Abstract
Introduction
Oncological remission along with high postoperative functionality [continence and erectile function (EF)] are the main aspects of prostate cancer (PCa) treatment. The aim of this study was to compare functional and oncological treatment results achieved after a nerve-sparing radical prostatectomy (RP) via transperitoneal (TPRP), extraperitoneal (EPRP) and robot-assisted (RARP) approach.
Material and methods
From March 2015 to March 2016, 507 RP were performed at the Institute for Urology and Reproductive Health (Moscow, Russia). A total of 264 patients with localized (cТ1а–2с) prostate cancer [prostate-specific antigen (PSA) <20 ng/ml, Gleason score ≤7], intact prostate capsule (according to MRI), International Index of Erectile Function (IIEF-5) ≥19 and a life expectancy >10 years were included into the retrospective study. All the surgeries were performed by a single surgeon. The outcomes were evaluated after urethral catheter removal and 3–6–12 months after RP.
Results
Nerve preservation (NP) was performed for 153 patients without significant distinctions in time (р = 0.064) and blood loss (р = 0.073). The International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-SF) score was lower for NP: 9.23 ±6.59 and 3.86 ±5.38 after 3 and 12 months respectively compared with continence after RP without nerve preservation (NP): 14.27 ±5.1 vs. 6.15 ±4.76 (р <0.001). Continent was 52.2% vs. 83.3% vs. 81.8% in TPRP, RARP and EPRP groups; р <0.001. IIEF-5 scores were 14.67 ±9.4, 4.2 ±4.26 and 4.0 ±2.07 after RARP, TPRP and EPRP respectively (р = 0.002). After 12 months the PSA: TPRP = 0.11 ±0.19, RARP = 0.03 ±0.05 and EPRP = 0.53 ±1.87 ng/ml (р <0.001). Outcomes depend on surgical approach and was better in the RARP-group (AUC = 0.768 ±0.034 (CI 95% 0,701–0.834; р <0.001).
Conclusions
We suggest RARP with NP as a method of choice for treatment of prostate cancer in patients interested in preservation of EF and quality of life in general.
Keywords: continence, erectile function, extraperitoneal, laparoscopy, prostate cancer, robot-assisted radical prostatectomy, robotic surgery, intrafascial nerve-sparing
INTRODUCTION
Prostate cancer (PCa) is the most common malignancy in males [1]. Radical prostatectomy (RP) is the gold standard for treatment of localized PCa [2]. RP can be performed by open, laparoscopic [transperitoneal (TPRP), extraperitoneal (EPRP)] or robot-assisted (RARP) approach.
The recommendations of the European Association of Urology emphasize the modern tendencies, in which RARP continues to actively become the intervention of choice [3].
The aim of this study was to compare functional and oncological treatment results achieved after a TPRP, EPRP and RARP.
MATERIAL AND METHODS
Research design and patient characteristics
From March 2015 to March 2016, 507 RP were performed. A total of 264 patients with localized (cT1a-2c) PCa (PSA <20 ng/ml, Gleason score ≤7) [2] and a life expectancy of over 10 years were included into the prospective study. The following negative prognostic factors served as criteria for exclusion from the study: tumor progression stage ≥ pT2c (n = 16), verified lymphatic metastasis (n = 2), positive surgical margins (n = 3), adjuvant therapy during 12 months of observation (n = 7), lack of compliance with regimen in PDE-5 (tadalafil), daily dosing of 5 mg (n = 1) and PSA control every 3 months (n = 1). Patients with history of androgen deprivation therapy (n = 3) were not included in the study. All the surgeries were performed by a single experienced surgeon. In order to assess co-morbidity, the following parameters were evaluated: patient age, body mass index (BMI) and potential postoperative complications risk via the ASA-PS scale after anesthesiologist consultation [4].
An extended pelvic lymph node dissection (PLND) was performed on patients with intermediate oncological risk (PSA = 10–20 ng/ml or Gleason score = 7, or cT2b) and lymphatic pathology risk of 5% according to the Briganti nomogram [2].
To assess early oncologic treatment effect, PSA dynamics were assessed with an interval of 3 months. An increase of PSA over 0.2 ng/ml in two consecutive measurements was established as a biochemical recurrence [5].
A nerve-sparing surgery was performed if a patient opted to preserve erectile function and had an absence of oncological contraindications [6, 7]. Robotic operations were performed by means of the Da Vinci System.
After removal of the urethral catheter, and at 3, 6, 12 months follow-up data from the DRIP-test and International Consultation on Incontinence Questionnaire-Urinary Incontinence Short Form (ICIQ-SF) were assessed. Continence was defined as the use of no pads [3].
Preoperative erectile function was tested in all patients according to the International Index of Erectile Function (IIEF-5) [8, 9]. Erectile dysfunction was diagnosed if IIEF-5 was below 19. None of the patients had diabetes or stroke in their history [10]. Quality of life of urological patients was assessed according to the Quality of Life (QoL) scale [11].
As a matter of prophylaxis and correction of erectile function postoperatively, patients were administered PDE-5 inhibitors. During discharge, patients received recommendations to perform Kegel exercises and come for an obligatory consultation 3 months after PSA analysis or earlier in case of complaint manifestation.
Surgical technique
Surgical treatment was performed via one of three approaches: TPRP, EPRP and RARP. In order to create a surgical working ground for the extraperitoneoscopic approach, a balloon-dissector was inserted into the Retzius retropubic space. A structural port was later installed along with 4 working ports (11 mm, 10 mm, two 5 mm). The laparoscopic approach accounted for the above-mentioned ports, but they were located 2–3 cm above. Next, excision of anterior prostatic fat was performed, which allowed us to visualize the border between the prostate and urinary bladder (UB). Next, the traction of the UB was performed, depending on the approach method: traction by means of Kelly forceps in the cranial direction in case of TPRP and EPRP or through traction made manually by grasper hold if RARP was performed. After dissection of the UB cervix the balloon-catheter was deflated. The prostate was lifted via catheter traction and a ligature, placed inside the catheter. The catheter was released into the suprapubic area and fixated with a clamp when performing a TPRP and EPRP, and in case of RARP a similar procedure was performed using a third robotic arm.
UB cervix dissection during a robot-assisted RP was performed using a monopolar dissector, for EPRP and TPRP – via an ultrasound scalpel.
The prostate was extracted without cutting the pelvic fascia or puboprostatic ligaments [12]. Nerve-sparing was done intrafascially without thermal cutting towards the urethra. Prostate cruxes were clipped by non-absorbable polymer clips and a double titanium clip. Dissection of the prostate from the dorsal venous complex and the urethra was performed with an intraabdominal pressure of 20 mm Hg. The dorsal venous complex was stitched with a polyglycolic acid thread (3/0, 15–23 cm). The urethra was cut using cold scissors. The preservation of the urethra was a priority during all stages of treatment. RARP was combined with a posterior Rocco reconstruction. The anastomosis was formed between the urethra and urinary UB cervix via monocryl (3/00, suture material with two needles (5/8), length was 26 cm, for robot-assisted surgery – monofilament incision thread (3/0, two threads 15 cm with 5/8 double needle ends).
After anastomosis, the tendinous arch was sutured to the UB. The specimen was extracted through a container via a trocar incision in the paraumbilical space. ‘Surgicel’ hemostatic sponges were used when necessary. Active drainage was removed postoperatively within 24 hours. The urethral catheter was removed depending on the vesicourethral anastomosis durability during cystographic examination.
According to the world-standards all the morphological specimens were investigated to reveal/exclude positive surgical margin [13, 14].
Statistical analysis
Non-parametric Kruskal-Wallis criteria was used for multiple intergroup comparisons. In case of statistically proven differences between groups, the Mann-Whitney test was conducted. Non-parametric Wilcoxon test was used to analyze dynamic change in matched samples. Relevance of observed differences in categorical data was analyzed by the Pearson chi-squared test. Spearman rank correlation coefficient was used to analyze the correlation between approaches, nerve-sparing technique and functional and oncological treatment results. Multiple factor analysis was performed by the logistic regression model. To analyze recurrence-free period (RFP) Kaplan-Meier estimator was applied. The Mantel-Cox log-ranking test and Breslau test were used to analyze statistically significant differences in RFP rates after intergroup categorization. When analyzing RFP, censored cases were considered when patients lived with no signs of oncologic recurrence during the 12 months observation period. Non-censored cases were considered in case of recurrence within 12 months postoperatively. To determine precursors of statistically significant RFP – function change, the Cox regression analysis model was used. All tests were two-sided, and p values ≤0.05 were considered statistically significant. All statistical analysis was performed using SPSS v.17.0 software.
RESULTS
All acquired data was comparable, with no statistically significant distinctions among the groups in age, body mass index, prostate size, transrectal ultrasonic examination (TRUS), PSA level and Gleason index (p >0.05) (Table 1).
Table 1.
Demographic, clinical, perioperative and morphological data of patients depending on surgical approach
| Total, n = 231 | TPRP, n = 75 | RARP, n = 90 | ERP, n = 63 | P value | |
|---|---|---|---|---|---|
| Age, years | 63.0 (60.0–67.0) | 65.0 (62.0–68.0) | 63.0 (59.0–67.0) | 64.0 (61.0–67.0) | 0.075 |
| Body mass index, kg/m2 | 28.0 (26.26–30.0) | 27.78 (26.0–30.1) | 28.0 (26.0–30.0) | 28.94 (27.76–29.86) | 0.070 |
| TRUS prostate volume, cm3 | 36.00 (27.0–50.0) | 42.0 (27.0–56.0) | 33.5 (27.0–40.0) | 30.5 (28.0–50.0) | 0.120 |
| Procedure time, min | 180.0 (145.0–205.0) | 180.0 (130.0–235.0) | 200.0 (180.0–210.0) | 147.5 (120.0–160.0) | <0.001 |
| Blood loss, mL | 200.00 (100.0–300.0) | 200.00 (150.0–400.0) | 200.00 (100.0–300.0) | 200.00 (150.0–300.0) | 0.017 |
| Nerve sparing, % – bilateral – none |
66.2 33.8 |
13.7 69.2 |
56.9 3.8 |
29.4 26.9 |
<0.001 |
| Lymph node dissection, % – extended – none |
50.6 49.4 |
60.0 40.0 |
63.3 37.7 |
27.7 73.3 |
<0.001 |
| Cathether removal, day | 8.0 (8.0–9.0) | 8.0 (8.0–10.0) | 8.0 (7.0–9.0) | 8.0 (8.0–8.0) | 0.001 |
| Pad-test | 0.0 (0.0–1.0) | 1.0 (0–1.0) | 0 (0.0–0.0) | 0.0 (0.0–1.0) | <0.001 |
| ICIQ–score SF – 3 months – 12 months |
13.0 (5.0–15.0) 3.0 (0.0–8.0) |
6.0 (0–9.0) 1.0 (0–1.0) |
10.0 (0–14.0) 0.0 (0–6.0) |
13.5(10.0–15.0) 4.0 (3.0–7.0) |
<0.001 <0.001 |
| Continence, % | 72.7 | 52.0 | 83.3 | 81.8 | <0.001 |
| IIEF–5 score – Preoperative – 12 months* |
20.0* (19.0–21.0) 5.0 (0–19.0) |
19.0 (19.0–20.0) 3.0 (0–8.0) |
20.0 (19.0–20.0) 19.0 (8.0–21.0) |
21.0 (20.0–21.0) 5.0 (5.0–5.0) |
0.020 0.001 |
| QoL | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 2.0 (1.0–2.0) | 0.019 |
| Tumor stage, % – pT1с – pT2a – pT2b – pT2c |
45.5 18.2 5.2 31.2 |
24.0 24.0 16.0 36.0 |
50.0 23.3 0.0 26.7 |
63.6 4.5 0.0 31.8 |
<0.001 |
| Pathologic Gleason score | 6.0 (6.0–7.0) | 6.0 (6.0–7.0) | 6.0 (6.0–7.0) | 6.5 (6.0–7.0) | 0.041 |
| PSA, ng/mL – Preoperative – 3 months – 6 months – 12 months |
8.0 (5.88–10.0) 0.01 (0.008–0.05) 0.02 (0.08–0.8) 0.03 (0.08–0.8) |
8.0 (6.0–9.0) 0.25 (0.008–0.8) 0.3 (0.008–0.8) 0.3 (0.008–0.8) |
8.8 (5,8–12,24) 0.0095 (0.001–0.02) 0.0095 (0.001–0.19) 0.01 (0.004–0.04) |
7.0 (5.0–8.0) 0.0225 (0.01–0.1) 0.075 (0.025–0.12) 0.08 (0.02–0.2) |
<0.001 <0.001 <0.001 <0.001 |
ICIQ – International Consultation on Incontinence Questionnaire, TRUS – transrectal ultrasound, IIEF-5 – International Index of Erectile Function, QoL – Quality of Life, PSA – prostate-specific antigen
Average operating time was 143.9 ±34.9 minutes for EPRP, 185.0 ±63.5 – for TPRP and 203.0 ±33.8 – for RARP (p ≤0.005).
Nerve-sparing RP was performed in 153 patients. This operating technique was not accompanied by significant distinctions in surgery time (p = 0.064) and average blood loss (p = 0.073).
One half of patients received a PLND (50.6%): robotic approach (63.3%) or laparoscopic approach (60.0%) were used more often than extraperitoneoscopic approach (22.7%). In the postoperative period, three complications were observed: one patient manifested lymphocele after enhanced pelvic laparoscopic PLND, lump drainage was necessary (Clavien grade 3a). Another patient after an extraperitoneoscopic approach surgery manifested with a hematoma, which was managed conservatively (Clavien grade 1). One patient after TPRP showed medium contrast leakage as a result of anastomosis partial failure. When examining morphological data, the presence of tumor cells in the anastomosis area was negative (negative surgical margin, R0). Absence of malignant invasion into surrounding structures was verified – capsule was not penetrated (up to pT2c).
The urethral catheter was removed on the 8.82 ±2.55 day: 9.05 ±2.8 day if EPRP was performed; 9.52 ±2.97 – in case of TPRP and 8.07 ±1.62 – after RARP (p <0.001).
The urethral catheter was removed on 8th (8.0–9.0) day in case of nerve-sparing RP, and in case of RP without nerve preservation (NP) – on the 8th (8.0–10.0) day (p = 0.087).
Continence outcomes
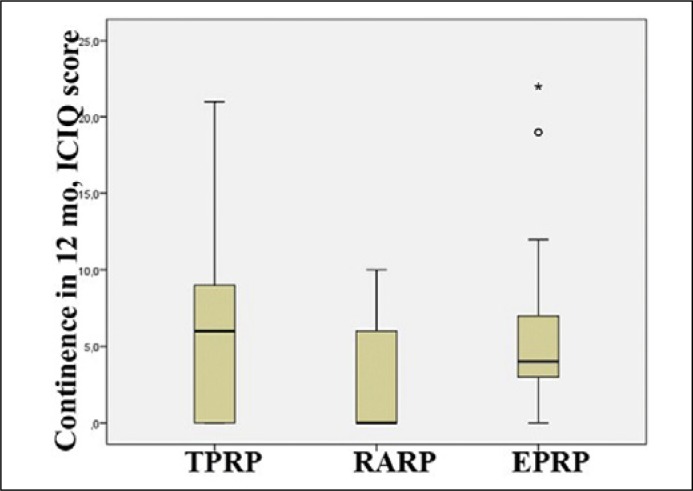
As per the data of ICIQ-SF scale after 3 months, distinctions in regard to urine continence in the observation groups were as follows: incontinence manifestation after TPRP and EPRP was severe; average grade was 13.24 ±5.25 and 13.05 ±5.13 respectively. At 3 months after RARP, the score did not exceed the average values (7.47 ±7.0, p <0.05). At 12 months after TPRP (Figure 1), the grades were 6.20 ±5.77, after EPRP – 5.74 ±0.0 and after RARP 2.73 ±3.77, for moderate and mild incontinence respectively.
Figure 1.
Continence at 12 months after radical prostatectomy, ICIQ score.
TPRP – transperitoneal, RARP – robot-assisted, EPRP – extraperitoneal
In accordance with survey data of 24-hour pad-test one year after surgery, patients tend to use 1.14 ±1.7 urological pads after TPRP; 0.37 ±0.99 – after RARP and 0.96 ±2.24 – after EPRP. Paired intergroup comparison was performed: EPRP and RARP (p = 0.07), RARP and TPRP (p <0.0001), TPRP and EPRP (p = 0.006).
Urinary continence at 12 months (0 pads) was achieved for 72.7% patients. In TPRP group 52.2% patients, were continent compared to 83.3% in RARP and 81.8% in EPRP group (p <0.001).
Multiple logistic regression was used to predict complete continence in the surgical treatment of PC. Factors reducing possibility of full urine continence are patient age (CI 95% 0.866–0.983; p = 0.013) and volume of prostate (CI 95% 0.976–1.0; p = 0.045). Nerve-sparing increases possibility of urinary continence (CI 95% 1.27–6.549; p = 0.011). Application of robotic technology was also a significant factor (p = 0.049).
Better scores in ICIQ-SF were observed with nerve-sparing: 9.23 ±6.59 and 3.86 ±5.38 after 3 and 12 months respectively, which is significantly better than after RP without nerve-sparing: 14.27 ±5.1 and 6.15 ±4.76 scores (p <0.001). Number of pads at 12 months is lower in the group with nerve-sparing RP (0.71 ±1.74 vs. 0.94 ±1.58; p <0.006).
Erectile function
When analyzing data acquired via IIEF-5, the results of 57 patients were included, whose data in the preoperative period was ≥19 scores (TPRP, n = 15; RARP, n = 15; EPRP, n = 27). Initial data of selected patients before prostatectomy did not manifest any statistically significant distinctions: 19.4 ±0.5 scores in case of TPRP; 20.2 ±1.65 – in case of RARP and 20.8 ±0.78 – in case of EPRP (p = 0.154).
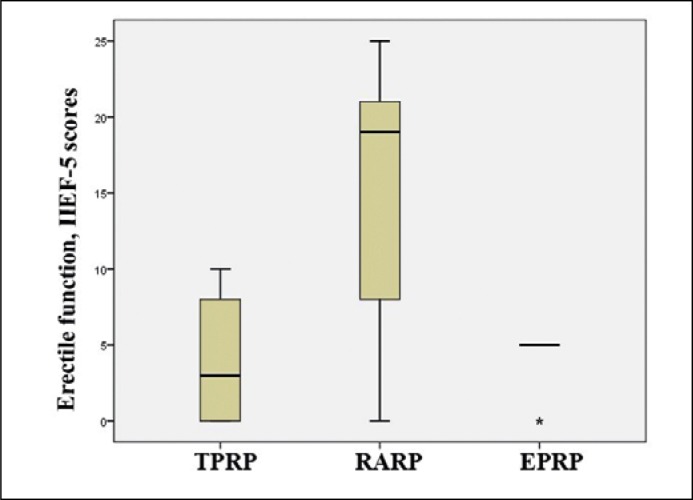
At 12 months post-op, the IIEF-5 score for these patients was 14.67 ±9.4, 4.2 ±4.26 and 4.0 ±2.07 scores after RARP, TPRP and TPRP, respectively (p = 0.002) (Figure 2).
Figure 2.
Erectile function as per IIEF-5 score after surgery.
TPRP – transperitoneal, robot-assisted, EPRP – extraperitoneal
When sorting the groups according to nerve-sparing technique application, it was discovered that patients after RP without NP had significant erectile dysfunction or complete loss of this function, when compared with patient groups with NP (5.0 (0–10.0) vs. 6.5 (0.8–19.0) score; p = 0.271): 96.2% vs. 72.2% (p <0.001).
Oncologic results
When assessing early oncologic results, the share of ‘censored’ cases (cases without biochemical recurrence) was 92.2%. Average recurrence-free period for all the patients was 11.57 ±0.11 months (CI 95% 11.35–11.79). Kaplan-Meier curve shows more than 90% probability of RFP before expiration of the annual observation term in all patients who underwent RPE.
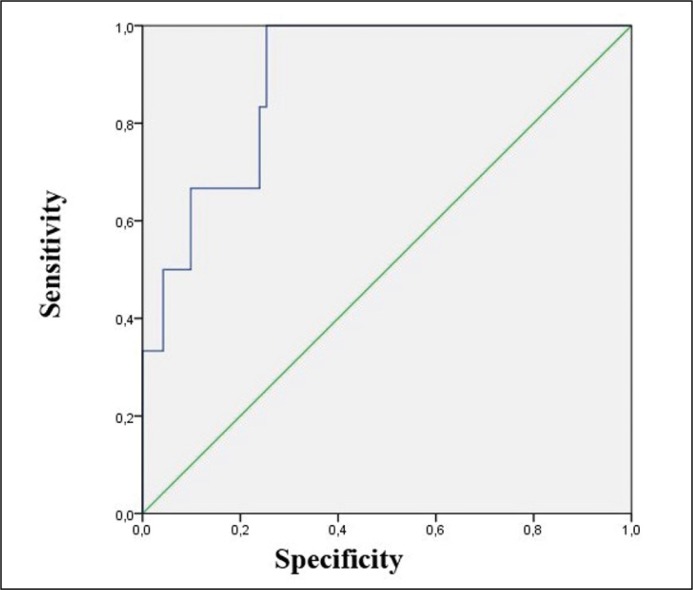
In the Cox regression model, the following factors were set as statistically significant predictors of RFP influence after PC surgical treatment: laparoscopic approach, NP, T-stage, high PSA level before surgery, Gleason score, as per the data used for ROC-curve plotting AUC value was 0.894 ±0.028 (CI 95% 0.839–0.950) which shows the high quality of this prognostic model (Figure 3).
Figure 3.
Receiver operating characteristic curve of Cox regression model of recurrence-free period after radical prostatectomy.
Method of multiple logistic regression analysis was applied for forecast of biochemical relapse after annual observation period. The following factors showed statistically significant influence on relapse cases among tentative predictors: surgical approach (p = 0.005), NP (p <0.001) and PSA level before surgery (p = 0.012). As per the data of ROC-curve plotting AUC value was equal to 0.934 ±0.021 (CI 95% 0.893–0.975) (p <0.001), and this proves the high quality of the prognostic model.
There was no recurrence in RARP group, which was significant comparing to other methods (p = 0.001). Average RFP in cases of TPRP and EPRP were equal to 11.16 ±0.28 months and 11.46 ±0.21 months, respectively, and they did not manifest statistically significant distinctions from each other.
PSA after 3, 6 and 12 months of observation were: after TPRP PSA levels – 0.16 ±0.54, 0.07 ±0.012 and 0.11 ±0.19; after RARP the values – 0.02 ±0.04, 0.02 ±0.04 and 0.03 ±0.05; after EPRP – 0.06 ±0.08, 0.1 ±0.12 and 0.53 ±1.87 (p <0.01). PSA levels were higher in patients after EPRP, and were minimal for patients after RARP (p <0.001).
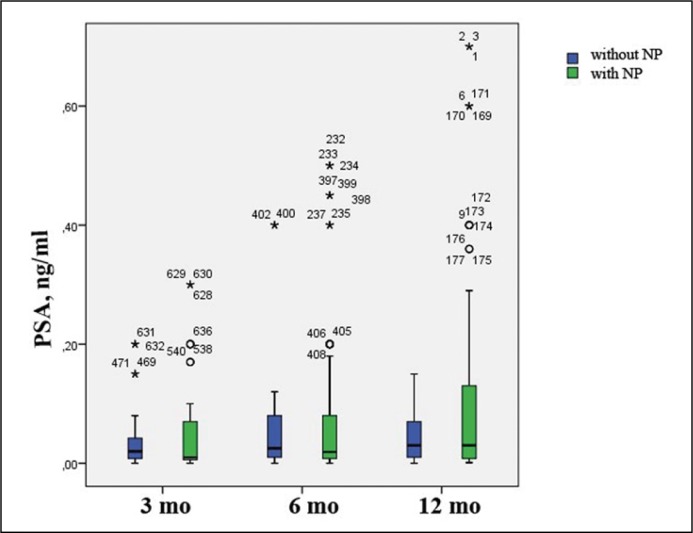
After RP with NP – 0.093 ±0.36, 0.066 ±0.11 and 0.105 ±0.17 respectively; after RP without NP – 0.035 +/-0.05, 0.051 ±0.08 and 0.389 ±1.73 (p = 0.94) (Figure 4).
Figure 4.
Dynamic changes of prostate-specific antigen (PSA) levels (ng/ml) 3, 6 and 12 months after surgery depending on nerve preservation (NP).
When assessing patient RFP depending on NP, censored cases account for 94.9% for procedures without NP, and 90.2% after surgery with NP. Difference in RFP in cases of nerve sparing RP and RP without NP were statistically insignificant: 11.47 ±0.16 vs. 11.77 ±0.15 mo (p = 0.217).
Significant difference in the duration of the recurrence-free period, without elevation of PSA, dependent on NP was obtained among patients who underwent TPRP. Censored cases after TPRP with NP account for 98.1%, for cases without NP – 42.9%. All the patients without NP survived without recurrence of the pathological process during the first 12 months post-op. In case of NP, recurrence-free period was 9.00 ±0.82 (CI 65% 7.40–10.60) months (p <0.001). In case of EPRP, statistically significant distinctions between surgery with NP and without NP were not found: 11.60 ±0.22 vs. 11.14 ±0.21 mo (p = 0.320).
Biochemical recurrence occurred more often after TPRP (16.0%) and EPRP (9.1%; p = 0.05). Positive treatment results were more persistent with RARP: there were no patients with early biochemical recurrence (p = 0.001).
A year after surgery, satisfactory life quality was achieved in 94.8% of respondents (according to QoL). The best QoL improvement was after RARP (p = 0.002) (40.0% – ‘good’). In TPRP and EPRP ‘generally satisfactory’ prevailed (56.0% and 27.3%, respectively, p <0.001). NP correlated with a statistically significant improvement of life quality: 1.63 ±1.16 vs. 1.88 ±1.02 (p = 0.035).
DISCUSSION
As a result of in-group comparison, blood loss volume was significantly less in case of TPRP surgery than after RARP (p = 0.017), but when compared to EPRP, the difference was insignificant (p = 0.878).
According to the ICIQ-SF survey of urine continence, a tendency to progressive improvement of results can be seen: higher rates of insignificant and mild incontinence towards the 3rd–12th months post-op, less incidence of severe incontinence. Noticeable differences from other approach methods can be seen in the robotic-approach (p <0.001) – only after a RARP mild incontinence (2.73 ±3.77 scores) is present, and 60.0% of patients did not have any signs of incontinence at all (p <0.0001) [15].
When analyzing the effect of operative approach on the restoration of erectile function, we obtained volatile data. The IIEF-5, at 12 months post-op, was 14.67 ±9.4 for 15 patients who received robot-assisted prostatectomy, 4.2 ±4.26 for 15 patients after TPPE, 4 ±2.07 for 27 patients after EPRP (p = 0.002). This shows a greater retention of erectile function in patients after a robot-assisted prostatectomy. Bowel complications are usually associated with languid intestinal motility, more expressive pain and lead to longer hospital stay. In the present study, we did not encounter any of these complications. Several factors influence the achievement of optimal postoperative results: laparoscopic approach, NP, T-stage, PSA levels preoperatively, and Gleason score. These factors have a high prognostic value in regard to tumor recurrence (ROC curve). The fact that a laparoscopic approach accounts for slightly less quality of functional results can be due to the fact that trocars were placed 2 cm higher than the traditionally used port localization. This leads to less mobility for the surgeon when performing the prostatectomy (with or without NP), which can have a negative effect on urine continence and erectile function as well as the ability of precise dissection of the prostate apex, where the positive surgical margin is most often located.
Since the primary goal of NP is retention of the erectile function, not only is high precision a priority, but also minimal electrosurgical intervention in the cavernous nerve zone is required. This is why several authors [16] have noted that implementation of this technique requires longer operative time and greater blood loss. When analyzing general operative data such as blood loss (p = 0.073) and operative time (p = 0.064), we did not find any statistically significant difference.
Due to classification of neurovascular bundle preservation (full/partial/no-preservation), we subjectively suppose, that intrafascial NP provides for a complete bundle conservation.
In our study, NP influenced the functional postoperative results. Initially, NP was implemented to save erectile function in eligible patients, however, this endpoint was achieved fully (IIEF-5 ≥19) only in 12.0% of patients (p <0.001), and a complete loss of erectile function after a RP 12 months postoperatively did not occur. More so, NP in patients with complete neurovascular bundle conservation correlated with lower risk of erectile dysfunction (46.0% vs. 88.5%, p< 0.001).
Influence of NP on urine continence is still a topic of discussion [15, 17, 18]. Our data suggest that complete urine continence was achieved in the group of patients who underwent nerve-sparing RP (82.4% vs. 53.8%, p <0.001). More so, patients in this group who manifested with incontinence, had mild and moderate incontinence according to the ICIQ-SF-score, when severe and very severe incontinence were characteristic of RP without NP (p <0.001). Nerve-sparing RP positively improved integral patient quality of life characteristics (p = 0.035).
Despite the fact that most authors point out a lack of correlation between method of approach and oncological treatment results, our data provides a basis to propose a possible improvement of oncological treatment results with the RARP. Minimal PSA levels of 0.03 ±0.05 (p <0.01) and a minimal incidence of biochemical recurrence was noted for the patient group with robot-assisted prostatectomy. Meanwhile there was no significant correlation between NP and PSA levels (p = 0.94), median recurrence-free period was not statistically different (p = 0.217).
Our study has some limitations; it was a single-centre, single-surgeon, short series with short follow-up research and a retrospective design.
CONCLUSIONS
In our study, the nerve-sparing technique correlated with higher urine continence and erectile function. These advantages are achieved without loss of operative radicalism, which allows us to suggest that the nerve-sparing technique is an advisable and viable method of incontinence and erectile dysfunction prevention when performing a RP in select patient groups. The most visible positive dynamics are characteristic of the RARP group, in which patient continence scores were more favorable. Most patients in the RARP group showed quicker rehabilitation times and generally better postoperative results.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Mottet N, Bellmunt J, Bolla M, et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Mottet N, Bellmunt J, Briers E, et al. Guidelines on Prostate Cancer. 2015.
- 4.Sankar A, Johnson SR, Beattie WS, Tait G, Wijeysundera DN. Reliability of the American Society of Anesthesiologists physical status scale in clinical practice. Br J Anaesth. 2014;113:424–432. doi: 10.1093/bja/aeu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidenreich A, Bastian PJ, Bellmunt J, et al. Guidelines on prostate EAU, Part II: cancer. treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 6.Eifler JB, Feng Z, Lin BM, et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int. 2013;111:22–29. doi: 10.1111/j.1464-410X.2012.11324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schoots IG, Petrides N, Giganti F, et al. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. 2015;67:627. doi: 10.1016/j.eururo.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 8.Mandel P, Kretschmer A, Chandrasekar T, et al. The effect of BMI on clinicopathologic and functional outcomes after open radical prostatectomy. Urol Oncol. 2014;32:297–302. doi: 10.1016/j.urolonc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Michl U, Tennstedt P, Feldmeier L, et al. Nerve-sparing surgery technique, not the preservation of the neurovascular bundles, leads to improved long-term continence rates after radical prostatectomy. Eur Urol. 2016;69:584–589. doi: 10.1016/j.eururo.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Burkhard FC, Lucas MG, Berghmans LC, et al. EAU Guidelines on Urinary Incontinence in Adults. 2016.
- 11.Wei J, Dunn R, Litwin M, Sandler H, Sanda M. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56:899–905. doi: 10.1016/s0090-4295(00)00858-x. [DOI] [PubMed] [Google Scholar]
- 12.Student V, Vidlar A, Grepl M, et al. Advanced Reconstruction of Vesicourethral Support (ARVUS) during Robot-assisted Radical Prostatectomy: One-year Functional Outcomes in a Two-group Randomised Controlled Trial. Eur Urol. 2017;71:822–830. doi: 10.1016/j.eururo.2016.05.032. [DOI] [PubMed] [Google Scholar]
- 13.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, Grading Committee (2016) The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am J Surg Path. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Shigemura K, Hinata N, Muramaki M, Miyake H, Fujisawa M. Histological evaluation of nerve sparing technique in robotic assisted radical prostatectomy. Indian J Urol. 2014;30:268–272. doi: 10.4103/0970-1591.128500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ficarra V, Novara G, Rosen RC, et al. Systematic review and meta-analysis of studies reporting urinary continence recovery after robot-assisted radical prostatectomy. Eur Urol. 2012;62:405–417. doi: 10.1016/j.eururo.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 16.Tavukçu HH, Aytac O, Atug F. Nerve-sparing techniques and results in robot-assisted radical prostatectomy. Investig Clin Urol. 2016;57(Suppl 2):S172–184. doi: 10.4111/icu.2016.57.S2.S172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima Y, Hamakawa T, Kubota Y, et al. Bladder neck sling suspension during robot-assisted radical prostatectomy to improve early return of urinary continence: A comparative analysis. Urology. 2014;83:632–639. doi: 10.1016/j.urology.2013.09.059. [DOI] [PubMed] [Google Scholar]
- 18.Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol. 2012;62:418–430. doi: 10.1016/j.eururo.2012.05.046. [DOI] [PubMed] [Google Scholar]