Abstract
Background:
Pre-exposure prophylaxis (PrEP) greatly reduces the risk of HIV acquisition, but its optimal delivery strategy remains uncertain. Clinics for sexually transmitted infections (STIs) can provide an efficient venue for PrEP delivery.
Methods:
To quantify the added value of STI clinic–based PrEP delivery, we used an agent-based simulation of HIV transmission among men who have sex with men (MSM). We simulated the impact of PrEP delivery through STI clinics compared with PrEP delivery in other community-based settings. Our primary outcome was the projected twenty-year reduction in HIV incidence among MSM.
Results:
Assuming PrEP uptake and adherence of 60% each, evaluating STI clinic attendees and delivering PrEP to eligible MSM reduced HIV incidence by 16% [95% uncertainty range: 14% – 18%] over 20 years, an impact that was 1.8 [1.7 – 2.0] times as great as that achieved by evaluating an equal number of MSM recruited from the community. Comparing strategies where an equal number of MSM received PrEP in each strategy (i.e., evaluating more individuals for PrEP in the community-based strategy, since MSM attending STI clinics are more likely to be PrEP eligible), the reduction in HIV incidence under the STI clinic-based strategy was 1.3 [1.3 – 1.4] times as great as that of community-based delivery.
Conclusions:
Delivering PrEP to MSM who attend STI clinics can improve efficiency and effectiveness. If high levels of adherence can be achieved in this population, STI clinics may be an important venue for PrEP implementation.
Keywords: HIV Infections, Sexually Transmitted Diseases, Pre-Exposure Prophylaxis, Homosexuality, Male, Computer Simulation
Short summary:
Clinics for sexually transmitted infections are highly effective venues for implementation of pre-exposure prophylaxis (PrEP) among men who have sex with men by improving efficiency of screening and effectiveness of PrEP.
INTRODUCTION
With approximately 40,000 new HIV infections among adolescents and adults across the United States in 2015, prevention of HIV transmission remains a national priority.1 In recent years, men who have sex with men (MSM) account for more than 60% of these infections.2 Pre-exposure prophylaxis (PrEP) is a promising prevention strategy to reduce the risk of HIV transmission and is recommended by the U.S. Centers for Disease Control and Prevention (CDC) for those at high risk of HIV infection3, including those reporting an ongoing sexual relationship with an HIV-positive male partner, unprotected anal intercourse in the past 6 months, or recent diagnosis with another sexually transmitted infection (STI). Despite its proven efficacy at the individual level4, PrEP uptake remains low5–8, in part because of challenges in implementation.9 For example, most clinicians providing HIV care are more experienced in PrEP initiation than clinicians who do not provide HIV care, but most HIV-negative MSM for whom PrEP is indicated do not get clinical care from HIV-care providers.10 One potential solution to this problem is to focus efforts to increase PrEP initiation among MSM (for whom it is indicated) in clinics for sexually transmitted infections (STIs), where such men are more likely to seek clinical care.11 Implementation of PrEP at STI clinics could both increase the efficiency of screening (as most HIV-negative MSM attendees will be eligible for PrEP) and target PrEP to the highest-risk individuals (i.e., patients who are infected with other STIs).12 However, the added value of delivering PrEP through STI clinics, in terms of population-level impact on HIV incidence and prevalence, remains unknown. We therefore extended an existing agent-based simulation model of HIV among MSM to include PrEP delivery in STI clinics.
METHODS
We calibrated our model to the MSM population of Baltimore City13, a city with an HIV epidemic concentrated among blacks. The model accounts for individual-level characteristics including age, race, geography, HIV serostatus, and (among infected men) HIV disease stage and use of antiretroviral therapy (ART). HIV natural history is modeled as consisting of three disease states representing acute, chronic and late infection (Figure 1, bottom panel). Each state carries a different average viral load that is used to determine HIV infectiousness in that state. The continuum of care is modeled as a series of individual probabilities for testing, linkage to care, ART initiation, and disengagement/re-engagement in care. HIV transmission is simulated over a network of sexual contacts consisting of stable/long-term (average duration of 4 years1) and casual/short-term (duration of one week) partnerships. Individuals can engage in a maximum of one stable and one casual partnership at any given time2. Partnerships are updated on a weekly basis. Upon reaching partnership dissolution time, a partnership is broken and individuals are eligible to engage in new partnerships with other people in the model. New partnerships are assumed as assortative according to age, race, and location. Individual’s likelihood of forming new partnerships is modeled as a function of current partnership status, individual’s sexual activity class, and age.14 HIV transmission is modeled as a per-act probability among serodiscordant partnerships according to the infected partner’s infectiousness (based on clinical stage and ART status), the uninfected partner’s PrEP status, condom use and sexual positioning (insertive/receptive anal sex). The core simulation model is calibrated against literature estimates (Table 1), publically available census data, annual HIV surveillance reports15, and the National HIV Behavioral Surveillance in Baltimore.16 For additional details regarding the model and calibration, see sections 1 and 2 of the Appendices.
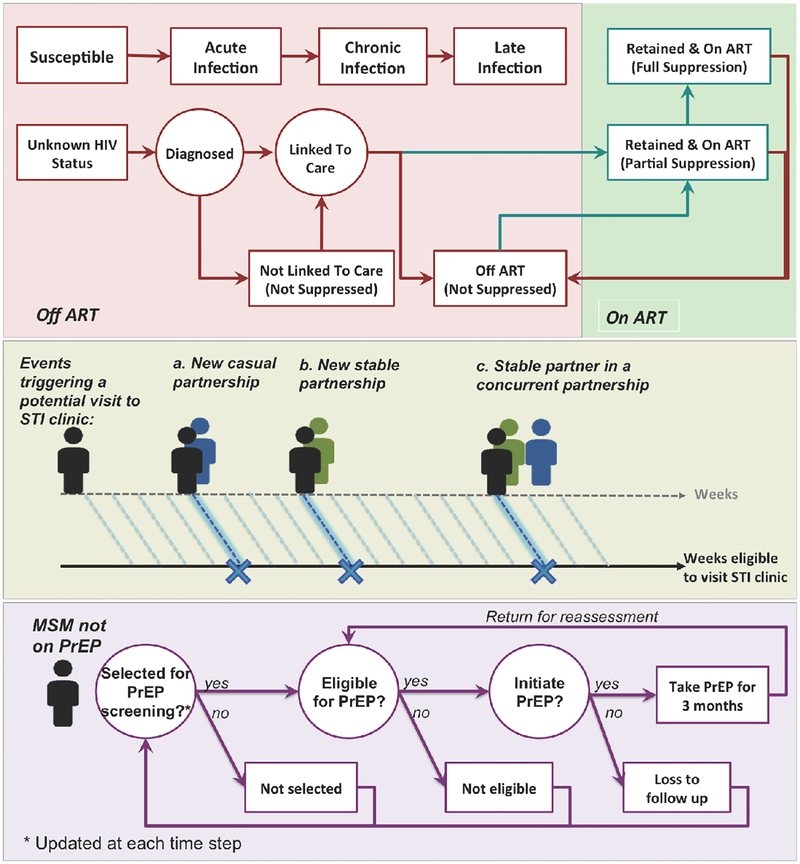
Figure 1: Simulation overview.
This figure illustrates the schematic simulation logic for modeling HIV natural history and the cascade of care (top panel), individuals’ presentation to STI clinics (middle panel) and PrEP procedure (bottom panel). Top Panel: HIV natural history is modeled through 3 main disease states associated with an increase in viral load (with parameters given in Table 1). The cascade of care – also evaluated on a weekly basis – represents processes of diagnosis, linkage to care, ART initiation, and retention in care. Middle panel: “Eligibility” for presenting to an STI clinic is evaluated at the end of each week: individuals have a defined probability of presenting to an STI clinic during any week in which they start a new partnership or their stable partner starts a concurrent partnership. Bottom panel: MSM are selected for PrEP assessment at the time of attending STI-clinics (strategy 1), or randomly from the community at large (strategy 2). The eligibility criteria for PrEP are set according to CDC guidelines. Eligible MSM who accept PrEP (according to PrEP “uptake”) initiate PrEP immediately and will experience a fixed level of protection against HIV transmission (“adherence”) while on PrEP.
Table 1: List of selected simulation parameters.
STI, sexually transmitted infection; PrEP, pre-exposure prophylaxis for HIV.
| Model Parameters | Value/[Range]1 | Reference |
|---|---|---|
| HIV disease state duration | ||
| Acute infection | [6, 9] weeks | 1S–3S |
| Chronic infection | [8, 10] years | 1S,4S |
| Late infection2 | [1, 3] years | 1S,2S,4S |
| Time from ART initiation to full viral suppression | [3, 6] months | 5S |
| Time from ART discontinuation to pre-ART CD4 nadir3 | [3, 9] months | 6S–9S |
| HIV mortality rate, acute and chronic HIV, no ART | 5 per 1000 person years | 10S–12S |
| Reduction in HIV mortality due to ART | 0.58 * Mortality rate in chronic state | |
| Probability of ART discontinuation | (20%,50%,90%) by the end of (1st,2nd, 8th) year, 50% per year afterward4 | 13S |
| Average viral load (log10 copies/mL) | ||
| Acute, no ART | 6.5 | |
| Chronic, no ART | 4.5 | |
| Late, no ART | 5 | |
| On ART, partially suppressed | 3.5 | |
| On ART, fully suppressed | 1.5 | |
| Infectiousness per sexual contact | 2.45(log(VL)-4.5) | 1S |
| Annual number STI clinic visits among MSM | 966 | 1S |
| PrEP reassessment period | 3 months | 14S |
| PrEP uptake | 60% [0–100%] | |
| PrEP adherence5 | 60% [0–100%] |
Values generated via uniform distributions over the specified range unless stated otherwise
Duration reflects the mortality rate due to late HIV disease
Infectiousness assumed equal to that of the chronic state
Values adjusted via a simulation coefficient (p=0.7) for calibration to Baltimore data
PrEP is 100% effective if adhered to
We incorporated a simulated STI clinic into the model above, as the point of PrEP delivery to HIV-negative MSM. Our research aim is to assess the population-level impact of PrEP delivery in STI clinics on HIV incidence. Our primary outcome is the percent reduction in HIV incidence among MSM after implementation of PrEP in the modeled STI clinic (“Strategy 1”), measured relative to a baseline with no PrEP and also against a PrEP delivery strategy implemented in the community at large (“Strategy 2”).
Strategy 1: STI clinic-based PrEP delivery
Individual MSM may present to STI clinics for a variety of reasons, including symptoms of STIs other than HIV (e.g., penile discharge) or general concern about their sexual health. While we are not modeling other STIs explicitly, we assume that such infections can be acquired from new or concurrent sexual partnerships.17–19 Consequently, we let the STI clinic visits occur after one of two events: (a) starting a new (stable or casual) partnership, (b) one’s stable partner engaging in a concurrent partnership with another individual (Figure 1, top panel). After each of these events, individual MSM experience a defined probability of seeking care in the STI clinic. We estimated this probability based on the reported number of annual MSM visits to the two public STI clinics in Baltimore City.
Upon presentation at the STI clinic, MSM are assessed for PrEP eligibility, and eligible MSM who accept PrEP (net uptake [initiation – immediate discontinuation] ranging 0% to 100%) are assumed to start PrEP immediately. According to CDC guidelines, we assume that all HIV-negative MSM reporting a casual partnership in the last six months or those in a stable sexual partnership with an HIV positive person are eligible to receive PrEP.3 Those on PrEP are assumed to return every 3 months for eligibility reassessment3 with those who no longer meet the eligibility criteria discontinuing PrEP. We model adherence3 to PrEP as the percentage of days on which the individual is protected against infection, ranging from 0% to 100%.
Strategy 2: Community-based PrEP delivery
In order to evaluate the added value of STI clinic-focused PrEP delivery, we compare this strategy against a comparison scenario in which PrEP is delivered to MSM selected at random from the community (e.g., among attendees of community-based events unrelated to STI clinic visits). This comparison scenario was designed primarily to approximate a baseline strategy for PrEP delivery to MSM against which to quantify the added value of STI clinic-based delivery (or any other targeted PrEP delivery strategy). In this strategy, all MSM, regardless of their partnership history, experience a defined weekly probability of being evaluated for PrEP. Those selected are assessed for PrEP eligibility according to the same criteria as above, and in order to isolate the effect of screening high-risk populations alone, eligible MSM in the community-based strategy were assumed to have received the same clinical evaluation and follow-up support enabling them to initiate PrEP with the same levels of uptake and adherence as in Strategy 1.
Simulation and analytic approach
In the absence of PrEP, we first develop a large series of independent simulations. Each simulation is first carried out over a “burn-in” phase until equilibrium is reached among a population equal in size and HIV prevalence to Baltimore’s estimated 3,300 MSM living with HIV in 2014.15 After this baseline experience without PrEP is achieved, we carry each simulation forward for an additional 20 years under each PrEP strategy described above.
We compare the community-based strategy to the STI clinic-based strategy in two ways: (a) assuming the same number of MSM are assessed for PrEP eligibility every year under each strategy, and (b) assuming the same number of MSM start PrEP every year under each strategy (i.e., more assessments in the community-based strategy since fewer people are eligible to receive PrEP than in the STI clinic-based strategy). The analysis is repeated for different levels of PrEP adherence (range [0%-100%]) and uptake (range [0%-100%]) in each PrEP strategy. For every adherence/uptake combination, we perform roughly 500 independent simulations. The number of simulation replications was selected to provide a precision of at worst +/-5% around each main simulation outcome. The primary outcomes are the projected reduction in HIV incidence and prevalence, relative to the baseline levels in the absence of PrEP. The relative impact of STI clinic-based delivery was calculated as the mean reduction in incidence over simulations with STI clinic-based delivery, divided by the mean reduction in incidence over those same simulations, assuming community-based PrEP delivery.
RESULTS
Population overview
At baseline, the simulation models a population of 15,000 MSM aged 15—75 years (median 33 [95% uncertainty range: 32 – 35]). HIV prevalence was calibrated to 23% of the MSM population at baseline15, which corresponded to an average incidence of 213 [212 – 214] cases per year. Within this population, we estimated an average of 966 annual visits to STI clinics (one visit per 15 person-years), based on unpublished data (personal communication, Susan Tuddenham, Baltimore City Health Department) from 2011 to 2015 indicating a combined total of 4834 visits to STI clinics by men who identified their sexual orientation as same sex or bisexual. Our model estimated that 54% [48% – 58%] of annual visits to the STI clinic were made by MSM younger than 30 years old, although these individuals accounted for only 39% [35% – 42%] of the general MSM population. Overall, 69% [66% – 72%] of all simulated STI clinic attendees were HIV-negative, 23% [21% – 26%] were in a serodiscordant partnership (compared to 11% [10% – 12%] of the general MSM population), and 90% [88% – 92%] reported at least one casual partnership in the six months (compared to 64% [63% – 65%] of the general population).
STI clinic-based versus community-based PrEP: Equal numbers evaluated
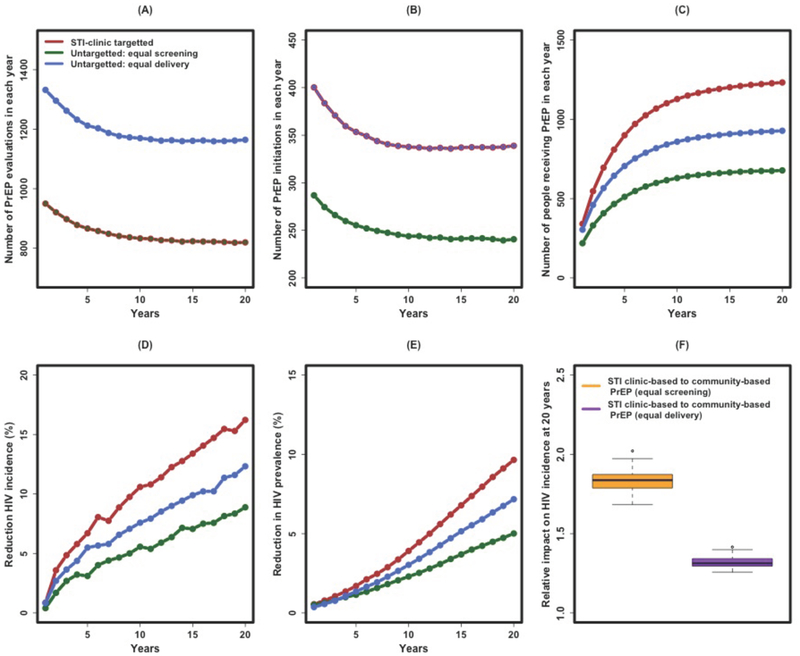
At baseline and in the absence of PrEP, 51% [49% – 52%] of simulated MSM were eligible to receive PrEP. Assuming 60% adherence and 60% uptake in both strategies, PrEP delivery through STI clinics was projected to reduce HIV incidence by 16% [14% – 16%] over 20 years (Figure 2D, red line). This reduction was 1.8 [1.7 – 2.0] times as great as that achieved by randomly screening an equal number of MSM for PrEP (Figure 2D, green line, and Figure 2F, green box). This reflects higher likelihood of PrEP eligibility among STI clinic attendees (63% [60% – 65%]), resulting in a higher rate of PrEP initiation (Figure 2B) and coverage over time, such that by the end of 20 years, a projected 1232 [1155 – 1315] MSM were on PrEP under the STI clinic delivery strategy (corresponding to 10.6% of all HIV-negative MSM) compared to 678 [626 – 734] (corresponding to 5.9% of all HIV-negative MSM) under the community engagement approach (Figure 2C).
Figure 2: Impact of STI clinic-based versus community-based PrEP delivery.
Shown on the y-axes are the projected annual number of MSM screened for PrEP (A), initiating PrEP (B), and receiving PrEP (C) in a given year, as well as the projected reduction in HIV incidence (D), reduction in HIV prevalence (E), and the relative impact of STI clinic-based PrEP versus community-based PrEP on HIV incidence at 20 years (F). For Panels A through E, the quantities are shown in each year after enacting a PrEP campaign of sufficient size to evaluate all MSM estimated to present to the STI clinic every year (966 visits per year, red line), a comparable number of MSM randomly screened for PrEP every year (green line), or a comparable number of eligible MSM randomly starting PrEP (blue line) in any given week of the program. Thus, in Panel A, the red and green lines overlap (equal number of MSM screened for PrEP every year), and in Panel B, the red and blue lines overlap (equal number initiating PrEP every year). Panel F depicts the relative impact of STI clinic-based PrEP versus community-based PrEP, measured as the projected reduction in HIV incidence at the 20th year of implementation. These figures assume 60% PrEP uptake and 60% adherence to PrEP once initiated. STI, sexually transmitted infection; PrEP, pre-exposure prophylaxis for HIV.
STI clinic-based versus community-based PrEP: Equal numbers receiving PrEP
We also compared the STI clinic-based PrEP campaign to a community-based strategy that was assumed to screen more individuals, enough to place the same number of MSM on PrEP in each week (Figure 2, blue lines). Compared against this scenario, the STI clinic-based strategy reduced HIV incidence 1.3 [1.3 – 1.4] times as great as than the community-based comparison (Figure 2F, blue box). Despite an equal number of MSM initiating PrEP in both strategies (Figure 2B), implementation of PrEP at the STI clinic nevertheless resulted in a higher number of MSM on PrEP over time (Figure 2C): 1232 [1155 – 1315] MSM on PrEP at 20 years (as above) versus 928 [869 – 987] (corresponding to 8.1% of all HIV-negative MSM) in the community-based comparison, reflecting the longer duration of PrEP eligibility (estimated at 2.04 [0.26 – 8.07] years) among STI clinic attendees (a younger and more sexually active population) compared to MSM in the general population (i.e., estimated at 1.53 [0.26 – 7.0] years).
Sensitivity analysis
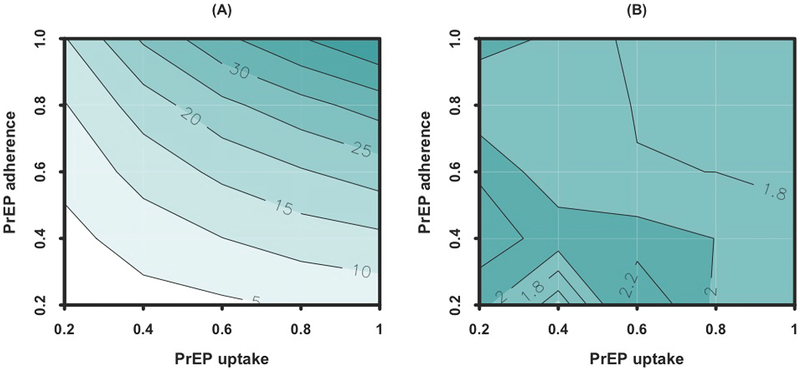
The population-level impact of PrEP delivery was sensitive to uptake and adherence (Figure 3A). For example, the reductions in HIV incidence after 20 years of implementing an STI clinic-based campaign with 80% uptake and 80% adherence, 40% uptake and 80% adherence, and 80% uptake and 40% adherence were projected at 28% [27% – 29%], 17% [16% – 18%] and 12% [11% – 14%] respectively. The impact of PrEP delivery via STI clinics was largely proportional to the number of individuals who successfully took PrEP, but was limited to the population of MSM presenting to STI clinics. Even at 100% uptake and adherence, STI clinic-based PrEP was projected to reduce HIV incidence by 47% [47% – 48%], reflecting the fact that half of HIV infections occur in people who do not access (or stay connected to) STI clinics. The relative impact of an STI clinic-based PrEP delivery strategy over community-based PrEP did not vary greatly at different levels of uptake and adherence (Figure 3B).
Figure 3: Sensitivity analysis of the impact of STI clinic-based PrEP delivery on HIV incidence according to uptake and adherence.
Panel A shows the percent reduction in HIV incidence after 20 years of STI clinic-based PrEP delivery, as a function of PrEP uptake (on the x-axis) and adherence (on the y-axis, modeled as the percentage of days with immunity to HIV infection). Panel B represents the relative impact of an STI clinic-based PrEP delivery strategy over community-based PrEP, assuming equal levels of screening (equivalent to the green scenarios in Figure 2).
In one-way sensitivity analysis, the projected impact of PrEP targeted to those attending STI clinics (primary scenario) was sensitive to variation of parameters relating to sexual activity (including the probabilities of starting new casual partnerships and the level of sexual activity in the most sexually active class). Under fixed levels of screening, the relative impact of STI clinic-based versus community-based PrEP delivery was sensitive to one-way variation in transmission related parameters (e.g., probability of condom use, per act risk of transmission) and was reduced as the force of infection was increased in the general population. However, this sensitivity was not observed at similar levels of PrEP delivery (see section 4 of the Appendices).
DISCUSSION
We have used an agent-based model of HIV epidemic in a simulated population of MSM calibrated to Baltimore City, Maryland (with regard to population size, HIV epidemiology, HIV risk factors, and STI-related medical care environment) to demonstrate that STI clinics can provide an efficient venue for PrEP services. Specifically, compared against a simulated community engagement approach, delivering PrEP to the same number of MSM attending STI clinics could nearly double the impact on HIV incidence. This reflects that MSM recruited from STI clinics for PrEP evaluation are: (1) more likely than those who are selected from community settings to be eligible for PrEP, and (2) more likely to engage in HIV-related high-risk behaviors that lead to future HIV infection. If STI clinics can be made the preferred source of PrEP for MSM, then STI clinics can have a substantial impact on HIV prevention in cities like Baltimore, Maryland.
A number of key findings from this analysis are worth highlighting. First, PrEP delivery must be sustained for about 20 years to have the sizeable community-level impact on HIV incidence and prevalence found in this study (Figure 2). Sustaining PrEP delivery in STI clinics or other community settings will require sustained engagement of patients and continued training of providers in adhering to PrEP guidelines. Second, about half of the added value of STI clinic-based PrEP delivery comes from the increased efficiency of screening, with the remainder derived from the higher risk profile of STI clinic attendees (see Figure 2F). Third, the relative impact of STI clinic-based delivery did not differ substantially by adherence, number of people presenting to care, or uptake – suggesting that this may be an efficient strategy for PrEP delivery regardless of these characteristics in any underlying population. Finally, STI clinic-based PrEP campaigns of sufficient scope (either large numbers of individuals started on PrEP, or maintained for long times) can have a major impact on HIV incidence and prevalence in high-risk MSM communities – up to a 30% reduction or more. If combined with programs to augment ART delivery and strengthen the continuum of HIV care, high-efficiency PrEP delivery may advance efforts to end local HIV epidemics.
Previous studies have evaluated PrEP delivery in various settings – including STI clinics11,20, primary care clinics21, and community-based organizations with linkage to clinical services.22 Each setting faces operational challenges.23 For example, while STI clinics provide access to a high-risk population, most do not offer primary care services and may lack an established system for ongoing clinical monitoring. Primary care clinics can provide ongoing care, but may be much less efficient in identifying eligible individuals, and general practitioners may have relatively little experience in prescribing and monitoring PrEP.24 While our results suggest that STI clinic-based PrEP delivery may lead to substantial gains in efficiency and impact, these gains are conditional on successful linkage, effective maintenance of adherence, and ongoing clinical monitoring. For example, only 5% of PrEP eligible patients identified at the primary STI clinic in Denver, Colorado, ultimately accessed PrEP services at the main referral clinic and filled at least one PrEP prescription.25 In our model, we assumed that those receiving PrEP also received full clinical monitoring over time (i.e., undergoing a 3-month reassessment of eligibility to continue PrEP). In the absence of this assumption, our results may overestimate the population impact of PrEP-delivery via STI clinics. Future research should evaluate innovative models of PrEP delivery, such as initiation in STI clinics with immediate linkage either to PrEP clinics with higher volumes of PrEP delivery, or to primary care clinics with convenient locations and good reputations within the local MSM community.
As with any modeling analysis, our findings are limited by necessary simplifying assumptions. In the absence of other STIs, we modeled the probability of presentation at the STI clinic as a function of recent sexual partnerships, as relevant in the case of STIs with short incubation periods such as gonorrhea, chlamydia, or herpes. We may therefore overestimate the efficiency of STI clinics in identifying PrEP-eligible MSM. On the other hand, infection with STIs other than HIV can increase the risk of HIV infection (in the absence of PrEP); this was not explicitly included in our analysis. Furthermore, our primary findings may be conservatively biased, to the extent that STI clinic attendance predicts higher sexual activity in the future. These results also assume full retention in PrEP care, which may be particularly challenging for high-risk subpopulations. Future studies should incorporate more explicit representation of the co-dynamics of other STIs and HIV, as well as potential correlations between STI clinic attendance and level of sexual activity to assess more fully the relationship between STI control and HIV prevention through PrEP. We also assumed immediate PrEP uptake by eligible individuals, with fixed levels of ongoing adherence. Previous studies have suggested heterogeneous patterns of PrEP uptake and adherence among high-risk MSM26–29, but also that PrEP awareness was associated with increased uptake in the STI clinic setting.20,24 Furthermore, in absence of informative data on differential rates of PrEP discontinuation among MSM attending STI clinics versus general community, we did not include the likelihood of PrEP discontinuation in our primary analysis. However, additional sensitivity analysis suggested that the relative impact of STI-clinic targetted PrEP compared to community-based screening is not sensitive to variation in rate of dropout (see Section 4.3 in the Appendices). As more empirical data from such implementation studies become available, future studies can incorporate more realistic models of the PrEP cascade and investigate scenarios for improving PrEP awareness, linkage, uptake, adherence and discontinuation.25 Moreover, as new data emerge on the natural history of HIV (e.g., higher viral load and relatively short duration of acute infection30), our modeling results can be refined. Finally, in the absence of individual-level data, our results are limited by simplifying assumptions used in modeling sexual partnership networks (such as limited level of partnership concurrency) and calibration to aggregate data available via public sources. In this sense, our modeling results should not be interpreted as evaluating specific interventions in Baltimore, but rather as illustrative of general principles regarding the efficiency of PrEP delivery through STI clinics as key venues.
In conclusion, PrEP has the potential to dramatically decrease the risk of HIV acquisition on both the individual and community levels. Delivering PrEP at STI clinics can nearly double its relative impact on incidence by improving the efficiency of screening and targeting a population at particularly high risk of HIV acquisition. Future research should evaluate the impact of linkage and retention of these individuals in PrEP care, the co-dynamics of HIV with other STIs, and the combined impact of intervention packages that include STI clinic-based PrEP delivery. Ultimately, by quantifying the likely impact of this intervention in high-risk MSM communities, these results can help local decision makers appropriately prioritize clinical venues for PrEP delivery including STI clinics.
Supplementary Material
Acknowledgments
Funding: This work was supported by U.S. Centers for Disease Control and Prevention (Enhancing Models of HIV, Viral Hepatitis, STIs, and Tuberculosis to Inform and Improve Public Health Impact, U38PS004646) – [PK,MSS,ESR,JP,DWD]
This work was supported by Johns Hopkins University Center for AIDS Research (P30AI094189) – [PK]
Footnotes
Random partnership durations generated through a geometric distribution with mean of 4 years.
An assumption based on data from Baltimore City suggesting that fewer than 10% of MSM report having more than one primary partnership in the preceding 12 months.16
While adherence, as defined here, is not empirically measurable, it may be estimated, for example, using serum drug levels. Since we do not explicitly model discontinuation within each 3-month period, adherence as defined for this exercise will be lower than what might be measured among cohorts of MSM who have not discontinued PrEP.
Conflict of interest: All authors report no potential conflicts of interest.
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the Center for Disease Control and Prevention.
REFERENCES
- 1.Centers for Disease Control and Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas, 2015. HIV Surveill Rep. 2015;27. [Google Scholar]
- 2.Centers for Disease Control Prevention. HIV Among Gay and Bisexual Men [Internet]. 2015. Available from: http://www.cdc.gov/hiv/group/msm/
- 3.Centers for Disease Control Prevention. Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2014 Clinical Practice Guideline. 2014. [Google Scholar]
- 4.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnold EA, Hazelton P, Lane T, Christopoulos KA, Galindo GR, Steward WT, et al. A qualitative study of provider thoughts on implementing pre-exposure prophylaxis (PrEP) in clinical settings to prevent HIV infection. PLoS One. 2012;7(7):e40603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saberi P, Gamarel KE, Neilands TB, Comfort M, Sheon N, Darbes LA, et al. Ambiguity, ambivalence, and apprehensions of taking HIV-1 pre-exposure prophylaxis among male couples in San Francisco: a mixed methods study. PLoS One. 2012;7(11):e50061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuchs JD, Sobieszczyk ME, Madenwald T, Grove D, Karuna ST, Andrasik M, et al. Intentions to use preexposure prophylaxis among current phase 2B preventive HIV-1 vaccine efficacy trial participants. J Acquir Immune Defic Syndr. 2013;63(3):259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krakower DS, Mimiaga MJ, Rosenberger JG, Novak DS, Mitty JA, White JM, et al. Limited awareness and low immediate uptake of pre-exposure prophylaxis among men who have sex with men Using an internet social networking site. PLoS One. 2012. January;7(3):e33119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansergh G, Koblin BA, Sullivan PS. Challenges for HIV Pre-Exposure Prophylaxis among Men Who Have Sex with Men in the United States. PLoS Med. Public Library of Science; 2012. August 21;9(8):e1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remien RH, Bauman LJ, Mantell JE, Tsoi B, Lopez-Rios J, Chhabra R, et al. Barriers and facilitators to engagement of vulnerable populations in HIV primary care in New York City. J Acquir Immune Defic Syndr [Internet]. NIH Public Access; 2015. May 1 [cited 2017 Oct 27];69 Suppl 1(0 1):S16–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25867774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen SE, Liu AY, Bernstein KT, Philip S. Preparing for HIV pre-exposure prophylaxis: lessons learned from post-exposure prophylaxis. Am J Prev Med. 2013. January;44(1 Suppl 2):S80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barash EA, Golden M. Awareness and use of HIV pre-exposure prophylaxis among attendees of a seattle gay pride event and sexually transmitted disease clinic. AIDS Patient Care STDS. 2010;24(11):689–91. [DOI] [PubMed] [Google Scholar]
- 13.Kasaie P, Pennington J, Berry SA, Shah MS, German D, Flynn CP, et al. The impact of pre-exposure prophylaxis among men who have sex Withmen in Baltimore City: An agent-based simulation. AIDS (In submission). 2015; [Google Scholar]
- 14.Beyrer C, Baral SD, van Griensven F, Goodreau SM, Chariyalertsak S, Wirtz AL, et al. Global epidemiology of HIV infection in men who have sex with men. Lancet. 2012;380(9839):367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maryland Department of Health and Mental Hygiene, MDHMH. 2012 Baltimore City Annual HIV Epidemiological Profile. Baltimore; 2012. [Google Scholar]
- 16.German D BESURE Study: National HIV Behavioral Surveillance Study in Baltimore [Internet]. Available from: http://www.jhsph.edu/faculty/research/map/US/1413/8192 [Google Scholar]
- 17.Rosenberg M, Gurvey J, Adler N. Concurrent sex partners and risk for sexually transmitted diseases among adolescents. Sex Transm Dis. 1999;264(208–212). [DOI] [PubMed] [Google Scholar]
- 18.Drumright L, Gorbach P. Do people really know their sex partners?: Concurrency, knowledge of partner behavior, and sexually transmitted infections within partnerships. Sex Transm Dis. 2004;31(7):437–42. [DOI] [PubMed] [Google Scholar]
- 19.Gorbach P, Drumright L. Discord, discordance, and concurrency: comparing individual and partnership-level analyses of new partnerships of young adults at risk of sexually transmitted. Sex Transm. 2005; [DOI] [PubMed] [Google Scholar]
- 20.Cohen SE, Vittinghoff E, Bacon O, Doblecki-Lewis S, Postle BS, Feaster DJ, et al. High interest in preexposure prophylaxis among men who have sex with men at risk for HIV infection: baseline data from the US PrEP demonstration project. J Acquir Immune Defic Syndr. 2015;68(4):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norton WE, Larson RS, Dearing JW. Primary care and public health partnerships for implementing pre-exposure prophylaxis. Am J Prev Med. 2013;44(1 Suppl 2):S77–9. [DOI] [PubMed] [Google Scholar]
- 22.Hosek SG. HIV pre-exposure prophylaxis diffusion and implementation issues in nonclinical settings. Am J Prev Med. 2013;44(1 Suppl 2):S129–32. [DOI] [PubMed] [Google Scholar]
- 23.Marcus JL, Volk JE, Pinder J, Liu AY, Bacon O, Hare CB, et al. Successful implementation of HIV preexposure prophylaxis: lessons learned from three clinical settings. Curr HIV/AIDS Rep. 2016; [DOI] [PubMed] [Google Scholar]
- 24.Liu A, Cohen S, Follansbee S, Cohan D, Weber S, Sachdev D, et al. Early experiences implementing pre-exposure prophylaxis (PrEP) for HIV prevention in San Francisco. PLoS Med. 2014;11(3):e1001613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marx GE, Bhatia R, Rietmeijer CA. An opportunity too good to miss: implementing human immunodeficiency virus preexposure prophylaxis in sexually transmitted diseases clinics. Sex Transm Dis. 2016;43(4):266–7. [DOI] [PubMed] [Google Scholar]
- 26.Aghaizu A, Mercey D, Copas A, Johnson AM, Hart G, Nardone A. Who would use PrEP? Factors associated with intention to use among MSM in London: a community survey. Sex Transm Infect. 2013;89(3):207–11. [DOI] [PubMed] [Google Scholar]
- 27.Lorente N, Fugon L, Carrieri MP, Andreo C, Le Gall J-M, Cook E, et al. Acceptability of an “on-demand” pre-exposure HIV prophylaxis trial among men who have sex with men living in France. AIDS Care. 2012;24(4):468–77. [DOI] [PubMed] [Google Scholar]
- 28.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu AY, Cohen SE, Vittinghoff E, Anderson PL, Doblecki-Lewis S, Bacon O, et al. Preexposure prophylaxis for HIV infection integrated with municipal- and community-based sexual health services. JAMA Intern Med. 2016;176(1):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robb ML, Eller LA, Kibuuka H, Rono K, Maganga L, Nitayaphan S, et al. Prospective study of acute HIV-1 infection in adults in east Africa and Thailand. N Engl J Med [Internet]. 2016. June 2 [cited 2016 Jun 13];374(22):2120–30. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1508952 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.