Significance
RIPK1 is a key signaling molecule controlling inflammation and cell death. Molecular targeting of RIPK1 is considered to be an attractive therapeutic strategy for inflammatory diseases or autoimmunity. However, the precise function of RIPK1 in human health and disease remains a matter of debate. Here, we report that human RIPK1 deficiency results in both immune and intestinal epithelial cell dysfunctions. Our studies provide insights into the pleiotropic functions of human RIPK1 and warrant awareness about potential toxicities of therapeutic strategies targeting RIPK1.
Keywords: primary immunodeficiency, inflammatory bowel diseases, rare diseases
Abstract
Receptor-interacting serine/threonine-protein kinase 1 (RIPK1) is a critical regulator of cell death and inflammation, but its relevance for human disease pathogenesis remains elusive. Studies of monogenic disorders might provide critical insights into disease mechanisms and therapeutic targeting of RIPK1 for common diseases. Here, we report on eight patients from six unrelated pedigrees with biallelic loss-of-function mutations in RIPK1 presenting with primary immunodeficiency and/or intestinal inflammation. Mutations in RIPK1 were associated with reduced NF-κB activity, defective differentiation of T and B cells, increased inflammasome activity, and impaired response to TNFR1-mediated cell death in intestinal epithelial cells. The characterization of RIPK1-deficient patients highlights the essential role of RIPK1 in controlling human immune and intestinal homeostasis, and might have critical implications for therapies targeting RIPK1.
Single-gene inborn errors of immunity underlie diverse pathologies such as infection, allergy, autoimmunity, autoinflammation, and malignancy. Until now, the discovery of more than 350 monogenic immune disorders has opened unprecedented insights into genes and pathways orchestrating differentiation and function of the human immune system (1). Very early onset inflammatory bowel diseases (VEO-IBDs) may also result from inborn errors of immunity, as evidenced by IL-10R deficiency (2). Although the spectrum of monogenic disorders affecting the intestinal immune homeostasis has recently expanded, most patients with VEO-IBDs lack a genetic diagnosis. It is of great therapeutic relevance to define underlying genetic defects: Whereas disorders of the hematopoietic system can be cured by allogeneic hematopoietic stem cell transplantation (HSCT), intrinsic defects in epithelial or stromal cells require other therapeutic strategies. The discovery of patients with monogenic diseases highlights the functional relevance of genes and pathways, provides a basis for the development of targeted therapies for both rare and common diseases, and may add to a critical appraisal of anticipated effects or side effects of therapies (3).
The receptor-interacting serine/threonine-protein kinase 1 (RIPK1) is a key signaling molecule controlling inflammation and cell death responses through both scaffolding- and kinase-specific functions. In particular, RIPK1 is known to mediate multimodal signaling downstream of TNFR1 depending on cell type and biological context (4). While TNF-α–induced NF-κB nuclear translocation promotes cell survival and inflammatory signaling, modulation of intracellular signaling cascades can also induce caspase-8 (CASP8)–mediated apoptosis or RIPK3-dependent necroptosis in the absence of CASP8 (4). The exact mechanisms controlling the multimodal transition switches from RIPK1-mediated cell survival and inflammation to cell death remain largely unknown.
Mice with constitutive deletion of Ripk1 die perinatally due to hyperinflammation and increased sensitivity to TNF-α–induced cell death and RIPK3-mediated necroptosis (5, 6). Depending on the context, murine RIPK1 deficiency might be associated with increased sensitivity to both RIPK3-dependent necroptosis and TNF-α– and/or CASP8-dependent apoptosis (5–7). Studies on conditional Ripk1 knockout (KO) mice have demonstrated that RIPK1 plays a critical role in controlling skin and intestinal inflammation, autoimmunity, and tissue fibrosis (8–11). RIPK3–MLKL–dependent necroptosis has been described as a common pathomechanism. However, the triggers and ligands relevant for activation of the necroptotic pathway in vivo remain poorly understood. Furthermore, RIPK1 has also been implicated in murine hematopoiesis (12), T and B cell homeostasis (13, 14), and inflammasome activity (5).
A pathogenic role of RIPK1 has been previously linked to multiple mouse models of disease, including colitis, skin inflammation, myocardial infarction, atherosclerosis, pancreatitis, and viral infections, as well as liver, retinal, and renal injury (15). Pharmacological inhibition of RIPK1 has been shown to block necroptosis and protect from ischemic organ damage (16). Small-molecule inhibitors of RIPK1 activity are currently being evaluated for patients with psoriasis, rheumatoid arthritis, and ulcerative colitis (17). Recently, RIPK1 has also been implicated in tumorigenesis and proposed as a therapeutic target in melanoma (18), colon cancer (19), and leukemia (20). However, the relevance of RIPK1 for human pathogenesis and the balance of anticipated effects and side effects of targeting RIPK1 are still discussed controversially.
Here, we report that biallelic loss-of-function mutations in human RIPK1 cause impaired innate and adaptive immunity and predispose to VEO-IBD.
Results
Identification of Patients with Biallelic Mutations in RIPK1.
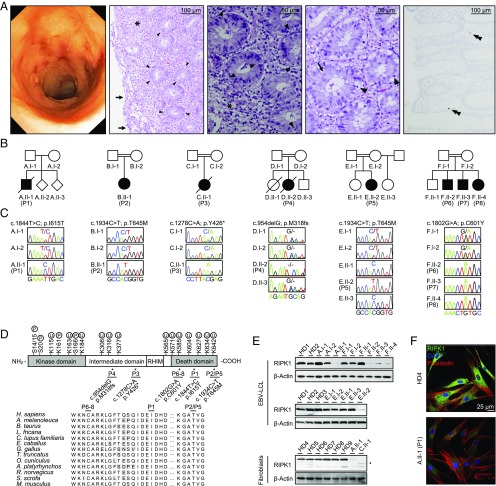
Our index patient (P)1 (A.II-1) born to Caucasian parents from Poland presented with VEO-IBD characterized by growth failure, abdominal pain, chronic mucous and bloody diarrhea, oral aphthous lesions, and perianal lesions at the age of 6 mo. Endoscopy confirmed the diagnosis of pancolitis with ulcers and granuloma (Fig. 1A, Left), esophagitis, and gastric ulcers. Histology of gastric and colonic biopsies revealed chronic-active inflammation with erosions (Fig. 1A, second panel from Left), increased apoptotic bodies within the cryptic bases (Fig. 1A, third and fourth panels from Left), and subtotal depletion of lamina propria plasma cells (Fig. 1A, Right). Extraintestinal manifestations included hepatosplenomegaly, maculopapular skin and transient atopic skin lesions, recurrent fever, and infections (pneumonia, conjunctivitis), including episodes of deep-seated infections and sepsis in the neonatal phase. He showed a refractory course despite amino acid-based formula, parenteral nutrition, antibiotics, steroids, azathioprine, and ileostomy and succumbed to septicemia at the age of 4 y. To decipher the molecular cause of disease in this patient, we have conducted whole-exome sequencing and found a rare homozygous missense mutation in the RIPK1 gene (NM_003804.3, c.1844T>C; NP_003795.2, p.I615T) (Fig. 1 B and C). Further screening for biallelic RIPK1 mutations in more than 1,942 patients with VEO-IBDs and/or primary immunodeficiencies identified another seven patients from five unrelated pedigrees with homozygous germ-line mutations in RIPK1 (Fig. 1 B and C). The sequence variants in RIPK1 have been deposited in the ClinVar database (21) (accession nos.: SCV000854770–SCV000854774). While P3 (c.1278C>A, p.Y426*) and P4 (c.954delG, p.M318IfsTer194) primarily manifested with combined immunodeficiency associated with lymphopenia, P2 (c.1934C>T, p.T645M), P5 (c.1934C>T, p.T645M), P6 (c.1802G>A, p.C601Y), P7 (c.1802G>A, p.C601Y), and P8 (c.1802G>A, p.C601Y) were primarily referred for genetic testing due to signs of VEO-IBD. All patients suffered from recurrent bacterial and/or viral infections and had episodes of diarrhea. Perianal disease was reported in all patients except for P3. Further clinical details for the patients are summarized in SI Appendix, Table S1. Segregation of the RIPK1 mutations with the disease phenotype could be confirmed by Sanger sequencing of available first-degree family members (Fig. 1C). In silico analysis using PolyPhen (22) and SIFT (23) predicted that the identified missense mutants in RIPK1 are deleterious. These homozygous mutations have not been previously reported in the Genome Aggregation Database (24). Sequence homology analysis revealed that the mutated amino acids in the death domain of RIPK1 are evolutionarily conserved (Fig. 1D). Immunoblotting of EBV-transformed lymphoblastoid cell lines (EBV-LCLs) from pedigrees A (P1), E (P5), and F (P6, P7, P8) and primary fibroblasts from pedigrees A (P1) and C (P3) (Fig. 1E), as well as confocal immunofluorescence microscopy of fibroblasts from pedigree A (P1) (Fig. 1F), demonstrated a reduced protein expression of mutated RIPK1. P3 carrying a frameshift mutation in RIPK1 showed reduced expression of a truncated protein.
Fig. 1.
Identification of biallelic RIPK1 mutations in patients with combined immunodeficiency and pediatric inflammatory bowel disease. (A) Colonoscopy showing pancolitis with ulcers and granuloma in P1 (Left). Histology of colonic biopsies from P1 revealed chronic-active inflammation (asterisk) with erosions of the mucosal surface (arrow) (second image from Left) and epithelial degeneration. Higher magnification displays epithelial regeneration with increased mitotic activity (arrow) and apoptotic bodies (arrowhead) within the crypt epithelium (third and fourth images from the Left). Multiple myeloma oncogene 1 immunohistochemistry indicated subtotal depletion of plasma cells (Right, double arrowhead). (B) Pedigrees of six families (A to F) with patients (P1 to P8) presenting with primary immunodeficiencies and/or VEO-IBDs. (C) Sanger sequencing confirmed segregation of the biallelic RIPK1 mutations with the disease phenotype in available first-degree relatives. (D) Schematic illustration of the RIPK1 protein domain architecture (NM_003804.3, NP_003795.2). Alignment of the human RIPK1 protein sequence showed that the mutated amino acids are conserved in orthologs. RHIM, RIP homotypic interaction motif. (E) Immunoblotting of three independent experiments revealing reduced protein expression of RIPK1 in patient-derived EBV-transformed B cells (P1, P5, P6, P7, P8) or fibroblast cell lines (P1, P3) in contrast to healthy donors (HDs) or patients’ relatives. Truncated RIPK1 protein expression in P3 is indicated by an asterisk. (F) Representative confocal immunofluorescence microscopy images confirming reduced expression of RIPK1 in fibroblasts from P1, compared with HDs.
P3 and P4 presented with lymphopenia affecting T and B cells (SI Appendix, Table S2). Immunophenotyping of peripheral blood mononuclear cells from P1, P6, P7, and P8 showed a decreased frequency of CD45RO+CCR7+ central memory and CD45RO+CCR7− effector memory CD4+ and CD8+ T cells (SI Appendix, Fig. S1 A and B), CD45RO+HLA-DR+ memory activated regulatory T cells (SI Appendix, Fig. S1C), and CXCR3+CCR6− T-helper 1 (Th1) and CXCR3−CCR6+ T-helper 17 (Th17) populations (SI Appendix, Fig. S1D), as well as IgD−CD27+ class-switched B cells (SI Appendix, Fig. S1E), whereas P5 exhibited no measurable changes in these parameters (SI Appendix, Table S3). These data suggest that RIPK1 deficiency may lead to combined T and B cell dysfunction. However, T cell proliferation, activation, and cell death in response to anti-CD3, anti-CD3/CD28, or anti-PMA/ionomycin were normal. In addition, we could not observe a significant difference in cell death in RIPK1-deficient Jurkat cells upon treatment with FAS ligand, TNF-α ± BV6 (the second mitochondrial activator of apoptosis mimetic), or TNF-α ± cycloheximide in comparison with RIPK1 wild-type (WT) reconstituted cells (SI Appendix, Fig. S2).
Defective TNF-α–Mediated NF-κB Signaling in RIPK1-Deficient Cells.
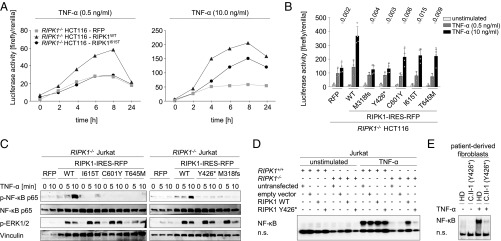
RIPK1 regulates multimodal signaling downstream of TNFR1 in a cell- and context-dependent manner (25). To assess the consequences of identified mutations for RIPK1 downstream signaling, we engineered colon carcinoma-derived HCT-116 cells with a CRISPR/Cas9-mediated RIPK1 KO and subsequent lentiviral overexpression of WT or mutant RIPK1 variants. NF-κB luciferase reporter assays showed that cells expressing the RIPK1 variant I615T (identified in P1) exhibited impaired NF-κB activity in response to TNF-α, compared with cells with WT RIPK1 (Fig. 2A). Similarly, we could detect reduced luciferase activity for all five identified RIPK1 mutants after TNF-α stimulation (Fig. 2B). Correspondingly, immunoblotting revealed reduced phosphorylation of the NF-κB p65 subunit (Ser536) in Jurkat cells with RIPK1 KO or expression of mutant RIPK1 (Fig. 2C), whereas phosphorylation of ERK1/2 (Thr202/Tyr204) was normal. Electrophoretic mobility-shift assays confirmed reduced NF-κB DNA-binding activity in Jurkat cells expressing the RIPK1 mutant Y426* (Fig. 2D) and fibroblasts of P3 (Fig. 2E) in response to TNF-α, compared with WT RIPK1 reconstituted Jurkat cells and healthy donor fibroblasts, respectively. These data indicate that the identified mutations in RIPK1 are associated with impaired TNF-α–induced NF-κB signaling.
Fig. 2.
Biallelic loss-of-function RIPK1 mutations lead to impaired NF-κB–mediated signaling. (A and B) Representative luciferase reporter assays showed reduced NF-κB activation upon TNF-α stimulation in HCT-116 cells with RIPK1 KO or lentiviral-mediated overexpression of RIPK1 mutants, compared with WT RIPK1. Data shown represent the mean ± SD. (C) Representative immunoblotting (n = 3) of serum-starved RIPK1−/− Jurkat cells with transgenic expression of mutant RIPK1 variants revealed decreased phosphorylation of the NF-κB p65 subunit (Ser536) in response to TNF-α (50 ng/mL), whereas phosphorylation of ERK1/2 was normal. (D and E) Representative EMSA (n = 3) showed reduced DNA-binding activity of the NF-κB p65 subunit in Jurkat cells overexpressing the RIPK1 mutation Y426* (D) or fibroblasts derived from P3 (E) after TNF-α stimulation (50 ng/mL) for 30 min. n.s., nonspecific bands.
Altered Inflammasome Activity in RIPK1-Deficient Macrophages.
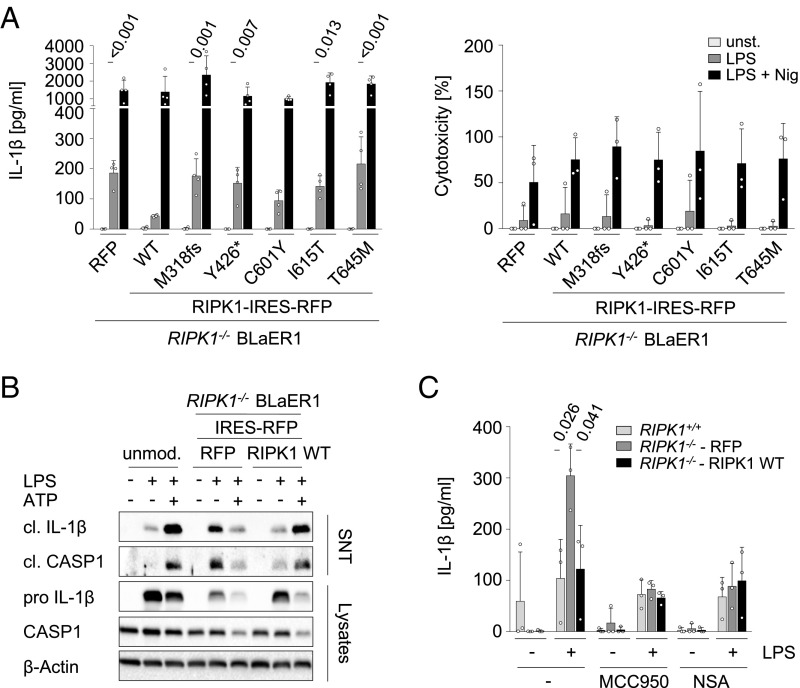
Previous studies have documented an altered inflammasome activity in conditional Ripk1 KO mice (5, 26). To examine effects of the identified RIPK1 mutations on inflammasome activity, we have adapted a BLaER1 monocyte cell model with KO of CASP4 and RIPK1 (27) and reconstituted the patients’ mutations by lentiviral gene transfer. In contrast to cells with reconstitution of WT RIPK1, cells with KO of RIPK1 or overexpression of the RIPK1 mutants (M318fs, Y426*, I615T, and T645M) showed increased IL-1β secretion without the requirement of a secondary stimulus for the processing of mature IL-1β (Fig. 3A). Increased inflammasome activity in RIPK1-deficient macrophages was not associated with increased cytotoxicity upon LPS priming for 3 h, as indicated by the LDH assay (Fig. 3A). Of note, no difference of IL-1β secretion could be observed upon addition of nigericin between cells with overexpression of WT and RIPK1 mutants (Fig. 3A). Immunoblotting confirmed increased release of mature IL-1β upon treatment with LPS in RIPK1-deficient macrophages (Fig. 3B). To test whether the altered IL-1β release is associated with increased NLPR3 activity and/or MLKL-dependent necroptosis in human RIPK1 deficiency, we assessed the inflammasome activation upon treatment with small-molecule inhibitors of NLRP3 (MCC950) and MLKL (NSA) (Fig. 3C). The inhibitors reduced IL-1β secretion in LPS-stimulated RIPK1-deficient macrophages, suggesting that both pathways might be implicated in dysregulation of proinflammatory responses. Taken together, our results suggest that human RIPK1 plays a critical role in regulating LPS-mediated inflammasome activation.
Fig. 3.
RIPK1 deficiency is associated with increased inflammasome activity upon LPS priming. (A) ELISA showed increased release of IL-1β upon LPS priming (20 ng/mL, 3 h) in conditioned media from heterologous BLaER1 cells with RIPK1 KO or overexpression of RIPK1 mutant variants. P values are analyzed in comparison with cells expressing WT RIPK1 (Left). No difference in inflammasome activation of RIPK1-deficient macrophages has been observed upon LPS + nigericin in contrast to WT RIPK1. Data are representative of four independent experiments. LDH release assays of three independent experiments showed no difference of cytotoxicity between RIPK1 WT and mutant BLaER1 cells upon stimulation with LPS ± nigericin (Right). (B) Representative immunoblotting (n = 4) of heterologous RIPK1-deficient BLaER1 cells confirmed increased secretion of the mature IL-1β and cleavage of CASP1 in comparison with unmodified or WT RIPK1-expressing cells (LPS, 200 ng/mL, 14 h). (C) Analysis of IL-1β release in RIPK1−/− BLaER1 cells overexpressing RFP or WT RIPK1 upon treatment with LPS ± small-molecule inhibitors for NLPR3 (MCC950) or MLKL (NSA) (three independent experiments). Data shown in A and C represent the means ± SD. cl., cleaved; SNT, supernatant.
Impaired TNF-α–Mediated Cell Death Responses in RIPK1-Deficient Epithelial Cells.
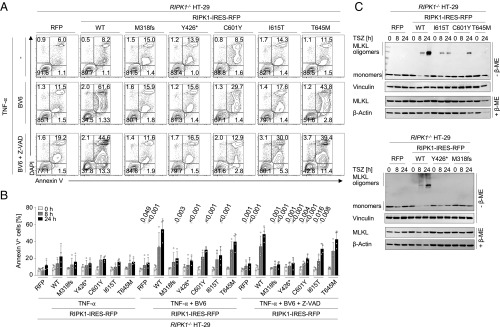
RIPK1 and RIPK3 are critical regulators of cell death (28). To study the effect of patients’ mutations on TNF-α–mediated cell death responses in epithelial cells, we engineered HT-29 colon carcinoma cells with KO of RIPK1 and lentiviral reconstitution of WT or mutant RIPK1 variants. No alteration of cell death could be observed upon treatment with TNF-α in RIPK1-deficient HT-29 cells (Fig. 4 A and B). However, cell death responses were impaired upon treatment with TNF-α and BV6 ± the pan caspase inhibitor Z-VAD-FMK in cells expressing mutated RIPK1 variants (M318fs, Y426*, C601Y, and I615T) compared with cells overexpressing WT RIPK1 (Fig. 4 A and B). Correspondingly, immunoblotting showed reduced MLKL oligomerization in RIPK1-deficient HT-29 cells in response to TNF-α, BV6, and Z-VAD-FMK, suggesting impaired necroptosis under conditions of RIPK1 deficiency (Fig. 4C).
Fig. 4.
RIPK1-deficient intestinal epithelial cells show altered cell death responses. (A) Representative FACS analysis of Annexin V/DAPI staining (n = 4) in HT-29 cells expressing mutant RIPK1 upon 24 h of treatment with TNF-α ± BV6 ± Z-VAD-FMK. (B) Graphical representation (n = 4) showing decreased frequencies of Annexin V+ RIPK1-deficient cells compared with cells with WT RIPK1 after stimulation with TNF-α + BV6 ± Z-VAD-FMK. (C) Representative SDS/PAGE under nonreducing conditions (n = 3) revealed reduced MLKL oligomerization upon treatment with TNF-α + BV6 + Z-VAD-FMK in RIPK1-deficient cells. Data shown represent the means ± SD. ME, mercaptoethanol; TSZ, TNF-α + BV6 (SMAC mimetic) + Z-VAD-FMK.
Discussion
The functional relevance of RIPK1 in human disease has been controversially discussed. We report RIPK1 deficiency as a Mendelian disorder predisposing to immunodeficiency and severe colitis. Whereas it may appear counterintuitive at first sight to associate immunodeficiency and hyperinflammatory responses, several monogenic diseases have a poorly understood Janus-faced appearance, for example autoimmune lymphoproliferative syndrome caused by TNFRSF6 (29) and CASP10 (30) deficiency or lymphoproliferation and autoimmunity caused by IL2RA null mutations (31).
Constitutive Ripk1 KO mice appear to exhibit no developmental defects but show perinatal mortality associated with systemic multiorgan inflammation and apoptosis in lymphoid and adipose tissues (32). A potential role of RIPK1 in pathogenesis has been documented in several models of inflammation and tissue damage (16). In particular, conditional ablation of Ripk1 has been reported to result in severe intestinal and skin inflammation associated with FADD-CASP8–dependent apoptosis of intestinal epithelial cells and ZBP1-RIPK3–MLKL–dependent necroptosis of keratinocytes, respectively (8, 11). Our patients with homozygous mutations in RIPK1 showed no obvious developmental defects, and predominantly presented with immunodeficiency and diarrhea or colitis. Whereas children with complete loss of function of RIPK1 (P3, stop-gain mutation; P4, frameshift mutation) primarily manifested with combined immunodeficiency and diarrhea, patients with missense mutations in the death domain of RIPK1 were referred for genetic testing due to IBD-like conditions. Differences in clinical manifestation might be reflective of genotype–phenotype correlations and incomplete penetrance, or may be due to secondary factors such as genetic modifiers, infections, and treatment. Emerging evidence suggests that kinase-independent RIPK1 functions are critical in controlling intestinal epithelial homeostasis (5, 6, 8, 11). None of our identified patients had mutations directly affecting the kinase domain of RIPK1, but the identified mutations perturbed total protein expression. Therefore, our study cannot unequivocally define whether the abrogated kinase activity is critical in mediating intestinal inflammation in our patients.
Mice with Ripk1 KO in intestinal epithelial cells develop colitis accompanied by disrupted tissue architecture and increased apoptosis (8, 11). In parallel investigations, Cuchet-Lourenço et al. (33) identified four patients with loss-of-function mutations in RIPK1 causing combined immunodeficiency and intestinal inflammation due to altered cytokine secretion and necroptosis of immune cells. Whereas these authors concluded that allogeneic HSCT may constitute a curative therapy, our studies suggest that RIPK1 plays a critical role in controlling cell death of the intestinal epithelium, and thus warrant awareness that HSCT might dampen intestinal inflammation but not rescue intrinsic intestinal phenotypes of human RIPK1 deficiency, similar to NF-kappa-B essential modulator deficiency (34). The exact triggers perturbing epithelial integrity in RIPK1 deficiency could not be fully determined in our studies or mouse models yet. Further studies are required to shed light on cell- and context-dependent functions of RIPK1 in controlling intestinal inflammation in vivo.
Necroptosis has been previously linked to the pathogenesis in various disease models such as atherosclerosis, myocardial infarction, ischemic brain injury, systemic inflammation, liver injury, and neurodegeneration (16). Targeting RIPK1 and RIPK3 represents an attractive therapeutic strategy for diseases with increased necroptotic activity. Necrostatin-1 allosterically inhibits RIPK1 activity and has been shown to block necroptosis in mouse models of ischemia (16). Recently, a small molecule (GSK2982772) has been developed as an inhibitor of RIPK1 to treat plaque-type psoriasis, rheumatoid arthritis, and ulcerative colitis in phase 2a clinical studies (17). The beneficial effects of this therapeutic strategy in patients still remain unclear. Inhibition of RIPK1 activity might be considered in patients with severe or refractory inflammatory or autoinflammatory diseases. Our study on RIPK1-deficient patients highlights that human RIPK1 has pleiotropic cell- and context-specific functions and thus warrants awareness about potential toxicities of targeting RIPK1.
Taken together, we report that patients with biallelic RIPK1 deficiency present with life-threatening combined immunodeficiency and/or intestinal inflammation associated with impaired lymphocyte functions, increased inflammasome activity, and altered TNF-α–mediated epithelial cell death responses. Thus, our study highlights the central role of RIPK1 in controlling human immunity and intestinal homeostasis.
Materials and Methods
Patients.
Peripheral blood and skin biopsies from patients, first-degree family members, and healthy donors were acquired upon written consent. The study was approved by the respective institutional review boards of the University of Ulm, Necker Medical School, and University Hospital, LMU Munich and conducted in accordance with current ethical and legal frameworks.
Genetic, Immunologic, and Biochemical Analyses.
Methods of genetic analyses, immunological studies, and biochemical and cell biological assays as well as statistics are described in SI Appendix.
Supplementary Material
Acknowledgments
We are grateful to the interdisciplinary medical staff. We acknowledge the assistance of the Flow Cytometry and Care-for-Rare Genomics Core Facility at the Dr. von Hauner Children’s Hospital, as well as the FACS facility of the Department of Pediatrics and Department of Molecular Diagnostics of the Institute for Clinical Transfusion Medicine and Immunogenetics Ulm and the Genomics and Bioinformatics Core Facility at the Imagine Institute and UFR Necker. We thank Dr. T. Graf (Center for Genomic Regulation, Barcelona) for providing BLaER1 cells, and Genentech for supplying BV6. Further, we acknowledge bioinformatics support by Dr. Jacek Puchalka. The work has been supported by The Leona M. and Harry B. Helmsley Charitable Trust, DFG (Gottfried-Wilhelm-Leibniz Program, Collaborative Research Consortium SFB1054 project A05), PID-NET (BMBF), BioSysNet, the Care-for-Rare Foundation, and INSERM. Y. Li has been supported by the China Scholarship Council, E.B. received a scholarship from the Care-for-Rare Foundation, and M. Führer received a scholarship from the “Landesgraduiertenförderungsgesetz.” D.K. has been a scholar funded by the Daimler und Benz Stiftung, Reinhard-Frank Stiftung, and Else-Kröner-Fresenius Stiftung.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The identified RIPK1 mutations reported in this paper have been deposited in the ClinVar database, https://www.ncbi.nlm.nih.gov/clinvar/ (accession nos.: SCV000854770–SCV000854774). Information on the raw whole-exome sequencing data is not published to protect research participant privacy.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813582116/-/DCSupplemental.
References
- 1.Bousfiha A, et al. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2018;38:129–143. doi: 10.1007/s10875-017-0465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glocker EO, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein C, Gahl WA. Patients with rare diseases: From therapeutic orphans to pioneers of personalized treatments. EMBO Mol Med. 2018;10:1–3. doi: 10.15252/emmm.201708365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasparakis M, Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 5.Rickard JA, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Dillon CP, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaiser WJ, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci USA. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dannappel M, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin J, et al. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature. 2016;540:124–128. doi: 10.1038/nature20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Donnell JA, et al. Dendritic cell RIPK1 maintains immune homeostasis by preventing inflammation and autoimmunity. J Immunol. 2018;200:737–748. doi: 10.4049/jimmunol.1701229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi N, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 12.Roderick JE, et al. Hematopoietic RIPK1 deficiency results in bone marrow failure caused by apoptosis and RIPK3-mediated necroptosis. Proc Natl Acad Sci USA. 2014;111:14436–14441. doi: 10.1073/pnas.1409389111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowling JP, Cai Y, Bertin J, Gough PJ, Zhang J. Kinase-independent function of RIP1, critical for mature T-cell survival and proliferation. Cell Death Dis. 2016;7:e2379. doi: 10.1038/cddis.2016.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang H, et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linkermann A, Green DR. Necroptosis. N Engl J Med. 2014;370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silke J, Rickard JA, Gerlic M. The diverse role of RIP kinases in necroptosis and inflammation. Nat Immunol. 2015;16:689–697. doi: 10.1038/ni.3206. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, et al. Discovery of a first-in-class receptor interacting protein 1 (RIP1) kinase specific clinical candidate (GSK2982772) for the treatment of inflammatory diseases. J Med Chem. 2017;60:1247–1261. doi: 10.1021/acs.jmedchem.6b01751. [DOI] [PubMed] [Google Scholar]
- 18.Liu XY, et al. RIP1 kinase is an oncogenic driver in melanoma. Cancer Res. 2015;75:1736–1748. doi: 10.1158/0008-5472.CAN-14-2199. [DOI] [PubMed] [Google Scholar]
- 19.Zeng F, et al. RIPK1 binds MCU to mediate induction of mitochondrial Ca2+ uptake and promotes colorectal oncogenesis. Cancer Res. 2018;78:2876–2885. doi: 10.1158/0008-5472.CAN-17-3082. [DOI] [PubMed] [Google Scholar]
- 20.Xin J, et al. Sensitizing acute myeloid leukemia cells to induced differentiation by inhibiting the RIP1/RIP3 pathway. Leukemia. 2017;31:1154–1165. doi: 10.1038/leu.2016.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landrum MJ, et al. ClinVar: Public archive of interpretations of clinically relevant variants. Nucleic Acids Res. 2016;44:D862–D868. doi: 10.1093/nar/gkv1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaser R, Adusumalli S, Leng SN, Sikic M, Ng PC. SIFT missense predictions for genomes. Nat Protoc. 2016;11:1–9. doi: 10.1038/nprot.2015.123. [DOI] [PubMed] [Google Scholar]
- 24.Lek M, et al. Exome Aggregation Consortium Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727–736. doi: 10.1038/nrm3683. [DOI] [PubMed] [Google Scholar]
- 26.Lawlor KE, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaidt MM, et al. Human monocytes engage an alternative inflammasome pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 28.Newton K. RIPK1 and RIPK3: Critical regulators of inflammation and cell death. Trends Cell Biol. 2015;25:347–353. doi: 10.1016/j.tcb.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Martin DA, et al. Defective CD95/APO-1/Fas signal complex formation in the human autoimmune lymphoproliferative syndrome, type Ia. Proc Natl Acad Sci USA. 1999;96:4552–4557. doi: 10.1073/pnas.96.8.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang J, et al. Inherited human Caspase 10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 31.Goudy K, et al. Human IL2RA null mutation mediates immunodeficiency with lymphoproliferation and autoimmunity. Clin Immunol. 2013;146:248–261. doi: 10.1016/j.clim.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelliher MA, et al. The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 33.Cuchet-Lourenço D, et al. Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science. 2018;361:810–813. doi: 10.1126/science.aar2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miot C, et al. Hematopoietic stem cell transplantation in 29 patients hemizygous for hypomorphic IKBKG/NEMO mutations. Blood. 2017;130:1456–1467. doi: 10.1182/blood-2017-03-771600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.