Abstract
Cardiovascular health declines with age, increasing the risk of hypertension and elevated heart rate in middle and old age. Here, we used multivariate techniques to investigate the associations between cardiovascular health (diastolic blood pressure, systolic blood pressure, and heart rate) and white matter macrostructure (lesion volume and number) and microstructure (as measured by diffusion-weighted imaging) in the cross-sectional, population-based Cam-CAN cohort (N = 667, aged 18–88). We found that cardiovascular health and age made approximately similar contributions to white matter health and explained up to 56% of variance therein. Lower diastolic blood pressure, higher systolic blood pressure, and higher heart rate were each strongly, and independently, associated with white matter abnormalities on all indices. Body mass and exercise were associated with white matter health, both directly and indirectly via cardiovascular health. These results highlight the importance of cardiovascular risk factors for white matter health across the adult lifespan and suggest that systolic blood pressure, diastolic blood pressure, and heart rate affect white matter health via separate mechanisms.
Keywords: White matter lesion, Cardiovascular health, Blood pressure, Heart rate, Diffusion weighted imaging, Aging
Highlights
-
•
Cardiovascular health is related to white matter lesion burden and diffusivity.
-
•
Low diastolic pressure, high systolic pressure, and higher heart rate contribute independently.
-
•
Cardiovascular health and age explain up to 56% of variance in white matter health.
-
•
The uncinate fasciculus, inferior fronto-occipital fasciculus, and forceps minor show most sensitivity.
-
•
Lower BMI and more exercise may have protective effects.
1. Introduction
Cardiovascular function changes over the lifespan, with an increased risk of elevated blood pressure and heart rate in middle and old age and a lifetime risk for hypertension of 90% (Vasan et al., 2002). Such declines in cardiovascular health are also major risk factors for clinically silent brain injuries such as white matter lesions (including the white matter hyperintensities evident on magnetic resonance imaging scans; Iadecola and Davisson, 2008, Maniega et al., 2015, Verhaaren et al., 2013). White matter lesions, in turn, are associated with cognitive impairment and the risk of dementia, starting as early as the fifth decade (Knopman et al., 2001, Prins and Scheltens, 2015). This raises the possibility that the high prevalence of cardiovascular ill-health in middle and old age contributes to the decline in cognitive functioning during later life (Kievit et al., 2016, Price et al., 2017, Prins and Scheltens, 2015). Unlike other risk factors of dementia such as variants of the APOE gene (Erikson et al., 2016), hypertension and elevated resting heart rate are preventable or potentially modifiable through medication and through addressing behavioral risk factors such as high body mass and lack of exercise (Gustafson et al., 2004, Ronan et al., 2016, Strömmer et al, 2018, Torres et al., 2015, Xu et al., 2013). At present, however, the relationship between cardiovascular and white matter health remains poorly understood, with most studies relying on a single indicator of white matter and cardiovascular health. To address this issue, we here used multivariate techniques to investigate the association between multiple measures of cardiovascular health and white matter macro- and microstructure.
Most studies of cardiovascular health have investigated either hypertensive status or heart rate but seldom both. There is cross-sectional and longitudinal evidence linking high systolic blood pressure to the development of white matter lesions (van Dijk et al., 2004, Verhaaren et al., 2013). For diastolic blood pressure, the evidence is inconclusive, with some studies finding that higher diastolic blood pressure predicts white matter lesions (Guo et al., 2009), whereas others find that lower diastolic blood pressure is associated with neurological abnormalities (Jochemsen et al., 2013). Elevated heart rate is also associated with cardiovascular problems, independent of blood pressure and physical activity (Fox et al., 2007, Woodward et al., 2012). However, few studies have directly investigated the association between heart rate and neurological disorders (e.g., Yamaguchi et al., 2015), and little is known about the relationship between heart rate and white matter macrostructure.
White matter degeneration is a continuous process, with microstructural changes occurring well before macrostructural changes (Maillard et al., 2013, Maillard et al., 2014). Diffuse changes in white matter microstructure can be assessed using diffusion-weighted imaging (DWI), which yields measures of fractional anisotropy (FA), mean diffusivity (MD), and mean kurtosis (MK). Although none of these measures have a clear one-to-one mapping to histopathological processes (such as myelination or axonal density), they show exquisite sensitivity to white matter microstructure (Jones et al., 2013, Maniega et al., 2015).
Most DWI studies on cardiovascular health have used FA, which measures the directionality of water diffusion in the brain (Jones et al., 2013). These studies have linked hypertension to decreased FA, which likely indicates poor microstructural integrity (Hoptman et al., 2009, Maillard et al., 2012, Pfefferbaum et al., 2000). Notably, these microstructural changes may be particularly pronounced in frontal areas, modifiable through lifestyle factors such as body mass index (BMI) and exercise, and predictive of cognitive aging (Maillard et al., 2012, Raz et al., 2003, Strömmer et al, 2018).
Relatively few studies have investigated the effect of cardiovascular health on MD and MK (Maniega et al., 2015, Shimoji et al., 2014). However, these measures may provide complimentary information to FA. MD is particularly sensitive to vascular-driven white matter changes and correctly identifies up to 95% of white matter lesions, whereby outperforming FA (Maniega et al., 2015). MK, unlike FA and MD, is sensitive to tissue microstructure and diffusion restrictions in regions with crossing or fanning fibers and can therefore aid the assessment of brain-wide local tissue microstructure (Fieremans et al., 2011, Neto Henriques et al., 2015).
This review of the literature highlights that most studies to date have relied on single indicators of both white matter and cardiovascular health and used mainly univariate statistical techniques. This has yielded fragmentary and partly inconsistent results, and hampered translation into clinical practice. To systematically address these issues, we here used structural equation modeling (SEM), a technique ideally suited to the examination of complex, multivariate relationships (Goldberger, 1972, McArdle, 2008). SEM combines the strengths of path modeling (an extension of multiple regression) and latent variable modeling (McArdle, 2008). This method allowed us to assess the relationship between multiple indicators of cardiovascular health (systolic blood pressure, diastolic blood pressure, and heart rate), white matter macrostructure (white matter lesion volume and number) and microstructure (FA, MD and MK in 10 white matter tracts), and lifestyle factors (BMI and exercise) in an adult lifespan sample of 667 participants aged 18–88 years.
We hypothesized that (1) cardiovascular health is associated with white matter lesion burden and microstructure (Guo et al., 2009, Maillard et al., 2012); (2) systolic blood pressure, diastolic blood pressure, and heart rate are each linked to white matter macrostructure and microstructure (Guo et al., 2009, Maillard et al., 2012, van Dijk et al., 2004, Yamaguchi et al., 2015); (3) BMI and exercise are associated with both white matter and cardiovascular health (Gustafson et al., 2004, Torres et al., 2015).
2. Methods
2.1. Sample
A healthy, population-based, adult lifespan sample was collected as part of the Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study, as described in detail elsewhere (Shafto et al., 2014, Taylor et al., 2017). Exclusion criteria included low Mini–Mental State Examination (≤25) score, poor English, poor vision, poor hearing, magnetic resonance imaging (MRI) or magnetoencephalography (MEG) contraindications (e.g., ferromagnetic metallic implants, pacemakers, or recent surgery), self-reported substance abuse, and other current serious health conditions (e.g., major psychiatric conditions). One participant was excluded because their diastolic blood pressure was recorded to be higher than their systolic blood pressure. The final sample consisted of N = 667 cognitively healthy adults (Ncardiovascular measures = 579, Nwhite matter microstructure = 646, Nwhite matter macrostructure = 272, 50.52% female). See Table 1 for demographic information. Raw data can be requested from https://camcan-archive.mrc-cbu.cam.ac.uk/dataaccess/, and the covariance matrix and analyses scripts can be downloaded from https://osf.io/zwk5p/.
Table 1.
Demographic information
| Variable | Min | Max | Mean | SD |
|---|---|---|---|---|
| Age (years) | 18 | 88 | 54.64 | 18.53 |
| Mini–Mental State Examination | 25 | 30 | 28.82 | 1.37 |
| Systolic blood pressure | 79.33 | 178.33 | 120.51 | 16.69 |
| Diastolic blood pressure | 49 | 118.67 | 73.00 | 10.27 |
| Heart rate | 39 | 107.67 | 65.73 | 10.42 |
| Lesion number | 0 | 34 | 3.77 | 4.94 |
| Lesion volume (mL) | 0 | 49.83 | 2.15 | 5.52 |
| Variable | Percentage of participants |
|---|---|
| Known hypertension | 19.34 |
| Antihypertensive medication | 15.59 |
| Diabetes | 4.35 |
| Stroke | 0.75 |
| Myocardial infarct | 0.90 |
| Hypercholesterolemia | 13.79 |
| Other cardiovascular disease | 7.65 |
2.2. Cardiovascular health
Heart rate and systolic and diastolic blood pressure were measured using the A&D Medical Digital Blood Pressure Monitor (UA-774), an automated sphygmomanometer. Measurements were carried out while participants were seated, and the arm resting comfortably. Blood pressure and heart rate are known to undergo fluctuation in response to stress and movement (Parati, 2005). We addressed this issue in 2 ways: First, to decrease the likelihood of confounding by recent movement, measurements were taken no sooner than 10 minutes after participants had been seated and repeated 3 times in succession with an approximately 1-minute gap between measures. Second, latent variable scores were estimated from each of these measurements (see Section 2.6.). Estimating latent variables from each measurement score can reduce measurement error and estimation bias and increase precision, compared with averaging over measurement occasions (Little et al., 2002, Little et al., 1999).
2.3. White matter lesion burden
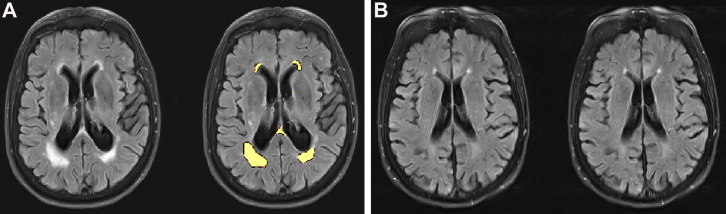
White matter lesion volume and number were estimated automatically using the lesion growth algorithm provided by the LST toolbox for SPM (Schmidt et al., 2012): www.statistical-modelling.de/lst.html (Fig. 1). This algorithm segments T2 hyperintense lesions using T1 and FLAIR images (see Supplementary Material for details of MRI data acquisition). FLAIR images were collected as part of in-depth neurocognitive testing of a subsample of Cam-CAN participants. Therefore, lesion burden was estimated for 272 participants only (Shafto et al., 2014). LST is a reliable, open-source, toolbox that demonstrates good agreement with manual tracing methods and very good specificity (García-Lorenzo et al., 2013, Schmidt et al., 2012). The algorithm also meets general standards for automated lesion detection such as the use of both multimodal and spatial information (García-Lorenzo et al., 2013). The algorithm requires user specification of a threshold for transformed intensities κ. As recommended by Schmidt et al. (2012), the threshold intensity parameter κ = 0.7 was chosen by visually inspecting lesion maps for different κ in 4 participants before modeling the data. We ran supplementary analyses with other thresholds κ to assess whether this choice impacted our results. We note that although the direction of effects and effect sizes for lesion volume were consistent across different κ, the effect sizes for lesion number were lower for κ = 0.1 and κ = 0.3 (Supplementary Table 1).
Fig. 1.
Estimating lesion burden. Panel A shows a participant with an estimated lesion number of 11 and volume of 29.16 mL. Panel B shows an age- and sex-matched control participant with a lesion number of 6 and volume of 0.72 mL. The left image in each panel shows T1-weighted images only, and the right image shows T1-weighted images with lesion maps overlaid in yellow. Images were obtained using the LST toolbox for SPM (Schmidt et al., 2012).
2.4. White matter microstructure
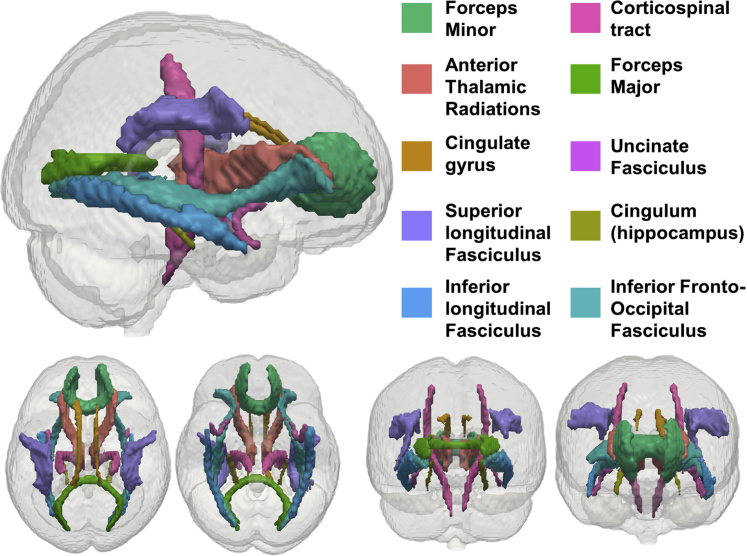
To assess the relationship between cardiovascular health and white matter microstructure, we modeled mean FA, MD, and MK for 10 tracts of the Johns Hopkins University (JHU) white matter tractography atlas averaged over the hemispheres (Fig. 2). See Supplementary Methods for details of MRI data acquisition and preprocessing.
Fig. 2.
JHU white matter tracts modeled in our analysis.
Adapted from Kievit et al. (2016).
2.5. Protective factors: exercise and BMI
We modeled exercise and BMI as potential protective factors for cardiovascular and white matter health in an exploratory analysis. We assessed exercise using the European Physical Activity Questionnaire (Wareham et al., 2002). Four measures of physical activity energy expenditure in kJ/d/kg were calculated from self-reported physical activities at home, during leisure, at work, and during the commute. Both paid employment and regular volunteering in the last 12 months were classified as work. BMI was calculated as weight (kg)/height (m)2. Height and weight were measured using portable scales (Seca 875).
2.6. Structural equation modeling
We modeled the relationship between cardiovascular health and white matter using Confirmatory Factor Analysis and SEM in R's (R core team, 2015) lavaan package (Rosseel, 2012). All models were fitted using maximum likelihood estimation with robust (Huber-White) standard errors and a scaled test statistic. The residual variance of all observed variables was freely estimated. All available data were used. Missing data were estimated using the full information maximum likelihood method for all models. This method yields unbiased estimates when data are missing at random or missing completely at random, and as such is preferable to alternative techniques such as complete-case analyses or imputation methods (Enders and Bandalos, 2001). Model fit was inspected using the χ2 test, the Root Mean Square Error of Approximation (RMSEA) and its confidence interval, the Comparative Fit Index (CFI), and the Standardized Root Mean Square Residual (SRMR). We report the scaled test statistics. Good fit was defined as approximately RMSEA < 0.05, CFI > 0.97, and SRMR < 0.05, acceptable fit as approximately RMSEA = 0.05–0.08, CFI = 0.95–0.97, and SRMR = 0.05–0.10 (Schermelleh-Engel et al., 2003). Nested models were compared using a χ2 test. Effect sizes were evaluated by inspecting R2 for cardiovascular health and age overall and standardized parameter estimates for the individual effects of blood pressure and heart rate. Absolute estimates above 0.10 were defined as small effects, 0.20 as typical and 0.30 as large (Gignac and Szodorai, 2016).
3. Results
3.1. Measurement models
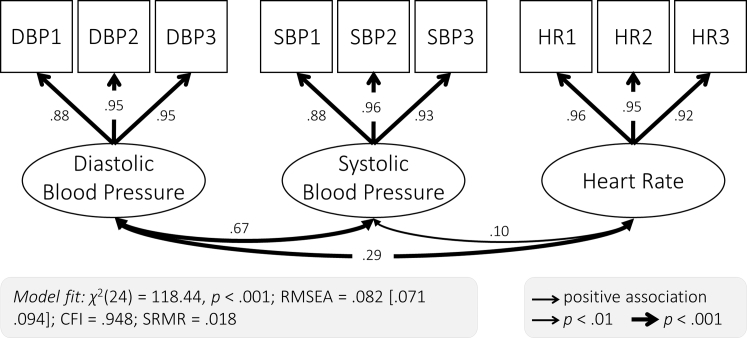
To establish the relationship between cardiovascular and white matter health, we first used Confirmatory Factor Analysis to specify a measurement model for our cardiovascular health measures (Fig. 3). Estimating latent variables has 2 benefits: first, it reduces measurement error in estimates of cardiovascular health (Little et al., 1999), and second, it allows for examining whether cardiovascular health is best represented as a single factor, which is theoretically plausible, or multiple, partially independent factors (here systolic blood pressure, diastolic blood pressure, and heart rate). We first fit a single-factor model, which represents cardiovascular health as a single latent dimension. This model did not fit well χ2(27) = 984.28, p < 0.001; RMSEA = 0.247 [0.240 0.255]; CFI = 0.474; SRMR = 0.218. We found that a three-factor model with heart rate and diastolic and systolic blood pressure as separate factors showed adequate fit (Fig. 3; Supplementary Table 2) and fit better than the single-factor model (Supplementary Table 3; Δχ2(3) = 181.71, p < 0.001). We therefore used the three-factor measurement model in all subsequent analyses. The first measurement for both diastolic and systolic blood pressure showed a lower factor loading than the latter 2 measurements. This is consistent with the notion that the first measurement was less reliable, potentially due to movement or adaptation to the testing setting, and highlights the value of latent variables for reducing measurement error.
Fig. 3.
Three-factor measurement model of cardiovascular health. Each of the 3 cardiovascular health factors was extracted from 3 measurements (diastolic blood pressure: DBP1, DBP2, DBP3; systolic blood pressure: SBP1, SBP2, SBP3; and heart rate: HR1, HR2, HR3). Standardized parameter estimates are shown.
We also assessed fit of a single-factor measurement model of white matter microstructure, by estimating a single latent variable from white matter microstructure in 10 white matter tracts. We found that this single-factor model did not fit well for FA (χ2(35) = 418.66, p < 0.001; RMSEA = 0.130 [0.120–0.140]; CFI = 0.879; SRMR = 0.062), MD (χ2(35) = 1825.69, p < 0.001; RMSEA = 0.281 [0.271–0.292]; CFI = 0.766; SRMR = 0.090), or MK (χ2(35) = 283.51, p < 0.001; RMSEA = 0.105 [0.097–0.112]; CFI = 0.840; SRMR = 0.054). This indicates that white matter microstructure cannot be adequately captured by a single factor in our cohort. We therefore modeled each of the 10 white matter tracts as a separate indicator.
We were not able to assess model fit of a single-factor model of white matter lesion burden. There were only 2 indicators of lesion burden (total lesion volume and number), making a single-factor model just identified. Just-identified models do not yield meaningful fit statistics. We therefore modeled each of the lesion burden measures as separate indicators in all subsequent analyses.
3.2. White matter lesion burden
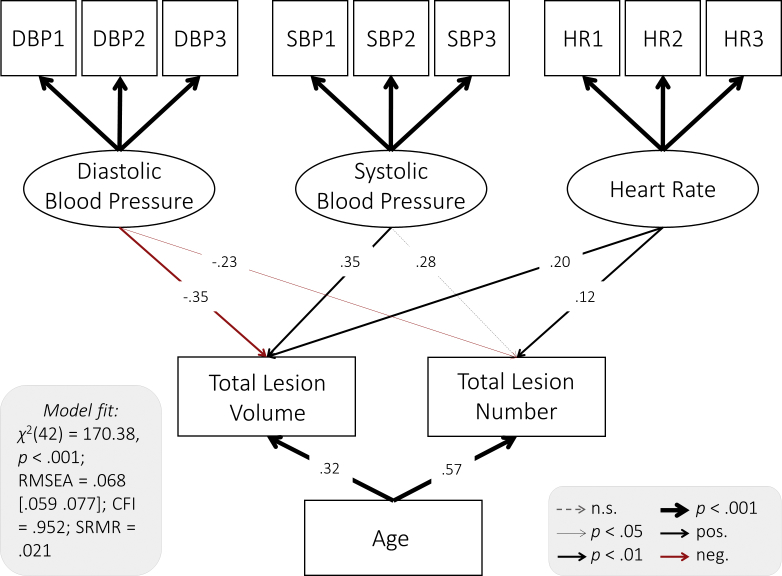
We examined the relationship between cardiovascular health and white matter lesion burden using structural equation models in which diastolic blood pressure, systolic blood pressure, and heart rate were modeled as predictors for total lesion volume and number. To account for potential confounding with age, this variable was additionally included as a covariate. The full model showed good fit (Fig. 4). All regression paths, apart from the relationship between systolic blood pressure and lesion number, were significant (Fig. 4), indicating that each cardiovascular measure made partially independent contributions to white matter lesion burden. Lower diastolic blood pressure, higher systolic blood pressure, and heart rate each predicted greater total lesion volume and a higher number of lesions above and beyond age (Fig. 4, Supplementary Table 1).
Fig. 4.
Path model of the relationship between cardiovascular health white matter lesion burden. Diastolic blood pressure, systolic blood pressure, and heart rate were modeled as latent variables (Fig. 3). Age and total lesion volume and number were modeled as manifest variables. Standardized parameter estimates are shown. Residual covariances between cardiovascular factors, lesion burden measures, and age were allowed but are not shown for simplicity.
Next, we examined whether diastolic blood pressure, systolic blood pressure, and heart rate each showed a specific link to white matter health, by comparing the freely estimated model shown in Fig. 4 with a model in which the parameter estimates for paths between cardiovascular health and each of the lesion burden measures are constrained to be equal (e.g., paths between lesion number and systolic blood pressure, diastolic blood pressure, and heart rate). We found that the constrained model fit worse than the freely estimated model (Δχ2(4) = 31.49, p < 0.001). This indicates that diastolic blood pressure, systolic blood pressure, and heart rate differed in their relationship to white matter lesion burden, with diastolic and systolic blood pressure showing greater effects than heart rate (for standardized parameter estimates, see Fig. 4).
We took the same approach to test whether lesion volume and lesion number showed different sensitivity to cardiovascular health. We found that a model in which parameter estimates for paths from each cardiovascular health measure to total lesion volume and number (e.g., paths between diastolic blood pressure and lesion volume and number) were constrained to be equal did not differ significantly in fit from a model in which these parameters were freely estimated (Δχ2(3) = 2.40, p = 0.494). This indicates that lesion volume and number showed a similar sensitivity to cardiovascular health. The total effect size of cardiovascular health and age on white matter lesion burden was considerable, with an R2 = 0.51 for lesion number and R2 = 0.30 for volume.
To better understand the negative association between diastolic blood pressure and lesion burden, we reran the model and included diastolic blood pressure as the only exogenous variable. We found that the association between diastolic blood pressure and total lesion volume and number became nonsignificant in this model (Supplementary Table 4). This indicates that the effects of diastolic blood pressure are conditional on other cardiovascular factors and age. This finding is compatible with the notion that pulse pressure, the difference between systolic and diastolic pressure, is a sensitive indicator of cardiovascular health (Kim et al., 2011, Strandberg and Pitkala, 2003). Exploratory models including pulse pressure and either systolic or diastolic blood pressure showed that higher pulse pressure was related to higher lesion burden overall (standardized coefficients ranging from 0.20 to 0.39, Supplementary Tables 5 and 6). Both systolic and diastolic blood pressure remained significant predictors of lesion burden over and above pulse pressure for lesion volume but not number (Supplementary Tables 5 and 6). This indicates that although pulse pressure is a sensitive indicator, absolute diastolic and systolic blood pressure can provide additional information about white matter health.
3.3. White matter microstructure
Next, we examined the link between cardiovascular health and nonclinical metrics of white matter microstructure. Specifically, we tested the relationship between cardiovascular health and FA, MD, and MK in 3 separate models. For each of these models, diastolic blood pressure, systolic blood pressure, and heart rate were modeled as simultaneous exogenous variables. Age was included as a covariate. We found largely converging results across FA, MD, and MK. For all models, blood pressure and heart rate made partially independent contributions to white matter microstructure. Consistently, lower diastolic blood pressure, higher systolic blood pressure, and higher heart rate were each associated with lower FA, higher MD, and lower MK (Fig. 5, Table 2, Supplementary Figs 1 and 2). Of our 3 measures of white matter microstructure, MD was generally most sensitive to cardiovascular health (Supplementary Table 7). Cardiovascular health and age explained up to 56% of the variance in MD (Supplementary Table 7), with absolute standardized parameter estimates indicating small to large effect sizes of cardiovascular health above and beyond age (Table 2). Effect sizes were generally larger for blood pressure than heart rate (Table 2). Although DWI metrics are only indirect measures of white matter microstructure (Jones et al., 2013), the associations reported here are compatible with an interpretation of adverse associations between cardiovascular ill health and white matter microstructure (Falangola et al., 2008, Jones et al., 2013, Madden et al., 2012).
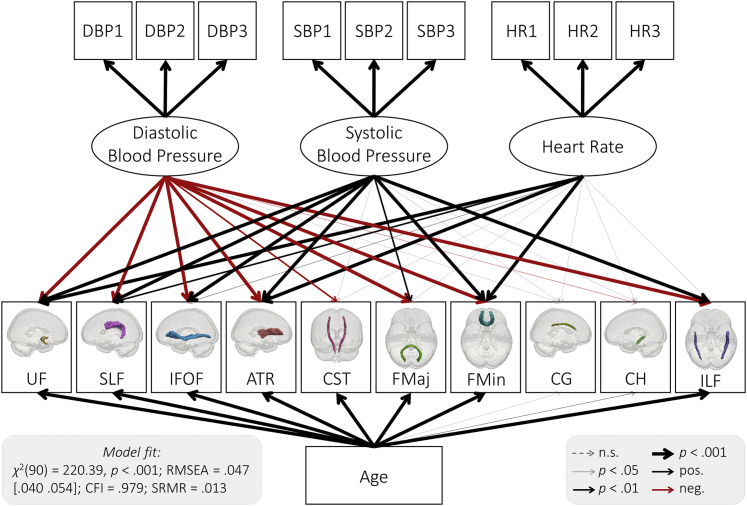
Fig. 5.
Path model of the relationship between cardiovascular health and MD. Diastolic blood pressure, systolic blood pressure, and heart rate were modeled as latent variables (Fig. 3). Age and MD in 10 white matter tracts were modeled as manifest variables. See Supplementary Table 9 for parameter estimates. Residual covariances between cardiovascular factors, lesion burden measures, and age were allowed but are not shown for simplicity. Abbreviations: UF, uncinate fasciculus; SLF, superior longitudinal fasciculus; IFOF, inferior fronto-occipital fasciculus; ATR, anterior thalamic radiations; CST, cerebrospinal tract; FMaj, forceps major; FMin, forceps minor; CG, dorsal cingulate gyrus; CH, ventral cingulate gyrus; ILF, inferior longitudinal fasciculus; MD, mean diffusivity.
Table 2.
Standardized parameter estimates for the three tracts most affected for FA, MD, and MK
| DBP | SBP | HR | Age | |
|---|---|---|---|---|
| FA | ||||
| Uncinate fasciculus | 0.19 | −0.26 | −0.15 | −0.23 |
| Anterior thalamic radiation | 0.18 | −0.15 | −0.15 | −0.40 |
| Inferior fronto-occipital fasciculus | 0.14 | −0.20 | −0.09 | −0.46 |
| MD | ||||
| Uncinate fasciculus | −0.36 | 0.38 | 0.17 | 0.38 |
| Superior longitudinal fasciculus | −0.30 | 0.26 | 0.11 | 0.55 |
| Forceps minor | −0.25 | 0.23 | 0.12 | 0.62 |
| MK | ||||
| Uncinate fasciculus | 0.38 | −0.32 | −0.21 | 0.11 |
| Inferior fronto-occipital fasciculus | 0.36 | −0.32 | −0.14 | −0.32 |
| Forceps minor | 0.35 | −0.30 | −0.17 | −0.34 |
See Supplementary Tables 8–10 for parameter estimates for all tracts.
Key: DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
To probe the specificity of the association between cardiovascular health and white matter microstructure, we again compared a series of models with different equality constraints. For all measures of white matter microstructure, the full model fit better than a model in which the parameter estimates of all cardiovascular factors were constrained to be equal (FA: Δχ2(20) = 49.53, p < 0.001; MD: Δχ2(20) = 118.66, p < 0.001; and MK: Δχ2(20) = 53.16, p < 0.001). This highlights that diastolic blood pressure, systolic blood pressure, and heart rate differed in their relationship to white matter microstructure.
In terms of tract specificity, we found that the freely estimated FA and MD (but not MK) models fit better than models in which white matter tracts were constrained to be equally vulnerable (FA: Δχ2(27) = 54.80, p = 0.001; MD: Δχ2(27) = 44.59, p = 0.018; and MK: Δχ2(27) = 30.54, p = 0.290), indicating that for FA and MD, white matter tracts differed in their sensitivity to cardiovascular health. The tracts most affected across our DWI measures were the uncinate fasciculus, inferior fronto-occipital fasciculus, and forceps minor (Table 2).
Similar to lesion burden, the negative association between diastolic blood pressure and white matter microstructure was conditional on the effects of other cardiovascular health factors and age (Supplementary Table 11) and exploratory models including pulse pressure (systolic minus diastolic blood pressure), and either systolic or diastolic blood pressure showed that higher pulse pressure was related to poorer white matter microstructure (standardized estimates ranging from 0.10 to 0.41, Supplementary Tables 12 and 13). However, both systolic and diastolic blood pressure remained significant predictors of white matter microstructure over and above pulse pressure for most tracts (Supplementary Tables 12 and 13).
3.4. Modeling protective factors
In an exploratory analysis, we examined whether 2 potential protective factors for cardiovascular health and white matter health—BMI and self-reported exercise—affected cardiovascular and white matter health. We modeled exercise and BMI in separate models and assessed their direct and indirect (via cardiovascular health) effects on MD, the indicator of white matter health in our analyses, which was most sensitive to cardiovascular health (Supplementary Table 7). For both exercise and BMI, a full model containing both direct and indirect effects on MD fit best (exercise: Δχ2(12) = 47.65, p < 0.001; BMI: Δχ2(3) = 128.67, p < 0.001). This indicates that exercise and BMI were associated with white matter microstructure both directly and indirectly, via cardiovascular health.
3.4.1. Exercise
The full model including exercise showed acceptable fit overall (χ2(117) = 577.52, p < 0.001; RMSEA = 0.077 [0.071 0.083]; CFI = 0.941; SRMR = 0.163). Exercise at home and during leisure showed no clear association with either MD or cardiovascular health (Supplementary Table 14). Exercise during commute showed no effect on MD but was the most significant predictor of cardiovascular health. More exercise during the commute correlated with lower diastolic blood pressure (standardized coefficient = −0.17, p = 0.001), systolic blood pressure (standardized coefficient = −0.20, p < 0.001), and heart rate (standardized coefficient = −0.13, p = 0.001), indicating that more energy spent while commuting was associated with better cardiovascular health overall. Exercise at work showed no clear relationship to cardiovascular health but was the most significant predictor of MD. There was a weak, negative correlation between exercise and MD in 7 tracts with standardized coefficients ranging from −0.04 to −0.12 (Supplementary Table 14). This indicates that more exercise at work was associated with lower MD in these tracts, likely reflecting better white matter health.
3.4.2. Body mass index
The model including BMI showed mediocre fit overall (χ2(99) = 516.44, p < 0.001; RMSEA = 0.080 [0.073 0.086]; CFI = 0.938; SRMR = 0.201). Higher BMI was associated with higher diastolic blood pressure (standardized coefficient = 0.29, p < 0.001), systolic blood pressure (standardized coefficient = 0.23, p < 0.001), and heart rate (standardized coefficient = 0.16, p < 0.001), thus predicting reduced cardiovascular health overall. Higher BMI was weakly correlated with lower MD in 3 tracts (Supplementary Table 15). This apparent negative effect of BMI was likely due to suppression. In a model where BMI alone was regressed onto MD, all but one nonsignificant association became positive. Here, lower BMI was significantly associated with lower MD and therefore increased white matter microstructure, in 4 tracts (forceps minor, superior longitudinal fasciculus, inferior fronto-occipital fasciculus, and anterior thalamic radiation), with standardized coefficients ranging from 0.09 to 0.18 (Supplementary Table 16).
3.5. Testing for potential confounds
Using multigroup models, we carried out a series of supplementary analyses of white matter lesion burden and microstructure to examine whether our results could be explained by possible differences between sexes, participants taking or not taking antihypertensive mediation and with and without known cardiovascular risk factors (diabetes, hypercholesterolemia, etc., see Table 1 for full list). Results were invariant across groups for all of these factors (see Supplementary Analyses for details). Finally, we investigated whether social and lifestyle factors confounded our results by rerunning our model and including covariates for social class, education levels, smoking, and alcohol consumption. The inclusion of these variables did not meaningfully change the directionality or significance level of the effects (see Supplementary Analyses for details).
4. Discussion
Here, we show a link between common clinical measures of cardiovascular health and imaging indices of white matter health, in terms of both macrostructure and microstructure observed using multimodal MRI in a population-based sample of healthy aging adults. Lower diastolic blood pressure, higher systolic blood pressure, and higher heart rate were each strongly and independently associated with poorer white matter health on all indices—over and above the effects of age. The link between cardiovascular and white matter health was robust across genders, in people taking and not taking antihypertensive medication, with and without known cardiovascular risk factors (diabetes, elevated cholesterol levels, etc.), and when controlling for social class, education levels, alcohol consumption, and smoking.
4.1. Systolic hypertension and diastolic hypotension
We found that systolic hypertension predicted poorer white matter macrostructure and microstructure on all indices, in line with previous studies showing bivariate links between high systolic blood pressure and lower FA, higher MD, and greater white matter lesion volume and number (Maillard et al., 2012, van Dijk et al., 2004, Verhaaren et al., 2013). Systolic blood pressure is known to increase more steeply with age than diastolic blood pressure and has been argued to be a better predictor of cardiovascular and neurological outcomes (Strandberg and Pitkala, 2003). However, we found that lower diastolic blood pressure had a similarly detrimental effect as higher systolic blood pressure. This negative correlation was not evident in a single-indicator model including diastolic blood pressure alone, showing that low diastolic blood pressure is likely to be particularly detrimental at moderate to high levels of systolic blood pressure. This conditional effect may capture the difference between systolic and diastolic blood pressure, a measure known as pulse pressure (Kim et al., 2011, Strandberg and Pitkala, 2003). Pulse pressure is often argued to be a particularly good measure of cardiovascular health in older populations because systolic and diastolic blood pressure widen with age (Supplementary Fig. 3), increasing pulse pressure and contributing to the high prevalence of isolated systolic hypertension in older adults (Huang et al., 2004, Strandberg and Pitkala, 2003). Exploratory models showed that pulse pressure was indeed a sensitive indicator of white matter microstructure and macrostructure and showed greater effect sizes than either systolic or diastolic blood pressure on their own. However, both systolic and diastolic blood pressure remained significant predictors of white matter health after controlling for pulse pressure, indicating that they each capture information about cardiovascular health over and above pulse pressure. Pathophysiologically, isolated systolic hypertension in combination with diastolic hypotension may reflect reduced vessel compliance and increased arterial stiffness, one of the hallmarks of vascular aging (Vlachopoulos et al., 2010).
These findings also highlight the importance of assessing and modeling systolic and diastolic blood pressure, rather than just hypertensive status. The use of multivariate models reveals the existence of conditional effects, which can remain undetected when using univariate techniques. This pattern of results is also relevant to clinical practice. Current UK treatment guidelines include only upper limits for blood pressure (NICE, 2016), but our findings indicate that lower limits for diastolic blood pressure, or pulse pressure, may provide crucial complementary information about cardiovascular health and its consequences.
4.2. Elevated heart rate
Elevated heart rate was associated with poorer white matter health, independent of age and blood pressure. Previous research showed that high heart rate predicts cardiovascular problems independent of blood pressure, physical activity, and comorbidities (Cooney et al., 2010, Fox et al., 2007, Woodward et al., 2012). Nighttime heart rate has also been implicated in white matter lesions and stroke (Yamaguchi et al., 2015). Here, we demonstrate that higher heart rate during the day is associated with poorer white matter microstructure and macrostructure, although effect sizes were somewhat smaller for heart rate than they were for blood pressure. The etiology and impact of elevated heart rate remain poorly understood but may be related to sympathetic nervous system hyperactivity and endothelial dysfunction, which, in turn, may put stress of vascular architecture (Palatini, 2011).
4.3. Body mass and exercise
Body mass and exercise were related to white matter health, both directly and indirectly via cardiovascular health. Higher BMI was associated with poorer cardiovascular health and higher MD, indicating reduced white matter integrity, although effect sizes were relatively small. This finding replicates previous studies showing a negative association of BMI and white matter health (Gustafson et al., 2004, Ronan et al., 2016) and indicates that both cardiovascular and white matter health are likely modifiable by controlling BMI. For exercise, exercise at work and exercise during the commute were significant, albeit weak, predictors. Exercise at home and during leisure showed no clear effect. This dissociation may reflect the regularity of exercise: Exercise at work and during the commute is likely more regular than exercise at home and during leisure. It may also reflect factors that are not, or not solely, related to physical activity. The exercise questionnaire used here classed regular volunteering as work (Wareham et al., 2002). Therefore, exercise at work and during commute may reflect social and intellectual engagement and their protective effects—particularly during retirement (James et al., 2011). Future research will have to disentangle these potential mechanisms of cardiovascular and white matter health and include in-depth analyses of physical and mental health, as well as social and cognitive engagement, and their links to cardiovascular and brain health. This will help inform interventions to promote healthy cognitive aging.
4.4. Limitations
Despite strengths of this study, such as the large sample size, multivariate methodology, and a broad set of cardiovascular and neural measures, several methodological limitations should be noted. First, given the inclusion criteria for Cam-CAN imaging (see Taylor et al., 2017), our sample are likely more healthy than the average population. Although this limits generalizability, it suggests that the negative effects of poor cardiovascular health are observed even in healthy, nonclinical samples.
Furthermore, blood pressure and heart rate are known to undergo fluctuation in response to stress and movement (Parati, 2005). Although we measured heart rate and blood pressure 3 times and modeled them as latent variables, which can reduce measurement error and increase precision (Little et al., 1999), ambulatory, 24-h assessment of blood pressure and heart rate may be even more sensitive. This method may be able to pick up additional disease symptoms such as variability in blood pressure or a lack of nocturnal nondipping of heart rate (Fox et al., 2007, Rothwell et al., 2010, Yamaguchi et al., 2015). Nonetheless, our findings indicate that even simple measurements of heart rate and blood pressure, as may be taken during a single health assessment, may have considerable prognostic value.
Similarly, our findings of the effects of exercise depend on limitations of questionnaire measures in accurately assessing levels of exercise (Prince et al., 2008). Future research may want to use direct assessments of physical activity, like accelerometers, which are generally more reliable than self-report measures (Prince et al., 2008). This will help tease apart the effects of exercise from other lifestyle factors such as social and intellectual engagement.
Finally, although previous longitudinal research in humans (Dufouil et al., 2001, Gustafson et al., 2004) and experimental work in animal models (Kaiser et al., 2014, Zuo et al., 2017) suggests causal links between cardiovascular and white matter health, our study was cross-sectional. As such, we cannot establish the temporal order of particular cardiovascular symptoms and changes in white matter micro- and macrostructure. We included age as a covariate in all structural equation models, thus accounting for possible linear age associations. Although nonlinear associations can be estimated using structural equation models, we limit ourselves here to more parsimonious linear associations only. We cannot, however, completely rule out other alternative explanations such as cohort effects. Our findings should therefore ideally be interpreted alongside interventional and longitudinal studies to further establish causal links. Future experimental studies will also need to investigate possible disease pathways, which, at present, are only partially understood (Iadecola and Davisson, 2008).
4.5. Implications for cognitive aging
We found that the uncinate fasciculus, forceps minor, and inferior fronto-occipito fasciculus were the 3 white matter tracts most affected by cardiovascular health. Although we did not explicitly model cognitive function in the present study, we note that these 3 tracts have been consistently linked to cognitive aging in previous research. The uncinate fasciculus is thought to play a major role in the formation of mnemonic associations and episodic memory (von der Heide et al., 2013; but see Henson et al., 2016) and has been implicated in the development of cognitive impairment in dementia (Serra et al., 2012). The forceps minor has been shown to predict processing speed and fluid intelligence in the Cam-CAN cohort (Kievit et al., 2016). The inferior fronto-occipital fasciculus has been linked to linguistic processing (Dick and Tremblay, 2012) and set-shifting (Perry et al., 2009). This pattern of results is therefore consistent with the notion that cardiovascular ill health may contribute to cognitive decline during aging.
5. Conclusion
Our multivariate analyses show that lower diastolic blood pressure, higher systolic blood pressure, and higher heart rate were each strongly associated with poorer white matter macrostructure and microstructure in a large sample of healthy, community-dwelling adults. These adverse effects were largely additive—diastolic blood pressure, systolic blood pressure, and heart rate each made partially independent contributions to white matter health. These findings highlight the value of concurrently assessing multiple indicators for cardiovascular health and indicate that systolic blood pressure, diastolic blood pressure, and heart rate may each affect neurological health via separate disease mechanisms.
Disclosure statement
The authors declare no competing financial interests.
Acknowledgements
The authors would like to thank Carol Brayne, Simon Davis, and Rik Henson for helpful comments on the study design and imaging techniques. DF, DN, JBR, DP, and RAK are supported by the UK Medical Research Council. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) was supported by the Biotechnology and Biological Sciences Research Council (grant number BB/H008217/1). JBR is supported by the Wellcome Trust (grant number 103838). RAK is supported by the Sir Henry Wellcome Trust (grant number 107392/Z/15/Z) and MRC Programme Grant SUAG/014 RG91365. This project has also received funding from the European Union's Horizon 2020 research and innovation programme (grant agreement number 732592).
The Cam-CAN corporate author consists of the project principal personnel: Lorraine K Tyler, Carol Brayne, Edward T Bullmore, Andrew C Calder, Rhodri Cusack, Tim Dalgleish, John Duncan, Richard N Henson, Fiona E Matthews, William D Marslen-Wilson, James B Rowe, and Meredith A Shafto; research associates: Karen Campbell, Teresa Cheung, Simon Davis, Linda Geerligs, Rogier Kievit, Anna McCarrey, Abdur Mustafa, Darren Price, David Samu, Jason R Taylor, Matthias Treder, Kamen Tsvetanov, Janna van Belle, and Nitin Williams; research assistants: Lauren Bates, Tina Emery, Sharon Erzinlioglu, Andrew Gadie, Sofia Gerbase, Stanimira Georgieva, Claire Hanley, Beth Parkin, and David Troy; affiliated personnel: Tibor Auer, Marta Correia, Lu Gao, Emma Green, and Rafael Henriques; research interviewers: Jodie Allen, Gillian Amery, Liana Amunts, Anne Barcroft, Amanda Castle, Cheryl Dias, Jonathan Dowrick, Melissa Fair, Hayley Fisher, Anna Goulding, Adarsh Grewal, Geoff Hale, Andrew Hilton, Frances Johnson, Patricia Johnston, Thea Kavanagh-Williamson, Magdalena Kwasniewska, Alison McMinn, Kim Norman, Jessica Penrose, Fiona Roby, Diane Rowland, John Sargeant, Maggie Squire, Beth Stevens, Aldabra Stoddart, Cheryl Stone, Tracy Thompson, and Ozlem Yazlik; and administrative staff: Dan Barnes, Marie Dixon, Jaya Hillman, Joanne Mitchell, and Laura Villis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neurobiolaging.2018.10.005.
Contributor Information
Delia Fuhrmann, Email: Delia.Fuhrmann@mrc-cbu.cam.ac.uk.
Cam-CAN:
Lorraine K. Tyler, Carol Brayne, Edward T. Bullmore, Andrew C. Calder, Rhodri Cusack, Tim Dalgleish, John Duncan, Richard N. Henson, Fiona E. Matthews, William D. Marslen-Wilson, James B. Rowe, Meredith A. Shafto, Karen Campbell, Teresa Cheung, Simon Davis, Linda Geerligs, Rogier Kievit, Anna McCarrey, Abdur Mustafa, Darren Price, David Samu, Jason R. Taylor, Matthias Treder, Kamen Tsvetanov, Janna van Belle, Nitin Williams, Lauren Bates, Tina Emery, Sharon Erzinlioglu, Andrew Gadie, Sofia Gerbase, Stanimira Georgieva, Claire Hanley, Beth Parkin, David Troy, Tibor Auer, Marta Correia, Lu Gao, Emma Green, Rafael Henriques, Jodie Allen, Gillian Amery, Liana Amunts, Anne Barcroft, Amanda Castle, Cheryl Dias, Jonathan Dowrick, Melissa Fair, Hayley Fisher, Anna Goulding, Adarsh Grewal, Geoff Hale, Andrew Hilton, Frances Johnson, Patricia Johnston, Thea Kavanagh-Williamson, Magdalena Kwasniewska, Alison McMinn, Kim Norman, Jessica Penrose, Fiona Roby, Diane Rowland, John Sargeant, Maggie Squire, Beth Stevens, Aldabra Stoddart, Cheryl Stone, Tracy Thompson, Ozlem Yazlik, Dan Barnes, Marie Dixon, Jaya Hillman, Joanne Mitchell, and Laura Villis
Appendix A. Supplementary data
References
- Cooney M.T., Vartiainen E., Laakitainen T., Juolevi A., Dudina A., Graham I.M. Elevated resting heart rate is an independent risk factor for cardiovascular disease in healthy men and women. Am. Heart J. 2010;159:612–619.e3. doi: 10.1016/j.ahj.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Dick A.S., Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135:3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Dufouil C., de Kersaint–Gilly A., Besancon V., Levy C., Auffray E., Brunnereau L., Alperovitch A., Tzourio C. Longitudinal study of blood pressure and white matter hyperintensities the EVA MRI cohort. Neurology. 2001;56:921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- Enders C.K., Bandalos D.L. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct. Equc. Model. 2001;8:430–457. [Google Scholar]
- Erikson G.A., Bodian D.L., Rueda M., Molparia B., Scott E.R., Scott-Van Zeeland A.A., Topol S.E., Wineinger N.E., Niederhuber J.E., Topol E.J., Torkamani A. Whole-genome sequencing of a healthy aging cohort. Cell. 2016;165:1002–1011. doi: 10.1016/j.cell.2016.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falangola M.F., Jensen J.H., Babb J.S., Hu C., Castellanos F.X., Di Martino A., Ferris S.H., Helpern J.A. Age-related non-Gaussian diffusion patterns in the prefrontal brain. J. Magn. Reson. Imaging. 2008;28:1345–1350. doi: 10.1002/jmri.21604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fieremans E., Jensen J.H., Helpern J.A. White matter characterization with diffusional kurtosis imaging. NeuroImage. 2011;58:177–188. doi: 10.1016/j.neuroimage.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox K., Borer J.S., Camm A.J., Danchin N., Ferrari R., Lopez Sendon J.L., Steg P.G., Tardif J.-C., Tavazzi L., Tendera M. Resting heart rate in cardiovascular disease. J. Am. Coll. Cardiol. 2007;50:823–830. doi: 10.1016/j.jacc.2007.04.079. [DOI] [PubMed] [Google Scholar]
- García-Lorenzo D., Francis S., Narayanan S., Arnold D.L., Collins D.L. Review of automatic segmentation methods of multiple sclerosis white matter lesions on conventional magnetic resonance imaging. Med. Image Anal. 2013;17:1–18. doi: 10.1016/j.media.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Gignac G.E., Szodorai E.T. Effect size guidelines for individual differences researchers. Personal. Individ. Differ. 2016;102:74–78. [Google Scholar]
- Goldberger A.S. Structural equation methods in the social sciences. Econometrica. 1972;40:979–1001. [Google Scholar]
- Guo X., Pantoni L., Simoni M., Bengtsson C., Björkelund C., Lissner L., Gustafson D., Skoog I. Blood pressure components and changes in relation to white matter lesions: a 32-year prospective population study. Hypertension. 2009;54:57–62. doi: 10.1161/HYPERTENSIONAHA.109.129700. [DOI] [PubMed] [Google Scholar]
- Gustafson D.R., Steen B., Skoog I. Body mass index and white matter lesions in elderly women. An 18-year longitudinal study. Int. Psychogeriatr. 2004;16:327–336. doi: 10.1017/s1041610204000353. [DOI] [PubMed] [Google Scholar]
- Henson R.N., Campbell K.L., Davis S.W., Taylor J.R., Emery T., Erzinclioglu S., Kievit R.A. Multiple determinants of lifespan memory differences. Sci. Rep. 2016;6:32527. doi: 10.1038/srep32527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman M.J., Gunning-Dixon F.M., Murphy C.F., Ardekani B.A., Hrabe J., Lim K.O., Etwaroo G.R., Kanellopoulos D., Alexopoulos G.S. Blood pressure and white matter integrity in geriatric depression. J. Affect. Disord. 2009;115:171–176. doi: 10.1016/j.jad.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Wildman R.P., Gu D., Muntner P., Su S., He J. Prevalence of isolated systolic and isolated diastolic hypertension subtypes in China. Am. J. Hypertens. 2004;17:955–962. doi: 10.1016/j.amjhyper.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Iadecola C., Davisson R.L. Hypertension and cerebrovascular dysfunction. Cell Metab. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James B.D., Wilson R.S., Barnes L.L., Bennett D.A. Late-life social activity and cognitive decline in old age. J. Int. Neuropsychol. Soc. 2011;17:998–1005. doi: 10.1017/S1355617711000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochemsen H.M., Muller M., Visseren F.L., Scheltens P., Vincken K.L., Mali W.P., van der Graaf Y., Geerlings M.I., SMART Study Group Blood pressure and progression of brain atrophy: the SMART-MR study. JAMA Neurol. 2013;70:1046–1053. doi: 10.1001/jamaneurol.2013.217. [DOI] [PubMed] [Google Scholar]
- Jones D.K., Knösche T.R., Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. NeuroImage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Weise G., Möller K., Scheibe J., Pösel C., Baasch S., Gawlitza M., Lobsien D., Diederich K., Minnerup J., Kranz A., Boltze J., Wagner D.-C. Spontaneous white matter damage, cognitive decline and neuroinflammation in middle-aged hypertensive rats: an animal model of early-stage cerebral small vessel disease. Acta Neuropathol. Commun. 2014;2:169. doi: 10.1186/s40478-014-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit R.A., Davis S.W., Griffiths J., Correia M.M., CamCAN, Henson R.N. A watershed model of individual differences in fluid intelligence. Neuropsychologia. 2016;91:186–198. doi: 10.1016/j.neuropsychologia.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.K., Lee S.-H., Kim B.J., Ryu W.-S., Yoon B.-W. Age-independent association of pulse pressure with cerebral white matter lesions in asymptomatic elderly individuals. J. Hypertens. 2011;29:325–329. doi: 10.1097/HJH.0b013e3283408ffb. [DOI] [PubMed] [Google Scholar]
- Knopman D., Boland L.L., Mosley T., Howard G., Liao D., Szklo M., McGovern P., Folsom A.R. Cardiovascular risk factors and cognitive decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- Little T.D., Lindenberger U., Nesselroade J.R. On selecting indicators for multivariate measurement and modeling with latent variables: when “good” indicators are bad and “bad” indicators are good. Psychol. Methods. 1999;4:192–211. [Google Scholar]
- Little T.D., Cunningham W.A., Shahar G., Widaman K.F. To parcel or not to parcel: Exploring the question, weighing the merits. Struct. Equ. Model. Multidiscip. J. 2002;9:151–173. [Google Scholar]
- Madden D.J., Bennett I.J., Burzynska A., Potter G.G., Chen N.-K., Song A.W. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim. Biophys. Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P., Seshadri S., Beiser A., Himali J.J., Au R., Fletcher E., Carmichael O., Wolf P.A., DeCarli C. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol. 2012;11:1039–1047. doi: 10.1016/S1474-4422(12)70241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P., Carmichael O., Harvey D., Fletcher E., Reed B., Mungas D., DeCarli C. FLAIR and diffusion MRI signals are independent predictors of white matter hyperintensities. Am. J. Neuroradiol. 2013;34:54–61. doi: 10.3174/ajnr.A3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard P., Fletcher E., Lockhart S.N., Roach A.E., Reed B., Mungas D., DeCarli C., Carmichael O.T. White matter hyperintensities and their penumbra lie along a continuum of injury in the aging brain. Stroke. 2014;45:1721. doi: 10.1161/STROKEAHA.113.004084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniega S.M., Valdés Hernández M.C., Clayden J.D., Royle N.A., Murray C., Morris Z., Aribisala B.S., Gow A.J., Starr J.M., Bastin M.E., Deary I.J., Wardlaw J.M. White matter hyperintensities and normal-appearing white matter integrity in the aging brain. Neurobiol. Aging. 2015;36:909–918. doi: 10.1016/j.neurobiolaging.2014.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J.J. Latent variable modeling of differences and changes with longitudinal data. Annu. Rev. Psychol. 2008;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- Neto Henriques R., Correia M.M., Nunes R.G., Ferreira H.A. Exploring the 3D geometry of the diffusion kurtosis tensor - impact on the development of robust tractography procedures and novel biomarkers. NeuroImage. 2015;111:85–99. doi: 10.1016/j.neuroimage.2015.02.004. [DOI] [PubMed] [Google Scholar]
- NICE . National Institute for Health and Care Execellence; London, UK: 2016. Hypertension in Adults: Diagnosis and Management. https://www.nice.org.uk/guidance/cg127. [PubMed] [Google Scholar]
- Palatini P. Role of elevated heart rate in the development of cardiovascular disease in hypertension. Hypertension. 2011;58:745. doi: 10.1161/HYPERTENSIONAHA.111.173104. [DOI] [PubMed] [Google Scholar]
- Parati G. Blood pressure variability: its measurement and significance in hypertension. J. Hypertens. 2005;23:S19–S25. doi: 10.1097/01.hjh.0000165624.79933.d3. [DOI] [PubMed] [Google Scholar]
- Perry M.E., McDonald C.R., Hagler D.J., Gharapetian L., Kuperman J.M., Koyama A.K., Dale A.M., McEvoy L.K. White matter tracts associated with set-shifting in healthy aging. Neuropsychologia. 2009;47:2835–2842. doi: 10.1016/j.neuropsychologia.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A., Sullivan E.V., Hedehus M., Lim K.O., Adalsteinsson E., Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn. Reson. Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Price D., Tyler L.K., Neto Henriques R., Campbell K.L., Williams N., Treder M.S., Taylor J.R., Henson R.N.A. Age-related delay in visual and auditory evoked responses is mediated by white- and grey-matter differences. Nat. Commun. 2017;8:15671. doi: 10.1038/ncomms15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince S.A., Adamo K.B., Hamel M.E., Hardt J., Gorber S.C., Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int. J. Behav. Nutr. Phys. Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins N.D., Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol. 2015;11:157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- R Foundation for Statistical Computing; Vienna, Austria: 2015. R Core Team, R: A Language and Environment for Statistical Computing. http://www.R-project.org/ [Google Scholar]
- Raz N., Rodrigue K.M., Acker J.D. Hypertension and the brain: vulnerability of the prefrontal regions and executive functions. Behav. Neurosci. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Ronan L., Alexander-Bloch A.F., Wagstyl K., Farooqi S., Brayne C., Tyler L.K., Fletcher P.C. Obesity associated with increased brain age from midlife. Neurobiol. Aging. 2016;47:63–70. doi: 10.1016/j.neurobiolaging.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y. Lavaan: an R package for structural equation modeling. J. Stat. Softw. 2012;48:1–36. [Google Scholar]
- Rothwell P.M., Howard S.C., Dolan E., O’Brien E., Dobson J.E., Dahlof B., Sever P.S., Poulter N.R. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- Schermelleh-Engel K., Moosbrugger H., Müller H. Evaluating the fit of Structural Equation Models: Tests of significance and descriptive goodness-of-fit measures. Methods Psychol. Res. 2003;8:23–74. [Google Scholar]
- Schmidt P., Gaser C., Arsic M., Buck D., Förschler A., Berthele A., Hoshi M., Ilg R., Schmid V.J., Zimmer C., Hemmer B., Mühlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. NeuroImage. 2012;59:3774–3783. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Serra L., Cercignani M., Basile B., Spano B., Perri R., Fadda L., Marra C., Giubilei F., Caltagirone C., Bozzali M. White matter damage along the uncinate fasciculus contributes to cognitive decline in AD and DLB. Curr. Alzheimer Res. 2012;9:326–333. doi: 10.2174/156720512800107555. [DOI] [PubMed] [Google Scholar]
- Shafto M.A., Tyler L.K., Dixon M., Taylor J.R., Rowe J.B., Cusack R., Calder A.J., Marslen-Wilson W.D., Duncan J., Dalgleish T., Henson R.N., Brayne C., Matthews F.E. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: a cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014;14:204. doi: 10.1186/s12883-014-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji K., Uka T., Tamura Y., Yoshida M., Kamagata K., Hori M., Motoi Y., Watada H., Kawamori R., Aoki S. Diffusional kurtosis imaging analysis in patients with hypertension. Jpn. J. Radiol. 2014;32:98–104. doi: 10.1007/s11604-013-0275-8. [DOI] [PubMed] [Google Scholar]
- Strandberg T.E., Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr. Opin. Nephrol. Hypertens. 2003;12:293–297. doi: 10.1097/00041552-200305000-00011. [DOI] [PubMed] [Google Scholar]
- Strömmer J.M., Davis S.W., Henson R.N., Tyler L.K., Cam-CAN, Campbell K.L. Physical Activity Mitigates Age-related Differences in Frontal White Matter. J Gerontol. 2018 doi: 10.1093/gerona/gly220. gly220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J.R., Williams N., Cusack R., Auer T., Shafto M.A., Dixon M., Tyler L.K., Cam-CAN, Henson R.N. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. Neuroimage. 2017;144:262–269. doi: 10.1016/j.neuroimage.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres E.R., Strack E.F., Fernandez C.E., Tumey T.A., Hitchcock M.E. Physical activity and white matter hyperintensities: a systematic review of quantitative studies. Prev. Med. Rep. 2015;2:319–325. doi: 10.1016/j.pmedr.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk E.J., Breteler M.M.B., Schmidt R., Berger K., Nilsson L.-G., Oudkerk M., Pajak A., Sans S., Ridder M. de, Dufouil C., Fuhrer R., Giampaoli S., Launer L.J., Hofman A. The association between blood pressure, hypertension, and cerebral white matter lesions. Hypertension. 2004;44:625–630. doi: 10.1161/01.HYP.0000145857.98904.20. [DOI] [PubMed] [Google Scholar]
- Vasan R.S., Beiser A., Seshadri S., Larson M.G., Kannel W.B., D’Agostino R.B., Levy D. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287:1003–1010. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- Verhaaren B.F.J., Vernooij M.W., de Boer R., Hofman A., Niessen W.J., van der Lugt A., Ikram M.A. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61:1354–1359. doi: 10.1161/HYPERTENSIONAHA.111.00430. [DOI] [PubMed] [Google Scholar]
- Vlachopoulos C., Aznaouridis K., Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- von der Heide R.J., Skipper L.M., Klobusicky E., Olson I.R. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wareham N.J., Jakes R.W., Rennie K.L., Mitchell J., Hennings S., Day N.E. Validity and repeatability of the EPIC-Norfolk physical activity questionnaire. Int. J. Epidemiol. 2002;31:168–174. doi: 10.1093/ije/31.1.168. [DOI] [PubMed] [Google Scholar]
- Woodward M., Webster R., Murakami Y., Barzi F., Lam T.-H., Fang X., Suh I., Batty G.D., Huxley R., Rodgers A. The association between resting heart rate, cardiovascular disease and mortality: evidence from 112,680 men and women in 12 cohorts. Eur. J. Prev. Cardiol. 2012;21:719–726. doi: 10.1177/2047487312452501. [DOI] [PubMed] [Google Scholar]
- Xu J., Li Y., Lin H., Sinha R., Potenza M.N. Body mass index correlates negatively with white matter integrity in the fornix and corpus callosum: a diffusion tensor imaging study. Hum. Brain Mapp. 2013;34:1044–1052. doi: 10.1002/hbm.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Wada M., Sato H., Nagasawa H., Koyama S., Takahashi Y., Kawanami T., Kato T. Impact of nocturnal heart rate variability on cerebral small-vessel disease progression: a longitudinal study in community-dwelling elderly Japanese. Hypertens. Res. 2015;38:564–569. doi: 10.1038/hr.2015.38. [DOI] [PubMed] [Google Scholar]
- Zuo S., Pan P., Li Q., Chen Y., Feng H. White matter injury and recovery after hypertensive intracerebral hemorrhage. Biomed. Res. Int. 2017;2017:6138424. doi: 10.1155/2017/6138424. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.