Significance
Gram-negative bacterial cells such as Escherichia coli contain a relatively rigid outer membrane, and cross-linked peptidoglycan in their periplasm, giving them the rigidity and stability to survive independently in harsh environments. To dismantle these strong bacterial cell envelopes, enzymatic processes need to be used. In contrast, human cell membranes are much more fragile, making it possible to dismantle them more easily by relatively mild mechanical disruption. Once these membranes are dismantled, they can be coassembled with synthetic phospholipid mimics, named Janus dendrimers, into cell-like hybrids. This method stabilizes the delicate human cell membranes, introducing the potential for the study of human cell membranes and of their constituents in vitro in a more robust environment.
Keywords: mammalian cell, bacterial membrane, hybrid vesicles, coassembly, bacterial adhesin
Abstract
Cell-like hybrids from natural and synthetic amphiphiles provide a platform to engineer functions of synthetic cells and protocells. Cell membranes and vesicles prepared from human cell membranes are relatively unstable in vitro and therefore are difficult to study. The thicknesses of biological membranes and vesicles self-assembled from amphiphilic Janus dendrimers, known as dendrimersomes, are comparable. This feature facilitated the coassembly of functional cell-like hybrid vesicles from giant dendrimersomes and bacterial membrane vesicles generated from the very stable bacterial Escherichia coli cell after enzymatic degradation of its outer membrane. Human cells are fragile and require only mild centrifugation to be dismantled and subsequently reconstituted into vesicles. Here we report the coassembly of human membrane vesicles with dendrimersomes. The resulting giant hybrid vesicles containing human cell membranes, their components, and Janus dendrimers are stable for at least 1 y. To demonstrate the utility of cell-like hybrid vesicles, hybrids from dendrimersomes and bacterial membrane vesicles containing YadA, a bacterial adhesin protein, were prepared. The latter cell-like hybrids were recognized by human cells, allowing for adhesion and entry of the hybrid bacterial vesicles into human cells in vitro.
The membranes of human cells are mechanically fragile and chemically unstable in vitro (1). Therefore, the investigation of the functions of biological membranes outside the in vivo natural cellular environment represents a significant challenge. Liposomes assembled from naturally occurring phospholipids (2) and their chemically modified versions (3, 4) are also unstable. Exceptions are stealth liposomes (5, 6), which are vesicles coassembled from phospholipids and water-soluble polymers conjugated to phospholipids. The first series of vesicles assembled from synthetic lipids (7, 8) did not solve this stability problem. Amphiphilic block copolymers (9) were the first amphiphiles that assembled in stable vesicles named polymersomes. However, block copolymers are not always biocompatible, and the thickness of the polymersome bilayers is larger than that of liposomes and of natural biological membranes. Amphiphilic Janus dendrimers (JDs) (10, 11) self-assemble into stable and monodisperse vesicles with bilayer thickness similar to that of liposomes (12). Since JDs are prepared from naturally occurring phenolic acids (13), they are also biocompatible (10, 11).
Phospholipids and amphiphilic block copolymers can be self-assembled into mixed hybrid phospholipid/block copolymer vesicles (14–17). The limited miscibility and the different thicknesses of the phospholipids and the hydrophobic part of the block copolymers create complex vesicle morphologies, sometimes with dissimilar bilayer membranes produced by phase separation. A positive outcome of the lack of miscibility and length similarity between phospholipids and hydrophobic parts of the block copolymers is that the phase-separated fragments of phospholipid could accommodate transmembrane proteins in the monolayers containing phospholipids and block copolymer (18, 19). Also, three-component hybrid vesicles from block copolymer−phospholipid−glycolipid mixtures could be made (20). The negative aspect of this issue is that the immiscibility between phospholipids and block copolymers does not contribute to the stabilization of the phospholipid fragments of the hybrid vesicles, and therefore a continuous reorganization of the structure of hybrid vesicles occurs (19, 21). None of these hybrid coassemblies used bacterial or mammalian cell membranes containing native components (17). Transmembrane proteins such as aquaporin were incorporated in a single block copolymer-derived polymersome rather than in a hybrid phospholipids−block copolymer vesicle (22). A single attempt by our laboratory to coassemble bacterial membranes with block copolymers failed (23).
Dendrimersomes (DSs) (10, 11) and glycodendrimersomes (GDSs) (24) self-assembled from monodisperse, amphiphilic JDs and Janus glycodendrimers were recently advanced as models of biological membranes with tunable size (25), structural organization (26, 27), and functional surfaces (28). DSs and GDSs allow for the design of specific interactions, such as glycan−lectin binding, to be investigated without interference from other functional groups present on the biological membrane (29). DSs and GDSs exhibit bilayer thicknesses similar to that of liposomes (∼4 nm) assembled from phospholipids (8, 9) and excellent stability in buffer at room temperature for several years (10). In comparison, phospholipid-based liposomes or stealth liposomes are stable under the same conditions for less than 1 wk, and phospholipids must be stored at −20 °C, while our JDs can be stored at room temperature. DSs and GDSs were successfully coassembled into giant hybrid vesicles with the membrane and the membrane components of Gram-negative bacterium Escherichia coli (23). Transmembrane proteins, such as channel proteins, and lipids from E. coli, as well as fluorescent labels and glycans from DSs or GDSs, were incorporated by coassembly into these hybrid cell-like vesicles. Therefore, this method overcomes the problems associated with incorporating cell components, including phospholipids and transmembrane proteins, into polymersomes or other synthetic membranes with mismatched thickness or other biocompatibility issues (30). Although human cell membranes are expected to be amenable to similar coassembly with synthetic components from DSs or GDSs, they have never been incorporated into synthetic vesicles, despite great interest in the elucidation of the functions of human cells. Indeed, due to the lack of cell walls and cross-linked glycans such as peptidoglycan available in bacterial membranes and cells (31), this coassembly should not only be more facile than for Gram-negative bacterial membranes but is also expected to provide a platform to stabilize human cell membranes and thus enable applications of human cell membranes in vitro.
Results and Discussion
Coassembly of Giant DSs with Human Membrane Vesicles.
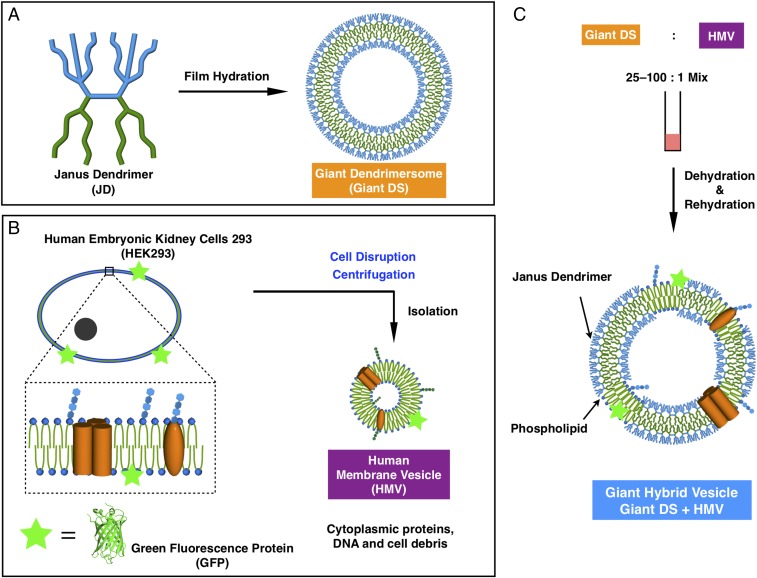
Giant DSs (diameter ∼2 μm to 50 μm) were prepared, using previously reported methods, by hydration of amphiphilic JD films on Teflon sheets with ultrapure water or PBS (Fig. 1) (10, 23, 32, 33). Selected JDs containing hydrophobic 3,5-di-dodecyl benzoic ester minidendrons and hydrophilic 3,4,5-Tris-triethylele glycol benzoic ester minidendrons were demonstrated to form stable giant DSs in water or buffer (10, 23, 32, 33). Giant DSs prepared by this method were coassembled with human membrane vesicles (HMVs) via the dehydration–rehydration technique similar to that described recently for bacterial membrane vesicles (BMVs) prepared from Gram-negative bacterium E. coli, except that the enzymatic degradation by lysosome/EDTA and sonication of the outer cross-linked membrane of E. coli is not required (23, 34). Only weak mechanical disruption such as centrifugation is required to prepare HMVs. Eukaryotic membranes, including human plasma membranes, differ from E. coli membranes in their composition of phospholipids as well as the lack of lipopolysaccharides (32). Eukaryotic/mammalian cell membranes contain cholesterol and glycolipids, which modulate the fluidity of the membrane (35–38) and may affect their ability to coassemble with DSs. To test the feasibility of this coassembly process, HMVs from green fluorescent-labeled HEK293 cells grown in cell culture were prepared. The label on HEK293 cells was added via the expression of GFP-CAAX protein, where CAAX is a prenylation motif of Ras GTPase protein (CMSCKCVLS) (39, 40), which targets GFP-CAAX protein to the plasma membrane. These HMVs enriched with GFP-CAAX together with red fluorescent DSs allow the coassembly process to be monitored by fluorescence microscopy.
Fig. 1.
Schematic illustration of (A) the preparation of giant DSs, (B) the preparation of HMV from human kidney cells 293 (HEK293), and (C) coassembly of giant hybrid vesicles from giant DSs, and HMV from HEK293 labeled with GFP.
Visualization of Giant DSs, and Their Coassembly with GFP-Labeled HMVs by Dual Color Imaging.
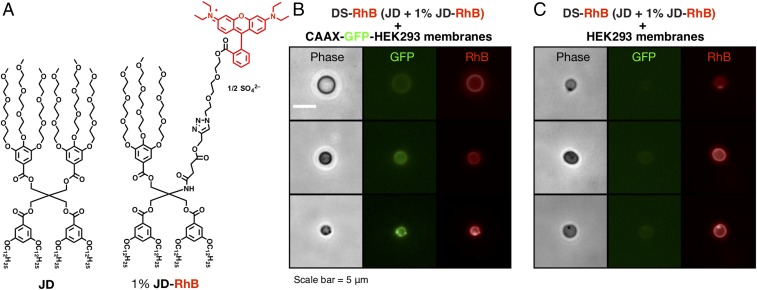
To independently visualize DSs and GFP, coassembled DSs from (3,5)12G1-PE-(3,4,5)-3EO-G1-(OCH3)3 (JD) (23, 32, 33) and 1% (wt/wt) of red fluorescent-labeled (3,5)12G1-Tris(3,4,5)-3EO-G1-(OCH3)3-RhB (JD-RhB) (32, 33), shortly named DS-RhB, were utilized. The giant hybrid vesicles were successfully coassembled from DS-RhB and HMVs containing GFP-CAAX by the dehydration–rehydration protocol (25∼100:1 mass ratio of DS-RhB and HMVs) and are comparable in size to giant DSs reported previously (23). These hybrid vesicles showed robust green and red fluorescence signals along the boundary, from GFP and JD-RhB, respectively, indicating that these vesicles consist of both amphiphilic JDs and human membrane components (Fig. 2). The mechanism of coassembly and the structure of the hybrid bilayers are not known, and hence Figs. 1 and 3 show a possible intrabilayer segregation of the phospholipids and the JD fragments. Furthermore, these giant vesicles are stable for at least 1 y, based on a negligible change in their sizes, fluorescence localization, and fluorescence intensity as determined by microscopy.
Fig. 2.
Coassembly of giant hybrid vesicles from DS-RhB and HMV obtained from GFP labeled HEK293. (A) JD with 1% of JD-RhB coassembles into DS-RhB. Representative microscopy images of giant hybrid vesicles containing (B) CAAX-GFP HEK293 and (C) HEK293 without CAAX-GFP. A phase-contrast image was first acquired, followed by the fluorescence image by successive exposures on the same vesicle. Scale is identical in B and C.
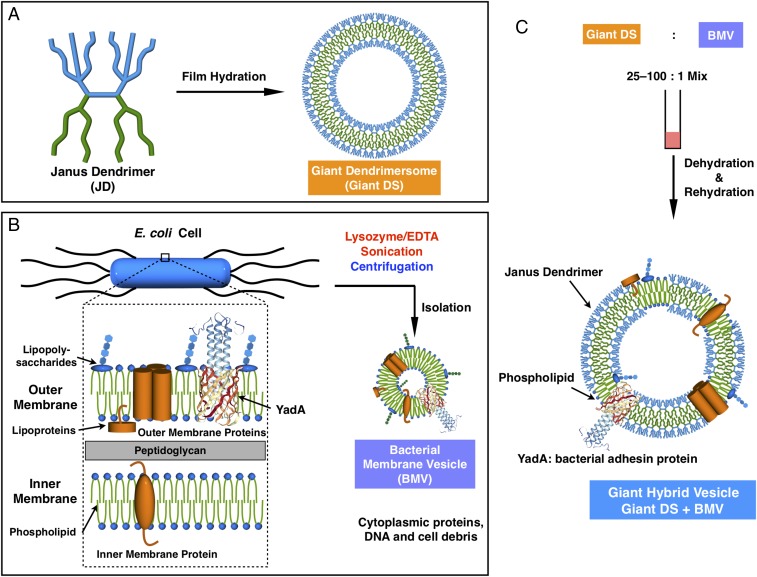
Fig. 3.
Illustration of (A) the preparation of giant DSs, (B) the preparation of BMV expressing YadA bacterial adhesin protein, and (C) coassembly of giant hybrid vesicles from giant DSs and E. coli BMV expressing YadA bacterial adhesin protein.
Bioactive Hybrid Vesicles Containing Bacterial Adhesion Protein (YadA) Bind to HeLa Cells.
Pathogenic bacteria commonly express proteins on their surface that have affinity for the mammalian extracellular matrix (ECM) proteins, such as collagens, laminin, fibronectin, etc. (34). YadA belongs to a family of outer membrane proteins called the trimeric autotransporter adhesins that are commonly found in Gram-negative bacteria (41–43). Autotransporter adhesins play an important role in the virulence of many bacterial pathogens by mediating adhesion to host cells and tissues. YadA from Yersinia pseudotuberculosis and Yersinia enterocolitica is crucial for adhesion and uptake into the host cell via binding to the mammalian ECM components fibronectin, laminin, and collagen (41–43). To show the utility of hybrid DSs as a potential cell-targeting agent, YadA from Y. pseudotuberculosis in the E. coli outer membrane was expressed (32). As described before (23), the outer membrane of E. coli was dismantled by enzymatic lysosome/EDTA followed by sonication. The YadA-containing BMVs (BMV-YadA) and giant DSs were then coassembled to produce giant hybrid cell-like vesicles (Fig. 3).
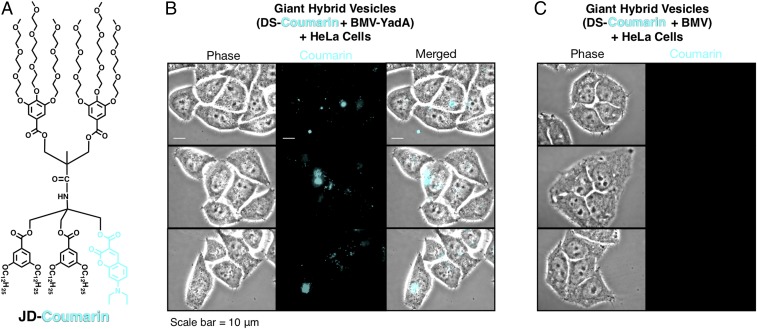
Previous fluorescence microscopy studies on the coassembly of DSs labeled with coumarin and BMVs labeled with mCherry demonstrated their coassembly into giant hybrid vesicles (23), which were analogous to the giant hybrid cell-like vesicles coassembled from DSs and HMVs and discussed earlier (Fig. 2). For better visualization of the location of giant hybrid DSs, a cyan-color coumarin labeled JD (3,5)12-2G1-coumarin-Tris(3,4,5)-3EO-G2-(OCH3)6 (JD-Coumarin) (23) was selected for self-assembly of DSs.
HeLa cells grown in cell culture were treated with coassembled hybrid DSs and visualized by fluorescence microscopy. After an overnight incubation, HeLa cells were thoroughly washed with PBS solution to remove any unbound vesicles before microscopy. Giant DSs generated by film hydration of JD-Coumarin are shortly named DS-Coumarin. Giant hybrid vesicles coassembled by hydration from giant DS-Coumarin and BMV-YadA were found to specifically associate with HeLa cells as depicted by cyan fluorescence (Fig. 4B), whereas control giant hybrid vesicles from DS-Coumarin and BMV without YadA did not bind to HeLa cells and no cyan fluorescence was observed (Fig. 4C).
Fig. 4.
Giant hybrid vesicles coassembled from DS-Coumarin with BMV or BMV-YadA. BMV-YadA represents BMV containing the YadA bacterial adhesion protein. (A) Structure of JD-Coumarin, which self-assembles into DS-Coumarin. (B) Encapsulation of giant hybrid vesicles (DS-Coumarin + BMV-YadA) into HeLa cells. (C) Lack of encapsulation of giant hybrid vesicles (DS-Coumarin + BMV) into HeLa cells due to the absence of YadA, as the control experiment for B. Scale is identical in B and C.
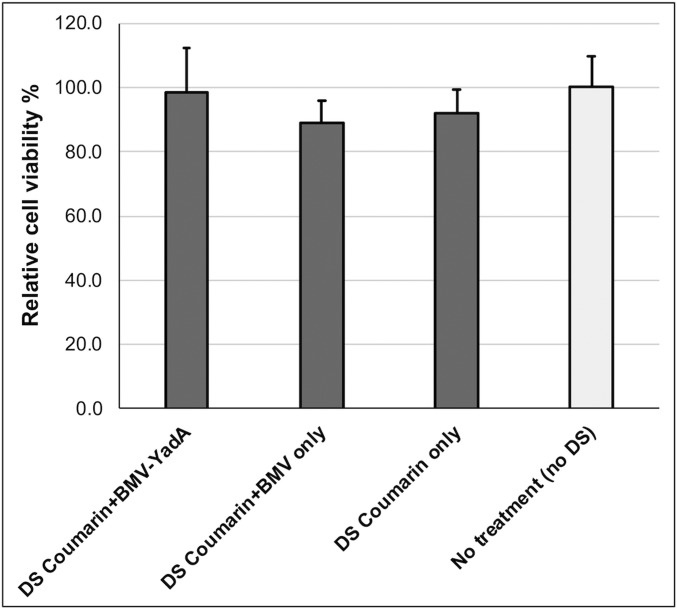
To check whether cyan DSs are potentially toxic to the cells, we assessed cell viability by crystal violet staining, a commonly used method to assess drug cytotoxicity (44). Cell viability was measured for cells treated with giant hybrid vesicles containing either BMV-YadA or BMV only, as well as giant DSs alone. We did not see a significant difference in cell viability between treated and untreated control cells (Fig. 5), indicating that the hybrid vesicles as well as cyan DSs alone exhibit negligible cytotoxicity.
Fig. 5.
Crystal violet cytotoxicity assay. HEK293 cells were treated with either giant hybrid vesicles (DS-Coumarin + BMV-YadA, DS-Coumarin + BMV only), or DS-Coumarin only. Relative cell viability was measured by crystal violet quantification. Data represent averages and SDs of six independent measurements.
Localization of Giant Hybrid Vesicles in the Human Cells in Grown Culture.
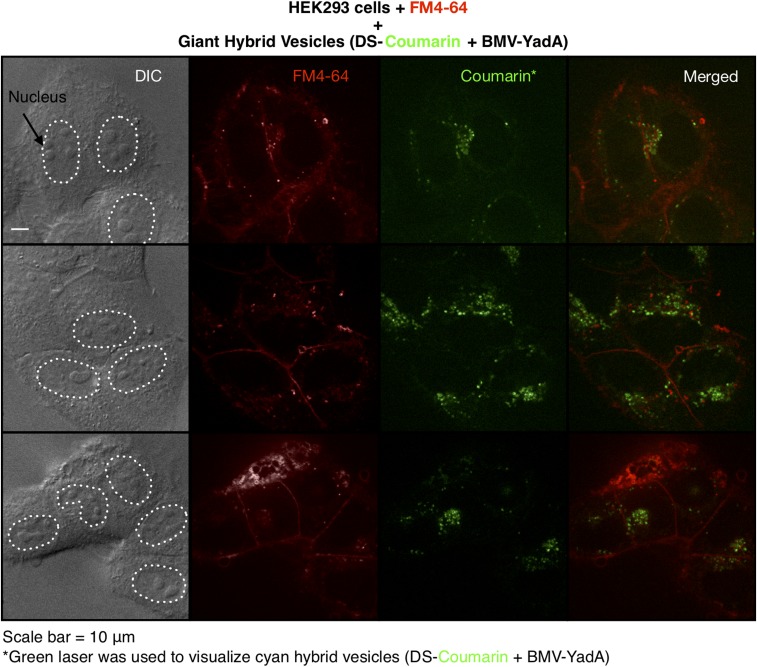
Confocal microscopy experiments were employed to determine if the giant hybrid vesicles (DS-Coumarin + BMV-YadA) associated with HeLa cells were able to enter the cytoplasm. HeLa cells grown in cell culture were incubated overnight with coassembled hybrid vesicles and washed before the microscopy experiments. Cyan fluorescent giant hybrid vesicles (DS-Coumarin + BMV-YadA) were visualized via the green fluorescence channel, and membranes/boundaries of HeLa cells were visualized by staining with the red dye FM4-64. Green fluorescence within the cytoplasm of HeLa cells was observed by confocal microscopy (Fig. 6). This indicates that giant hybrid vesicles (DS-Coumarin + BMV-YadA) not only bind to HeLa cells but are also able to enter the cells. It is not possible to ascertain by confocal microscopy whether the vesicle remains intact once it has entered the cell. Interestingly, green fluorescence in the nucleus (depicted by dotted lines in Fig. 6) was not observed, suggesting that the nuclear membrane may be impermeable to these giant hybrid vesicles. Based on the cellular architecture of cells treated with giant hybrid vesicles and untreated cells, it appeared that the HeLa cells were alive and were able to divide.
Fig. 6.
Representative confocal microscopy images of giant hybrid vesicles (DS-Coumarin + BMV-YadA) localizing to the cytoplasm in HeLa cells. HeLa cell membrane was stained with red FM4-64.
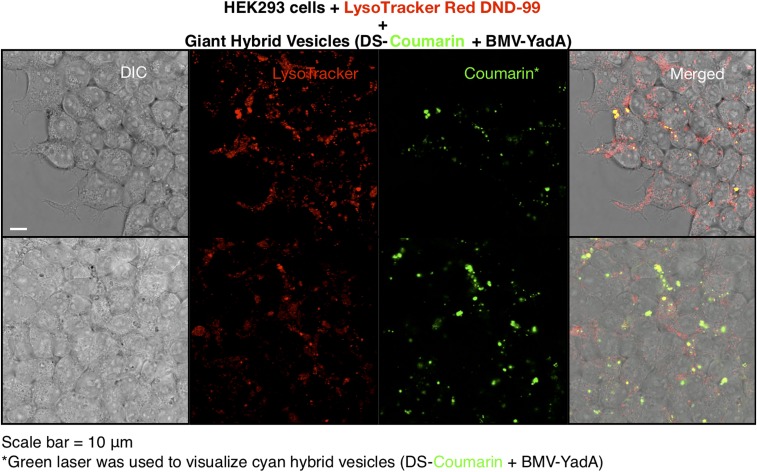
In addition, we utilized LysoTracker Red as a probe to label lysosomes (and other acidic organelles) in live cells, together with localization of giant hybrid vesicles in HEK293 cells. Cyan fluorescent giant hybrid vesicles were visualized via the green fluorescence channel, and lysosomes labeled by LysoTracker Red dye were visualized via red fluorescence by confocal microscopy. As shown in Fig. 7, red fluorescence was distributed throughout the cytoplasm. Cyan fluorescence from the giant hybrid vesicles was also only in the cytoplasm, and always appeared colocalized with the red fluorescence, suggesting that the hybrid DSs localize to lysosomes.
Fig. 7.
Representative confocal microscopy images of giant hybrid vesicles (DS-Coumarin + BMV-YadA) localizing to the cytoplasm in HEK293 cells. The lysosomes within cell cytoplasm were stained with LysoTracker Red DND-99 dye.
Bioactive Hybrid Vesicles Containing Human Cell Membranes Bind Bacterial Cells Expressing the Adhesion Protein YadA.
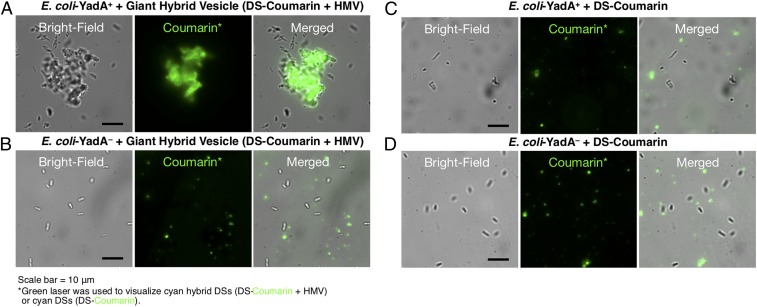
The interactions of YadA protein with human cells can also be used to demonstrate the bioactivity of hybrid cell-like coassembly with DSs and HMVs rather than BMVs. Giant hybrid vesicles (DS-Coumarin + HMV) consisting of cyan JD coassembled with HEK293 human cell membranes vesicles were mixed with E. coli bacterial cells expressing the YadA protein (E. coli-YadA+) as a 1:1 (vol/vol) mixture (Fig. 8A). As a control, giant hybrid vesicles (DS-Coumarin + HMV) were mixed with E. coli cells lacking YadA (E. coli-YadA−) (Fig. 8B). E. coli cells and vesicles were monitored in bright-field and fluorescence images, respectively. Control experiments with E. coli-YadA− did not show any discernable interaction with giant hybrid vesicles (DS-Coumarin + HMV) (Fig. 8B). In contrast, E. coli-YadA+ cells mixed with giant hybrid vesicles (DS-Coumarin + HMV) showed significant clumping with fluorescent vesicles (Fig. 8A), similar to the agglutination reaction observed for antigen−antibody interactions. Additional control experiments (Fig. 8 C and D), consisting of fluorescent giant vesicles (DS-Coumarin) mixed with either E. coli-YadA+ or E. coli-YadA−, did not show interaction between bacterial cells and giant vesicles.
Fig. 8.
Representative confocal microscopy images of E. coli incubated with (A and B) giant hybrid vesicle (DS-Coumarin + HMV) or (C and D) giant vesicle (DS-Coumarin) E. coli cells expressing YadA (E. coli-YadA+, in A and C) or control E. coli cells without YadA (E. coli-YadA−, in B and D). HMVs were prepared by HEK293 human cells. E. coli cells and vesicles were monitored in bright-field and fluorescence images, respectively.
Conclusions
The methodology for the preparation of bioactive giant hybrid vesicles coassembled from DSs and E. coli BMVs (23) was adapted to a much-simplified coassembly of DSs and HMVs. The lack of peptidoglycan in the human (mammalian) cell (HEK293 kidney cell) membrane allowed for easy disruption without the need for enzymatic treatment with lysozyme to form HMVs, which were coassembled with DSs by simplified methods. Giant hybrid vesicles were confirmed by visualization of GFP (green) labels on the human cells and RhB (red) labels on DSs. As demonstrated by fluorescence microscopy, these giant hybrid vesicles are stable for at least 1 y and represent a substantial advance toward a simple preparation of hybrid cells and protocells containing mammalian cell components. By expressing YadA, a bacterial adhesin protein in E. coli’s inner membrane, the giant hybrid vesicles containing giant DSs and BMVs could be recognized by the living human cells (HeLa cells). Confocal microscopy images indicated that giant hybrid vesicles were delivered into the cytoplasm of HeLa cells. Similarly, in a reverse experiment, giant hybrid vesicles containing DSs and HMVs bind and aggregate E. coli bacterial cells expressing YadA protein, demonstrating the bioactivity of hybrid vesicles with human cell membranes. This approach can be applied to targeted delivery and nanomedicine (45–49). The methodologies demonstrated here are expected to enable the design of synthetic and hybrid (synthetic–natural) protocell capsules to perform cell-like functions of recognition, signaling, and delivery.
Methods
Preparation of DSs.
A solution or mixed solution of JDs (10 mg·mL−1, 200 μL) in THF was deposited on the top surface of a roughened Teflon sheet (1 cm2), placed in a flat-bottom vial, followed by evaporation of the solvent for 12 h in darkness. PBS (1×, pH = 7.4, 2.0 mL) was added to submerge the JDs film on the Teflon sheet, and the vial was placed in a 60 °C oven for 12 h for hydration. The sample was then mixed using a vortex mixer for 30 s with a final concentration of 1 mg·mL−1.
Cell Culture and Transfection.
HEK293 and HeLa cells were grown in Dulbecco’s Modified Eagle Medium (with 4.5 g/L glucose, 25 mM Hepes from Gibco) supplemented with 10% FBS/Glutamax/Penicillin-Streptomycin (Gibco) at 37 °C in a humidified atmosphere of 5% CO2, 95% air. HEK293 cells were transfected with plasmid pEGFP-CAAX using Lipofectamine 2000 reagent (ThermoFisher Scientific) and selected using Geneticin (G418 sulfate; Sigma-Aldrich). CAAX is a prenylation motif of Ras GTPase protein (CMSCKCVLS), which targets GFP-CAAX protein to the human plasma membrane. Transfected cells were identified by visualization of the green fluorescence protein.
HEK293 cells and LysoTracker Red DND-99 (ThermoFisher Scientific) were gifts from Dejian Ren, University of Pennsylvania; HeLa cells, and plasmid pEGFP-CAAX were gifts from Michael Lampson and Wei Guo, respectively, both at University of Pennsylvania, Philadelphia.
Bacterial Strains and Growth Media.
E. coli K-12 strain MG1655 was transformed with plasmid pPD284 expressing the YadA adhesin protein from Y. pseudotuberculosis. YadA is a bacterial outer membrane protein which promotes attachment to eukaryotic cell surface and subsequent internalization into the eukaryotic cells. A single colony of the strain MG1655/pPD284 was inoculated in LB (Lysogeny broth; Fisher Scientific) medium for overnight growth at 37 °C on a roller drum for aeration. A saturated culture of this strain was diluted 1:200 in 50 mL of LB medium supplemented with ampicillin (100 μg·mL−1) in a 250-mL culture flask. Following growth for 1.5 h at 37 °C with aeration, YadA expression was induced by the addition of arabinose to a final concentration of 0.5%. Protein induction was carried out for 3.5 h, and then cells were harvested at 7,500 × g for 10 min at 4 °C. The cell pellets were washed in Tris(hydroxymethyl)aminomethane·HCl (Tris·HCl) buffer (50 mM, pH 8.0) and then frozen at −80 °C. Plasmid pPD284 was a gift from Petra Dersch, Helmholtz Centre for Infection Research, Braunschweig, Germany.
Preparation of Human and Bacterial Cell Membranes.
For preparation of HMVs, HEK293 cells expressing GFP-CAAX were trypsinized using 0.05% trypsin-EDTA (Gibco) and harvested by centrifugation at 300 × g for 5 min. The cells were resuspended in 1 mL of a solution of sucrose (20%) and Tris·HCl (30 mM, pH 8.0) and homogenized using 1 mL of Dounce tissue grinder (Fisher Scientific). Cells were spun at 4 °C for 5 min at 7,500 × g, and the supernatant was transferred to a fresh microcentrifuge tube to isolate the membranes from the cytoplasmic fraction and cell debris. This supernatant was then spun at 4 °C for 30 min at 40,000 × g. The membrane fraction was obtained as a pellet following the high-speed centrifugation, and the supernatant was discarded. The membrane vesicles were resuspended in 500 μL of buffer composed of Tris·HCl (30 mM, pH 8.0), sucrose (20%), and EDTA (0.1 mM). For BMVs, cells were grown and collected as described above. Frozen cells were processed and membrane vesicles were prepared as outlined, using previously reported protocols (23).
Coassembly of Giant Hybrid DSs with Human and Bacterial Cell Membranes.
Red fluorescent DSs (JD + 1% JD-RhB) were coassembled with HEK293-derived human cell membranes enriched with either GFP (GFP-CAAX-HEK293 HMV) or no GFP control (HMV only). Cyan fluorescent DSs (JD-coumarin) were coassembled with E. coli membranes enriched with either YadA cell adhesion protein (BMV-YadA) or no protein control (BMV only). Coassembly was performed and coassembled giant hybrid DSs containing human or bacterial cell membranes were imaged as described previously (23).
Binding of Coassembled Giant Hybrid Vesicles to Human Cells.
For BMV adhesion/uptake, HeLa or HEK293 cell monolayers were grown to ∼50% confluency (24 h to 48 h) in cell culture-treated 35-mm fluorodishes (ThermoFisher Scientific), then gently washed three times with 1× PBS pH 7.4 and incubated in binding buffer (Roswell Park Memorial Institute medium 1640 supplemented with 20 mM Hepes pH 7.0, 5% FBS, and 0.4% BSA) for 1 h before the addition of giant hybrid DSs containing bacterial vesicles (42). Then 4 μL of the giant hybrid vesicles coassembled with cyan fluorescent DSs (JD-Coumarin) and E. coli membranes enriched with either (i) cell adhesion protein YadA (BMV-YadA) or (ii) no protein control (BMV only) were incubated with HeLa or HEK293 cells for 18 h to 24 h at 37 °C in binding buffer. Cells were then thoroughly washed with 1× Dulbecco’s PBS pH 7.4 and visualized by fluorescence microscopy. For membrane labeling using FM4-64 dye, cells treated with giant hybrid DSs were gently washed three times with HBSS (Gibco). Prewarmed HBSS medium containing FM4-64 to a final concentration of 4 μM was then added to the cells. To label lysosomes in live cells, cells treated with hybrid DSs were washed with HBSS followed by the addition of prewarmed HBSS containing LysoTracker Red dye at a working concentration of 40 nM. For both the staining procedures, cells were incubated at 37 °C and 5% CO2 for ∼30 min, and then imaged by fluorescence microscopy.
Crystal Violet Cytotoxicity Assay.
Crystal violet staining to determine cell viability was adapted from a published method (44). Cells were seeded in a 24-well plate and grown as mentioned in Cell Culture and Transfection. Binding of giant hybrid DSs was performed as outlined in Binding of Coassembled Giant Hybrid Vesicles to Human Cells. Then 0.5% crystal violet staining solution was added to each well, and the cells were incubated at room temperature for 15 min. The staining solution was then aspirated, each well was gently washed twice with 1× PBS to remove excess stain, and the plate was air-dried without the lid for 3 h. After drying, the crystal violet stain was solubilized using methanol for 20 min at room temperature, and optical density of each sample was measured at 570 nm using a spectrophotometer. Relative cell viability was calculated as a percentage derived from the average OD570 of each sample relative to the average OD570 of untreated cells.
Fluorescence Microscopy.
To visualize the location of giant hybrid DSs within the human cells, confocal microscopy and image acquisition was carried out using a DM4000 spinning disk confocal microscope (Leica) equipped with 488- and 593-nm diode lasers (Spectral Applied Research) controlled by MetaMorph software (Molecular Devices), as described previously (50). Cyan fluorescent vesicles were visualized via green fluorescence channel, and FM4-64 dye was used to stain the HeLa cell membrane, which was visualized by the red fluorescence. For lysosome staining using LysoTracker Red dye and localization of giant hybrid DSs within the human cells, image acquisition was carried out using a confocal laser scanning microscope Leica TCS SP8 (Leica) equipped with 488- and 552-nm diode lasers (Spectral Applied Research) with a 100× objective controlled by the Leica Application Suite X software. Cyan fluorescent vesicles were visualized via green fluorescence, and the LysoTracker Red dye was visualized by the red fluorescence.
Binding of E. Coli-YadA+ Cells to Hybrid DSs + HMVs.
HEK293 cells were grown in cell culture and HMVs were prepared as described in Methods. E. coli cells expressing YadA (YadA+) and control cells (no YadA, YadA−) were grown as described above. Then 500 μL of cells was harvested, washed, and resuspended in 1× PBS (pH 7.4). E. coli cells and HMVs were mixed in a 1:1 ratio in 1× PBS (pH 7.4) and 0.4% BSA and then incubated at 37 °C for ∼1.5 h before imaging. All controls were treated in the same manner. Fluorescence was observed using an Axioplan II upright epifluorescence microscope (Carl Zeiss), a 100× 1.4 NA PlanApo objective. Bright-field and fluorescence images were captured using an ORCA charge‐coupled device camera (Hamamatsu) and iVision software (Biovision Technologies).
Acknowledgments
We thank M. Lampson’s laboratory at University of Pennsylvania, the Waksman Confocal Microscope Core Facility at Rutgers University for access to the confocal fluorescence microscope, and Christopher Rongo’s laboratory at Rutgers University for access to the Zeiss epifluorescence microscope. This work was supported by National Science Foundation Grants DMR-1066116 and DMR-1807127 (to V.P.), the P. Roy Vagelos Chair at the University of Pennsylvania (V.P.), the Humboldt Foundation (V.P.), Vagelos Integrated Program in Energy Research (W.D.H.), National Science Foundation Grants DMR-1120901 (to M.L.K., M.G., and V.P.) and DMR-1720530 (to M.G. and V.P.), and National Institutes of Health Grant R01-GM080279 (to M.G.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Mohandas N, Evans E. Mechanical properties of the red cell membrane in relation to molecular structure and genetic defects. Annu Rev Biophys Biomol Struct. 1994;23:787–818. doi: 10.1146/annurev.bb.23.060194.004035. [DOI] [PubMed] [Google Scholar]
- 2.Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13:238–252. doi: 10.1016/s0022-2836(65)80093-6. [DOI] [PubMed] [Google Scholar]
- 3.Ringsdorf H, Schlarb B, Venzmer J. Molecular architecture and function of polymeric oriented systems: Models for the study of organization, surface recognition, and dynamics of biomembranes. Angew Chem Int Ed Engl. 1988;27:113–158. [Google Scholar]
- 4.Thomas JL, Tirrell DA. Polyelectrolyte-sensitized phospholipid vesicles. Acc Chem Res. 1992;25:336–342. [Google Scholar]
- 5.Allen TM, Cullis PR. Drug delivery systems: Entering the mainstream. Science. 2004;303:1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- 6.Immordino ML, Dosio F, Cattel L. Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomedicine. 2006;1:297–315. [PMC free article] [PubMed] [Google Scholar]
- 7.Kunitake T. Synthetic bilayer membranes: Molecular design, self-organization, and application. Angew Chem Int Ed Engl. 1992;31:709–726. [Google Scholar]
- 8.Guo X, Szoka FC., Jr Chemical approaches to triggerable lipid vesicles for drug and gene delivery. Acc Chem Res. 2003;36:335–341. doi: 10.1021/ar9703241. [DOI] [PubMed] [Google Scholar]
- 9.Discher BM, et al. Polymersomes: Tough vesicles made from diblock copolymers. Science. 1999;284:1143–1146. doi: 10.1126/science.284.5417.1143. [DOI] [PubMed] [Google Scholar]
- 10.Percec V, et al. Self-assembly of Janus dendrimers into uniform dendrimersomes and other complex architectures. Science. 2010;328:1009–1014. doi: 10.1126/science.1185547. [DOI] [PubMed] [Google Scholar]
- 11.Sherman SE, Xiao Q, Percec V. Mimicking complex biological membranes and their programmable glycan ligands with dendrimersomes and glycodendrimersomes. Chem Rev. 2017;117:6538–6631. doi: 10.1021/acs.chemrev.7b00097. [DOI] [PubMed] [Google Scholar]
- 12.Weiss M, et al. Sequential bottom-up assembly of mechanically stabilized synthetic cells by microfluidics. Nat Mater. 2018;17:89–96. doi: 10.1038/nmat5005. [DOI] [PubMed] [Google Scholar]
- 13.Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 14.Ruysschaert T, et al. Hybrid nanocapsules: Interactions of ABA block copolymers with liposomes. J Am Chem Soc. 2005;127:6242–6247. doi: 10.1021/ja043600x. [DOI] [PubMed] [Google Scholar]
- 15.Nam J, Beales PA, Vanderlick TK. Giant phospholipid/block copolymer hybrid vesicles: Mixing behavior and domain formation. Langmuir. 2011;27:1–6. doi: 10.1021/la103428g. [DOI] [PubMed] [Google Scholar]
- 16.Le Meins J-F, Schatz C, Lecommandoux S, Sandre O. Hybrid polymer/lipid vesicles: State of the art and future perspectives. Mater Today. 2013;16:397–402. [Google Scholar]
- 17.Schulz M, Binder WH. Mixed hybrid lipid/polymer vesicles as a novel membrane platform. Macromol Rapid Commun. 2015;36:2031–2041. doi: 10.1002/marc.201500344. [DOI] [PubMed] [Google Scholar]
- 18.Thoma J, et al. Membrane protein distribution in composite polymer-lipid thin films. Chem Commun (Camb) 2012;48:8811–8813. doi: 10.1039/c2cc32851h. [DOI] [PubMed] [Google Scholar]
- 19.Kowal J, Wu D, Mikhalevich V, Palivan CG, Meier W. Hybrid polymer-lipid films as platforms for directed membrane protein insertion. Langmuir. 2015;31:4868–4877. doi: 10.1021/acs.langmuir.5b00388. [DOI] [PubMed] [Google Scholar]
- 20.Schulz M, Werner S, Bacia K, Binder WH. Controlling molecular recognition with lipid/polymer domains in vesicle membranes. Angew Chem Int Ed Engl. 2013;52:1829–1833. doi: 10.1002/anie.201204959. [DOI] [PubMed] [Google Scholar]
- 21.Dao TPT, et al. Modulation of phase separation at the micron scale and nanoscale in giant polymer/lipid hybrid unilamellar vesicles (GHUVs) Soft Matter. 2017;13:627–637. doi: 10.1039/c6sm01625a. [DOI] [PubMed] [Google Scholar]
- 22.Kumar M, Grzelakowski M, Zilles J, Clark M, Meier W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein aquaporin Z. Proc Natl Acad Sci USA. 2007;104:20719–20724. doi: 10.1073/pnas.0708762104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiao Q, et al. Bioactive cell-like hybrids coassembled from (glyco)dendrimersomes with bacterial membranes. Proc Natl Acad Sci USA. 2016;113:E1134–E1141. doi: 10.1073/pnas.1525589113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Percec V, et al. Modular synthesis of amphiphilic Janus glycodendrimers and their self-assembly into glycodendrimersomes and other complex architectures with bioactivity to biomedically relevant lectins. J Am Chem Soc. 2013;135:9055–9077. doi: 10.1021/ja403323y. [DOI] [PubMed] [Google Scholar]
- 25.Peterca M, Percec V, Leowanawat P, Bertin A. Predicting the size and properties of dendrimersomes from the lamellar structure of their amphiphilic Janus dendrimers. J Am Chem Soc. 2011;133:20507–20520. doi: 10.1021/ja208762u. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, et al. Self-assembly of amphiphilic Janus dendrimers into uniform onion-like dendrimersomes with predictable size and number of bilayers. Proc Natl Acad Sci USA. 2014;111:9058–9063. doi: 10.1073/pnas.1402858111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Q, et al. Onion-like glycodendrimersomes from sequence-defined Janus glycodendrimers and influence of architecture on reactivity to a lectin. Proc Natl Acad Sci USA. 2016;113:1162–1167. doi: 10.1073/pnas.1524976113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao Q, et al. Why do membranes of some unhealthy cells adopt a cubic architecture? ACS Cent Sci. 2016;2:943–953. doi: 10.1021/acscentsci.6b00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao Q, et al. Exploring functional pairing between surface glycoconjugates and human galectins using programmable glycodendrimersomes. Proc Natl Acad Sci USA. 2018;115:E2509–E2518. doi: 10.1073/pnas.1720055115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Copper GM. The cell: A molecular approach. In: Meyer R, editor. Structure of the Plasma Membrane. 2nd Ed. Sinauer Assoc; Sunderland, MA: 2000. pp. 321–235. [Google Scholar]
- 31.Alberts B, et al. The Lipid Bilayer-Molecular Biology of the Cell. 4th Ed Garland Sci; New York: 2002. Membrane proteins. [Google Scholar]
- 32.Xiao Q, et al. Self-sorting and coassembly of fluorinated, hydrogenated, and hybrid Janus dendrimers into dendrimersomes. J Am Chem Soc. 2016;138:12655–12663. doi: 10.1021/jacs.6b08069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Q, et al. Janus dendrimersomes coassembled from fluorinated, hydrogenated, and hybrid Janus dendrimers as models for cell fusion and fission. Proc Natl Acad Sci USA. 2017;114:E7045–E7053. doi: 10.1073/pnas.1708380114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goulian M, et al. Gramicidin channel kinetics under tension. Biophys J. 1998;74:328–337. doi: 10.1016/S0006-3495(98)77790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper RA. Influence of increased membrane cholesterol on membrane fluidity and cell function in human red blood cells. J Supramol Struct. 1978;8:413–430. doi: 10.1002/jss.400080404. [DOI] [PubMed] [Google Scholar]
- 36.Raffy S, Teissié J. Control of lipid membrane stability by cholesterol content. Biophys J. 1999;76:2072–2080. doi: 10.1016/S0006-3495(99)77363-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Meyer F, Smit B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc Natl Acad Sci USA. 2009;106:3654–3658. doi: 10.1073/pnas.0809959106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khatibzadeh N, Gupta S, Farrell B, Brownell WE, Anvari B. Effects of cholesterol on nano-mechanical properties of the living cell plasma membrane. Soft Matter. 2012;8:8350–8360. doi: 10.1039/C2SM25263E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choy E, et al. Endomembrane trafficking of ras: The CAAX motif targets proteins to the ER and Golgi. Cell. 1999;98:69–80. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 40.Wright LP, Philips MR. Thematic review series: Lipid posttranslational modifications. CAAX modification and membrane targeting of Ras. J Lipid Res. 2006;47:883–891. doi: 10.1194/jlr.R600004-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Pizarro-Cerdá J, Cossart P. Bacterial adhesion and entry into host cells. Cell. 2006;124:715–727. doi: 10.1016/j.cell.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Eitel J, Dersch P. The YadA protein of Yersinia pseudotuberculosis mediates high-efficiency uptake into human cells under environmental conditions in which invasin is repressed. Infect Immun. 2002;70:4880–4891. doi: 10.1128/IAI.70.9.4880-4891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heise T, Dersch P. Identification of a domain in Yersinia virulence factor YadA that is crucial for extracellular matrix-specific cell adhesion and uptake. Proc Natl Acad Sci USA. 2006;103:3375–3380. doi: 10.1073/pnas.0507749103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feoktistova M, Geserick P, Leverkus M. Crystal violet assay for determining viability of cultured cells. Cold Spring Harb Protoc. 2016;2016:pdb.prot087379. doi: 10.1101/pdb.prot087379. [DOI] [PubMed] [Google Scholar]
- 45.Wei T, et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc Natl Acad Sci USA. 2015;112:2978–2983. doi: 10.1073/pnas.1418494112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 47.Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: Design considerations for nanomedical applications. Drug Discov Today. 2010;15:171–185. doi: 10.1016/j.drudis.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 48.Kannan RM, Nance E, Kannan S, Tomalia DA. Emerging concepts in dendrimer-based nanomedicine: From design principles to clinical applications. J Intern Med. 2014;276:579–617. doi: 10.1111/joim.12280. [DOI] [PubMed] [Google Scholar]
- 49.Jishkariani D, et al. Self-interrupted synthesis of sterically hindered aliphatic polyamide dendrimers. Proc Natl Acad Sci USA. 2017;114:E2275–E2284. doi: 10.1073/pnas.1700922114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yadavalli SS, et al. Antimicrobial peptides trigger a division block in Escherichia coli through stimulation of a signalling system. Nat Commun. 2016;7:12340. doi: 10.1038/ncomms12340. [DOI] [PMC free article] [PubMed] [Google Scholar]