Abstract
Background: The prevalence of diabetes mellitus is rapidly increasing in China, but the secular trends in incidence and mortality remain unknown. This study aims to examine time trends from 1990 to 2017 and the net age, period, and cohort effects on diabetes incidence and mortality. Methods: Incidence and mortality rates of diabetes (1990–2017) were collected for each 5-year age group (from 5–9 to 80–84 age group) stratified by gender from the Global Burden of Disease 2017 Study. The average annual percentage changes in incidence and mortality were analyzed by joinpoint regression analysis; the net age, period, and cohort effects on the incidence and mortality were estimated by age-period-cohort analysis. Results: The joinpoint regression analysis showed that age-standardized incidence significantly rose by 0.92% (95% CI: 0.6%, 1.3%) in men and 0.69% in women (95% CI: 0.3%, 1.0%) from 1990 to 2017; age-standardized mortality rates rose by 0.78% (95% CI: 0.6%, 1.0%) in men and decreased by 0.12% (95% CI: −0.4%, 0.1%) in women. For age-specific rates, incidence increased in most age groups, with exception of 30–34, 60–64, 65–69 and 70–74 age groups in men and 25–29, 30–34, 35–39 and 70–74 age groups in women; mortality in men decreased in the younger age groups (from 20–24 to 45–49 age group) while increased in the older age groups (from 50–54 to 80–84 age group), and mortality in women decreased for all age groups with exception of the age group 75–79 and 80–84. The age effect on incidence showed no obvious changes with advancing age while mortality significantly increased with advancing age; period effect showed that both incidence and mortality increased with advancing time period while the period trend on incidence began to decrease since 2007; cohort effect on incidence and mortality decreased from earlier birth cohorts to more recent birth cohorts while incidence showed no material changes from 1982–1986 to 2012–2016 birth cohort. Conclusions: Mortality decreased in younger age groups but increased in older age groups. Incidence increased in most age groups. The net age or period effect showed an unfavorable trend while the net cohort effect presented a favorable trend. Aging likely drives a continued increase in the mortality of diabetes. Timely population-level interventions aiming for obesity prevention, healthy diet and regular physical activity should be conducted, especially for men and earlier birth cohorts at high risk of diabetes.
Keywords: incidence, mortality, diabetes mellitus, joinpoint regression analysis, age-period-cohort effect, trends
1. Introduction
Diabetes mellitus is a global health challenge in the 21st century [1,2,3]. As reported, global age-standardized prevalence of diabetes in men increased from 4.3% in 1980 to 9.0% in 2014 and in women increased from 5.0% to 7.9% [4]. In China alone, the age-standardized prevalence of diabetes increased to 5.04% for both men and women in 2017 [5]. Due to its large population, there were an estimated 3,338,131 new cases and 153,184 deaths of diabetes in China in 2017, accounting for 14.55% and 11.18% of all new cases and all deaths of diabetes worldwide, respectively [5,6]. To understand and control the burden of diabetes, it is necessary to analyzed the trends in the incidence and mortality of diabetes. China has undergone a rapid aging transmission, medical care and socioeconomic development. All these changes may impact the incidence or mortality rates differently for different age groups. Thus, we examined the trends in diabetes incidence and mortality rates for each 5-year age group stratified by gender from 1990 to 2017.
2. Materials and Methods
2.1. Data Source
The incidence and mortality rates of diabetes were obtained from the Global Burden of Diseases 2017 (GBD 2017) Study, which provided a comprehensive assessment of incidence, prevalence, and years lived with disability (YLDs) for 354 causes in 195 countries and territories from 1990 to 2017. Diabetes mellitus includes type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) in this study. The incidence and mortality of diabetes mellitus for all ages in different provinces was age-standardized by the GBD 2017 global age-standard population [5]. The original data, which GBD adapted to estimate incidence and mortality of diabetes mellitus in China, was mainly from the Cause of Death Reporting System of the Chinese Center for Disease Control and Prevention (CDC), Disease Surveillance Points (DSPs) and the Maternal and Child Surveillance System, which are considered to be nationally representative [7,8,9,10,11].
2.2. Joinpoint Regression Analysis
The identification of changes in time trend is an important issue in the analysis of mortality and incidence data, and such changes were described by joinpoint regression analysis. In this analysis, logarithmic transformation of the rates was carried out and the standard errors were calculated based on binomial approximation. To determine the magnitude of the time trends in incidence and mortality rates of diabetes, the average annual percent change (AAPC) and corresponding 95% confidence interval (CI) was evaluated using joinpoint regression analysis [12]. AAPC was calculated as a geometrically weighted average of various annual percent change (APC) values from the regression analysis [13]. This analysis was performed using ‘Joinpoint’ software from the Surveillance Research Program of the US National Cancer Institute.
2.3. Age-Period-Cohort (APC) Analysis
Incidence and mortality reflect not only the incidence and death risks experienced by the population in a current year but also the accumulation of health risks since birth. Common statistical analysis could not decompose these risks and health risks when estimating incidence and mortality [14,15]. APC analysis is developed to estimate net age, period, and cohort effects on incidence and mortality trends simultaneously [8,16]. APC model with intrinsic estimator (IE) method can be used to decompose three temporal trends and provides unbiased and relatively efficient estimation results [17,18]. In APC-IE model, the age-specific rates were appropriately recoded into successive 5-year age groups (5–9, 10–14, …, 80–84), consecutive 5-year periods from 1990 to 2017, and correspondingly consecutive 5-year birth cohort groups (1912–1916, 1917–1921, …, 2012–2016) to estimate net age, period, and cohort effects on incidence and mortality of diabetes. In this model, the groups under 5 years old for incidence were excluded, and groups under 20 years old and groups above 85 years old for mortality were excluded. The APC model can be expressed as:
| Yj = µ + αagej + βperiodj + γcohortj + εi | (1) |
where Yj denotes the response variable—the net effect on incidence or mortality of diabetes for group j, α, β and γ denote the coefficients of age, period and cohort of the APC model, respectively, and μ denotes the intercept of the model. εi denotes the residual of the APC model.
APC model was performed through the Stata 12.0 software (StataCorp, College Station, TX, USA). Deviance, Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) were used to estimate the degree of model fitting.
3. Results
3.1. Descriptive Analysis of Incidence and Mortality Rates of Diabetes
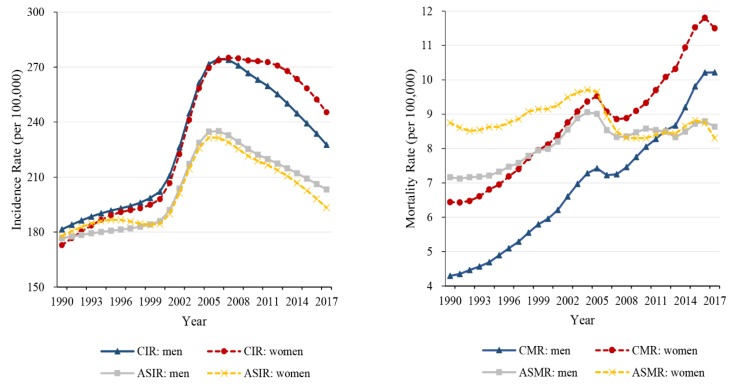
Trends in the crude incidence rate (CIR), age-standardized incidence rate (ASIR), crude mortality rate (CMR) and age-standardized mortality rate (ASMR) in men and women at all ages for diabetes from 1990 to 2017 are depicted in Figure 1. The age-standardized incidence rates of diabetes increased from 1990 to 2006 and subsequently decreased from 2006 to 2017, and mortality experienced a slight increase in men and did not change materially in women during 1990–2017.
Figure 1.
Trends in the crude rates and age-standardized rates for diabetes mellitus in men and women from 1990–2017, at all ages. CIR, crude incidence rate; ASIR, age-standardized incidence rate; CMR, crude mortality rate; ASMR, age-standardized mortality rate.
3.2. Trends in Age-Specific Incidence and Mortality Rates Using Joinpoint Regression Analysis
Table 1 shows the average annual percent change (APCC) in the incidence and mortality of diabetes for both men and women in China from 1990 to 2017. Age-standardized incidence rate rose by 0.92% (95% CI: 0.6%, 1.3%) in men and 0.69% (95% CI: 0.3%, 1.0%) in women, and age-standardized mortality rate rose by 0.78% (95% CI: 0.6%, 1.0%) in men and declined by 0.12% (95% CI: −0.4%, 0.1%) in women over the last decades. For age-specific rates, incidence increased in most age groups (from age group 10–14 to 80–84), with exception of 30–34, 60–64, 65–69 and 70–74 age groups in men, and 25–29, 30–34, 35–39 and 70–74 age groups in women; mortality in men decreased for the younger age groups (from 20–24 to 45–49 age group) and increased for the older age groups (from 50–54 to 80–84 age group). Mortality in women almost decreased for all age groups with exception of the age group 75–79 (AAPC = 0.73%, 95% CI: 0.4–1.0%) and 80–84 (AAPC = 2.34%, 95% CI: 2.0–2.7%) during the period.
Table 1.
The average annual percent changes (AAPC) in incidence and mortality of diabetes, 1990–2017.
| Age-Group (Year) | Incidence (95% CI) | Mortality (95% CI) | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| ASR | 0.92 (0.6, 1.3) | 0.69 (0.3, 1.0) | 0.78 (0.6, 1.0) | −0.12 (−0.4, 0.1) |
| 5–9 | −0.63 (−0.8, −0.5) | −0.65 (−0.9, −0.4) | ||
| 10–14 | 0.05 (−0.4, 0.5) | 0.63 (0.3, 0.9) | ||
| 15–19 | 3.27 (2.7, 3.9) | 3.10 (2.5, 3.7) | ||
| 20–24 | 2.17 (1.8, 2.5) | 1.79 (1.4, 2.1) | −3.98 (−4.7, −3.3) | −5.37 (−6.3, −4.5) |
| 25–29 | 0.01 (−0.1, 0.1) | −0.75 (−0.9, −0.6) | −3.39 (−4.4, −2.4) | −4.88(−5.9, −3.8) |
| 30–34 | −0.02 (−0.3, 0.3) | −1.07 (−1.3, −0.8) | −3.45 (−4.3, −2.6) | −4.97 (−5.9, −4.1) |
| 35–39 | 0.59 (−0.0, 1.2) | −0.76 (−1.1, −0.5) | −2.25 (−2.8, −1.7) | −3.77 (−4.4, −3.2) |
| 40–44 | 0.92 (0.2, 1.6) | 0.29 (−0.2, 0.7) | −2.42 (−2.8, −2.0) | −3.66 (−4.2, −3.1) |
| 45–49 | 1.11 (0.5, 1.7) | 1.25 (0.6, 1.9) | −0.52 (−0.9, −0.2) | −3.22 (−3.8, −2.6) |
| 50–54 | 0.89 (0.3, 1.5) | 1.26 (0.6, 1.9) | 0.69 (0.3, 1.1) | −1.70 (−2.4, −1.0) |
| 55–59 | 0.16 (−0.4, 0.8) | 0.73 (0.1, 1.3) | 0.21 (−0.3, 0.7) | −1.99 (−2.7, −1.2) |
| 60–64 | −0.36 (−0.9, 0.2) | 0.36 (−0.1, 0.8) | 0.80 (0.6, 1.0) | −1.36 (−1.6, −1.2) |
| 65–69 | −0.55 (−1.0, −0.1) | 0.09 (−0.1, 0.3) | 0.97 (0.6, 1.3) | −0.20 (−0.6, 0.2) |
| 70–74 | −0.41 (−0.8, −0.0) | −0.01 (−0.2, 0.2) | 0.49 (0.2, 0.8) | −0.14 (−0.5, 0.2) |
| 75–79 | 0.20 (−0.0, 0.4) | 0.38 (0.1, 0.6) | 1.41 (1.0, 1.8) | 0.73 (0.4, 1.0) |
| 80–84 | 0.55 (0.5, 0.6) | 0.62 (0.3, 1.0) | 1.83 (1.4, 2.3) | 2.34 (2.0, 2.7) |
CI, Confidence interval; ASR, age-standardized rate. Incidence and mortality for diabetes mellitus was age-standardized by the GBD 2017 global age-standard population.
3.3. The Age, Period, and Cohort Effects on Incidence and Mortality Using Age-Period-Cohort Analysis
The APC-IE analysis estimated coefficients for the age, period, and cohort effects (Table A1). These coefficients were then calculated to their exponential value (exp(coef.) = ecoef.) that denoted the incidence and mortality relative risk (RR) of a particular age, period, or birth cohort relative to each average level [19] (Table 2). Figure 2 was also plotted to reflect the age, period, and cohort effect based on Table 2.
Table 2.
The incidence and mortality relative risks of diabetes due to age, period, and cohort effects.
| Factor | Incidence (RR 95% CI) | Mortality (RR 95% CI) | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Age | ||||
| 5–9 | 0.03 (0.02–0.04) | 0.04 (0.03–0.06) | ||
| 10–14 | 0.16 (0.14–0.19) | 0.11 (0.09–0.14) | ||
| 15–19 | 1.70 (1.57–1.83) | 1.39 (1.27–1.52) | ||
| 20–24 | 2.79 (2.61–3.00) | 2.16 (2.00–2.34) | 0.13 (0.04–0.49) | 0.19 (0.06–0.61) |
| 25–29 | 2.09 (1.95–2.24) | 1.39 (1.29–1.51) | 0.19 (0.07–0.48) | 0.20 (0.08–0.52) |
| 30–34 | 1.94 (1.82–2.08) | 1.24 (1.15–1.34) | 0.28 (0.13–0.60) | 0.21 (0.09–0.50) |
| 35–39 | 2.18 (2.05–2.32) | 1.45 (1.35–1.56) | 0.29 (0.15–0.58) | 0.21 (0.10–0.47) |
| 40–44 | 2.20 (2.07–2.33) | 2.00 (1.88–2.13) | 0.45 (0.26–0.77 | 0.32 (0.17–0.59) |
| 45–49 | 2.05 (1.94–2.17) | 2.81 (2.66–2.96) | 0.60 (0.39–0.94) | 0.52 (0.33–0.84) |
| 50–54 | 1.82 (1.72–1.92) | 2.94 (2.80–3.09) | 0.86 (0.60–1.24) | 0.82 (0.57–1.20) |
| 55–59 | 1.49 (1.41–1.58) | 2.57 (2.45–2.70) | 1.23 (0.91–1.66) | 1.33 (1.00–1.78) |
| 60–64 | 1.25 (1.18–1.32) | 2.04 (1.94–2.14) | 1.95 (1.53–2.47) | 2.39 (1.91–3.00) |
| 65–69 | 1.07 (1.01–1.14) | 1.36 (1.29–1.44) | 3.09 (2.51–3.80) | 3.68 (2.99–4.53) |
| 70–74 | 0.88 (0.83–0.94) | 0.91 (0.85–0.97) | 4.77 (3.86–5.90) | 5.48 (4.36–6.88) |
| 75–79 | 0.69 (0.64–0.74) | 0.65 (0.61–0.70) | 6.47 (5.06–8.28) | 7.47 (5.66–9.85) |
| 80–84 | 0.49 (0.45–0.53) | 0.44 (0.40–0.48) | 9.12 (6.76–12.31) | 8.78 (6.24–12.37) |
| Period | ||||
| 1992 | 0.80 (0.77–0.83) | 0.80 (0.77–0.83) | 0.78 (0.63–0.98) | 0.97 (0.77–1.22) |
| 1997 | 0.88 (0.85–0.91) | 0.87 (0.84–0.90) | 0.83 (0.71–0.98) | 0.94 (0.80–1.10) |
| 2002 | 1.04 (1.01–1.07) | 1.00 (0.97–1.03) | 0.99 (0.89–1.11) | 1.02 (0.92–1.13) |
| 2007 | 1.19 (1.16–1.23) | 1.17 (1.14–1.20) | 1.04 (0.93–1.16) | 0.95 (0.85–1.05) |
| 2012 | 1.11 (1.08–1.15) | 1.13 (1.10–1.17) | 1.16 (1.00–1.35) | 1.03 (0.88–1.21) |
| 2017 | 1.03 (1.00–1.07) | 1.09 (1.05–1.13) | 1.28 (1.04–1.58) | 1.11 (0.89–1.39) |
| Cohort | ||||
| 1912–1916 | 1.62 (1.35–1.95) | 1.79 (1.48–2.17) | 1.96 (1.24–3.09) | 1.57 (0.97–2.53) |
| 1917–1921 | 1.55 (1.36–1.76) | 1.67 (1.46–1.90) | 1.95 (1.34–2.85) | 1.81 (1.22–2.68) |
| 1922–1926 | 1.46 (1.32–1.62) | 1.54 (1.39–1.71) | 2.02 (1.46–2.80) | 2.04 (1.46–2.85) |
| 1927–1931 | 1.37 (1.26–1.49) | 1.42 (1.30–1.55) | 2.08 (1.56–2.79) | 2.26 (1.68–3.04) |
| 1932–1936 | 1.30 (1.20–1.40) | 1.33 (1.23–1.43) | 1.97 (1.49–2.60) | 2.34 (1.77–3.09) |
| 1937–1941 | 1.21 (1.12–1.30) | 1.27 (1.19–1.36) | 1.79 (1.35–2.38) | 2.20 (1.65–2.94) |
| 1942–1946 | 1.10 (1.02–1.18) | 1.20 (1.13–1.29) | 1.59 (1.15–2.20) | 1.94 (1.40–2.70) |
| 1947–1951 | 1.00 (0.93–1.08) | 1.15 (1.08–1.23) | 1.51 (1.04–2.20) | 1.85 (1.25–2.72) |
| 1952–1956 | 0.93 (0.86–1.00) | 1.11 (1.03–1.19) | 1.56 (1.01–2.41) | 1.81 (1.15–2.86) |
| 1957–1961 | 0.90 (0.83–0.97) | 1.07 (1.00–1.15) | 1.35 (0.81–2.25) | 1.43 (0.83–2.48) |
| 1962–1966 | 0.91 (0.84–0.98) | 1.05 (0.97–1.13) | 1.07 (0.59–1.94) | 1.03 (0.53–1.99) |
| 1967–1971 | 0.89 (0.83–0.97) | 0.97 (0.90–1.06) | 1.08 (0.56–2.09) | 1.03 (0.49–2.15) |
| 1972–1976 | 0.83 (0.76–0.90) | 0.84 (0.77–0.92) | 0.79 (0.37–1.69) | 0.75 (0.32–1.75) |
| 1977–1981 | 0.74 (0.68–0.80) | 0.72 (0.66–0.79) | 0.54 (0.21–1.41) | 0.52 (0.17–1.58) |
| 1982–1986 | 0.68 (0.62–0.75) | 0.65 (0.58–0.72) | 0.41 (0.12–1.39) | 0.37 (0.09–1.56) |
| 1987–1991 | 0.71 (0.64–0.78) | 0.66 (0.59–0.73) | 0.35 (0.08–1.56) | 0.31 (0.05–1.77) |
| 1992–1996 | 0.83 (0.75–0.92) | 0.74 (0.66–0.83) | 0.28 (0.03–2.34) | 0.24 (0.02–2.35) |
| 1997–2001 | 0.94 (0.84–1.05) | 0.82 (0.72–0.92) | 0.17 (0.00–16.78) | 0.14 (0.00–14.53) |
| 2002–2006 | 0.98 (0.86–1.12) | 0.81 (0.69–0.95) | ||
| 2007–2011 | 0.89 (0.64–1.24) | 0.65 (0.42–1.00) | ||
| 2012–2016 | 0.85 (0.33–2.18) | 0.66 (0.26–1.68) | ||
| Deviance | 73.51 8.55 −182.09 |
105.89 8.87 −149.71 |
1.84 4.74 −189.85 |
3.04 4.74 −188.66 |
| AIC | ||||
| BIC | ||||
RR: Relative risk [RR = exp(coefficient)]; CI: Confidence interval; AIC: Akaike Information Criterions; BIC: Bayesian Information Criterions.
Figure 2.
The incidence and mortality relative risks of diabetes due to (a) age; (b) period; and (c) cohort effects.
3.3.1. Age Effect
After controlling for period and cohort effects, the net age effect on diabetes showed that the RR of mortality continuously increased with advancing age (from 20 to 84 years) (Figure 2a, Table 2); the RR of incidence increased with advancing age in men for the younger age groups (from 10–14 to 20–24 age groups) while slightly decreased for the older age groups (from 20–24 to 80–84 age groups) (Figure 2a), and in women, the changes could be divided into two slight decreases and two slight increases. The net age effect on diabetes indicated that its mortality significantly increased with advancing age, and no material changes in its incidence. From 5–9 to 80–84 age group, the RR of incidence increased by 16.38 times and 11.18 times in men and women, respectively; from age group 20–24 to 80–84, the RR of mortality increased by 69.59 times and 45.98 times in men and women, respectively.
3.3.2. Period Effect
The net period effect presented slight increasing incidence and mortality trends in men and women (Figure 2b, Table 2). During the period of observation, the RR of diabetes incidence increased by 1.29 and 1.36 times in men and women, respectively, which indicated that the incidence increased with advancing time period. The changes of period effect on the incidence could be divided into one accelerating increase from 1992 to 2007 and one slight decrease from 2007 to 2017. The RR of mortality increased by 1.64 and 1.15 times in men and women, respectively; for men, the mortality continuously increased with time period, while women showed two slight decreases and two slight increases during the period 1992–2017. Overall, the net period effect on diabetes showed the incidence and mortality of diabetes increased with advancing time period.
3.3.3. Birth Cohort Effect
The cohort effect presented the incidence risk continuously decreased from 1912–1916 to 1982–1986 birth cohort and subsequently showed no material changes from 1982–1986 to 2012–2016 birth cohort in both men and women (Figure 2c, Table 2); the mortality risk decreased from 1927–1931 to 1997–2001 birth cohort in men, while increased from 1912–1916 to 1932–1936 birth cohort and subsequently decreased to the 1997–2001 birth cohort among women. From the earlier birth cohorts to more recent birth cohorts, the RR of diabetes incidence decreased by 47.76% and 63.22% in men and women, respectively; the RR of mortality decreased by 91.16% and 90.99% in men and women, respectively.
4. Discussion
The present study used longitudinal data from the Global Burden of Disease Study to investigate age, period, and cohort effects of diabetes mellitus incidence and mortality trends in China between 1990 to 2017. The present study found, that diabetes mortality decreased in younger age groups and increased in older age groups, while diabetes incidence increased in most age groups during the last decades in China. Through APC model, the net age effect showed no material changes with advancing age for the incidence of diabetes while the mortality increased with advancing age both in men and women; the net period effect showed both the incidence and mortality of diabetes increased with advancing time period but the period trend on the incidence began to decrease since 2007; the net cohort effect presented the incidence and mortality decreased from earlier birth cohorts to more recent birth cohorts while the incidence showed no material changes from 1982–1986 to 2012–2016 birth cohort. Therefore, the three trends on the incidence and mortality of diabetes were discussed preliminarily in the following section, which could provide epidemiology evidences for understand on reasons of increasing prevalence of diabetes.
4.1. Age Effect
Age effect on the mortality of diabetes showed it increased with advancing age for both men and women, China’s aging transition may intensify this situation [20], and Chinese rapid aging is observed from 1980 to 2010 in China [21]. Previous studies have reported that older people with high-risk diabetes mellitus mortality [22], frailty is associated with increased mortality as diabetes mellitus and frailty are two conditions that frequently occur concurrently and are increasingly prevalent in the older patients [23]. Moreover, diabetes complications and co-morbidities are more frequent in old diabetics compared to their young counterparts, and the most frequent are cardiovascular diseases [24,25] and the most bothersome are visual and cognitive impairments [26]. All these factors may impact the net age effect on mortality that substantially increased with advancing age (from 20–24 to 80–84 years). However, it’s worth noting that the age effect on incidence is not significant in both men and women.
4.2. Period Effect
Period effect is usually influenced by a complex set of historical events and environmental factors. Our study reported for the first time that the incidence and mortality of diabetes increased over the last decades. Our findings were compatible with the many studies reporting an increasing prevalence of diabetes in China over the past decades [27,28,29,30,31].
Risk factors of diabetes mainly included family history of diabetes mellitus, age, obesity, and physical inactivity [32,33,34,35]. Nutritional changes and sedentary lifestyles are reported to be the main causes of the epidemic of diabetes in China [28]. Obesity is a known characteristic closely related to diabetes [27,32,33,34,36]. In addition, China seems to be at highest risk of type 2 diabetes in Asian countries [3,37], despite there has also been significant improvement in screening [38,39], treatment [40,41,42] and/or prevention of diabetes [43]. The reason for the increasing period trend was possibly related to the increasing prevalence of obesity in China. The China Health and Nutrition Survey showed that 184 million (14.7%) of Chinese were overweight and another 31 million (2.6%) were obese in 2002, out of a total population of 1.3 billion [44], and the prevalence of overweight has tripled in men and doubled in women from 1989 to 1997 among pre-school children [45], and the prevalence of obesity has also increased over the last decades among Chinese children and adolescents [46,47]. According to GBD 2017 study, the ASMR for diabetes attributable to high body-mass index shows a significantly increasing trend from 1990 to 2017 in China, while it sharply increased in global [5]. The trend in ASMR for diabetes attributable to high body-mass index through 1990–2017 was also plotted in the present study (Figure A1). The prevalence of obesity was very likely to contribute to the rising period trends in incidence and mortality of diabetes in China over the last three decades.
Apart from the obesity, family history of diabetes mellitus, age, physical inactivity [32], and occupational chemical exposure [48], alcohol consumption may also be the risk factor for diabetes mellitus incidence and mortality [49]. Engler et al. [50] illustrated that excessive alcohol consumption not only negatively impacts diabetes self-care adherence but also affects the course of diabetes, and drinkers are likely to have poor treatment adherence, leading to increased morbidity and mortality. Cullmann et al.’s study showed total alcohol consumption and binge drinking increased the risk of pre-diabetes and type 2 diabetes in men, while low consumption decreased diabetes risk in women [51]. Therefore, alcohol consumption is likely to be one of the factors explaining the period trend of diabetes.
Diabetes has also become a public health problem in rural China. The levels of awareness, treatment, and control of diabetes were relatively low [52]; moreover, the awareness and treatment were positively associated with age and were high in adults with a family history of diabetes and those who exercise frequently, but low for cigarette smokers and alcohol drinkers in China [40]. Treatment improvements didn’t seem to reduce the period trends in the incidence and mortality of diabetes, which was probably related to the differential diagnosis and management in China [53].
Finally, we found that the incidence of diabetes decreased from 2007 to 2017 in both men and women. The possible reason is worth being studied. A recent study reported that incidence of diabetes in Hong Kong appeared to be stable and there have been slight decreases through 2006 to 2014. This trend was also observed in other developed western and Asian countries [54], and again in keeping with patterns seen in the UK, USA, Korea and Denmark [55,56,57,58], and with a small reduction in obesity in Hong Kong over the same period [59]. The majority (94%) of the population in Hong Kong is ethnically Chinese as mostly second- or third-generation immigrants are from the southern Chinese province of Guangdong [60], which shows possible future trends in the incidence of diabetes for mainland China. Moreover, the decline in the incidence of diabetes in Hong Kong also suggests improved health assessment. As Hong Kong has a mixed public-private healthcare economy, which is based on the British National Health Service model with 94.2% of the funding derived from government general revenue [61], all residents can use public health care services at highly subsidized rates, who are provided services by the Hospital Authority which provides the majority of inpatient care (90% total bed-days and 80% of admissions), and 50% of specialist outpatient care, despite the private sector providing 70% of first-contact outpatient services [61,62]. These changes could possibly explain the decline in diabetes incidence since 2007.
4.3. Cohort Effect
Cohort effect represents variations, across groups of individuals born in the same year or years. These variations may arise when each succeeding cohort carries the imprints of physical and social exposures from gestation to old age [63,64]. Cohort effect on the incidence and mortality of diabetes showed a decreasing trend from earlier birth cohorts to more recent birth cohorts while the incidence showed no material changes from 1982–1986 to 2012–2016 birth cohort. The probable reason was that more recent birth cohorts received good education and had a stronger awareness of health and disease prevention, compared with earlier birth cohorts [65]. In addition, more studies reported that chemical exposure was associated with the risk of diabetes mellitus [48,66,67,68,69]. With the rapid industrialization in China in the last decades, the birth cohorts from 1982–1986 to 2012–2016 might have experienced a higher risk of diabetes due to increasing exposures to chemicals. The more recent cohorts for the incidence of diabetes showed no decreasing trends, which was possibly attributable to their high exposure to chemicals during the last decades.
This study has limitations. APC analysis with IE method is as an ecological study. We were thus unable to make causal inference. We only tried to bring forth scientific hypotheses regarding the causality of these trends in the incidence and mortality of diabetes, based on the available data and existing literatures. In addition, most patients with diabetes die of complications. We do not have details of diabetic complications when examining the trends in mortality rate from 1990 to 2017.
5. Conclusions
This study shows the mortality of diabetes decreased in younger age groups while increased in older age groups, but the incidence increased in most age groups. The net age effect showed the mortality increased with advancing age while no material changes are observed for its incidence; the net period effect generally showed both the incidence and mortality increased with advancing time period; the net cohort effect presented them decreased from earlier birth cohorts to more recent cohorts, but not for some individuals in more recent cohorts for the incidence. Overall, aging likely drives a continued increase in the incidence and mortality of diabetes. Timely population-level interventions aiming for obesity prevention, healthy diet and regular physical activity should be conducted, especially for men and earlier birth cohorts at high risk of diabetes.
Appendix A
Table A1.
Diabetes mellitus incidence and mortality rates estimated coefficients for the age, period and cohort effects.
| Factor | Incidence (Coef.) | Death (Coef.) | ||
|---|---|---|---|---|
| Men | Women | Men | Women | |
| Age | ||||
| 5–9 | −3.510167 | −3.244566 | ||
| 10–14 | −1.807966 | −2.189139 | ||
| 15–19 | 0.528192 | 0.329081 | ||
| 20–24 | 1.02728 | 0.7703547 | −2.031919 | −1.655243 |
| 25–29 | 0.7372475 | 0.3325766 | −1.66746 | −1.599612 |
| 30–34 | 0.6644725 | 0.2171844 | −1.263897 | −1.573054 |
| 35–39 | 0.7810942 | 0.3725534 | −1.222644 | −1.542911 |
| 40–44 | 0.7868705 | 0.69302 | −0.7980116 | −1.137536 |
| 45–49 | 0.7183525 | 1.03148 | −0.5081929 | −0.6463694 |
| 50–54 | 0.5961038 | 1.077748 | −0.1480281 | −0.1927196 |
| 55–59 | 0.3996364 | 0.943903 | 0.2057711 | 0.2868395 |
| 60–64 | 0.2202215 | 0.711614 | 0.6654444 | 0.8731999 |
| 65–69 | 0.0704756 | 0.3065629 | 1.128641 | 1.30325 |
| 70–74 | −0.1246894 | −0.0960116 | 1.562543 | 1.700852 |
| 75–79 | −0.3730952 | −0.4259169 | 1.867112 | 2.010437 |
| 80–84 | −0.7140285 | −0.8304447 | 2.21064 | 2.172865 |
| Period | ||||
| 1992 | −0.2226325 | −0.2242129 | −0.2451134 | −0.0345336 |
| 1997 | −0.129069 | −0.1417353 | −0.1820466 | −0.0624061 |
| 2002 | 0.0391526 | −0.000973 | −0.0053663 | 0.0187235 |
| 2007 | 0.1743509 | 0.1577314 | 0.0370973 | −0.0560221 |
| 2012 | 0.1050478 | 0.1246121 | 0.1479796 | 0.0310282 |
| 2017 | 0.0331502 | 0.0845777 | 0.2474493 | 0.1032101 |
| Cohort | ||||
| 1912–1916 | 0.4822343 | 0.583342 | 0.672919 | 0.4480838 |
| 1917–1921 | 0.4357266 | 0.511082 | 0.6686099 | 0.5945071 |
| 1922–1926 | 0.3800227 | 0.4330906 | 0.7027365 | 0.7141181 |
| 1927–1931 | 0.3147905 | 0.3491688 | 0.7344632 | 0.8168712 |
| 1932–1936 | 0.2587044 | 0.2818655 | 0.6757107 | 0.8488769 |
| 1937–1941 | 0.1899287 | 0.2398806 | 0.5830051 | 0.7903227 |
| 1942–1946 | 0.0923892 | 0.185763 | 0.4661998 | 0.664695 |
| 1947–1951 | −0.0000148 | 0.1417224 | 0.4134285 | 0.6131105 |
| 1952–1956 | −0.0712591 | 0.1038608 | 0.4431983 | 0.5943092 |
| 1957–1961 | −0.104813 | 0.0707695 | 0.300206 | 0.3604245 |
| 1962–1966 | −0.0991281 | 0.0457361 | 0.0653768 | 0.0246938 |
| 1967–1971 | −0.112517 | −0.0258342 | 0.0775096 | 0.0288989 |
| 1972–1976 | −0.1894786 | −0.1702079 | −0.2347566 | −0.2896645 |
| 1977–1981 | −0.3018504 | −0.3255781 | −0.6212781 | −0.6509558 |
| 1982–1986 | −0.3834765 | −0.4333095 | −0.8883387 | −0.9827572 |
| 1987–1991 | −0.3430538 | −0.4220359 | −1.048479 | −1.173499 |
| 1992–1996 | −0.1827672 | −0.307167 | −1.257226 | −1.443751 |
| 1997–2001 | −0.0620815 | −0.2038927 | −1.753285 | −1.958284 |
| 2002–2006 | −0.0173308 | −0.2080546 | ||
| 2007–2011 | −0.1188886 | −0.4334115 | ||
| 2012–2016 | −0.1671372 | −0.41679 | ||
Coef.: Coefficient.
Figure A1.
The trend in ASMR for diabetes attributable to high body-mass index from 1990 to 2017.
Author Contributions
X.L. drafted the article. X.L., C.Y., Y.W., Y.B., Y.L., and Z.-J.Z. have contributed to the conception and design of the study, revising the article critically for important intellectual content and given the final approval of this version to be submitted.
Funding
This work was supported by the National Natural Science Foundation of China (Grant 81641123, 81773552) and the Fundamental Research Funds for the Central Universities (grant 2042017kf0193).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Zimmet P.Z., Magliano D.J., Herman W.H., Shaw J.E. Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol. 2014;2:56–64. doi: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P., Alberti K.G., Magliano D.J., Bennett P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016;12:616–622. doi: 10.1038/nrendo.2016.105. [DOI] [PubMed] [Google Scholar]
- 3.Tuomi T., Santoro N., Caprio S., Cai M., Weng J., Groop L. The many faces of diabetes: A disease with increasing heterogeneity. Lancet. 2014;383:1084–1094. doi: 10.1016/S0140-6736(13)62219-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhou B., Lu Y., Hajifathalian K., Bentham J., Di Cesare M., Danaei G., Bixby H., Cowan M.J., Ali M.K., Taddei C., et al. Worldwide trends in diabetes since 1980: A pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou M., Wang H., Zhu J., Chen W., Wang L., Liu S., Li Y., Wang L., Liu Y., Yin P., et al. Cause-specific mortality for 240 causes in China during 1990–2013: A systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387:251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang L., Yu C., Liu Y., Wang J., Li C., Wang Q., Wang P., Wu S., Zhang Z.J. Lung Cancer Mortality Trends in China from 1988 to 2013: New Challenges and Opportunities for the Government. Int. J. Environ. Res. Public Health. 2016;13:1052. doi: 10.3390/ijerph13111052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei M., Zhou W., Bi Y., Wang H., Liu Y., Zhang Z.J. Rising mortality rate of cervical cancer in younger women in urban China. J. Gen. Intern. Med. 2019 doi: 10.1007/s11606-018-4732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X., Bi Y., Wang H., Meng R., Zhou W., Zhang G., Yu C., Zhang Z.J. Different trends in colorectal cancer mortality between age groups in China: An age-period-cohort and joinpoint analysis. Public Health. 2019;166:45–52. doi: 10.1016/j.puhe.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Li Z., Wang P., Gao G., Xu C., Chen X. Age-period-cohort analysis of infectious disease mortality in urban-rural China,1990–2010. Int. J. Equity Health. 2016;15:55. doi: 10.1186/s12939-016-0343-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000;19:335–351. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Clegg L.X., Hankey B.F., Tiwari R., Feuer E.J., Edwards B.K. Estimating average annual per cent change in trend analysis. Stat. Med. 2009;28:3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo G., Zhang Y., Guo P., Wang L., Huang Y., Li K. Global patterns and trends in stomach cancer incidence: Age, period and birth cohort analysis. Int. J. Cancer. 2017;141:1333–1344. doi: 10.1002/ijc.30835. [DOI] [PubMed] [Google Scholar]
- 15.Wang J.Y., Bai Z.Q., Wang Z.K., Yu C.H. Comparison of secular trends in cervical cancer mortality in china and the united states: An age-period-cohort analysis. Int. J. Environ. Res. Public Health. 2016;13:1148. doi: 10.3390/ijerph13111148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y. Social inequalities in happiness in the united states, 1972 to 2004: An age-period-cohort analysis. Am. Sociol. Rev. 2008;73:204–226. doi: 10.1177/000312240807300202. [DOI] [Google Scholar]
- 17.Yang Y., Schulhofer-Wohl S., Fu W.J.J., Land K.C. The intrinsic estimator for age-period-cohort analysis: What it is and how to use it. Am. J. Sociol. 2008;113:1697–1736. doi: 10.1086/587154. [DOI] [Google Scholar]
- 18.Fu W.J.J. Ridge estimator in singular design with application to age-period-cohort analysis of disease rates. Commun. Stat.-Theory Methods. 2000;29:263–278. doi: 10.1080/03610920008832483. [DOI] [Google Scholar]
- 19.Keyes K.M., Miech R. Age, period, and cohort effects in heavy episodic drinking in the US from 1985 to 2009. Drug Alcohol Depend. 2013;132:140–148. doi: 10.1016/j.drugalcdep.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanasi E., Ayilavarapu S., Jones J. The aging population: Demographics and the biology of aging. Periodontol 2000. 2016;72:13–18. doi: 10.1111/prd.12126. [DOI] [PubMed] [Google Scholar]
- 21.Lutz W., Sanderson W., Scherbov S. The coming acceleration of global population ageing. Nature. 2008;451:716–719. doi: 10.1038/nature06516. [DOI] [PubMed] [Google Scholar]
- 22.Chi M.J., Liang C.K., Lee W.J., Peng L.N., Chou M.Y., Chen L.K. Association of new-onset diabetes mellitus in older people and mortality in Taiwan: A 10-year nationwide population-based study. J. Nutr. Health Aging. 2017;21:227–232. doi: 10.1007/s12603-016-0751-9. [DOI] [PubMed] [Google Scholar]
- 23.Cobo A., Vazquez L.A., Reviriego J., Rodriguez-Manas L. Impact of frailty in older patients with diabetes mellitus: An overview. Endocrinol. Nutr. 2016;63:291–303. doi: 10.1016/j.endonu.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z.J., Zhao G., Song Y. Attention is needed for women in the control of diabetic complications in China. Acta Diabetol. 2014;51:1081–1082. doi: 10.1007/s00592-014-0589-8. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z.J., Wu Y.M., Jiang P., Zhao G.M. Study on risk factors of diabetes mellitus complications in a natural population. Chin. Gen. Pract. 2001;4:970–972. [Google Scholar]
- 26.Chentli F., Azzoug S., Mahgoun S. Diabetes mellitus in elderly. Indian J. Endocrinol. Metab. 2015;19:744–752. doi: 10.4103/2230-8210.167553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan X.R., Yang W.Y., Li G.W., Liu J. Prevalence of diabetes and its risk factors in China, 1994. Diabetes Care. 1997;20:1664–1669. doi: 10.2337/diacare.20.11.1664. [DOI] [PubMed] [Google Scholar]
- 28.Yang W.Y., Lu J.M., Weng J.P., Jia W.P., Ji L.N., Xiao J.Z., Shan Z.Y., Liu J., Tian H.M., Ji Q.H., et al. Prevalence of diabetes among men and women in China. N. Engl. J. Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y., Wang L., He J., Bi Y., Li M., Wang T., Wang L., Jiang Y., Dai M., Lu J., et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z.J., Li S.B., Zhao G.M., Han M. Analysis of disease burden of diabetes mellitus in 1971–2000 in Shanghai. Zhonghua Yu Fang Yi Xue Za Zhi. 2004;38:338. [PubMed] [Google Scholar]
- 31.Zhang Z.J., Zhao G., Yu C., Bi Y., Zhang Q., Song Y. Diabetic women suffer more years of life lost than diabetic men. Int. J. Endocrinol. 2014;2014:208369. doi: 10.1155/2014/208369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fletcher B., Gulanick M., Lamendola C. Risk factors for type 2 diabetes mellitus. J. Cardiovasc. Nurs. 2002;16:17–23. doi: 10.1097/00005082-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Polsky S., Ellis S.L. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr. Opin. Endocrinol. Diabetes Obes. 2015;22:277–282. doi: 10.1097/MED.0000000000000170. [DOI] [PubMed] [Google Scholar]
- 34.Volaco A., Cavalcanti A.M., Pecoits R., Precoma D.B. Socioeconomic status: The missing link between obesity and diabetes mellitus? Curr. Diabetes Rev. 2018;14:321–326. doi: 10.2174/1573399813666170621123227. [DOI] [PubMed] [Google Scholar]
- 35.Altobelli E., Petrocelli R., Verrotti A., Chiarelli F., Marziliano C. Genetic and environmental factors affect the onset of type 1 diabetes mellitus. Pediatr. Diabetes. 2016;17:559–566. doi: 10.1111/pedi.12345. [DOI] [PubMed] [Google Scholar]
- 36.Servan P.R. Obesity and Diabetes. Nutr. Hosp. 2013;28:138–143. doi: 10.3305/nh.2013.28.sup5.6929. [DOI] [PubMed] [Google Scholar]
- 37.Ali M.K., Siegel K.R., Chandrasekar R., Tandon R., Montoya P.A., Mbanya J.C., Chan J., Zhang P., Narayan K.M. Diabetes: An update on the pandemic and potential solutions. In: Prabhakaran D., Anand S., Gaziano T.A., Mbanya J.C., Wu Y., Nugent R., editors. Cardiovascular, Respiratory, and Related Disorders. 3rd ed. World Bank Publications; Washington, DC, USA: 2017. [PubMed] [Google Scholar]
- 38.Stadler M., Frohlich-Reiterer E., Prager R. Type 2 Diabetes mellitus-screening and prevention: Update 2016. Wien. Klin. Wochenschr. 2016;128(Suppl. 2):S41–S44. doi: 10.1007/s00508-016-0971-3. [DOI] [PubMed] [Google Scholar]
- 39.Zhao Z., Yu L. High-throughput screening in general population for type 1 diabetes. Diabetes Technol. Ther. 2016;18:674–676. doi: 10.1089/dia.2016.0324. [DOI] [PubMed] [Google Scholar]
- 40.Wang C., Yu Y., Zhang X., Li Y., Kou C., Li B., Tao Y., Zhen Q., He H., Kanu J.S., et al. Awareness, treatment, control of diabetes mellitus and the risk factors: Survey results from northeast China. PLoS ONE. 2014;9:e103594. doi: 10.1371/journal.pone.0103594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Z.J., Bi Y., Li S., Zhang Q., Zhao G., Guo Y., Song Q. Reduced risk of lung cancer with metformin therapy in diabetic patients: A systematic review and meta-analysis. Am. J. Epidemiol. 2014;180:11–14. doi: 10.1093/aje/kwu124. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Z.J., Li S. The prognostic value of metformin for cancer patients with concurrent diabetes: A systematic review and meta-analysis. Diabetes Obes. Metab. 2014;16:707–710. doi: 10.1111/dom.12267. [DOI] [PubMed] [Google Scholar]
- 43.Xu T., He Y., Dainelli L., Yu K., Detzel P., Silva-Zolezzi I., Volger S., Fang H. Healthcare interventions for the prevention and control of gestational diabetes mellitus in China: A scoping review. BMC Pregnancy Childbirth. 2017;17:171. doi: 10.1186/s12884-017-1353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y. Overweight and obesity in China. BMJ. 2006;333:362–363. doi: 10.1136/bmj.333.7564.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo J., Hu F.B. Time trends of obesity in pre-school children in China from 1989 to 1997. Int. J. Obes. 2002;26:553–558. doi: 10.1038/sj.ijo.0801944. [DOI] [PubMed] [Google Scholar]
- 46.Cai Y., Zhu X., Wu X. Overweight, obesity, and screen-time viewing among Chinese school-aged children: National prevalence estimates from the 2016 Physical Activity and Fitness in China-The Youth Study. J. Sport Health Sci. 2017;6:404–409. doi: 10.1016/j.jshs.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cui Z., Huxley R., Wu Y., Dibley M.J. Temporal trends in overweight and obesity of children and adolescents from nine Provinces in China from 1991-2006. Int. J. Pediatr. Obes. 2010;5:365–374. doi: 10.3109/17477166.2010.490262. [DOI] [PubMed] [Google Scholar]
- 48.Leso V., Capitanelli I., Lops E.A., Ricciardi W., Iavicoli I. Occupational chemical exposure and diabetes mellitus risk. Toxicol. Ind. Health. 2017;33:222–249. doi: 10.1177/0748233715624594. [DOI] [PubMed] [Google Scholar]
- 49.Polsky S., Akturk H.K. Alcohol consumption, diabetes risk, and cardiovascular disease within diabetes. Curr. Diabetes Rep. 2017;17:136. doi: 10.1007/s11892-017-0950-8. [DOI] [PubMed] [Google Scholar]
- 50.Engler P.A., Ramsey S.E., Smith R.J. Alcohol use of diabetes patients: The need for assessment and intervention. Acta Diabetol. 2013;50:93–99. doi: 10.1007/s00592-010-0200-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cullmann M., Hilding A., Ostenson C.G. Alcohol consumption and risk of pre-diabetes and type 2 diabetes development in a Swedish population. Diabet. Med. 2012;29:441–452. doi: 10.1111/j.1464-5491.2011.03450.x. [DOI] [PubMed] [Google Scholar]
- 52.Yang F., Qian D., Chen J., Hu D., Hou M., Chen S., Wang P., Group L.W.S.P. Prevalence, awareness, treatment and control of diabetes mellitus in rural China: Results from Shandong Province. Diabet. Med. 2016;33:454–458. doi: 10.1111/dme.12842. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y.H., Crimmins E.M., Hu P.F., Shen Y., Smith J.P., Strauss J., Wang Y.F., Zhang Y. Prevalence, diagnosis, and management of diabetes mellitus among older Chinese: Results from the China Health and Retirement Longitudinal Study. Int. J. Public Health. 2016;61:347–356. doi: 10.1007/s00038-015-0780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quan J., Li T.K., Pang H., Choi C.H., Siu S.C., Tang S.Y., Wat N.M.S., Woo J., Johnston J.M., Leung G.M. Diabetes incidence and prevalence in Hong Kong, China during 2006–2014. Diabet. Med. 2017;34:902–908. doi: 10.1111/dme.13284. [DOI] [PubMed] [Google Scholar]
- 55.Nichols G.A., Schroeder E.B., Karter A.J., Gregg E.W., Desai J., Lawrence J.M., O’Connor P.J., Xu S., Newton K.M., Raebel M.A., et al. Trends in diabetes incidence among 7 million insured adults, 2006–2011 The SUPREME-DM Project. Am. J. Epidemiol. 2015;181:32–39. doi: 10.1093/aje/kwu255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Holden S.H., Barnett A.H., Peters J.R., Jenkins-Jones S., Poole C.D., Morgan C.L., Currie C.J. The incidence of type 2 diabetes in the United Kingdom from 1991 to 2010. Diabetes Obes. Metab. 2013;15:844–852. doi: 10.1111/dom.12123. [DOI] [PubMed] [Google Scholar]
- 57.Carstensen B., Kristensen J.K., Ottosen P., Borch-Johnsen K., Register S.G.N.D. The Danish National Diabetes Register: Trends in incidence, prevalence and mortality. Diabetologia. 2008;51:2187–2196. doi: 10.1007/s00125-008-1156-z. [DOI] [PubMed] [Google Scholar]
- 58.Koo B.K., Lee C.H., Yang B.R., Hwang S.S., Choi N.K. The incidence and prevalence of diabetes mellitus and related atherosclerotic complications in Korea: A national health insurance database study. PLoS ONE. 2014;9:e110650. doi: 10.1371/journal.pone.0110650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Centre for Health Protection . Statistics on Behavioural Risk Factors: Body Mass Index (BMI) Distribution. SAR, Government of the HKSAR; Hong Kong, China: 2014. [Google Scholar]
- 60.Leung G.M., Wong I.O.L., Chan W.S., Choi S., Lo S.V., Grp H.C.F.S. The ecology of health care in Hong Kong. Soc. Sci. Med. 2005;61:577–590. doi: 10.1016/j.socscimed.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 61.Tin K.Y., Tsoi P.K., Lee Y.H., Chong D.S., Lam D.W., Yeung A.Y., Ma E.S., Maw C.K. Hong Kong domestic health spending: Financial years 1989/90 to 2011/12. Hong Kong Med. J./Xianggang Yi Xue Za Zhi. 2015;21:1–24. [PubMed] [Google Scholar]
- 62.Census and Statistics Department . Thematic Household Survey Report No. 45. SAR, Census and Statistics Department; Hong Kong, China: 2010. [Google Scholar]
- 63.Janssen F., Kunst A.E., Demograph N.E. Cohort patterns in mortality trends among the elderly in seven European countries, 1950–99. Int. J. Epidemiol. 2005;34:1149–1159. doi: 10.1093/ije/dyi123. [DOI] [PubMed] [Google Scholar]
- 64.Yang Y. Trends in US adult chronic disease mortality, 1960-1999: Age, period, and cohort variations. Demography. 2008;45:387–416. doi: 10.1353/dem.0.0000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cohen A.K., Syme S.L. Education: A missed opportunity for public health intervention. Am. J. Public Health. 2013;103:997–1001. doi: 10.2105/AJPH.2012.300993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Trasande L., Lampa E., Lind L., Lind P.M. Population attributable risks and costs of diabetogenic chemical exposures in the elderly. J. Epidemiol. Community Health. 2017;71:111–114. doi: 10.1136/jech-2016-208006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruiz D., Becerra M., Jagai J.S., Ard K., Sargis R.M. Disparities in environmental exposures to endocrine-disrupting chemicals and diabetes risk in vulnerable populations. Diabetes Care. 2018;41:193–205. doi: 10.2337/dc16-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uemura H. Associations of exposure to dioxins and polychlorinated biphenyls with diabetes: Based on epidemiological findings. Nihon Eiseigaku Zasshi. 2012;67:363–374. doi: 10.1265/jjh.67.363. [DOI] [PubMed] [Google Scholar]
- 69.Tseng C.H., Tseng C.P., Chiou H.Y., Hsueh Y.M., Chong C.K., Chen C.J. Epidemiologic evidence of diabetogenic effect of arsenic. Toxicol. Lett. 2002;133:69–76. doi: 10.1016/S0378-4274(02)00085-1. [DOI] [PubMed] [Google Scholar]