Abstract
Background
The “EMERGE” study, aimed to capture real-life management patterns and outcomes in metastatic breast cancer (MBC) in Greece, also accounting for hormone (HR) and human epidermal growth factor receptor 2 (HER2) status.
Methods
“EMERGE” was a multicenter, retrospective cohort study of adult MBC patients diagnosed between 01-Janaury-2010 and 30-June-2012, either de novo or having progressed from a non-metastatic state. Patient data, including treatment patterns and outcomes, were mainly abstracted through medical chart review.
Results
386 patients were enrolled by 16 hospital-based oncologists between 12-March-2013 and 31-March-2015. The median look-back period was 29.1 months. At MBC diagnosis, 56.1% of the patients were HR+/HER2−, 16.6% HR+/HER2+, 14.5% HR−/HER2−, and 12.8% HR−/HER2+. In the first line setting, chemotherapy, targeted therapy and endocrine therapy were received by 76.7, 52.4, and 28.3% of the overall population, and by 66.5/36.2/42.0%, 80.4/80.4/28.6%, 88.4/90.7/0.0, and 95.6%/56.5/6.5% of the HR+/HER2−, HR+/HER2+, HR−/HER2+, HR−/HER2− subpopulations, respectively. In the overall population, the disease progression incidence rate was 0.57 [95% confidence interval (CI): 0.48–0.67] per person-year; median progression-free survival (PFS) was 22.4 (95% CI: 20.4–24.7) and overall survival (OS) was 45.0 (95% CI: 40.9–55.0) months. Median PFS was 24.6 (95% CI: 21.3–27.9) in HR+/HER2−, 19.7 (95% CI: 12.9–25.9) in HR+/HER2+, 23.0 (95% CI: 16.6–29.7) in HR−/HER2+ and 18.3 (95% CI: 10.0–24.7) months in HR−/HER2− subpopulations. A multivariable Cox proportional hazards model, adjusted among other factors for age and duration of diagnosis, HR and HER2 status, demonstrated that in the overall population PFS was better among those receiving first line endocrine therapy (hazard ratio: 0.70; 95%CI: 0.51–0.95; p = 0.024).
Conclusions
“EMERGE” demonstrates differences between HR/HER2 subtypes in clinical outcomes and divergence from evidence-based guideline recommendations for MBC management, especially as it pertains to the HR+/HER2− patients in which chemotherapy was favored over endocrine therapy in the first line setting.
Study registration
The study has been registered on the electronic Registry of Non-Interventional Studies (RNIS) posted on the website of the Hellenic Association of Pharmaceutical Companies (SFEE): https://www.dilon.sfee.gr/studiesp_d.php?meleti_id=NIS-OGR-XXX-2012/1
Keywords: Metastatic breast cancer, Hormone receptor, Human epidermal growth factor receptor 2, Treatment patterns, Progression-free survival, Overall survival, Greece
Background
Breast cancer is the most frequently diagnosed cancer worldwide, conferring 523,000 deaths and 15.1 million disability-adjusted life-years in women in 2015 [1]. The estimated age-standardized incidence and mortality rate of breast cancer among females in Greece for 2012, was 58.6 and 21.0 per 100,000, respectively, thus, being the leading cause of death from cancer among Greek women [2].
The past two decades have witnessed significant advances in awareness, screening and molecular understanding of breast cancer. Nevertheless, 6–10% of all women still present with distant and 30% with regional lymph node metastases [3]. Additionally, an estimated 20–50% of women diagnosed with early stage breast cancer will eventually develop metastatic disease (MBC) [4, 5]. While the 5-year survival rate for patients diagnosed with localized disease is 98.8%, the rate drops to 85.2% among women diagnosed with regional and to 26.3% for those diagnosed with distant metastases [3]. Median overall survival (OS) in the MBC setting is 2 to 3 years [6–8]. Age at diagnosis is considered one of the main prognostic factors of survival from MBC [9]. Additionally, patient comorbidities and menopausal status, tumor histology and pathology, hormone receptor (HR) and human epidermal growth factor receptor 2 (HER2) status, sites of metastatic involvement, number of involved axillary lymph nodes and de novo metastatic disease presentation are also considered prognostic factors of survival and treatment response [9–11]. HR and HER2 are key elements guiding selection of an individualized treatment strategy. Additional factors, taken into consideration when deciding on the optimal treatment, include the length of disease-free interval since primary diagnosis, presence of visceral crisis, menopausal status, patient preference and prior treatments with special challenges posed by the development of endocrine or anti-HER2 resistance [9, 10, 12, 13].
Evidence regarding the clinical management and outcomes of MBC in Greece largely stems from registries including patients participating in clinical trials, and to a lesser extent from studies conducted in the routine care [7, 14, 15]. This retrospective cohort study aimed to provide a snapshot of MBC disease burden, clinical course and healthcare resource utilization as well as to depict the management patterns in relation to HR and HER2 status in a representative population of MBC patients treated under real life clinical conditions in Greece.
Methods
Study design and setting
“EMERGE” was a multicenter, national, retrospective cohort study. Patient data abstraction was mainly carried out through medical chart review, but also from databases developed and maintained by co-operative groups for their research activities. As a prerequisite, the selected databases contained an adequate number of potential candidates that met the inclusion/exclusion criteria, and captured only non-identifiable patient information including the study variables. All required information for the purposes of the study was collected using paper case report forms. Chemotherapy and endocrine therapy (ET) for MBC management have been grouped using the Anatomical Therapeutic Chemical Drug Classification dictionary by the World Health Organization Collaborating Centre for Drug Statistics Methodology ‘chemical subgroup’, while targeted therapies (TT) have been presented as ‘anti-HER2 agents’, ‘antiangiogenic agents’ and ‘mTOR inhibitor’. Supportive therapies (other than radiotherapy), such as bisphosphonates have not been captured in the context of this study.
Site selection was carried out through a documented and constructed feasibility assessment process that accounted, among others, for the expertise of the investigators in clinical study conduct, their intent to comply with the study procedures and their ability to enroll the pre-specified number of patients, as well as for the availability of patients’ complete medical records. The study was conducted in accordance with the Declaration of Helsinki and all applicable local requirements.
Study population
According to the final study protocol eligible patients were comprised of females aged ≥18 years, diagnosed with MBC between 01-Janaury-2010 and 30-June-2012, either de novo or having progressed from a non-metastatic state. Patients with a history of concurrent or other primary malignancies (except curatively resected non-melanoma skin cancer or in situ cervical cancer) were excluded from the study.
Study objectives and endpoints
The primary endpoint of the study was the estimation of disease progression incidence rate (IR) per patient-year, for MBC patients on first line treatment. The secondary objectives of the study were to estimate the overall survival (OS), the progression-free survival (PFS) and the time to progression (TTP) and to estimate the overall all-cause mortality and MBC-related mortality rate. Furthermore, the study aimed to record clinical and pathological characteristics of newly diagnosed MBC patients and to describe the management patterns of MBC, as well as the healthcare utilization associated with the disease, in Greece.
Statistical methods
The disease progression IR for patients receiving first line treatment, expressed in patient-years has been calculated by dividing the number of patients with progression in the first line setting by the overall time of follow-up (i.e. the sum of first line treatment duration of each patient). The Kaplan-Meier method has been used, in order to assess the OS, PFS and TTP [16]. PFS time has been defined as the time from first line treatment onset to the first documented disease progression or death due to any cause during the patients’ overall look-back period. Differences in the OS and PFS between subgroups have been examined by the log-rank test. Hazard ratios (95% confidence interval) between subgroups of interest have been derived by univariate Cox regression analysis. Likewise, a multivariable Cox proportional hazard model has been used to examine the association of PFS time with diagnosis of MBC de novo, presence of liver and bone metastases, receipt of endocrine therapy in the first line setting, HR and HER2 status, age at MBC diagnosis and MBC duration at first line treatment onset. Mortality rate, hospitalization as well as the emergency room and hospital outpatient visit rates (per patient-year) have been calculated by dividing the number of events by the sum of person-time (in years) of patient observation since MBC diagnosis. No imputation of missing data has been performed with the exception of partial dates.
Sample size evaluation was based on the maximum acceptable margin error that has been set at less than 5%. Under this consideration, a sample size of 400 patients, with a significance level a = 0.05 and power 80%, provided a margin of error of no more than 0.049. All statistical tests were two-sided and were performed at a 0.05 significance level. Statistical analysis has been conducted using SAS® v9.3 (SAS Institute, Cary, NC).
Results
Patient characteristics
A total of 386 patients diagnosed with MBC between 8-January-2010 and 27-June-2012 were enrolled in the study from 12-March-2013 to 31-March-2015, by oncologists practicing in 16 hospital-based sites distributed in 4 regions of Greece. The 12 sites located in Attica enrolled 77.5% of the patients. Of the enrolled patients, 7 did not meet all inclusion criteria. The median study look-back period of the eligible population was 29.1 [interquartile range (IQR): 19.2–37.7] months.
Eligible patients (N = 379) had been diagnosed with MBC at a mean ± SD age of 60.4 ± 12.8 years. At MBC diagnosis, 77.6% (256/330) were postmenopausal, 81.4% (206/253) had at least one term pregnancy and 54.8% (119/217) had a positive nursing history, while 92.0% (344/374) had an ECOG PS of 0 or 1. The primary tumor was histologically classified as invasive ductal carcinoma (IDC) in 76.0% of patients, as invasive lobular carcinoma in 7.9%, and for the remaining patients the tumor had characteristics of IDC with other histological features. The primary tumor differentiation was grade I or I/II in 3.4%, grade II or III or II/III in 81.5%, while for 15.0% the grade was unknown. Τhe most common metastatic sites were the bones (207/369; 56.1%), lungs (138/369; 37.4%;) and liver (28.7%; 106/369) (Table 1).
Table 1.
Clinical and primary tumor characteristics of the overall population at MBC diagnosis (N = 379)
| Characteristic | N (%) | |
|---|---|---|
| Caucasians | 371 (99.2) | |
| Median age at MBC diagnosis, years | 61.2 (50.7–70.9) | |
| BMI at MBC diagnosis (kg/m2), median (IQR) (N = 320) | 27.0 (23.9–31.4) | |
| BMI category at MBC diagnosis (N = 320) | Underweight (BMI < 18.5 kg/m 2 ) | 2 (0.6) |
| Normal (BMI ≥ 18.5 and BMI < 25 kg/m 2 ) | 117 (36.6) | |
| Overweight (BMI ≥ 25 and BMI < 30 kg/m 2 ) | 102 (31.9) | |
| Obese (BMI ≥ 30 kg/m 2 ) | 99 (30.9) | |
| Menopausal status at MBC diagnosis (N = 330) | Postmenopausal | 256 (77.6) |
| Premenopausal | 66 (20.0) | |
| Perimenopausal | 8 (2.5) | |
| Use of oral contraceptives (N = 236) | 9 (3.8) | |
| Use of hormone replacement therapy (N = 238) | 10 (4.2) | |
| Family history of breast cancer (N = 323) | 47 (14.6) | |
| Family history of ovarian cancer (N = 322) | 5 (1.6) | |
| Comorbidities at MBC diagnosisa (N = 379) | Vascular disorders | 61 (16.1) |
| Cardiac disorders | 51 (13.5) | |
| Endocrine disorders | 50 (13.2) | |
| Psychiatric disorders | 24 (6.3) | |
| Metabolism and nutrition disorders | 22 (5.8) | |
| ECOG performance status at MBC diagnosis (N = 374) | PS 0–1 | 344 (92.0) |
| PS 2–3 | 30 (8.0) | |
| Primary tumor location (N = 376) | Left breast | 189 (50.3) |
| Right breast | 179 (47.6) | |
| Both breasts | 8 (2.1) | |
| Primary tumor size (N = 301) | Tumor size ≤ 2 cm | 110 (36.5) |
| 2 cm < tumor size ≤ 5 cm | 148 (49.2) | |
| Tumor size > 5 cm | 43 (14.3) | |
| Number of metastases at MBC diagnosis, median (IQR) (N = 264 b) | 2.0 (1.0–3.0) | |
| Distant metastatic sites (N = 369)a | Bones | 207 (56.1) |
| Lung | 138 (37.4) | |
| Liver | 106 (28.7) | |
| Lymph nodes | 70 (19.0) | |
| Brain | 35 (9.5) | |
| Pleural effusion | 29 (7.9) | |
aOnly those present in at least 5% of the population have been included
bPatients for whom the precise number of metastases was known
IQR, interquartile range (Q25-Q75); N, number of patients with known data for each variable
Patient disposition based on HR/HER2 status and on de novo diagnosis or progression from an earlier stage
At the time of MBC diagnosis, nearly half of the patients (49.9%; 189/379) were HR+/HER2−, while 14.8% (56/379) were HR+/HER2+, 12.9% (49/379) triple negative, and 11.3% (43/379) HR−/HER2+. Classification could not be performed in the remaining patients (11.1%; 42/379). Additionally, 59.9% (227/379) of the eligible population had been first diagnosed at an earlier breast cancer stage (a mean ± SD of 5.2 ± 5.3 years prior to MBC diagnosis), while the remaining 40.1% (152/379) presented with de novo metastatic disease.
MBC management patterns
Of the eligible patients (n = 379), 99.5% had been exposed to systemic therapy, 32.2% had received radiotherapy and 13.2% had undergone surgery for MBC management. Of the patients exposed to systemic treatment (n = 377), 374 had received first line therapy [median exposure 5.5 (IQR: 3.7–11.0) months], 254 proceeded to second line [median 4.4 (IQR: 2.7–7.9) months], 175 to third line [median 3.5 (IQR: 1.9–5.7) months] and 105 to fourth line treatment. Information for patients of each subpopulation per HR/HER2 status that received first line treatment and those that advanced to the second, third and beyond the third line is indicated in Table 2.
Table 2.
Systemic treatment management patterns per HR/HER2 status
| Overall | HR+/HER2− | HR+/HER2+ | HR−/HER2+ | HR−/HER2− | |
| Patients receiving first line treatment | N = 374 | N = 188 | N = 56 | N = 43 | N = 46 |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Taxanes & Antiangiogenic agents | 54 (14.4) | 35 (18.6) | 4 (7.1) | 1 (2.3) | 6 (13.0) |
| Aromatase inhibitors only | 41 (11.0) | 36 (19.1) | 2 (3.6) | – | 1 (2.2) |
| Platinum compounds & Taxanes & Antiangiogenic agents | 19 (5.1) | 5 (2.7) | – | – | 13 (28.3) |
| Taxanes & Anti-HER2 agents | 18 (4.8) | 1 (0.5) | 11 (19.6) | 6 (14.0) | – |
| Pyrimidine analogues & Anti-HER2 agents | 15 (4.0) | 2 (1.1) | 4 (7.1) | 9 (20.9) | – |
| Other chemotherapy & Anti-HER2 agents | 9 (2.4) | – | 3 (5.4) | 6 (14.0) | – |
| Patients receiving second line treatment | N = 254 | N = 125 | N = 36 | N = 35 | N = 33 |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Aromatase inhibitors only | 21 (8.3) | 18 (14.4) | 1 (2.8) | – | 1 (3.0) |
| Anti-estrogens only | 20 (7.9) | 17 (13.6) | – | – | – |
| Aromatase inhibitors & mTOR inhibitors | 20 (7.9) | 17 (13.6) | – | – | 2 (6.1) |
| Pyrimidine analogues & anti-HER2 agents | 20 (7.9) | 1 (0.8) | 7 (19.4) | 10 (28.6) | 2 (6.1) |
| Anti-HER2 agents only | 11 (4.3) | 1 (0.8) | 1 (2.8) | 8 (22.9) | 1 (3.0) |
| Other chemotherapy & Anti-HER2 agents | 6 (2.4) | – | 2 (5.6) | 4 (11.4) | – |
| Platinum compounds & Pyrimidine analogues | 6 (2.4) | 1 (0.8) | – | – | 4 (12.1) |
| Aromatase inhibitors & Anti-HER2 agents | 6 (2.4) | – | 6 (16.7) | – | – |
| Patients receiving third line treatment | N = 175 | N = 89 | N = 28 | N = 24 | N = 18 |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Taxanes only | 15 (8.6) | 12 (13.5) | 1 (3.6) | 1 (4.2) | 1 (5.6) |
| Pyrimidine analogues & Anti-HER2 agents | 13 (7.4) | – | 4 (14.3) | 8 (33.3) | 1 (5.6) |
| Pyrimidine analogues only | 11 (6.3) | 10 (11.2) | 1 (3.6) | – | – |
| Aromatase inhibitors only | 11 (6.3) | 10 (11.2) | – | – | – |
| Anthracyclines & Other chemotherapy | 7 (4.0) | 4 (4.5) | – | 1 (4.2) | 2 (11.1) |
| Other chemotherapy & Anti-HER2 agents | 6 (3.4) | 1 (1.1) | 3 (10.7) | 1 (4.2) | 1 (5.6) |
| Pyrimidine analogues & Antiangiogenic agents | 5 (2.9) | 2 (2.2) | – | – | 3 (16.7) |
| Anti-HER2 agents only | 5 (2.9) | – | – | 5 (20.8) | – |
| Patients receiving beyond the third line treatment | N = 105 | N = 49 | N = 17 | N = 18 | N = 11 |
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Pyrimidine analogues only | 6 (5.7) | 5 (10.2) | 1 (5.9) | – | – |
| Anti-estrogens only | 5 (4.8) | 5 (10.2) | – | – | – |
| Anthracyclines & Anti-HER2 agents | 3 (2.9) | – | – | 2 (11.1) | 1 (9.1) |
| Other chemotherapy & Anti-HER2 agents | 3 (2.9) | – | – | 3 (16.7) | – |
| Pyrimidine analogues & Anti-HER2 agents | 2 (1.9) | – | – | 2 (11.1) | – |
| Pyrimidine analogues & Other chemotherapy & Anti-HER2 agents | 2 (1.9) | – | – | 2 (11.1) | – |
| Pyrimidine analogues & Taxanes & Other chemotherapy | 2 (1.9) | – | – | – | 2 (18.2) |
Only systemic treatment patterns utilized in at least 10.0% of one or more of the four subpopulations per HR/HER2 status are shown. Taxanes (docetaxel, paclitaxel, nab-paclitaxel); platinum compounds (carboplatin, cisplatin); pyrimidine analogues (capecitabine, fluorouracil, gemcitabine); anthracyclines (doxorubicin, liposomal and pegylated doxorubicin, epirubicin); aromatase inhibitors (anastrozole, exemestane, letrozole); anti-estrogens (tamoxifen, fulvestrant); other chemotherapy (cyclophosphamide, eribulin, methotrexate, vinorelbine); anti-HER2 agents (trastuzumab, lapatinib); antiangiogenic agents (bevacizumab, sorafenib), mTOR inhibitor (everolimus). N, number of patients analyzed for each outcome; n, number of patients who received the particular treatment
The median time elapsed from MBC diagnosis to first line treatment onset was 0.6 (IQR: 0.2–1.4) months (373 patients with available data). Chemotherapy was administered in 76.7% (287/374) of the overall population in the first line setting; TT (i.e. anti-HER2 agents, antiangiogenic agents and mTOR inhibitor) in 52.4% (196/374) and ET in 28.3% (106/374); additionally, chemotherapy, TT and ET were administered in 65.0% (165/254), 46.5% (118/254) and 36.6% (93/254) of patients in the second line; in 73.1% (128/175), 42.3% (74/175) and 28.6% (50/175) of patients in the third line setting; and in 87.6% (92/105), 42.9% (45/105), 36.2% (38/105) of patients receiving systemic fourth line treatment and beyond.
Systemic treatment patterns in terms of therapeutic drug classes administered in the first, second and third line setting of the overall population and subpopulations per HR/HER2 status are displayed in Fig. 1. Similarly, first, second, third and beyond the third line treatment patterns per therapeutic agent(s) subgroup administered in at least 10% of any of the subpopulations per HR/HER2 status are displayed in Table 2.
Fig. 1.
Treatment patterns of systemic therapy in the overall population and subpopulations per HR/HER2 status. Treatment patterns of chemotherapy (CT), endocrine therapy (ET) and targeted therapy (TT) utilized in the (a) first (b) second and (c) third line setting among patients of the overall population and in the subpopulations per HR/HER2 status
In regards to the treatment patterns (as displayed in Fig. 1) utilized from the first to the second and third line of therapy in the overall population, no single dominant pattern emerged. It shall be noted that a total of 30.2% (57/189), 25.0% (14/56), 58.1% (25/43) and 34.7% (17/49) of the HR+/HER2−, HR+/HER2+, HR−/HER2+ and HR−/HER2− patients had received radiotherapy in the MBC setting.
Clinical outcomes: Disease progression incidence rate, progression-free survival, overall survival and mortality rate
The disease progression IR in the overall population over a cumulative 256.7 years of follow-up in the first line setting, was 0.57 (95% CI: 0.48–0.67) per person-year. The IR was the lowest among HR+/HER2− (0.47; 95% CI: 0.37–0.58) patients. Additionally, the IR was higher among patients primarily diagnosed at an earlier stage (0.85; 95% CI: 0.69–1.01) rather than de novo metastatic (0.29; 95% CI: 0.20–0.38) (Table 3).
Table 3.
Disease progression incidence rate, PFS time and OS time
| Disease progression incidence rate | Kaplan-Meir estimated progression-free survival | Kaplan-Meier-estimated overall survival | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Patients with disease progression assessment | Total follow up time in first line treatment (years) | Incidence Rate (95% CI) (person-years) |
N | 12-month PFS | Median PFS (95% CI) (months) | Hazard Ratio (95% CI); p-value, univariate analysis |
N | 12-month survival rate | Median OS(95% CI) (months) | Hazard Ratio (95% CI); p-value; univariate analysis | |
| Overall patient population | ||||||||||||
| 357 | 147 | 256.7 | 0.57 (0.48–0.67) |
373 | 76.0 (71.2–80.1) |
22.4 (20.4–24.7) |
n/a | 379 | 88.8 (85.1–91.6) |
45.0 (40.9–55.0) |
n/a | |
| Patient subpopulations per MBC diagnosis state | ||||||||||||
| First diagnosed at an earlier stage | 208 | 110 | 129.1 | 0.85 (0.69–1.01) |
221 | 68.1 (59.7–75.1) |
20.4 (17.8–22.8) |
1.42 (1.12–1.81); p = 0.004 |
227 | 87.9 (82.9–91.6) |
41.0 (36.9–48.4) |
1.37 (0.97–1.93); p = 0.073 |
| de novo Diagnosis | 149 | 37 | 127.6 | 0.29 (0.20–0.38) |
152 | 82.7 (75.5–88.0) |
26.8 (21.8–30.3) |
152 | 90.1 (84.1–93.9) |
not reached | ||
| Patient subpopulations per HR/HER2 status | ||||||||||||
| HR+/HER2− | 182 | 76 | 161.0 | 0.47 (0.37–0.58) |
187 | 79.2 (72.5–84.5) |
24.6 (21.3–27.9) |
HR+/HER2+ vs. HR+/HER2− 1.48 (1.05–2.09); p = 0.027 HR−/HER2+ vs. HR+/HER2− 1.21 (0.82–1.77); p = 0.337 HR−/HER− vs. HR+/HER2− 1.52 (1.06–2.16); p = 0.023 |
189 | 94.1 (89.6–96.7) |
48.4 (36.3–61.0) |
HR+/HER2+ vs. HR+/HER2−1.00 (0.60–1.68); p = 0.992 HR−/HER2+ vs. HR+/HER2−1.71 (1.05–2.78); p = 0.032 HR−/HER− vs. HR+/HER2−1.79 (1.12–2.87); p = 0.015 |
| HR+/HER2+ | 51 | 21 | 29.7 | 0.71 (0.40–1.01) |
55 | 67.5 (53.0–78.4) |
19.7 (12.9–25.9) |
56 | 89.3 (77.7–95.0) |
45.6 (21.5–65.3) |
||
| HR−/HER2+ | 43 | 22 | 25.5 | 0.86 (0.50–1.22) |
42 | 85.6 (70.8–93.3) |
23.0 (16.6–29.7) |
43 | 92.9 (79.5–97.6) |
34.0 (25.5–45.6) |
||
| HR−/HER2− | 45 | 16 | 19.9 | 0.80 (0.41–1.20) |
47 | 62.2 (46.3–74.6) |
18.3 (10.0–24.7) |
49 | 72.9 (58.1–83.3) |
40.4 (18.4–55.0) |
||
Disease progression incidence rate, and the Kaplan-Meier estimated PFS time and OS time along with the 95% confidence intervals and hazard ratios of univariate analyses (p-values in bold indicated statistical significance) are displayed in the overall population and subpopulations per state of MBC diagnosis and HR/HER2 status. CI, confidence interval; N, number of patients analyzed for each outcome; n/a, not applicable
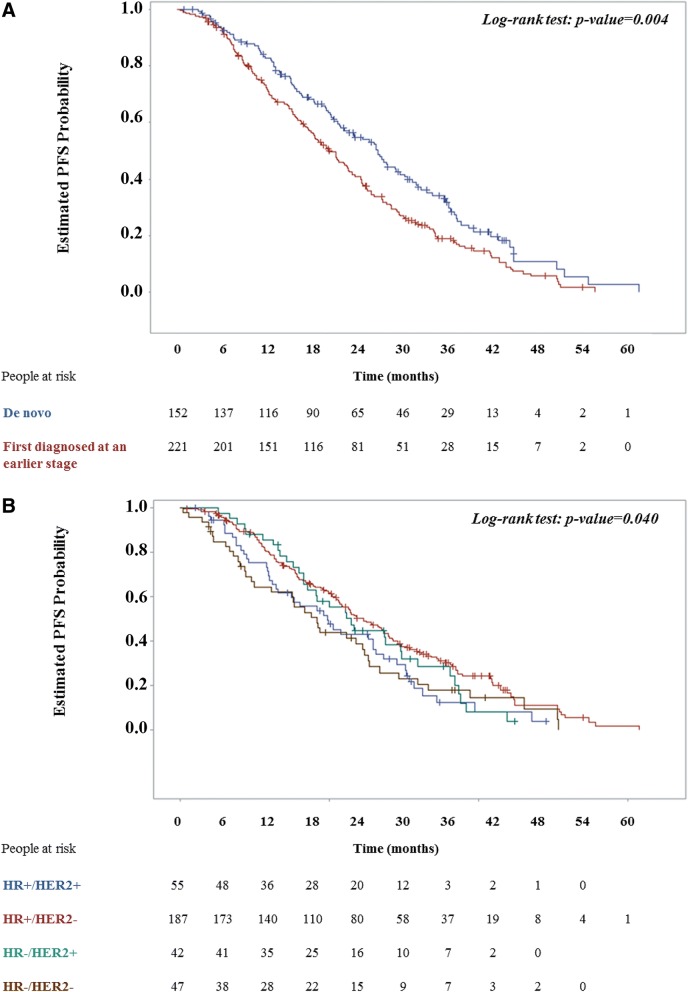
The Kaplan-Meier estimated median PFS of the overall population over the study observation period was 22.4 months (95% CI: 20.4–24.7) while the median TTP was 22.8 months (95% CI: 20.8–25.2). The estimated median PFS in the subpopulation primarily diagnosed at an earlier stage was shorter than that of patients diagnosed with de novo MBC (log-rank p-value: 0.004) (Fig. 2A and Table 3). A statistically significant difference was also detected in the median PFS of the subpopulations per HR/HER2 status (log-rank: p-value = 0.040) (Fig. 2B). Specifically, the median PFS of the HR+/HER2+ subpopulation was 19.7 months (95% CI: 12.9–25.9) and that of the HR−/HER2− 18.3 months (95% CI: 10.0–24.7), both shorter than that of the HR+/HER2− subpopulation (24.6 months, 95% CI: 21.3–27.9).
Fig. 2.
Kaplan-Meier progression-free survival (PFS) plots. PFS plots and the relevant log-rank test p-value is shown for MBC patients (a) diagnosed de novo versus at an earlier stage and (b) per HR/HER2 status
Among the factors (Table 4) examined by a multivariable Cox proportional hazards model in regards to their association with disease progression, median PFS was estimated to be higher among patients receiving ET as part of the first line treatment [HR: 0.70 (95% CI: 0.51–0.95; p = 0.024)], and lower in patients first diagnosed at an earlier breast cancer stage [HR: 1.42 (95% CI: 1.09–1.85; p = 0.009)] as well as among those with liver metastases [HR: 1.45 (95% CI: 1.10–1.93); p = 0.009].
Table 4.
Multivariable Cox proportional hazard model to identify factors associated with PFS in the overall population
| Multivariable Cox proportional hazard model (N = 319) | Hazard ratio | 95% confidence interval | p-value |
|---|---|---|---|
| First diagnosed at an earlier stage vs. de novo MBC diagnosis | 1.42a | 1.09–1.85 | 0.009 |
| Presence of liver metastases vs. No liver metastases | 1.45b | 1.10–1.93 | 0.009 |
| Presence of bone metastases vs. No bone metastases | 0.91c | 0.69–1.20 | 0.509 |
| Receipt of endocrine therapy vs. No endocrine therapy as part of first line treatment | 0.70d | 0.51–0.95 | 0.024 |
| Negative vs. Positive HER2 status | 0.91e | 0.68–1.22 | 0.531 |
| Positive vs. Negative HR status | 1.01f | 0.74–1.37 | 0.970 |
| Age at MBC diagnosis (years) | 1.00g | 0.99–1.01 | 0.953 |
| Time from MBC diagnosis to first line treatment (months) | 0.97h | 0.93–1.01 | 0.154 |
aAdjusted for presence of liver and bone metastases, receipt of endocrine therapy in the first line setting, HR and HER2 status, age at MBC diagnosis, and MBC duration at first line treatment onset
bAdjusted for diagnosis of MBC de novo, presence of bone metastases, receipt of endocrine therapy in the first line setting, HR and HER2 status, age at MBC diagnosis, and MBC duration at first line treatment onset
cAdjusted for diagnosis of MBC de novo, presence of liver metastases, receipt of endocrine therapy in the first line setting, HR and HER2 status, age at MBC diagnosis, and MBC duration at first line treatment onset
dAdjusted for diagnosis of MBC de novo, presence of liver and bone metastases, HR and HER2 status, age at MBC diagnosis, and MBC duration at first line treatment onset
e Adjusted for diagnosis of MBC de novo, presence of liver and bone metastases, receipt of endocrine therapy in the first line setting, HR status, age at MBC diagnosis, and MBC duration at first line treatment onset
fAdjusted for diagnosis of MBC de novo, presence of liver and bone metastases, receipt of endocrine therapy in the first line setting, HER2 status, age at MBC diagnosis, and MBC duration at first line treatment onset
gAdjusted for diagnosis of MBC de novo, presence of liver and bone metastases, receipt of endocrine therapy in the first line setting, HR and HER2 status, and MBC duration at first line treatment onset
hAdjusted for diagnosis of MBC de novo, presence of liver and bone metastases, receipt of endocrine therapy in the first line setting, HR and HER2 status, and age at MBC diagnosis
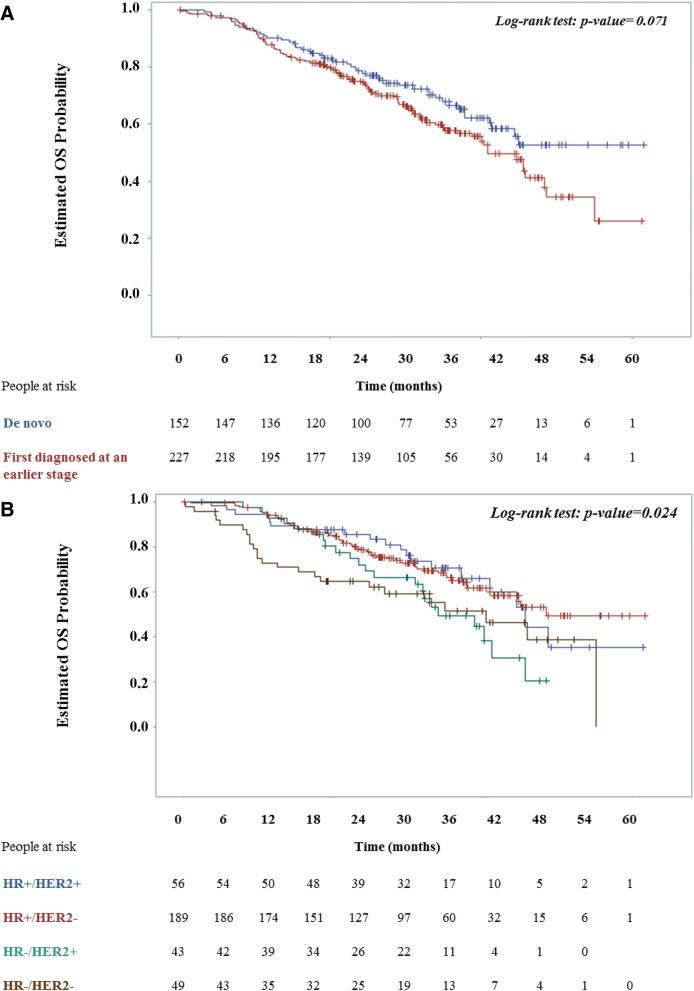
In regards, to the all-cause mortality rate, 143 deaths (37.7% of the overall population) due to any cause were recorded over a cumulative study observation period of 905.0 years, yielding a rate of 0.16 per person-year. The respective MBC-related mortality rate was 0.15 per person-year. The Kaplan-Meier estimated median OS was 45.0 months (95% CI: 40.9–55.0) (Table 3). The relative risk of death did not significantly differ between patients first diagnosed at an earlier breast cancer stage vs. those with a diagnosis of de novo MBC (Fig. 3A and Table 3). The relative risk of death was significantly higher for HR−/HER2+ vs. HR+/HER2− and for triple negatives vs. HR+/HER2− [HR: 1.71 (95% CI: 1.05–2.78; p = 0.032); and HR: 1.79 (95% CI: 1.12–2.87; p = 0.015), respectively] (Fig. 3B and Table 3).
Fig. 3.
Kaplan-Meier overall survival (OS) plots. OS plots and the relevant log-rank test p-value is shown for MBC patients (a) diagnosed de novo versus at an earlier stage and (b) per HR/HER2 status
Healthcare resource utilization
Over a cumulative observation period of 660.5 years, the all-cause hospitalization incidence rate was 0.74 per person-year. The hospitalization incidence rate due to treatment-related toxicity and due to other reasons was 0.21 (cumulative observation period: 724.0 years) and 0.51 (cumulative observation period: 652.6 years) per person-year, respectively.
Similarly, the all-cause emergency room and hospital outpatient visit rate over a cumulative follow-up of 605.4 years was 2.67 per person-year. The emergency room and hospital outpatient visit rate for treatment-related toxicity was 0.58 (cumulative observation period: 638.1 years), while the respective rate due to other reasons was 2.02 (cumulative observation period: 618.3 years).
Discussion
This study has yielded evidence on the clinicopathological characteristics, management patterns and treatment outcomes in the real-life clinical practice of Greece of 379 MBC patients. In addition to the overall population, treatments and clinical outcomes have been presented in the subpopulations diagnosed de novo and having progressed from a non-metastatic state and in the four subpopulations by joint HR and HER2 status. Although microarray gene-expression profiling constitutes a promising tool for prognostication and disease management, HR and HER2 status remains widely used for guiding treatment decision in the routine care [9, 10, 17, 18]. Overall, of the 337 MBC patients with known HR/HER2 status enrolled in the study, 56% were HR+/HER2−, while a nearly equal percentage were HR+/HER2+ (17%) and triple negative (15%), matching the distribution reported elsewhere [18, 19].
The disease progression IR in the overall population was 0.57 (95% CI: 0.48–0.67) per person-year; the median OS was 45.0 months, i.e. higher than the general estimates of about 2 years reported elsewhere [6–8], but similar (44 months) to that reported in a study of similar design conducted in a single institution in Germany [20]. The lowest disease progression IR and the longest median PFS and OS were observed in HR+/HER2− patients, in alignment with other studies demonstrating a better prognosis for this breast cancer entity [5]. Despite the fact that the guideline-recommended treatment of choice for HR+/HER2− disease is sequential ET, only 42% of the subpopulation in the present study had received ET-containing first line therapy and the remaining 58% had received chemotherapy-based regimens (with or without TT). Of further concern is the fact that herein only 54% received ET in the second line setting. Suboptimal rates of ET in HR+/HER2− patients have also been reported from data from US databases covering the decade of 2002–2012, with 60% receiving ET in the first line, and about three quarters not receiving a second ET [21]. Collectively, these observations suggest that in the real-world practice of Greece, chemotherapy is favored over ET in HR+/HER2− patients, despite the fact that as demonstrated in this and other studies [10, 22], ET was shown to be associated with longer PFS. The factors contributing to the administration of chemotherapy over ET cannot be inferred, since the characteristics of this subpopulation have not been examined separately, and the presence of visceral crisis, the only condition under which the guidelines recommend administration of chemotherapy [9, 10], was not collected. Clarification of the reasons for this divergence from the guidelines in the routine care of Greece would be an interesting topic for further study.
In regards to triple negative cancer, the present study also supports reports that this subpopulation exhibits the worst clinical outcomes [23, 24]. Treatment for nearly all triple negative patients was comprised of chemotherapeutic regimens (96% in the first line setting), while paradoxically about 4% in the first line setting and 6% in the second line setting were exclusively treated with ET.
Furthermore, even though most studies indicate that among patients overexpressing HER2, survival and disease-free interval is better among those with a positive versus a negative HR status [25, 26], in the present study no statistical significance in the clinical outcomes of the two groups could be established, likely due to the relatively small number of patients in these two groups. In line with the evidence-based management guidelines [10], TT was used in the majority of patients overexpressing HER2; specifically, 80% of the triple positive and 93% of the HR−/HER2+ subpopulation had received TT as part of their first line therapy.
In addition, the present study adds to the observations that patients diagnosed de novo compared to those that progressed from an earlier disease state have better clinical outcomes (PFS and OS). Importantly, the survival advantage of de novo MBC patients compared to those with recurrence from an earlier stage has been linked to a short disease-free interval (≤24 months in one study [8], and < 5 years in another [11]). Furthermore, in the present study, the most common sites of metastasis were the bones, followed by the lungs, liver and brain, in line with other reports [27, 28]. Herein, the presence of liver metastasis, but not bone metastases, was shown to be associated with a shorter PFS confirming once again the perception that liver involvement in MBC signals the end of the natural course of the disease, while, on the other hand, bone involvement points out a more indolent disease course [28].
Apart from the study limitations inherent in its retrospective design, the following limitations should be acknowledged. First, for about 11% of the patients the HR/HER2 status was unclassified, due mainly to unknown HER2 status; as a result a bias of unknown magnitude and direction may have been introduced in the patient distribution per HR/HER2 status. Second, healthcare utilization was unknown for a relatively large number of patients (approximately 21% in regards to hospitalizations and 37% for emergency room and outpatient visits). Third, recording of management patterns did not extend to the utilization of supportive therapies, such as bisphosphonates, but are restricted to radiotherapy, chemotherapy, endocrine and targeted therapies in the MBC setting. In addition, regarding PFS and TTP, it should further be noted that due to the observational retrospective nature of the study, no specific response criteria or schedule of response assessments were dictated by the protocol; therefore, tumor progression assessment may have been performed less frequently than it would be in a clinical trial setting, delaying identification of disease progression, which could have led to an upward bias of these outcomes. Notably, this inherent limitation of real-world PFS hinders comparability with data generated in other studies. Furthermore, due to the large number of censored data in the OS analysis should be taken into consideration when appraising the robustness of the estimated median OS of the overall population. In contrast, the strengths of the present study lie in its medical chart review design which aided the depiction of normal clinical practice settings under real-life conditions, and in enrollment of the study population by 16 study sites geographically distributed across four regions of Greece, which allows for variations in local standards of care to be reflected.
Conclusions
In conclusion, “EMERGE” has yielded clinically-relevant real-world data for a diverse patient population with a disease of complex and heterogeneous biology, such as MBC. The findings clearly demonstrate differences between the subtypes per HR/HER2 status in clinical outcomes and mortality and identify areas of divergence from evidence-based guideline recommendations for MBC management, especially as it pertains to the HR+/HER2− subpopulation. In order to achieve the maximum benefit from existing, but also from promising novel treatment options for breast cancer management, further studies that specifically examine the factors guiding the physician’s treatment decision-making in routine care, should be considered.
Acknowledgements
The authors wish to thank Andriana Papaconstantinou and Agoritsa Bismpiroula from the Clinical Research Organization Qualitis Ltd. for medical writing support and statistical analysis of the study data.
Funding
The study was sponsored by AstraZeneca Greece. The study Sponsor was involved in the study design, but not in the collection, analysis or interpretation of the data included in this publication. AstraZeneca also funded the medical writing support for this publication.
Availability of data and materials
The full clinical study report supporting the data and conclusions of this article is available on the Hellenic electronic Registry of Non-Interventional Studies and can be downloaded from: https://www.dilon.sfee.gr/studiesp_d.php?meleti_id=NIS-OGR-XXX-2012/1. Row Datasets are available from the authors upon reasonable request and with permission of the sponsor.
Abbreviations
- CI
confidence interval
- ET
endocrine therapy
- HER2
human epidermal growth factor receptor 2
- HR
hormone receptor
- IDC
invasive ductal carcinoma
- IQR
interquartile range
- IR
incidence rate
- MBC
metastatic breast cancer
- OS
overall survival
- PFS
progression-free survival
- TT
targeted therapy
- TTP
time to progression
Authors’ contributions
AK, AA, GK, ES, and AP have made substantial contributions to acquisition and interpretation of the data and were involved in revising the manuscript critically for important intellectual content. Furthermore, AK, AA, GK, ES, and AP have given final approval of the version to be published, take public responsibility for the content, agree to be accountable for all aspects of the work and vouch for its accuracy and integrity. CP drafted the original manuscript, made substantial contributions to acquisition and interpretation of the data, has given final approval of the version to be published, takes public responsibility for the content, agrees to be accountable for all aspects of the work and vouches for the accuracy and integrity of the data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and all applicable local requirements. The original study protocol was reviewed and approved by the competent institutional review boards of the participating hospital sites before the enrollment of any patient into the study and the performance of any study-related procedure. The IRBs which approved the conduct of this study were those of the: General Hospital of Athens “Alexandra”, Athens, Greece; Prefecture General Hospital for Cancer Treatment “Agioi Anargyri”, Athens, Greece (two principal investigators); University General Hospital of Ioannina, Ioannina, Greece; General University Hospital of Heraklion, Crete, Greece; Regional Hospital for Cancer Treatment “Agios Savvas”, Athens, Greece (two principal investigators); 251 Air Force Hospital, Athens, Greece; “Mitera” Maternity Hospital, Athens, Greece; “IASO” General Hospital, Athens, Greece; General Hospital of Chania “Agios Georgios”, Crete, Greece; Piraeus Regional General Hospital for Cancer Treatment “Metaxa”, Piraeus, Greece; General Hospital of Patras “Ag. Andreas”, Patras, Greece; “Metropolitan” Hospital, Piraeus, Greece; Diagnostic Centre of Athens “Hygeia”, Athens, Greece; and “Attikon” University Hospital, Athens, Greece. All subsequent amendments of the study protocol were also approved by the competent institutional review boards. Due to the retrospective chart review design that included the collection of secondary data only and in order to minimize the risk of biasing the clinical outcomes by exclusion from study participation of deceased subjects or patients not able to provide consent, informed consent requirement was not applied.
Consent for publication
Not applicable.
Competing interests
AK has nothing to disclose. AA has received honoraria for consultancy in advisory boards from Pfizer, Novartis and Roche. ES has received speaker honoraria from Novartis, BMS, AstraZeneca, Genesis, MSD, Amgen, Merck and Roche. GK has received honoraria for consultancy in advisory boards from Novartis, BMS, AstraZeneca, Genesis, MSD and Roche. AP has received honoraria for consultancy in advisory boards from AstraZeneca, Novartis and Roche and research grants from BMS. CP has received speaker honoraria and honoraria for consultancy in advisory boards from Novartis, AstraZeneca, Genesis, MSD, Amgen, Merck and Roche and research grants from BMS and Roche.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Athanasios Kotsakis, Email: kotsakis@med.uoc.gr.
Alexandros Ardavanis, Email: ardavanis@yahoo.com.
Georgios Koumakis, Email: gkoumak@otenet.gr.
Epameinondas Samantas, Email: epsam@otenet.gr.
Amanta Psyrri, Email: psyrri237@yahoo.com.
Christos Papadimitriou, Phone: +30 210 7286305, Phone: +30 697 4441501, Email: chr_papadim@yahoo.gr.
References
- 1.Global Burden of Disease Cancer Collaboration. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3:524–548. doi: 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Surveillance Epidemiology and End Results. SEER Stat Fact Sheets: Breast Cancer. SEER Cancer Statistics Review 2006–2012. Available at https://seer.cancer.gov/statfacts/html/breast.html. Accessed December 6, 2016.
- 4.Lu J, Steeg PS, Price JE, Krishnamurthy S, Mani SA, Reuben J, et al. Breast cancer metastasis: challenges and opportunities. Cancer Res. 2009;69:4951–4953. doi: 10.1158/0008-5472.CAN-09-0099. [DOI] [PubMed] [Google Scholar]
- 5.Bonotto M, Gerratana L, Poletto E, Driol P, Giangreco M, Russo S, et al. Measures of outcome in metastatic breast cancer: insights from a real-world scenario. Oncologist. 2014;19:608–615. doi: 10.1634/theoncologist.2014-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiely BE, Soon YY, Tattersall MH, Stockler MR. How long have I got? Estimating typical, best-case, and worst-case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: a systematic review of recent randomized trials. J Clin Oncol. 2011;29:456. doi: 10.1200/JCO.2010.30.2174. [DOI] [PubMed] [Google Scholar]
- 7.Dafni U, Grimani I, Xyrafas A, Eleftheraki AG, Fountzilas G. Fifteen-year trends in metastatic breast cancer survival in Greece. Breast Cancer Res Treat. 2010;119:621. doi: 10.1007/s10549-009-0630-8. [DOI] [PubMed] [Google Scholar]
- 8.Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br J Cancer. 2015;112:1445–1451. doi: 10.1038/bjc.2015.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, Version 2.2016. Available at: www.nccn.com. Accessed 8 Dec 2016.
- 10.Cardoso F, Costa A, Senkus E, Aapro M, André F, Barrios CH, et al. 3rd ESO-ESMO international consensus guidelines for advanced breast Cancer (ABC 3) Ann Oncol. 2017;28:16–33. doi: 10.1093/annonc/mdx447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010;21:2169–2174. doi: 10.1093/annonc/mdq220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giuliano M, Trivedi MV, Schiff R. Bidirectional crosstalk between the estrogen receptor and human epidermal growth factor receptor 2 signaling pathways in breast cancer: molecular basis and clinical implications. Breast Care (Basel) 2013;8:256–262. doi: 10.1159/000354253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Angus L, Beije N, Jager A, Martens JW, Sleijfer S. ESR1 mutations: moving towards guiding treatment decision-making in metastatic breast cancer patients. Cancer Treat Rev. 2017;52:33–40. doi: 10.1016/j.ctrv.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Pentheroudakis G, Fountzilas G, Kalofonos HP, Golfinopoulos V, Aravantinos G, Bafaloukos D, et al. Hellenic cooperative oncology group. Palliative chemotherapy in elderly patients with common metastatic malignancies: a Hellenic cooperative oncology group registry analysis of management, outcome and clinical benefit predictors. Crit Rev Oncol Hematol. 2008;66:237–247. doi: 10.1016/j.critrevonc.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Pentheroudakis G, Fountzilas G, Bafaloukos D, Koutsoukou V, Pectasides D, Skarlos D, et al. Metastatic breast cancer with liver metastases: a registry analysis of clinicopathologic, management and outcome characteristics of 500 women. Breast Cancer Res Treat. 2006;97:237–244. doi: 10.1007/s10549-005-9117-4. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 17.Colombo PE, Milanezi F, Weigelt B, Reis-Filho JS. Microarrays in the 2010s: the contribution of microarray-based gene expression profiling to breast cancer classification, prognostication and prediction. Breast Cancer Res. 2011;13:212. doi: 10.1186/bcr2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parise CA, Caggiano V. Breast cancer survival defined by the ER/PR/HER2 subtypes and a surrogate classification according to tumor grade and immunohistochemical biomarkers. J Cancer Epidemiol. 2014;469251. [DOI] [PMC free article] [PubMed]
- 19.Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst. 2014;106 pii: dju055. [DOI] [PMC free article] [PubMed]
- 20.Geiger S, Cnossen JA, Horster S, DiGioia D, Heinemann V, Stemmler HJ. Long-term follow-up of patients with metastatic breast cancer: results of a retrospective, single-center analysis from 2000 to 2005. Anti-Cancer Drugs. 2011;22:933–939. doi: 10.1097/CAD.0b013e32834860af. [DOI] [PubMed] [Google Scholar]
- 21.Swallow E, Zhang J, Thomason D, Tan RD, Kageleiry A, Signorovitch J. Real-world patterns of endocrine therapy for metastatic hormone-receptor-positive (HR+)/human epidermal growth factor receptor-2-negative (HER2-) breast cancer patients in the United States: 2002-2012. Curr Med Res Opin. 2014;30:1537–1545. doi: 10.1185/03007995.2014.908829. [DOI] [PubMed] [Google Scholar]
- 22.Lobbezoo DJ, van Kampen RJ, Voogd AC, Dercksen MW, van den Berkmortel F, Smilde TJ, et al. In real life, one-quarter of patients with hormone receptor-positive metastatic breast cancer receive chemotherapy as initial palliative therapy: a study of the Southeast Netherlands Breast Cancer Consortium. Ann Oncol. 2016;27:256–262. doi: 10.1093/annonc/mdv544. [DOI] [PubMed] [Google Scholar]
- 23.Sharma P. Biology and Management of Patients With Triple-Negative Breast Cancer. Oncologist. 2016;21:1050–1062. doi: 10.1634/theoncologist.2016-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hennigs A, Riedel F, Gondos A, Sinn P, Schirmacher P, Marmé F, et al. Prognosis of breast cancer molecular subtypes in routine clinical care: A large prospective cohort study. BMC Cancer. 2016;16:734. doi: 10.1186/s12885-016-2766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HJ, Park IA, Park SY, Seo AN, Lim B, Chai Y, et al. Two histopathologically different diseases: hormone receptor-positive and hormone receptor-negative tumors in HER2-positive breast cancer. Breast Cancer Res Treat. 2014;145:615–623. doi: 10.1007/s10549-014-2983-x. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Sun T, Wan D, Sheng L, Li W, Zhu H, et al. Hormone receptor status predicts the clinical outcome of human epidermal growth factor 2-positive metastatic breast cancer patients receiving trastuzumab therapy: a multicenter retrospective study. Onco Targets Ther. 2015;8:3337–3348. doi: 10.2147/OTT.S91166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berman AT, Thukral AD, Hwang WT, Solin LJ, Vapiwala N. Incidence and patterns of distant metastases for patients with early-stage breast cancer after breast conservation treatment. Clin Breast Cancer. 2013;13:88–94. doi: 10.1016/j.clbc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Cheng YC, Ueno NT. Improvement of survival and prospect of cure in patients with metastatic breast cancer. Breast Cancer. 2012;19:191–199. doi: 10.1007/s12282-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The full clinical study report supporting the data and conclusions of this article is available on the Hellenic electronic Registry of Non-Interventional Studies and can be downloaded from: https://www.dilon.sfee.gr/studiesp_d.php?meleti_id=NIS-OGR-XXX-2012/1. Row Datasets are available from the authors upon reasonable request and with permission of the sponsor.