal bovine serum, 1% penicillin/streptomycin, 2 μM insulin, 0.5 mM IBMX, 1 nM dexamethasone, and 20 nM rosiglitazone. After 96 h, BMSCs were switched to DMEM high glucose media supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, 2 μM insulin, and 20 nM rosiglitazone. After 7 d, cultures were washed once with PBS and placed in serum-free media containing different doses of PTH (1–34 aa; Bachem Americas). The media were collected after 6 h. To determine glycerol release, the glycerol cell–based assay kit was purchased and performed according to the manufacturer’s protocol with the conditioned medium samples (Cayman Chemicals, Ann Arbor, MI, USA).
Free fatty acid release
BMSCs were plated in 12-well plates at a density of 2.5 × 105 cells/well and cultured in adipogenic medium for 7 d. On d 7 of adipogenic differentiation, medium was switched to HBSS, and the cells were treated with Veh (Ctrl) or 100 nM PTH for 2 h. Supernatant was then collected, and free fatty acids were determined by using a commercially available kit (700310; Cayman Chemicals) according to the manufacturer’s protocol.
Mitochondrial stress test
BMSCs were plated in Seahorse XF96 plates (Agilent Technologies, Santa Clara, CA, USA) at a density of 1.5 × 104 cells/well and cultured in adipogenic medium for 7 d. On d 7 of adipogenic differentiation, cells were treated without (Veh-Con) or with 100 nM PTH for 45 (C) or 90 (D) min subjected to Seahorse XF96 Flux analysis. Briefly, the mitochondrial stress tests were performed in Seahorse XF Base Medium (without Phenol Red) supplemented with 10 mM glucose, 2 mM glutamine, and 1 mM pyruvate. Mitochondrial respiration was measured and presented as the oxygen consumption rate (OCR) under basal conditions and after acute, sequential injections of the following: 1) 2 µM oligomycin A; 2) 2 µM carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP); and 3) 1 µM/1 µM rotenone/antimycin. Data were then normalized to protein (µg) per well.
Donor/acceptor experiment
BMSCs were plated on fibronectin-coated coverslips in adipogenic medium and cultured for 7 d. On d 7 of adipogenic differentiation, a fluorescently labeled fatty acid analogue (BODIPY558/568 or Red C12; 2 µM) was pulsed in to the culture along with oleic acid (0.6 mM) for 16 h. These bone marrow adipocytes would serve as the donor cells. In parallel, BMSCs in a separate flask were labeled with 5 µM CellTracker Green (CMFDA) for 45 min. These green-labeled acceptor cells were washed, trypsinized, and coplated with the donor cells in complete DMEM for 24 h. In this coculture system, cells were then treated with Veh (Ctrl) or 100 nM PTH for 2 h in HBSS. Cells were then fixed with PFA, stained for DAPI, and mounted on coverslips. Confocal microscopy was then used to survey each coverslip (n = 3) to image green cells and determine whether they also contained red.
Statistical analysis
The data are expressed as means ± sem. Results were analyzed for statistically significant differences by using Prism 6 statistical software (GraphPad Software, Inc., La Jolla, CA, USA). A 2-way ANOVA was performed followed by Bonferroni’s multiple comparison post hoc tests with statistical significance set at P < 0.05. For mice cohorts, we powered (β = 0.95) the study for n = 10 mice per experimental group for DXA and tissue collection to complete all assays with an sd of 1% in the DXA measurement tool for an α (P < 0.01). Numbers of mice (n) used in specific experiments are indicated in each figure. ANCOVA analysis was performed by using JMP statistical software v.13.2 (SAS Institute, Cary, NC, USA).
RESULTS
PTH partially protects against the effects of calorie restriction on postnatal growth
The study aimed to determine whether PTH could reverse or prevent calorie restriction–induced marrow fat expansion in mice and identify the mechanism that underlies that process. Previously, we showed that 30% calorie restriction in mice leads to a significant increase in BMAT (27). Therefore, to test our hypothesis, we designed a study (Fig. 1A) in which mice were Ctrl-fed or CR for 4 wk and then injected with either Veh (Ctrl/Veh, CR/Veh) or PTH (Ctrl/PTH, CR/PTH) for 4 more weeks. To test whether PTH could prevent marrow fat accumulation in CR mice, we injected mice at the same time that calorie deprivation started (CR + Veh, CR + PTH).
After 4 wk of calorie restriction, mice lost an average of 18% of their initial body weight, whereas Ctrl-fed mice gained 6% of their initial body weight (Fig. 1B). Similarly, CR mice that were simultaneously injected with Veh (CR + Veh) or PTH (CR + PTH) lost 13 and 14% of their initial body weight, respectively. At the end of the study, the CR groups all had significant decreases in body weight compared with the Ctrl-fed groups. However, there were no differences in body weight between Veh- and PTH-treated mice (Fig. 1C). Body and femur lengths were measured at the end of the study to determine potential effects on longitudinal growth. CR mice were markedly shorter than Ctrl-fed mice independent of treatment (Fig. 1D). Interestingly, simultaneous CR and PTH (CR + PTH) was able to protect against this stunting in mice; however, it was unable to reverse CR-induced attenuation of longitudinal bone growth in the other cohort (CR/PTH) (Fig. 1D, E).
As expected, at 12 wk of age, fat and lean mass were significantly lower in all CR groups compared with Ctrl, regardless of Veh or PTH treatments (Supplemental Fig. S1A–D). However, at 16 wk of age, fat mass was significantly higher in CR/PTH mice compared with CR/Veh mice (Supplemental Fig. S1B). Food consumption did not differ between the Veh or PTH treatment groups (Supplemental Fig. S1E).
PTH can rescue bone loss caused by calorie restriction
DXA analysis revealed that CR mice had a significant decrease in both areal bone mineral density (aBMD) and areal bone mineral content at 12 wk of age compared with Ctrl mice (Fig. 2A, B). However, CR + PTH mice had significantly higher aBMD and areal bone mineral content compared with CR + Veh mice. The same increase was observed in 16-wk-old mice that were CR/PTH-Ctrl/PTH-fed for 4 wk before injections started. These data suggest that PTH is able to exert an enhanced bone anabolic effect in CR mice.
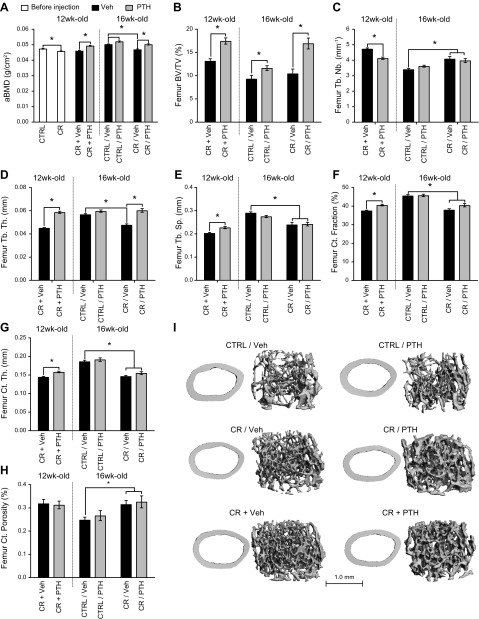
Figure 2 .
PTH prevents and rescues bone loss caused by calorie restriction. A) DXA was performed on 12- and 16-wk-old mice to measure aBMD. Microarchitecture of the femora was investigated by using μCT imaging at 12 and 16 wk of age. B–E) Trabecular parameters [trabecular volume (B), number (C), thickness (D), and separation (E)] were measured in the distal femora metaphysis. F–H) Cortical parameters (cortical fraction (F), thickness (G), and porosity (H) were measured at the midshaft of the femora. I) Representative images of the cortices and trabecular compartment reconstructed based on the scans used for the analysis. Tb. Nb., trabecular number; Tb. Th., trabecular thickness; Tb. Sp., trabecular separation; Ct., cortical; Ct. Th., cortical thickness. *P < 0.05 (n = 10/group for DXA; n = 8/group for µCT imaging; 2-way ANOVA).
To examine whether the microarchitecture of the bone was affected by PTH and calorie restriction, μCT imaging was performed on femurs and vertebrae at 12 and 16 wk of age (Fig. 2B–I). Calorie restriction did not influence femoral trabecular BV/TV (Fig. 2B) or vertebral trabecular BV/TV (Supplemental Fig. S2A); however, PTH was able to increase trabecular BV/TV in all groups (CR + PTH, CR/PTH, Ctrl/PTH) both in the distal femur metaphysis (Fig. 2C) and vertebral body regardless of when injections started or dietary intervention. With calorie restriction, trabecular number was moderately higher in femora but not in vertebrae (Supplemental Fig. S2B). PTH treatment decreased trabecular number in the femora of CR + PTH mice but increased trabecular number in vertebrae of all treated mice. Femoral trabecular thickness and separation were significantly lower in CR vs. Ctrl, and PTH treatment significantly increased those parameters (Fig. 2D, E). Similar trends were observed at the vertebral level (Supplemental Fig. S2C, D). Cortical fraction and thickness were lower in CR mice compared with Ctrl mice (Fig. 2F, G), whereas cortical porosity was significantly increased (Fig. 2H). Notably, both cortical fraction and thickness were significantly higher in CR + PTH mice compared with CR + Veh mice. Although PTH also tended to increase cortical fraction and thickness in CR/PTH compared with CR/Veh, the difference did not reach statistical significance (Fig. 2F, G).
PTH effects on global metabolism
To investigate the effects of PTH and CR on metabolism, mice were placed in metabolic cages to measure their EE and their respiratory quotient.
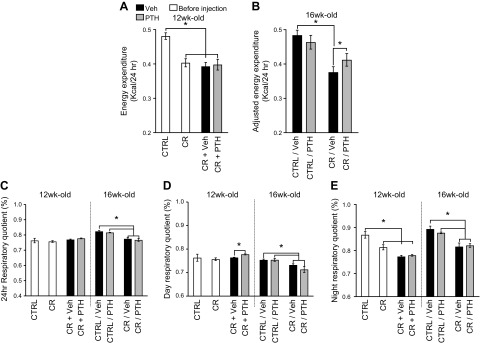
The initial 4 wk of calorie restriction caused a significant decrease in EE, which was not rescued by concomitant PTH injections at 12 wk of age (Fig. 3A). However, at 16 wk of age, PTH led to a modest but significant increase in adjusted EE in CR/PTH mice compared with CR/Veh mice (Fig. 3B). At 16 wk of age, EE was found to be correlated with body composition and was adjusted as such.
Figure 3 .
Metabolic changes in CR and PTH-treated mice. Mice were placed in metabolic cages and allowed to acclimate for 24 h. A, B) Energy expenditure was measured over a 24-h period, and if correlation was found with body composition, EE was adjusted for it. C–E) Respiratory quotient was measured over 24 h (C), during the day (D), or the night cycles (E). *P < 0.05 (n = 8 for the Ctrl, CR, CR + Veh, and CR + PTH groups; n = 4 for the Ctrl/Veh, Ctrl/PTH, CR/Veh and CR/PTH groups; 2-way ANOVA).
No difference was recorded between the 24 h respiratory quotient of Ctrl mice and that of the CR groups at 12 wk of age (Fig. 3C). However, there was a significant decrease in respiratory quotient with CR in the 16-wk-old cohorts. PTH seemed to have no significant effects on the 24 h respiratory quotient of the Ctrl or CR groups; however, when separating the data according to day and night, CR + PTH exhibited a mild but significant increase compared with CR + Veh during the day (Fig. 3D) but not during the night (Fig. 3E). PTH had no effect on the Ctrl/PTH or CR/PTH cohorts despite the stratification by day or night.
Effects of PTH on bone marrow adipose tissue and osteoblast number
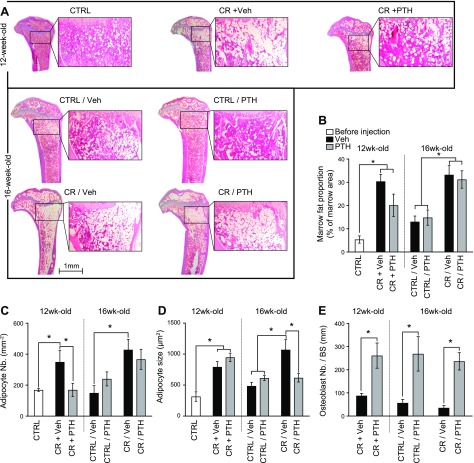
The effect of PTH on bone marrow adipocytes was assessed on histologic sections of tibia. After the initial 4 wk of calorie restriction, a significant increase was observed in marrow fat proportion in the proximal tibias of all the CR cohorts compared with the Ctrl cohorts (Fig. 4A, B). In CR mice concomitantly treated with PTH, adipocyte number was considerably lower (CR + Veh vs. CR + PTH) (Fig. 4C), but adipocyte size was similar to Veh-treated mice (Fig. 4D). However, mice that were injected with PTH 4 wk after the calorie restriction started (CR/Veh or CR/PTH) exhibited a different effect; that is, a smaller adipocyte size with only slightly lower adipocyte number compared with Veh-treated mice (Fig. 4C). Hence, the major effect of PTH, once calorie restriction was established (i.e., a reduction in marrow adipose volume), was principally due to a marked decrease in adipocyte size (Fig. 4D) compared with CR/Veh. Thus, depending on the timing of PTH administration, the response of BMAT differed. Moreover, in Veh-treated CR mice, aBMD was inversely associated with marrow adipose volume (r = −0.62, P = 0.03), a finding noted in other human and mouse studies (28–30) (Supplemental Fig. S3A). However, this association was not present in PTH-treated mice (Supplemental Fig. S3B).
Figure 4 .
PTH reduces BMAT of CR mice. A) Tibias were sectioned and stained with hematoxylin and eosin to observe BMAT. B–E) Marrow fat proportion (B), adipocyte number (C), and size (D) were assessed by using Bioquant software in a region of interest extending down for ∼1.5 mm. The region of interest was located 50 μm down from the growth plate and 50 μm from the cortices. Marrow fat proportion was calculated as the proportion of marrow area occupied by adipocytes. Adipocyte number (Nb.) was calculated as number of adipocyte per square millimeter of marrow area, and adipocyte size represents the average area occupied by each adipocyte. Osteoblast number (E) was quantified on tetrachrome-stained plastic sections of proximal tibias in a region of interest similar to where the adipocytes were counted. Osteoblast number is presented as number of osteoblast per square millimeter of bone surface (BS). *P < 0.05 (n = 3–10/group; 2-way ANOVA).
In addition, we analyzed the histologic response to PTH according to group by using dynamic histomorphometry. Osteoblast numbers were significantly increased in all groups treated with PTH independent from diet manipulation (Fig. 4E). Mice treated with PTH concomitantly with calorie restriction showed a 3-fold increase in mineralizing surface per bone surface (MS/BS) (Table 1) as adipocyte number declined (Fig. 4C). Conversely, mice that were CR and then treated with PTH exhibited no change in MS/BS but a decrease in adipocyte volume (Fig. 4D). PTH increased MS/BS by 50% in Ctrl/PTH mice compared with Ctrl/VEH mice. Interestingly, we were unable to detect a second label in mice treated with PTH at the same time as calorie restriction was initiated and therefore could not estimate bone formation rates.
TABLE 1.
Mineralizing surface was quantified in proximal tibias as percentage of calcein-labeled surface over bone surface
| Group | MS/BS (%) |
|---|---|
| Ctrl/Veh | 36.5 ± 7 |
| Ctrl/PTH | 54.7 ± 8 |
| CR/Veh | 33.5 ± 1 |
| CR/PTH | 31.3 ± 1 |
| CR + Veh | 9.5 ± 5 |
| CR + PTH | 27 ± 5 |
Data are presented as averages ± se. Each group, n = 3–4.
PTH regulates gene expression and bone histology depending on timing of treatment
We hypothesized that PTH activated the lipolytic pathway of mature adipocytes in mice that were CR for 4 wk before injections started (CR/PTH) but acted on progenitor cells in mice that were simultaneously injected and calorie deprived (CR + PTH). To address this hypothesis, gene expression analysis was performed at the end of the study on whole femora to investigate specific genes involved in the lipolytic and the mesenchymal differentiation pathways.
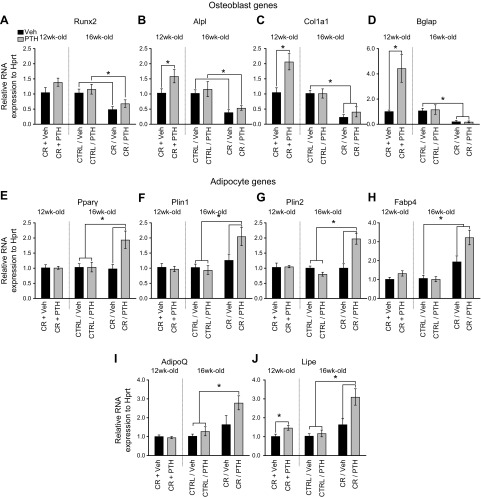
Osteoblastic genes such as Alpl, Bglap, Col1a1, and Runx2 were all significantly down-regulated with CR (Fig. 5A–D). PTH did not influence the expression of those genes in mice that were CR and then injected (CR/PTH) or in Ctrl-fed (Ctrl/PTH) mice. However, mice that were simultaneously injected and CR (CR + PTH) had considerable up-regulations of those genes compared with CR + Veh mice.
Figure 5 .
PTH affects gene expression in femurs of CR mice. RNA was isolated from femurs and converted to cDNA to perform quantitative PCR. Expression levels of genes involved in osteoblast (A–D) and in adipocyte (E–J) differentiation and function were assessed with normalization to expression of Hprt. *P < 0.05 (n = 6/group; 2-way ANOVA).
Adipogenic genes such as Pparγ, Plin1 and 2, Fapb4, AdipoQ, and Lipe were all up-regulated in CR/PTH mice compared with CR/Veh mice (Fig. 5E–J). In contrast with osteoblastic genes, adipogenic gene expression was similar in the CR + PTH cohort compared with the CR + Veh cohort (Fig. 5E–I), albeit for Lipe, which was up-regulated (Fig. 5J). PTH had no effect on Ctrl/Veh vs. Ctrl/PTH mice for either osteoblastic (Fig. 5A–D) or adipogenic (Fig. 5E–J) gene expression.
PTH induces lipolysis in adipocytes derived from BMSCs
To address whether PTH could directly induce lipolysis on adipocytes in the bone marrow, we differentiated BMSCs into adipocytes before treating the cultures with different doses of PTH. BMSCs were isolated from 8-wk-old female mice and culture for 2 d before being differentiated into adipocytes for 7 d. Differentiated cultures were serum-starved for 2 h before being treated with different doses of PTH for 12 h.
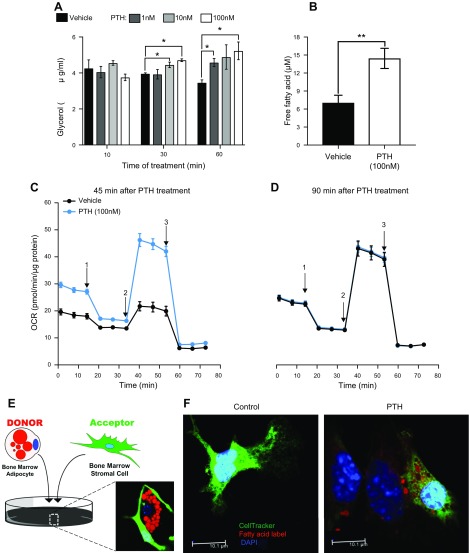
Glycerol and fatty acids released in the conditioned media were measured as an output of lipolysis. PTH treatment induced a dose-dependent release of glycerol in the medium (Fig. 6A) after 30 and 60 min of treatment. Similarly, free fatty acid concentration in media was significantly increased with PTH treatment (Fig. 6B).
Figure 6 .
PTH induces lipolysis in adipocytes to regulate their internal metabolism and the transfer of fatty acids to neighboring cells in vitro. BMSCs were differentiated into adipocytes for 7 d before being treated with different doses of PTH. A, B) Media were collected after 12 h to measure glycerol output (A) and free fatty acids (B) representative of lipolytic activity. C, D) Mitochondrial stress tests were performed on adipocytes using Seahorse XF96 to quantify OCR 45 min (C) and 90 min (D) after Veh or PTH treatment under basal conditions and after acute, sequential injections of the following: 1) 2 µM oligomycin A; 2) 2 µM FCCP; and 3) 1 µM/1 µM rotenone/antimycin. Data were normalized to quantity of protein (µg) per well. E) Donor/acceptor of free fatty acid experiment was performed on adipocytes that received a pulse of fluorescently labeled FA (Red C12) and oleic acid for 16 h and then cocultured with CellTracker green-labeled BMSCs for 24 h before being treated with 100 nM PTH or Veh. F) Confocal microscopy was then used to capture representative images. *P < 0.05 (2-way ANOVA), **P < 0.05 (Student’s t test) (n = 3 wells/treatment).
To test if mitochondrial metabolism was affected by PTH, we subjected BMSC-derived adipocytes to mitochondrial stress testing. Basal OCR was markedly increased with PTH treatment compared with Veh Ctrl at 45 min (Fig. 6C). OCR in PTH-treated adipocytes remained elevated in the presence of oligomycin, FCCP, or rotenone/antimycin. Conversely, by 90 min, there were no differences in OCR between PTH and Veh (Fig. 6D), potentially implicating export of fatty acids rather than utilization.
Next, we hypothesized that fatty acids released in the media upon treatment with PTH could be utilized by neighboring cells. To examine this theory, BMSC-derived adipocytes were cultured with red-labeled fatty acids. After they incorporated the labeled lipids, we cocultured them with green-labeled stromal cells (as shown on Fig. 6E). After treatment with PTH, incorporation of the red label into the green neighboring cells was observed (Fig. 6F), indicating that fatty acids could be mobilized and transferred from adipocytes to stromal cells upon treatment with PTH.
DISCUSSION
The current study found that intermittent PTH simultaneously increases bone formation and bone mass and suppresses BMAT even in the presence of inadequate caloric intake. Moreover, we showed in vitro and in vivo that PTH can work directly on bone marrow adipocytes and their progenitors in at least 2 ways: 1) through greater osteoprogenitor recruitment; and 2) activation of a lipolysis program. In addition, we postulate that active lipolysis might serve to provide greater fuel availability for the working osteoblast, particularly during states of increased bone formation.
To test how PTH regulates bone marrow adipocytes, we chose a calorie restriction mouse model using a specific diet that leads to reduced carbohydrate intake but no change in fat or protein. Thirty percent calorie restriction leads to blunting of growth between 8 and 16 wk of age, and an increase in BMAT (27). Interestingly, we found that PTH injections increased body and femur lengths but only when treatment started at the same time as the calorie restriction (CR + PTH mice) and not 4 wk after the diet manipulation (CR/PTH). PTH is known to be involved in postnatal growth plate elongation (31); our data suggest, however, that timing of treatment is important, almost certainly due to greater progenitor recruitment. Indeed, mice are still growing between 8 and 16 wk of age, and thus it is likely that early PTH injections (from 8 to 12 wk) can partially rescue the effects of calorie restriction on longitudinal growth. However, injecting PTH in older animals (from 12 to 16 wk) can increase bone mass but is too late for PTH to have a significant effect on growth.
Some data indicate that PTH can induce greater bone mass increments in older vs. younger animals despite an age-related reduction in osteoprogenitor recruitment (32). Similarly, in a small pilot study of humans, Fazeli et al. (17) showed that the 6-mo increase in lumbar spine BMD with PTH was greater in anorexic women than that observed in postmenopausal women. In both of these conditions, marrow adiposity is high, suggesting there may be an additive effect of adipocytic lipolysis on bone formation with PTH. In our study, PTH increased bone mass in Ctrl mice, but femoral and lumbar BV/TV remained higher in the calorie-deprived cohorts regardless of when PTH was started. We also noted that calorie restriction reduced only cortical bone mass and not the trabecular bone volume fraction. A previous study showed that a 50% calorie restriction caused a decreased in trabecular and cortical bone (33). Another study showed that a 40% dietary restriction did not cause alterations of BV/TV in 12-wk-old mice and was in fact protective against trabecular bone loss in older mice (34). Therefore, it is possible that our 30% calorie restriction model was not sufficient to affect trabecular bone.
BMAT is believed to be negatively associated with bone mass because of a switch in mesenchymal lineage differentiation and by production of adipokines inhibiting bone formation and driving bone resorption (21, 28–30, 35, 36). However, this association is not always consistent. For example, in a mouse model depleted in bone marrow adipocytes, bone loss was still observed with ovariectomy (37). Moreover, in C3H/HeJ mice, high BMAT is associated with high trabecular and cortical bone mass (30). In addition, in certain conditions, it has been suggested that adipocytes could promote bone formation. Overexpression of FoxC2, a transcription factor that sensitizes cells to the β-adrenergic cAMP-PKA pathway, can induce beige-like characteristics in adipocytes, which in turn secrete factors promoting bone formation (38). Similarly, it has been shown that PTH can act on mouse adipocytes via the PKA pathway to activate hormone-sensitive lipase (9). Thus, there could be a synergistic effect of marrow adipocytic lipolysis on PTH-induced bone formation either through release of fatty acids from lipolysis or by generation of adipokines that further stimulate osteogenesis.
In the current study, whole body metabolism of the C57BL/6J mice was profoundly affected by calorie restriction but not very much by PTH. EE was drastically reduced in CR mice, and PTH induced a modest increase in CR/PTH. The respiratory quotient of 12-wk-old CR mice did not vary over 24 h, whereas that of Ctrl mice was increased at night. This finding indicates a switch from fatty acid to carbohydrate utilization at night in Ctrl mice but not in CR mice. The metabolic circadian rhythm was also altered in 16-wk-old mice that first underwent calorie restrictions before being injected with Veh or PTH. In those cohorts, during the day, respiratory quotients were closer to 0.7% and lower than in the 12-wk-old CR cohorts, thus showing a more robust fatty acid utilization in the mice that underwent calorie restrictions for a longer period of time (i.e., 8 wk) compared with those with calorie restrictions for 4 wk only. Overall, PTH had only a minimal effect on whole body metabolism or body fat of the mice. This outcome may be due to differences in expression of the PTH1R between peripheral adipocytes (low expression) and those in the bone marrow (high expression). These findings are also consistent with earlier studies from our group showing that marrow adipocytes have a unique set of adipocyte and osteoblast markers, possibly making them more responsive to PTH treatment (7) (unpublished data).
Our histologic analysis revealed that PTH reduced the number of adipocytes with little effect on their size in mice that were CR and injected at the same time, whereas the opposite effect was observed in mice that were first CR and then injected with PTH. This reduction in BMAT with PTH treatment is not surprising. Our findings are concordant with published data showing that PTH can decrease bone marrow adiposity observed after ovariectomy in rats and in men with idiopathic osteoporosis (7, 39, 40).
Previously, Rickard et al. (41) found that intermittent PTH could prevent adipogenesis of human mesenchymal stem cells though the PKA pathway. More recently, Balani et al. (8) showed that PTH rapidly affects lineage allocation and prevents adipogenesis, and previous research from our group also showed that PTH added early during in vitro adipogenesis could block full differentiation (7). Because PTH is known to induce osteoblast activation, primarily by stimulating bone lining cells and mesenchymal marrow progenitors to differentiate, it seems likely that this anabolic factor could act at several different levels.
In the current study, PTH regulated adipocyte function or differentiation to remodel BMAT depending on the timing of the PTH intervention. Gene analysis revealed that if PTH is injected at the same time calorie restriction starts, expressions of osteoblastic genes are strongly up-regulated but not those of adipogenesis-related genes. However, after 4 wk of calorie restriction, BMAT has already drastically expanded in the proximal tibia and continued to expand from the proximal to the distal part of the tibia after 8 wk of diet manipulation. Thus, PTH intervention starting 4 wk after calorie restriction can no longer prevent adipogenesis because the adipocytes have already developed in the marrow space. Hence, no change in adipocyte number or osteoblastic gene expression was observed in our CR/PTH cohort. However, in this cohort, adipogenic genes were up-regulated, notably the expression of Lipe, the gene for hormone-sensitive lipase. This lipase is a key factor for mobilizing fatty acids in adipocytes and is known to be activated through the PKA pathway (42, 43), a downstream signaling node for PTH. Importantly, the increase in Lipe expression is concordant with our findings of a reduction in the size of the adipocytes in CR/PTH treated mice. Accordingly, in the CR + PTH cohort that did not show differences in adipocyte size but rather number, the up-regulation of Lipe expression was much lower compared with that seen in CR/PTH mice.
The effects of PTH on white adipose tissue have received scant attention since an early report by Sinha et al. (44), although recently Larsson et al. (9) noted that PTH could induce lipolysis through the PKA pathway. Our in vitro and in vivo studies also support a role for PTH in adipogenic lipolysis, particularly within the bone marrow niche. We established that PTH could induce oxidative phosphorylation in BMSCs grown in adipogenic media, that PTH could also stimulate release of fatty acids and glycerol, and that, importantly, PTH could induce transfer of fatty acids to the osteoblast. We propose that the induction of lipolysis by PTH in CR mice may be a mechanism whereby energy can be provided for osteoblastic function, despite continuation of caloric deprivation. Importantly, PTH-induced lipolysis is relatively limited to BMAT and does not occur to a significant degree in peripheral adipocytes, almost certainly because PTH receptor expression is higher in bone marrow adipocytes compared with inguinal, visceral, or ear adipocytes (7).
Our studies have some limitations. First, our experiments showing transfer of fatty acids to osteoblasts were performed in vitro and therefore may not reflect in vivo conditions within the marrow niche. Second, our measurements of marrow adipocyte size reflect the staining of the “ghost” of the adipocyte, not the cell itself. There is no current method that allows us to measure marrow fat in vivo in the mouse, although MRI and osmium μCT imaging can provide quantitative evidence of fat volume in bone marrow cells. Third, our gene expression data should be viewed within the context of temporal specificity and because we cannot currently measure only marrow adipocyte gene expression. Techniques such as TRAP, that assess tissue-specific gene expression, are currently being developed for marrow but are not yet available.
In summary, our study provides new insights into the mechanism of action of PTH within the bone marrow by showing synergy between adipocytes and osteoblasts for new bone acquisition. These data also support previous findings from Fazeli et al. (17) that PTH can improve skeletal mass and fragility in teens and adults even in the face of profound calorie restriction. More studies are needed to define the precise extra-osteogenic mechanisms in vivo that drive bone formation in response to PTH administration.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank D.B. for performing the μCT analyses. All grants are supported by the U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS). The authors thank Dr. Vicki Rosen (Harvard School of Dental Medicine) for the use of her equipment. This project was primarily supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grants DK092759 (to C.J.R.) and DK112374 (to C.J.R.). Additional support was provided by the NIH National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR072123 (to E.R.-R.). The experiments were performed using the Physiology, Molecular Phenotyping, and Histopathology Cores. These cores are supported by the following NIH NIGMS grants: Physiology and Histopathology Core are supported by Grants P30GM106391 [Centers of Biomedical Research Excellence (COBRE) in Stem Cell Biology and Regenerative Medicine; R. Friesel, Principal Investigator], P20GM121301 (COBRE in Metabolic Networks; L. Liaw, Principal Investigator) and U54GM115516 (Northern New England Clinical and Translational Research Network; C.J.R., Principal Investigator). The Molecular Phenotyping Core is supported by Grants P30GM106391 and U54GM115516. The authors declare no conflicts of interest.
Glossary
- μCT
microcomputed tomography
- AN
anorexia nervosa
- BMAT
bone marrow adipose tissue
- BMD/aBMD
bone mineral density/areal bone mineral density
- BMSC
bone marrow stromal cell
- BV/TV
bone volume/total volume
- CR
calorie-restricted
- Ctrl
control
- DXA
dual X-ray absorptiometry
- EE
energy expenditure
- FCCP
carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone
- MS/BS
mineralizing surface per bone surface
- OCR
oxygen consumption rate
- PTH
parathyroid hormone
- Veh
vehicle
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
D. E. Maridas, E. Rendina-Ruedy, and C. J. Rosen designed the study and wrote the manuscript; experiments and data analyses were performed by D. E. Maridas, E. Rendina-Ruedy, R. C. Helderman, V. E. DeMambro, and D. Brooks; A. R. Guntur, B. Lanske, and M. L. Bouxsein contributed analytic tools and instruments; and D. E. Maridas performed the µCT analyses.
REFERENCES
- 1.Silva, B. C., Bilezikian, J. P. (2015) Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 22, 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang, J., Wang, X., Holz, J. D., Rutkowski, T., Wang, Y., Zhu, Z., Dong, Y. (2013) Runx1 is critical for PTH-induced onset of mesenchymal progenitor cell chondrogenic differentiation. PLoS One 8, e74255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim, S. W., Pajevic, P. D., Selig, M., Barry, K. J., Yang, J. Y., Shin, C. S., Baek, W. Y., Kim, J. E., Kronenberg, H. M. (2012) Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J. Bone Miner. Res. 27, 2075–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Esen, E., Lee, S. Y., Wice, B. M., Long, F. (2015) PTH promotes bone anabolism by stimulating aerobic glycolysis via IGF signaling. J. Bone Miner. Res. 30, 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ben-awadh, A. N., Delgado-Calle, J., Tu, X., Kuhlenschmidt, K., Allen, M. R., Plotkin, L. I., Bellido, T. (2014) Parathyroid hormone receptor signaling induces bone resorption in the adult skeleton by directly regulating the RANKL gene in osteocytes. Endocrinology 155, 2797–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wein, M. N., Liang, Y., Goransson, O., Sundberg, T. B., Wang, J., Williams, E. A., O’Meara, M. J., Govea, N., Beqo, B., Nishimori, S., Nagano, K., Brooks, D. J., Martins, J. S., Corbin, B., Anselmo, A., Sadreyev, R., Wu, J. Y., Sakamoto, K., Foretz, M., Xavier, R. J., Baron, R., Bouxsein, M. L., Gardella, T. J., Divieti-Pajevic, P., Gray, N. S., Kronenberg, H. M. (2017) Corrigendum: SIKs control osteocyte responses to parathyroid hormone. Nat. Commun. 8, 14745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fan, Y., Hanai, J. I., Le, P. T., Bi, R., Maridas, D., DeMambro, V., Figueroa, C. A., Kir, S., Zhou, X., Mannstadt, M., Baron, R., Bronson, R. T., Horowitz, M. C., Wu, J. Y., Bilezikian, J. P., Dempster, D. W., Rosen, C. J., Lanske, B. (2017) Parathyroid hormone directs bone marrow mesenchymal cell fate. Cell Metab. 25, 661–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balani, D. H., Ono, N., Kronenberg, H. M. (2017) Parathyroid hormone regulates fates of murine osteoblast precursors in vivo. J. Clin. Invest. 127, 3327–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larsson, S., Jones, H. A., Göransson, O., Degerman, E., Holm, C. (2016) Parathyroid hormone induces adipocyte lipolysis via PKA-mediated phosphorylation of hormone-sensitive lipase. Cell. Signal. 28, 204–213 [DOI] [PubMed] [Google Scholar]
- 10.Hoek, H. W. (2006) Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr. Opin. Psychiatry 19, 389–394 [DOI] [PubMed] [Google Scholar]
- 11.Fazeli, P. K., Klibanski, A. (2014) Anorexia nervosa and bone metabolism. Bone 66, 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fazeli, P. K., Bredella, M. A., Freedman, L., Thomas, B. J., Breggia, A., Meenaghan, E., Rosen, C. J., Klibanski, A. (2012) Marrow fat and preadipocyte factor-1 levels decrease with recovery in women with anorexia nervosa. J. Bone Miner. Res. 27, 1864–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bredella, M. A., Fazeli, P. K., Miller, K. K., Misra, M., Torriani, M., Thomas, B. J., Ghomi, R. H., Rosen, C. J., Klibanski, A. (2009) Increased bone marrow fat in anorexia nervosa. J. Clin. Endocrinol. Metab. 94, 2129–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cawthorn, W. P., Scheller, E. L., Learman, B. S., Parlee, S. D., Simon, B. R., Mori, H., Ning, X., Bree, A. J., Schell, B., Broome, D. T., Soliman, S. S., DelProposto, J. L., Lumeng, C. N., Mitra, A., Pandit, S. V., Gallagher, K. A., Miller, J. D., Krishnan, V., Hui, S. K., Bredella, M. A., Fazeli, P. K., Klibanski, A., Horowitz, M. C., Rosen, C. J., MacDougald, O. A. (2014) Bone marrow adipose tissue is an endocrine organ that contributes to increased circulating adiponectin during caloric restriction. Cell Metab. 20, 368–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misra, M., Klibanski, A. (2014) Anorexia nervosa and bone. J. Endocrinol. 221, R163–R176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Towell, D. B., Woodford, S., Reid, S., Rooney, B., Towell, A. (2001) Compliance and outcome in treatment-resistant anorexia and bulimia: a retrospective study. Br. J. Clin. Psychol. 40, 189–195 [DOI] [PubMed] [Google Scholar]
- 17.Fazeli, P. K., Wang, I. S., Miller, K. K., Herzog, D. B., Misra, M., Lee, H., Finkelstein, J. S., Bouxsein, M. L., Klibanski, A. (2014) Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J. Clin. Endocrinol. Metab. 99, 1322–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeMambro, V. E., Clemmons, D. R., Horton, L. G., Bouxsein, M. L., Wood, T. L., Beamer, W. G., Canalis, E., Rosen, C. J. (2008) Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinology 149, 2051–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouxsein, M. L., Boyd, S. K., Christiansen, B. A., Guldberg, R. E., Jepsen, K. J., Müller, R. (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 25, 1468–1486 [DOI] [PubMed] [Google Scholar]
- 20.DeMambro, V. E., Le, P. T., Guntur, A. R., Maridas, D. E., Canalis, E., Nagano, K., Baron, R., Clemmons, D. R., Rosen, C. J. (2015) Igfbp2 deletion in ovariectomized mice enhances energy expenditure but accelerates bone loss. Endocrinology 156, 4129–4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasparrini, M., Rivas, D., Elbaz, A., Duque, G. (2009) Differential expression of cytokines in subcutaneous and marrow fat of aging C57BL/6J mice. Exp. Gerontol. 44, 613–618 [DOI] [PubMed] [Google Scholar]
- 22.Pack, A. I., Galante, R. J., Maislin, G., Cater, J., Metaxas, D., Lu, S., Zhang, L., Von Smith, R., Kay, T., Lian, J., Svenson, K., Peters, L. L. (2007) Novel method for high-throughput phenotyping of sleep in mice. Physiol. Genomics 28, 232–238 [DOI] [PubMed] [Google Scholar]
- 23.Kaiyala, K. J., Schwartz, M. W. (2011) Toward a more complete (and less controversial) understanding of energy expenditure and its role in obesity pathogenesis. Diabetes 60, 17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arch, J. R., Hislop, D., Wang, S. J., Speakman, J. R. (2006) Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int. J. Obes. 30, 1322–1331 [DOI] [PubMed] [Google Scholar]
- 25.Kaiyala, K. J., Morton, G. J., Leroux, B. G., Ogimoto, K., Wisse, B., Schwartz, M. W. (2010) Identification of body fat mass as a major determinant of metabolic rate in mice. Diabetes 59, 1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maridas, D. E., Rendina-Ruedy, E., Le, P. T., Rosen, C. J. (2018) Isolation, culture, and differentiation of bone marrow stromal cells and osteoclast progenitors from mice. J. Vis. Exp. 131 (abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devlin, M. J., Cloutier, A. M., Thomas, N. A., Panus, D. A., Lotinun, S., Pinz, I., Baron, R., Rosen, C. J., Bouxsein, M. L. (2010) Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J. Bone Miner. Res. 25, 2078–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bredella, M. A., Fazeli, P. K., Daley, S. M., Miller, K. K., Rosen, C. J., Klibanski, A., Torriani, M. (2014) Marrow fat composition in anorexia nervosa. Bone 66, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwartz, A. V., Sigurdsson, S., Hue, T. F., Lang, T. F., Harris, T. B., Rosen, C. J., Vittinghoff, E., Siggeirsdottir, K., Sigurdsson, G., Oskarsdottir, D., Shet, K., Palermo, L., Gudnason, V., Li, X. (2013) Vertebral bone marrow fat associated with lower trabecular BMD and prevalent vertebral fracture in older adults. J. Clin. Endocrinol. Metab. 98, 2294–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fazeli, P. K., Horowitz, M. C., MacDougald, O. A., Scheller, E. L., Rodeheffer, M. S., Rosen, C. J., Klibanski, A. (2013) Marrow fat and bone—new perspectives. J. Clin. Endocrinol. Metab. 98, 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu, T., Xian, L., Crane, J., Wen, C., Hilton, M., Lu, W., Newman, P., Cao, X. (2015) PTH receptor signaling in osteoblasts regulates endochondral vascularization in maintenance of postnatal growth plate. J. Bone Miner. Res. 30, 309–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knopp, E., Troiano, N., Bouxsein, M., Sun, B. H., Lostritto, K., Gundberg, C., Dziura, J., Insogna, K. (2005) The effect of aging on the skeletal response to intermittent treatment with parathyroid hormone. Endocrinology 146, 1983–1990 [DOI] [PubMed] [Google Scholar]
- 33.Ahn, H., Seo, D. H., Kim, H. S., Choue, R. (2014) Calorie restriction aggravated cortical and trabecular bone architecture in ovariectomy-induced estrogen-deficient rats. Nutr. Res. 34, 707–713 [DOI] [PubMed] [Google Scholar]
- 34.Behrendt, A. K., Kuhla, A., Osterberg, A., Polley, C., Herlyn, P., Fischer, D. C., Scotland, M., Wree, A., Histing, T., Menger, M. D., Müller-Hilke, B., Mittlmeier, T., Vollmar, B. (2016) Dietary restriction-induced alterations in bone phenotype: effects of lifelong versus short-term caloric restriction on femoral and vertebral bone in C57BL/6 mice. J. Bone Miner. Res. 31, 852–863 [DOI] [PubMed] [Google Scholar]
- 35.Justesen, J., Stenderup, K., Ebbesen, E. N., Mosekilde, L., Steiniche, T., Kassem, M. (2001) Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology 2, 165–171 [DOI] [PubMed] [Google Scholar]
- 36.Verma, S., Rajaratnam, J. H., Denton, J., Hoyland, J. A., Byers, R. J. (2002) Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J. Clin. Pathol. 55, 693–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwaniec, U. T., Turner, R. T. (2013) Failure to generate bone marrow adipocytes does not protect mice from ovariectomy-induced osteopenia. Bone 53, 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman, S., Lu, Y., Czernik, P. J., Rosen, C. J., Enerback, S., Lecka-Czernik, B. (2013) Inducible brown adipose tissue, or beige fat, is anabolic for the skeleton. Endocrinology 154, 2687–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kulkarni, N. H., Wei, T., Kumar, A., Dow, E. R., Stewart, T. R., Shou, J., N’cho, M., Sterchi, D. L., Gitter, B. D., Higgs, R. E., Halladay, D. L., Engler, T. A., Martin, T. J., Bryant, H. U., Ma, Y. L., Onyia, J. E. (2007) Changes in osteoblast, chondrocyte, and adipocyte lineages mediate the bone anabolic actions of PTH and small molecule GSK-3 inhibitor. J. Cell. Biochem. 102, 1504–1518 [DOI] [PubMed] [Google Scholar]
- 40.Dempster, D. W., Cosman, F., Kurland, E. S., Zhou, H., Nieves, J., Woelfert, L., Shane, E., Plavetić, K., Müller, R., Bilezikian, J., Lindsay, R. (2001) Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J. Bone Miner. Res. 16, 1846–1853 [DOI] [PubMed] [Google Scholar]
- 41.Rickard, D. J., Wang, F. L., Rodriguez-Rojas, A. M., Wu, Z., Trice, W. J., Hoffman, S. J., Votta, B., Stroup, G. B., Kumar, S., Nuttall, M. E. (2006) Intermittent treatment with parathyroid hormone (PTH) as well as a non-peptide small molecule agonist of the PTH1 receptor inhibits adipocyte differentiation in human bone marrow stromal cells. Bone 39, 1361–1372 [DOI] [PubMed] [Google Scholar]
- 42.Trzeciak, W. H., Boyd, G. S. (1974) Activation of cholesteryl esterase in bovine adrenal cortex. Eur. J. Biochem. 46, 201–207 [DOI] [PubMed] [Google Scholar]
- 43.Holm, C. (2003) Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 31, 1120–1124 [DOI] [PubMed] [Google Scholar]
- 44.Sinha, T. K., Thajchayapong, P., Queener, S. F., Allen, D. O., Bell, N. H. (1976) On the lipolytic action of parathyroid hormone in man. Metabolism 25, 251–260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.