Significance
Argonaute (Ago) proteins function in host defense against foreign mRNA. The Ago-mediated mRNA target cleavage necessitates glutamate finger insertion. Two positively charged residues locate symmetrically around the plugged-in finger, but their functional roles are unclear for Thermus thermophilus Ago (TtAgo), a prokaryotic Ago protein. Surprisingly, our simulations and site-mutagenesis experiments indicated that, in contrast to the equivalent roles of symmetrical positively charged residues in Kluyveromyces polysporus Ago (a eukaryotic Ago protein), in TtAgo, only R545 is critical for mRNA cleavage as a structural anchor to stabilize the plugged-in conformation, whereas R486 plays a negligible role. These differences provide a molecular basis of the distinct glutamate finger functions of Ago proteins in different organisms.
Keywords: bacterial Argonaute, QM/MM simulations, DNA cleavage
Abstract
Bacterium Thermus thermophilus Argonaute (Ago; TtAgo) is a prokaryotic Ago (pAgo) that acts as the host defense against the uptake and propagation of foreign DNA by catalyzing the DNA cleavage reaction. The TtAgo active site consists of a plugged-in glutamate finger with two arginine residues (R545 and R486) located symmetrically around it. An interesting challenge is to understand how they can collaboratively facilitate enzymatic catalysis. In Kluyveromyces polysporus Ago, a eukaryotic Ago, the evolutionarily symmetrical residues are arginine and histidine, both of which function to stabilize the plugged-in catalytic tetrad conformation. Surprisingly, our simulation results indicated that, in TtAgo, only R545 is involved in the cleavage reaction by serving as a critical structural anchor to stabilize the catalytic tetrad Asp-Glu-Asp-Asp that is completed by the insertion of the glutamate finger, whereas R486 is not involved in target cleavage. The TtAgo-mediated target DNA cleavage occurs in a substrate-assisted mechanism, in which the pro-Rp (Rp, a tetrahedral phosphorus center with “R-type” chirality) oxygen of scissile phosphate acts as a general base to activate the nucleophilic water. Our unexpected theoretical findings on distinct roles played by R545 and R486 in TtAgo catalysis have been validated by single-point site-mutagenesis experiments, wherein the target cleavage is abolished for all mutants of R545. In sharp contrast, the cleavage activity is maintained for all mutants of R486. Our work provides mechanistic insights on the catalytic specificity of Ago proteins and could facilitate the design of new gene-editing tools in the long term.
Argonaute (Ago) proteins are critical components of the RNA-induced silencing complex that play an essential role in guide strand-mediated target RNA recognition and cleavage (1–15). The prokaryotic Ago (pAgo) proteins are characterized to serve as host defense against the uptake and propagation of foreign RNA/DNA through RNA/DNA interference (16–20), whereas eukaryotic Ago (eAgo) proteins carry out this process of host defense through RNA interference (20, 21). Molecular insights into target DNA/RNA cleavage have emerged from structural (13) and chemical studies (16, 22), with potential application as gene manipulation against a range of diseases (9, 21, 23–32).
Bacterium Thermus thermophilus Ago (TtAgo), as reported in our previous structural work (13), features four domains (PIWI, MID, PAZ, N) and two connecting linkers (L1, L2) as found in other Ago proteins (13, 16, 19, 21). The PIWI domain adopts an RNase H fold, in which the catalytic Asp-Glu-Asp-Asp tetrad contributes to the slicer activity (13, 21, 33–37). Previous phosphorothioate substitution cleavage kinetic studies (5, 13) also suggested that TtAgo-mediated target DNA cleavage might follow the RNase H-mediated two-metal-ion catalysis cleavage pathway (38–46). However, distinct from RNase H, whose catalytic tetrad is formed during initial folding, Ago proteins require insertion of the glutamic acid residue on loop PL2 (termed “glutamate finger”) into the catalytic pocket when it is bound to the guide/target nucleotide strand (13, 21, 37). This difference is likely a result of the high catalytic specificity of Ago; to the contrary, RNase H exerts a nonspecific role in RNA–DNA hybrid cleavage (37). Also, the TtAgo-mediated target DNA cleavage is a typical phosphoryl transfer reaction (45, 47–52) that requires a general acid to protonate the leaving group and a general base to deprotonate the inline nucleophilic-attacking water under neutral pH (13, 16, 40, 44). Importantly, it is unclear whether this plugged-in glutamate finger could act as a general acid to directly participate in cleavage reaction or only act as a structural anchor to impart the catalytic specificity of Ago. In addition, it is unknown how the two-metal-ion catalysis and pro-Rp (Rp, a tetrahedral phosphorus center with “R-type” chirality) oxygen atoms achieve substrate specificity of TtAgo (45).
The Ago-mediated cleavage generally necessitates glutamate finger insertion, and this plugged-in conformation can be stabilized through a hydrogen-bond network in pAgo and eAgo proteins as observed in the reported crystal structures (13, 21, 37, 53–55). Interestingly, the two symmetric positively charged residues that form hydrogen bonds with plugged-in glutamate finger are arginine in pAgo (13), such as R545 and R486 in TtAgo (13), whereas, in eAgo (37, 53, 54), they are arginine and histidine, both of which function to stabilize the plugged-in conformation of Kluyveromyces polysporus Ago (KpAgo) (37). It is unknown from the previously reported ternary structure (13) whether the two symmetrical arginines in TtAgo play the same roles as the corresponding arginine and histidine in KpAgo (37). Furthermore, an intriguing question is whether this structural difference has mechanistic consequences that explain the evolutionary differences between prokaryotic and eAgo proteins.
Interestingly, in the present work, we found that, in contrast to the equivalent roles of symmetrical Arg and His residues in KpAgo (37), the two seemingly symmetric residues in TtAgo, R545 and R486, play distinct roles during target cleavage. Specifically, R545 is a key structural anchor in stabilizing the catalytic plugged-in conformation for target cleavage, whereas R486 is not involved in cleavage reaction. Through extensive ab initio quantum mechanics (QM)/molecular mechanics (MM) molecular dynamics (MD; aiQM/MM-MD) simulations with periodic boundary conditions (56), we characterized a substrate-assisted (57, 58) reaction pathway for this TtAgo-mediated target DNA cleavage (the system contains more than 170,000 atoms in MM region and 67 atoms in QM region; Fig. 1A). Our results revealed that the plugged-in glutamate finger (E512) does not act as a general acid to protonate the 3′ leaving group, but only serves as a critical structural anchor to impart the catalytic specificity of TtAgo. Our theoretical predictions on the roles of critical residues have been further validated by in vitro cleavage assays performed on R545 and R486 single-point mutants.
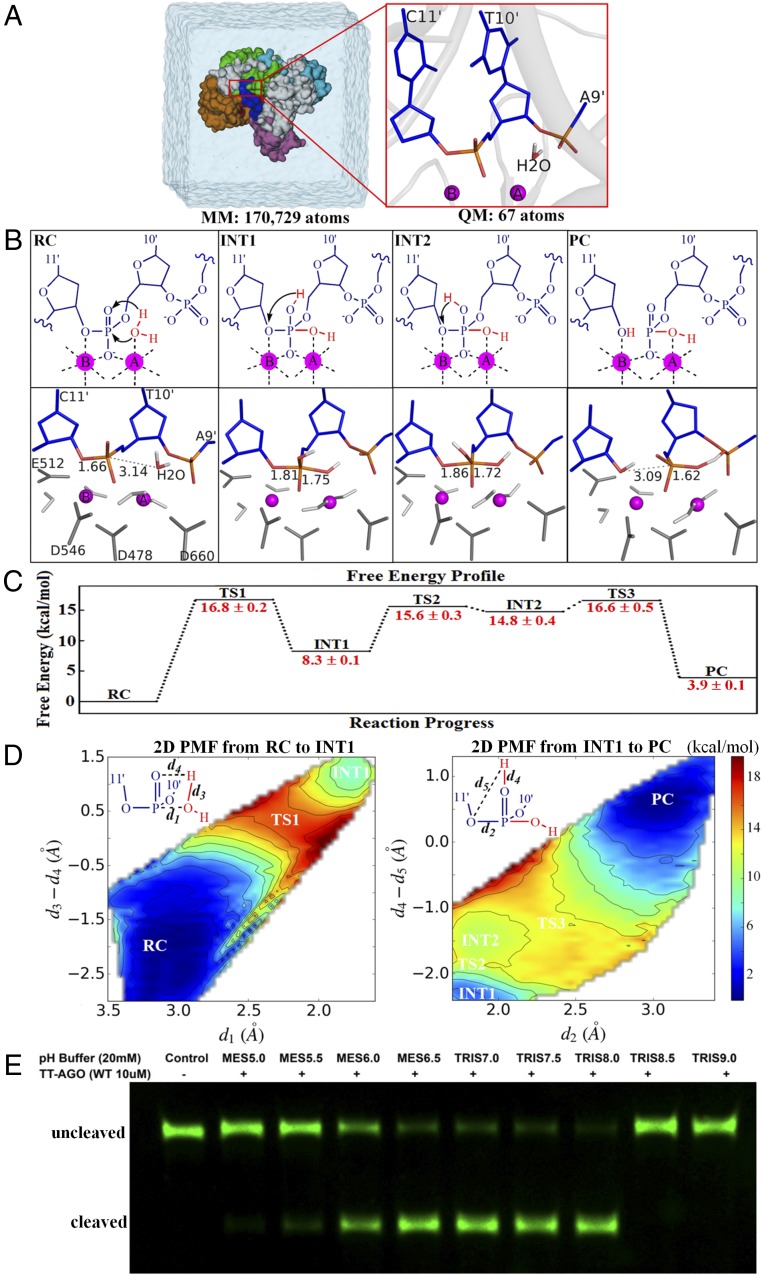
Fig. 1.
The substrate-assisted target DNA cleavage mechanism. (A) Overview of the setup used for our simulations for TtAgo-mediated target DNA cleavage. Snapshot of the simulation box (Left) and detailed view of the QM subsystem in the precleavage state (Right; PDB ID code 4NCB). (B) Detailed cleavage mechanism (Top) and respective critical structures (Bottom). Movies S1 and S2 show the mechanism details. The coordinating amino acid residues D546, D478, D660, and E512 are colored dark gray, and the coordinating waters that do not participate in the cleavage reaction are colored light gray. INT1, first intermediate; INT2, second intermediate; PC, product complex, i.e., postcleavage state; RC, reactant complex, i.e., precleavage state; TS1, first transition state; TS2, second transition state; TS3, third transition state. (C) Calculated free energy changes with their statistical errors along the reaction progress (in kilocalories per mole). Approximately 430 windows were selected to perform a total of approximately 6.5 ns B3LYP(6-31G*) QM/MM-MD simulations to generate the free energy profile. (D) Calculated 2D PMF along the reaction progress (in kilocalories per mole). The reaction coordinate d1 involves the nucleophilic attack of water to form a new P–O bond, reaction coordinate d3 − d4 is the hydrogen transfer from nucleophilic water to the pro-Rp phosphate oxygen of target nucleotide T10′, reaction coordinate d2 is the breaking of P–O bond between target nucleotides T10′ and C11′, and reaction coordinate d4 − d5 is the proton transfer from the pro-Rp oxygen of nucleotide T10′ to the 3′ leaving oxygen of target nucleotide C11′. (E) pH-dependent experimental measurements for the cleaved product of WT.
Results and Discussion
In this work, we carried out B3LYP(6-31G*) QM/MM-MD (56) simulations coupled with experimental measurements of cleavage activity for selective mutants to characterize the detailed reaction mechanism for the TtAgo-mediated target DNA cleavage and identify the catalytic roles of some critical residues, such as E512, R545, R486, and K575. Given that R486 and R545 are located symmetrically around the plugged-in glutamate finger E512 in the precleavage state (13), we discovered that they played distinct catalytic roles during target cleavage.
TtAgo-Mediated DNA Target Cleavage Follows a Substrate-Assisted Cleavage Pathway.
Our aiQM/MM-MD simulation results revealed that the TtAgo-mediated target DNA cleavage exhibits a substrate-assisted (57, 58) mechanism (Fig. 1B and C), in which the scissile phosphate acts as the general base to deprotonate the nucleophilic water and the protonation of 3′ leaving oxygen is accomplished by shuttling the attacking water proton through the pro-Rp oxygen of scissile phosphate, similar to the two-metal-ion catalysis used by RNase H (45, 59) and ribozyme (46, 60). Our calculations indicated pentacovalent phosphorus as intermediates experimentally (61–63) and theoretically (38, 44). Also, the free energy profiles (Fig. 1 C and D and SI Appendix, Fig.S1) computed from umbrella sampling (64, 65) found that the rate-determining step is the nucleophilic attack of water to form the pentacovalent phosphorus intermediate, with a free energy barrier of 16.8 ± 0.2 kcal/mol (kcat = 112.3 ± 0.8 s−1), similar to that of RNase H-mediated RNA cleavage (38, 44, 66, 67). In addition, our pH-dependent experimental results (Fig. 1E) indicated that the TtAgo-mediated target DNA cleavage occurs at neutral pH condition, which strongly supports our calculated substrate-assisted mechanism that does not require any amino acid residues of the enzyme to have specific protonation state to support catalytic activity. Our proposed detailed cleavage mechanism explains how substrate specificity for the Ago-mediated DNA/RNA cleavage is achieved.
We decipher from the previous structure (13) a “substrate-as-base” mechanism (42, 45, 46, 57, 58, 68, 69) for the target DNA cleavage reaction, and our simulation results showed that it is the pro-Rp oxygen of scissile phosphate, rather than the pro-Rp oxygen of phosphate group 3′ to scissile bond, that acts as a general base for the target cleavage. This is because it is not energetically feasible for the pro-Rp oxygen of phosphate group 3′ to scissile bond to deprotonate the nucleophilic water (SI Appendix, Fig. S2). Instead, the role of pro-Rp oxygen of phosphate group 3′ to scissile bond is to orient and stabilize the attacking hydroxide ion through hydrogen bond networks (Fig. 1B). In addition, when the pro-Rp oxygen of scissile phosphate was substituted to sulfur, the energy barrier for the target DNA cleavage reaction was much higher (SI Appendix, Fig. S3). These calculation results are in accordance with the previous reported experimental observations that the cleavage rate was reduced by 200 fold for phosphorothioate substitution of pro-Rp oxygen at the scissile phosphate, whereas the rate was reduced by only 15 fold for phosphorothioate substitution of pro-Rp oxygen at the phosphate group 3′ to scissile bond (5).
It is obvious from our simulation results that the TtAgo-mediated target DNA cleavage has no apparent general acid to donate the proton for the 3′ leaving group of cleavage at neutral pH (13, 45) (SI Appendix, Figs. S4 and S5). Neither of the two remaining candidates for general acid, the plugged-in glutamate finger E512 and the positive charged K575, could protonate the 3′ leaving group. It is because the carboxylic hydrogen of protonated E512 does not form a hydrogen bond with bridging waters (SI Appendix, Fig. S4B), and the energy barrier is too high for the proton transfer from protonated E512 to the 3′ leaving oxygen through bridging water (SI Appendix, Fig. S4C). Additionally, K575, a better candidate than E512 because it locates closer to 3′ leaving oxygen, cannot act as a general acid because it is not energetically feasible for the proton transfer from K575 to the 3′ leaving oxygen (SI Appendix, Fig. S5).
The Plugged-In Glutamate Finger E512 only Acts as a Critical Structural Anchor to Form Catalytic Tetrad Conformation.
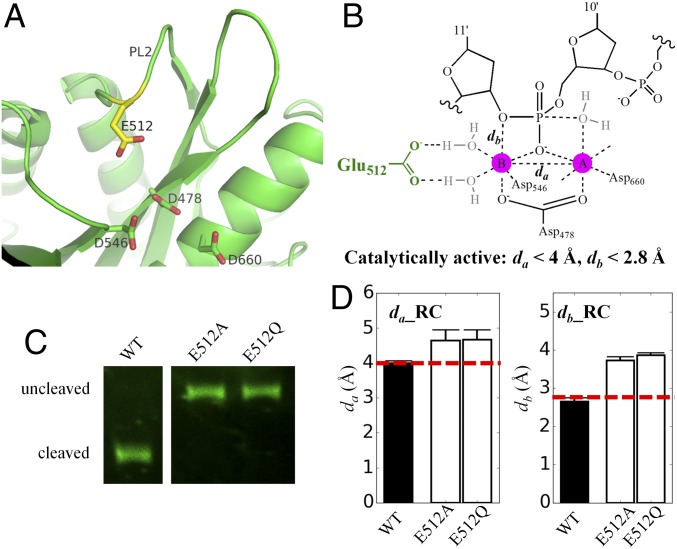
As discussed here earlier, the plugged-in glutamate finger does not act as a general acid. Instead, the highly conserved glutamate finger E512 is a crucial structural anchor for the formation of catalytic tetrad from the previously reported structures (Fig. 2A and SI Appendix, Fig. S6) (13), and our aiQM/MM-MD simulation results indicated that the plugged-in catalytic tetrad conformation is well maintained throughout the whole target cleavage process (Fig. 1B and SI Appendix, Table S1). To further validate the role of glutamate finger in structural anchoring, we performed classical MD simulations and DNA-cleavage experiments for mutants E512A and E512Q. The simulation results show that the catalytic active site configuration is disrupted for mutants E512A and E512Q in the precleavage state, as the distances between Mg2+ A and B (Fig. 2 B and D and SI Appendix, Table S2) are larger than 4.5 Å, which exceeds the separation distance (<4.0 Å) required for the cleavage reaction to occur (45). Furthermore, in the two mutants, the two Mg2+ ions are no longer coordinating with nucleotide phosphate oxygen atoms, as the distances between Mg2+ ions and nucleotide phosphate oxygen atoms (Fig. 2 B and D and SI Appendix, Fig. S7C and Table S2) are larger than 3.0 Å, which exceeds the coordination distance (<2.8 Å) needed for cleavage reaction (45, 70, 71). Overall, the mutants E512A and E512Q are enzymatically inactive, consistent with our experimental observations (Fig. 2C and SI Appendix, Fig. S8) that DNA cleavage is abolished for these two mutants. In addition to E512, our simulation and experimental results show that other residues in the catalytic tetrad (i.e., D478, D546, D660) are also critical structural anchors, as the cleavage activities are abolished for the mutants of these residues (SI Appendix, Fig. S9) (5, 6, 13). Hence, we conclude that the plugged-in glutamate finger is only a critical structural anchor to complete the catalytic tetrad to impart catalytic specificity for Ago-mediated target cleavage.
Fig. 2.
The simulation measuring active site stability and experimental results for glutamate finger mutants of TtAgo in comparison with that of WT. (A) Catalytic tetrad that includes the plugged-in glutamate finger (yellow sticks) of TtAgo (PDB ID code 4NCB). (B) Critical distance to describe the stability of the active site. (C) Experimental measurements for the cleaved products at 120 min. Original figures are shown in SI Appendix, Fig. S8. (D) Average distance between Mg2+ cation A and B (da), average distance between Mg2+ cation B and 3′-hydroxyl oxygen of target nucleotide C11′ (db), and their statistical errors calculated in the precleavage state (RC). The statistical errors were computed by bootstrapping the 10 independent trajectories 10 times with replacement. The precleavage crystal structure (PDB ID code 4NCB) complex with guide and target DNA is used for our simulation.
Two Symmetric Arginine Residues Play Distinct Roles: R545, Not R486, Is Involved in Target Cleavage as an Important Stabilizing Structural Support for the Catalytic Tetrad Conformation.
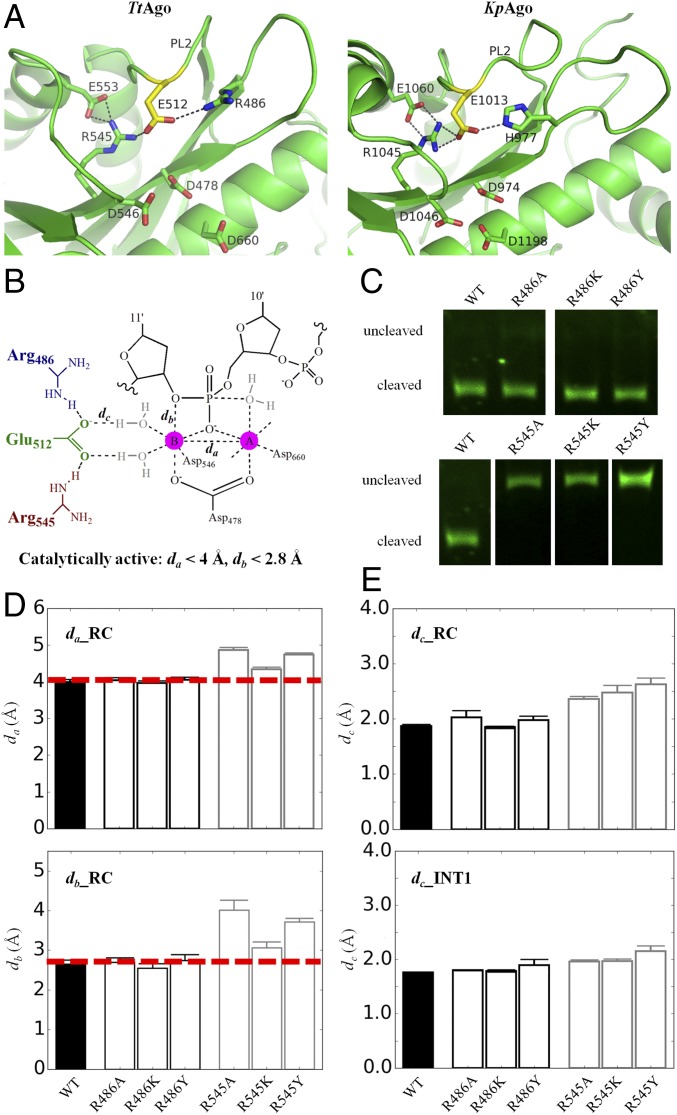
Our simulation and experimental results show that R545 is involved in target cleavage as a crucial stabilizing residue for the plugged-in catalytic tetrad conformation. In the precleavage state, for mutants R545A, R545K, and R545Y, the simulated distances between Mg2+ A and B (Fig. 3 B and D and SI Appendix, Table S2) are much larger than the separation distance (<4.0 Å) (45) suitable for triggering cleavage reaction. Additionally, the two Mg2+ ions flip away from the nucleotide phosphate oxygen atoms, as the coordination distance (Fig. 3 B and D and SI Appendix, Fig. S7C and Table S2) for the mutants is larger than the value (<2.8 Å) for triggering cleavage (45, 70, 71). Moreover, to validate the role of R545 throughout the entire cleavage reaction process, we generated R545 mutants by using the first pentacovalent intermediate (INT1) state structure obtained from our aiQM/MM-MD calculations to perform classic MD simulations (detailed in Methods). The results show that, for R545Y mutant, the active site configuration is almost not maintained because the hydrogen bond networks between glutamate finger E512 and two bridge waters are nearly broken (Fig. 3 B and E). Overall, all three mutants of R545 are catalytically inactive, and this observation is consolidated by our experiments showing that the cleavage is completely eliminated for these mutants (Fig. 3C and SI Appendix, Fig. S8). In fact, R545 permanently locates close to the catalytic pocket, even in the cleavage-incompatible state (13). Therefore, it is conceivable that R545 serves as a crucial structural anchor to stabilize the plugged-in catalytic active site conformation.
Fig. 3.
The simulation measuring active site stability and experimental results for mutants of the two arginines that locate symmetrically and form a hydrogen bond with glutamate finger in comparison with that of WT. (A) Comparison of surrounding residues and hydrogen bond networks for the plugged-in glutamate finger between TtAgo (PDB ID code 4NCB) and KpAgo (PDB ID code 4F1N). Glutamate finger is shown as yellow sticks, and hydrogen bond networks are indicated by black dashed lines. (B) Important distance to describe the stability of the active site of TtAgo. (C) Experimental measurements for the cleaved products at 120 min. Original figures are shown in SI Appendix, Fig. S8. (D) Average distance between Mg2+ cation A and B (da), average distance between Mg2+ cation B and 3′-hydroxyl oxygen of target nucleotide C11′ (db), and their statistical errors calculated in the precleavage state (RC). (E) Average hydrogen bond distance between glutamate finger and coordination waters (dc) with their statistical errors calculated in the precleavage (RC) state (Top) and first intermediate (i.e., INT1) state (Bottom) obtained from B3LYP(6-31G*) QM/MM-MD simulations. The statistical errors were computed by bootstrapping the 10 independent trajectories 10 times with replacement. The precleavage crystal structure (PDB ID code 4NCB) complex with guide and target DNA is used for our simulation.
Surprisingly, we discovered that R486 is not involved in target DNA cleavage. As shown in Fig. 3 and SI Appendix, Fig. S7 and Table S2, all distances characterizing the active site conformation remain almost unchanged in the precleavage state for all of the R486 mutants (including R486A, R486K, and R486Y mutants). For example, the distance between Mg2+ A and B and both of their coordination geometries are mostly maintained. Furthermore, the hydrogen bonds between glutamate finger and bridge waters are also almost maintained in precleavage state and INT1 state obtained from aiQM/MM-MD simulations. Consistently, our experimental results show that, in contrast to R545, there remained DNA cleavage activities for these R486 mutants (Fig. 3C and SI Appendix, Fig. S8). Therefore, our results suggest that, drastically differently than R545, the seemingly symmetric R486 may not act as a critical structure anchor to maintain plugged-in conformation.
Interestingly, in contrast to RNase H, Ago-mediated cleavage requires glutamate finger insertion to complete the catalytic tetrad and impart catalytic specificity (21, 37). In pAgo, such as TtAgo (13, 21), the glutamate finger is inserted within the DDD triad in the guide-target bound structure following guide-target base pairing (55), and both of the two symmetric arginine residues may play roles in stabilizing the catalytic tetrad conformation from the previously reported crystal structure (Fig. 3A) (13). However, from aiQM/MM-MD and MD simulations, we show that, surprisingly, only R545 is involved in the cleavage reaction by serving as a critical structural anchor to stabilize the plugged-in conformation, whereas R486 is not directly involved in cleavage reaction. Our experimental observations also prove that substitution of R545 will completely eliminate the target cleavage, whereas the cleavage activity is retained for R486 substitutions. In addition, when we mutated R486 to histidine, the cleavage activity was also retained for this R486H mutant from our simulation results (SI Appendix, Fig. S7).
We postulate that, in TtAgo, R486 may play the role of facilitating mRNA target binding and glutamate finger insertion because R486 flips close to the catalytic pocket together with the insertion of the glutamate finger loop. As shown in the crystal structure before finger insertion (SI Appendix, Fig. S10C) (13), R486 has already formed stable interactions with the glutamate finger via the hydrogen bonds but locates far from the active site. During finger insertion, R486 moves along with the glutamate finger loop (especially E512) close to the active site (SI Appendix, Fig. S10A). In sharp contrast, R545 always stays close to the active site during the entire process. We thus speculate that R486 may facilitate the insertion of the glutamate finger loop, which subsequently leads to the formation of the catalytically active conformation. In contrast, in eAgo, such as KpAgo (37), the glutamate finger has already been inserted at the stage of the siRNA (i.e., guide) bound complex formation before the recognition of the mRNA target. This sequential order of events suggests that the glutamate finger insertion is unlikely to be correlated with the mRNA target recognition in eAgo. Even for the siRNA-bound complex formation, the two symmetric positive charge residues, R1045 and H977, are not likely to facilitate the glutamate finger insertion because they are already located close to the catalytic pocket before the glutamate finger insertion as shown in the apoenzymatic structure of the eAgo Neurospora crassa QDE-2 (SI Appendix, Fig. S10D) (72). On the contrary, R1045 and H977 (Fig. 3A and SI Appendix, Fig.S10B) have been shown to be critical for catalysis, as their mutants (R1045A and H977A) lead to significant reduction of the cleavage activities based on the fluorescence measurements (37). Therefore, it is striking that the functions of R545 and R486 in TtAgo are different from those of R1045 and H977 in KpAgo; the difference between R486 and H977 is especially pronounced. We speculate that differences in the functions of the arginine in TtAgo and histidine in KpAgo (37) may result in the distinct insertion motions of glutamate finger between pAgo and eAgo, and therefore could shed light on the evolution of Ago proteins. Consistently, our sequence alignment results also show that R545 is highly conserved among different eAgo and eAgo proteins, whereas R486 is not conserved (SI Appendix, Fig. S6).
K575 Acts as a Structural Anchor to Maintain the Active Site Conformation.
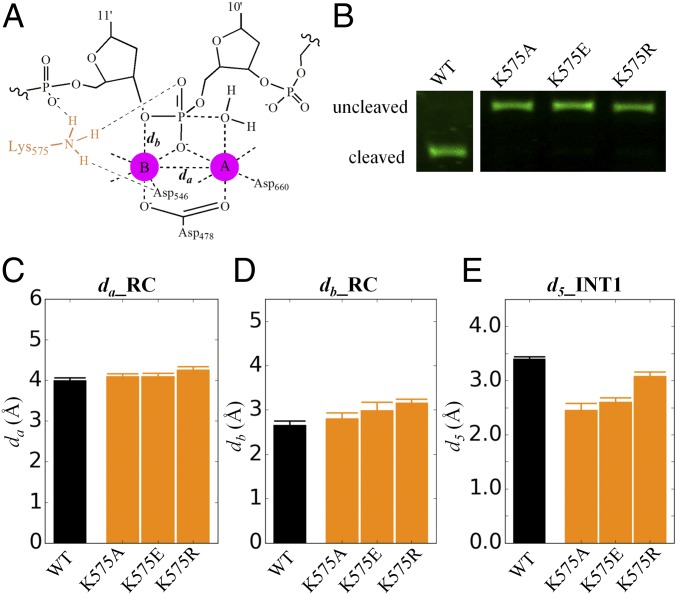
As discussed, K575 does not act as a general acid for target DNA cleavage. Instead, we postulate that it acts as a structural anchor to maintain the catalytic active conformation through hydrogen bonds with D546 and phosphates of target nucleotide (Fig. 4A) (13). When K575 is mutated to ALA (Alanine), GLU (Glutamine), and ARG (Arginine) in the precleavage state, simulation results (Fig. 4 C and D) showed that their active site configuration fluctuates much larger than that of WT. In addition, when we mutate K575 in the INT1 state, the transferred water hydrogen spontaneously flips close to the 3′-hydroxyl oxygen of nucleotide 11′ (Fig. 4E), disrupting the active site configurations of these three mutants. Consistently, experiments showed that DNA cleavage is shut down for these three mutants (Fig. 4B and SI Appendix, Fig. S8). Based on these results, we conclude that the role of K575 is to maintain the active site configuration through its hydrogen bond network during the whole cleavage process.
Fig. 4.
The simulation measuring active site stability and experimental results of K575 mutants in comparison with that of WT. (A) Critical distance to describe the stability of the active site of TtAgo. (B) Experimental measurements for the cleaved products at 120 min. Original figures are shown in SI Appendix, Fig. S8. (C) Average distance between Mg2+ cation A and B (da), (D) average distance between Mg2+ cation B and 3′-hydroxyl oxygen of target nucleotide C11′ (db), and their statistical errors are calculated in the precleavage state (RC). (E) Average distance between phosphate hydroxyl hydrogen and 3′-hydroxyl oxygen of target nucleotide C11′ (d5; see Fig. 1D for definition) and their fluctuations calculated in INT1 state. The statistical errors were computed by bootstrapping 10 independent trajectories 10 times with replacement. The precleavage crystal structure (PDB ID code 4NCB) complexed with guide and target DNA is used for our simulation.
Conclusions
Intriguingly, we discovered the distinct roles of two seemingly symmetric arginines, R486 and R545, for TtAgo-mediated target cleavage. Previous research focused on the active site structural feature of Ago (1, 3, 5–7, 21, 73), and the corresponding R1045 and H977 in KpAgo were both reported to be involved in cleavage (37) and proposed to stabilize the plugged-in catalytic tetrad conformation. In the present work, our simulation and experimental results revealed that, in TtAgo, it is R545, rather than R486, that is involved in target cleavage and acts as a crucial structural anchor in stabilizing the plugged-in catalytic tetrad conformation, given that the precleavage state is inactive and the cleavage is abolished for R545A, R545K, and R545Y mutants. In contrast, R486 is not involved in cleavage reaction because the cleavage activity of R486A, R486K, and R486Y mutants are all maintained. By extensive B3LYP(6-31G*) QM/MM-MD simulations, we also showed that the target DNA cleavage is characterized by a substrate-assisted mechanism with a barrier height of approximately 16.8 kcal/mol, and the plugged-in glutamate finger only acts as a critical structural anchor for the catalytic tetrad formation rather than a general acid to protonate the 3′ leaving group. Our substrate-assisted mechanism and validation of catalytic role of the glutamate finger could impart the catalytic specificity of Ago proteins. Overall, our finding of unique roles for the two positively charged residues in TtAgo in contrast to the equivalent roles for the corresponding residues in KpAgo may provide a molecular basis for the differences in glutamate finger insertion motion between pAgo and eAgo proteins. In pAgo, the glutamate finger plugged-in catalytic tetrad conformation is formed following guide-target base pairing, whereas, in eAgo, the plugged-in conformation is formed before guide-target base pairing. This information could illuminate the evolutionary journey from pAgo to eAgo proteins.
Methods
Based on our previous reported ternary crystal structures (13) of TtAgo complex with guide and 19-mer target DNA, we performed classic MD simulations and then obtained proper snapshots for the subsequent aiQM/MM simulations. We employed B3LYP/6–31G(d) QM/MM-MD (56, 74) simulations with umbrella sampling (65, 75, 76), a computational tour de force to study biochemical reactions. This state-of-the-art computational approach provides a first-principles description of chemical bond formation/breaking and dynamics of the enzyme active site while properly incorporating the effects of heterogeneous and fluctuating protein environment, and has been demonstrated to be powerful in characterizing the reaction mechanism for a number of complex systems (64, 74, 77–87). In addition, we also performed classic MD simulations for selective mutants of reactant and pentacovalent intermediate to identify the catalytic roles of key charged residues near the active site. More computational details and experimental measurement methods are presented in the following subsections.
Structure Preparation for Simulation.
The ternary crystal structure [Protein Data Bank (PDB) ID code (13) 4NCB] of TtAgo complex with guide and 19-mer target DNA in cleavage-compatible states was the basis for our enzyme-substrate model. The missing residues and atoms were added by using MODELER (88–90), and the PO2 group of DC5 in target DNA chain D was deleted because it was unsolved and far away from active site. The partial charges for the 5′-phosphorylated terminal basis DT5 of guide DNA were fitted with HF/6–31G(d) calculations by using the restrained electrostatic potential module (91) in the Amber package. The protonation states of the ionizable residues were determined at pH 7 based on pKa calculations via PROPKA (92–95) and H++ (96, 97) programs. If these two programs produced inconsistent predictions, the local hydrogen bonding network would be taken into account. As a result, the histidine residues HIS379, HIS500, and HIS621 were protonated as HIP in the following MD and QM/MM simulations for TtAgo complex with guide and 19-mer target DNA.
Classical MD Simulation.
For the classical MD simulations of TtAgo complex with guide and 19-mer target DNA, the starting model was subjected to minimization of the hydrogen atoms that were added by LEAP module of the Amber 12 simulation package (98) with 600 steps of steepest descent followed by an additional 600 steps of conjugate gradient minimization. Then, the whole system was solvated into explicit TIP3P water (99) molecules by using a cubic box with a 15-Å buffer distance between the box wall and its nearest solute atom, and five Na+ ions were added to neutralize the charge. As a result, the whole system contains ∼170,000 atoms. The subsequent energy minimizations and equilibration MD simulations followed the same state-of-the-art protocol as in our previous studies (74, 78). First, the solvent and counter ions were minimized with 2,500 steps of steepest descent followed by a 2,500-cycle conjugate gradient minimization by restraining the protein, DNA, and Mg2+ atoms with a restraint force constant of 50 kcal·mol−1·Å−2. While gradually reducing this restraint to 25 kcal·mol−1·Å−2, the solvent and counter ions were first minimized (1,000 steps of steepest descent followed by 1,000-cycle conjugate gradient), then equilibrated with a 100-ps NVT [constant number (N), volume (V), and temperature (T)] MD simulation (temperature, 10 K) followed by another 100-ps NPT [constant number (N), pressure (P), and temperature (T)] (pressure, 1 atm) MD simulation. Next, the system was heated from 10 K to 340 K with a 200-ps NVT simulation and equilibrated with a 100-ps NPT simulation, during which the restraint force constant was reduced to 10 kcal·mol−1·Å−2. Then, two sequential 100-ps NPT equilibration simulations were performed with looser restraint force constants of 1 kcal·mol−1·Å−2 and 0.1 kcal·mol−1·Å−2, respectively. Finally, a 50-ns production simulation was conducted at a temperature of 340 K with the Berendsen thermostat method (100) and a constant pressure of 1 atm coupled with isotropic position scaling. Five independent equilibration and production MD simulations were carried out in total with different initial velocities. In all MD simulations, the SHAKE algorithm (101) for bond constraint and a time step of 1 fs were used, the long-range electrostatic interactions were treated with particle mash Ewald (PME) (102, 103) method, and a 12-Å cutoff was used for van der Waals (vdW) and short-range PME interactions. All MD simulations were performed by using the Amber 12 MD package (98), the Amber99SB-ILDN force field (104) was employed for protein, and Amber99SB force field (91, 105, 106) with modification by parmbs0 (107) was used for DNA. In addition, to validate the convergence of our MD simulations, we conducted another five independent production simulations by GROMACS 5.0.4 (108, 109). Thus, a total of 10 50-ns product MD trajectories were used for our data analysis (SI Appendix, Fig. S11).
To further determine the roles for critical amino acid residues that stabilize the local structure of the active site, several classical MD simulations for mutants of reactant and first pentacovalent phosphorane intermediate (i.e., INT1) were carried out. As the intermediate contains five covalent bonds, an Amber-compatible force field has to be developed to simulate this state. A new residue (INT1; Fig. 1 B and D) composed of the phosphated nucleotide T10′ of target DNA strand was defined. This residue (INT1; Fig. 1 B and D) contained six new atom types for amber force field of MD simulations: the pentacovalent phosphorus of nucleotide T10′, two hydroxyl groups connected to pentacovalent phosphorus, and the 3′-hydroxyl oxygen atom of nucleotide C11′ that connects to the pentacovalent phosphorus. The geometries, topology assignments, and partial charge parameters (Tables S3 and S4) were characterized on the basis of the QM/MM-MD calculations for the INT1 state of unmodified TtAgo complex (78, 110). The force constants were chosen to be large enough to maintain the bond lengths, bond angles, and dihedral angles in the INT1 state of unmodified TtAgo complex (78, 110). All MD protocols for mutants were the same as classical MD for unmodified TtAgo complex with guide and 19-mer target DNA. In total, 10 independent 50-ns production MD trajectories were collected for each mutant (SI Appendix, Figs. S12–S14).
QM/MM Simulation.
The initial structure for QM/MM calculations was a snapshot chosen from one 50-ns production MD simulation trajectory for unmodified TtAgo complex with guide and 19-mer target DNA. The choice of QM subsystem is usually based on the proposed reaction schemes, and includes fragments directly participating in the reaction (111). The QM subsystem of TtAgo system includes the nucleotide bases T10′ and C11′ of target DNA, the nucleophilic water, and two Mg2+ ions treated by B3LYP (112–114) functional with 6–31G(d) (115–117) basis set. The QM/MM interface was described by the improved pseudobond approach (118, 119). All other atoms were described by the same molecular mechanical force field used in the classical MD simulations. In our QM/MM calculations, the recently developed periodic boundary condition with Ewald method was applied to reliably describe the long-range interactions and dynamics (56, 120–122). The 12-Å cutoff was used for vdW and short-range PME interactions, and there was no cutoff for electrostatic interactions between QM and MM regions. The selected initial structure was first minimized and then employed to map out the minimal energy path for the investigated mechanisms by using the reaction coordinate driving method (78, 123). For each of the 430 determined structures selected along the path, an 800-ps MD simulation with MM force field was carried out to equilibrate the MM subsystem with the QM subsystem being frozen. Finally, the resulting snapshot was used as the starting structure for Born–Oppenheimer QM/MM MD simulation with umbrella sampling (65, 75, 76) that applied a harmonic potential to constrain the reaction coordinate at the successive values. To ensure sufficient overlap between the successive windows, a force constant of 150 kcal·mol−1·Å−2 was employed for each window. Each of the 430 windows was simulated for at least 15 ps, and the active site dynamics and those of the surroundings were simulated on an equal footing. The potential of mean force (PMF) was obtained from the probability distributions along a reaction coordinate by using the weighted histogram analysis method (124–126). The statistical error of the calculated free energy change was estimated by computing the average deviation between the calculated free energy change using half of sampling data for each umbrella window (5–10 ps or 10–15 ps) and the free energy change calculated using data from the whole sampling period for each umbrella window (5–15 ps). The first 5-ps simulation was considered as equilibration for each umbrella window. All aiQM/MM calculations were performed with modified Q-Chem (127) and Amber 12 (98) programs (56).
In our aiQM/MM-MD simulations for the substrate-assisted mechanism, we included only the nucleotides and nucleophilic water that directly coordinated with the Mg ions in the QM region. We did not include the coordinated ligands that are not involved in the bond forming or breaking of the cleavage reaction, such as the catalytic triad ASP residues D478, D546, and D660. This is because our aiQM/MM-MD calculations would become quite expensive and less effective when the QM region becomes large. In addition, in the aiQM/MM studies of enzymatic reactions with transition metal atom Mg, it is quite common to include only ligands that directly participate in the reaction to save the computational cost because MM can describe such classical electrostatics interactions (64, 78, 128).
Protein Expression and Purification.
The gene encoding full-length TtAgo was inserted into a sumo-PET vector (Invitrogen) with N-terminal His6-SUMO tag following an ubiquitin-like protease (ULP1) cleavage site. Recombinant protein was overexpressed in Escherichia coli Rosetta 2 (DE3; Novagen) strain in lysogeny broth medium. The cells were grown at 37 °C until OD600 reached 0.6 and then induced with 0.1 mM isopropyl β-d-1-thiogalactopyranoside at 18 °C for 12 h. Cell pellets were resuspended in buffer A (20 mM Tris⋅HCl, pH 7.5, 0.5 M NaCl, and 2 mM MgCl2) and then lysed by French press and centrifuged at 39,191 × g (Beckman Coulter, Avanti J-26 XP Centrifuge, Rotor ID: 25.50) for 40 min at 4 °C. The supernatant containing TtAgo was loaded to a 10-mL HisTrap Fast Flow column (GE Healthcare) preequilibrated in buffer A and eluted with buffer A supplemented with 200 mM imidazole. The His6-SUMO tag was removed by ULP1 and during dialysis against buffer A. The TtAgo protein was further purified by HisTrap Fast Flow column preequilibrated with buffer A. The purified TtAgo protein was concentrated to 25 mg/mL in buffer A, snap-frozen in liquid nitrogen, and stored at −80 °C.
In Vitro Cleavage Assays of TtAgo.
The 5′-phosphorylated (5′-phos-TGAGGTAGTAGGTTGTATAGT) 21-base DNA guide and 5′-Cys–labeled (5′-Cys-AATTAACCAAATATCAATATACAACCTACT ACCTCAGT-3′) 38-nt DNA target with the complementary sequence to the guide strand were purchased from Sangon Biotech. The cleavage reaction was performed by mixing TtAgo protein (1.0 μM) and guide DNA at the molar ratio of 1:1 and incubated in buffer (10 mM Hepes-KOH, pH 7.5, 150 mM NaCl, 5 mM MgCl2) for 30 min at 42 °C in a final volume of 10 μL. The reaction buffer Hepes-KOH, pH 7.5, changed with Mes-NaOH, pH 5.0–6.5, Tris⋅HCl, pH 7.0–9.0, in pH-dependent cleavage reaction. Next, 1.0 μM 5′-Cy3–labeled DNA target was added and incubated at 60 °C for the indicated times. Reactions were terminated by addition of an equal volume of stop solution containing 8 M urea and 50 mM EDTA. The cleavage products were heated for 15 min at 95 °C, resolved on 20% denaturing polyacrylamide gels, and visualized by Multi Green using FluorChem M (ProteinSimple).
Supplementary Material
Acknowledgments
This work was supported by Hong Kong Research Grant Council (Hong Kong University of Science and Technology) Grants C6009-15G, 16318816, 16302214, AoE/P-705/16, and T31-605/18-W; King Abdullah University of Science and Technology (KAUST) Office of Sponsored Research (OSR) Grant OSR-2016-CRG5-3007; Innovation and Technology Commission Grants ITCPD/17-9 and ITC-CNERC14SC01; National Natural Science Foundation of China Grants 31725008, 31571335, and 31630015; and National Institutes of Health Grant R35-GM127040. This research made use of the resources of the Supercomputing Laboratory at KAUST. X.H. is the Padma Harilela Associate Professor of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1817041116/-/DCSupplemental.
References
- 1.Hutvagner G, Simard MJ. Argonaute proteins: Key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32. doi: 10.1038/nrm2321. [DOI] [PubMed] [Google Scholar]
- 2.Peters L, Meister G. Argonaute proteins: Mediators of RNA silencing. Mol Cell. 2007;26:611–623. doi: 10.1016/j.molcel.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Wilson RC, Doudna JA. Molecular mechanisms of RNA interference. Annu Rev Biophys. 2013;42:217–239. doi: 10.1146/annurev-biophys-083012-130404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meister G. Argonaute proteins: Functional insights and emerging roles. Nat Rev Genet. 2013;14:447–459. doi: 10.1038/nrg3462. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, et al. Nucleation, propagation and cleavage of target RNAs in Ago silencing complexes. Nature. 2009;461:754–761. doi: 10.1038/nature08434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Sheng G, Juranek S, Tuschl T, Patel DJ. Structure of the guide-strand-containing Argonaute silencing complex. Nature. 2008;456:209–213. doi: 10.1038/nature07315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y, et al. Structure of an Argonaute silencing complex with a seed-containing guide DNA and target RNA duplex. Nature. 2008;456:921–926. doi: 10.1038/nature07666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zander A, Holzmeister P, Klose D, Tinnefeld P, Grohmann D. Single-molecule FRET supports the two-state model of Argonaute action. RNA Biol. 2014;11:45–56. doi: 10.4161/rna.27446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaya E, et al. A bacterial Argonaute with noncanonical guide RNA specificity. Proc Natl Acad Sci USA. 2016;113:4057–4062. doi: 10.1073/pnas.1524385113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arribas-Hernández L, et al. Theslicer activity of ARGONAUTE1 is required specifically for the phasing, not production, of trans-acting short interfering RNAs in Arabidopsis. Plant Cell. 2016;28:1563–1580. doi: 10.1105/tpc.16.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swarts DC, et al. Argonaute of the archaeon Pyrococcus furiosus is a DNA-guided nuclease that targets cognate DNA. Nucleic Acids Res. 2015;43:5120–5129. doi: 10.1093/nar/gkv415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyoshi T, Ito K, Murakami R, Uchiumi T. Structural basis for the recognition of guide RNA and target DNA heteroduplex by Argonaute. Nat Commun. 2016;7:11846. doi: 10.1038/ncomms11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheng G, et al. Structure-based cleavage mechanism of Thermus thermophilus Argonaute DNA guide strand-mediated DNA target cleavage. Proc Natl Acad Sci USA. 2014;111:652–657. doi: 10.1073/pnas.1321032111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerstberger S, Hafner M, Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet. 2014;15:829–845. doi: 10.1038/nrg3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fareh M, et al. TRBP ensures efficient Dicer processing of precursor microRNA in RNA-crowded environments. Nat Commun. 2016;7:13694. doi: 10.1038/ncomms13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swarts DC, et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature. 2014;507:258–261. doi: 10.1038/nature12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogel J. Biochemistry. A bacterial seek-and-destroy system for foreign DNA. Science. 2014;344:972–973. doi: 10.1126/science.1252962. [DOI] [PubMed] [Google Scholar]
- 18.Hur JK, Olovnikov I, Aravin AA. Prokaryotic Argonautes defend genomes against invasive DNA. Trends Biochem Sci. 2014;39:257–259. doi: 10.1016/j.tibs.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swarts DC, et al. Autonomous generation and loading of DNA guides by bacterial Argonaute. Mol Cell. 2017;65:985–998.e6. doi: 10.1016/j.molcel.2017.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hegge JW, Swarts DC, van der Oost J. Prokaryotic Argonaute proteins: Novel genome-editing tools? Nat Rev Microbiol. 2018;16:5–11. doi: 10.1038/nrmicro.2017.73. [DOI] [PubMed] [Google Scholar]
- 21.Swarts DC, et al. The evolutionary journey of Argonaute proteins. Nat Struct Mol Biol. 2014;21:743–753. doi: 10.1038/nsmb.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaynor JW, Campbell BJ, Cosstick R. RNA interference: A chemist’s perspective. Chem Soc Rev. 2010;39:4169–4184. doi: 10.1039/b920362c. [DOI] [PubMed] [Google Scholar]
- 23.Esquela-Kerscher A, et al. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–764. doi: 10.4161/cc.7.6.5834. [DOI] [PubMed] [Google Scholar]
- 24.Broderick JA, Zamore PD. MicroRNA therapeutics. Gene Ther. 2011;18:1104–1110. doi: 10.1038/gt.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hafner M, et al. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landgraf P, et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell. 2007;129:1401–1414. doi: 10.1016/j.cell.2007.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zander A, et al. Guide-independent DNA cleavage by archaeal Argonaute from Methanocaldococcus jannaschii. Nat Microbiol. 2017;2:17034. doi: 10.1038/nmicrobiol.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willkomm S, et al. Structural and mechanistic insights into an archaeal DNA-guided Argonaute protein. Nat Microbiol. 2017;2:17035. doi: 10.1038/nmicrobiol.2017.35. [DOI] [PubMed] [Google Scholar]
- 29.Doxzen KW, Doudna JA. DNA recognition by an RNA-guided bacterial Argonaute. PLoS One. 2017;12:e0177097. doi: 10.1371/journal.pone.0177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willkomm S, Zander A, Grohmann D, Restle T. Mechanistic insights into archaeal and human Argonaute substrate binding and cleavage properties. PLoS One. 2016;11:e0164695. doi: 10.1371/journal.pone.0164695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olovnikov I, Chan K, Sachidanandam R, Newman DK, Aravin AA. Bacterial Argonaute samples the transcriptome to identify foreign DNA. Mol Cell. 2013;51:594–605. doi: 10.1016/j.molcel.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koh HR, Ghanbariniaki A, Myong S. RNA stem structure governs coupling of dicing and gene silencing in RNA interference. Proc Natl Acad Sci USA. 2017;114:E10349–E10358. doi: 10.1073/pnas.1710298114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker JS, Roe SM, Barford D. Crystal structure of a PIWI protein suggests mechanisms for siRNA recognition and slicer activity. EMBO J. 2004;23:4727–4737. doi: 10.1038/sj.emboj.7600488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song J-J, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 35.Yuan Y-R, et al. Crystal structure of A. aeolicus Argonaute, a site-specific DNA-guided endoribonuclease, provides insights into RISC-mediated mRNA cleavage. Mol Cell. 2005;19:405–419. doi: 10.1016/j.molcel.2005.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rivas FV, et al. Purified Argonaute2 and an siRNA form recombinant human RISC. Nat Struct Mol Biol. 2005;12:340–349. doi: 10.1038/nsmb918. [DOI] [PubMed] [Google Scholar]
- 37.Nakanishi K, Weinberg DE, Bartel DP, Patel DJ. Structure of yeast Argonaute with guide RNA. Nature. 2012;486:368–374. doi: 10.1038/nature11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sgrignani J, Magistrato A. QM/MM MD simulations on the enzymatic pathway of the human flap endonuclease (hFEN1) elucidating common cleavage pathways to RNase H enzymes. ACS Catal. 2015;5:3864–3875. [Google Scholar]
- 39.Palermo G, et al. Catalytic metal ions and enzymatic processing of DNA and RNA. Acc Chem Res. 2015;48:220–228. doi: 10.1021/ar500314j. [DOI] [PubMed] [Google Scholar]
- 40.Rosta E, Nowotny M, Yang W, Hummer G. Catalytic mechanism of RNA backbone cleavage by ribonuclease H from quantum mechanics/molecular mechanics simulations. J Am Chem Soc. 2011;133:8934–8941. doi: 10.1021/ja200173a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ho MH, De Vivo M, Dal Peraro M, Klein ML. Understanding the effect of magnesium ion concentration on the catalytic activity of ribonuclease H through computation: Does a third metal binding site modulate endonuclease catalysis? J Am Chem Soc. 2010;132:13702–13712. doi: 10.1021/ja102933y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elsässer B, Fels G. Atomistic details of the associative phosphodiester cleavage in human ribonuclease H. Phys Chem Chem Phys. 2010;12:11081–11088. doi: 10.1039/c001097a. [DOI] [PubMed] [Google Scholar]
- 43.Rosta E, Woodcock HL, Brooks BR, Hummer G. Artificial reaction coordinate “tunneling” in free-energy calculations: The catalytic reaction of RNase H. J Comput Chem. 2009;30:1634–1641. doi: 10.1002/jcc.21312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Vivo M, Dal Peraro M, Klein ML. Phosphodiester cleavage in ribonuclease H occurs via an associative two-metal-aided catalytic mechanism. J Am Chem Soc. 2008;130:10955–10962. doi: 10.1021/ja8005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang W, Lee JY, Nowotny M. Making and breaking nucleic acids: Two-Mg2+-ion catalysis and substrate specificity. Mol Cell. 2006;22:5–13. doi: 10.1016/j.molcel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Steitz TA, Steitz JA. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci USA. 1993;90:6498–6502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roston D, Demapan D, Cui Q. Leaving group ability observably affects transition state structure in a single enzyme active site. J Am Chem Soc. 2016;138:7386–7394. doi: 10.1021/jacs.6b03156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roston D, Cui Q. Substrate and transition state binding in alkaline phosphatase analyzed by computation of oxygen isotope effects. J Am Chem Soc. 2016;138:11946–11957. doi: 10.1021/jacs.6b07347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lassila JK, Zalatan JG, Herschlag D. Biological phosphoryl-transfer reactions: Understanding mechanism and catalysis. Annu Rev Biochem. 2011;80:669–702. doi: 10.1146/annurev-biochem-060409-092741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Allen KN, Dunaway-Mariano D. Phosphoryl group transfer: Evolution of a catalytic scaffold. Trends Biochem Sci. 2004;29:495–503. doi: 10.1016/j.tibs.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Mildvan AS. Mechanisms of signaling and related enzymes. Proteins. 1997;29:401–416. [PubMed] [Google Scholar]
- 52.Wong KY, et al. Characterization of the reaction path and transition states for RNA transphosphorylation models from theory and experiment. Angew Chem Int Ed Engl. 2012;51:647–651. doi: 10.1002/anie.201104147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elkayam E, et al. The structure of human Argonaute-2 in complex with miR-20a. Cell. 2012;150:100–110, and correction (2012) 150:233. doi: 10.1016/j.cell.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schirle NT, MacRae IJ. The crystal structure of human Argonaute2. Science. 2012;336:1037–1040. doi: 10.1126/science.1221551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsumoto N, et al. Crystal structure of silkworm PIWI-clade Argonaute Siwi bound to piRNA. Cell. 2016;167:484–497.e9. doi: 10.1016/j.cell.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Y, Wang S, Li Y, Zhang Y. Born-Oppenheimer ab initio QM/MM molecular dynamics simulations of enzyme reactions. Methods Enzymol. 2016;577:105–118. doi: 10.1016/bs.mie.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schweins T, Langen R, Warshel A. Why have mutagenesis studies not located the general base in ras p21. Nat Struct Biol. 1994;1:476–484. doi: 10.1038/nsb0794-476. [DOI] [PubMed] [Google Scholar]
- 58.Schweins T, et al. Substrate-assisted catalysis as a mechanism for GTP hydrolysis of p21ras and other GTP-binding proteins. Nat Struct Biol. 1995;2:36–44. doi: 10.1038/nsb0195-36. [DOI] [PubMed] [Google Scholar]
- 59.Nowotny M, Gaidamakov SA, Crouch RJ, Yang W. Crystal structures of RNase H bound to an RNA/DNA hybrid: Substrate specificity and metal-dependent catalysis. Cell. 2005;121:1005–1016. doi: 10.1016/j.cell.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 60.Strater N, Lipscomb WN, Klabunde T, Krebs B. Two-metal ion catalysis in enzymatic acyl- and phosphoryl-transfer reactions. Angew Chem Int Ed Engl. 1996;35:2024–2055. [Google Scholar]
- 61.Tremblay LW, Zhang G, Dai J, Dunaway-Mariano D, Allen KN. Chemical confirmation of a pentavalent phosphorane in complex with beta-phosphoglucomutase. J Am Chem Soc. 2005;127:5298–5299. doi: 10.1021/ja0509073. [DOI] [PubMed] [Google Scholar]
- 62.Lahiri SD, Zhang G, Dunaway-Mariano D, Allen KN. The pentacovalent phosphorus intermediate of a phosphoryl transfer reaction. Science. 2003;299:2067–2071. doi: 10.1126/science.1082710. [DOI] [PubMed] [Google Scholar]
- 63.Lahiri SD, Zhang G, Dunaway-Mariano D, Allen KN. Caught in the act: The structure of phosphorylated beta-phosphoglucomutase from Lactococcus lactis. Biochemistry. 2002;41:8351–8359. doi: 10.1021/bi0202373. [DOI] [PubMed] [Google Scholar]
- 64.Rooklin DW, Lu M, Zhang Y. Revelation of a catalytic calcium-binding site elucidates unusual metal dependence of a human apyrase. J Am Chem Soc. 2012;134:15595–15603. doi: 10.1021/ja307267y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu H, Yang W. Free energies of chemical reactions in solution and in enzymes with ab initio quantum mechanics/molecular mechanics methods. Annu Rev Phys Chem. 2008;59:573–601. doi: 10.1146/annurev.physchem.59.032607.093618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Patel N, et al. Flap endonucleases pass 5′-flaps through a flexible arch using a disorder-thread-order mechanism to confer specificity for free 5′-ends. Nucleic Acids Res. 2012;40:4507–4519. doi: 10.1093/nar/gks051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Syson K, et al. Three metal ions participate in the reaction catalyzed by T5 flap endonuclease. J Biol Chem. 2008;283:28741–28746. doi: 10.1074/jbc.M801264200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamerlin SCL, Williams NH, Warshel A. Dineopentyl phosphate hydrolysis: Evidence for stepwise water attack. J Org Chem. 2008;73:6960–6969. doi: 10.1021/jo801207q. [DOI] [PubMed] [Google Scholar]
- 69.Langen R, Schweins T, Warshel A. On the mechanism of guanosine triphosphate hydrolysis in ras p21 proteins. Biochemistry. 1992;31:8691–8696. doi: 10.1021/bi00152a002. [DOI] [PubMed] [Google Scholar]
- 70.Harding MM. The geometry of metal-ligand interactions relevant to proteins. Acta Crystallogr D Biol Crystallogr. 1999;55:1432–1443. doi: 10.1107/s0907444999007374. [DOI] [PubMed] [Google Scholar]
- 71.Harding MM. The geometry of metal-ligand interactions relevant to proteins. II. Angles at the metal atom, additional weak metal-donor interactions. Acta Crystallogr D Biol Crystallogr. 2000;56:857–867. doi: 10.1107/s0907444900005849. [DOI] [PubMed] [Google Scholar]
- 72.Boland A, Huntzinger E, Schmidt S, Izaurralde E, Weichenrieder O. Crystal structure of the MID-PIWI lobe of a eukaryotic Argonaute protein. Proc Natl Acad Sci USA. 2011;108:10466–10471. doi: 10.1073/pnas.1103946108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schirle NT, Sheu-Gruttadauria J, MacRae IJ. Structural basis for microRNA targeting. Science. 2014;346:608–613. doi: 10.1126/science.1258040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lei J, Zhou Y, Xie D, Zhang Y. Mechanistic insights into a classic wonder drug–aspirin. J Am Chem Soc. 2015;137:70–73. doi: 10.1021/ja5112964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roux B. Thecalculation of the potential of mean force using computer-simulations. Comput Phys Commun. 1995;91:275–282. [Google Scholar]
- 76.Boczko EM, Brooks CL. Constant-temperature free-energy surfaces for physical and chemical processes. J Phys Chem. 1993;97:4509–4513. [Google Scholar]
- 77.Zhou Y, Xie D, Zhang Y. Amide rotation hindrance predicts proteolytic resistance of cystine-knot peptides. J Phys Chem Lett. 2016;7:1138–1142. doi: 10.1021/acs.jpclett.6b00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lior-Hoffmann L, et al. Preferred WMSA catalytic mechanism of the nucleotidyl transfer reaction in human DNA polymerase κ elucidates error-free bypass of a bulky DNA lesion. Nucleic Acids Res. 2012;40:9193–9205. doi: 10.1093/nar/gks653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng Y, Zhang Y, McCammon JA. How does the cAMP-dependent protein kinase catalyze the phosphorylation reaction: An ab initio QM/MM study. J Am Chem Soc. 2005;127:1553–1562. doi: 10.1021/ja0464084. [DOI] [PubMed] [Google Scholar]
- 80.Cheng Y, Zhang Y, McCammon JA. How does activation loop phosphorylation modulate catalytic activity in the cAMP-dependent protein kinase: A theoretical study. Protein Sci. 2006;15:672–683. doi: 10.1110/ps.051852306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ke Z, Smith GK, Zhang Y, Guo H. Molecular mechanism for eliminylation, a newly discovered post-translational modification. J Am Chem Soc. 2011;133:11103–11105. doi: 10.1021/ja204378q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lu X, et al. QM/MM free energy simulations: Recent progress and challenges. Mol Simul. 2016;42:1056–1078. doi: 10.1080/08927022.2015.1132317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roston D, Cui Q. QM/MM analysis of transition states and transition state analogues in metalloenzymes. Methods Enzymol. 2016;577:213–250. doi: 10.1016/bs.mie.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nam K, Gao J, York DM. Quantum mechanical/molecular mechanical simulation study of the mechanism of hairpin ribozyme catalysis. J Am Chem Soc. 2008;130:4680–4691. doi: 10.1021/ja0759141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee TS, et al. Role of Mg2+ in hammerhead ribozyme catalysis from molecular simulation. J Am Chem Soc. 2008;130:3053–3064. doi: 10.1021/ja076529e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao Y, Cao ZX. Global simulations of enzymatic catalysis. Acta Phys -Chim Sin. 2017;33:691–708. [Google Scholar]
- 87.Wu R, Wang S, Zhou N, Cao Z, Zhang Y. A proton-shuttle reaction mechanism for histone deacetylase 8 and the catalytic role of metal ions. J Am Chem Soc. 2010;132:9471–9479. doi: 10.1021/ja103932d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol. 1993;234:779–815. doi: 10.1006/jmbi.1993.1626. [DOI] [PubMed] [Google Scholar]
- 89.Fiser A, Do RKG, Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9:1753–1773. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martí-Renom MA, et al. Comparative protein structure modeling of genes and genomes. Annu Rev Biophys Biomol Struct. 2000;29:291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- 91.Wang JM, Cieplak P, Kollman PA. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem. 2000;21:1049–1074. [Google Scholar]
- 92.Søndergaard CR, Olsson MHM, Rostkowski M, Jensen JH. Improved treatment of ligands and coupling effects in empirical calculation and rationalization of pKa values. J Chem Theory Comput. 2011;7:2284–2295. doi: 10.1021/ct200133y. [DOI] [PubMed] [Google Scholar]
- 93.Olsson MHM, Søndergaard CR, Rostkowski M, Jensen JH. PROPKA3: Consistent treatment of internal and surface residues in empirical pKa predictions. J Chem Theory Comput. 2011;7:525–537. doi: 10.1021/ct100578z. [DOI] [PubMed] [Google Scholar]
- 94.Bas DC, Rogers DM, Jensen JH. Very fast prediction and rationalization of pKa values for protein-ligand complexes. Proteins. 2008;73:765–783. doi: 10.1002/prot.22102. [DOI] [PubMed] [Google Scholar]
- 95.Li H, Robertson AD, Jensen JH. Very fast empirical prediction and rationalization of protein pKa values. Proteins. 2005;61:704–721. doi: 10.1002/prot.20660. [DOI] [PubMed] [Google Scholar]
- 96.Anandakrishnan R, Aguilar B, Onufriev AV. H++ 3.0: Automating pK prediction and the preparation of biomolecular structures for atomistic molecular modeling and simulations. Nucleic Acids Res. 2012;40:W537–W541. doi: 10.1093/nar/gks375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gordon JC, et al. H++: A server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 2005;33:W368–W371. doi: 10.1093/nar/gki464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Case DA, et al. AMBER 12. Univ California; San Francisco: 2012. [Google Scholar]
- 99.Price DJ, Brooks CL., 3rd A modified TIP3P water potential for simulation with Ewald summation. J Chem Phys. 2004;121:10096–10103. doi: 10.1063/1.1808117. [DOI] [PubMed] [Google Scholar]
- 100.Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR. Molecular-dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–3690. [Google Scholar]
- 101.Ryckaert JP, Ciccotti G, Berendsen HJC. Numerical-integration of cartesian equations of motion of a system with constraints–molecular-dynamics of N-alkanes. J Comput Phys. 1977;23:327–341. [Google Scholar]
- 102.Darden T, York D, Pedersen L. Particle mesh Ewald–An N.log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 103.Essmann U, et al. A smooth particle mesh Ewald method. J Chem Phys. 1995;103:8577–8593. [Google Scholar]
- 104.Lindorff-Larsen K, et al. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins. 2010;78:1950–1958. doi: 10.1002/prot.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hornak V, et al. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cornell WD, et al. A 2nd generation force-field for the simulation of proteins, nucleic-acids, and organic-molecules. J Am Chem Soc. 1995;117:5179–5197. [Google Scholar]
- 107.Pérez A, et al. Refinement of the AMBER force field for nucleic acids: Improving the description of alpha/gamma conformers. Biophys J. 2007;92:3817–3829. doi: 10.1529/biophysj.106.097782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Abraham MJ, Van Der Spoel D, Lindahl E, Hess B. the GROMACS Development Team 2014 GROMACS User Manual Version 5.0.4. Available at www.gromacs.org. Accessed October 6, 2015.
- 109.Pronk S, et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatic s. 2013;29:845–854. doi: 10.1093/bioinformatics/btt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lior-Hoffmann L, Ding S, Geacintov NE, Zhang Y, Broyde S. Structural and dynamic characterization of polymerase κ’s minor groove lesion processing reveals how adduct topology impacts fidelity. Biochemistry. 2014;53:5683–5691. doi: 10.1021/bi5007964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shi Y, Zhou Y, Wang S, Zhang Y. Sirtuin deacetylation mechanism and catalytic role of the dynamic cofactor binding loop. J Phys Chem Lett. 2013;4:491–495. doi: 10.1021/jz302015s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raghavachari K. Perspective on “Density functional thermochemistry. III. The role of exact exchange” - Becke AD (1993) J Chem Phys 98:5648-52. Theor Chem Acc. 2000;103:361–363. [Google Scholar]
- 113.Becke AD. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A Gen Phys. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 114.Lee C, Yang W, Parr RG. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B Condens Matter. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 115.Montgomery JA, Ochterski JW, Frisch MJ, Petersson GA. A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J Chem Phys. 1999;110:2822–2827. [Google Scholar]
- 116.Petersson GA, Tensfeldt TG, Montgomery JA. A complete basis set model chemistry. III. The complete basis set-quadratic configuration-interaction family of methods. J Chem Phys. 1991;94:6091–6101. [Google Scholar]
- 117.Petersson GA, Allaham MA. A complete basis set model chemistry.II. Open-shell systems and the total energies of the 1st-row atoms. J Chem Phys. 1991;94:6081–6090. [Google Scholar]
- 118.Zhang Y. Improved pseudobonds for combined ab initio quantum mechanical/molecular mechanical methods. J Chem Phys. 2005;122:024114. doi: 10.1063/1.1834899. [DOI] [PubMed] [Google Scholar]
- 119.Zhang YK, Lee TS, Yang WT. A pseudobond approach to combining quantum mechanical and molecular mechanical methods. J Chem Phys. 1999;110:46–54. [Google Scholar]
- 120.Walker RC, Crowley MF, Case DA. The implementation of a fast and accurate QM/MM potential method in Amber. J ComputChem. 2008;29:1019–1031. doi: 10.1002/jcc.20857. [DOI] [PubMed] [Google Scholar]
- 121.Nam K, Gao J, York DM. An efficient linear-scaling Ewald method for long-range electrostatic interactions in combined QM/MM calculations. J Chem Theory Comput. 2005;1:2–13. doi: 10.1021/ct049941i. [DOI] [PubMed] [Google Scholar]
- 122.Giese TJ, York DM. Ambient-potential composite Ewald method for ab initioquantum mechanical/molecular mechanical molecular dynamics simulation. J Chem Theory Comput. 2016;12:2611–2632. doi: 10.1021/acs.jctc.6b00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang YK, Liu HY, Yang WT. Free energy calculation on enzyme reactions with an efficient iterative procedure to determine minimum energy paths on a combined ab initio QM/MM potential energy surface. J Chem Phys. 2000;112:3483–3492. [Google Scholar]
- 124.Souaille M, Roux B. Extension to the weighted histogram analysis method: Combining umbrella sampling with free energy calculations. Comput Phys Commun. 2001;135:40–57. [Google Scholar]
- 125.Kumar S, Bouzida D, Swendsen RH, Kollman PA, Rosenberg JM. The weighted histogram analysis method for free-energy calculations on biomolecules. I. The method. J Comput Chem. 1992;13:1011–1021. [Google Scholar]
- 126.Ferrenberg AM, Swendsen RH. New Monte Carlo technique for studying phase transitions. Phys Rev Lett. 1988;61:2635–2638. doi: 10.1103/PhysRevLett.61.2635. [DOI] [PubMed] [Google Scholar]
- 127.Shao Y, et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys Chem Chem Phys. 2006;8:3172–3191. doi: 10.1039/b517914a. [DOI] [PubMed] [Google Scholar]
- 128.Ke Z, Wang S, Xie D, Zhang Y. Born-Oppenheimer ab initio QM/MM molecular dynamics simulations of the hydrolysis reaction catalyzed by protein arginine deiminase 4. J Phys Chem B. 2009;113:16705–16710. doi: 10.1021/jp9080614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.