Significance
Most ion channels are multimeric (typically comprising 3–5 subunits). The subunits are encoded by homologous members of a gene family, generating an enormous set of possible heteromeric combinations. In this study, we provide evidence that the preferred target of conopeptide κM-RIIIJ is a heteromeric Kv1 channel consisting of three Kv1.2 subunits and one Kv1.1 or Kv1.6 subunit. We define the molecular interaction of κM-RIIIJ with these asymmetric Kv1 channels and show that in dorsal root ganglia (DRG) neurons, different κM-RIIIJ concentrations can distinguish discrete subpopulations of neurons. Our results highlight the potential of natural products and venom components, such as conopeptides, to generally elucidate native physiological roles of specific heteromeric ion channel isoforms at the cellular, circuit, and systems level.
Keywords: heteromeric Kv-channels, conotoxin kappaM-RIIIJ, Kv1.2
Abstract
The vast complexity of native heteromeric K+ channels is largely unexplored. Defining the composition and subunit arrangement of individual subunits in native heteromeric K+ channels and establishing their physiological roles is experimentally challenging. Here we systematically explored this “zone of ignorance” in molecular neuroscience. Venom components, such as peptide toxins, appear to have evolved to modulate physiologically relevant targets by discriminating among closely related native ion channel complexes. We provide proof-of-principle for this assertion by demonstrating that κM-conotoxin RIIIJ (κM-RIIIJ) from Conus radiatus precisely targets “asymmetric” Kv channels composed of three Kv1.2 subunits and one Kv1.1 or Kv1.6 subunit with 100-fold higher apparent affinity compared with homomeric Kv1.2 channels. Our study shows that dorsal root ganglion (DRG) neurons contain at least two major functional Kv1.2 channel complexes: a heteromer, for which κM-RIIIJ has high affinity, and a putative Kv1.2 homomer, toward which κM-RIIIJ is less potent. This conclusion was reached by (i) covalent linkage of members of the mammalian Shaker-related Kv1 family to Kv1.2 and systematic assessment of the potency of κM-RIIIJ block of heteromeric K+ channel-mediated currents in heterologous expression systems; (ii) molecular dynamics simulations of asymmetric Kv1 channels providing insights into the molecular basis of κM-RIIIJ selectivity and potency toward its targets; and (iii) evaluation of calcium responses of a defined population of DRG neurons to κM-RIIIJ. Our study demonstrates that bioactive molecules present in venoms provide essential pharmacological tools that systematically target specific heteromeric Kv channel complexes that operate in native tissues.
Ion channels open to allow ion flow across an otherwise impermeable membrane. The functional channel protein may consist of a single α-subunit with multiple similarly organized domains (e.g., voltage-gated Na+ and Ca2+ channels) or by the assembly of monomeric α-subunits that associate noncovalently to form the functional pore. Thus, several homologous genes may encode the monomeric α-subunits that comprise a functional ion channel. Examples include TRP (transient receptor potential), Kv (voltage-gated potassium channels), and the ligand-gated channels. In these families, combinations of multiple α-subunit isoforms give rise to a vast array of functional channels.
More than 70 different genes encode mammalian K+ channel α-subunits constituting the most diverse ion channel family with thousands of potential multimeric arrangements. A central question in integrating the molecular/cellular knowledge into systems neuroscience is which specific complexes of these diverse families are physiologically relevant?
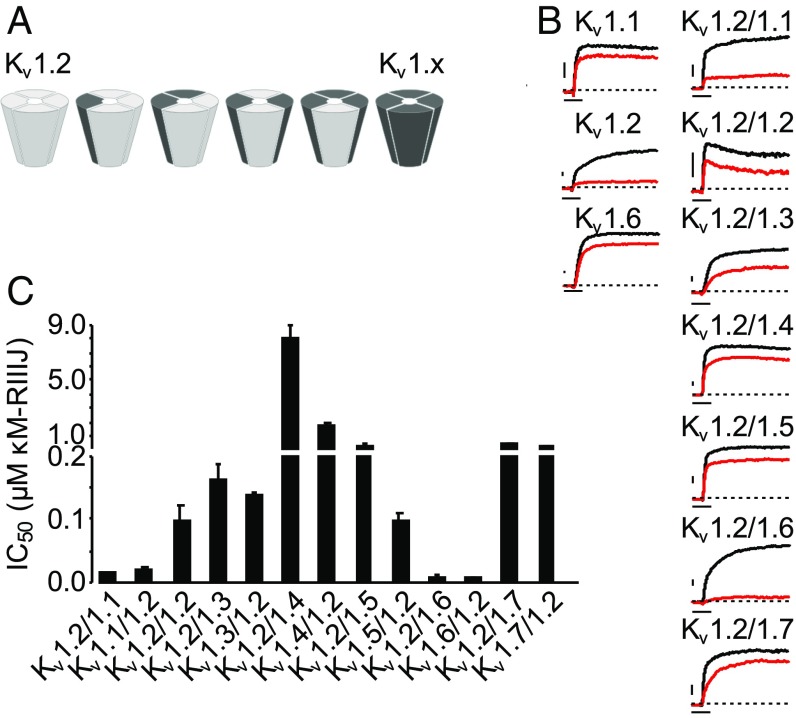
The problem addressing this fundamental question is schematically illustrated in Fig. 1A. There, the combination of only two different Kv1 subunits produces six alternative channels with varying levels of functional specialization. Theoretically, the heteromerization of the seven members of the Kv1 family (Kv1.1–1.7) investigated here potentially generate hundreds of tetrameric complexes that are virtually indistinguishable in native tissues as the biophysical properties often overlap. The straightforward approach of ablating individual α-subunits (gene knockouts) would result in the functional deletion of all of the different complexes containing that subunit, complicating the interpretation of the resulting phenotypes.
Fig. 1.
κM-RIIIJ blocks homomeric and heteromeric Kv1 channels. (A) Schematic representation of all possible combinations between two different Kv1 subunits. Homomeric and heteromeric channels containing Kv1.2 α-subunits and a second other Kv1 family (Kv1.x) subunit are displayed. Similar combinations are possible for all Kv1.x subunits with up to four different subunits forming a functional channel. (B) κM-RIIIJ block of homo- and heterodimeric Kv1 channels. Current traces in control (black) and in the presence of κM-RIIIJ (red) are shown. (Left) At 1 µM, κM-RIIIJ blocks homomeric Kv1.1 and Kv1.6 with low apparent affinity (IC50 ∼ 3–5 µM), whereas it displays ∼10× higher potency for Kv1.2 homomers (IC50 ∼ 300 nM). (Right) Representative dimeric channel-mediated currents in control and 100 nM κM-RIIIJ evidencing stronger block of Kv1.2/1.1 and Kv1.2/1.6 channels. Due to extreme differences in apparent affinity, 50 nM κM-RIIIJ is shown for Kv1.2/1.1 and 2 μM for Kv1.2/1.4. Dashed lines indicate zero current. (Scale bars, 500 pA and 10 ms.) (C) κM-RIIIJ affinity toward Kv1.2-containing dimeric Kv1 channels. The bar diagram presented summarizes κM-RIIIJ IC50s for dimeric constructs in forward or reversed order. In comparison with homomeric Kv1.2 channels, κM-RIIIJ has a 10- to 20-fold higher affinity for heterodimeric channels composed of Kv1.2 and either Kv1.1 or Kv1.6 subunits. All other dimeric constructs are blocked similarly or less strongly than homomeric Kv1.2. The order of the subunits in the concatemers bears no major influence on κM-RIIIJ’s activity.
A large body of literature suggests that most neuronal Kv channels are in fact heteromeric combinations of one to four different α-subunits of loosely defined molecular identity. Here, we devised a neuropharmacological strategy to systematically dissect the contribution of single Kv1 α-subunits to heteromeric complexes by exploiting venom peptides that presumably evolved to alter prey behavior and appear “surgically” targeted to specific heteromeric K+ channel complexes. We have examined the specificity of κM-conotoxin RIIIJ from the venom of piscivorous cone snail Conus radiatus, which was initially described as a Kv1.2 antagonist (1). The current study provides a thorough assessment of its activity against a variety of Kv1.2 containing heteromeric Kv1 channels. The potency and exquisite selectivity of κM-RIIIJ suggests that this peptide evolved to target asymmetrically arranged heteromeric Kv channels instead of the symmetric Kv1.2 homomer as previously reported.
Thus, this study has important general implications: if natural products like venom-derived peptides evolved to target native voltage-gated K+ channels, their highest-affinity targets may well be heteromeric combinations. Accordingly, naturally evolved K+ channel-targeted ligands like κM-RIIIJ may be key to determining the composition and function of heteromeric voltage-gated K+ channels in biological systems.
Results
κM-RIIIJ Preferentially Targets Heteromeric Kv Channels.
Block of homomeric Kv1 channels.
As previously noted, assessing the vast diversity of potential Kv1 channel combinations is a formidable endeavor, as represented by the schematic (Fig. 1A) of the combinations possible from only two different subunits. In this work we investigated the effects of κM-RIIIJ on evoked K+ currents of homotetrameric hKv1.1–7 channels and their heterodimeric combinations by patch clamp on HEK293 cells. Whole-cell current recordings of homomeric channels revealed that κM-RIIIJ affects Kv1.2 channels with an IC50 of about 300 nM, whereas all other Kv1 channels showed IC50s in the µM range or were not blocked at all (Kv1.4 and Kv1.5; SI Appendix, Table S1). These results are in agreement with those reported previously from two-electrode voltage clamp in Xenopus oocytes (1) and demonstrate that κM-RIIIJ displays ∼10-fold higher apparent affinity for homomeric Kv1.2 channels than for other Kv1 channels including Kv1.1 and Kv1.6 (IC50s ∼ µM).
Block of Kv1 heterodimeric channels.
Dimeric concatemers composed of one Kv1.2 α-subunit linked to a Kv1.x (x = Kv1.1–7) were constructed. The stoichiometry and arrangement of subunits was controlled by generating two sets of concatemers, in which Kv1.2 provided either the free N-terminal of the dimer (Kv1.2/Kv1.x) or the free C-terminal of the dimer (Kv1.x/Kv1.2), thus forming heterodimeric channels (A/B-A/B or B/A-B/A). Whole-cell patch-clamp experiments evidenced robust expression of all constructs in HEK293 cells (Fig. 1B) after 24–48 h. As readily seen in Fig. 1B (Right), 100 nM κM-RIIIJ (red traces) blocked the various Kv1.2/Kv1.x heterodimeric constructs with remarkable differences in potency.
From the summary bar graph in Fig. 1C, various important points emerge.
-
i)
Steric constraints imposed by the concatemerization, per se, have negligible effects on κM-RIIIJ binding, based on the similar IC50s of homotetramers formed by monomeric Kv1.2 α-subunits and those assembled by the linked homodimers (98.3 ± 24.3 nM, n = 7; see SI Appendix, Table S2).
-
ii)
For certain heterodimer pairs, reversing monomer order has minimal effect. Kv1.2/1.3 and Kv1.3/1.2 dimers show comparable IC50 values of 165 ± 22 nM (n = 5) and 138 ± 3 nM (n = 2), respectively, as well as Kv1.2/1.7 (370 ± 19 nM, n = 4) and Kv1.7/1.2 (267 ± 38 nM, n = 3). This suggests that reversing order of monomers within these heterodimers yields similar binding surfaces for the κM-RIIIJ. Similarly, Kv1.2/1.5 dimers were blocked slightly less potently than those formed by Kv1.5/1.2 (287 ± 163 nM, n = 3, vs. 99 ± 11 nM, n = 4, respectively), suggesting κM-RIIIJ’s minimal ability to distinguish between these two different arrangements. Furthermore, κM-RIIIJ displayed modest block of heterodimers of Kv1.2/1.4 and Kv1.4/1.2 (IC50: 8.1 ± 2 µM, n = 3 and 1.6 ± 2.6 µM, n = 5, respectively), similar to the homomeric channel screen.
-
iii)
In striking contrast, the apparent affinity of κM-RIIIJ for heterodimers Kv1.2/1.1 (14.3 ± 2.6 nM, n = 7) and Kv1.1/1.2 (18.4 ± 6 nM, n = 10) was significantly increased in comparison with their homotetramers. Similarly, the heterodimeric Kv1.2/1.6 and Kv1.6/1.2 were strongly blocked by κM-RIIIJ with IC50s of 8.7 ± 2.1 nM (n = 14) and 6.1 ± 1.7 nM (n = 9). Thus, the apparent affinity of κM-RIIIJ toward heterodimers containing Kv1.2 is greatly influenced by the subunit composition in the heterodimeric complexes. Furthermore, the observed increase in the apparent affinity of κM-RIIIJ for some of the channels generated as linked concatemers provides a functional readout implying their correct assembly in the plasma membrane.
In summary, heterodimerization of Kv1.3–5 and Kv1.7 α-subunits with Kv1.2 results in ∼10-fold increase in κM-RIIIJ’s apparent affinity for the complex. Most dramatically, K+ currents mediated by dimers of Kv1.2 with either Kv1.1 or Kv1.6 are blocked >100-fold more potently than those flowing through their homomeric counterparts.
Block of Kv1 “asymmetric” heterotetramers.
The higher affinity of κM-RIIIJ for heterodimeric Kv1.2/Kv1.1 and Kv1.2/Kv1.6 channels, relative to their homomeric counterparts, demonstrates that relatively small differences in the binding surface of the target channel are critical to κM-RIIIJ’s activity. Functional Kv channels are formed by four independent α-subunits; therefore, a binomial arrangement of two α-subunits will have any of those subunits occupying any position of the tetramer. Thus, “symmetric” (2:2 = AABB or ABAB) or “asymmetric” channels composed of 3xA and 1xB (and vice versa) are possible. This flexibility would result in significantly different molecular recognition surfaces exposed to peptide toxins like κM-RIIIJ, which we set out to explore by generating binomial concatemers of Kv1.2 and either Kv1.1 or Kv1.6, in 2:2 and 3:1 stoichiometry, and linked in different orders.
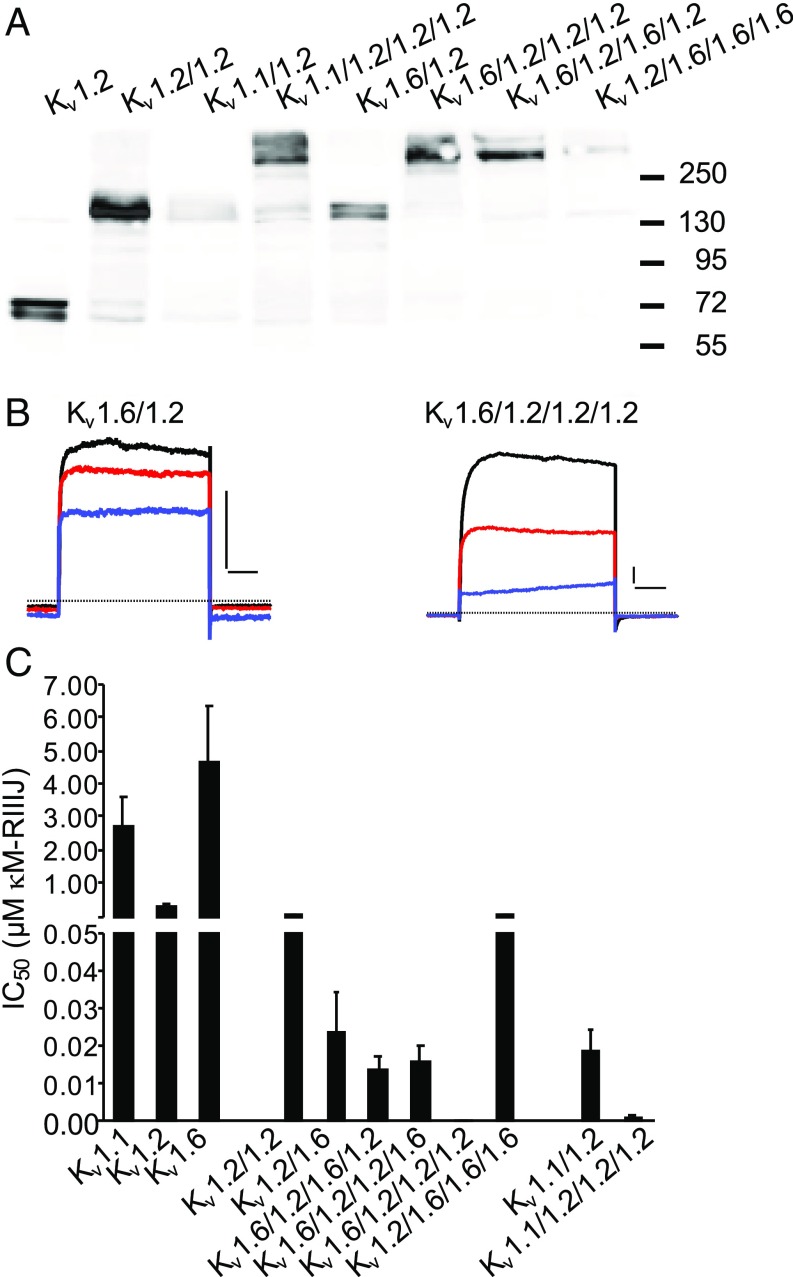
The correct and complete synthesis of the constructs used throughout this work was verified by immunodetection by an anti-Kv1.2 antibody in Western blot experiments. Fig. 2A shows an experiment, in which the protein products of representative constructs expressed in HEK293 cells were electrophoresed and immunoblotted. In this figure, clear bands at 75, 150, and 300 kDa report on monomeric, dimeric, and tetrameric channels, respectively, of expected molecular weight, as each homomer is ∼75 kDa.
Fig. 2.
κM-RIIIJ displays enhanced apparent affinity toward asymmetric Kv1 channels. (A) Western blot analysis of selected concatemeric constructs expressed in HEK293. Bands corresponding to 75-, 150-, and 300-kDa protein products were recognized by an anti-Kv1.2 antibody evidencing expression of monomeric, dimeric, and tetrameric channels, respectively. (B) Representative current traces of symmetric heterodimeric and asymmetric heterotetrameric channels composed of Kv1.2 and Kv1.6. The effect of 0.5 nM (red) and 5 nM (blue) κM-RIIIJ on evoked K+ currents from Kv1.6/1.2 dimer and tetrameric concatemers composed of three Kv1.2 subunits and one Kv1.6 subunit are shown (black: control). The zero current level is marked by the dashed line. (Scale bars, 1 nA and 50 ms.) (C) IC50s of κM-RIIIJ block of homomeric and heteroconcatemeric Kv1 channels formed by Kv1.1, Kv1.2, and Kv1.6 subunits in different combinations. κM-RIIIJ displayed extremely high apparent affinity (sub-nM) for asymmetric concatemers composed of one Kv1.1 or Kv1.6 subunit together with three Kv1.2 subunits (SI Appendix, Table S2).
Fig. 2B exhibits representative currents of binomial constructs composed of Kv1.2 and Kv1.6 channels of different stoichiometry and arrangement. The colored traces were recorded in the presence of 0.5 nM (red) and 5 nM (blue) κM-RIIIJ, showing that those currents mediated by the asymmetric (3:1) channel (Right) were preferentially blocked in comparison with the symmetric dimer of dimers (2:2; Left).
The bar graph in Fig. 2C condenses our analysis of symmetric and asymmetric channels exposed to conotoxin κM-RIIIJ. The equal inhibition of symmetric Kv1.6/1.2/1.6/1.2 and Kv1.6/1.2/1.2/1.6 channels (IC50: 13.6 ± 3.6 nM, n = 4 and 15.9 ± 4.2 nM, n = 3, respectively) shows that the “order” of the two subunits does not alter κM-RIIIJ binding. In contrast, analysis of the tetrameric concatemers that presented an asymmetric surface (3:1 stoichiometry) revealed that such configuration was determinant to high-affinity κM-RIIIJ binding. Hence, the apparent affinity of κM-RIIIJ for Kv1.1/1.2/1.2/1.2 channels was 1.2 ± 0.3 nM (n = 8), while only 0.5 ± 0.1 nM (n = 14) κM-RIIIJ was sufficient to block 50% of the Kv1.6/1.2/1.2/1.2 mediated currents (Fig. 2 B and C). These results demonstrate that the apparent affinity of κM-RIIIJ for heteromeric Kv1 channels consisting of Kv1.2 and either Kv1.1 or Kv1.6 is higher when the tetrameric channel contains only one subunit of either Kv1.1 or Kv1.6 as opposed to two. This shows that although the apparent affinity to homomers of Kv1.1 or Kv1.6 is lower by one order of magnitude compared with those of Kv1.2, the sole presence of one such subunit, either Kv1.1 or Kv1.6, is sufficient to cause a >100-fold enhancement in the affinity of κM-RIIIJ for the Kv1 complex. The observed increased sensitivity of the linked concatemeric proteins to κM-RIIIJ provides a functional readout of their correct assembly in the plasma membrane.
Furthermore, these findings show that the asymmetric surface of Kvs composed of three Kv1.2 and one Kv1.1/Kv1.6 is highly favorable to κM-RIIIJ binding. Thus, the graded effects of κM-RIIIJ are an accurate correlate to the channel composition and stoichiometry, representing a valuable tool in the study of neuronal voltage-gated Kv channels.
Molecular Insights into κM-RIIIJ Binding to Asymmetric Kv1 Heteromers.
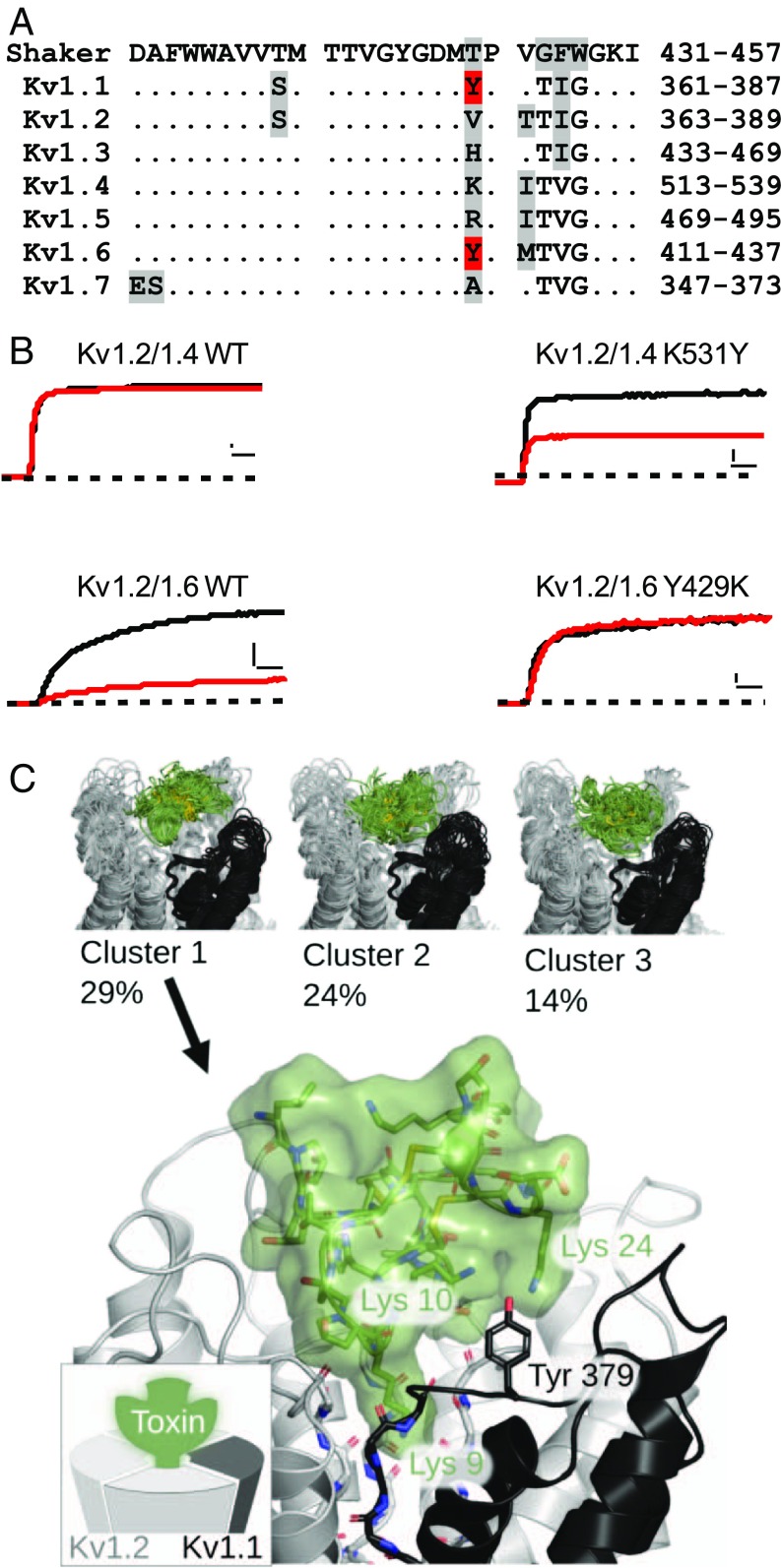
Functionally, inclusion of one subunit of either Kv1.1 or Kv1.6 leads to a profound increase in κM-RIIIJ’s affinity for Kv1.2-containing channels. Sequence alignment of the Shaker and Kv1 pore regions is shown in Fig. 3A. Visual inspection reveals that Kv1.1 and Kv1.6 distinguish themselves from the other Kv1s in that a tyrosine (Y379 and Y429, respectively) resides in the homologous position of Shaker T449, known to be critical for extracellular binding of several substances including tetraethylammonium (2). Previous studies on κM-RIIIK (a close relative of κM-RIIIJ) described it as a pore-blocking peptide (3–5), and therefore we surmised κM-RIIIJ would exert its inhibitory actions by interacting with the same channel region. Hence, a tyrosine residue was introduced in the homologous position of Kv1.4 (K531Y) within the heterodimer Kv1.2/Kv1.4, and the reciprocal mutation was made in Kv1.6 in Kv1.2/Kv1.6. Fig. 3B provides examples of current traces of the wild-type and pore mutant dimers Kv1.2/Kv1.4-K531Y and Kv1.2/Kv1.6-Y429K. Exposure to 10 nM κM-RIIIJ (red) clearly highlights the importance of such tyrosine within the heteromeric pores. Thus, Kv1.2/Kv1.4-K531Y is blocked with an IC50 of 9 ± 0.7 nM (n = 5), in stark contrast with its wild-type counterpart (8.1 ± 2 μM, n = 5). Accordingly, the mutant heterodimer Kv1.2/Kv1.6-Y429K significantly lost sensitivity to κM-RIIIJ (IC50 2.0 ± 0.2 μM, n = 4). Thus, an apparent affinity increase of ∼1,000-fold for Kv1.2/Kv1.4-K531Y and the ∼250-fold decrease in binding affinity for Kv1.2/Kv1.6-Y429K demonstrate that (i) a tyrosine residue within the heteromeric pore is a major determinant of κM-RIIIJ’s potency; and (ii) κM-RIIIJ intimately interacts with the Kv channel pore.
Fig. 3.
Binding of κM-RIIIJ to the Kv1 channel pore. (A) Pore region sequence alignment of Shaker and Kv1 channel subunits. Observe that in Kv1.1 and Kv1.6 there is a tyrosine (Y379 and Y429, respectively, in red) homologous to Shaker T449. Identical amino acids to Shaker are shown as dots. Amino acids that differ from the majority are shaded in gray. (B) Tyrosine incorporation into a κM-RIIIJ–resistant dimer Kv1.2/1.4 renders it highly sensitive. The 10 nM κM-RIIIJ hardly affects Kv1.2/1.4 mediated currents (Top Left), whereas the same [κM-RIIIJ] leads to a profound block of Kv1.2/1.4-K531Y mediated currents (Top Right). Accordingly, Kv1.2/1.6 mediated currents are potently blocked by 10 nM of κM-RIIIJ, whereas sensitivity to the same [κM-RIIIJ] was abolished in the reciprocal mutant Kv1.2/1.6-Y429K. These results underscore the importance of the bulkier aromatic residue (tyrosine) at this position for κM-RIIIJ’s high-affinity binding. Zero current is indicated by the dashed line. [Scale bars, 2 nA (dimeric-WT), 200 pA (dimeric mutants), and 10 ms.] (C) Molecular dynamics simulation for κM-RIIIJ binding to Kv1.2/1.2/1.2/1.1 heterotetramer. Trajectories have been clustered to reveal different κM-RIIIJ docking positions. Visualization of the largest cluster (constituting 29% of the observed poses) is shown in more detail. Kv1.2 subunits are colored gray in cartoon representation, while the Kv1.1 subunit is black. κM-RIIIJ is represented by the green licorice and surface. κM-RIIIJ-Lys9 interacts with the channel selectivity filter (blue) occluding the permeation pathway, while Lys10 and Lys24 interact with Kv1.1-Tyr379 (homologous to Kv1.6-Tyr429).
Introducing the equivalent K/Y mutation in the asymmetric Kv1.2/1.2/1.2/1.4 concatemer results in 200-fold higher affinity of κM-RIIIJ for Kv1.2/1.2/1.2/1.4-K531Y channels, corroborating the crucial role of this amino acid for peptide-binding strength to asymmetric channel complexes (SI Appendix, Fig. S1).
A global perspective of κM-RIIIJ association with asymmetric heterotetramers was obtained by molecular dynamics simulations. For this, the biological assembly of Kv1.2’s crystal structure (3LUT, pore residues 311–421) served as a template where the asymmetry-imposing subunits (Kv1.6 and Kv1.1) were homology-modeled and incorporated. The structure of κM-RIIIJ was based on similarity to κM-RIIIK (Methods), and interaction to Kv1.6/1.2/1.2/1.2 and Kv1.1/1.2/1.2/1.2 was assessed in silico. As depicted in Fig. 3C, molecular dynamic simulation returned three major clusters (29–14% likelihood, Top) where the likely orientation of κM-RIIIJ within the asymmetric pore indicates that lysine 9 (from κM-RIIIJ) orients itself into the channel’s permeation pathway. Fig. 3C, Bottom, provides an expanded view of Cluster 1 where K24 and K10 (κM-RIIIJ) are in close vicinity of the critical pore tyrosine from Kv1.1 (Y379) and Kv1.6 (Y429) in agreement with the functional data compiled here.
In addition, the observed role of positively charged Lys residues in κM-RIIIJ affords a mechanistic explanation toward weak binding to most of the channels containing Kv1.4 or Kv1.5 subunits. Homomeric Kv1.4 and Kv1.5, as well as heterodimers of Kv1.2 and Kv1.4, are modestly blocked by κM-RIIIJ, with the exception of heterodimers including Kv1.5, toward which κM-RIIIJ displayed higher affinity. These subunits have basic amino acids at the homologous position of Tyr in Kv1.1 and Kv1.6 (Kv1.4 K531 and Kv1.5 R487; Fig. 3A), which may exert repulsive forces over the toxin’s positively charged moieties. As shown by the electrophysiological experiments, eliminating the charge of this position in heterodimeric and tetrameric constructs restores the toxin affinity for the heteromeric constructs, further underpinning the significance of electrostatic interactions in κM-RIIIJ binding.
κM-RIIIJ Distinguishes Between DRG Neuron Subtypes.
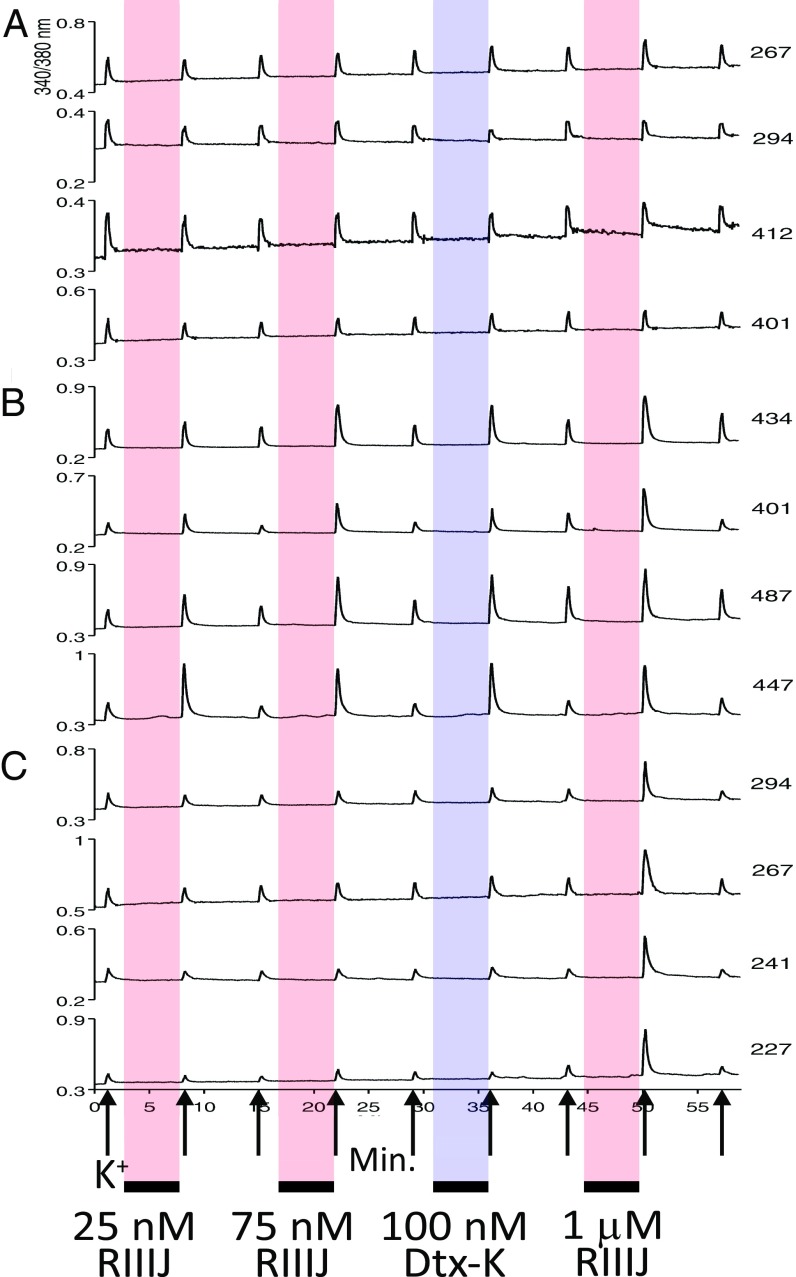
Sensory neurons in the dorsal root ganglia (DRG) express various members of the Kv1 family of K+ channels. However, the functional and pharmacological similarities between homomeric K+ channels mediated by close family relatives like Kv1.1, Kv1.2, and Kv1.6 has hindered the assignment of precise molecular correlates to the native currents observed in DRGs. An exquisitely tuned peptide like κM-RIIIJ provides the opportunity to distinguish neurons in which homo- or heteromeric Kv1 channel populations are expressed, based on their sensitivity to this conotoxin. We took advantage of the high-content Ca2+-imaging approach known as constellation pharmacology (6, 7) to explore the question posed. Fig. 4 provides a snapshot of one such experiment, in which dissociated mouse DRG neurons were loaded with Fura-2-AM and subjected to various concentrations of κM-RIIIJ and the Kv1.1-selective blocker, Dtx-K, before depolarizing stimuli. Fig. 4A displays stereotyped Ca2+ responses to depolarization with high [K+]0 that return to baseline on K+ removal, from four different neurons where neither of the toxins caused apparent changes in the Ca2+ signal. The four neurons from Fig. 4B (each had a cross-sectional cell area ∼450 µm2), however, robustly responded to the presence of three different concentrations of κM-RIIIJ (low: 25 nM and 75 nM; high: 1 µM), as well as to 100 nM Dtx-K by displaying enhanced Ca2+ peaks. The reaction of these neurons to low [κM-RIIIJ] indicates that in these cells, Kv currents are likely mediated by heteromeric channels composed of Kv1.2 subunits and those with Dtx-K sensitive Kv1.1 subunits, as a plausible partner. Therefore, we can postulate that this medium-diameter DRG neuronal cell population expresses Kv1.1/1.2 heteromeric channels. Furthermore, the Ca2+ responses shown in Fig. 4C were obtained from small (each had a cross-sectional cell area ∼250 µm2) DRG cells that only responded to the high dose of κM-RIIIJ suggesting a population where Kv1.2 homotetramers or low-affinity heterotetramers are the dominant channels expressed.
Fig. 4.
κM-RIIIJ can distinguish between different sensory neuron subtypes. Representative calcium-imaging traces from three DRG neuronal subclasses displaying distinct responses to κM-RIIIJ and Dtx-K. (A) Neurons unaffected by κM-RIIIJ or Dtx-K: Four representative neurons unaltered by the application of κM-RIIIJ (25 nM, 75 nM, and 1 μM) and Dtx-K (100 nM), suggesting the lack of functional expression of Kv1.1 and Kv1.2 channels in these neurons. (B) Neurons responsive to the application of low [κM-RIIIJ]. The examples shown correspond to neurons that displayed an increase in peak height following a depolarizing stimulus in the presence of 25 nM, 75 nM, and 1 μM κM-RIIIJ and 100 nM Dtx-K, indicating the presence of both Kv1.1 and Kv1.2 channels in these neurons. (C) Neurons exclusively responsive to high [κM-RIIIJ]. These neurons were not affected by the application of low doses (25 and 75 nM) of κM-RIIIJ nor 100 nM Dtx-K but displayed increases in peak height after exposure to 1 μM κM-RIIIJ. The low sensitivity to κM-RIIIJ and insensitivity to Dtx-K suggest the presence of homotetrameric Kv1.2 channels and the absence of homomeric Kv1.1 or heteromeric Kv1.1/1.2 complexes in this cell population. Each trace corresponds to individual cells. The x axis is time (in minutes), and the y axis tracks the ratio of relative fluorescence change (ΔF/F) excited at 340 and 380 nM. Upward arrows mark the application of the depolarizing stimulus (20 mM [K+]o, ∼15 s). Colored boxes (red: κM-RIIIJ; blue: dendrotoxin-K) indicate the time of peptide exposure (∼6 min) in between the applications of depolarizing stimulus. Numbers to the right indicate cell area in μm2.
Discussion
The physiological role of heteromeric Kv channels has rarely been explored due to the lack of reliable pharmacological tools. From the multiple cone snail toxins that interact with Kv channels, κM-RIIIJ was shown to interact with homomeric Kv1.2 channels in Xenopus oocytes. Here, we offer evidence of κM-RIIIJ’s preferential (>100-fold) inhibition of heteromeric channels composed of three Kv1.2 and one Kv1.1 or Kv1.6 α-subunits. We have identified crucial molecular interactions between κM-RIIIJ and the Kv1 channel pore that provide a rationale for the exquisite selectivity and potency of κM-RIIIJ for asymmetric Kv1 heteromers. Finally, use of κM-RIIIJ in constellation pharmacology (6, 7) experiments pinpointed identifiable subpopulations of mouse DRG neurons with distinct functional Kv1.2-containing channel subsets.
Heteromeric Kv Channels.
Although different Kv1 subunits have been identified in various cell types, most studies rely on immunodetection of channel proteins or mRNA (qPCR, hybridization probes), which do not provide accurate information about the actual composition of multisubunit complexes like heteromeric Kv1 channels at the single-cell level. In the CNS, several heteromeric combinations of Kv1.2 with either Kv1.1 or Kv1.6 have been proposed. An elegant but laborious immunoprecipitation study used antibodies to systematically deplete individual Kv subunits from tissue homogenates, revealing the presence of multiple heteromeric Kv channels in the human CNS (8). In this study, the existence of Kv1.1/Kv1.2 heteromeric channels in cortical gray matter, white matter, and spinal cord (8) was inferred in good agreement with our Ca2+-imaging results.
Additional studies using specific antibodies against various Kv1s demonstrated that Kv1 channels are predominantly found in neuronal cells within the mammalian brain. The Kv1 subunits most abundantly detected in human brain are Kv1.1, Kv1.2, and Kv1.4 [for review, see Trimmer and Rhodes (9) and Vacher et al. (10)], and often Kv1.1 colocalizes with either Kv1.2 or Kv1.4 in support of the existence of heteromeric Kv1 channels in the brain. Examples of colocalization of Kv1.1 and Kv1.2 in the absence of Kv1.4 occur in cerebellar basket cell terminals, the juxtaparanodal membrane (node of Ranvier), and in axon terminal segments. Kv1.6 seems to be found less abundantly and predominantly in interneurons. However, throughout the whole brain, some Kv1.6 staining appears on principal cell dendrites where Kv1.2 and Kv1.1 are also present (11).
Little is known about heteromeric Kv1 complexes in nonneuronal tissue and, particularly, in the heart the functional importance of heteromeric Kv1s has received little attention. A previous report from our laboratory showed that, unlike κM-RIIIJ, κM-RIIIK is cardioprotective in a rodent model of ischemia/reperfusion, despite its comparatively weaker block of homomeric Kv1.2 (1). Differential activity of these peptides on Kv1.2 heterodimeric channels provided a plausible explanation for disparate effects in such closely related peptides. The results presented in this study corroborate our previous findings in that the higher-affinity targets of κM-RIIIJ are composed of subunits not abundantly found in cardiomyocytes [for overview, see Nerbonne and Kass (12)]. Curiously, Kv1.1, Kv1.2, and Kv1.6 have been immunodetected in sinoatrial node cells in ferret hearts (13).
Our data suggest that the likely “natural” targets of κM-RIIIJ actions are neurons containing Kv1.2/Kv1.1 or Kv1.2/Kv1.6 heteromers, which would be congruent with the potential role of κM-RIIIJ in prey capture as a part of the “lightning strike cabal” (14). Our experiments identifying different subpopulations of DRG neurons by their responses to either low or high [κM-RIIIJ] strongly support the notion of Kv1.2 subunits as part of hetero- and homomeric K+ channels in the peripheral nervous system.
Asymmetric Kv1 Channel.
It is remarkable that κM-RIIIJ preferentially inhibits asymmetric Kv channels with 1:3 stoichiometry. Other channel families that assemble in tetramers composed of two related subunits in 3:1 stoichiometry may be of physiological importance. Such is the case of the modulatory Kv α-subunit Kv9.3, which does not form functional homotetramers, but participates in heteromeric arrangements of 3xKv2.1 and 1xKv9.3 subunits (15). The molecular determinants of heteromeric cyclic nucleotide-gated (CNG) channels of 3xCNGA1 and 1xCNGB1 have been described elsewhere (16, 17). Thus, it is plausible to anticipate that many other heteromeric K+ channels of asymmetric composition exist in nature. This would imply that such nonsymmetric arrangement of functional channels delineates a correspondingly asymmetric permeation pathway of significant pharmacological standing. In line with our findings, previous work showed that the affinity of TEA to Kv1.1/Kv1.2 concatemers is affected by the subunit ratio (18). Therefore, asymmetric ion channel compositions should be taken into account in the development of Kv channel-targeted substances of therapeutic potential.
κM-RIIIJ: A Tool for the Study of Heteromeric Kv1 Channels.
Our work in heterologous systems underpins the potential of κM-conotoxin RIIIJ as a valuable diagnostic tool for the identification of functional Kv1 channel complexes according to their sensitivity to this conotoxin. We verified κM-RIIIJ’s ability to distinguish among peripheral neuron subclasses expressing distinct heteromeric Kv channels by constellation pharmacology. We used the Kv1.1-selective blocker Dendrotoxin-K to identify DRG neuronal cells in which heteromeric channels formed by Kv1.1 and Kv1.2 constitute a major component of the IKDR in these cells. Results presented throughout this paper highlight the advantages of using conotoxins and, particularly, κM-RIIIJ, as pharmacological tools to enable the study of functioning heteromeric Kv channels in living cells.
Materials and Methods
Conotoxin κM-RIIIJ was synthesized as described by Chen et al. (1). The Kv1 channels used here correspond to the human isoforms: Kv1.1 (hKCNA1; NM_000217), Kv1.2 (hKCNA2; NM_004974), Kv1.3 (hKCNA3; NM_002232), Kv1.4 (hKCNA4; NM_002233), Kv1.5 (hKCNA5; NM_002234), Kv1.6 (hKCNA6; NM_002235), and Kv1.7 (hKCNA7; NM_031886).
Molecular Biology.
cDNAs coding hKv1.1–7 α-subunits were subcloned into pcDNA3.1 for expression in HEK293 cells. Dimeric concatenated Kv1 subunits were generated by replacement of the stop (anterior subunit) and start codons (posterior subunit) by AatII restriction sites according to the QuikChange Site directed Mutagenesis method (Stratagene). Tetrameric concatemers were built by fusion of dimeric constructs, in which the stop (anterior dimer) and start codons (posterior dimer) were replaced by an XhoI restriction site. All constructs were verified by sequencing.
Cell Culture and Electrophysiological Recordings.
All electrophysiological measurements were done with transiently transfected HEK293 cells in the whole-cell configuration of the patch-clamp technique at room temperature. For more detail, see SI Appendix, Supplementary Methods.
Western Blot.
Western blotting was performed by using monoclonal antibodies to Kv1.2 (NeuroMab monoclonal mouse K14/16; #75–008; 1:20,000). For more detail, see SI Appendix, Supplementary Methods.
Molecular Dynamics Simulations.
All simulations were done with GROMACS 4.6 (19–22), the amber99sb force field (23), and the SPC/E water model (24), at constant 320 K [v-rescale thermostat (25)] and pressure of 1 bar [Berendsen Barostat (26)]. Parameters used for lipids were from Berger et al. (27), and the appropriate ions were from Joung and Cheatham (28).
Kv channel models were based on the full-length Kv1.2 crystal structure [PDB ID: 3LUT (29)]; the pore region (aa 311–421) of chimeric channels was modeled with Modeler9.8 (30, 31) by exchanging one Kv1.2 monomer with the aligned sequence of Kv1.1 and Kv1.6, respectively. The model of κM-conotoxin RIIIJ was generated by Modeler9.8 from the solution NMR structure of κM-RIIIK (4). For more detail, see SI Appendix, Supplementary Methods.
Constellation Pharmacology: Calcium Imaging.
The experimental methods of constellation pharmacology have been described in detail previously (6, 7, 32, 33). For more detail, see SI Appendix, Supplementary Methods.
Data Analysis.
IC50 values were calculated according to IC50 = [toxin]/(Ictrl/Itoxin − 1) where [toxin] is the applied concentration of toxin; Ictrl is the current amplitude before toxin; and Itoxin is the current after toxin application. All data are presented as mean ± SEM (n), where n is the number of biological replicates.
Supplementary Material
Acknowledgments
This work was supported by the University of Kiel and by Grant GM 48677 (to B.M.O.) from the National Institute of General Medical Sciences. This work was also supported by Canadian Institutes of Health Research, CIHR MOP-10053, and National Science and Engineering Research Council (Canada) NSERC RGPIN-2012-418658-2012 (to R.J.F.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1813161116/-/DCSupplemental.
References
- 1.Chen P, Dendorfer A, Finol-Urdaneta RK, Terlau H, Olivera BM. Biochemical characterization of kappaM-RIIIJ, a Kv1.2 channel blocker: Evaluation of cardioprotective effects of kappaM-conotoxins. J Biol Chem. 2010;285:14882–14889. doi: 10.1074/jbc.M109.068486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacKinnon R, Yellen G. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 1990;250:276–279. doi: 10.1126/science.2218530. [DOI] [PubMed] [Google Scholar]
- 3.Ferber M, Al-Sabi A, Stocker M, Olivera BM, Terlau H. Identification of a mammalian target of kappaM-conotoxin RIIIK. Toxicon. 2004;43:915–921. doi: 10.1016/j.toxicon.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 4.Al-Sabi A, et al. KappaM-conotoxin RIIIK, structural and functional novelty in a K+ channel antagonist. Biochemistry. 2004;43:8625–8635. doi: 10.1021/bi0495681. [DOI] [PubMed] [Google Scholar]
- 5.Verdier L, et al. Identification of a novel pharmacophore for peptide toxins interacting with K+ channels. J Biol Chem. 2005;280:21246–21255. doi: 10.1074/jbc.M502376200. [DOI] [PubMed] [Google Scholar]
- 6.Teichert RW, et al. Functional profiling of neurons through cellular neuropharmacology. Proc Natl Acad Sci USA. 2012;109:1388–1395. doi: 10.1073/pnas.1118833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teichert RW, et al. Characterization of two neuronal subclasses through constellation pharmacology. Proc Natl Acad Sci USA. 2012;109:12758–12763. doi: 10.1073/pnas.1209759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coleman SK, Newcombe J, Pryke J, Dolly JO. Subunit composition of Kv1 channels in human CNS. J Neurochem. 1999;73:849–858. doi: 10.1046/j.1471-4159.1999.0730849.x. [DOI] [PubMed] [Google Scholar]
- 9.Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- 10.Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes KJ, et al. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nerbonne JM, Kass RS. Molecular physiology of cardiac repolarization. Physiol Rev. 2005;85:1205–1253. doi: 10.1152/physrev.00002.2005. [DOI] [PubMed] [Google Scholar]
- 13.Brahmajothi MV, Morales MJ, Campbell DL, Steenbergen C, Strauss HC. Expression and distribution of voltage-gated ion channels in ferret sinoatrial node. Physiol Genomics. 2010;42A:131–140. doi: 10.1152/physiolgenomics.00049.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olivera BM. E.E. Just Lecture, 1996. Conus venom peptides, receptor and ion channel targets, and drug design: 50 million years of neuropharmacology. Mol Biol Cell. 1997;8:2101–2109. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerschensteiner D, Soto F, Stocker M. Fluorescence measurements reveal stoichiometry of K+ channels formed by modulatory and delayed rectifier alpha-subunits. Proc Natl Acad Sci USA. 2005;102:6160–6165. doi: 10.1073/pnas.0500468102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuart NG, Haitin Y, Camp SS, Black KD, Zagotta WN. Molecular mechanism for 3:1 subunit stoichiometry of rod cyclic nucleotide-gated ion channels. Nat Commun. 2011;2:457. doi: 10.1038/ncomms1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron. 2002;36:881–889. doi: 10.1016/s0896-6273(02)01098-x. [DOI] [PubMed] [Google Scholar]
- 18.Al-Sabi A, Kaza SK, Dolly JO, Wang J. Pharmacological characteristics of Kv1.1- and Kv1.2-containing channels are influenced by the stoichiometry and positioning of their α subunits. Biochem J. 2013;454:101–108. doi: 10.1042/BJ20130297. [DOI] [PubMed] [Google Scholar]
- 19.Hess B, Kutzner C, van der Spoel D, Lindahl E. GROMACS 4: Algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 20.Pronk S, et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics. 2013;29:845–854. doi: 10.1093/bioinformatics/btt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Der Spoel D, et al. GROMACS: Fast, flexible, and free. J Comput Chem. 2005;26:1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 22.Abraham MJ, et al. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. [Google Scholar]
- 23.Hornak V, et al. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berendsen HJC, Grigera JR, Straatsma TP. The missing term in effective pair potentials. J Phys Chem. 1987;91:6269–6271. [Google Scholar]
- 25.Bussi G, Donadio D, Parrinello M. Canonical sampling through velocity rescaling. J Chem Phys. 2007;126:014101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 26.Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. Molecular dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–3690. [Google Scholar]
- 27.Berger O, Edholm O, Jähnig F. Molecular dynamics simulations of a fluid bilayer of dipalmitoylphosphatidylcholine at full hydration, constant pressure, and constant temperature. Biophys J. 1997;72:2002–2013. doi: 10.1016/S0006-3495(97)78845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joung IS, Cheatham TE., 3rd Determination of alkali and halide monovalent ion parameters for use in explicitly solvated biomolecular simulations. J Phys Chem B. 2008;112:9020–9041. doi: 10.1021/jp8001614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Wang Q, Ni F, Ma J. Structure of the full-length Shaker potassium channel Kv1.2 by normal-mode-based X-ray crystallographic refinement. Proc Natl Acad Sci USA. 2010;107:11352–11357. doi: 10.1073/pnas.1000142107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fiser A, Do RK, Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9:1753–1773. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 32.Smith NJ, et al. Comparative functional expression of nAChR subtypes in rodent DRG neurons. Front Cell Neurosci. 2013;7:225. doi: 10.3389/fncel.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teichert RW, Memon T, Aman JW, Olivera BM. Using constellation pharmacology to define comprehensively a somatosensory neuronal subclass. Proc Natl Acad Sci USA. 2014;111:2319–2324. doi: 10.1073/pnas.1324019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.