Abstract
Introduction
The aim of this study was to independently evaluate idiopathic urinary retention and other known Fowler's syndrome (FS) descriptions in 24 women treated for posterior fornix syndrome (PFS) by reinforcement of the uterosacral ligaments (USL) using the tissue fixation system (TFS).
Material and methods
The main inclusion criterion was: idiopathic urinary retention with post-void residual urines (PVR) >100 ml.
Results
The mean patient age was 63 years (range 32–87). Except for peak urine flow, features typical of FS were statistically improved (p 0.015 to <0.0001), pre-op mean with post-op mean in brackets: PVR 272 ml (34 ml); abnormal emptying symptoms n = 24 (18/24 cured or 80% improved); natural bladder volume 598 ml (301 ml); emptying time 50 seconds (20 seconds): peak flow 42 ml/sec (37 ml/sec); chronic pelvic pain n = 18 (14/18 >80% improved); maximal urethral closure pressure >90 cm (n = 4) 93 cm H2O (75 cm); frequency (14/14 improved); nocturia 110 episodes (33 episodes).
Conclusions
Functional disorders typical of FS, also present in posterior fornix syndrome, principally idiopathic urinary retention, were cured/improved by USL sling repair. Suggested anatomical pathway: lax USLs weaken the backward muscular forces, unbalancing bladder neck and urethral closure. Compensatory forward-acting closure muscles narrow the distal urethra, causing urinary flow difficulties, and retention. This functional imbalance can be relieved by posterior sling repair. We suggest that, rather than spasm from the (weak) rhabdosphincter (Fowler's syndrome), USL weakness is the most likely cause of idiopathic urinary retention in women.
Keywords: Fowler's syndrome, idiopathic urinary retention, posterior fornix syndrome, chronic pelvic pain, uterosacral ligament repair, Integral Theory
INTRODUCTION
In 1988 Fowler et al. [1] suggested that urinary retention or voiding difficulty in young women, a disorder of hitherto obscure causation, was associated with a characteristic electromyographic (EMG) abnormality in the striated urethral sphincter muscle consisting of complex repetitive discharges and repetitive bursts of motor unit activity. Polycystic ovarian disease (PCOD) was noted in 14 of the 17 women described [1], but subsequently PCOD was no longer considered an associated feature of the syndrome: only 3/62 patients with difficulty voiding had ovarian cysts [2]. There were a number of additional features; 3 also had urodynamic detrusor instability; 4 had after ‘unstable bladders which are usually associated with urge, frequency and nocturia; 12 had low flow rates and high residual urine volumes after voiding; 3 had ‘bladder failure’, presumably meaning an ‘underactive bladder’ problem; 5 could not void and were said to have chronic urinary retention. It was not stated whether these 5 women self-catheterized or simply could not void on the day of testing.
In a later report, chronic pelvic pain was a feature of 50% of patients [2], with half of these on opiates; indeed, opiate abuse was then suggested as a possible risk factor. In 2018, Panicker et al. noted that of 61 women referred to the National Hospital in Queen Square, London, only a third were confirmed as having the syndrome, a diagnosis based on an abnormal urinary sphincter EMG, although the urinary difficulties were similar in those with or without EMG abnormality [3]. However, the EMG features described by Fowler in idiopathic urinary retention in young women have since been found in 53% of a group of normal healthy women, when the recordings were made in the luteal phase of the menstrual cycle. It can therefore no longer be regarded as a diagnostic abnormality [4].
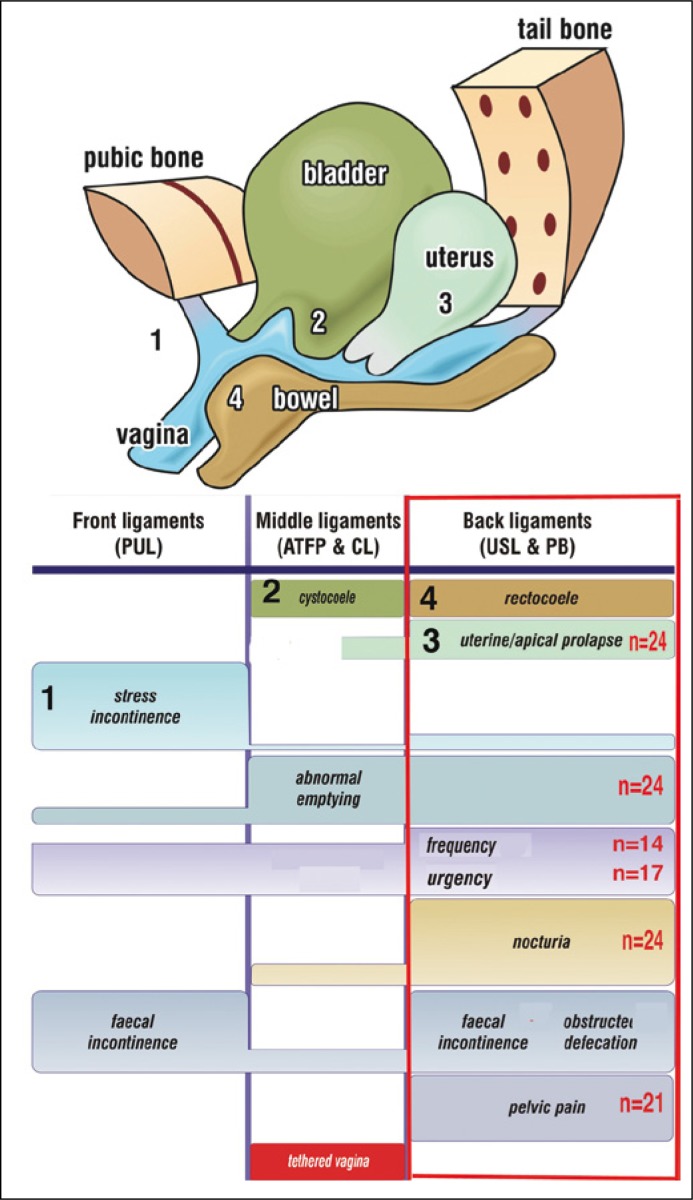
Abnormal emptying (‘obstructive micturition’, ‘dysuria’, ‘urinary retention’) is an essential component of the posterior fornix syndrome (PFS). PFS was first described in 1993 as part of the 2nd iteration of the Integral Theory of Female Urinary Incontinence [5, 6]. The PFS comprised 4 main symptoms: urge, nocturia, abnormal bladder emptying and chronic pelvic pain, shown in Figure 1, due to uterosacral ligament weakness or incompetence [5]. They resemble key features of FS. These symptoms can be improved or cured by plication of the uterosacral ligaments [6], or, more reliably, by USL reinforcement with posterior slings, as described in a recent multicenter report of 611 patients [7]. The PFS can also be associated with obstructed defecation syndrome and fecal incontinence (Figure 1) [8, 9, 10]. Mechanical support of USLs with speculum, roll gauze or pessary can relieve PFS symptoms [6, 8]. A similar direct test of USL causation for obstructed micturition utilizes a cylindrical ‘ProDry’ pessary 3x3x6 cm (Innocept, Gladbeck, Germany) placed into the posterior vaginal fornix; this results in an improvement in urine flow in most cases. Since obstructed micturition, pelvic pain, and overactive bladder constitute 3 of the 4 core features of the posterior fornix syndrome (PFS) and also 3 of the major features of FS, this suggested to us that urinary retention or obstructed micturition in FS and PFS may be functionally related disorders, dependent on the incompetence of the USLs.
Figure 1.
The Pictorial Diagnostic Algorithm – co-occurrence of symptoms with uterosacral ligament (USL) damage.
The size of the bar correlates broadly with the site and probability of symptom causation. The posterior zone (red rectangle) indicates the symptoms associated with USL looseness. These occur in predictable groupings (red rectangle). The red numbers indicate the co-occurrence of symptoms in the group of 24 patients who had post-void residual urines of 100 ml or more. The main ligaments are indicated in capital letters: PUL ‘pubourethral ligament’; ATFP ‘arcus tendineus fascia pelvis’, CL ‘cardinal ligament’; USL ‘uterosacral ligament,’ PB ‘perineal body’.
Here we compare features typical of FS found in 24 women from a Perth Australia data base diagnosed as PFS and the effect on these features following USL surgical reconstruction using tissue fifixation system (TFS) tensioned tapes.
MATERIAL AND METHODS
We analysed clinical data from 24 patients in our database, managed in Perth Australia, who had undergone cardinal ligament and uterosacral ligament sling repair for a diagnosis of PFS. All had symptomatic difficulty in bladder emptying with post-void residual urine volumes of 100 ml or more on urodynamic testing; all had uterine or apical prolapse. There were no specific exclusion criteria. The 24 patients were tested pre- and post-operatively with a validated symptom questionnaire for symptoms of bladder emptying, urgency, frequency, nocturia [11], and by transperineal ultrasound and urodynamics according to ICS definitions [12]. Abnormal bladder emptying symptoms were: Do you feel that your bladder isn't emptying properly? Do you ever have difficulty starting off your stream? Is it a slow stream? Does it stop and start involuntarily? Improvement in emptying symptoms and chronic pelvic pain of unknown origin was assessed with a self-administered visual analog scale (VAS). Pre- and post-op urodynamic measures assessed natural bladder volume, emptying time, post-void residual volume, peak flow rate, maximal urethral closure pressure and detrusor overactivity. The data analysis required for the study was approved by the Ethics Committee of the University of Western Australia (RA/4/20/4676).
Statistics
The GraphPad Quickcalcs platform (http://graphpad.com/quickcalcs/mcNemar1/) was used for this analysis.
The sample size (https://www.statstodo.com/SSiz McNemar_Pgm.php) was deemed sufficient to assume a Chi-squared distribution. Chi-square was calculated with one degree of freedom. P-values quoted are for 2 tailed tests with a confidence interval (CI) of 95%.
RESULTS
The results are listed in Table 1. The mean age was 63 years (range 32–87), parity 3 (0–6) and BMI 26 (range 18–45). Half of the 24 women had 1st degree and half had 2nd degree or greater uterine/apical prolapse according to the Baden-Walker classification. The 24 patients all had post-void residual (PVR) urine volumes of 100 ml or more (mean pre-operative PVR 272 ml; range 100 to 630 ml), mean post-operative PVR was 34 ml (range 0–50 ml; p <0.0001). Two patients who were self-catheterizing pre-operatively were restored to normal micturition, with postoperative residuals of 50 ml and 32 ml. The former was 87 years old with no cystocele, 3rd degree apical prolapse, 2nd degree rectocele. The latter was 82 years old, with 2nd degree uterine prolapse and 1st degree cystocele. No ovarian cysts or other pathology was revealed by 2D transperineal ultrasound studies.
Table 1.
Pre-operative and post-operative results (after tissue fixation system reinforcement of uterosacral ligaments) in 24 women with posterior fornix syndrome. PVR – postvoid residual urine
| Pre-op | Post-op | P value | |
|---|---|---|---|
| Postvoid residual urine | 272 ml (100–630) | 34 ml (0–150) | <0.0001 |
| Abnormal bladder emptying (24 patients) | 100% | 9 100% cured 9 80% cured 2 50% improved 3 failed |
<0.0001 |
| Natural bladder volume | Mean 598 ml | Mean 301 ml | 0.0001 |
| Voiding time | Mean 50 sec | Mean 20 sec | 0.006 |
| Peak urine flow rate | 42 ml/sec | 37 ml/sec | NS |
| Chronic pelvic pain VAS scale assessment |
18/24 | 14 improved 80% | <0.0001 |
| Max urethral closure pressure (4 patients) | >93 cm water | 75 cm water | NS |
| Urge | 17/24 3–4 wet episodes/day (range 1–8/day) |
13/17 cured 3 episodes/day range (1–8) in 4 patients |
<0.0015 |
| Frequency | Mean 12/day (range 5–21) 14 voided >14 times/day |
Mean 8.5/day (range 5–13) 8 voided > 8/day |
0.015 |
| Nocturia | 2 or more/night in all 24 patients. Mean 4.6 (range 2–9) | 13 cured 10 failures mean 2.7/night (range 2–6). 1 lost to F/U. | <0.0001 |
| Excess detrusor activity | 4 patients | 1 patient | – |
DISCUSSION
Our main finding was that other than apical prolapse*, which may or may not be present in FS, symptoms and signs ascribed to 'Fowler's syndrome' were almost identical to those constituting the 'posterior fornix syndrome', in particular, urinary retention. In a group of 24 women who had symptoms of urinary retention (including two who were self-catheterizing), we were able to cure or improve almost every historical manifestation of FS by reinforcement of USLs with tensioned tapes: high post-void residual urine, high natural bladder volumes, very high urethral pressures, prolonged voiding times, urge, nocturia, frequency, and chronic pelvic pain. Furthermore, we were able to explain almost all these manifestations by reference to previous experiments, which we analyse further below.
Absent from our analysis was testing for the rhabdosphincter EMG abnormality, a key pillar of ‘Fowler's syndrome’ which was originally thought to cause the urinary retention reported in young women [1]. However, as it is now established to be present in normal women, the cause of FS requires a different explanation. The only possible causes are isolated detrusor muscle failure, or weakened directional muscle forces acting on the urethral closure/opening mechanisms (Figures 2 and 3) [6, 8]. Detrusor muscle failure can be excluded because the retention was cured by USL repair. This leaves weakened directional muscle forces shown to act during urethral closure and micturition [6, 8].
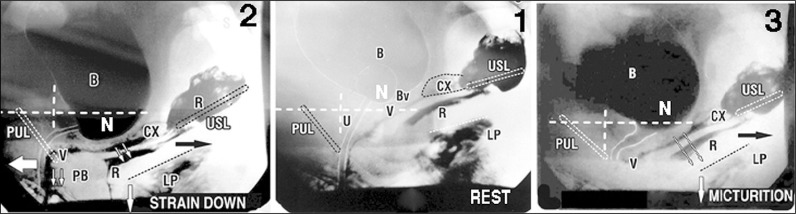
Figure 2.
External directional muscle vector forces open and close the urethra
Same patient. White lines represent vertical and horizontal bony co-ordinates.
1. Middle figure. At rest, asymptomatic patient. Slow twitch muscle forces and inherent elasticity combine to close urethra and prevent activation of the micturition reflex. N – stretch receptors for micturition reflex; LP – levator plate; U – urethra; B – bladder; U – urethra; CX – cervix; Bv – attachment of bladder base to anterior vagina; PUL – pubourethral ligament; USL – uterosacral ligament; R – rectum.
2. Left figure. Straining – urethral closure. The PUL, USL do not stretch but are angulated downwards. With reference to the vertical bony co-ordinates, fast twitch muscles forces stretch the distal vagina forwards against PUL (arrow) to close distal urethra; backward/downward vectors (arrows) contract against USL to stretch and rotate the proximal urethra, proximal vagina and rectum downwards around PUL, to effect bladder neck closure. PB – perineal body.
3. Right figure. Micturition. The ligaments do not stretch. USL is angulated downwards. There is absence of a forward vector which has been relaxed by the micturition reflex. The distal vagina and urethra can now be unrestrictedly stretched backwards behind the vertical bony co-ordinate. With reference to the horizontal co-ordinate, proximal vagina and rectum are stretched backwards/downwards, against USL to open out the posterior wall of urethra (arrows), exponentially reducing the internal resistance to flow. After Petros PE, The Female Pelvic Floor, 3rd Ed Springer 2010.
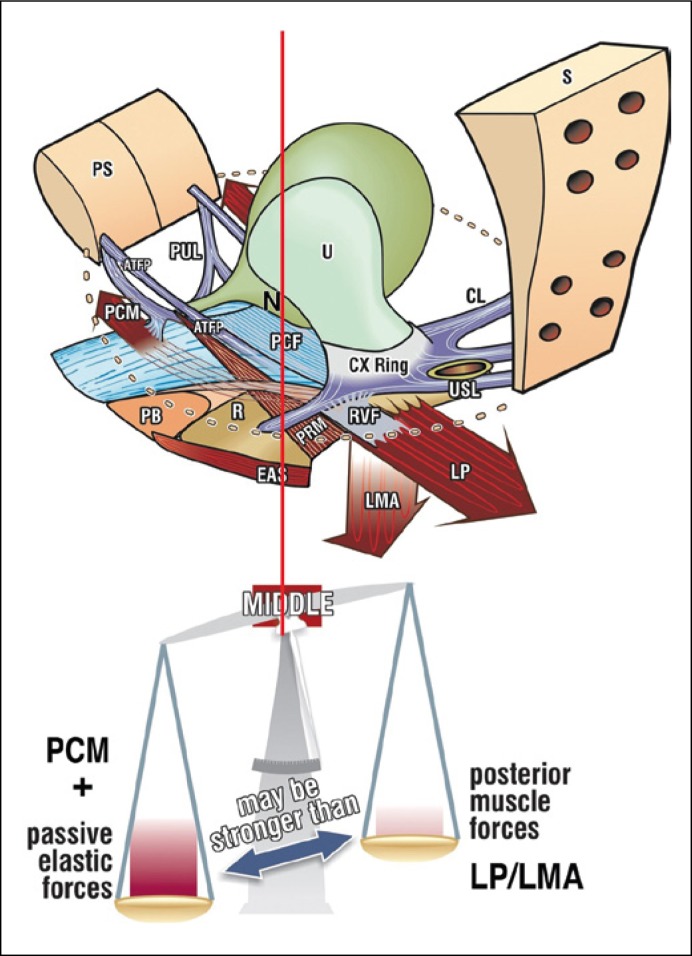
Figure 3.
The opposite directional vector forces are always in balance in the normal patient (red vertical line). The posterior vectors require a firm uterosacral ligament (USL) insertion point. If USL is loose, the posterior vectors weaken and the system loses balance, moving to the left of the red vertical line. PCM compensates by contracting forwards. N – bladder base stretch receptors; PCM – pubococcygeus muscle; LP – levator plate; LMA longitudinal muscle of the anus; PUL – pubourethral ligament. After Petros PE, The Female Pelvic Floor, 3rd Ed Springer 2010.
*Apical prolapse must be assessed in the operating room as it may not be evident on outpatient testing, as even 1st degree prolapse may cause PFS.
Critical analysis of diagnostic criteria for Fowler's syndrome
One of the two key diagnostic elements of FS, characteristic EMG changes in the rhabdosphincter (RS), was found by Tawadros et al. [4] to occur in normal women, usually around the luteal phase of their menstrual cycle. PCOD, initially said to be a frequent concomitant feature of FS, but subsequently retracted [2, 3], is not accompanied by a luteal phase as PCOD patients, by definition, do not ovulate. Thus, of the original criteria for FS, only 'obstructed micturition' or urinary retention remains as the one constant diagnostic feature. ‘Obstructed micturition' symptoms are core components of the surgically curable posterior fornix syndrome [5, 6]. We suggest that these two described entities, FS and PFS, may have a similar pathogenesis, USL laxity.
Critical analysis of mechanisms proposed for urinary retention in Fowler's syndrome
FS retention was at first assumed to occur from urinary rhabdosphincter spasm or contraction due to aberrant electrical activity – complex repetitive discharges and repetitive motor unit bursts – in rhabdosphincter (RS) muscle fibers [1, 3]. However, this EMG activity occurs in normal women [4]. Anatomically, RS is a small, weak muscle concentrated in the middle 1/3 of the urethra. It does not fully encircle the urethra [6, 8]. We submit that such a weak muscle is not capable of closing the urethra alone, especially as it is severely atrophied in older women [13], a conclusion first advanced by Huisman [13]. Huisman placed electrodes in the pelvic floor and in the dorsal striated urethral RS muscle [13]. He studied its EMG activity during coughing, standing, and straining and during unilateral and bilateral pudendal nerve blockade. He found that after unilateral pudendal nerve blockade, spontaneous EMG activity in the RS striated muscle continued, and the intra-urethral pressure was maintained. Continence was maintained during provocation tests. However, after bilateral pudendal nerve blockade incontinence occurred during cough-induced stress even though there was a >500% increase in EMG activity in the RS. Petros & Ulmsten recorded pressures inside and in equivalent positions outside the urethra in patients undergoing a midurethral sling procedure under local anaesthesia [14]. When the suburethral vagina was dislocated from the pubococcygeal muscles, massive loss of urine occurred during coughing. Despite this, the intraurethral pressure rose: the maximum mid-urethral pressure increase recorded in 4 patients was 78%, 94%, 112%, and 170%. After the vaginal flaps were closed, continence was restored. These observations suggest that RS is not the major muscle required for urinary continence. Rather, these observations can only be explained by a musculo-elastic mechanism acting to close the urethra as described in the various iterations of the Integral Theory System [6, 8, 14, 15, 16] and by Figures 2, 3, 5.
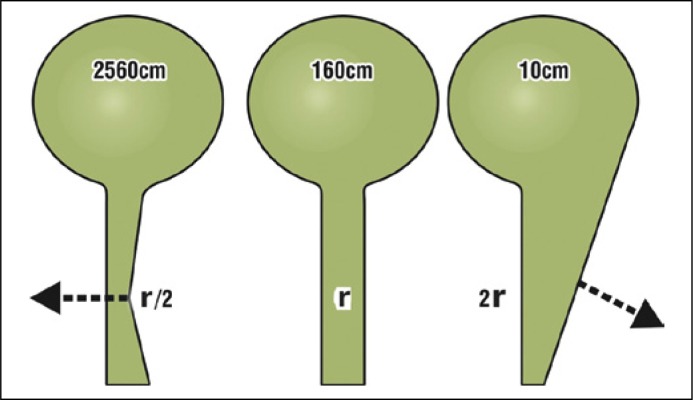
Figure 5.
Exponential effect of the external striated muscle mechanism on urethral resistance explains Fowler's syndrome (FS) retention. The arrows represent simplified vector forces which close the urethra during continence (forward arrow) or open it during micturition (backward arrow). The indicated pressures required to drive the urine through the changed diameter, are based on a nominal pressure of 160 cm for radius ‘r’. Pressures to expel through diameters r/2 (closure) and 2r (micturition) are calculated using the 4th power law of Poiseuille. Halving the radius (r/2) increases the expulsion force required by a factor of 16, to 2560 cm H2O. Doubling the radius (2r), decreases that force by a factor of 16 to 10 cm H2O. If we take the front arrow to represent overcompensation by the distal urethral closure mechanism in FS patients, very little extra activity is required to close the urethra sufficiently to make it impassable to urine flow. This explains the clinical ‘retention’ of FS. After Petros PE, The Female Pelvic Floor, 3rd Ed Springer 2010.
Unbalancing of closure mechanisms explains ‘idiopathic’ urinary retention
As long ago as 1997 [15, 16], video x-ray myogram, EMG and urodynamic studies showed that in the resting closed phase (‘1’, Figure 2) urethral geometry and closure are maintained by tonically active muscle forces [6, 8]. During coughing or straining (‘2’, Figure 2), a fast-twitch forward muscle vector pulls the distal vagina forward against the pubourethral ligament (PUL), thus closing the distal urethra; at the same time, backward muscle forces stretch the bladder base posteriorly against the pubo-urethral ligament (PUL) and the downward vector (arrow) contracts against the uterosacral ligament (USL) to rotate the now tensioned vagina and bladder base around PUL so as to 'kink the urethra' at the bladder neck [15, 16], the main continence mechanism [8], See Video 1. At rest, the forces due to tonic contraction of the forward and backward muscle vectors must be in balance [17], Figure 3. These opposite forces are modulated by integration in the spinal cord of sensory afferent information from muscle spindle stretch receptors and Golgi tendon organ tension receptors, and from the bladder base and proximal urethra. At the onset of normal micturition, the forward muscle vector, which presses the distal vagina forward to close the distal part of the posterior urethral wall, Figure 3, relaxes. This is evident on comparing the fig 2 xrays for rest‘1’ and straining ‘2’, with ‘3’(micturition): the backward/downward vectors (arrows) unrestrictedly open out the posterior urethral wall, reducing resistance to urinary flow and therefore the head of pressure required for urine evacuation See Video 2. It is important to recognize that these muscle forces all act against pelvic floor ligaments (Figures 2 and 3). These active forces are evident in the two videos available online (Videos 1 and 2).
If the USLs, the insertion points of the posterior muscle vectors (LP/LMA), are stretched and incompetent (weak), the posterior muscle vectors' ability to close the bladder neck is compromised. In an attempt to compensate, the forward muscle vectors stretch distal vagina forward beyond the vertical red line shown in Figure 3 to close (constrict) the distal urethra beyond what is normally required; at the same time, the rhabdosphincter contractility increases and this is recorded as an increase in urethral pressure, Table1. These events explain the 'catheter grabbing' oft described in FS [1, 3] and also, Fowler’s descriptions of x-ray video studies carried out in 17 women, which showed urethral obstruction at the region of the striated sphincter in all 12 who could still void.
Role of urethral resistance in idiopathic urinary retention
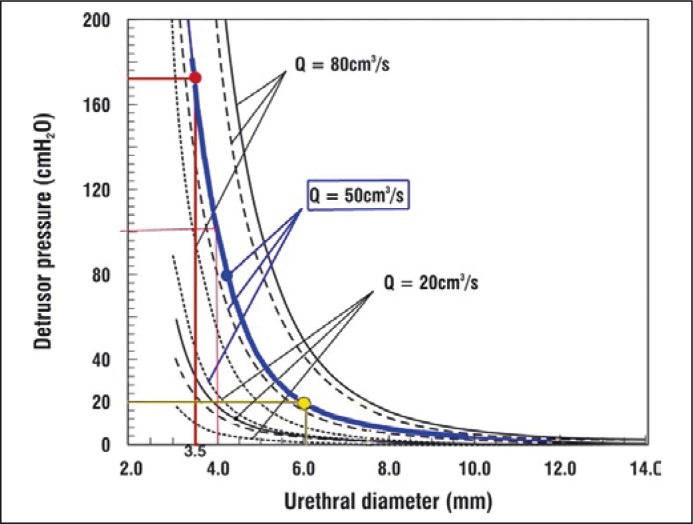
It is our view that idiopathic ‘urethral obstruction’, ‘urinary retention’, ‘obstructed micturition’ should be considered in terms of urethral resistance to urine flow. This has been studied by Bush and colleagues in a series of laboratory bench tests [18] and by using mathematical models [19, 20, 21]. They found that active opening of the posterior urethral wall (Figure 2, micturition) inversely lowered the urethral resistance to flow by the 4th power of the urethral radius, as predicted by Poiseuille's Law, thus facilitating rapid evacuation of urine. The pressure-flow graph (Figure 4) indicates that even a small increase in urethral diameter, e.g., from 3.5 to 4 mm, almost halves the head of pressure required for the detrusor to expel urine from the bladder. Conversely, Poiseuille's Law also explains the excessive urethral closure pressures found in the patients described by Fowler's group, and by ourselves in PFS, Table 1. Even a small compensatory narrowing of distal urethral diameter, from 4 mm to 3.5 mm, would increase the head of pressure required to drive the urine from the bladder from 100 cm to 172 cm water (Figure 4). We emphasize that only about half the patients described by Panicker et al. [3] as ‘Fowler's syndrome’ showed the EMG abnormality that was at first thought to be both characteristic and causative [1]. Since this feature is now recognized in about 30% of normal women [22] during the luteal phase of their menstrual cycles [4], this cannot be the sole cause of the syndrome of urinary retention in young women. It is likely that this EMG activity, is induced in connexion with hormonal influences, by ephaptic transmission from nearby and intermingled smooth muscle fibers in the internal urinary sphincter and in the pelvic fascial tissue around the RS, but it is not to be an abnormal phenomenon in this muscle.
Figure 4.
Exponential nature of urine flow is related to urethral diameter [26, 27]. For a flow rate of 50 ml/sec (thick blue line), opening the urethral diameter from 3.5 mm to 4 mm reduces the head of pressure required by the detrusor to expel urine from the bladder from 172 to 100 cm H2O. Expanding to 6 mm (yellow lines), reduces the head of pressure to 20 cm H2O. The blue line represents the total urethral resistance to flow. The broken lines are dynamic and frictional flow components.
Obstructive micturition occurring in young nulliparous women could be due to hormonally-induced changes in the collagen content of the pelvic ligaments to cause not only urinary emptying problems, but other symptoms also, fig1. Although we recognize that this concept is not fully understood, we draw attention to the studies of Downing and Sherwood [23] who analyzed the role of relaxin, oestrogen, progesterone, and prostaglandins in cervical softening, the cervix being a pure collagenous structure. They found that the inter-relationship of these hormones was a critical factor in collagen softening, including the relative ratios of glycosaminoglycans, and that this affected collagen strength.
Chronic pelvic pain
Recent studies [24, 25] have confirmed Heinrich Martius's assertion, put forward in 1938, that laxity in the USLs causes chronic pelvic pain because of inability to support the plexus of Frankenhauser (T11–L2) and the sacral plexus (S2–4). In a multicenter trial [26], reinforcing the USLs with a tensioned TFS sling cured or greatly improved pelvic pain in 79% of 197 patients along with other PFS symptoms such as urge incontinence: n = 320 (86%); frequency: n = 313 (84%); nocturia: n = 257 (69%); fecal incontinence: n = 93 (65%). This marked improvement in co-morbidities of chronic pelvic pain after restoration of the uterosacral ligament anatomy by TFS implies that these symptoms are likely to be due to the underlying anatomical disorder rather than of secondary psychogenic causation [27].
CONCLUSIONS
We show that uterosacral ligament (USL) reconstruction can cure or improve the pelvic floor dysfunctions of the posterior fornix syndrome. We suggest that, since the urethral sphincter spasm theory for Fowler's syndrome has been rejected, the two syndromes may have similar underlying abnormalities in terms of ligamentous dysfunction or incompetence.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Fowler CJ, Christmas TJ, Chapple CR, Parkhouse HF, Kirby AS, Jacobs HS. Abnormal electromyographic activity of the urethral sphincter, voiding dysfunction, and polycystic ovaries: a new syndrome? BMJ. 1988; 297:1436–1438. doi: 10.1136/bmj.297.6661.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeritzauer I, Stone J, Fowler C, Elneil-Coker S, Canon A, Panicker J. Fowler's syndrome of urinary retention: a retrospective study of co-morbidity. Neurourol Urodyn. 2016;35:601–603. doi: 10.1002/nau.22758. [DOI] [PubMed] [Google Scholar]
- 3.Panicker JN, Pakzad M, Fowler Cl. Fowler's syndrome: a primary disorder of urethral sphincter relaxation. Obstet Gynaecol. 2018;20:95–100. [Google Scholar]
- 4.Tawadros C, Burnett G, Derbyshire LF, Tawadros T, Clarke NW, Betts CD. External urinary sphincter electromyography in asymptomatic women and the influence of the menstrual cycle. BJU Int. 2015;116:423–431. doi: 10.1111/bju.13042. [DOI] [PubMed] [Google Scholar]
- 5.Petros PE, Ulmsten U. The posterior syndrome: a multiple symptom complex of pelvic pain and abnormal urinary symptoms deriving from laxity in the posterior fornix. Scand J Urol Nephrol. 1993;27(Suppl 153):89–93. [Google Scholar]
- 6.Petros PE, Ulmsten U. An Integral Theory and its Method, for the Diagnosis and Management of female urinary incontinence. Scand J Urol Nephrol Suppl. 1993;153:1–93. [PubMed] [Google Scholar]
- 7.Liedl B, Inoue H, Sekiguchi Y, et al. Is overactive bladder in the female surgically curable by ligament repair? Cent European J Urol. 2017;70:53–59. doi: 10.5173/ceju.2017.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petros PE, Ulmsten U. An Integral Theory of female urinary incontinence. Acta Obstet Gynecol Scand Suppl. 1990;153:1–79. doi: 10.1111/j.1600-0412.1990.tb08027.x. [DOI] [PubMed] [Google Scholar]
- 9.Abendstein B, Petros PEP, Richardson PA, Goeschen K, Dodero D. The surgical anatomy of rectocele and anterior rectal wall intussusception. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:705–710. doi: 10.1007/s00192-007-0513-7. [DOI] [PubMed] [Google Scholar]
- 10.Abendstein B, Brugger BA, Furtschegger A, Rieger M, Petros PE. Role of the uterosacral ligaments in the causation of rectal intussusception, abnormal bowel emptying, and fecal incontinence. A prospective study. Pelviperineology. 2008;27:118–121. [Google Scholar]
- 11.Wagenlehner FM, Fröhlich O, Bschleipfer T, Weidner W, Perletti G. The Integral Theory System Questionnaire: an anatomically directed questionnaire to determine pelvic floor dysfunctions in women. World J Urol. 2014;32:769–781. doi: 10.1007/s00345-013-1150-z. [DOI] [PubMed] [Google Scholar]
- 12.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–126. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 13.Huisman AB. Aspects on the anatomy of the female urethra with special relation to urinary continence. Contrib Gynecol Obstet. 1983;10:1–31. [PubMed] [Google Scholar]
- 14.Petros, Ulmsten U. Urethral pressure increase on effort originates from within the urethra, and continence from musculo-vaginal closure. Neurourol Urodyn. 1995;14:337–346. doi: 10.1002/nau.1930140406. [DOI] [PubMed] [Google Scholar]
- 15.Petros PE, Ulmsten U. Role of the pelvic floor in bladder neck opening and closure: I muscle forces. Int J Urogynecol J Pelvic Floor Dysfunct. 1997;8:74–80. doi: 10.1007/BF02764822. [DOI] [PubMed] [Google Scholar]
- 16.Petros PE, Ulmsten U. Role of the pelvic floor in bladder neck opening and closure: II vagina. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:69–73. doi: 10.1007/BF02764821. [DOI] [PubMed] [Google Scholar]
- 17.Petros PE. The Anatomy and Dynamics of Pelvic Floor Function and Dysfunction in The Female Pelvic Floor: Function, Dysfunction and Management according to the Integral Theory. Vol. 2. Heidelberg: Springer-verlag; 2010. pp. 57–58. [Google Scholar]
- 18.Bush MB, Petros PEP, Barrett-Lennard BR. On the flow through the human urethra. J Biomech. 1997;30:967–969. doi: 10.1016/s0021-9290(97)00050-x. [DOI] [PubMed] [Google Scholar]
- 19.Petros PE, Bush MB. A mathematical model of micturition gives new insights into pressure measurement and function. Urogynecol Pelvic Floor Dysfunct. 1998;9:103–107. doi: 10.1007/BF01982218. [DOI] [PubMed] [Google Scholar]
- 20.Bush MB, Moron C, Messner-Pellenc L, Petros PE, Millard RA. Mechanical model for the opening of the human female urethra. In: Adlassnig K-P, Bracale M, editors. Proceedings of Biomedical Engineering. Austria: Acta Press; 2005. pp. 210–213. [Google Scholar]
- 21.Ramm O, Mueller ER, Brubaker L, Lowenstein L, Kenton K. Complex repetitive discharges: a feature of the continence mechanism or a pathological finding? J Urol. 2012;187:2140–2143. doi: 10.1016/j.juro.2012.01.118. [DOI] [PubMed] [Google Scholar]
- 22.Partanen JV. Ephaptic transmission from Type ll afferents to static alpha and beta efferents causes complex repetitive discharges: a hypothesis. Muscle Nerve. 2016;53:508–512. doi: 10.1002/mus.25056. [DOI] [PubMed] [Google Scholar]
- 23.Downing SJ, Sherwood OD. The physiological role of relaxin in the pregnant rat IV. The influence of relaxin on cervical collagen and glycosaminoglycans. Endocrinology. 1986;118:471–479. doi: 10.1210/endo-118-2-471. [DOI] [PubMed] [Google Scholar]
- 24.Weintraub AY, Petros PE. Dedicated to Professor Heinrich Martius, pioneer in the ligamentous origin of chronic pelvic pain in the female. Pelviperineology. 2017;36:66. [Google Scholar]
- 25.Goeschen K. Role of uterosacral ligaments in the causation and cure of chronic pelvic pain syndrome. Pelviperineology. 2015;34:2–20. [Google Scholar]
- 26.Sekiguchi Y, Inoue H, Liedl B, et al. Is chronic pelvic pain in the female surgically curable by uterosacral/cardinal ligament repair? Pelviperineology. 2017;36:74–78. [Google Scholar]
- 27.McCredie JP, Skilling PM. Are 'psychiatric' findings in patients with Chronic Pelvic Pain primary or secondary? Pelviperineology. 2018;37:24–27. [Google Scholar]