Abstract
Recent studies have demonstrated an effect of neurotrophins, particularly brain-derived neurotrophic factor (BDNF), on airway contractility [via increased airway smooth muscle (ASM) intracellular calcium [Ca2+]i] and remodeling (ASM proliferation and extracellular matrix formation) in the context of airway disease. In the present study, we examined the role of BDNF in allergen-induced airway inflammation using 2 transgenic models: 1) tropomyosin-related kinase B (TrkB) conditional knockin (TrkBKI) mice allowing for inducible, reversible disruption of BDNF receptor kinase activity by administration of 1NMPP1, a PP1 derivative, and 2) smooth muscle–specific BDNF knockout (BDNFfl/fl/SMMHC11Cre/0) mice. Adult mice were intranasally challenged with PBS or mixed allergen (Alternaria alternata, Aspergillus fumigatus, house dust mite, and ovalbumin) for 4 wk. Our data show that administration of 1NMPP1 in TrkBKI mice during the 4-wk allergen challenge blunted airway hyperresponsiveness (AHR) and reduced fibronectin mRNA expression in ASM layers but did not reduce inflammation per se. Smooth muscle–specific deletion of BDNF reduced AHR and blunted airway fibrosis but did not significantly alter airway inflammation. Together, our novel data indicate that TrkB signaling is a key modulator of AHR and that smooth muscle–derived BDNF mediates these effects during allergic airway inflammation.—Britt, R. D., Jr., Thompson, M. A., Wicher, S. A., Manlove, L. J., Roesler, A., Fang, Y.-H., Roos, C., Smith, L., Miller, J. D., Pabelick, C. M., Prakash, Y. S. Smooth muscle brain-derived neurotrophic factor contributes to airway hyperreactivity in a mouse model of allergic asthma.
Keywords: TrkB, neurotrophin, fibrosis, extracellular matrix
Airway hyperresponsiveness (AHR) and remodeling are major aspects of structural and functional changes in asthma that contribute to exaggerated airway narrowing and resistance to airflow. Airway smooth muscle (ASM) contributes to the increased contractility of AHR, whereas remodeling involves epithelial thickening, increased ASM mass, and fibrosis characterized by altered extracellular matrix (ECM) composition and content (1–6). Understanding the mechanisms that contribute to AHR and remodeling in asthma is thus relevant to developing novel strategies. In this regard, there is increasing recognition that resident cells, such as epithelium and ASM, secrete growth factors and inflammatory mediators that have autocrine and paracrine effects that can promote and sustain the effects of initial insults thus prolonging the changes that occur in asthma (1, 7–14). Recent studies, including our own, have identified neurotrophins (NTs) as noncanonical local factors in the airway (15).
Although well known in the nervous system (16–19), NTs and their receptors have been shown to be expressed by structural cells of the lung, including ASM, and thus have the potential to influence airway structure and function (20–25). For example, sputum and bronchoalveolar lavage (BAL) fluid from patients with asthma show increased levels of the NT brain-derived neurotrophic factor (BDNF) (24, 26–29). Using in vitro models of human ASM, we previously showed that exogenous BDNF enhances intracellular calcium ([Ca2+]i) regulation and contractility (20, 21, 30), proliferation (31), and fibrosis (32). We recently found that ASM is a not only a target but also a source of BDNF, with increased BDNF secretion after inflammation (33, 34) and oxidative stress (35). Furthermore, such elevated BDNF secretion leads to autocrine effects on ASM (32, 34). Thus, it appears that NTs such as BDNF could contribute to altered airway structure and function. However, beyond in vitro studies such as our own, there are currently no in vivo data demonstrating the importance of BDNF expression or signaling in the context of airway inflammation and asthma.
The effects of BDNF are mediated through its high-affinity receptor, tropomyosin-related kinase B (TrkB), which is expressed by ASM (35, 36). TrkB, a tyrosine kinase receptor, activates multiple signaling pathways to regulate several downstream processes, including proliferation, differentiation, [Ca2+]i regulation, and migration (15). BDNF-TrkB signaling activates several signaling cascades, including PKC, MAPK, and NF-κB, that overlap with proinflammatory cytokine signaling (15).
To assess the role of BDNF-TrkB signaling in allergic airway inflammation, we used 2 transgenic mouse models. TrkB knockin (TrkBKI) mice that express a F616A mutation in the TrkB ATP binding pocket (37) such that TrkB expression is always normal and its function remains normal until administration of a PP1 derivative, 1NMPP1, which inhibits TrkB tyrosine kinase activity (37). We further assessed the role of smooth muscle–derived BDNF in a conditional smooth muscle–specific BDNF knockout mouse. Both transgenic models were used to test the hypothesis that disruption of BDNF/TrkB signaling particularly in smooth muscle will reduce AHR and remodeling in allergen-challenged mice, highlighting the role of ASM BDNF per se.
MATERIALS AND METHODS
Mouse models
Animal protocols were approved by the Mayo Clinic Institutional Animal Care and Use Committee and conducted in accordance to guidelines from the Guide for the Care and Use of Laboratory Animals [U.S. National Institutes of Health (NIH), Bethesda, MD, USA]. Mouse colonies were maintained and housed at Mayo Clinic St. Mary’s Hospital vivarium. All mice were provided food and water ad libitum. TrkBF616A mice (on C57Bl/6+129 background) were a gift from Dr. David D. Ginty (Harvard University, Cambidge, MA, USA). Mice were administered vehicle (0.001% DMSO) or 25 μM 1NMPP1 (529581; MilliporeSigma, Burlington, MA, USA) in drinking water for 4 wk (prevention strategy) or for the latter 2 wk (reversal strategy) of the mixed allergen (MA) challenge. The rationale for imitating 1NMPP1 after initial MA sensitization was to assess whether interference with TrkB signaling can blunt ongoing allergen-induced functional and particularly structural changes. Pilot studies demonstrated the ability of 1NMPP1 to inhibit TrkB kinase activity in the lung and confirmed that 1NMPP1 does not alter TrkB expression per se or TrkB activity in wild-type (WT) C57Bl/6J mice. In the absence of 1NMPP1, TrkBF616A mice had no abnormalities in lung function. In control experiments, 1NMPP1 was also administered to WT C57Bl/6J mice for 4 wk during allergen challenge.
To generate smooth muscle–specific conditional BDNF knockout mice [via smooth muscle myosin heavy chain (SMMHC11)], female BDNFfl/fl mice with loxP sites inserted into the coding exon of BDNF (004339; The Jackson Laboratory, Bar Harbor, ME, USA) were bred with male SMMHC11-CreERT2,Cre/0 mice that express Cre recombinase (Cre) under the control of a mutated human estrogen receptor (ERT2) (019079; The Jackson Laboratory) to generate BDNFfl/+/SMMHC11Cre/0 founders. Intercrossing these mice led to littermate matching of BDNF+/+, BDNFfl/+, and BDNFfl/fl mice that carry (SMMHC11Cre/0) or do not carry (SMMHC110/0) Cre recombinase. Subsequent breeding involved crossing BDNFfl/+/SMMHC11Cre/0 male mice with BDNFfl/+/SMMHC110/0 female mice to generate several genotypes: BDNF+/+/SMMHC11Cre/0, BDNFfl/+/SMMHC11Cre/0, BDNFfl/fl/SMMHC11Cre/0, BDNF+/+/SMMHC110/0, BDNFfl/+/SMMHC110/0, or BDNFfl/fl/SMMHC110/0. The ERT2 agonist 4-hydroxytamoxifen (4OHTM) was used to induce CreERT2 nuclear translocation and Cre recombinase activity in SMMHC11-expressing cells. Male SMMHC11Cre/0/BDNFfl/fl (Cre-positive) or SMMHC110/0/BDNFfl/fl (Cre-negative) mice were intraperitoneally injected with vehicle (20% ethanol/80% sunflower seed oil) or 1 mg 4OHTM for 5 consecutive days prior to allergen challenge (or interference with BDNF expression). Mouse genotype was identified by isolating DNA from tails and PCR using primers specific for SMMHC11-CreERT2 or BDNFfl/fl. Data from BDNFfl/fl/SMMHC11Cre/0 (sensitive to 4OHTM conditional deletion) and BDNFfl/fl/SMMHC110/0 (resistant 4OHTM conditional deletion) mice are presented.
Allergen exposure
For MA challenge in adult mice (38, 39), 12- to 16-wk-old male mice were intranasally challenged with 10 μg Alternaria alternata (Greer Laboratories, Lenior, NC, USA), 10 μg Aspergillus fumigatus (Greer Laboratories), 10 μg house dust mite (Greer Laboratories), and 10 μg ovalbumin (MilliporeSigma) 3 times per week for 4 wk. Twenty-four hours after the last MA challenge, mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (20 mg/kg). Lung function was assessed, and tissues were harvested for histologic and molecular analyses.
Lung function
Lung function assessments were performed using the FlexiVent (Scireq, Montreal, QC, Canada) (40). Mouse trachea were cannulated with a 19G blunt tip cannula and ventilated with positive pressure on a 37°C heated pad. Lung dynamic resistance and compliance were measured at baseline and after administration of nebulized methacholine at increasing doses (PBS, 6.3, 12.5, 25, and 50 mg/ml).
Bronchoalveolar lavage
After functional measurements were performed, the lung was lavaged with 1 ml PBS 3 times. Recovered BAL fluid was centrifuged at 10,000 rpm for 10 min at 4°C. Cell pellets were resuspended in PBS, and BAL total cell counts were measured using a hemacytometer. For differential analysis, BAL cells were Cytospun onto microscope slides and stained with Wright-Giemsa stain (MilliporeSigma). Eosinophils, macrophages, lymphocytes, and neutrophils were counted until a total of 300 cells was obtained, and the number of each cell type was calculated based on total BAL cell count.
Histology
Mouse lungs were inflated with 4% paraformaldehyde at 25 cm H2O pressure, and the fixed lungs were paraffin embedded and cut into 5 μm sections. To assess airway inflammation and gross airway changes, lung sections were stained with hematoxylin and eosin (H&E) and Periodic-acid Schiff (PAS). Photomicrographs of all airways in a lung section were acquired using light microscopy at ×200 or 400 magnification (Nikon Instruments, Melville, NY, USA). ImageJ (NIH) software was used to measure airway area and perimeter and to calculate airway thickness. To quantify mucus production in airways, airway epithelial cells and cells positive for PAS staining (blue) were manually counted. The percentage of PAS-positive cells was subsequently calculated. Airway analyses were performed by a blinded investigator.
Immunofluorescence
Lung sections were exposed overnight to polyclonal rabbit anti-BDNF (Abcam, Cambridge, MA, USA) and/or monoclonal mouse anti–α-smooth muscle actin (A2547; MilliporeSigma) antibodies (1 μg/ml each), washed, and then incubated with Alexa 488 donkey anti-rabbit and Alexa 647 donkey anti-mouse secondary antibodies (Thermo Fisher Scientific, Waltham, MA, USA). Nuclei were counterstained with DAPI. Samples were imaged at ×200 or 400 magnification using a Nikon Eclipse Ti microscope. To quantify α-smooth muscle actin, immunofluorescence intensity was measured using Nikon NIS-Elements software and normalized to airway area.
ELISA
Lung tissue was homogenized in sucrose buffer and protease inhibitors. Homogenates were centrifuged at 12,000 rpm for 10 min at 4°C, and supernatants were collected. Expression of IL-4, IL-5, and IL-13 was measured using Quantikine ELISA kits (R&D Systems, Minneapolis, MN, USA). Cytokine levels were determined from a standard curve and normalized to protein concentration.
Laser capture microdissection
Under RNase-free conditions, lungs were harvested, immediately frozen in liquid nitrogen, and stored at −80°C. Frozen lungs were cut into 10-μm sections and processed as previously described (40, 41). Laser capture microdissection (LCM) was performed using an Arcturus XT microdissection system (Molecular Devices, Sunnyvale, CA, USA). Small airways (300–350 µm diameter) were visualized under light microscopy (×200 magnification). ASM and airway epithelial layers were visually identified and distinguished. Using an infrared laser, layers were microdissected and captured onto CapSure Macro LCM caps. Total RNA was isolated from caps for cDNA synthesis and quantitative RT-PCR (Roche LightCycler 96 System; Roche Diagnostics, Indianapolis, IN, USA). Expression of mRNA was calculated using the ΔΔCt method, where GAPDH was used as the reference gene. mRNA expression of α-smooth muscle actin and E-cadherin was used to validate relative purity of ASM vs. airway epithelial layers. Primers used for quantitative RT-PCR are listed in Table 1. Quantitech Primers for IP3R (QT00316736), Orai1 (QT00285775), and α-smooth muscle actin (QT00140119) from were purchased from Qiagen (Germantown, MD, USA).
TABLE 1.
Mouse primer sequences used in LCM analysis
| Gene | Primer sequence, 5′–3′ |
|
|---|---|---|
| Forward | Reverse | |
| Collagen I | GAAGTCAGCTGCATACACAATGGCCT | GATACGTATTCTTCCGGGCAGAAAGC |
| Collagen III | CTGTAACATGGAAACTGGGGAAA | CCATAGCTGAACTGAAAACCACC |
| E-Cadherin | AACAGGCCAGAGTTTACCCAGGAGG | GGTGTAGGCGATGGCAGCGTT |
| Fibronectin | TTCGTGCTGGCAAAGAAAGCCATCAC | CTGTATTTCCACCAGATCATCCTCGG |
| M3ACHR | TTGCACAGTAACAGTACAACCTCG | TGTTGCCGATGATGGTCACCAATG |
| S16 | TGCAGGTCTTCGGACGCAAGAAAA | CGAATATCCACACCAGCAAATCGC |
| Stim1 | GGTTCAACGCCATAAATGT | GAGATTGTGTCGCCCT |
Statistical analysis
Statistical analyses were performed using GraphPad Prism Software (La Jolla, CA, USA). Data were analyzed by an unpaired Student’s t test or 2-way ANOVA with Bonferroni corrections for multiple comparisons. Statistical significance is indicated by P < 0.05. Values are presented as means ± se.
RESULTS
Effect of 1NMPP1 in TrkBKI mice
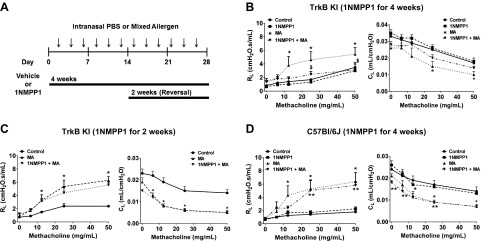
TrkBKI mice were administered vehicle or 25 μM 1NMPP1 in drinking water and intranasally challenged with PBS or MA (3×/wk) for 4 wk (Fig. 1A). MA-exposed mice that were administered vehicle showed significantly increased lung resistance after 12.5, 25, or 50 mg/ml methacholine, whereas compliance was significantly reduced at 25 mg/ml methacholine (Fig. 1B). In contrast, lung resistance was significantly reduced in mice administered 1NMPP1 during the 4 wk MA challenge (i.e., the preventative protocol) (Fig. 1B). To determine if 1NMPP1 could reverse the effects of MA, 1NMPP1 was administered to TrkBKI mice during the latter 2 wk of MA challenge (Fig. 1A). In contrast to 1NMPP1 for 4 wk, 1NMPP1 for the latter 2 wk did not blunt the effects of MA on lung resistance or compliance (Fig. 1C). In control experiments, the effects of 1NMPP1 on MA-induced increases in lung resistance and decreases in lung compliance were absent in WT C57Bl/6J mice (Fig. 1D), demonstrating the specificity of 1NMPP1 for TrkBKI mice.
Figure 1 .
Treatment with 1NMPP1 for 4 wk inhibits resistance in MA-challenged mice. A) Experimental design of MA challenge and 1NMPP1 administration to TrkBKI or C57Bl/6J mice. B) The effects of MA on resistance and compliance during methacholine challenge are inhibited in TrkBKI mice treated with 1NMPP1. C, D) The effects of 1NMPP1 on resistance and compliance are absent in TrkBKI mice treated with 1NMPP1 for the last 2 wk (C) and C57Bl/6J mice treated with 1NMPP1 for 4 wk (D). Data are presented as means ± sem (n = 6–12 for TrkBKI and n = 7–8 C57Bl/6J mice/group). *P < 0.05, significant difference between control and MA; **P < 0.05, significant difference between control and MA + 1NMPP1; $P < 0.05, significant difference between MA and 1NMPP1 + MA.
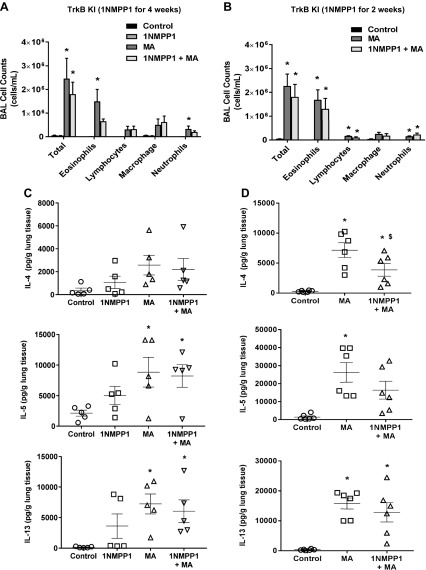
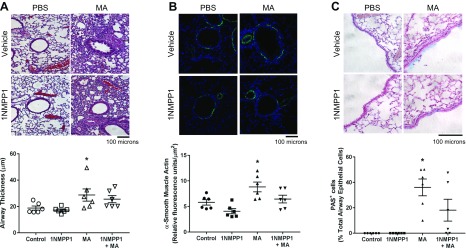
Next, we tested the effect of 1NMPP1 on allergen-induced airway inflammation by assessing the portfolio of immune cells and expression of cytokines in lung tissue. We performed differential analyses of cells collected from BAL. MA increased total BAL eosinophils, lymphocytes, and neutrophils (Fig. 2A, B). Treatment with 1NMPP1 for 4 wk reduced eosinophils, but lymphocytes and neutrophils were not affected (Fig. 2A). 1NMPP1 in the latter 2 wk of MA challenge did not affect eosinophils, lymphocytes, or neutrophils (Fig. 2B). MA significantly increased lung tissue expression of T helper (Th)2 cytokines, IL-4, IL-5, and IL-13 (Fig. 2C, D). Although 1NMPP1 for 2 wk significantly reduced IL-4 expression, these effects were not observed in mice that received 1NMPP1 for 4 wk (Fig. 2C, D). H&E and PAS staining revealed significant inflammation, remodeling, and mucin expression around airways in MA-challenged mice (Fig. 3). 1NMPP1 administration for 4 wk did not significantly reduce airway inflammation and thickness, smooth muscle actin staining, or mucin expression (Fig. 3).
Figure 2 .
Effect of 1NMPP1 on immune cell infiltration and Th2 cytokine levels. BAL cell counts of eosinophils, lymphocytes, and neutrophils we increased by MA challenge. A) 1NMPP1 for 4 wk reduced eosinophils in MA-challenged TrkBKI mice, whereas lymphocytes and neutrophils remained increased. B) BAL eosinophils, lymphocytes, and neutrophils remained increased in MA mice treated with 1NMPP1 for 2 wk. C, D) Expression of IL-4, IL-5, and IL-13 was increased in lung tissue from MA-challenged mice, whereas 1NMPP1 treatment for 4 (C) and 2 (D) wk did not affect their levels. Data are presented as mean ± sem (n = 4–8 mice/group). *P < 0.05, significant difference between control and MA; $P < 0.05, significant difference between MA and 1NMPP1 + MA.
Figure 3 .
Histology of airway inflammation in TrkBKI mice. H&E and PAS stained lung sections show airway inflammation, α-smooth muscle actin expression, and mucus production in MA-challenged mice. Airway inflammation and thickness (A), α-smooth muscle actin expression (B), and mucin expression (C) were not significantly reduced in mice administered 1NMPP1. Original magnification: ×200 (A, B), ×400 (C). Data are presented as means ± sem (n = 6 mice/group). *P < 0.05, significant difference between control and MA.
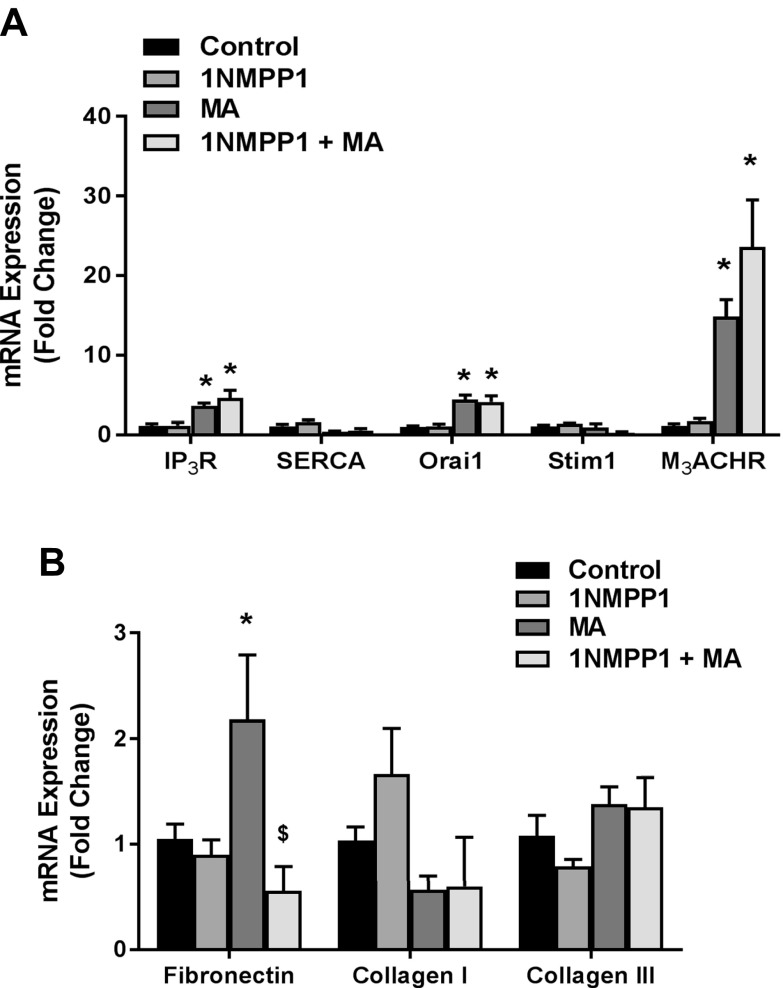
To determine the effects of 1NMPP1 on ASM, we examined mRNA changes in LCM samples of the ASM layer. MA significantly increased mRNA expression of the Ca2+ regulatory proteins IP3R, Orai1, and M3ACHR in MA and in 1NMPP1 (4 wk) + MA mice (Fig. 4A). Although expression of ECM proteins collagen I and collagen III protein was not affected by MA or 1NMPP1, expression of fibronectin was significantly increased by MA and reduced in 1NMPP1 + MA mice (Fig. 4B).
Figure 4 .
Airway smooth muscle expression of Ca2+ regulatory and ECM proteins. Laser capture microdissection was used to isolate mRNA from the airway smooth muscle layer. A) MA challenge increased mRNA expression of Ca2+ regulatory proteins IP3R, Orai1, and M3ACHR, which remained increased in 1NMPP1-treated mice. B) Treatment with 1NMPP1 reduced MA-induced fibronectin, but not collagen I and III, mRNA expression. Data are presented as means ± sem (n = 6 mice/group). *P < 0.05, significant difference between control and MA; $P < 0.05, significant difference between MA and 1NMPP1 + MA.
Effect of smooth muscle–specific conditional BDNF knockout
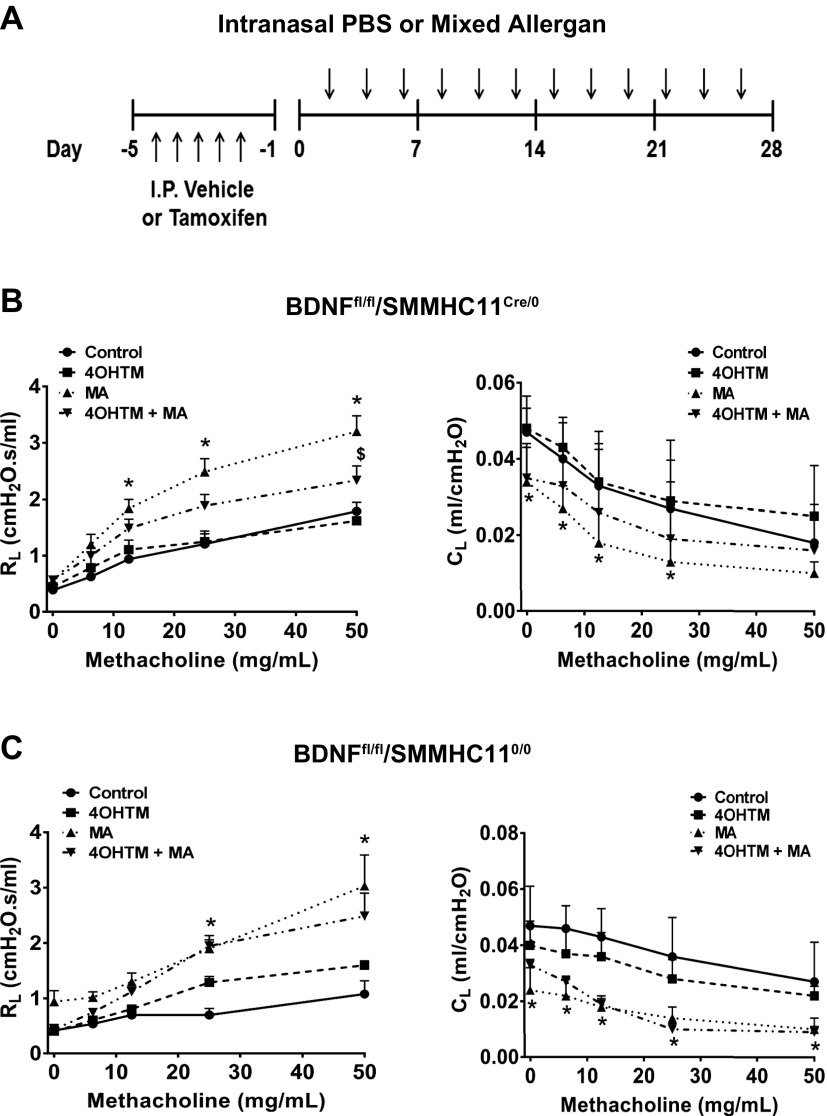
To examine the role of ASM-derived BDNF during allergen challenge, we generated mice to conditionally excise BDNF from cells expressing smooth muscle myosin heavy chain 11 (SMMHC11) (Fig. 5). Genotype was identified by PCR (Fig. 5A), and confirmation of BDNF deletion from the ASM was by immunofluorescence staining of lung tissue sections. SMMHC11Cre/0/BDNFfl/fl (Cre-positive) or SMMHC110/0/BDNFfl/fl (Cre-positive) mice were intraperitoneally injected with vehicle or 1 mg 4OHTM for 5 d prior to 4 wk of MA challenge (Fig. 6A). Vehicle-injected SMMHC11Cre/0/BDNFfl/fl mice that were challenged with MA showed increased resistance and decreased compliance in response to 12.5, 25, and 50 mg/ml methacholine (Fig. 6B). These effects were blunted in SMMHC11Cre/0/BDNFfl/fl MA mice injected with 4OHTM (Fig. 6B). However, the effects of MA on resistance and compliance remained increased in SMMHC110/0/BDNFfl/fl mice injected with 4OHTM (Fig. 6C).
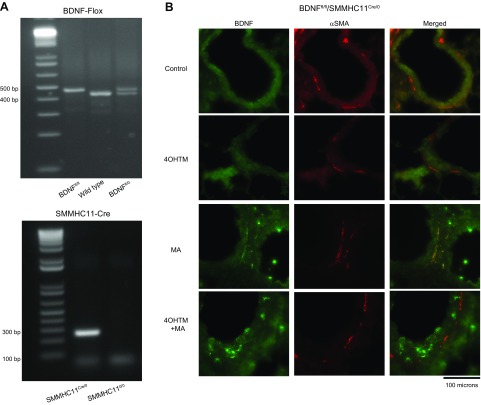
Figure 5 .
Generation of conditional smooth muscle–specific BDNF knockout mice. BDNFfl/fl/SMMHC11Cre/0 and BDNFfl/fl/SMMHC110/0 were generated for the present studies. A) Wild-type (437 bp), BDNFfl/fl (487 bp), BDNFfl/0 (437 and 487 bp), and SMMHC11Cre/0 (287 bp) genotypes were assessed by PCR. B) BDNFfl/fl/SMMHC110/0 treated with 1 mg 4OHTM for 5 consecutive days led to deletion of BDNF in airway smooth muscle (identified by α-smooth muscle actin). Original magnification, ×400.
Figure 6 .
Conditional smooth muscle deletion of BDNF reduces resistance in MA-challenged mice. A) BDNFfl/fl/SMMHC11Cre/0 and BDNFfl/fl/SMMHC110/0 mice were intraperitoneally injected with 1 mg 4OHTM for 5 consecutive days prior to MA challenge for 4 wk. B) MA-challenged BDNFfl/fl/SMMHC11Cre/0 mice injected with 4OHTM had reduced resistance and improved compliance. C) The effects of 4OHTM were absent in BDNFfl/fl/SMMHC110/0 MA-challenged mice. *P < 0.05, significant difference between control and MA; $P < 0.05, significant difference between MA and 4OHTM + MA.
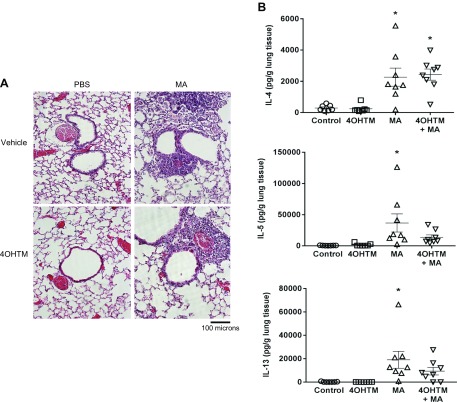
Similar to the TrkBKI model, airway inflammation remained increased in MA-challenged SMMHC110/0/BDNFfl/fl mice injected with vehicle and 4OHTM (Fig. 7A). Additionally, expression of IL-4, IL-5, and IL-13 remained elevated in MA-exposed SMMHC110/0/BDNFfl/fl mice injected with vehicle and 4OHTM (Fig. 7B).
Figure 7 .
Airway inflammation and cytokine levels in BDNFfl/fl/SMMHC11Cre/0 mice. A) H&E-stained lung sections show airway inflammation in MA-challenged mice, whereas airway inflammation remained in BDNFfl/fl/SMMHC11Cre/0 mice administered 4OHTM. Original magnification, ×200. B) Expression of IL-4, IL-5, and IL-13 was increased in lung tissue from MA-challenged mice. Data are presented as means ± sem (n = 7–8 mice/group). *P < 0.05, significant difference between control and MA.
DISCUSSION
Although the role of BDNF in human ASM contractility and remodeling has been explored in recent years, its role in allergic airway inflammation in vivo has not been examined. Two transgenic mouse models were used to examine the role of BDNF/TrkB signaling in adult allergic airway inflammation. In the TrkBKI model, where administration of a small molecule inhibitor (1NMPP1) disrupts TrkB kinase activity (37), 4 wk of 1NMPP1 prevented AHR and improved compliance compared with mice treated with MA alone. This effect was specific for TrKBKI mice that carry the TrkBF616A mutation. In contrast, treatment with 1NMPP1 2 wk after starting MA challenge was unable to reduce AHR or improve compliance, suggesting an important role for BDNF/TrkB in the initiation of changes to the airway in response to allergic inflammation. We further investigated the role of ASM-derived BDNF in a smooth muscle–specific conditional BDNF knockout mouse model (SMMHC11Cre/0/BDNFfl/fl). Results showed reduced MA-induced AHR in the preventative model, whereas this effect was absent in Cre-negative mice (SMMHC110/0/BDNFfl/fl). Together, these novel findings demonstrate that BDNF/TrkB signaling contributes to AHR. In this regard, ASM-derived BDNF is important.
Initially recognized in the nervous system, several NTs, including BDNF, and their receptors (including TrkB) have been localized to non-neuronal tissues, including the lung (17, 20–22, 24, 33, 42). Although it has been less clear what the sources vs. targets of BDNF are in the lung [with intracellular BDNF not necessarily reflecting extracellular active levels, given that NTs are secreted in a proform that need to be extracellularly cleaved (42, 43)], our previous studies (33, 44) have demonstrated ASM-derived levels of BDNF to be comparable to circulating and brain levels (45–47), highlighting the role of ASM as a source of BDNF. Here, given its functional TrkB receptor, ASM also becomes a target.
The relevance of BDNF in the airway lies in evidence that exogenous BDNF significantly increases airway contractility and ASM proliferation (21, 31, 35, 48). BDNF has been found to be elevated in the sputum and/or BAL fluid from patients with allergic rhinitis, cough, and asthma (24, 26–28, 49–51). Elevated BDNF in sputum from patients with asthma has also been linked to airway epithelium secretions and asthma severity (24).
[Ca2+]i regulation is central to modulation of ASM contraction/relaxation and to ASM hypercontractility observed in patients with asthma (52). In addition to several inflammatory cytokines that are known to enhance baseline levels and [Ca2+]i responses to bronchoconstrictor agonists, BDNF has emerged as a key regulator of contractility in human ASM (30, 35). Exposure to exogenous BDNF, acutely (15–30 min) and chronically (24–48 h), enhances human ASM [Ca2+]i response to bronchoconstrictors, acting via several [Ca2+]i regulatory mechanisms, such as store operated Ca2+ entry and sarcoplasmic reticulum calcium release (20, 48). These effects on [Ca2+]i in human ASM cells are corroborated by contractile studies in human bronchial strips showing increased force in response to BDNF (20). The effects of BDNF on ASM [Ca2+]i regulation are mediated through TrkB and are more pronounced than other neurotrophins, such as NT3 and NT4 (20, 48). BDNF enhances [Ca2+]i regulation by stimulating IP3 release and increasing expression of [Ca2+]i regulatory proteins, such as IP3R, Orai1, and Stim1 (30, 53). Our previous studies have highlighted how BDNF can enhance [Ca2+]i and contractility. Consistent with these in vitro results, our present study demonstrating a blunting of AHR in vivo (induced by MA treatment) in the absence of TrkB signaling (TrkBKI mice) underlines the functional efficacy of BDNF in the airway. Here, our results showing that TrkBKI has minimal influences in non-MA mice but influences MA mice suggest that inflammation per se promotes BDNF/TrkB signaling. Indeed, we have previously shown that proinflammatory cytokines, such as TNF-α (34), and oxidant stress (35) can promote ASM BDNF as well as TrkB expression, thus sensitizing the airway to BDNF effects.
Expression of [Ca2+]i regulatory proteins, such as those for SR Ca2+ release and Ca2+ influx, are known to be up-regulated in ASM of patients with asthma or with inflammatory mediators (52). In the present study, using LCM, we examined mRNA expression of several [Ca2+]i regulatory proteins in the ASM layer. IP3R, Orai1, and M3ACHR mRNA expression was increased in MA-challenged mice. Given our previous data on the ASM mechanisms by which BDNF could increase contractility, airway BDNF in MA mice could thus have greater effects due to increased expression of Ca2+ regulatory pathways. In MA-challenged mice administered 1NMPP1, expression of these regulatory pathways was not suppressed, suggesting that BDNF/TrkB itself does not have an effect in enhancing their expression. This aspect of our results is in contrast to previous data in human ASM, where we found that prolonged BDNF exposure in fact increases expression of multiple Ca2+ regulatory proteins. The reasons for this discrepancy are not clear and need to be resolved in models with BDNF overexpression particularly within the ASM.
Loss of BDNF signaling and smooth muscle expression had no effect on MA-induced airway inflammation. Lung expression levels of Th2 cytokines, IL-4, IL-5, and IL-13 was increased in MA-exposed TrkBKI and SMMHC110/Cre/BDNFfl/fl mice treated with 1NMPP1 and 4OHTM, respectively. Similarly, histologic sections (H&E and PAS staining) and differential BAL cell counts show that airway inflammation remains largely unchanged. However, 1NMPP1 treatment for 4 wk reduced BAL eosinophil counts in MA-challenged mice. In addition to ASM, 1NMPP1 could potentially affect eosinophils. There is evidence that eosinophils express TrkB and that BDNF regulates allergic responses in the skin of atopic subjects by affecting eosinophil apoptosis and chemotaxis (54, 55). However, it is unclear how BDNF affects eosinophils that infiltrate the lung during allergic airway inflammation. Furthermore, any potential effect on eosinophils is mitigated by the significant inflammation and cytokine expression that remains in 1NMPP1-treated, MA-challenged mice. Thus, given the substantial reduction in AHR in the TrkBKI and smooth muscle BDNF knockout mice, it is likely that “local” influences of BDNF at the level of resident airway cells, such as ASM, are more important in modulating AHR.
We also assessed the effect of 1NMPP1 on airway remodeling in MA-challenged TrkBKI mice. Using LCM, we measured mRNA expression of ECM proteins in the ASM layer and found that, although mRNA expression of collagen I and III was not altered by MA or 1NMPP1, treatment with 1NMPP1 blunted MA-induced increases in fibronectin. Although the data on fibronectin are consistent with our findings in human ASM cells showing an enhancing effect of BDNF (32), we had previously found that BDNF also increases collagens in human ASM. There are several explanations for the differences between human ASM and our mouse data. However, it is likely that, unlike in the ASM cell model where only a single cell type is present, and BDNF, which may or may not influence other ASM-derived factors, MA in vivo may enhance other factors (including cytokines as suggested by BAL data). Furthermore, disruption of TrkB or even smooth muscle BDNF may not be sufficient to overcome the influence of these other cytokines or growth factors on airway remodeling aspects. We have previously noted increased expression of IL-13, which is known to enhance remodeling in ASM and airway epithelial cells (56). Additionally, expression of growth factors, such as PDGF and/or TGF-β, could also be elevated and promote airway remodeling mechanisms. Furthermore, the role of BDNF in remodeling could intrinsically differ between mice and humans. Nonetheless, our findings suggest that BDNF has an important role in airway remodeling.
Understanding of the role of BDNF in the lung has emerged in recent years, yet its role in airway disease pathogenesis is lacking. Our study used 2 transgenic approaches to examine the role of BDNF in allergic airway inflammation. We found that inhibition of TrkB kinase activity and conditional deletion of BDNF from smooth muscle cells blunted AHR and improved compliance induced by MA challenge. These effects were observed while airway inflammation remained elevated, suggesting that BDNF has a central role in modulating AHR but not the inflammatory response induced by allergen exposure. Exploration into the role of BDNF in other cells types, such as the airway epithelium (24) and nerves (57), will be important to further understand how BDNF can affect asthma pathogenesis. As such, these 2 transgenic mouse models and more complex cell-type specific models will be very useful for elucidating the effects of ASM-derived BDNF on these other cell types. Here, it is interesting to note the increased mucin staining in WT MA mice. The lack of effect of 1NMPP1 on epithelial mucin in the smooth muscle-BDNF knockout per se suggests that, even with suppressed ASM-derived BDNF, the epithelium responds to MA, suggesting the potential validity of this allergic inflammation model to explore epithelial changes. Whether epithelium-derived BDNF plays an autocrine role will need to be determined in epithelium-specific knockout mice. It will also be necessary to determine whether epithelial TrkB expression and functionality are altered with MA.
In the present study, we explored 12- to 16-wk-old mice in the context of BDNF effects and MA. “Classical” models of allergic asthma, such as the OVA model, use 6-wk-old mice that are in fact representative of human adolescence (58). We planned our studies to reflect an adult onset model of asthma and thus chose mice that were 3–4 mo old. This age group was not chosen to explore aging per se, although it would be interesting to explore aging in the context of BDNF and the aging lung. Nonetheless, the importance of our studies using mice from the 12–16 wk age group lies in establishing that BDNF/TrkB signaling is maintained in the adult bronchial airways and contributes to airway reactivity. Previous studies, including our own, have shown that BDNF/TrkB signaling occurs early in life and could contribute to neonatal/pediatric airway disease (36, 59, 60). The present study establishes the role of ASM BDNF in the adult domain.
ACKNOWLEDGMENTS
The authors thank Jacob Teske (Mayo Clinic) for technical assistance in the smooth muscle and airway thickness analyses. This work was supported by U.S. National Institutes of Health, National Heart, Lung, and Blood Institute Grants HL088029 and HL56470 (to Y.S.P.), K99 HL131682 (to R.D.B., Jr.), and T32 HL105355 (to S.A.W.). The authors declare no conflicts of interest.
Glossary
- 4OHTM
4-hydroxytamoxifen
- AHR
airway hyperresponsiveness
- ASM
airway smooth muscle
- BAL
broncholaveolar lavage
- BDNF
brain-derived neurotrophic factor
- Cre
Cre recombinase genotype
- ECM
extracellular matrix
- H&E
hematoxylin and eosin
- MA
mixed allergen
- NT
neurotrophin
- PAS
Periodic-acid Schiff
- SMMHC
smooth muscle myosin heavy chain
- Th
T helper
- TrkB
tropomyosin-related kinase B
- TrkBKI
TrkB knockin
- WT
wild type
AUTHOR CONTRIBUTIONS
R. D. Britt, Jr., M. A. Thompson, J. D. Miller, C. M. Pabelick, and Y. S. Prakash designed the research; R. D. Britt, Jr., M. A. Thompson, C. Roos, and L. Smith performed the mouse breeding for transgenic lines; R. D. Britt, Jr., M. A. Thompson, S. A. Wicher, L. J. Manlove, A. Roesler, Y.-H. Fang, C. Roos, and L. Smith performed the research; R. D. Britt, Jr., M. A. Thompson, S. A. Wicher, L. J. Manlove, and A. Roesler analyzed the data; and R. D. Britt, Jr., M. A. Thompson, and Y. S. Prakash wrote the manuscript.
REFERENCES
- 1.Holgate, S. T. (2008) Pathogenesis of asthma. Clin. Exp. Allergy 38, 872–897 [DOI] [PubMed] [Google Scholar]
- 2.Rydell-Törmänen, K., Risse, P. A., Kanabar, V., Bagchi, R., Czubryt, M. P., Johnson, J. R. (2013) Smooth muscle in tissue remodeling and hyper-reactivity: airways and arteries. Pulm. Pharmacol. Ther. 26, 13–23 [DOI] [PubMed] [Google Scholar]
- 3.Royce, S. G., Cheng, V., Samuel, C. S., Tang, M. L. (2012) The regulation of fibrosis in airway remodeling in asthma. Mol. Cell. Endocrinol. 351, 167–175 [DOI] [PubMed] [Google Scholar]
- 4.Prakash, Y. S. (2013) Airway smooth muscle in airway reactivity and remodeling: what have we learned? Am. J. Physiol. Lung Cell. Mol. Physiol. 305, L912–L933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess, J. K., Ceresa, C., Johnson, S. R., Kanabar, V., Moir, L. M., Nguyen, T. T., Oliver, B. G., Schuliga, M., Ward, J. (2009) Tissue and matrix influences on airway smooth muscle function. Pulm. Pharmacol. Ther. 22, 379–387 [DOI] [PubMed] [Google Scholar]
- 6.Hirota, N., Martin, J. G. (2013) Mechanisms of airway remodeling. Chest 144, 1026–1032 [DOI] [PubMed] [Google Scholar]
- 7.Churg, A., Zhou, S., Wright, J. L. (2012) Series “matrix metalloproteinases in lung health and disease”: matrix metalloproteinases in COPD. Eur. Respir. J. 39, 197–209 [DOI] [PubMed] [Google Scholar]
- 8.Lagente, V., Boichot, E. (2010) Role of matrix metalloproteinases in the inflammatory process of respiratory diseases. J. Mol. Cell. Cardiol. 48, 440–444 [DOI] [PubMed] [Google Scholar]
- 9.Halwani, R., Al-Muhsen, S., Al-Jahdali, H., Hamid, Q. (2011) Role of transforming growth factor-β in airway remodeling in asthma. Am. J. Respir. Cell Mol. Biol. 44, 127–133 [DOI] [PubMed] [Google Scholar]
- 10.Al-Muhsen, S., Johnson, J. R., Hamid, Q. (2011) Remodeling in asthma. J. Allergy Clin. Immunol. 128, 451–462; quiz 463–454 [DOI] [PubMed] [Google Scholar]
- 11.Hamid, Q., Tulic, M. (2009) Immunobiology of asthma. Annu. Rev. Physiol. 71, 489–507 [DOI] [PubMed] [Google Scholar]
- 12.Dekkers, B. G., Maarsingh, H., Meurs, H., Gosens, R. (2009) Airway structural components drive airway smooth muscle remodeling in asthma. Proc. Am. Thorac. Soc. 6, 683–692 [DOI] [PubMed] [Google Scholar]
- 13.Davies, D. E. (2009) The role of the epithelium in airway remodeling in asthma. Proc. Am. Thorac. Soc. 6, 678–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elshaw, S. R., Henderson, N., Knox, A. J., Watson, S. A., Buttle, D. J., Johnson, S. R. (2004) Matrix metalloproteinase expression and activity in human airway smooth muscle cells. Br. J. Pharmacol. 142, 1318–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prakash, Y. S., Martin, R. J. (2014) Brain-derived neurotrophic factor in the airways. Pharmacol. Ther. 143, 74–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skaper, S. D. (2008) The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol. Disord. Drug Targets 7, 46–62 [DOI] [PubMed] [Google Scholar]
- 17.Reichardt, L. F. (2006) Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao, M. V., Rajagopal, R., Lee, F. S. (2006) Neurotrophin signalling in health and disease. Clin. Sci. (Lond.) 110, 167–173 [DOI] [PubMed] [Google Scholar]
- 19.Hempstead, B. L. (2015) Brain-derived neurotrophic factor: three ligands, many actions. Trans. Am. Clin. Climatol. Assoc. 126, 9–19 [PMC free article] [PubMed] [Google Scholar]
- 20.Prakash, Y. S., Iyanoye, A., Ay, B., Mantilla, C. B., Pabelick, C. M. (2006) Neurotrophin effects on intracellular Ca2+ and force in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 291, L447–L456 [DOI] [PubMed] [Google Scholar]
- 21.Prakash, Y., Thompson, M. A., Meuchel, L., Pabelick, C. M., Mantilla, C. B., Zaidi, S., Martin, R. J. (2010) Neurotrophins in lung health and disease. Expert Rev. Respir. Med. 4, 395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piedimonte, G. (2003) Contribution of neuroimmune mechanisms to airway inflammation and remodeling during and after respiratory syncytial virus infection. Pediatr. Infect. Dis. J. 22, S66–S74; discussion S74–S75 [DOI] [PubMed] [Google Scholar]
- 23.Rochlitzer, S., Nassenstein, C., Braun, A. (2006) The contribution of neurotrophins to the pathogenesis of allergic asthma. Biochem. Soc. Trans. 34, 594–599 [DOI] [PubMed] [Google Scholar]
- 24.Watanabe, T., Fajt, M. L., Trudeau, J. B., Voraphani, N., Hu, H., Zhou, X., Holguin, F., Wenzel, S. E. (2015) Brain-derived neurotrophic factor expression in asthma. Association with severity and type 2 inflammatory processes. Am. J. Respir. Cell Mol. Biol. 53, 844–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao, Q., Haxhiu, M. A., Zaidi, S. I., Liu, S., Jafri, A., Martin, R. J. (2005) Hyperoxia enhances brain-derived neurotrophic factor and tyrosine kinase B receptor expression in peribronchial smooth muscle of neonatal rats. Am. J. Physiol. Lung Cell. Mol. Physiol. 289, L307–L314 [DOI] [PubMed] [Google Scholar]
- 26.Braun, A., Lommatzsch, M., Mannsfeldt, A., Neuhaus-Steinmetz, U., Fischer, A., Schnoy, N., Lewin, G. R., Renz, H. (1999) Cellular sources of enhanced brain-derived neurotrophic factor production in a mouse model of allergic inflammation. Am. J. Respir. Cell Mol. Biol. 21, 537–546 [DOI] [PubMed] [Google Scholar]
- 27.Braun, A., Lommatzsch, M., Renz, H. (2000) The role of neurotrophins in allergic bronchial asthma. Clin. Exp. Allergy 30, 178–186 [DOI] [PubMed] [Google Scholar]
- 28.Hoyle, G. W. (2003) Neurotrophins and lung disease. Cytokine Growth Factor Rev. 14, 551–558 [DOI] [PubMed] [Google Scholar]
- 29.Virchow, J. C., Julius, P., Lommatzsch, M., Luttmann, W., Renz, H., Braun, A. (1998) Neurotrophins are increased in bronchoalveolar lavage fluid after segmental allergen provocation. Am. J. Respir. Crit. Care Med. 158, 2002–2005 [DOI] [PubMed] [Google Scholar]
- 30.Abcejo, A. J., Sathish, V., Smelter, D. F., Aravamudan, B., Thompson, M. A., Hartman, W. R., Pabelick, C. M., Prakash, Y. S. (2012) Brain-derived neurotrophic factor enhances calcium regulatory mechanisms in human airway smooth muscle. PLoS One 7, e44343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aravamudan, B., Thompson, M., Pabelick, C., Prakash, Y. S. (2012) Brain-derived neurotrophic factor induces proliferation of human airway smooth muscle cells. J. Cell. Mol. Med. 16, 812–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeman, M. R., Sathish, V., Manlove, L., Wang, S., Britt, R. D., Jr., Thompson, M. A., Pabelick, C. M., Prakash, Y. S. (2017) Brain-derived neurotrophic factor and airway fibrosis in asthma. Am. J. Physiol. Lung Cell. Mol. Physiol. 313, L360–L370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vohra, P. K., Thompson, M. A., Sathish, V., Kiel, A., Jerde, C., Pabelick, C. M., Singh, B. B., Prakash, Y. S. (2013) TRPC3 regulates release of brain-derived neurotrophic factor from human airway smooth muscle. Biochim. Biophys. Acta 1833, 2953–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aravamudan, B., Thompson, M. A., Pabelick, C. M., Prakash, Y. S. (2016) Mechanisms of BDNF regulation in asthmatic airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L270–L279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sathish, V., Vanoosten, S. K., Miller, B. S., Aravamudan, B., Thompson, M. A., Pabelick, C. M., Vassallo, R., Prakash, Y. S. (2013) Brain-derived neurotrophic factor in cigarette smoke-induced airway hyperreactivity. Am. J. Respir. Cell Mol. Biol. 48, 431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson, M. A., Britt, R. D., Jr., Kuipers, I., Stewart, A., Thu, J., Pandya, H. C., MacFarlane, P., Pabelick, C. M., Martin, R. J., Prakash, Y. S. (2015) cAMP-mediated secretion of brain-derived neurotrophic factor in developing airway smooth muscle. Biochim. Biophys. Acta 1853(10 Pt A), 2506–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen, X., Ye, H., Kuruvilla, R., Ramanan, N., Scangos, K. W., Zhang, C., Johnson, N. M., England, P. M., Shokat, K. M., Ginty, D. D. (2005) A chemical-genetic approach to studying neurotrophin signaling. Neuron 46, 13–21 [DOI] [PubMed] [Google Scholar]
- 38.Iijima, K., Kobayashi, T., Hara, K., Kephart, G. M., Ziegler, S. F., McKenzie, A. N., Kita, H. (2014) IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J. Immunol. 193, 1549–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarova, P. L., Stewart, A. L., Sathish, V., Britt, R. D., Jr., Thompson, M. A., P. Lowe, A. P., Freeman, M., Aravamudan, B., Kita, H., Brennan, S. C., Schepelmann, M., Davies, T., Yung, S., Cholisoh, Z., Kidd, E. J., Ford, W. R., Broadley, K. J., Rietdorf, K., Chang, W., Bin Khayat, M. E., Ward, D. T., Corrigan, C. J., T. Ward, J. P., Kemp, P. J., Pabelick, C. M., Prakash, Y. S., Riccardi, D. (2015) Calcium-sensing receptor antagonists abrogate airway hyperresponsiveness and inflammation in allergic asthma. Sci. Transl. Med. 7, 284ra60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faksh, A., Britt, R. D., Jr., Vogel, E. R., Kuipers, I., Thompson, M. A., Sieck, G. C., Pabelick, C. M., Martin, R. J., Prakash, Y. S. (2016) Effects of antenatal lipopolysaccharide and postnatal hyperoxia on airway reactivity and remodeling in a neonatal mouse model. Pediatr. Res. 79, 391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aravamudan, B., VanOosten, S. K., Meuchel, L. W., Vohra, P., Thompson, M., Sieck, G. C., Prakash, Y. S., Pabelick, C. M. (2012) Caveolin-1 knockout mice exhibit airway hyperreactivity. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L669–L681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang, J., Siao, C. J., Nagappan, G., Marinic, T., Jing, D., McGrath, K., Chen, Z. Y., Mark, W., Tessarollo, L., Lee, F. S., Lu, B., Hempstead, B. L. (2009) Neuronal release of proBDNF. Nat. Neurosci. 12, 113–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Greenberg, M. E., Xu, B., Lu, B., Hempstead, B. L. (2009) New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. 29, 12764–12767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, S. Y., Freeman, M. R., Sathish, V., Thompson, M. A., Pabelick, C. M., Prakash, Y. S. (2016) Sex steroids influence brain-derived neurotropic factor secretion from human airway smooth muscle cells. J. Cell. Physiol. 231, 1586–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song, X. Y., Li, F., Zhang, F. H., Zhong, J. H., Zhou, X. F. (2008) Peripherally-derived BDNF promotes regeneration of ascending sensory neurons after spinal cord injury. PLoS One 3, e1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein, A. B., Williamson, R., Santini, M. A., Clemmensen, C., Ettrup, A., Rios, M., Knudsen, G. M., Aznar, S. (2011) Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int. J. Neuropsychopharmacol. 14, 347–353 [DOI] [PubMed] [Google Scholar]
- 47.Zuccato, C., Marullo, M., Vitali, B., Tarditi, A., Mariotti, C., Valenza, M., Lahiri, N., Wild, E. J., Sassone, J., Ciammola, A., Bachoud-Lèvi, A. C., Tabrizi, S. J., Di Donato, S., Cattaneo, E. (2011) Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS One 6, e22966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prakash, Y. S., Thompson, M. A., Pabelick, C. M. (2009) Brain-derived neurotrophic factor in TNF-alpha modulation of Ca2+ in human airway smooth muscle. Am. J. Respir. Cell Mol. Biol. 41, 603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhuri, R., McMahon, A. D., McSharry, C. P., Macleod, K. J., Fraser, I., Livingston, E., Thomson, N. C. (2005) Serum and sputum neurotrophin levels in chronic persistent cough. Clin. Exp. Allergy 35, 949–953 [DOI] [PubMed] [Google Scholar]
- 50.Nassenstein, C., Kerzel, S., Braun, A. (2004) Neurotrophins and neurotrophin receptors in allergic asthma. Prog. Brain Res. 146, 347–367 [DOI] [PubMed] [Google Scholar]
- 51.Braun, A., Lommatzsch, M., Neuhaus-Steinmetz, U., Quarcoo, D., Glaab, T., McGregor, G. P., Fischer, A., Renz, H. (2004) Brain-derived neurotrophic factor (BDNF) contributes to neuronal dysfunction in a model of allergic airway inflammation. Br. J. Pharmacol. 141, 431–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prakash, Y. S. (2016) Emerging concepts in smooth muscle contributions to airway structure and function: implications for health and disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 311, L1113–L1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amaral, M. D., Pozzo-Miller, L. (2007) TRPC3 channels are necessary for brain-derived neurotrophic factor to activate a nonselective cationic current and to induce dendritic spine formation. J. Neurosci. 27, 5179–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raap, U., Goltz, C., Deneka, N., Bruder, M., Renz, H., Kapp, A., Wedi, B. (2005) Brain-derived neurotrophic factor is increased in atopic dermatitis and modulates eosinophil functions compared with that seen in nonatopic subjects. J. Allergy Clin. Immunol. 115, 1268–1275 [DOI] [PubMed] [Google Scholar]
- 55.Namura, K., Hasegawa, G., Egawa, M., Matsumoto, T., Kobayashi, R., Yano, T., Katoh, N., Kishimoto, S., Ohta, M., Obayashi, H., Ose, H., Fukui, M., Nakamura, N., Yoshikawa, T. (2007) Relationship of serum brain-derived neurotrophic factor level with other markers of disease severity in patients with atopic dermatitis. Clin. Immunol. 122, 181–186 [DOI] [PubMed] [Google Scholar]
- 56.Ingram, J. L., Kraft, M. (2012) IL-13 in asthma and allergic disease: asthma phenotypes and targeted therapies. J. Allergy Clin. Immunol. 130, 829–842; quiz 843–844 [DOI] [PubMed] [Google Scholar]
- 57.Aven, L., Paez-Cortez, J., Achey, R., Krishnan, R., Ram-Mohan, S., Cruikshank, W. W., Fine, A., Ai, X. (2014) An NT4/TrkB-dependent increase in innervation links early-life allergen exposure to persistent airway hyperreactivity. FASEB J. 28, 897–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chapman, D. G., Tully, J. E., Nolin, J. D., Janssen-Heininger, Y. M., Irvin, C. G. (2014) Animal models of allergic airways disease: where are we and where to next? J. Cell. Biochem. 115, 2055–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sopi, R. B., Martin, R. J., Haxhiu, M. A., Dreshaj, I. A., Yao, Q., Jafri, A., Zaidi, S. I. (2008) Role of brain-derived neurotrophic factor in hyperoxia-induced enhancement of contractility and impairment of relaxation in lung parenchyma. Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L348–L355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.García-Suárez, O., Pérez-Pinera, P., Laurà, R., Germana, A., Esteban, I., Cabo, R., Silos-Santiago, I., Cobo, J. L., Vega, J. A. (2009) TrkB is necessary for the normal development of the lung. Respir. Physiol. Neurobiol. 167, 281–291 [DOI] [PubMed] [Google Scholar]