Abstract
Introduction
To characterize sperm dynamic motility patterns and chromatin integrity in infertile men with leukocytospermia.
Material and methods
Fifty patients with primary infertility and oligoasthenoteratozoospermia included in this prospective, controlled, blind study. All patients underwent clinical evaluation, semen peroxidase stain, computer aided semen analysis (CASA), sperm DNA integrity evaluation with acridine orange test (AOT) and fluorescence in situ hybridization (FISH) analysis of 18, X and Y chromosomes. Pregnancy outcomes were documented following antibiotic treatment of patients with leukocytospermia.
Results
Infertile men with leukocytospermia had significantly lower progressive and total sperm motility percentages compared to the control group. Sperm dynamic motility parameters by CASA including curvilinear, straight line and average pathway velocities, straightness and amplitude of lateral head displacement were significantly lower in leukocytospermia. Sperm DNA fragmentation index was significantly higher in leukocytospermia. Percentages of sperm with disomy XY and 18 were significantly higher. These changes in sperm motility parameters and DNA integrity correlated with the number of peroxidase positive leukocytes. Follow-up of 23 of the 25 patients with leukocytospermia after antibiotic treatment revealed significantly higher pregnancy rates in cured patients than in those with persistent leukocytospermia.
Conclusions
Leukocytospermia has a significant impact on sperm dynamic motility patterns, DNA and chromosomal integrity in infertile men which can adversely affect the likelihood of a successful pregnancy.
Keywords: leukocytospermia, computer aided semen analysis, sperm DNA, FISH
INTRODUCTION
Leukocytospermia is defined by the World Health Organization (WHO) as ≥1 × 106 WBC/ml of semen [1]. An elevated leukocyte count is found in the seminal fluid of up to 30% of infertile men, even in the absence of inflammatory symptoms or a seminal bacterial infection [2]. Evidence from several studies indicates that leukocytospermia could significantly contribute to male infertility by decreasing sperm motility, increasing sperm DNA damage and affecting the fertilizing ability. The great majority of these studies used manual semen analysis [3, 4, 5]. Fewer studies applied computer aided semen analysis (CASA) [6]. CASA technology has been applied widely in clinical andrology laboratories [7]. CASA helps provide precise and meaningful information about sperm motility fractions and dynamics, and carries out statistical analysis of the retrieved data [8].
Sperm DNA integrity is an objective parameter of sperm quality that correlates with both natural conception probability and the outcome of assisted reproductive procedures [9]. Despite some debate regarding its accuracy, acridine orange test (AOT) is a simple and rapid test that is still applied in the evaluation of sperm DNA integrity of infertile men with different etiologies. Its results are comparable to the other more advanced tests such as TUNEL and flowcytometry [10, 11].
Sperm from infertile men may be genetically defective at the chromosomal level, containing numerical and/or structural chromosomal aberrations [12]. Fluorescence in situ hybridization (FISH) is the most commonly used tool to determine the proportion of aneuploidy present in sex chromosomes and autosomes of sperm from infertile men [13].
The current study aims to characterize sperm dynamic motility patterns and chromatin integrity in infertile men with leukocytospermia.
MATERIAL AND METHODS
Twenty-five infertile men with oligoasthenoteratozoospermia and leukocytospermia were consecutively recruited from the Andrology clinic, Department of Dermatology and Andrology, Assiut University Hospital. Another 25 men with oligoasthenoteratozoospermia and no leukocytospermia were included as a control group. The study has been approved by the institutional review board and all patients signed an informed consent form. Exclusion criteria were history of smoking, occupational risk of exposure to sperm DNA toxins, genital disorders that impair reproductive capacity such as clinical varicocele, undescended testis and testicular atrophy, systemic diseases that may impair reproductive capacity such as hepatic, renal, endocrine and autoimmune diseases, azoospermia, administration of antioxidants, or presence of female partner factor infertility contributing to infertility. All patients underwent history taking, and thorough general medical and genital examination. Semen collection was carried out after an abstinence period of 2–7 days, with initial conventional analysis as per WHO 2010 guidelines [14]. Assessment of leukocytic count was carried out using peroxidase stain (LeucoScreen test, FertiPro, Belgium) [4]. Patients with leukocyte concentration in semen equal to or more than 10x6/ml were placed into the leukocytospermia group. Other patients with leukocytic concentration in semen less than 10x6/ml were placed in the non- leukocytospermia group [15].
Semen analysis
Following the assessment of semen content of leukocytes, further investigations were done in a blinded manner; thus, researchers were unaware which sample belonged to which study group. The evaluation of sperm concentration and motility was carried out using Mira-9000 CASA Quick Use system (MiraLab, Geneva, Switzerland). Different dynamic sperm motion parameters were assessed including curvilinear velocity (VCL), straight line velocity (VSL), average path velocity (VAP), LIN or linearity (VSL/VCL), STR or straightness (VSL/VAP), WOB or oscillation (VAP/VCL), ALH or lateral head displacement amplitude, BCF or beat cross frequency and MAD or mean angular displacement degree. Sperm morphology was evaluated using Diff-Quik staining (Polysciences Inc., Warrington, PA) and sperm deformity and teratozoospermia indices (SDI and TZI) were determined [1, 14].
Acridine orange test [16]
The AOT assay measures the ability of sperm nuclear DNA to denature in acid which forms a metachromatic shift of AO fluorescence from green (native DNA) to red (denatured DNA). The fluorochrome AO intercalates in the double-stranded DNA as a monomer, which binds to single-stranded DNA. The monomeric AO bound to native DNA fluoresce green, whereas the aggregated AO on denatured DNA fluoresces red. By using fluorescent microscopy, thick semen layers are fixed in fixative (methanol: acetic acid 3:1) for 2 hours. The slides are stained for 5 minutes and rinsed with water. The slides were washed with distilled water then covered with a glass cover and examined under a ZEISS mot plus (ZEISS, Germany) fluorescent microscope at the excitation wavelength of 450–490 nm. An average of 200 sperm cells was evaluated on each slide by the same examiner. Spermatozoa showing green fluorescence were interpreted as having a normal DNA content, whereas those displaying a spectrum of yellow-orange to red fluorescence were considered to have damaged DNA. The ratio between (yellow to red)/(green + yellow to red) fluorescence was considered as sperm DNA fragmentation index (DFI) percentages.
FISH analysis for sperm chromosomes 18, X and Y [17]
FAST FISH prenatal X, Y and 18 enumeration (Cytocell, Cambridge, UK) were carried out using a probe designed for the detection and quantification of these chromosomes in the sperm nuclei. FISH analysis involved hybridization of chromosome-specific DNA probes labeled with fluorochromes to complementary DNA sequences on target chromosomes, followed by detection of the bound probes under a fluorescence microscope. Spermatozoa were evaluated as normal when a single signal was detected for blue (chromosome 18) and either orange (Y chromosome) or green (X chromosome). The total number of signals for each chromosome were subsequently calculated and disomy, as well as nullisomy (loss) rates were calculated for X, Y and 18.
Statistical analysis
The data were analyzed and expressed as mean values ± standard deviations using SPSS version 17 program (SPSS, Inc., IBM, Armonk, NY). Unpaired t-test was used in comparisons of numerical parametric data between different groups. The Mann-Whitney test was used in comparison of numerical non-parametric data between groups. Fisher's exact test was used to compare percentages. Spearman correlation test was applied to analyze correlations between different quantitative variables. Values were considered significant when P values were equal to or less than 0.05.
Pregnancy outcomes
Patients with leukocytospermia received proper antibiotic treatment and were contacted for a telephone interview in June 2017 to inquire whether or not their partners had become pregnant.
RESULTS
Baseline characteristics showed no significant statistical difference between men with and without leukocytospermia; mean age was 32.1 ± 6.4 years vs. 33.7 ±7.7 years respectively, mean BMI was 22.0 ± 1.8 vs. 21.8 ±2.1 kg/m2, and mean duration of infertility was 4.2 ±2.1 years vs. 5.6 ±5.4 years respectively (P >0.05).
Infertile men with leukocytospermia had significantly higher semen liquefactive time, and percentages of viscous samples. They also had significantly lower progressive and total sperm motility percentages as compared to the non-leukocytospermia group (Table 1).
Table 1.
Comparison of physical and microscopic semen variables in leukocytospermia and non leukocytospermia group
| Semen variable | Leukocytospermia group (n = 25) | Non leukocytospermia group, (n = 25) | P-value |
|---|---|---|---|
| Semen volume (ml) Range Mean ±SD |
1–6.5 2.61 ±1.40 |
1–8 2.56 ±1.67 |
>0.05 |
| Liquefactive time (min) Range Mean ±SD |
20–60 39.00 ±12.75 |
20–45 28.60 ±8.10 |
<0.01 |
| Percentages of viscous samples (%) |
60 (15/25) |
28 (7/25) |
<0.05 χ2 |
| Sperm count (mil/ejaculate)* Range Median Mean ±SD |
5–258 64 81.90 ±87.26 |
3–239 61 72.63 ±85.00 |
>0.05 |
| Normal sperm morphology% Range Mean ±SD |
3–36 13.44 ±9.19 |
0–29 15.00 ±6.99 |
>0.05 |
| Progressive sperm motility % Range Mean ±SD |
12–37 22.04 ±8.67 |
20–50 39.08 ±11.41 |
<0.001 |
| Total sperm motility % Range Mean ±SD |
13–48 26.80 ±11.03 |
24–61 45.44 ±13.16 |
<0.001 |
| Semen leukocytic count (mil/ml) Range Mean ±SD |
1–8 3.52 ±2.49 |
0.1–0.8 0.34 ±0.18 |
<0.001 |
χ2 Chi-square test
The leukocytospermia group had significantly lower CASA sperm dynamic motility parameters including VCL, VSL, VAP, MAD, ALH and STR as compared to the non-leukocytospermia group. BCF and WOB were significantly higher with leukocytospermia indicating a negative effect on linearity (Table 2).
Table 2.
Comparison of dynamic sperm motility parameters between both groups
| Semen variable | Leukocytospermia group | Non leukocytospermia group | P-value |
|---|---|---|---|
| VCL (µm/sec) Range Mean ±SD |
21–44 36.08 ±6.00 |
32–64 55.96 ±9.01 |
<0.001 |
| VSL (µm/sec) Range Mean ±SD |
8–30 24.12 ±5.28 |
25–45 38.08 ±5.74 |
<0.001 |
| VAP (µm/sec) Range Mean ±SD |
10–34 27.28 ±5.17 |
27–46 40.88 ±5.45 |
<0.001 |
| MAD Range Mean ±SD |
36–68 46.32 ±8.47 |
31–63 53.00 ±10.92 |
<0.05 |
| ALH (µm) Range Mean ±SD |
0–5 2.60 ±1.41 |
1–5 3.10 ±0.89 |
<0.05 |
| BCF (Hz) Range Mean ±SD |
4–5 4.44 ±0.51 |
3–5 4.00 ±0.50 |
<0.01 |
| LIN Range Mean ±SD |
39–75 66.56 ±8.43 |
58–84 69.68 ±6.97 |
>0.05 |
| WOB Range Mean ±SD |
51–83 75.48 ±6.72 |
62–88 74.84 ±6.47 |
<0.05 |
| STR Range Mean ±SD |
62–90 85.52 ±6.04 |
83–96 91.56 ±4.06 |
<0.01 |
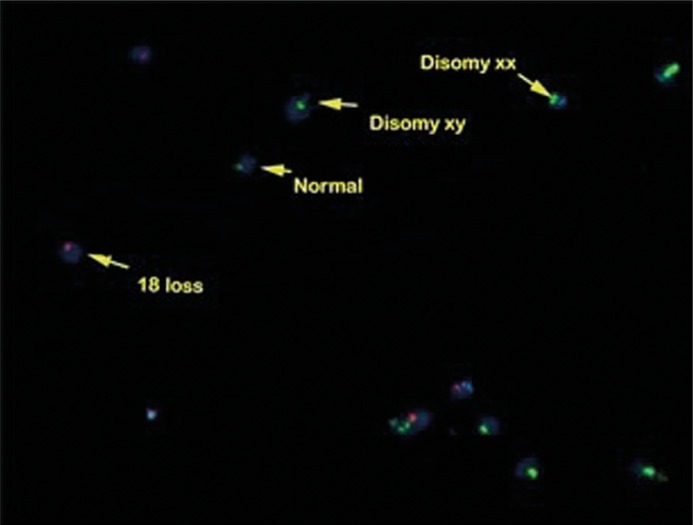
There were statistically significant increases in the percentages of the sperm DNA fragmentation index (DFI) by AOT, disomy XY, disomy 18, and the percentage of samples with disomy by FISH in the leukocytospermia group as compared to the non-leukocytospermia group (Table 3, Figure 1).
Table 3.
Comparison of sperm DNA and chromosomal integrity between the two groups
| Semen variable | Leukocytospermia group | Non leukocytospermia group | P-value |
|---|---|---|---|
| Sperm DFI (%) Range Mean ±SD |
25–70 43.60 ±13.43 |
20–35 30.20 ±5.10 |
<0.001 |
| Disomy XY (%) Range Mean ±SD |
0–0.1 0.017 ±0.027 |
0–0.05 0.004 ±0.012 |
<0.05 |
| Disomy XX (%) Range Mean ±SD |
0–0.1 0.009 ±0.025 |
30–70 50.1 ±9.68 |
>0.05 |
| Disomy YY (%) Range Mean ±SD |
0–0.02 0.002 ± 0.005 |
0–0.02 0.001 ±0.004 |
>0.05 |
| Disomy 18 (%) Range Mean ±SD |
0–0.2 0.032 ±0.048 |
0–0.05 0.005 ±0.011 |
<0.05 |
| Percentages of semen samples with disomy (%) | 68% (17/25) | 36% (9/25) | <0.05 |
Figure 1.
Disomy XY, XX and 18 chromosome nullisomy by FISH in a case of leukocytospermia.
Significant negative correlations were found between the number of peroxidase positive leukocytes and sperm motility parameters as evaluated by CASA, including progressive sperm motility (r = -0.580, P <0.001), VCL (r = -0.590, P <0.01), VSL (r = -0.526, P <0.01), VAP (r = -0.424, P <0.05). There was also significant positive correlation between semen leukocytic count and the percentage of sperm DFI (r = 0.767, P <0.001).
The 25 patients with leukocytospermia received proper antibiotic treatment, 23 of them were successfully followed-up, and two patients were lost during follow up. Thirteen of the 23 patients were cured and 10 had persistent leukocytospermia. Five of the 13 cured patients' partners reported clinical pregnancy while 1 of the uncured 10 reported clinical pregnancy, which, unfortunately, ended in a spontaneous abortion.
DISCUSSION
Leukocytospermia is a common finding in the semen of infertile men [1]. Apart from being a sign of an accessory gland or genitourinary infection, it may also be associated with numerous other risk factors for male subfertility including smoking, obesity, autoimmune disorders, exposure to environmental pollution and defective spermatogenesis [18, 19].
The changes in semen parameters in leukocytospermia may be a reflection of oxidative stress due to excessive ROS production by leukocytes, dysfunction of the male genital ducts and glands by an infectious process, or they are induced by the microorganisms causing an infection [20].
In our study, there was a statistically significant increase in semen hyperviscosity in the leukocytospermia group when compared with the non leukocytospermia group. This finding is in accordance with most previous studies reporting seminal hyperviscosity in leukocytospermia with different techniques [21, 22, 23]. Semen hyperviscosity is mostly attributed to male accessory gland infection that commonly associates leukocytospermia with inflammation, as well as dysfunction of the sex glands or even the immune system [21].
CASA provides an objective approach to routine manual semen analysis that can promote laboratory standardization and allows for more precise and detailed analysis of sperm motion criteria [24]. CASA results in our study showed a significantly higher decrease in progressive sperm motility percentage, total sperm motility percentage and many CASA sperm dynamic velocities such as VCL, VSL, VAP, MAD, ALH and STR in the leukocytospermia group than in the non leukocytospermia group. Also, there were significant negative correlations between the number of leukocytes using the peroxidase stain and progressive sperm motility percentage, total sperm motility percentage, VCL, VSL and VAP. Meanwhile, BCF and WOB were increased in the leukocytospermia group. This increase in BCF (which refers to the average rate at which the curvilinear path crosses the average path) and WOB (which refers to the oscillation of the actual path about the average path expressed as VAP/VCL) reflect the decrease in the sperm linearity. Since these dynamic motility patterns and velocities are important markers for sperm capacitation, the changes detected by CASA indicate that leukocytospermia may have a negative impact on important sperm functions. Our study is the first to characterize such changes in sperm dynamic motility patterns in leukocytospermia. Previous studies using CASA in leukocytospermia reported a significant negative correlation between the semen leukocyte count and the percentage of sperm motility but without a comment on other dynamic sperm motility parameters [25, 26]. On the other hand, another study found no significant difference in sperm motility between infertile patients with or without leukocytospermia [27]. Changes in sperm motility parameters detected by conventional semen analysis and CASA in leukocytospermia can be attributed to excessive ROS release, which causes peroxidation of lipids in sperm plasma membrane and dysfunction [20].
In our study, there was a statistically significant increase in the DFI in the leukocytospermia group as compared to the non-leukocytospermia group. Also there was a significant positive correlation between the number of pus cells by peroxidase stain and the sperm DNA fragmentation index by acridine orange test. Similarly, Saleh et al. [25], using the sperm chromatin structure assay (SCSA) to assess sperm DNA integrity reported a positive correlation between sperm DFI and leukocytospermia. A subsequent study reported a positive association between leukocytospermia and increased DNA fragmentation using the COMET technique [3]. More recently, Agarwal and associates measured sperm DNA damage using the TUNEL technique and found it to be significantly higher in the low leukocytospermia infertile group (0.1–10 x6/ml) as compared to the non leukocytospermia infertile group [27]. A later study also found a positive correlation between the number of seminal leukocytes and the number of spermatozoa with TUNEL-detected DNA fragmentation [26]. Leukocytospermia was hypothesized to play a major role in sperm DNA damage via oxidative stress and defective apoptosis [3]. On the other hand, an earlier study reported a very weak correlation between leukocytospermia and DNA fragmentation index measured with flow cytometry with acridine orange stain. The authors of this study mentioned that the role of leukocytes as the main source of reactive oxygen species in the pathology of sperm DNA damage may be overestimated [28].
To the best of our knowledge, this is the first study that used the FISH technique to demonstrate sperm chromosomal aneuploidy in the semen of infertile patients with leukocytospermia. FISH analysis in our study showed significant increases in the percentages of sperm with disomy X, Y and 18 and percentage of semen samples with disomy in the leukocytospermia. A possible explanation for the positive correlation between leukocytospermia and both sperm DNA damage and chromosomal aneuploidy may be related to an association between leukocytospermia induced oxidative stress and defective spermiogenesis [25]. Defective spermiogenesis results in an increased production of sperm with defective nuclear and membrane remodeling. It has been postulated that men with reduced spermatogenesis may not produce enough spermatozoa to trigger Sertoli cells to produce Fas ligand that is thought to stimulate the apoptosis process and thus, these abnormal, damaged sperms escape apoptosis [29]. Our data suggest that at least some sperm aneuploidies are acquired and may result from inflammation and/or excessive ROS production. These results are important considering the serious genetic consequences of these genetic abnormalities on increased incidence of congenital anomalies in the offspring of affected males and necessitate certain intervention such as antioxidant supplementation, which has been reported to reduce the incidence of aneuploidy in men with severe oligoasthenoteratospermia [30].
CONCLUSIONS
Sperm from infertile men with leukocytospermia showed remarkable changes in its dynamic motility patterns, DNA and chromosomal integrity. Further studies on larger patient groups are required to validate our findings.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
References
- 1.World Health Organization . WHO Laboratory manual for the examination and processing of human semen. 5th ed. Cambridge: Cambridge University Press; 2010. [Google Scholar]
- 2.Gambera L, Serafini F, Morgante G, Focarelli R, De Leo V, Piomboni P. Sperm quality and pregnancy rate after COX-2 inhibitor therapy of infertile males with abacterial leukocytospermia. Hum Reprod. 2007;22:1047. doi: 10.1093/humrep/del490. [DOI] [PubMed] [Google Scholar]
- 3.Arata de Bellabarba G, Tortolero I, Villarroel V, Molina CZ, Bellabarba C, Velazquez E. Non sperm cells in human semen and their relationship with semen parameters. Arch Androl. 2000;45:131. doi: 10.1080/01485010050193896. [DOI] [PubMed] [Google Scholar]
- 4.Fariello RM, Del Giudice PT, Spaine DM, Fraietta R, Bertolla RP, Cedenho AP. Effect of leukocytospermia and processing by discontinuous density gradient on sperm nuclear DNA fragmentation and mitochondrial activity. J Assist Reprod Genet. 2009;26:151–157. doi: 10.1007/s10815-008-9288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Domes T, Lo KC, Grober ED, Mullen JB, Mazzulli T, Jarvi K. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil Steril. 2012;97:1050–1055. doi: 10.1016/j.fertnstert.2012.01.124. [DOI] [PubMed] [Google Scholar]
- 6.Depuydt CE, Bosmans E, Zalata A, Schoonjans F, Comhaire FH. The relation between reactive oxygen species and cytokines in andrological patients with or without male accessory gland infection. 1996;17:699–707. [PubMed] [Google Scholar]
- 7.Lu JC, Huang YF, Lü NQ. Computer-aided sperm analysis: past, present and future. Andrologia. 2014;46:329–338. doi: 10.1111/and.12093. [DOI] [PubMed] [Google Scholar]
- 8.Lammers J, Splingart C, Barriere P, Jean M, Fréour T. Double-blind prospective study comparing two automated sperm analyzers versus manual semen assessment. J Assist Reprod Genet. 2014;31:35. doi: 10.1007/s10815-013-0139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57:78–85. doi: 10.3109/19396368.2010.515704. [DOI] [PubMed] [Google Scholar]
- 10.Zini A, Kamal K, Phang D, Willis J, Jarvi K. Biologic variability of sperm DNA denaturation in infertile men. Urology. 2001;58:258–261. doi: 10.1016/s0090-4295(01)01180-3. [DOI] [PubMed] [Google Scholar]
- 11.Mohammed EM, Mosad E, Zahran AM, et al. Acridine Orange and Flow Cytometry: Which Is Better to Measure the Effect of Varicocele on Sperm DNA Integrity? Adv Urol. 2015;2015:814150. doi: 10.1155/2015/814150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egozcue S, Blanco J, Vendrell JM, et al. Human male infertility: chromosome anomalies, meiotic disorders, abnormal spermatozoa and recurrent abortion. Hum Reprod Update. 2000;6:93–105. doi: 10.1093/humupd/6.1.93. [DOI] [PubMed] [Google Scholar]
- 13.Sarrate Z, Blanco J, Anton E, Egozcue S, Egozcue J, Vidal F. FISH studies of chromosome abnormalities in germ cells and its relevance in reproductive counseling. Asian J Androl. 2005;7:227–236. doi: 10.1111/j.1745-7262.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization . WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. 4th ed. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 15.Politch JA, Wolff H, Hill JA, Anderson DJ. Comparison of methods to enumerate white blood cells in semen. Fertil Steril. 1993;60:372–375. doi: 10.1016/s0015-0282(16)56116-0. [DOI] [PubMed] [Google Scholar]
- 16.Hoshi K, Katayose H, Yanagida K, Kimura Y, Sato A. The relationship between acridine orange fluorescence of sperm nuclei and the fertilizing ability of human sperm. Fertil Steril. 1996;66:634–639. doi: 10.1016/s0015-0282(16)58581-1. [DOI] [PubMed] [Google Scholar]
- 17.Muriel L, Goyanes V, Segrelles E, Gosálvez J, Alvarez JG, Fernández JL. Increased aneuploidy rate in sperm with fragmented DNA as determined by the sperm chromatin dispersion (SCD) test and FISH analysis. J Androl. 2007;28:38–49. doi: 10.2164/jandrol.106.000067. [DOI] [PubMed] [Google Scholar]
- 18.Jarvi K, Noss MB. Pyospermia and male infertility. Can J Urol. 1994;1:25–30. [PubMed] [Google Scholar]
- 19.Singer G, Granger DN. Inflammatory responses underlying the microvascular dysfunction associated with obesity and insulin resistance. Microcirculation. 2007;14:375–387. doi: 10.1080/10739680701283158. [DOI] [PubMed] [Google Scholar]
- 20.La Vignera S, Vicari E, Condorelli RA, D'Agata R, Calogero AE. Male accessory gland infection and sperm parameters (review) Int J Androl. 2011;34:330–347. doi: 10.1111/j.1365-2605.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 21.Elia J, Delfino M, Imbrogno N, et al. Human semen hyperviscosity: prevalence, pathogenesis and therapeutic aspects. Asian J Androl. 2009;11:609–615. doi: 10.1038/aja.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flint M, du Plessis SS, Menkveld R. Revisiting the assessment of semen viscosity and its relationship to leucocytospermia. Andrologia. 2014;46:837–841. doi: 10.1111/and.12157. [DOI] [PubMed] [Google Scholar]
- 23.Mahran Z, Saleh ME. Human semen hyperviscosity: prevalence and effects on physical and biochemical semen parameters in subfertile Egyptian men. Egyp J Dermatol Venereol. 2014;34:135. [Google Scholar]
- 24.Tomlinson MJ, Pooley K, Simpson T, et al. Validation of a novel computer-assisted sperm analysis (CASA) system using multitarget-tracking algorithms. Fertil Steril. 2010;93:1911–1920. doi: 10.1016/j.fertnstert.2008.12.064. [DOI] [PubMed] [Google Scholar]
- 25.Saleh RA, Agarwal A, Kandirali E, et al. Leukocytospermia is associated with increased reactive oxygen species production by human sperm. Fertil Steril. 2002;78:1215–1224. doi: 10.1016/s0015-0282(02)04237-1. [DOI] [PubMed] [Google Scholar]
- 26.Lobascio AM, De Felici M, Anibaldi M, et al. Involvement of seminal leukocytes, reactive oxygen species, and sperm mitochondrial membrane potential in the DNA damage of the human spermatozoa. Andrology. 2015;3:265–270. doi: 10.1111/andr.302. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal A, Mulgund A, Alshahrani S, et al. Reactive oxygen species and sperm DNA damage in infertile men presenting with low level leukocytospermia. Reprod Biol Endocrinol. 2014;12:126. doi: 10.1186/1477-7827-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moskovtsev SI, Willis J, White J, Mullen JB. Leukocytospermia: relationship to sperm deoxyribonucleic acid integrity in patients evaluated for male factor infertility. Fertil Steril. 2007;88:737–740. doi: 10.1016/j.fertnstert.2006.11.132. [DOI] [PubMed] [Google Scholar]
- 29.Francavilla S, D'abrizio P, Rucci N, et al. Fas and Fas ligand expression in fetal and adult human testis with normal or deranged spermatogenesis. J Clin Endocrinol Metab. 2000;85:2692–2700. doi: 10.1210/jcem.85.8.6723. [DOI] [PubMed] [Google Scholar]
- 30.Cavallini G, Magli MC, Crippa A, Ferraretti AP, Gianaroli L. Reduction in sperm aneuploidy levels in severe oligoasthenoteratospermic patients after medical therapy: a preliminary report. Asian J Androl. 2012;14:591–598. doi: 10.1038/aja.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]