Abstract
Pheochromocytomas have been shown to impair glucose tolerance and, rarely, to precipitate overt diabetes mellitus. We report here a case of a large pheochromocytoma in a woman with a recent diagnosis of diabetes mellitus that proved difficult to control despite high-dose insulin therapy who had complete resolution of her hyperglycemia following adrenalectomy. Her dramatic presentation demonstrates the need to consider this etiology in patients with new-onset insulin resistance and hypertension.
Keywords: Pheochromocytoma, Insulin resistance, Impaired glucose tolerance, Diabetes mellitus
1. Introduction
Pheochromocytomas are rare chromaffin tumors of adrenomedullary origin, with an estimated incidence of 0.2–0.6% in patients with hypertension [1]. However the true incidence is unknown, as up to 0.13% of autopsies revealed pheochromocytoma [1,2]. These tumors can be associated with impaired glucose tolerance or even overt diabetes in up to 20–40% of cases [2,3]. In our case report, we discuss a patient who was treated with more than 150 units of total daily insulin preoperatively and then did not require treatment post-operatively.
2. Case report
A 41-year-old female with a six-year history of hypertension and newly diagnosed diabetes was evaluated for six months of progressive abdominal pain in October 2017. She had uncontrolled hypertension documented during primary care visits despite therapy with amlodipine and metoprolol. Her past medical history was notable for a hysterectomy six years prior which was complicated by a perioperative hypertensive crisis. She also reported a long-standing history of palpitations and intermittent panic attacks accompanied by diaphoresis and pallor which were only partially controlled with intermittent alprazolam. For these symptoms, she had undergone a cardiac evaluation in 2011 including an ambulatory Holter monitor, a trans-thoracic echo-cardiogram and a cardiac stress test, that did not reveal any abnormalities. She had three pregnancies with uneventful vaginal deliveries and denied a history of gestational diabetes.
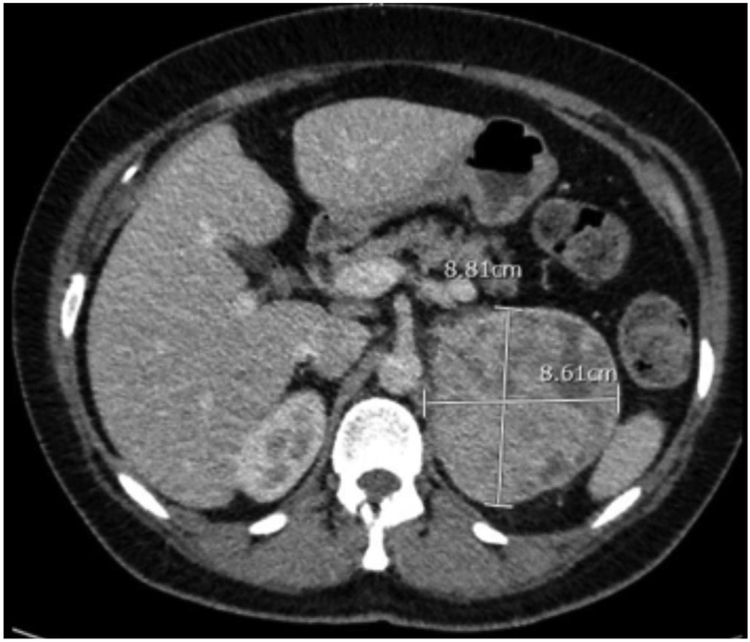
A screening abdominal ultrasound performed for her abdominal pain noted a large left adrenal mass. She underwent a contrast-enhanced computed-tomography (CT) scan of the abdomen and pelvis which identified an 8-cm, heterogeneous, enhancing left adrenal mass (Fig. 1). She was then referred to endocrinology and endocrine surgery in December 2017. A 24-h urine collection at that time showed a total catecholamine level of 2347 mcg/24hr (ref range: 26–121 mcg/24hr) and a total metanephrine level of 24,385 mcg/24h (ref range: 182–739 mcg/24hr). Given the high degree of concern for a pheochromocytoma, the decision was made to proceed with a left trans-abdominal adrenalectomy.
Fig. 1. Radiographic Image of the Left Adrenal Gland.
CT imaging of the abdomen shows an 8.8 × 8.6cm heterogeneous left sided adrenal mass.
She reported having been diagnosed with type 2 diabetes mellitus in August 2017 with a hemoglobin A1C of 9.4% and a random blood glucose over 400 without ketosis. She failed initial oral therapy with metformin 1000 mg daily and remained uncontrolled with the addition of saxagliptin and mealtime insulin. Upon referral to endocrinology in December 2017, she was transitioned to a multi-dose insulin regimen but remained difficult to control despite titration to a total daily dose of over 150 units of insulin up until the day of surgery (insulin glargine 68 units daily, insulin aspart 28 units three times daily).
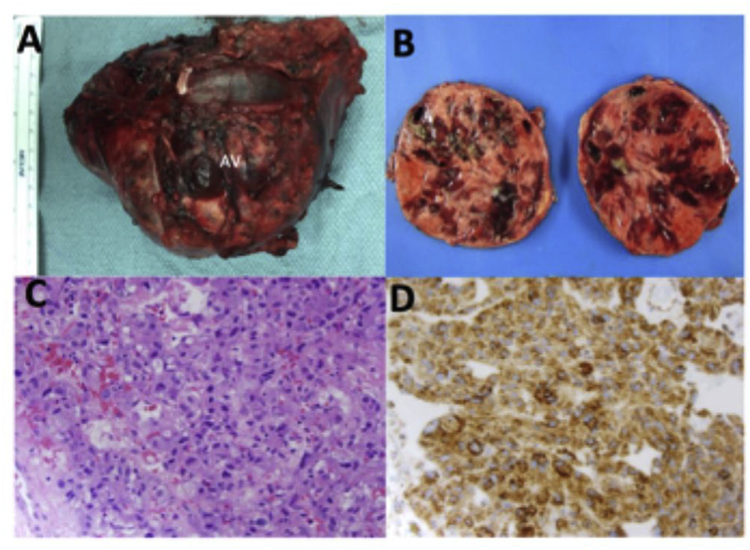
She underwent a left transabdominal open adrenalectomy in January 2018 with anticipated perioperative hypotension following ligation of the adrenal vein. Histology was consistent with pheochromocytoma with retained SDH-B staining (Fig. 2). An insulin drip was initiated perioperatively (up to 14 units per hour) but was stopped within a few hours. Surgical pathology revealed a 420-g, 12-cm pheochromocytoma without invasion into neighboring structures or metastases. The PASS score (Pheochromocytoma of the Adrenal Gland Scaled Score), used to distinguish between benign and malignant neoplasms [4], showed a benign score of 1.
Fig. 2. Gross pathology and histopathology of large pheochromocytoma.
(A) Gross image of left adrenal mass resected with a pronounced left adrenal vein (AV). (B) Gross image of a bisected left adrenal mass showed variegated, partially cystic and hemorrhagic cut surface. (C) H&E staining of the adrenomedullary tumor cells showing finely granular eosinophilic cytoplasm, round to oval nuclei with occasional prominent nucleolus and focal cytologic pleo-morphism characteristic of pheochromocytoma. Magnification, 400 ×. (D) Immunohistochemical staining of the left adrenal tumor reveals retained succinyl dehydrogenase B (SDH-B) expression (brown staining) in the tumor cells. Magnification, 400 ×.
She required minimal insulin postoperatively and was discharged without insulin or oral anti-hyperglycemic medications. With appropriate blood pressures, she was also instructed not to restart her home antihypertensive medications. During a follow-up clinic visit two weeks after surgery, a fasting glucose on a basic metabolic panel was 90 (Table 1). She reported premeal blood sugars in the low 100’s at home. She also reported improvement in blood pressures with improvement in subjective palpitations. Her blood pressure during the clinic visit was 104/70 with a regular heart rate of 72. Four months later, she continued to do well with a fasting glucose of 105 and resting blood pressure of 118/80.
Table 1.
Patient’s laboratory values before and after surgical resection of pheochromocytoma.
| November 2017 Preoperative (Ref. ranges) |
January 2018 Postoperative (Ref. ranges) |
|
|---|---|---|
| 24hr urine catecholamines, mcg/24hr |
2357 (26–121) | |
| 24hr urine metanephrines, mcg/24hr |
24385 (182–739) | |
| Fasting blood sugar, mg/dL | 220 (70–110) | 90 |
| Random blood sugar, mg/dL | 400 (< 200) | 90–100 |
| Hemoglobin A1C, % | 9.4% (4.0–5.7%) | |
| Insulin, total daily units | 152 | 0 |
3. Discussion
The pathogenesis of impaired glucose tolerance in pheochromocytoma is multifactorial. The benefit of impaired glucose tolerance in hyperadrenergism is believed to be maintenance of adequate glucose supply to promote survival in times of critical illness or stress [5]. Catecholamines produced by a pheochromocytoma act upon peripheral alpha- and beta-adrenergic receptors which are widely expressed in the body, including in the pancreas, muscle, adipose tissue, and liver [1,2]. Pheochromocytomas have been shown as early as the 1960’s to be associated with impaired insulin secretion due to systemic, hyperadrenergic tone on pancreatic alpha and beta cells, inhibiting insulin secretion and stimulating glucagon secretion [6–8]. Classic studies have shown that IV adrenaline and noradrenaline given to healthy individuals can reversibly impair insulin secretion [6]. Subsequent studies have shown that the increases in adrenergic receptor activation also act upon adipose tissue, muscle and liver to increase lipolysis and gluconeogenesis and alter glycogenolysis [9,10]. While it is difficult to retrospectively distinguish patients with pre-existing diabetes/pre-diabetes from patients whose diabetes is caused by hyperadrenergism, these effects in sum can result in precipitating overt diabetes mellitus or worsening pre-existing diabetes with remarkable improvement in insulin sensitivity and/or secretory ability following surgical cure of a pheochromocytoma [2,3,11].
The precise relationship between catecholamines, metanephrine levels, and insulin resistance remains to be determined, though elevations of epinephrine levels greater than 400pg/mL have been shown to mediate inhibitory effects of α2-adrenergic receptor-mediated insulin secretion [12]. While general practice has varied on the type of catecholamine and metabolites obtained for diagnosis of pheochromocytoma, areas for future research include identifying a signature of catecholamine levels which could predict diabetes onset and whether duration of disease could have an effect on predicting diabetes onset or postoperative resolution of diabetes.
We have reviewed the literature for case reports of pheochromocytomas with significant insulin resistance which improved postoperatively. Of those cases which reported preoperative and postoperative insulin doses, the total daily use of insulin typically ranged from 12 to 110 units per day. Postoperatively, ~50% of these cases could be taken off insulin entirely, while the remaining 50% remained on significantly lower doses of insulin or transitioned to oral anti-hyperglycemic medications only (Table 2). Thus, it remains unclear whether or not these patients had pre-existing diabetes or pre-diabetes.
Table 2.
References which report patients’ diabetes treatment regimens before and after surgical resection of pheochromocytoma.
| Reference | Preoperative diabetes Regimena |
Postoperative diabetes Regimena |
|---|---|---|
| Mesmar et al., Endocr Pract, 23(8):999, 2017 [2] | Case 1: Insulin 45U/day | Case 1: metformin |
| Case 2: Insulin 70U/day | Case 2: metformin + glipizide | |
| Hirai et al., Int Med, 55(2): 2985, 2016 [13] | Insulin 40U/day | None |
| Gallagher et al., Minerva Endocrine, 36(4): 341, 2011 [14] | Insulin 110U/dayb | Glipizide |
| Murao et al., Endocrine, 32(3): 350, 2007 [15] | Insulin 38U/day | Insulin 27U/day |
| Rofougaran et al., Am J Nephrol, 17(5):474, 1997 [16] | Insulin 40U/day | None |
| Isotani et al., Diabetes Research and Clin Practice, 34(1): 57, 1996 [17] | Insulin 52U/day | None |
| Edelman, Cleve Clin J Med, 59(4): 423, 1992 [11] | Insulin 44U/day | None |
| White et al., Neuro Neurosurg Psych, 49(12): 1449, 1986 [18] | Insulin 44U/day | Glibenclamide |
| Isles et al., Clin Endocrinol (Oxf), 18(1):37, 1983 [19] | Insulin 36U/day | None |
Insulin doses documented in total daily units.
History of steroid-induced diabetes on insulin prior to diagnosis of pheochromocytoma.
While it is well recognized that insulin resistance significantly improves after resection of a pheochromocytoma, our patient is unique in that she had new-onset diabetes with a significant insulin requirement (rapid uptitration to > 150U with fasting blood sugars remaining in the 150–180’s preoperatively) with complete resolution of her diabetes postoperatively. To our knowledge, our patient’s insulin requirements are the highest of those reported in the literature with complete resolution after treatment for a benign pheochromocytoma. While there are currently no recommendations for monitoring for diabetes onset in patients after resection of pheochromocytoma, we plan to continue to monitor her closely for hyperglycemia.
Overall, it is important for clinicians to consider secondary causes of diabetes mellitus or impaired glucose tolerance, including pheochromocytoma, in young patients with a new diagnosis of diabetes mellitus and difficult-to-control hypertension. Although rare, these tumors can promote reduced insulin secretion and severe peripheral insulin resistance which can be reversible, making early diagnosis important.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest
The authors declare they have no conflicts of interest.
References
- [1].Lenders JW, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab June 2014;99(6):1915–42. [DOI] [PubMed] [Google Scholar]
- [2].Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract August 2017;23(8):999–1005. [DOI] [PubMed] [Google Scholar]
- [3].Komada H, et al. Insulin secretion and insulin sensitivity before and after surgical treatment of pheochromocytoma or paraganglioma. J Clin Endocrinol Metab September 1 2017;102(9):3400–5. [DOI] [PubMed] [Google Scholar]
- [4].Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol May 2002;26(5):551–66. [DOI] [PubMed] [Google Scholar]
- [5].Soeters MR, Soeters PB. The evolutionary benefit of insulin resistance. Clin Nutr December 2012;31(6):1002–7. [DOI] [PubMed] [Google Scholar]
- [6].Porte D Jr., Graber AL, Kuzuya T, Williams RH. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest February 1966;45(2):228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Porte D Jr. A receptor mechanism for the inhibition of insulin release by epinephrine in man. J Clin Invest January 1967;46(1):86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wilber JF, Turtle JR, Crane NA. Inhibition of insulin secretion by a phaeochromocytoma. Lancet October 1 1966;2(7466):733. [DOI] [PubMed] [Google Scholar]
- [9].Rizza RA, Cryer PE, Haymond MW, Gerich JE. Adrenergic mechanisms for the effects of epinephrine on glucose production and clearance in man. J Clin Invest March 1980;65(3):682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rosengren AH, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science January 8 2010;327(5962):217–20. [DOI] [PubMed] [Google Scholar]
- [11].Edelman ER, Stuenkel CA, Rutherford JD, Williams GH. Diabetic ketoacidosis associated with pheochromocytoma. Cleve Clin J Med July-August 1992;59(4):423–7. [DOI] [PubMed] [Google Scholar]
- [12].Clutter WE, Cryer PE. Plasma dose-response studies with noradrenaline and adrenaline in man. Prog Biochem Pharmacol 1980;17:84–9. [PubMed] [Google Scholar]
- [13].Hirai H, Midorikawa S, Suzuki S, Sasano H, Watanabe T, Satoh H. Somatostatin-secreting pheochromocytoma mimicking insulin-dependent diabetes mellitus. Intern Med 2016;55(20):2985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gallagher EJ, Courgi R, Heiba S, Tamler R. Resolution of insulin-requiring diabetes in a liver transplant recipient after treatment of a pheochromocytoma: case report and review of literature. Minerva Endocrinol December 2011;36(4):341–5. [PubMed] [Google Scholar]
- [15].Murao K, et al. A case of pheochromocytoma complicated with slowly progressive type 1 diabetes mellitus and chronic thyroiditis. Endocrine December 2007;32(3):350–3. [DOI] [PubMed] [Google Scholar]
- [16].Rofougaran R, Mooraki A, Bastani B. Insulin-requiring diabetes mellitus, hyperlipidemia, and anginal chest pains as prominent features of pheochromocytoma. Am J Nephrol 1997;17(5):474–6. [DOI] [PubMed] [Google Scholar]
- [17].Isotani H, Fujimura Y, Furukawa K, Morita K. Diabetic ketoacidosis associated with the pheochromocytoma of youth. Diabetes Res Clin Pract September 1996;34(1):57–60. [DOI] [PubMed] [Google Scholar]
- [18].White PD, Lishman WA, Wyke MA. Phaeochromocytoma as a cause of reversible dementia. J Neurol Neurosurg Psychiatry December 1986;49(12):1449–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Isles CG, Johnson JK. Phaeochromocytoma and diabetes mellitus: further evidence that alpha 2 receptors inhibit insulin release in man. Clin Endocrinol (Oxf) January 1983;18(1):37–41. [DOI] [PubMed] [Google Scholar]