Abstract
Statement of the Problem:
The endodontically treated teeth usually suffer from the discoloration induced by endodontic materials, which can adversely affect the esthetical outcome of a treatment.
Purpose:
This study aimed to compare the discoloration caused by the silver nanoparticles coated with imidazolium as an irrigant, sodium hypochlorite (NaOCl), and chlorhexidine gluconate (CHX).
Materials and Method:
The root tips of 65 single-rooted human teeth were resected and root canal systems were chemomechanically prepared from the apical aspect. The specimens were randomly divided into three experimental (n=15) and two control groups (n=10). In the experimental groups, the substance was placed in the root canal for 30 minutes, and then washed with normal saline. Saline or blood alone was used in the control groups. The discoloration was assessed spectrophotometrically right after substance placement (T1), 1 week (T2), and 1 (T3) and 3 months (T4) after and color change values were calculated. Statistical analysis was performed using multi-sample repeated measures analysis of variance, Tukey's HSD, and Sidak tests.
Results:
In T1, there was no significant difference in color change between silver nano particle, blood and CHX (p> 0.05); but these three groups had significantly more ∆E value than NaOCl and Normal Saline (p< 0.05). NaOCl and normal saline had no significant difference in T1 (p> 0.05). In T2, T3 and T4 results were the same and showed blood and silver nano particle had significantly higher color change in comparison with NaOCl, CHX and normal saline (p< 0.05). There was no significant difference between NaOCl, CHX, and normal saline in tooth discoloration (p> 0.05). There was no significant difference between silver nano particle and blood in ability of tooth discoloration (p> 0.05).
Conclusion:
Within the limitation of this study, silver nano particle material could not be suggested as an intra-canal irrigant regarding its unfavourable tooth discoloration.
Keywords: Silver nano particle , Imidazolium , Tooth discoloration , Irrigant
Introduction
Among the common problems of endodontically treated teeth is the discoloration induced by endodontic materials, which influences the esthetical outcome of the treatment.[1] Tooth color is the contrast of light of dentine and the reflecting features of enamel; thus, any change in the composition of enamel, dentine, or coronal pulp can alter the reflected light, and consequently the tooth color.[2]
According to the investigations, most of endodontic materials such as sealers, intracanal medicaments, and Portland cement-based materials can cause tooth discoloration.[3-6] Several studies concerning the tooth discoloration associated with root canal irrigants proved the discolouring effect of NaOCl, especially in combination with materials such as MTAD and chlorhexidine (CHX). Evidence showed that CHX, as an irrigant, in combination with citric acid, ethylenediaminetetraacetic acid (EDTA) and NaOCl could change the color of enamel and dentine.[7-18]
Nanosilver is used in various fields of dentistry including endodontics, prosthodontics, implant and operative dentistry.[19-24] Advancements in science and technology resulted in introduction of nanosilver as antifungal and antimicrobial agent.[25-27] The antimicrobial properties of nanosilver is comparable to NaOCl 5.25%; thus, it may be used as an intracanal irrigant.[28] Sofi et al.[29] approved the bactericidal ability of nanosilver on a wide spectrum of organisms. They reported that nanosilver significantly reduced the attachment of Enterococcus faecalis to nano-particulate dentine.
According to Abbaszadegan et al.,[30] surface charge of the silver nanoparticles significantly affected the bactericidal activity. The positively charged nanoparticles showed the highest level of effectiveness against the organisms tested in their study.[30] In another study, Abbaszadegan et al.,[31] it was shown that silver nanoparticle, positively charged with imidazolium, was more effective against E.faecalis in lower concentrations, compared with NaOCl, CHX, and nanosilver.
Positively charging the silver nanoparticle with imidazolium might promote it to a suitable antibacterial choice as a promising root canal disinfectant in root canal therapy. We found no study about the effect of nanosilver as an irrigant on tooth discoloration; thus, the present study was conducted to evaluate tooth discoloration with silver nanoparticle coated with imidazolium as an irrigant in comparison with 5.25% NaOCl and 2% CHX.
Materials and Method
This experimental study adopted a closed system previously described by Felman et al.[32] The samples were 65 human single-rooted permanent maxillary anterior and mandibular premolar teeth, which were extracted due to periodontal disease or as part of orthodontic treatment plans. The teeth were fully formed and inspected to ensure absence of cracks, fractures, caries, and coronal restoration.
To disinfect the teeth, they were immersed in 5.25% NaOCl solution for 1 hour, and then stored in normal saline solution until used. The samples were cleansed with an ultrasonic scaler (Supprasson; Satelec, France), and polished with pumice and water to remove the extrinsic debris and stains. The root ends were resected to obtain standard specimens with 15-mm root length. The toot canals were prepared from the apical side to the most coronal part of the pulp chamber. Canal enlargement was initially performed with Protaper rotary files (Dentsply; Maillefer, Switzerland) in F1, F2, and F3 sequence, followed by #1-6 Gates Glidden drills (MANI, Tochigi-Ken, Japan). Between each instruments, the canals were irrigated with 2-mL 5.25% NaOCl solution (Cerkamed; Poland) by using a 27-G needle.
In order to remove any remaining pulp tissue at the end of root canal preparation, the root canals were irrigated with 10 mL 2.5% NaOCl by using an ultrasonic device (SUPPRASSON, Satelec, France) with #30 stainless steel file tip. To remove the smear layer, the canals were irrigated with 17% EDTA (Cerkamed; Poland) for 1 minute. The canals were finally irrigated with 10 mL of normal saline.
Blood collection
The fresh blood was collected from a healthy consenting volunteer by a trained member of medical staff. The blood collection tubes were sterile and coated with K2EDTA as an anticoagulant agent to prevent clotting during the experiment.
Experimental setup
The teeth were randomly allocated to three experimental (n=15) groups of 2% CHX, 5.25% NaOCl, and silver nanoparticle coated with Imidazolium. Two control groups (n=10) were considered consisting of blood as positive control and saline as negative control group. Then, 5 ml of the experimental materials was applied as irrigant for 30 minutes in each sample root canal, and was refreshed during this time. The canals were finally irrigated with 5 mL of normal saline. The samples were stored in airtight boxes with 100% humidity in an incubator at 37°C during the experiment.
Tooth color assessment
The tooth color was evaluated with a spectrophotometer (Spectroshade MHT S.p.A.; Verona, Italy) and the basic tooth color (L0,a0,b0) was determined right after canal preparation. Color changes were evaluated four times as right after material placement (T1), and 1 week (T2), 1 month (T3), and 3 months after material placement (T4). A mounting system was developed to allow reproducible tooth positioning. The measurements were done in a room with same light at different times. A single operator performed the color measurements in a region 3 mm above the buccal cementoenamel junction. The measurements were repeated three times for each sample and the mean values were calculated. Data were reported by using the Commission International de I’Eclairage’s (CIE) L*a*b* color system. The color change between two measurements was calculated through the following formula:
∆E* = [(L1−L0*)+(a1−a0*)+(b1−b0*)²] ½
Where L* values represent lightness, ranging from black (0) to white (100), a* and b* represent greenness/redness and blueness/yellowness, respectively. The proposed limit for color matching adopted in this study was set at 3.7 ∆E* units (perceptibility threshold). Differences beyond this limit were considered as clinically perceptible.[33]
Statistical analysis
Multi-sample repeated measures ANOVA was used to assess the effects of material type over time. Furthermore, pairwise comparisons between groups and within group were done by using Tukey’s HSD and Sidak tests, respectively. The level of statistical significance was set at 0.05.
Results
Changes in tooth discoloration during the study time in all groups are illustrated in Figure 1. Table 1 represents the mean and standard deviation (mean±SD) of color change (∆E) in all groups during the study time.
Figure1.
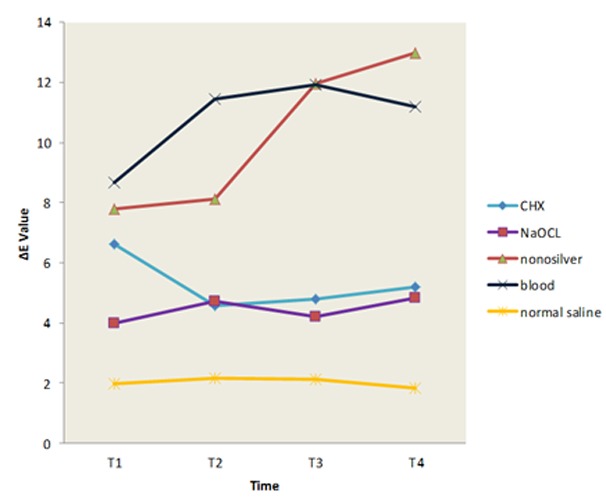
Colour change during the study time
Table 1.
The mean and standard deviation of color change (∆E) in all groups
| Time | ∆E1 | ∆E2 | ∆E3 | ∆E4 | N |
|---|---|---|---|---|---|
| CHX | 6.62 ± 3.44 | 4.57 ± 2.57 | 4.79 ± 1.81 | 5.18 ± 2.72 | 15 |
| NaOCl | 4.00 ± 1.63 | 4.71 ± 2.2 | 4.20 ± 1.17 | 4.81 ± 2.38 | 15 |
| Nanosilver | 7.77 ± 2.33 | 8.10 ± 3.04 | 11.9 ± 3.19 | 12.98 ± 5.81 | 15 |
| Blood | 8.68 ± 3.6 | 11.46 ± 6.44 | 11.94 ±5.81 | 11.19 ± 4.28 | 10 |
| Normal Saline | 1.98 ± 0.76 | 2.14 ± 0.82 | 2.10 ± 0.19 | 1.81 ± 0.56 | 10 |
In T1 (∆E between the base color and that measure right after substance application), no significant difference was observed in ∆E value between the silver nanoparticle, blood and CHX (p> 0.05). However, ∆E values in these three groups were significantly higher than that in NaOCl and normal saline groups. No significant difference was detected between the NaOCl and normal saline in T1 (p< 0.05). The results in T2, T3, and T4 were the same indicating that blood and silver nanoparticle had significantly higher ∆E value compared with NaOCl, CHX, and normal saline (p< 0.05). There was no significant difference between NaOCl, CHX, and normal saline in ∆E values (p> 0.05). Nor was any significant difference noted between the silver nanoparticle and blood in ∆E value (p> 0.05).
Discussion
Irrigation is one of the most important phases during endodontic therapy; thus, when choosing a suitable irrigating solution, one should consider the antimicrobial activity,[34-36] its biocompatibility with tissues,[37] and the changes that these irrigating solutions can make to the tooth surface.[38] Silver nanoparticle coated with imidazolium has been suggested as an intra-canal irrigant. However, some studies showed that the silver-based materials such as silver-containing sealers (e.g. AH26 and Kerr pulp canal sealer) could make grey to black tooth discoloration.[3,39-40] The present study aimed to analyse the color changes in enamel and dentine produced by silver nanoparticle coated with imidazolium as an irrigant in comparison with NaOCl, CHX, blood (as positive control group), and normal saline (as negative control group).
Spectrophotometry is more reliable than the subjective (visual) assessment of the tooth color changes.[33,41-42] Spectrophotometers are quite accurate devices, which can even show minute color changes that are not clinically observable.[33-34] Hence, it was decided to use spectrophotometric analysis in the present study for assessing tooth discoloration and determining the CIE L*A*B* variables and delta E (ΔE).
Several studies about tooth discoloration considered the blood as positive control, which means that blood can undoubtedly change the clinical color of tooth.[1,4,32] The present findings revealed that the nano silver irrigant adversely affected the tooth color; this discoloration was comparable to blood, which was considered as positive control group. Hernández-Sierra et al.[44] evaluated the tooth discoloration with silver nanoparticle per se and in combination with the bio-adhesive Gantrez S-97 as a tooth paste and found that silver nanoparticle could not change the tooth color. They used enamel blocks for evaluating the tooth discoloration and applied the substances paste on their samples with brush, whereas in the present study, liquid silver nanoparticles were applied in root canal for 30 minutes. The present findings are in contrast with Hernández-Sierra et al.’s study.[44] The penetration of silver nanoparticle as an irrigant into the permeable dentine is not comparable with its applying on enamel, which is an impermeable structure.
NaOCl with tissue dissolving ability was initially introduced as a bleaching agent.[45-46] Souza et al.[47] investigated the color changes of CHX and NaOCl on bovine dentinal blocks by dipping method. They did not detect any changes in the color of the tooth in comparison with normal saline as a negative control group. They also found that combination of NaOCl and CHX caused tooth discoloration, especially if CHX was used before NaOCl. These are in line with the present findings; although, we did not investigated the simultaneous use of NaOCl and CHX.
In another study, Koursoumis et al.[48] reported that NaOCl and CHX did not affect the tooth color. Measuring the L, a, b before and after the access cavity revealed the access cavity as a major influential factor in color change. The current study eliminated this contributing factor through retrograde application of the irrigants. According to the above-mentioned study, CHX could not change the tooth color over time. These finding are similar to what was found in the present study, except for the color at T1. It implies that compared with normal saline, CHX would not cause discoloration over time.
Several studies reported extrinsic tooth discoloration caused by CHX, which was related to factors such as presence of dental pellicle, proteins, and carbohydrate covering the tooth structures.[49-50] Inability to remove these agents and CHX from the tooth surfaces can result in tooth discoloration. Therefore, eliminating these factors can help reduce the discoloration ability of substances. The different findings about CHX at the initial measurements and thereafter might be due to incomplete removal of CHX after canal preparation. On the other hand, binding of CHX to dentin might reduce the discoloration ability of CHX in the long-term.
One the limitations of this in-vitro study was that the root canals were not prepared through the access cavity and some remnants of pulp tissue may remain in the pulp chamber especially in pulp area during the cleaning process of the canals that may contribute to tooth discoloration. Moreover, the role of filling materials was not investigated in tooth discoloration.
Conclusion
According to finding of this study, use of silver nanoparticle coated with imidazolium as an irrigant in root canal therapy can cause tooth discoloration the same as blood does. However, it cannot still be suggested as an intra-canal irrigant regarding its unfavourable tooth discoloration, and further investigations are required.
Acknowledgement
The authors thank the Vice-Chancellory of Shiraz University of Medical Science for supporting this research (Grant# 12456). This manuscript is based on the thesis by Dr. Moazami. The authors also thank Dr. Salehi of the Center for Research Improvement of the school of dentistry for the statistical analysis.
Conflict of Interest:The authors declare no conflicts of interest.
References
- 1.Lenherr P, Allgayer N, Weiger R, Filippi A, Attin T, Krastl G. Tooth discoloration induced by endodontic materials: a laboratory study. Int Endod J. 2012; 45: 942–949. doi: 10.1111/j.1365-2591.2012.02053.x. [DOI] [PubMed] [Google Scholar]
- 2.Akhavan H, Motalebi A, Mehrvarzfar P, Salari MH, Toursavadkouhi S, Sadighnia A. Comparison of discoloration induced on coronal dentine by AH PLUS and MTA Sealer in endodontically treatedd teeth. J Fundam Appl Sci. 2015; 5: 28–33. [Google Scholar]
- 3.Parsons JR, Walton RE, Ricks-Williamson L. In vitro longitudinal assessment of coronal discoloration from endodontic sealers. J Endod. 2001; 27: 699–702. doi: 10.1097/00004770-200111000-00012. [DOI] [PubMed] [Google Scholar]
- 4.Partovi M, Al-Havvaz AH, Soleimani B. In vitro computer analysis of crown discolouration from commonly used endodonticsealers. Aust Endod J. 2006; 32: 116–119. doi: 10.1111/j.1747-4477.2006.00034.x. [DOI] [PubMed] [Google Scholar]
- 5.Kim ST, Abbott PV, McGinley P. The effects of Ledermix paste on discolouration of immature teeth. Int Endod J. 2000; 33: 233–237. doi: 10.1046/j.1365-2591.2000.00277.x. [DOI] [PubMed] [Google Scholar]
- 6.Day PF, Duggal MS, High AS, Robertson A, Gregg TA, Ashley PF, et al. Discoloration of teeth after avulsion and replantation: results from a multicenterrandomized controlled trial. J Endod. 2011; 37: 1052–1057. doi: 10.1016/j.joen.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 7.Gutiérrez JH, Guzmán M. Tooth discoloration in endodontic procedures. Oral Surg Oral Med Oral Pathol. 1968; 26: 706–711. doi: 10.1016/0030-4220(68)90443-x. [DOI] [PubMed] [Google Scholar]
- 8.González-López S, Camejo-Aguilar D, Sanchez-Sanchez P, Bolaños-Carmona V. Effect of CHX on the decalcifying effect of 10% citric acid, 20% citric acid, or 17% EDTA. J Endod. 2006; 32: 781–784. doi: 10.1016/j.joen.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Akisue E, Tomita VS, Gavini G, Poli de, Figueiredo JA. Effect of the combination of sodium hypochlorite and chlorhexidine on dentinalpermeability and scanning electron microscopy precipitate observation. J Endod. 2010; 36: 847–850. doi: 10.1016/j.joen.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Krishnamurthy S, Sudhakaran S. Evaluation and prevention of the precipitate formed on interaction between sodiumhypochlorite and chlorhexidine. J Endod. 2010; 36: 1154–1157. doi: 10.1016/j.joen.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Tay FR, Mazzoni A, Pashley DH, Day TE, Ngoh EC, Breschi L. Potential iatrogenic tetracycline staining of endodontically treated teeth viaNaOCl/MTAD irrigation: a preliminary report. J Endod. 2006; 32: 354–358. doi: 10.1016/j.joen.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Torabinejad M, Cho Y, Khademi AA, Bakland LK, Shabahang S. The effect of various concentrations of sodium hypochlorite on the ability of MTAD to remove the smear layer. J Endod. 2003; 29: 233–239. doi: 10.1097/00004770-200304000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Tay FR, Hiraishi N, Schuster GS, Pashley DH, Loushine RJ, Ounsi HF, et al. Reduction in antimicrobial substantivity of MTAD after initial sodium hypochloriteirrigation. J Endod. 2006; 32: 970–975. doi: 10.1016/j.joen.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Basrani BR, Manek S, Sodhi RN, Fillery E, Manzur A. Interaction between sodium hypochlorite and chlorhexidine gluconate. J Endod. 2007; 33: 966–969. doi: 10.1016/j.joen.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Bui TB, Baumgartner JC, Mitchell JC. Evaluation of the interaction between sodium hypochlorite and chlorhexidine gluconate and its effect on root dentin. J Endod. 2008; 34: 181–185. doi: 10.1016/j.joen.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Rasimick BJ, Nekich M, Hladek MM, Musikant BL, Deutsch AS. Interaction between chlorhexidine digluconate and EDTA. J Endod. 2008; 34: 1521–1523. doi: 10.1016/j.joen.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 17.Nassar M, Awawdeh L, Jamleh A, Sadr A, Tagami J. Adhesion of Epiphany self-etch sealer to dentin treated with intracanal irrigating solutions. J Endod. 2011; 37: 228–230. doi: 10.1016/j.joen.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Marchesan MA, Pasternak B Jr, Freitas Afonso MM, Sousa-Neto MD, Paschoalato C. Chemical analysis of the flocculate formed by the association of sodium hypochlorite and chlorhexidine. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007; 103:e103– e105. doi: 10.1016/j.tripleo.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Samiei M, Aghazadeh M, Lotfi M, Shakoei S, Aghazadeh Z, Vahid Pakdel SM. Antimicrobial Efficacy of Mineral Trioxide Aggregate with and without SilverNanoparticles. Iran Endod J. 2013; 8: 166–170. [PMC free article] [PubMed] [Google Scholar]
- 20.Lotfi M, Vosoughhosseini S, Ranjkesh B, Khani S, Saghiri M, Zand V. Antimicrobial efficacy of nanosilver, sodium hypochlorite and chlorhexidine gluconate against Enterococcus faecalis. Afr J Biotechno. 2011; 10: 6799–6803. [Google Scholar]
- 21.Nam KY. In vitro antimicrobial effect of the tissue conditioner containing silver nanoparticles. J Adv Prosthodont. 2011; 3: 20–24. doi: 10.4047/jap.2011.3.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flores CY, Diaz C, Rubert A, Benítez GA, Moreno MS, Fernández Lorenzo de Mele MA, et al. Spontaneous adsorption of silver nanoparticles on Ti/TiO2 surfaces. Antibacterialeffect on Pseudomonas aeruginosa. J Colloid Interface Sci 2010; 350: 402–408. doi: 10.1016/j.jcis.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 23.Zhao L, Wang H, Huo K, Cui L, Zhang W, Ni H, et al. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials. 2011; 32: 5706–5716. doi: 10.1016/j.biomaterials.2011.04.040. [DOI] [PubMed] [Google Scholar]
- 24.Durner J, Stojanovic M, Urcan E, Hickel R, Reichl FX. Influence of silver nano-particles on monomer elution from light-cured composites. Dent Mater. 2011; 27: 631–636. doi: 10.1016/j.dental.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramírez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005; 16: 2346–2353. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 26.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009; 27: 76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Lok CN, Ho CM, Chen R, He QY, Yu WY, Sun H, et al. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006; 5: 916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- 28.Shantiaee Y, Dianat O, Khani HM, Baghban AA. Cytotoxicity comparison of nanosilver coated gutta-percha with Guttaflow and normal gutta-percha on L929 fibroblast with MTT assay. J Dent Sch Shahid Beheshti Univ Med Sci. 2011; 29: 62–68. [Google Scholar]
- 29.Sofi W, Gowri M, Shruthilaya M, Rayala S, Venkatraman G. Silver nanoparticles as an antibacterial agent for endodontic infections. BMC Infect Dis. 2012; 12(Suppl 1): P60. [Google Scholar]
- 30.Abbaszadegan A, Ghahramani Y, Gholami A, Hemmateenejad B, Dorostkar S, Nabavizadeh M, et al. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater. 2015; 16: 53. [Google Scholar]
- 31.Abbaszadegan A, Nabavizadeh M, Gholami A, Aleyasin ZS, Dorostkar S, Saliminasab M, et al. Positively charged imidazolium-based ionic liquid-protected silver nanoparticles: a promising disinfectant in root canal treatment. Int Endod J. 2015; 48: 790–800. doi: 10.1111/iej.12377. [DOI] [PubMed] [Google Scholar]
- 32.Felman D, Parashos P. Coronal tooth discoloration and white mineral trioxide aggregate. J Endod. 2013; 39: 484–487. doi: 10.1016/j.joen.2012.11.053. [DOI] [PubMed] [Google Scholar]
- 33.Johnston WM, Kao EC. Assessment of appearance match by visual observation and clinical colorimetry. J Dent Res. 1989; 68: 819–822. doi: 10.1177/00220345890680051301. [DOI] [PubMed] [Google Scholar]
- 34.Ferraz CC, Gomes BP, Zaia AA, Teixeira FB, Souza-Filho FJ. In vitro assessment of the antimicrobial action and the mechanical ability of chlorhexidine gel as an endodontic irrigant. J Endod. 2001; 27: 452–455. doi: 10.1097/00004770-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Mohammadi Z, Abbott PV. The properties and applications of chlorhexidine in endodontics. Int Endod J. 2009; 42: 288–302. doi: 10.1111/j.1365-2591.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- 36.Vianna ME, Gomes BP, Berber VB, Zaia AA, Ferraz CC, de Souza-Filho FJ. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004; 97: 79–84. doi: 10.1016/s1079-2104(03)00360-3. [DOI] [PubMed] [Google Scholar]
- 37.Vianna ME, Gomes BP. Efficacy of sodium hypochlorite combined with chlorhexidine against Enterococcus faecalis in vitro. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107: 585–589. doi: 10.1016/j.tripleo.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 38.Zehnder M. Root canal irrigants. J Endod. 2006; 32: 389–398. doi: 10.1016/j.joen.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Krastl G, Allgayer N, Lenherr P, Filippi A, Taneja P, Weiger R. Tooth discoloration induced by endodontic materials: a literature review. Dent Traumatol. 2013; 29: 2–7. doi: 10.1111/j.1600-9657.2012.01141.x. [DOI] [PubMed] [Google Scholar]
- 40.Van der Burgt TP, Mullaney TP, Plasschaert AJ. Tooth discoloration induced by endodontic sealers. Oral Surg Oral Med Oral Pathol. 1986; 61: 84–89. doi: 10.1016/0030-4220(86)90208-2. [DOI] [PubMed] [Google Scholar]
- 41.Colorimetry Official recommendations of the International Commission on Illumination. Paris: Commission Internationale de l'Èclairage [International Commission on Illumination. Available at: [http://cie.mogi.bme.hu/cie_arch/kee/div1/tc148.pdf. ] [Google Scholar]
- 42.Paul S, Peter A, Pietrobon N, Hämmerle CH. Visual and spectrophotometric shade analysis of human teeth. J Dent Res. 2002; 81: 578–582. doi: 10.1177/154405910208100815. [DOI] [PubMed] [Google Scholar]
- 43.Seghi RR, Johnston WM, O'Brien WJ. Spectrophotometric analysis of color differences between porcelain systems. J Prosthet Dent. 1986; 56: 35–40. doi: 10.1016/0022-3913(86)90279-9. [DOI] [PubMed] [Google Scholar]
- 44.Hernández-Sierra JF, Ruíz F, Castanedo-Cázares JP, Martinez-Ruiz V, Mandeville P, Pierdant-Pérez M, et al. In vitro determination of the chromatic effect of a silver nanoparticles solution linkedto the gantrez S-97 copolymer on tooth enamel. J Clin Pediatr Dent. 2010; 35: 65–68. doi: 10.17796/jcpd.35.1.f466p70100253643. [DOI] [PubMed] [Google Scholar]
- 45.Baumgartner JC, Cuenin PR. Efficacy of several concentrations of sodium hypochlorite for root canal irrigation. J Endod. 1992; 18: 605–612. doi: 10.1016/S0099-2399(06)81331-2. [DOI] [PubMed] [Google Scholar]
- 46.Cárdenas Flores A, Flores Reyes H, Gordillo Moscoso A, Castanedo Cázares JP, Pozos Guillén Ade J. Clinical efficacy of 5% sodium hypochlorite for removal of stains caused by dental fluorosis. J Clin Pediatr Dent. 2009; 33: 187–191. doi: 10.17796/jcpd.33.3.c6282t1054584157. [DOI] [PubMed] [Google Scholar]
- 47.Souza M, Cecchin D, Barbizam JV, Almeida JF, Zaia AA, Gomes BP, et al. Evaluation of the colour change in enamel and dentine promoted by the interactionbetween 2% chlorhexidine and auxiliary chemical solutions. Aust Endod J. 2013; 39: 107–111. doi: 10.1111/j.1747-4477.2011.00311.x. [DOI] [PubMed] [Google Scholar]
- 48.Koursoumis AD, Kerezoudis NP, Kakaboura A. In vitro assessment of tooth color alteration by two different types of endodonticirrigants. J Contemp Dent Pract. 2014; 15: 529–533. doi: 10.5005/jp-journals-10024-1574. [DOI] [PubMed] [Google Scholar]
- 49.Watts A, Addy M. Tooth discolouration and staining: a review of the literature. Br Dent J. 2001; 190: 309–316. doi: 10.1038/sj.bdj.4800959. [DOI] [PubMed] [Google Scholar]
- 50.Carpenter GH, Pramanik R, Proctor GB. An in vitro model of chlorhexidine-induced tooth staining. J Periodontal Res. 2005; 40: 225–230. doi: 10.1111/j.1600-0765.2005.00791.x. [DOI] [PubMed] [Google Scholar]


