Significance
By using a gene knockout that leads to T cell-specific deletion, we reveal the essential role of Ser/Thr phosphatase PP2A in TH17 differentiation. We also show that this works through the regulation of SMAD2/3 phosphorylation status, which elucidates molecular pathways by which PP2A modulates the expression of TH17 phenotypes. This finding extends our understanding of the close relationship between PP2A overexpression and inflammatory disease. PP2A is a Ser/Thr phosphatase shown to be capable of controlling TH17 differentiation via modulating R-SMADs activity. We also demonstrate the translational potential of these findings by showing a therapeutic effect of PP2A inhibitors in controlling autoimmune disease in the encephalomyelitis model.
Keywords: TH17, TGFβ, PP2A, SMAD2/SMAD3, EAE
Abstract
Phosphatase PP2A expression levels are positively correlated to the clinical severity of systemic lupus erythematosus (SLE) and IL17A cytokine overproduction, indicating a potential role of PP2A in controlling TH17 differentiation and inflammation. By generating a mouse strain with ablation of the catalytic subunit α of PP2A in peripheral mature T cells (PP2A cKO), we demonstrate that the PP2A complex is essential for TH17 differentiation. These PP2A cKO mice had reduced TH17 cell numbers and less severe disease in an experimental autoimmune encephalomyelitis (EAE) model. PP2A deficiency also ablated C-terminal phosphorylation of SMAD2 but increased C-terminal phosphorylation of SMAD3. By regulating the activity of RORγt via binding, the changes in the phosphorylation status of these R-SMADs reduced Il17a gene transcription. Finally, PP2A inhibitors showed similar effects on TH17 cells as were observed in PP2A cKO mice, i.e., decreased TH17 differentiation and relative protection of mice from EAE. Taken together, these data demonstrate that phosphatase PP2A is essential for TH17 differentiation and that inhibition of PP2A could be a possible therapeutic approach to controlling TH17-driven autoimmune diseases.
T helper type 17 (TH17) cells, a subset of CD4+ T cells defined by IL17, IL22, and IL21 production, are essential for control and clearance of extracellular bacterial and fungi (1, 2). However, excessive TH17 responses are involved in chronic inflammation and development of many human autoimmune diseases (3). Upon encountering antigen in the context of a local cytokine milieu including transforming growth factor β (TGFβ) and IL6, naïve CD4+ T cells undergo differentiation into effective TH17 cells. TGFβ is the principal, essential factor promoting the differentiation of TH17 cells (4, 5).
Through two related transmembrane Ser/Thr kinase receptors, TGFβ induces Ser/Thr signal cascades in activated T cells. Recent work including work from our laboratory has revealed the regulatory roles of some other Ser/Thr kinases in this process. For example, both MEKK2/3 and MINK1 suppress TH17 differentiation through direct phosphorylation of the TGFβ signaling components SMAD2 and SMAD3 (6, 7). Precise regulation of SMAD2/3 Ser/Thr phosphorylation status is thus important in driving TH17 differentiation (6–8). Dephosphorylation of SMAD2/3 is equally critical in this process, but the specific phosphatases that catalyze SMAD2/3 dephosphorylation remains unknown.
As one of the major Ser/Thr phosphatases in eukaryotes, phosphatase PP2A is critical for many cellular functions including cell survival, proliferation, activation, and differentiation (9). It has been reported that elevated PP2A expression levels are linked to the up-regulation of IL17A production by CD4+ T cells in human systemic lupus erythematosus patients (10). Studies in the PP2Ac transgenic mouse model also demonstrated the relationship and mechanism linking of PP2A and Il17-dependent immunopathology (11, 12). PP2A is composed of three polypeptide chains, the structural A, the regulatory B, and the catalytic C subunits (13). The heterodimer of the A subunit and the C subunit (PP2AA-PP2AC) forms the PP2A core enzyme that associates with one regulatory B subunit, thus determining the substrate specificity of the holoenzyme complex (13).
In TGFβ signaling, two related regulatory B subunits, Bα (Ppp2r2a) and Bδ (Ppp2r2d), opposingly modulate TGFβ/Activin/Nodal signaling (14), while carboxy terminal phosphorylation of MAD (the SMAD homolog protein in Drosophila) is negatively regulated by the PP2A inhibitor okadaic acid (OA) (15). By analogy, these observations suggest that PP2A might be a Ser/Thr phosphorylation modulator involved in controlling TH17 differentiation.
Here, we present data showing that TH17 cell polarization was largely impaired when Ppp2ca was ablated in mature T cells and rendered resistance toward MOG-induced experimental autoimmune encephalomyelitis (EAE). We also show that PP2A knockout led to altered activation of R-SMADs (specifically decreasing SMAD2 activation and increasing SMAD3 activation). This synergistically inhibited RORγt mediated Il17a transcription. This work thus reveals a specific role of PP2A in regulating the canonical TGFβ−R-SMADs–RORγt signaling process during TH17 differentiation and indicates a possible therapeutic approach for controlling TH17-driven autoimmune diseases via inhibition of PP2A.
Results
Normal T Cell Development in PP2A cKO Mice.
To explore the function of PP2A in peripheral T cells, we deleted the dominant PP2A Cα isoform of PP2A catalytic subunit (PP2Ac) in T cell by crossing disLck (dLck) Cre with Ppp2cafl/fl mice (in which exons 3–5 of Ppp2ca are loxP flanked) (16) to generate Ppp2cafl/fl dLck-Cre (termed PP2A cKO mice) and Ppp2cafl/+ dLck-Cre or Ppp2ca+/+ dLck-Cre mice (collectively called PP2A WT mice here) (SI Appendix, Fig. S1 A and B). The dLck-Cre was driven by the distal promoter of the lymphocyte protein tyrosine kinase (Lck) gene, enabling investigation of the Ppp2ca deletion after positive selection in T cells (17).
To assess deletion efficiency, Ppp2ca mRNA and protein levels were measured and showed clear reduction in peripheral T cells in PP2A cKO mice, while remaining normal in thymic subsets and splenic B cells (SI Appendix, Fig. S1 C and D). The catalytic subunit of PP2A has two isoforms, Cα and Cβ (encoded by Ppp2ca and Ppp2cb, respectively). Notably we didn’t observe compensatory overexpression of Ppp2cb (SI Appendix, Fig. S1C). PP2A activity in cKO CD4+ T cells was reduced to half of that measured in WT controls (SI Appendix, Fig. S1E).
Analysis of the numbers and frequencies of different T cell subsets in these mice showed that cKO mice exhibited normal T cell development in thymus (SI Appendix, Fig. S2 A–C) as well as in peripheral lymphoid organs (SI Appendix, Fig. S2 A, D, and E). The proportions of naïve/effective T cells in spleen and mesenteric lymph nodes (MLN) were also similar between WT and cKO littermates (SI Appendix, Fig. S2 F and G). The normal development of peripheral lymphocytes in PP2A cKO mice allowed further investigation of the role of PP2A in T cell differentiation.
TH17 Cell Numbers Are Reduced in PP2A cKO Mice.
To clarify whether PP2A is involved in T helper cell lineage commitment, we analyzed the populations of T helper subsets in vivo. Interestingly, CD4+ T cells from PP2A cKO mice only contained half the number of TH17 cells comparing to their WT littermates (SI Appendix, Fig. S3 A and B), while the numbers of TH1 and Treg CD4+ T cells were not affected in the peripheral lymphoid organs (SI Appendix, Fig. S3 A, C, D, and F). The frequency of Foxp3+ regulatory T cells in the thymus was also comparable between PP2A WT and cKO mice (SI Appendix, Fig. S3 E and F). Similarly, subsets analysis of the lamina propria of small intestine also showed a consistent reduction of TH17 cells (SI Appendix, Fig. S3 G and I). These data demonstrate that PP2A is involved in maintaining TH17 cell composition, while other T cell subsets, including Treg and TH1, appear unaffected.
PP2A Deletion Impairs TH17 Differentiation in Vitro.
To investigate whether reduced levels of TH17 cells in PP2A cKO mice result from impaired TH17 differentiation, we sorted naïve CD4+ T cells from both WT and cKO cells and polarized them under TH1, TH2, TH17, and iTreg conditions to compare their differentiation efficiencies. The results showed that only the generation of TH17 cells notably declined with the PP2A deficiency, while other T helper subsets were not affected (Fig. 1 A and B and SI Appendix, Fig. S4 A and B). The expression levels of Ppp2ca mRNA and PP2A Cα protein were more abundant in TH17 cells than in other T helper subsets (SI Appendix, Fig. S4 G and H).
Fig. 1.
PP2A deficiency specifically limits TH17 differentiation in vitro. (A and B) Flow cytometry (A) and quantification (B) of IL17A staining in naïve CD4+ T cells from PP2A WT and cKO mice differentiated under TH17 polarizing condition for 5 d. (C) ELISA of IL17A in the culture medium of each polarizing condition (n = 3 technical replicates). (D) RT-PCR analysis of TH17 signature genes (n = 3 technical replicates). Each symbol represents an individual mouse (n = 8); error bars show mean ± SEM. Data are representative of at least three independent experiments with similar results. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
p-PP2Ac (Y307) levels, a negative indicator for PP2A activity, are lower in TH17 and TH1 than in the other subsets, indicating higher PP2A activity in these two subsets (SI Appendix, Fig. S4I). Cytokine production showed similar results, confirming that PP2A deficiency specifically reduced TH17 differentiation (Fig. 1C and SI Appendix, Fig. S4C). We further tested the expression of several key TH17 signature genes and found notably decreased Il17a and Il17f expression and slightly reduced expression of Rora and Il21, while the expression of Rorc, Il22, and Il23r were not significantly affected (Fig. 1D). The alteration of the expression pattern of TH17 signature genes (including Il17a and Il17f) induced by PP2A deficiency was also confirmed by an RNAseq analysis (SI Appendix, Fig. S5 A and B and Datasets S1–S3).
The proliferation capacity of WT and cKO T cells was measured under TH17 conditions and was comparable 2 and 5 d after stimulation (SI Appendix, Fig. S4D). Upon PI and Annexin V staining, WT and cKO cells showed comparable apoptotic rates (SI Appendix, Fig. S4E). Thus, reduced IL17A+ CD4+ T cell levels and IL17A production were not due to either impaired proliferation or increased apoptosis in cKO cells. Furthermore, we did not observe an increased portion of IFNγ+ or Foxp3+ CD4+ T cells in cKO T cells under TH17 conditions, which ruled out the likelihood of TH17 cells converting to other cell subsets in these circumstances (Fig. 1A and SI Appendix, Fig. S4F). These findings therefore demonstrate a T cell intrinsic impairment of the TH17 polarization program upon PP2A deficiency, which is independent of proliferation, apoptosis, or subset conversion.
Reduced Severity of EAE in Mice with PP2A Deficiency.
Given the required role of PP2A in inducing normal TH17 polarization, the question of whether defective TH17 PP2A cKO cells also influence TH17-driven autoimmune disease was investigated in vivo using an EAE model. We therefore immunized PP2A WT and cKO mice with myelin oligodendrocyte glycoprotein peptide of amino acids 35–55 (MOG35–55) to induce EAE.
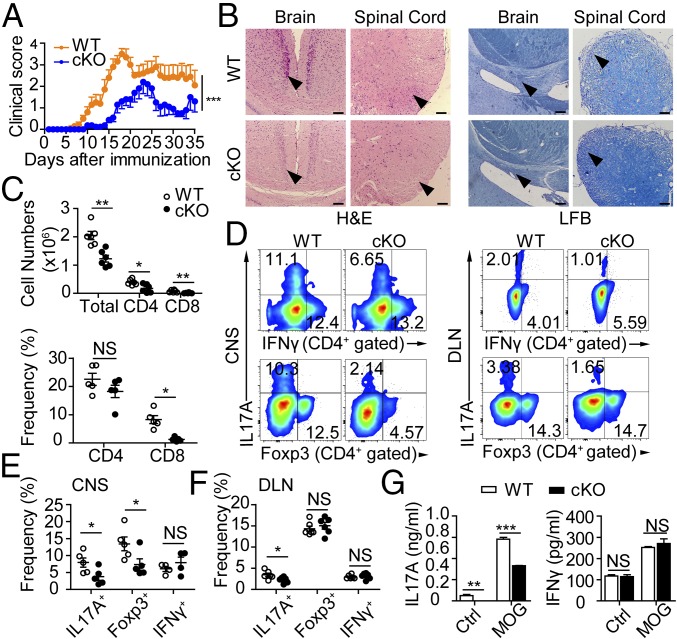
Clinical scoring showed that PP2A deficiency alleviated symptoms of autoimmunity (Fig. 2A). Histological examination showed significantly less mononuclear cell infiltration and demyelination in the cerebral and spinal cord of the cKO mice (Fig. 2B). Inflammatory cell infiltration into the central nervous system (CNS) was also greatly diminished in the cKO group (Fig. 2C). Although the proportion of CD4+ T cells was unchanged in the CNS of cKO mice, the total numbers of infiltrating CD4+ and CD8+ cells were significantly reduced (Fig. 2C). IL17A-producing CD4+ T cells were also present in significantly decreased numbers in both the CNS and draining lymph nodes (DLN), whereas the frequencies of IFN-γ–producing CD4+ T cells were unchanged. (Fig. 2 D–F). These data indicate that reduced IL17A production upon PP2A cKO results in less severe MOG-induced CNS inflammation. Indeed, in vitro MOG recall analysis also showed reduced IL17A production by PP2A cKO splenocytes, while IFN-γ production was unaffected (Fig. 2G). Intriguingly, Foxp3+ CD4+ T cell proportions were also reduced in the CNS but not in the DLN (Fig. 2 D–F). This might be explained by markedly milder inflammation in the CNS, which consequently recruited less regulatory cells. PP2A cKO mice are thus resistant to EAE, and this is strongly correlated with an observed significant reduction in TH17 cell induction.
Fig. 2.
Loss of PP2A protects mice from EAE by repressing IL17A production. (A) Mean clinical scores for EAE from each group. (B) Representative histology of the brain and spinal cord [hematoxylin and eosin (H&E) at Left and luxol fast blue (LFB) at Right] of mice after EAE induction (day 19). Arrowheads indicate inflammatory infiltration (Left) and demyelination (Right). (Scale bars: 100 μm.) (C) Number and frequency of mononuclear cells or CD4+ or CD8+ T cells infiltrated into CNS. (D) Flow cytometry of IL17A, IFN-γ, and Foxp3 staining from CNS (Left) or DLN (Right) CD4+ T cells. (E and F) Quantification of IL17A+, IFNγ+, and Foxp3+ CD4+ T cells in CNS (E) or DLN (F). (G) Splenocytes were rechallenged with MOG peptide (5 μg/mL) or control vehicle for 3 d, and cytokine production was measured by ELISA. Each symbol represents an individual mouse (n = 4–6); error bars show mean ± SEM. Data are representative of three independent experiments with similar results. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.
Intact TCR and IL6 Signaling in PP2A cKO CD4+ T Cells.
We next investigated how PP2A deficiency affected intracellular signaling in controlling TH17 cell differentiation. Intact TCR signaling is indispensable for TH17 commitment (18). However, our results showed that PP2A deficiency did not affect normal CD69 up-regulation (SI Appendix, Fig. S6A), cell proliferation (SI Appendix, Fig. S6B), or production of IL2 or IFN-γ (SI Appendix, Fig. S6C) following anti-CD3 stimulation. Interestingly, Western blot analysis showed up-regulation of pERK and pP38 in PP2A-deficient cells (SI Appendix, Fig. S6D), and these two pathways are known to play opposing roles in TH17 differentiation. Inhibition of MEK-ERK signaling enhances TH17 differentiation while activation of P38 is critical for optimal TH17 polarization (19–21). We found that use of an MEK inhibitor (inhibiting ERK, U0126) failed to restore TH17 differentiation due to PP2A deficiency (SI Appendix, Fig. S6E). Thus, subtle changes in MAPKs activation are not correlated with decreased TH17 differentiation due to PP2A deficiency. PP2A overexpression has been previously reported to up-regulate Il17a gene transcription by enhancing IRF4 activity (11). However, the observed mRNA, protein, and IRF4 activity did not support involvement of IRF4 in leading to diminished Il17a transcription (SI Appendix, Fig. S5 C–E).
By using the PP2A inhibitor OA, previous work has suggested a possible regulatory role of PP2A in regulating IL6 signaling, including promoting the stability of IL6 receptor gp130 (CD130) (22) and modulating STAT3 phosphorylation (23). However, our experiments did not show any alterations in either IL6 receptor expression (SI Appendix, Fig. S7 A and B) or STAT3 phosphorylation (Y705 and S727) after IL6 stimulation in cKO T cells (SI Appendix, Fig. S7C), thus excluding the possibility that the inhibition of TH17 differentiation upon PP2A deletion is due to TCR or IL6 signaling changes.
Differential Modulation of SMAD2/3 Activity by PP2A Deficiency Restrains TH17 Differentiation.
In TGFβ pathway, TGFβ RI and RII, which are reported to be opposingly regulated by two different PP2A B subunits (14), were similarly expressed in PP2A cKO T cells (Fig. 3 A and B). Meanwhile, we found that pSMAD2 (Ser465/467) level was decreased but pSMAD3 (Ser423/425) level was increased after TGFβ stimulation in PP2A cKO cells (Fig. 3C). In accordance, SMAD2 hyperphosphorylation and SMAD3 underphosphorylation were observed in PP2A Cα overexpressed 293FT cells after TGFβ stimulation (SI Appendix, Fig. S8 A and B). We next performed the Co-IP assay in Jurkat cells to explore the binding of R-SMADs to PP2Ac. We found that PP2Ac can form stable complex with SMAD2 and SMAD3, which does not depend on either TCR or TGFβ stimulation (SI Appendix, Fig. S8 C and D).
Fig. 3.
Insufficient SMAD2 activation and overactivated SMAD3 under TGFβ pathway restrains TH17 differentiation. (A and B) Histograms (A) and mean fluorescence intensity (MFI) quantification (B) of TGFβ receptor I and II staining on CD4-gated cells from splenocytes of PP2A WT and cKO mice. (C) Enriched CD4+ T cells from PP2A WT and cKO were stimulated with 10 ng/mL TGFβ as indicated, whole-cell lysates were probed with the indicated antibodies in the immunoblots. (D and E) Flow cytometry (D) and quantification (E) of TH17 polarization with ectopic expression of Vector, WT, 2SA, and 2SD of SMAD2 in WT naïve CD4+ T cells. GFP-expressing cells were gated for analysis on day 3. (F and G) Flow cytometry (F) and quantification (G) of TH17 polarization with ectopic expression of Vector, WT, 2SA, and 2SD of SMAD3 in WT naïve CD4+ T cells. GFP expressing cells were gated for analysis on day 3. Each symbol represents an individual mouse (n = 4). Error bars show mean ± SEM. Data are representative of three independent experiments (C) or two independent experiments with two replicates (D and F). *P < 0.05; **P < 0.01; NS, not significant.
In addition, in vitro dephosphorylation assay showed that PP2A can directly dephosphorylate pSMAD3 (SI Appendix, Fig. S8 E–G). Studies on SMAD2 and SMAD3 conditional knockout mice have revealed the opposite functions of these two molecules in inducing TH17 cells (24–27). We thus suspected that the altered activation of SMAD2/3 in PP2A cKO cells might serve as the major contributor toward decreased Il17a transcription. Indeed, when we expressed different activation forms of SMAD2/3 (WT, dominant negative form 2SA, constitutive active mutants 2SD) in naïve CD4+ T cells and analyzed cell differentiation in TH17 condition, the results clearly showed that insufficient activation of SMAD2 caused defective TH17 differentiation, while constitutively activated SMAD2 promoted optimal TH17 priming (Fig. 3 D and E). On the contrary, ectopic expression of SMAD3 dramatically repressed TH17 polarization. Interestingly, a SMAD3-2SA mutant also showed inhibitory activity to TH17 polarization although to a lesser degree (Fig. 3 F and G), indicating that suppression of TH17 differentiation by SMAD3 depends on both SMAD3 activation and on its overall expression level. To rule out alterations in iTreg cell skewing condition in the previous experiments, we also used a series of TGFβ doses for suboptimal iTreg priming but observed no difference between the PP2A cKO and WT groups (SI Appendix, Fig. S7D).
Changes in SMAD2/3 Activity Synergistically Down-Regulate Il17a Transcription via Reducing RORγt Activity.
We next asked how the shift of SMAD2/3 phosphorylation status affected Il17a transcription. We found that the protein expression levels of RORγt and RORα were not different in PP2A WT and cKO TH17 cells (Fig. 4 A and B). More importantly, retrovirus-mediated ectopic expression of RORγt could not completely restore TH17 potentiation in cKO T cells (Fig. 4 C and D). These data supported the hypothesis that PP2A-controlled TH17 differentiation is independent of RORγt protein expression. To address whether overactivation of SMAD3 and insufficient activation of SMAD2 could work cooperatively to suppress RORγt activity, ChIP analysis of RORγt occupancy of Il17a gene region was carried out. The result confirmed our hypothesis that with equal RORγt expression, its activity was largely reduced due to PP2A deficiency (Fig. 4E). Phosphorylation changes of SMAD2/3 affected their binding ability with RORγt. In cKO TH17 cells, RORγt binded more SMAD3 and less SMAD2 than observed in WT TH17 cells (Fig. 4F).
Fig. 4.
Differential modulation of SMAD2/3 inhibits RORγt-mediated Il17a transcription by forming complex with RORγt. (A) Flow cytometry of RORγt staining from naïve CD4+ T cells primed under TH17 polarizing condition for 2 d. (B) RORα was immunoblotted with whole-cell lysate of TH17 cells. (C and D) Flow cytometry (C) and quantification (D) of TH17 polarization with ectopic expression of Vector or RORγt in PP2A WT and cKO naïve CD4+ T cells. GFP-expressing cells were gated for analysis on day 3. (E) RORγt binding to the sites of the Il17a gene promoter was analyzed by using chromatin immunoprecipitation (ChIP) assay with RT-PCR. (F) Co-IP analysis of binding ability with RORγt among SMAD2/3 in WT and cKO TH17 cells. (G) Co-IP analysis of binding ability with RORγt among WT, 2SA, and 2SD mutant of SMAD2/3 in 293FT cells. (H) Flow cytometry of TH17 polarization with ectopic expression of Vector or SMAD2-2SD in PP2A WT and cKO naïve CD4+ T cells. (I) Ratio of IL17A frequency comparing VEC-cKO or SMAD2-2SD-cKO to VEC-WT. (J) Flow cytometry of TH17 polarization after suppressing SMAD3 expression by siRNA in naïve CD4+ T cells. (K) The ratio of IL17A frequency in cKO cells transfected with siRNA-SMAD3 or control to the frequency of WT cells transfected with control. Error bars show mean ± SEM. Data are representative of three (A, B, G, H, and J) or two (C–F) independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001.
Different activation forms of SMAD2/3 were then cotransfected with RORγt in the 293FT cell line. These experiments showed that the constitutively active SMAD2/3 preferentially interacts with RORγt over the inactive forms, and that SMAD3 is more accessible to bind RORγt than SMAD2 (Fig. 4G). These results suggest that phosphorylation changes in SMAD2/3 may inhibit RORγt activity by affecting the capacity of SMAD2/3 to form complexes with RORγt. Based on this hypothesis, we performed rescue experiments with SMAD2-2SD transfection or SMAD3 knockdown. Both approaches significantly improved TH17 polarization in cKO naïve CD4+ T cells (Fig. 4 H–K). SMAD3 knockdown efficiency by siRNA was verified by Western blot and RT-PCR (SI Appendix, Fig. S9 A and B).
PP2A Inhibitors Phenocopy Phosphorylation Changes of SMAD2/3 and Restrain TH17 Polarization in Vitro.
We observed that administration of PP2A inhibitors in 293FT cells phenocopied the SMAD2/3 activation changes in a dose-dependent manner (Fig. 5A). Further, the PP2A inhibitor Cantharidin (CAN) restrained RORγt-mediated Il17a promoter activation (Fig. 5B). More importantly, when administrating PP2A inhibitor CAN in TH17 culture medium, we also observed a dose-dependent inhibitory role at a concentration that had no effect on cell proliferation (Fig. 5 C–E). Another two PP2A inhibitors OA and Fostriecin (FOS) also showed similar effects upon TH17 differentiation (SI Appendix, Fig. S10 A–E). In addition, PP2A cKO T cells did not display suppression effects by PP2A inhibitors, ruling out the off-target effects might result in TH17 depression (Fig. 5F and SI Appendix, Fig. S10F).
Fig. 5.
PP2A inhibitors lead the same change of SMAD2/3 activation under TGFβ pathway and limit TH17 priming in vitro. (A) SMAD2/3 was overexpressed in the 293FT cells, and PP2A inhibitors were added in serum-free medium for 2 h followed by TGFβ (3 ng/mL) stimulation for 3 h. Western blot analysis using phospho-specific SMAD2/3 (p-SMAD2/3) and FLAG-tag (total-SMAD2/3) antibodies. (B) Il17a promoter and SMAD2/3 with or without RORγt was transfected into 293FT cells and then treated with PP2A inhibitors and TGFβ as A. Luciferase activity was measured and normalized based on Renilla luciferase gene. (C) Sorted naïve CD4+ T cells were polarized under TH17 priming condition with the indicated concentration of CAN for 3 d, and IL17A+ population was analyzed using flow cytometry on day 5. (D) The concentration of IL17A in the culture supernatant was measured by ELISA. (E) Histogram of CFSE fluorescence staining of cells in C. (F) WT naïve CD4+ T cells cultured with CAN (5 μM) for the first 3 d in TH17 priming condition and analyzed IL17A+ population by flow cytometry on day 5. Error bars show mean ± SEM. Data are representative of two independent experiments with similar results.
A PP2A Inhibitor Protects Mice from EAE.
We examined whether CAN can block TH17 cell-mediated inflammation in EAE. A significant reduction of disease severity was observed in CAN-treated PP2A WT mice compared with PBS-treated WT mice (Fig. 6A). Histological examination also showed significantly less mononuclear cell infiltration and demyelination in the spinal cord of the CAN-treated WT mice (Fig. 6B). Meanwhile, there was no observable aggravation of clinical symptoms by applying CAN in PP2A cKO mice, suggesting that the CAN dose was within a safe range (Fig. 6A). Fewer mononuclear lymphocytes and CD4+ T cells infiltrated into the CNS of CAN-treated WT mice than of the PBS WT group at the onset or peak of EAE, while the CD8+ and myeloid population was not significantly changed (Fig. 6 C and D). Additionally, the ratio of TH17 cells was lower in CAN-treated WT mice than in PBS-treated WT mice (P = 0.08) (Fig. 6E). The number of infiltrated TH17 cells was also significantly lower in the CNS. Treg cells and IFN-γ–producing cells were slightly decreased (Fig. 6F). Furthermore, CAN treatment in PP2A cKO groups did not alter the number of inflammatory lymphocytes infiltrated into CNS (Fig. 6 G and H). CAN-treated cKO groups showed no effects to EAE symptoms, suggesting the specific targeting of CAN to PP2A in T cells in this experimental setting. However, whether CAN can also act on other cells and contribute to the therapeutic effects is unclear. Collectively, data above showed that PP2A inhibitor CAN can limit EAE development principally by reducing TH17 differentiation.
Fig. 6.
A PP2A inhibitor suppresses EAE development. (A) PP2A WT and cKO mice were immunized with MOG(35-55) peptide. PP2A inhibitor CAN (0.6 μg/g) was administered i.p. daily from day 10 to day 12 and then was given once every 2 d. Mean clinical scores for EAE from each group. (B) Representative histology of the spinal cord (H&E at Left and LFB at Right) of mice after EAE induction (day 19). Arrowheads indicate inflammatory infiltration (Left) and demyelination (Right). (Scale bars: 100 μm.) (C) Quantification of total mononuclear cells, CD4+ T cells, CD8+ T cells, and myeloid cells that infiltrated into the CNS of WT mice at peak of the disease. (D) Ratio of CD4+ and CD8+ T cells (gated at CD45hi CD11b−) in the CNS of WT groups. (E and F) Ratio (E) and number (F) of IL17A+ or Foxp3+ or IFNγ+ CD4+ cells in the CNS of the WT groups at disease peak. (G) Quantification of total mononuclear cells, CD4+ T cells, CD8+ T cells, and myeloid cells that infiltrated into the CNS of the cKO groups. (H) Ratio of CD4+ and CD8+ T cells (gated at CD45hi CD11b−) that infiltrated into the CNS of the cKO groups. Each symbol represents an individual mouse (n = 5–7 per genotype); error bars show mean ± SEM. Data are representative of two independent experiments with similar results.
Discussion
By using peripheral T cell-specific KO mice, we have established the positive regulatory role of PP2A in TH17 differentiation as well as in inflammatory autoimmune diseases. We have also proved that underactivated SMAD2 and overactivated SMAD3 downstream of TGFβ signaling collectively reduced RORγt-mediated Il17a transcription in PP2A cKO mice. PP2A inhibitors also reduced TH17 polarization, indicating a promising therapeutic avenue for treating TH17 cell-mediated autoimmune diseases.
PP2A is one of the most abundant phosphatases and crucial for many key cellular events. A total knockout of the PP2A catalytic subunit a in mice is lethal (16). To our surprise, PP2A deficiency in T cell did not lead to fundamental changes in basic processes such as cell survival and proliferation. This is either because of the residual PP2A expression in our cKO mice or the stage and cell-specific functions of PP2A.
As shown by RNAseq and RT-PCR analysis, the transcriptional changes of TH17 signature genes induced in PP2A cKO were limited. Obvious changes were observed in Il17a and Il17f, but not in Rorc. Expression of RORγt was intact, which exclude the possibility that PP2A is involved in the pathways leading to RORγt induction. Therefore, intracellular signaling events downstream of RORγt and closely related to Il17a transcription might be the candidate targets of PP2A-mediated inhibition of TH17 differentiation.
SMAD2, SMAD3, and SMAD4 are all critical for TGFβ signaling and participate in TH17 or iTreg cell priming to induce balanced expression of Foxp3 and RORγt (24–26, 28–30). It is intriguing that SMAD2 knockout mice show reduced TH17 cell differentiation and ameliorated EAE, while a deficiency in SMAD3 has the opposite effects (24, 26–28). Furthermore, overexpression of SMAD2 and RORγt augments the differentiation of TH17 cells. However, SMAD3 binds to RORγt in Co-IP experiments and decreases its transcriptional activity (26). Moreover, R-SMAD activation is mainly via the phosphorylation of the C-terminal SSXS motif, which is critical for R-SMAD function. The active form of SMAD3 might enhance its binding affinity with RORγt, and it is known that SMAD3 can compete with SMAD2 for binding with RORγt (27). These results suggest that active SMAD2 plays a positive role and active SMAD3 plays a negative role in TH17 cell differentiation likely via dynamic interaction with RORγt (26, 27).
Our study confirmed that altered SMAD2/3 activation by PP2A affects TH17 differentiation, resulting in decreased Il17a transcription via forming complex with RORγt and reducing its activity and, thus, affecting TH17 differentiation. Importantly, this is a study that identifies PP2A as the critical phosphatase responsible for Ser/Thr dephosphorylation on R-SMADs and necessary for efficient TH17 differentiation.
It is important to know the precise dephosphorylation site on R-SMAD and the responsible kinases. Our previous work has elucidated the importance of threonine residue T324 in the α-helix 1 region of SMAD2 for regulation during TH17 differentiation (6). It is thus likely that PP2A up-regulates SMAD2 C-terminal phosphorylation via modulating MINK1 activity. However, the function of PP2A appears to be broader and more dominant than this single phosphorylation event, since we also observed increased SMAD3 phosphorylation in cKO CD4+ T cells, which also contributes to deceased TH17 differentiation. This observation is in accordance with a previous finding that the PP2A structural subunit PR65 could interact with SMAD3 (31).
It is also likely that PP2A directly regulates the phosphorylation status of RORγt. A recent study showed that two functional phosphorylation sites identified on RORγt played opposite roles in TH17 polarization. IKKα was discovered as an upper stream regulator for the phosphorylation change (32). Whether PP2A participates in the interaction with IKKα or RORγt in regulating RORγt activity remains to be elucidated.
The classical TGFβ pathway is also critical for iTreg differentiation (33). SMAD2 and SMAD3 double knockout mice showed a dramatic loss of Foxp3 induction (28). Then why is iTreg differentiation not affected by PP2A deficiency? It is most likely because of the redundant roles of SMAD2 and SMAD3 in TGFβ-induced iTreg plasticity (28). Overactivated SMAD3 may compensate for insufficient activation of SMAD2 in Foxp3 induction. In a recent study, PP2A was reported to be indispensable for the maintenance of the suppressive function of Treg cells via regulating the activity of the mTORC1 complex. Specific ablation of PP2A in Treg cells by Foxp3-YFP-Cre leads to autoimmunity with similar clinical features of scurfy mice (34). We also observed the same Treg phenotype in dLck-driven PP2A cKO mice, but the overall outcome of PP2A defect in peripheral T cells is dominated by TH17 differentiation impairment, which is demonstrated by their reduced susceptibility to autoimmune diseases induction.
Finally, in addition to finding the importance of PP2A to TH17 differentiation, we demonstrated the translational potential of this pathway by showing the therapeutic effect of PP2A inhibitor in controlling autoimmune diseases in the EAE model.
Materials and Methods
Mice.
Ppp2ca floxed mice were provided by X.G. Mice with Cre recombinase driven by the distal promoter of the gene encoding the kinase Lck were bought from the Jackson Laboratory. The experimental protocols were approved by the Review Committee of Zhejiang University School of Medicine and followed institutional guidelines.
EAE Induction.
EAE was induced as described (35). Briefly, mice aged 6–8 wk were immunized with 200 mg of MOG35–55 (Sangon, MEVGWYRSPFSRVVHLYRNGK) in an equal amount of Complete Freund’s Adjuvant (Chondrex, Inc.) and received 200 ng of pertussis toxin (List Biochemicals) i.v. on days 0 and 2 after induction. Clinical evaluation was assigned daily using a five-point scale: 1, flaccid tail; 2, impaired righting reflex and hindlimb weakness; 3, hindlimb paralysis; 4, hindlimb and forelimb paralysis; 5, moribund. Detailed materials and methods are presented fully in SI Appendix, SI Materials and Methods.
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism. The data were analyzed by Student’s t test. All P value less than 0.05 was considered significant (*P < 0.05; **P < 0.01; ***P < 0.001).
Supplementary Material
Acknowledgments
We thank Dr. Dawang Zhou for providing plasmids in luciferase assay; Dr. Xinhua Feng for providing ALK5(202D)-HA plasmid; Youfa Zhu, Yanwei Li, Jiajia Wang, and Yingying Huang from the core facilities (Zhejiang University School of Medicine) for technical assistance in histology and FACS analysis. This work was supported by National Natural Science Foundation of China Grants 31770954 and 31530019 (to L.L.), 81570013 (to S.W.), and 31500708 (to M.Z.); National Key R&D Program of China Grant 2018YFC1105102 (to L.L.); and Fundamental Research Funds for the Central Universities Grant 2018XZZX001-12 (to L.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.K. is a guest editor invited by the Editorial Board.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE119836).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1807484116/-/DCSupplemental.
References
- 1.Bai H, et al. Respective IL-17A production by γδ T and Th17 cells and its implication in host defense against chlamydial lung infection. Cell Mol Immunol. 2017;14:850–861. doi: 10.1038/cmi.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simonian PL, et al. IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis. J Immunol. 2009;182:6540–6549. doi: 10.4049/jimmunol.0900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong CK, et al. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: Implications for Th17-mediated inflammation in auto-immunity. Clin Immunol. 2008;127:385–393. doi: 10.1016/j.clim.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Mangan PR, et al. Transforming growth factor-β induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 5.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Fu G, et al. Suppression of Th17 cell differentiation by misshapen/NIK-related kinase MINK1. J Exp Med. 2017;214:1453–1469. doi: 10.1084/jem.20161120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang X, et al. The kinases MEKK2 and MEKK3 regulate transforming growth factor-β-mediated helper T cell differentiation. Immunity. 2011;34:201–212. doi: 10.1016/j.immuni.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 9.Seshacharyulu P, Pandey P, Datta K, Batra SK. Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett. 2013;335:9–18. doi: 10.1016/j.canlet.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsiari CG, Kyttaris VC, Juang Y-T, Tsokos GC. Protein phosphatase 2A is a negative regulator of IL-2 production in patients with systemic lupus erythematosus. J Clin Invest. 2005;115:3193–3204. doi: 10.1172/JCI24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apostolidis SA, Rauen T, Hedrich CM, Tsokos GC, Crispín JC. Protein phosphatase 2A enables expression of interleukin 17 (IL-17) through chromatin remodeling. J Biol Chem. 2013;288:26775–26784. doi: 10.1074/jbc.M113.483743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crispín JC, et al. Cutting edge: Protein phosphatase 2A confers susceptibility to autoimmune disease through an IL-17-dependent mechanism. J Immunol. 2012;188:3567–3571. doi: 10.4049/jimmunol.1200143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho US, Xu W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature. 2007;445:53–57. doi: 10.1038/nature05351. [DOI] [PubMed] [Google Scholar]
- 14.Batut J, et al. Two highly related regulatory subunits of PP2A exert opposite effects on TGF-β/Activin/Nodal signalling. Development. 2008;135:2927–2937. doi: 10.1242/dev.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneko S, et al. Smad inhibition by the Ste20 kinase Misshapen. Proc Natl Acad Sci USA. 2011;108:11127–11132. doi: 10.1073/pnas.1104128108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu P, Qi X, Zhou Y, Wang Y, Gao X. Generation of Ppp2Ca and Ppp2Cb conditional null alleles in mouse. Genesis. 2012;50:429–436. doi: 10.1002/dvg.20815. [DOI] [PubMed] [Google Scholar]
- 17.Zhang DJ, et al. Selective expression of the Cre recombinase in late-stage thymocytes using the distal promoter of the Lck gene. J Immunol. 2005;174:6725–6731. doi: 10.4049/jimmunol.174.11.6725. [DOI] [PubMed] [Google Scholar]
- 18.Molinero LL, Cubre A, Mora-Solano C, Wang Y, Alegre M-L. T cell receptor/CARMA1/NF-κB signaling controls T-helper (Th) 17 differentiation. Proc Natl Acad Sci USA. 2012;109:18529–18534. doi: 10.1073/pnas.1204557109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan AH-M, Lam K-P. Pharmacologic inhibition of MEK-ERK signaling enhances Th17 differentiation. J Immunol. 2010;184:1849–1857. doi: 10.4049/jimmunol.0901509. [DOI] [PubMed] [Google Scholar]
- 20.Noubade R, et al. Activation of p38 MAPK in CD4 T cells controls IL-17 production and autoimmune encephalomyelitis. Blood. 2011;118:3290–3300. doi: 10.1182/blood-2011-02-336552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Mitri D, et al. The p38 mitogen-activated protein kinase cascade modulates T helper type 17 differentiation and functionality in multiple sclerosis. Immunology. 2015;146:251–263. doi: 10.1111/imm.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitsuhashi S, et al. Protein phosphatase type 2A, PP2A, is involved in degradation of gp130. Mol Cell Biochem. 2005;269:183–187. doi: 10.1007/s11010-005-3089-x. [DOI] [PubMed] [Google Scholar]
- 23.Woetmann A, et al. Inhibition of protein phosphatase 2A induces serine/threonine phosphorylation, subcellular redistribution, and functional inhibition of STAT3. Proc Natl Acad Sci USA. 1999;96:10620–10625. doi: 10.1073/pnas.96.19.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon J-H, et al. Phosphorylation status determines the opposing functions of Smad2/Smad3 as STAT3 cofactors in TH17 differentiation. Nat Commun. 2015;6:7600. doi: 10.1038/ncomms8600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malhotra N, Robertson E, Kang J. SMAD2 is essential for TGF β-mediated Th17 cell generation. J Biol Chem. 2010;285:29044–29048. doi: 10.1074/jbc.C110.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez GJ, et al. Smad3 differentially regulates the induction of regulatory and inflammatory T cell differentiation. J Biol Chem. 2009;284:35283–35286. doi: 10.1074/jbc.C109.078238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martinez GJ, et al. Smad2 positively regulates the generation of Th17 cells. J Biol Chem. 2010;285:29039–29043. doi: 10.1074/jbc.C110.155820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takimoto T, et al. Smad2 and Smad3 are redundantly essential for the TGF-β-mediated regulation of regulatory T plasticity and Th1 development. J Immunol. 2010;185:842–855. doi: 10.4049/jimmunol.0904100. [DOI] [PubMed] [Google Scholar]
- 29.Tone Y, et al. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, et al. Reversing SKI-SMAD4-mediated suppression is essential for TH17 cell differentiation. Nature. 2017;551:105–109. doi: 10.1038/nature24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heikkinen PT, et al. Hypoxia-activated Smad3-specific dephosphorylation by PP2A. J Biol Chem. 2010;285:3740–3749. doi: 10.1074/jbc.M109.042978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He Z, et al. Regulation of Th17 differentiation by IKKα-dependent and -independent phosphorylation of RORγt. J Immunol. 2017;199:955–964. doi: 10.4049/jimmunol.1700457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou L, et al. TGF-β-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Apostolidis SA, et al. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat Immunol. 2016;17:556–564. doi: 10.1038/ni.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao Y, et al. Glatiramer acetate ameliorates inflammatory bowel disease in mice through the induction of Qa-1-restricted CD8+ regulatory cells. Eur J Immunol. 2013;43:125–136. doi: 10.1002/eji.201242758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.