Abstract
Background
Karoshi, which is sudden death associated with overwork, has become a serious problem in China. Many studies have examined the relationship between cardiovascular risks and karoshi, but there is little evidence that explains the exact mechanism by which overwork induces sudden death. In these cases, there are few obvious positive findings from forensic autopsies except for histories of overwork prior to death. Therefore, we assume that abnormalities, such as cardiac arrhythmia, rather than organic changes are the cause of karoshi.
Material/Methods
In the present study, the forced swim test (FST) was used to establish models of overwork. The myocardial tissues of SD rats taking FST (1 h per day, for 30 consecutive days) were collected. The arrhythmia-related molecule CX43 as well as its upstream regulation molecule Cav-1 and cSrc were tested by Western blot (WB) and immunohistochemistry (IHC). HE staining and Masson‘s staining were performed in the myocardium tissue section.
Results
We observed downregulation of caveolin-1 (Cav1) followed by cSrc activation, resulting in the decrease of connexin43 (Cx43) levels in overwork models. Myocardial interstitial fibrosis, which is associated with electrophysiological aberrances that result in arrhythmia, was also found in the overwork models.
Conclusions
These data provide a mechanistic explanation for the speculated link between karoshi and cardiac arrhythmias.
MeSH Keywords: Arrhythmias, Cardiac; Connexin 43; Fibrosis; Forensic Pathology
Background
Overwork-related disorders have become a major occupational and public health issue in some East Asian countries. Karoshi is thought to be caused by the stimulation of disorders associated with chronic fatigue after performing high-stress work for a long time, such as cerebrovascular/cardiovascular diseases (CCVD) and mental disorders. Karoshi is not only a clinical disease but also a social one, and remains a complex issue for forensic science [1]. Karoshi, a term coined to describe unexplained sudden death associated with overwork, was first reported in Japan in 1969. Japanese scholars have been studying karoshi since the 1980s, and in response to increased number of compensated cases of occupational mental disorders, the Japanese Government passed the “Act on Promotion of Preventive Measures against Karoshi and Other Overwork-Related Health Disorders” in June 2014 to promote preventive measures addressing overwork-related disorders [2]. The proportion of compensated karoshi cases versus claims has increased in Japan [3]. The 2014 Act defines karoshi as any of the following (translated from Japanese):
Death from cerebrovascular or ischemic heart disease due to excessive workload; or
Suicide caused by a mental disorder due to severe stress at work; or
Cerebrovascular or ischemic heart disease due to excessive workload or a mental disorder due to severe stress at work (defined by the onset of a condition and not necessarily leading to a fatality) [4].
In Japan, long working hours are defined as working more than 51 h per week or more than 45 h of overtime per month. Many studies have shown that working long hours induces a higher risk of stroke or heart disease than working standard hours [5–7]. Compared with standard working hours (35 to 40 h per week), working long hours (55 h per week) was associated with increases in the risk of incidence of coronary heart disease (13% increase) and stroke (33% increase) [8]. A recently case report described a middle-aged man with idiopathic ventricular fibrillation following a period of overwork and sleep deprivation [3]. However, it is difficult to prove a causative relationship between overwork and sudden cardiac arrest [3]. Furthermore, psychosocial stress from high-demand low-control work increases the incidence of cardiovascular diseases [9]. Psychological factors are also potential triggers for cardiac disease. These studies reveal a potential link between overwork and coronary heart disease and stroke.
Fatigue from overwork has been accepted as closely related to nervousness, anxiety, depression, and social or psychological problems, which over the long term lead to endocrine and metabolism disorders. Many previous studies have shown that overwork is associated with diseases such as hypertension [10,11], cardiovascular disease [12–14], and diabetes [15]. These studies argued that overwork could worsen existing physical diseases. However, in our practical and medicolegal expertise, we have found that some karoshi cases present no typical positive findings in the heart, brain, and other important tissues and organs. The commonalities in these cases were that the deceased had experienced overwork for several months. Excluding other possibilities such as allergies and poisoning, we highly suspect that arrhythmias lead to the death of the overworked person. Although previous reports have suggested that overwork might be a precipitating factor for idiopathic ventricular fibrillation [3], the exact mechanism by which overwork induces an underlying disease is unknown. Additionally, overwork may be an independent cause of malignant arrhythmia leading to karoshi. In the present study, we attempted to investigate the potential mechanism by which overwork could induce arrhythmia and to reveal the associations between overwork fatigue and karoshi.
Material and Methods
Animals
Adult male SD rats were used in the present study. The rats were aged 45–52 d, 18–22 cm in length, and had body weights of 280–330 g. All rats were maintained on a 16 h/8 h light/dark cycle (lights on at 06: 00 A.M.), at a temperature of 25°C and relative humidity of 50%. Rats were provided food and water ad libitum. All procedures were conducted according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the Southern Medical University Institutional Animal Care and Use Committees. The SD rats were randomly divided into a control group and a test group (n=5 each).
The forced swim test (FST) was performed in the test group [16]. Cylindrical plastic tanks (65 cm height; 35 cm diameter) were filled with water (50 cm depth, 25–26°C). Each rat was placed individually into the tank for 1 h (9: 00 a.m. to 10: 00 a.m.) of swimming. The overworking fatigue standard used was the observation of distinctly maladjusted actions while swimming and the nose tip at water level. When the rats submerged, more than 10 s would elapse before the rat would float up to the surface, and this occurred 3 consecutive times [16]. Then, the rats were returned to their home cages and allowed to eat food and drink ad libitum. Body weights were recorded the next morning.
Collection of cardiac tissue
Rats received FST 1 h per day for 30 d and then were sacrificed after anesthetization (1: 10 chloral hydrate, 3.5 mg/kg). The heart was immediately removed. Samples were immediately frozen using liquid nitrogen, and the tissue was stored at −80°C.
Immuno-histochemical staining, hematoxylin-eosin (HE) staining and Masson’s staining
Paraffin tissue sections were serially cut at 2 μm thickness and mounted on glass slides, which were placed in an incubator chamber at 37 °C overnight, then maintained at 65 °C for 2 h. Paraffin sections were dewaxed with water. Tissue sections were placed in a repair kit filled with antigen repair buffer, and antigen repair was performed in a microwave oven. To block endogenous peroxidase, the tissue sections were incubated in 3% hydrogen peroxide solution at room temperature for 10 min. Connexin43 antibody was diluted 1: 200 and incubated at 37°C for 1 h. The samples were treated 5 times for 2 min each time, then washed with 0.01 M PBS. The samples were then incubated with goat anti-rabbit IgG at 37°C for 30 min.
The DAB chromogenic process was performed according to the manufacturer’s instructions. Sections were re-stained by hematoxylin followed by dehydration in mounting ethanol. Finally, the sections were covered by neutral balata wraps. HE staining and Masson’s staining were performed similarly, using corresponding regent kits.
Western blot analysis
The ventricle tissue from SD rats in the control and FST groups was collected to determine relevant protein expression levels. Tissue was frozen in liquid nitrogen for 5 s and then transferred into a tissue grinder on ice, where the tissue was ground thoroughly in lysis buffer. Subsequently, the homogenates were pulse sonicated at 5 watts on ice for 15 min. The lysate was centrifuged at 14 000×g at 4°C for 15 min. Supernatant was collected and stored at −80°C.
Statistical analyses
Data are presented as the means ±SD for analyses and figures. A value of p<0.05 was considered to be statistically significant. The t test was used for comparisons of protein expression and the changes in organ coefficients between controls and FST groups.
Results
Cav1, p-cSrc, and Cx43 protein expression
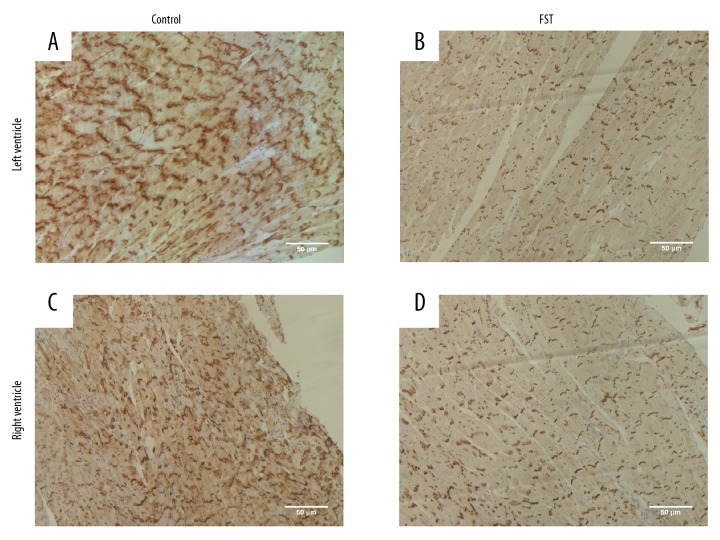
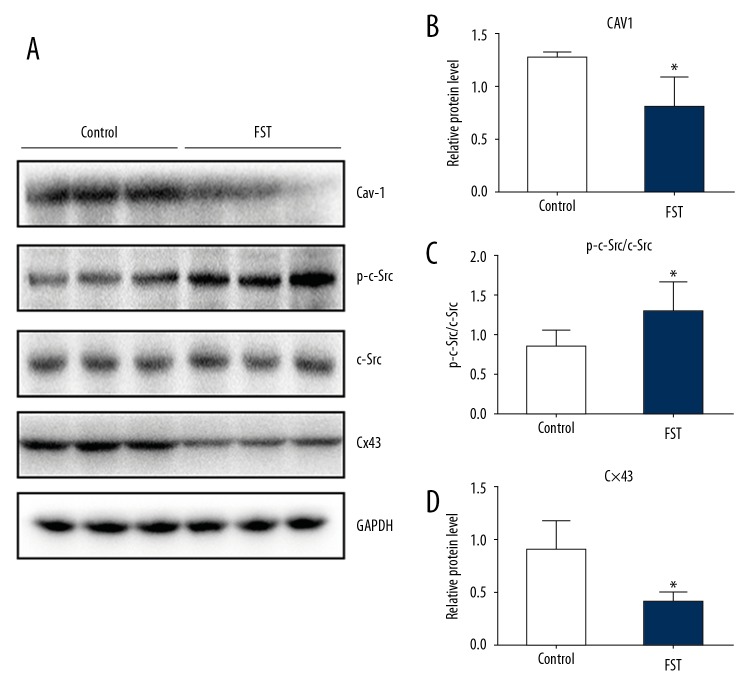
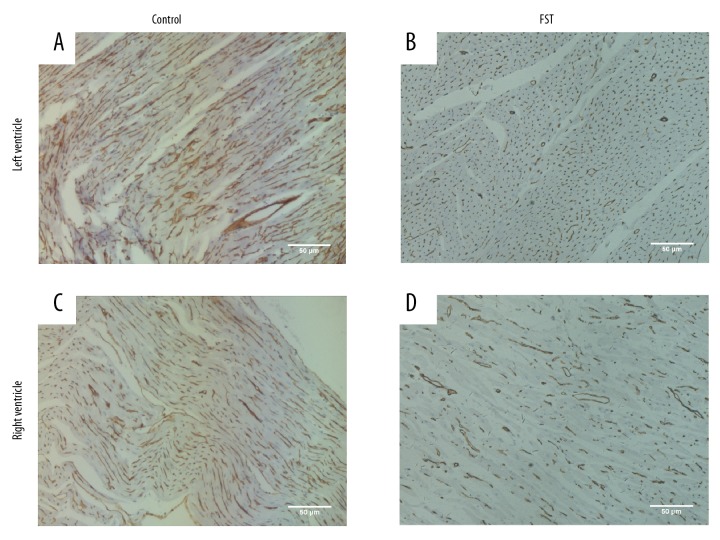
We hypothesized that overwork was associated with protein Cx43, which had been reported to be closely related to arrhythmia [17–19]. Immunohistochemical staining of ventricle tissue sections indicated that the expression of Cx43 decreased in the myocardial tissue of rats from the FST overwork model group (Figure 1B, 1D) compared with the control group (Figure 1A, 1C). Western blot analysis also indicated decreased expression of Cx43 (55.6% reduction) in the FST group (Figure 2A, 2D). We next attempted to investigate the pathways involved in the regulation of Cx43 during overwork. The loss of Cav1 leads to the activation of cSrc tyrosine kinase, resulting in the downregulation of Cx43 and subsequent electrical abnormalities [20]. Therefore, we tested the expression levels of Cav1 and p-cSrc (the activated form of cSrc) in FST overwork model rats. Western blot analysis showed that rats in the FST group (n=5) displayed significantly lower levels of Cav1 (38.5% reduction, p<0.05) relative to the control group (n=5, Figure 2A, 2B). Immunohistochemical staining also showed a reduction in the expression of Cav1 in rats in the FST group (Figure 3). However, expression of p-cSrc increased (by 1.8-fold, P<0.05, Figure 2A, 2C). Based on the above data, we deduced that overwork could be associated with the Cav1-related modulation of cardiac gap junctions through the regulation of cSrc tyrosine kinase, which could reasonably explain the downregulation of Cx43 in the FST overwork model.
Figure 1.
Overwork leads to decreased expression of Cx43 in myocardium. (A, C) Representative immunohistochemical staining showed expression of Cx43 in the myocardial tissue of rats in control group. (A for the left ventricle, C for the right ventricle). (B, D) Representative immunohistochemical staining showed expression of Cx43 in the myocardial tissue of rats in FST group. Brownish yellow granules indicated Cx43 positive molecules (B for the left ventricle, D for the right ventricle).
Figure 2.
Overwork down-regulates the expression of Cx43 via the activation of cSrc and decreased Cav-1. (A) Representative Western blot and (B–D) quantitative analyses of the expression levels of Cav-1, cSrc, p-cSrc, and Cx43 in the FST and control groups, respectively, are shown. GAPDH was used as a loading control. Data are presented as the means ±SD (n=5) and were analyzed with the t test. (* P<0.05)
Figure 3.
Overwork decreases the expression of Cav1 in the myocardium. (A, C) Representative immunohistochemical staining indicated expression of Cav1 in the myocardium of the control group (A for the left ventricle, C for the right ventricle). (B, D) Representative immunohistochemical staining showed expression of Cav1 in FST group. Brown granules indicated Cav1 positive molecule. (B for the left ventricle, D for the right ventricle).
Heart weight/body weight coefficient (HW/BW coefficient)
Structural changes in the heart were also noticed in the karoshi model rats. Organ coefficients were used in our study to reflect variations in heart tissue in the overwork model [21]. In the FST groups, overwork induced an evident increase in the HW/BW coefficient compared with the controls (0.41±0.09 vs. 0.32±0.02, p<0.05). The increased HW/BW coefficient implies that changes occurred in the structure of the heart that lead to organic lesions.
Myocardial fibrosis
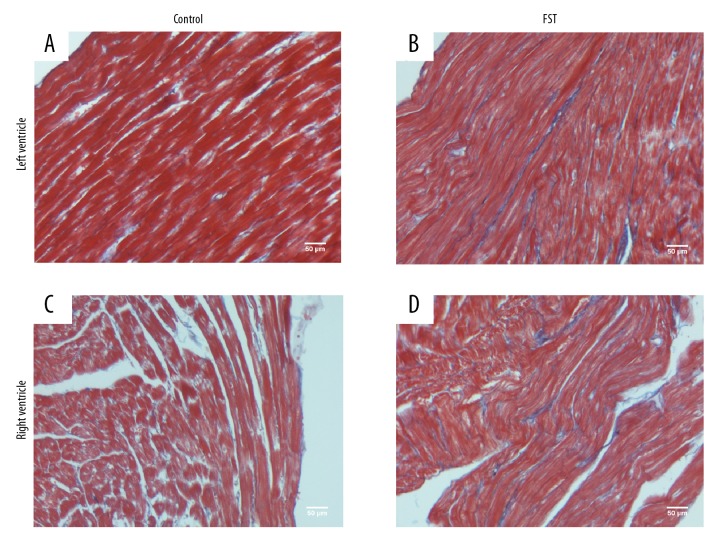
HE staining and Masson’s staining were used to highlight fibrosis in myocardial tissues, which provided proof of the occurrence of organic lesions in the heart during overwork (Figure 4 for Masson’s and Figure 5 for HE). A previous study showed that myocardial fibrosis could not only reduce myocardial contractility but also affect the conduction of the heart, causing arrhythmia and consequent cardiac dysfunction [22]. Compared with the controls (Figures 4A–4C, 5A–5C), dramatically increased myocardial fibrosis was observed in rats in the FST group (Figures 4B, 4D, 5B, 5D). This evident myocardial interstitial fibrosis was the consequence of the compensatory hyperplasia of fibroblasts in the damaged myocardium, which was consistent with the increased heart coefficients observed. These results suggest that acute heart failure and arrhythmia caused by overwork could be critical independent causes of karoshi.
Figure 4.
Enlarged interstitia and disordered arrangement of cardiomyocytes in overworked rats. (A, C). Representative HE staining of myocardial tissue in the control group (A for the left ventricle, C for the right ventricle). (B, D) Representative HE staining presented the enlarged myocardial interstitium and disordered myocardial fibers in the FST overwork models (B for the left ventricle, D for the right ventricle).
Figure 5.
Overwork facilitates myocardial interstitial fibrosis. Representative Masson’s staining indicated fibrosis of the myocardial interstitial in (A, C) control group and (B, D) FST group. Blue stripes represented fibrotic interstitial tissue (A, B for the left ventricle; C, D for the right ventricle).
Discussion
Substantial evidence has suggested that Cav1 is involved in the regulation of cardiac electrical function [23–26]. Significant associations of Cav1 variants with increased risk of cardiac arrhythmias have been proven by human genome-wide association studies [27,28]. In the present study, FST rat overwork models were built to investigate the association between overwork and the possibility of ventricular arrhythmias. Overwork induces the downregulation of Cav1 followed by the activation of cSrc (p-cSrc), resulting in the decreased expression of Cx43. At the intercalated disc, p-cSrc competes with Cx43 to bind with ZO-1 protein, promoting Cx43 internalization and degradation [29]. The Cx43 downregulation observed in overwork can be explained as a result of p-cSrc-mediated Cx43 depletion.
The loss of connexin43 could underlie arrhythmogenic ventricular cardiomyopathy [17, 30]. In a recent study, a 30% reduction in left ventricular conduction velocity accompanied by a ~50% reduction in Cx43 was observed in Cav1−/− mice [20]. Previous studies have shown that down-regulated Cx43 levels are accompanied by significant changes in the ventricular conduction velocity in the myocardium [31–33], suggesting that a modest reduction in gap-junction conduction can occur with significantly decreased conduction velocity in the myocardium. In addition, the electrophysiological abnormalities linked to Cx43 dysregulation were also shown to be associated with increased cardiac RAS activity [34,35]. In summary, the present study demonstrates that overwork can disrupt Cav1-cSrc interactions, resulting in the activation of cSrc, Cx43 reduction, and an increased propensity for ventricular arrhythmias. Enhanced cardiac renin-angiotensin system (RAS) signaling can reduce binding between Cav1 and cSrc by increasing the S-nitrosation of Cav1 upon increased Cav1-eNOS binding, resulting in cSrc activation and subsequent Cx43 downregulation [36]. Therefore, we hypothesized that overwork leads to Cx43 downregulation in a disordered RAS system, ultimately resulting in arrhythmias and death.
The HW/BW coefficients in the FST and control groups were significantly different, which suggests that the myocardium is associated with a relative increase in cardiac connective tissue [37]. Interstitial fibrosis interferes with myocardial electrophysiology by slowing action potential propagation, initiating reentry, promoting after-depolarizations, and increasing ectopic automaticity. Meanwhile, increasing numbers of myofibroblasts (the activated form of fibroblasts) undergo phenotypic changes with the expression of gap junctions and ion channels, thereby forming direct electrical couplings with cardiomyocytes, which can result in profound electrophysiological disturbances [22]. In the present study, myocardial interstitial fibrosis was found in overwork fatigue models, suggesting that karoshi is associated with electrophysiological aberrances, thereby resulting in arrhythmia.
According to the data, it is reasonable to conclude that fatigue caused by overwork not only induces or worsens existing diseases, but also independently leads to malignant arrhythmia, resulting in karoshi. However, the risk factors of karoshi, other than long working hours and aspects of work environment, are complicated [4]. Fatigue caused by overwork is influenced by many factors, including age, gender, physical condition, mental status, psychological conditions, personality type, life experience, and health status [38]. The complexities of the etiology and pathogenesis of karoshi present difficulties for researchers but require further attention and in-depth studies.
Conclusions
Long-term overwork disturbs heart rhythm through Cav-1/pSrc/Cx43 pathway and intensifies myocardial fibrosis, which may be one of the factors that initiate ventricular remodeling, revealing a mechanism for the speculated link among Karoshi, cardiac arrhythmias, and myocardial fibrosis.
Footnotes
Source of support: 1. The Key Laboratory of Forensic Pathology Program of Ministry of Public Security (GAFYBL201601). 2. Chinese National Natural Science Fund: 81772021
Conflict of interest
None.
References
- 1.Liu NG, Wang T, Huang P, et al. Karoshi related to labor intensity and risk of cardiovascular events: A case report. Fa Yi Xue Za Zhi. 2015;31:343–46. [PubMed] [Google Scholar]
- 2.Yamauchi T, Yoshikawa T, Takamoto M, et al. Overwork-related disorders in Japan: Recent trends and development of a national policy to promote preventive measures. Ind Health. 2017;55:293–302. doi: 10.2486/indhealth.2016-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong CW, Chan YH, Cheng YH, Lam CS. Is overwork a precipitant factor of idiopathic ventricular fibrillation? Int J Cardiol. 2016;223:218–19. doi: 10.1016/j.ijcard.2016.08.104. [DOI] [PubMed] [Google Scholar]
- 4.Eguchi H, Wada K, Smith DR. Recognition, compensation, and prevention of karoshi, or death due to overwork. J Occup Environ Med. 2016;58:e313–14. doi: 10.1097/JOM.0000000000000797. [DOI] [PubMed] [Google Scholar]
- 5.Cheng Y, Du CL, Hwang JJ, et al. Working hours, sleep duration and the risk of acute coronary heart disease: A case-control study of middle-aged men in Taiwan. Int J Cardiol. 2014;171:419–22. doi: 10.1016/j.ijcard.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 6.Kang MY, Cho SH, Yoo MS, et al. Long working hours may increase risk of coronary heart disease. Am J Ind Med. 2014;57:1227–34. doi: 10.1002/ajim.22367. [DOI] [PubMed] [Google Scholar]
- 7.Ke DS. Overwork, stroke, and karoshi-death from overwork. Acta Neurol Taiwan. 2012;21:54–59. [PubMed] [Google Scholar]
- 8.Kivimaki M, Jokela M, Nyberg ST, et al. Long working hours and risk of coronary heart disease and stroke: A systematic review and meta-analysis of published and unpublished data for 603,838 individuals. Lancet. 2015;386:1739–46. doi: 10.1016/S0140-6736(15)60295-1. [DOI] [PubMed] [Google Scholar]
- 9.Backe EM, Seidler A, Latza U, et al. The role of psychosocial stress at work for the development of cardiovascular diseases: A systematic review. Int Arch Occup Environ Health. 2012;85:67–79. doi: 10.1007/s00420-011-0643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi T, Kobayashi Y, Yamaoka K, Yano E. Effect of overtime work on 24-hour ambulatory blood pressure. J Occup Environ Med. 1996;38:1007–11. doi: 10.1097/00043764-199610000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Yoo DH, Kang MY, Paek D, et al. Effect of long working hours on self-reported hypertension among middle-aged and older wage workers. Ann Occup Environ Med. 2014;26:25. doi: 10.1186/s40557-014-0025-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uchiyama S, Kurasawa T, Sekizawa T, Nakatsuka H. Job strain and risk of cardiovascular events in treated hypertensive Japanese workers: Hypertension follow-up group study. J Occup Health. 2005;47:102–11. doi: 10.1539/joh.47.102. [DOI] [PubMed] [Google Scholar]
- 13.Kawada T. Long working hours and the risk of coronary heart disease. Am J Ind Med. 2016;59:336–37. doi: 10.1002/ajim.22524. [DOI] [PubMed] [Google Scholar]
- 14.Lee DW, Hong YC, Min KB, et al. The effect of long working hours on 10-year risk of coronary heart disease and stroke in the Korean population: The Korea National Health and Nutrition Examination Survey (KNHANES), 2007 to 2013. Ann Occup Environ Med. 2016;28:64. doi: 10.1186/s40557-016-0149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bannai A, Yoshioka E, Saijo Y, et al. The risk of developing diabetes in association with long working hours differs by shift work schedules. J Epidemiol. 2016;26:481–87. doi: 10.2188/jea.JE20150155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka M, Nakamura F, Mizokawa S, et al. Establishment and assessment of a rat model of fatigue. Neurosci Lett. 2003;352:159–62. doi: 10.1016/j.neulet.2003.08.051. [DOI] [PubMed] [Google Scholar]
- 17.Osbourne A, Calway T, Broman M, et al. Downregulation of connexin43 by microRNA-130a in cardiomyocytes results in cardiac arrhythmias. J Mol Cell Cardiol. 2014;74:53–63. doi: 10.1016/j.yjmcc.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagibin V, Egan BT, Viczenczova C, et al. Ageing related down-regulation of myocardial connexin-43 and up-regulation of MMP-2 may predict propensity to atrial fibrillation in experimental animals. Physiol Res. 2016;65(Suppl 1):S91–100. doi: 10.33549/physiolres.933389. [DOI] [PubMed] [Google Scholar]
- 19.Gutstein DE, Morley GE, Tamaddon H, et al. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res. 2001;88:333–39. doi: 10.1161/01.res.88.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang KC, Rutledge CA, Mao M, et al. Caveolin-1 modulates cardiac gap junction homeostasis and arrhythmogenecity by regulating cSrc tyrosine kinase. Circ Arrhythm Electrophysiol. 2014;7:701–10. doi: 10.1161/CIRCEP.113.001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang R, Zhang L, Jiang D, et al. Mouse organ coefficient and abnormal sperm rate analysis with exposure to tap water and source water in Nanjing reach of Yangtze River. Ecotoxicology. 2014;23:641–46. doi: 10.1007/s10646-014-1228-4. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen MN, Kiriazis H, Gao XM, Du XJ. Cardiac fibrosis and arrhythmogenesis. Compr Physiol. 2017;7:1009–49. doi: 10.1002/cphy.c160046. [DOI] [PubMed] [Google Scholar]
- 23.Darby PJ, Kwan CY, Daniel EE. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca(2+) handling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1226–35. doi: 10.1152/ajplung.2000.279.6.L1226. [DOI] [PubMed] [Google Scholar]
- 24.Schubert AL, Schubert W, Spray DC, Lisanti MP. Connexin family members target to lipid raft domains and interact with caveolin-1. Biochemistry. 2002;41:5754–64. doi: 10.1021/bi0121656. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Lin S, Choy PC, et al. The regulation of the cardiac potassium channel (HERG) by caveolin-1. Biochem Cell Biol. 2008;86:405–15. doi: 10.1139/o08-118. [DOI] [PubMed] [Google Scholar]
- 26.Massaeli H, Sun T, Li X, et al. Involvement of caveolin in low K+-induced endocytic degradation of cell-surface human ether-a-go-go-related gene (hERG) channels. J Biol Chem. 2010;285:27259–64. doi: 10.1074/jbc.M110.124909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holm H, Gudbjartsson DF, Arnar DO, et al. Several common variants modulate heart rate, PR interval and QRS duration. Nat Genet. 2010;42:117–22. doi: 10.1038/ng.511. [DOI] [PubMed] [Google Scholar]
- 28.Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. 2012;44:670–75. doi: 10.1038/ng.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kieken F, Mutsaers N, Dolmatova E, et al. Structural and molecular mechanisms of gap junction remodeling in epicardial border zone myocytes following myocardial infarction. Circ Res. 2009;104:1103–12. doi: 10.1161/CIRCRESAHA.108.190454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyon RC, Mezzano V, Wright AT, et al. Connexin defects underlie arrhythmogenic right ventricular cardiomyopathy in a novel mouse model. Hum Mol Genet. 2014;23:1134–50. doi: 10.1093/hmg/ddt508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kostin S, Rieger M, Dammer S, et al. Gap junction remodeling and altered connexin43 expression in the failing human heart. Mol Cell Biochem. 2003;242:135–44. [PubMed] [Google Scholar]
- 32.Glukhov AV, Fedorov VV, Kalish PW, et al. Conduction remodeling in human end-stage nonischemic left ventricular cardiomyopathy. Circulation. 2012;125:1835–47. doi: 10.1161/CIRCULATIONAHA.111.047274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guerrero PA, Schuessler RB, Davis LM, et al. Slow ventricular conduction in mice heterozygous for a connexin43 null mutation. J Clin Invest. 1997;99:1991–98. doi: 10.1172/JCI119367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donoghue M, Wakimoto H, Maguire CT, et al. Heart block, ventricular tachycardia, and sudden death in ACE2 transgenic mice with downregulated connexins. J Mol Cell Cardiol. 2003;35:1043–53. doi: 10.1016/s0022-2828(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 35.Xiao HD, Fuchs S, Campbell DJ, et al. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am J Pathol. 2004;165:1019–32. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang KC, Rutledge CA, Mao M, et al. Caveolin-1 modulates cardiac gap junction homeostasis and arrhythmogenecity by regulating cSrc tyrosine kinase. Circ Arrhythm Electrophysiol. 2014;7:701–10. doi: 10.1161/CIRCEP.113.001394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cunha DF, Cunha SF, Reis MA, Teixeira VP. Heart weight and heart weight/body weight coefficient in malnourished adults. Arq Bras Cardiol. 2002;78:382–87. doi: 10.1590/s0066-782x2002000400005. [DOI] [PubMed] [Google Scholar]
- 38.Finsterer J, Mahjoub SZ. Fatigue in healthy and diseased individuals. Am J Hosp Palliat Care. 2014;31:562–75. doi: 10.1177/1049909113494748. [DOI] [PubMed] [Google Scholar]