Abstract
Background:
Immediate open repair of acute type A aortic dissection (ATAAD) is traditionally recommended to prevent death from aortic rupture. However, organ failure due to malperfusion syndrome (MPS) might be the most imminent life-threatening problem for a subset of patients.
Methods:
From 1996–2017, among 597 ATAAD patients, 135 patients with MPS were treated with upfront endovascular reperfusion (fenestration/stenting) followed by delayed open repair (OR). We compared outcomes between the first and second decade, as well as observed mortalities with those expected with an “upfront OR for every patient” approach, determined using prognostic models from the literature (Verona, Leipzig-Halifax, Stockholm, Penn, and GERAADA models).
Results:
Overall, in-hospital mortality improved between the two decades (21.0% vs. 10.7%, p<0.001). In the second decade, for MPS patients initially treated with fenestration/stenting, mortality from aortic rupture decreased from 16% to 4% (p=0.05), the risk of dying from organ failure was 6.6 times higher than dying from aortic rupture (hazard ratio = 6.63, 95%CI 1.5–29, p=0.01), and 30-day mortality after OR for MPS patients was 3.7%. Compared to the expected mortalities with the “upfront OR for every patient” models, our observed 30-day and in-hospital mortalities (9% and 11%, respectively) of all ATAAD patients were significantly lower (p≤0.03).
Conclusions:
Immediate open repair is the strategy to prevent death from aortic rupture for the majority of ATAAD patients. However, relatively stable (no rupture, no tamponade) patients with MPS benefit from a staged approach: upfront endovascular reperfusion followed by open aortic repair at resolution of organ failure.
Related to an abstract (“Malperfusion Syndrome Management in Acute Type A Aortic Dissection: Two-Decade Experience”) presented at the AHA Scientific Sessions 2017 (Anaheim, CA, Nov 2017).
Keywords: aortic dissection, acute aortic syndrome, acute cardiac care, aortic disease, aortic surgery, endovascular fenestration/stenting, malperfusion, malperfusion syndrome
INTRODUCTION
Acute type A aortic dissection (ATAAD) patients presenting with organ necrosis and failure due to dissection-related malperfusion (malperfusion syndrome or MPS) have a worse prognosis with operative mortality ranging between 25%−45%1–16. Classic teaching1,4,17,18 predicates that every patient with ATAAD (even with MPS) be treated with immediate aortic repair to prevent death from aortic rupture, with the assumption that stabilizing the true lumen through aortic repair resolves the peripheral organ malperfusion. However, end-organ failure from MPS might be the most immediate life-threatening problem for relatively stable patients presenting with no evidence of aortic rupture or tamponade. Moreover, malperfusion due to static obstruction of branch vessels (such as thrombosis) may not be resolved by open aortic repair alone19,20.
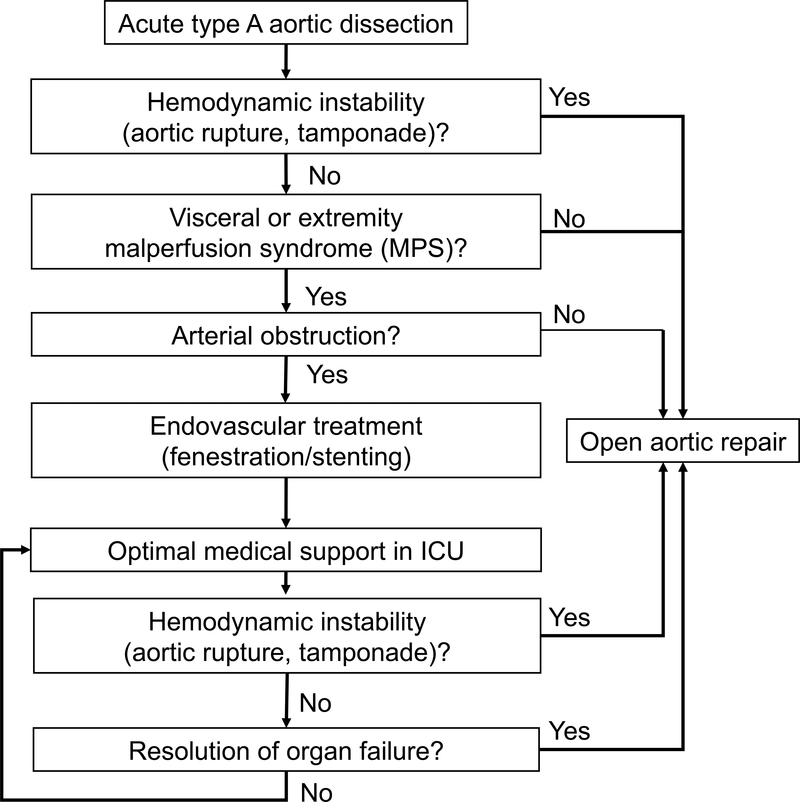
In 1996, the University of Michigan adopted a new paradigm of care for MPS patients21 (Figure 1), entailing upfront percutaneous endovascular revascularization through fenestration and/or stenting of the critically malperfused abdominal organs and/or extremities by interventional radiology (IR), followed by delayed open repair (OR) of the dissected proximal aorta. In 2008, we reported outcomes from the first decade (1997–2007) and found a significant improvement in operative mortality of ATAAD patients with MPS22. Other centers have subsequently adopted similar strategies to treat patients with MPS11,23–27. However, a staged approach does put patients at risk for aortic rupture while awaiting resolution of malperfusion-related organ failure prior to OR. Over the last decade, we continued to modify our strategy to improve clinical outcomes.
Figure 1. Algorithm for clinical decision making.
All patients with acute type A aortic dissection were managed through this algorithm, and only patients with MPS (tissue/organ necrosis and dysfunction) but no aortic rupture or cardiac tamponade were treated with upfront endovascular revascularization by interventional radiology followed by delayed open aortic repair.
ICU = intensive care unit. MPS = malperfusion syndrome.
In this study, we focus on the modifications in our strategy and outcomes in the second decade (2008–2017). We hypothesized that the observed mortality would be lower than the expected mortality following an “upfront surgical repair for every ATAAD patient” approach, according to prognostic models from the literature.
METHODS
The de-identified data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure at Michigan Medicine.
Patient population
This study was approved by the Institutional Review Board at the University of Michigan (Michigan Medicine, Ann Arbor, MI).
All patients (n=602) who presented to the University of Michigan with an ATAAD (defined as onset in ≤14 days of admission) between July 1996 and January 2017 were identified. Four patients received no form of intervention (i.e., endovascular fenestration/stenting or OR): 2 patients died after presentation to the emergency department, 1 patient presented with severe multiorgan failure and refused further intervention, and 1 patient suffered a massive hemorrhagic stroke and received palliative measures until demise. One patient underwent successful thoracic endovascular aortic repair of a retrograde ATAAD. Therefore, 597 patients were included in the analysis, including 243 patients from the first decade (1996–2007) and 354 patients from the second decade (2008–2017).
We obtained Society of Thoracic Surgeons (STS) data elements from the University of Michigan Cardiac Surgery Data Warehouse to identify the cohort and determine pre-, intra-, and post-operative characteristics. The National Death Index database through December 31, 2015 and medical record review were utilized to collect information about survival and other supplemental data.
Definition of malperfusion syndrome (MPS)
We defined malperfusion as inadequate blood flow to the end organs due to dissection-related obstruction of the aorta and/or its branches; and MPS as tissue necrosis and functional failure of vital organs (such as viscera or lower extremity) secondary to late stage malperfusion. The diagnosis of MPS requires both clinical features and laboratory findings (e.g., abdominal pain and tenderness to palpation, decreased urine output, elevated lactate, liver or pancreatic enzymes, bilirubin or creatinine, absence of peripheral pulses, motor or sensory deficit of the extremity, neurologic deficit) compatible with end-organ failure as well as radiographic findings demonstrating dynamic or static findings consistent with low or absent blood flow to the damaged end organs. Patients diagnosed with visceral and/or extremity MPS were considered candidates for endovascular fenestration/stenting unless there was an aortic rupture or cardiac tamponade.
Indications for IR procedures
The indications for angiographic evaluation of patients for endovascular fenestration/stenting in IR are clinical evidence of tissue/organ necrosis and dysfunction (MPS), not just malperfusion of branch vessels on imaging; specifically 1. celiac artery malperfusion on CT angiogram (CTA), plus abdominal pain, and increased liver enzymes; 2. superior mesenteric artery (SMA) malperfusion on CTA, plus abdominal pain, bloody diarrhea, or increased serum lactate and metabolic acidosis; 3. iliac or femoral artery malperfusion on CTA, plus loss of sensation and motor function of extremities, and loss of radial or femoral pulse, with or without increased serum lactate or creatinine kinase (CK). Frequently, patients have multiple vascular beds of malperfusion. As long as there is suspected MPS of any end organ (necrosis and dysfunction of the organ), we perform fenestration/stenting first. Isolated renal MPS used to be an indication but not in current practice. Isolated coronary artery, cerebral, and spinal cord MPS in ATAAD were not indications for IR and were treated with emergent open aortic repair. It should be noted that unlike leg malperfusion, where physical exam is available, or renal malperfusion, where abnormal renal enhancement suggests possible renal malperfusion, SMA malperfusion can be relatively hard to diagnose until hours after onset. The indications for angiographic treatment of ATAAD patients with fenestration/stenting during the IR procedure was angiographic, intravascular ultrasound (IVUS), and manometric evidence for ongoing arterial obstruction.
Over 2 decades, of 597 patients with ATAAD, 419 presented without MPS and 178 presented with MPS (Table 1): 43 had immediate OR because of hemodynamic instability from aortic rupture or cardiac tamponade (n=7) or isolated neurological or coronary malperfusion (n=36). The remaining 135 patients underwent upfront endovascular fenestration/stenting for suspected visceral or extremity MPS, including isolated celiac/mesenteric MPS 13 (10%), isolated renal MPS 11 (8%), isolated extremity MPS 15 (11%), and multiple sites of MPS 96 (71%).
Table 1.
General differences and initial management (all patients)
| Both decades (n = 597) | 1st decade (n = 243) | 2nd decade (n = 354) | p value (1st vs. 2nd decade) | |
|---|---|---|---|---|
| ATAAD patients/year | 26 (range: 12–59) | 17 (range: 12–46) | 41 (range: 26–59) | 0.004 |
| All patients with malperfusion syndrome, any type | 178 (30) | 93 (38) | 85 (24) | <0.001 |
| Type of MPS | 0.003 | |||
| Coronary | 17 (2.8) | 5 (2.1) | 12 (3.4) | |
| Cerebral | 31 (5.2) | 9 (3.7) | 22 (6.2) | |
| Spinal | 15 (2.5) | 7 (2.9) | 8 (2.3) | |
| Celiac/hepatic | 16 (2.7) | 11 (4.5) | 5 (1.4) | |
| Mesenteric | 82 (14) | 52 (21) | 30 (8.5) | |
| Renal | 74 (12) | 47 (19) | 27 (7.6) | |
| Lower extremity | 78 (13) | 48 (20) | 30 (8.5) | |
| Upper extremity | 1 (0.2) | 0 (0) | 1 (0.3) | |
| IR-amenable MPS | 135 (23) | 86 (35) | 49 (14) | < 0.001 |
| Type of IR-amenable MPS | 0.94 | |||
| Celiac/hepatic | 16 (12) | 11 (13) | 5 (10) | |
| Mesenteric | 82 (61) | 52 (60) | 30 (61) | |
| Renal | 74 (55) | 47 (55) | 27 (55) | |
| Extremity | 79 (59) | 48 (56) | 31 (63) | |
| Patients who underwent IR | 135 (23) | 86 (35) | 49 (14) | < 0.001 |
| Therapeutic IR | 112 (83) | 67 (78) | 45 (92) | 0.055 |
| Non-therapeutic IR | 23 (17) | 19 (22) | 4 (8.2) | |
| Time from IR to aortic rupture (days) | 2 (1–4) | 2.5 (2–4) | 0 (0–0) | 0.03 |
| Time from IR to OR (days) | 3 (1–12) | 4 (1–15.5) | 2 (1–6.5) | 0.19 |
| IR and/or OR | < 0.001 | |||
| IR only | 52 (8.7) | 30 (12) | 22 (6.2) | |
| Both IR and OR | 83 (14) | 56 (23) | 27 (7.6) | |
| OR only | 462 (77) | 157 (65) | 305 (86) | |
| Patients who underwent OR | 545 (91) | 213 (88) | 332 (94) | 0.01 |
Data expressed as number (percentage) or median (interquartile range), unless otherwise specified.
ATAAD = acute type A aortic dissection; IR = interventional radiology (endovascular treatment); MPS = malperfusion syndrome; OR = open repair.
Endovascular fenestration/stenting (IR procedures)
All fenestration/stenting was performed percutaneously in the angiography suite or hybrid room at the University of Michigan. Angiographic confirmation of treatable MPS was documented by a significant pressure gradient (>15 mmHg) between the ascending aorta true lumen and a branch artery. Fenestration and stenting were performed percutaneously by creating a tear in the dissection flap to equalize the blood pressure (BP) and permit flow between the true and false lumens as previously described21,22. Fenestration was most frequently created above the celiac artery for visceral malperfusion and below the lowest renal artery for iliac artery malperfusion. We measured the BP of the malperfused portion of the aorta or aortic branches before (baseline BP) and after fenestration/stenting of the aorta or aortic branches (completion BP). Selective branch arteriography (and, if necessary, intravascular ultrasound inspection) was performed in the SMA, renal, and iliac arteries to confirm branch artery obstruction, localize the terminal extent of a branch artery dissection, and to distinguish between true and false lumen thrombus. Branch artery pressure was measured just past the vessel origin of undissected arteries or in the distal undissected segment of dissected vessels. The time between baseline BP and completion BP was noted as the time needed for the intervention. Treatment of dynamic visceral artery obstruction began with fenestration slightly above the celiac origin, then a 16–18 mm Wallstent was deployed in the aortic true lumen such that it extended down to the rostral margin of the SMA. This procedure resolved dynamic obstruction of the celiac artery, SMA, and sometimes the renal arteries. Based on our experience in the second decade, the average time to perform aortic fenestration/stenting to resolve dynamic malperfusion was 30 minutes.
If, after correction of dynamic obstruction by aortic fenestration/stenting, the pressure gradient between the ascending aorta and a dissected branch vessel, such as the SMA, was still above 15 mmHg, bare stents were placed into the true lumen of the branch vessel down past the terminal extent of the dissection. In dissected vessels with thrombosed false lumens, gradients after stenting might exceed 15 mmHg, but, as long as absolute perfusion pressure was viable, i.e., >60 mmHg, post-dilation of stents was not performed. In non-dissected branch arteries, gradients persisting after proximal aortic fenestration and stenting usually indicates persistent dynamic obstruction, which generally respond to infrarenal aortic fenestration and aortoiliac stenting. Thrombolysis, thrombectomy, and embolectomy were occasionally performed to resolve thrombus in the true lumen of branch arteries, typically due to stasis distal to an obstructing flap or thrombosed false lumen. Renal artery stenting takes approximately 40 minutes, and infrarenal aortic fenestration and aortoiliac stenting approximately 90 minutes. Clearing SMA true lumen thrombus distal to an obstructing proximal SMA false lumen thrombosis takes 3 hours for tPA-thrombolysis, SMA stenting. Celiac artery stenting, which takes 60 minutes on average, is rarely necessary. We only study and treat the celiac trunk if the SMA is extensively dissected or if the liver enzymes are grossly elevated.
In those 135 ATAAD patients with suspected MPS, 3 (2.2%) had only fenestration of the aortic dissection flap, 2 (1.5%) had only aortic true lumen stenting, 11 (8%) had both fenestration and stenting of the aorta alone, 13 (9.6%) had fenestration/stenting of the aortic branch vessels alone without aortic intervention, and 83 (61%) had aortic fenestration/stenting plus fenestration/stenting of aortic branches. Seventeen (13%) patients had “additional” procedures for false lumen thrombosis of aortic branch vessels (thrombolysis, thrombectomy/embolectomy, coil embolization of ileal artery aneurysm) (See supplemental Table S1 for more details of IR procedures).
In terms of the order of treatment, iliac artery occlusion with a thrombosed TRUE lumen received highest priority. The reasoning behind this was that correcting dynamic obstruction before removing true lumen thrombus could result in inadvertent showering of thrombus distally to the legs. Therefore, true lumen thrombus was aspirated FIRST. Second level priority was given to SMA obstruction, third to renal obstruction, and fourth to extremity obstruction.
Delayed open aortic repair
After patients recovered from organ failure or shock and we felt the patient could tolerate an open aortic operation, the patients underwent delayed OR. In general, in the second decade we waited for downtrending rather than normalization of ischemic markers. For celiac (i.e., hepatic) MPS, we waited for liver function tests to improve. For mesenteric MPS, we waited for recovery from bowel resection; resolution of shock, sepsis, or acute respiratory distress syndrome (ARDS); and near-normalization of serum lactate. For isolated renal MPS, we performed open repair within 24 hours after the IR procedure (of note, there was only one isolated renal MPS in the second decade). For extremity MPS, we waited for patients to recover from shock, acidosis, ARDS, amputation if needed, and rhabdomyolysis. Most of these patients developed concomitant renal failure due to extremity MPS, but we did not wait for resolution of the renal failure before proceeding with OR. In the second decade, our vascular surgery colleagues took a more aggressive stance towards performing fasciotomies of ischemic extremities immediately following the IR procedure in order to relieve – or prevent – compartment syndrome and to assess viability of the muscle. If the muscle was non-viable, we waited for patients to recover from debridement of necrotic muscle or amputation. The operative strategy was similar in both immediate OR and delayed OR groups.
Our observed short-term (in-hospital or 30-day) mortality was compared with expected mortality following an “upfront OR for every patient” approach as determined by several prognostic models previously published in the literature:
Verona model28 – in-hospital mortality;
Source: Verona (Italy)
Studied patient population: all ATAAD patients (all comers) – including operable and non-operable patients.
Variables needed: age, tamponade, hypotension, mesenteric ischemia, acute renal failure, neurologic injury
Leipzig-Halifax model15 – in-hospital mortality;
Source: Leipzig (Germany) and Halifax (Canada)
Studied patient population: operable ATAAD patients
Variables needed: age, critical pre-operative state (inotropes, pre-operative mechanical ventilation or pre-operative cardiopulmonary resuscitation), coronary MPS, extremity MPS, visceral MPS, coronary artery disease
Stockholm model16 – in-hospital mortality;
Source: Stockholm (Sweden)
Studied patient population: operable ATAAD patients
Variables needed: critical pre-operative state (inotropes, pre-operative mechanical ventilation or pre-operative cardiopulmonary resuscitation), Penn class non-Aa, coronary artery disease
Penn model29 – 30-day mortality after OR;
Source: Philadelphia (United States)
Studied patient population: operable ATAAD patients
Variables needed: no ischemia (Penn class Aa) vs. localized ischemia (Penn class Ab) vs. generalized ischemia (Penn class Ac) vs. combined (localized & generalized) ischemia (Penn class Ab&c)
GERAADA model30 – 30-day mortality after OR;
Source: German Registry for Acute Aortic Dissection type A (Germany)
Studied patient population: operable ATAAD patients
Variables needed: number of pre-operative malperfused organ systems
For the Penn and GERAADA models (both predicting risk of mortality up to 30 days after surgery), we used our observed mortality after first intervention (IR or OR, whichever occurred first).
Statistical analysis.
All analyses were performed using the open source software for statistical computing and graphics R (https://www.r-project.org/) and SAS 9.4 (SAS Institute, Cary, NC). Chi-square test or Fisher exact test was used for categorical data. Wilcoxon rank sum test was used for non-parametric comparisons of continuous variables. Kaplan-Meier method and log-rank test were used for survival analysis. A competing risks model31,32 was used to analyze eventual outcome (survival without OR vs. death from aortic rupture vs. death from organ failure vs. survival to OR) in patients after IR. We used the associated hazard ratio to compare the risk of death from aortic rupture vs. death from organ failure and the proportional hazard assumption was tested using Schoenfeld residuals. Statistical significance set at p ≤ 0.05.
RESULTS
Short-term outcomes: Second decade vs. First decade
Patient population and distribution of MPS
In this study, we analyzed the outcome of all ATAAD patients who came to our institution, who were treated with either IR or OR, including all comers with celiac, mesenteric, renal, or extremity MPS which were IR-amenable, i.e., suitable for correction by endovascular techniques. (Supplemental Figure S1) Compared to the first decade (1996–2007), we treated more ATAAD patients in the second decade (2008–2017) (Table 1, Supplementary Figure S2). The percentage of total MPS patients significantly decreased in the second decade, but distribution of IR-amenable MPS was similar between the two decades. (Table 1). In the second decade, there were 4 isolated mesenteric MPS cases, 1 isolated renal MPS case, and 6 isolated extremity MPS cases. Thirty-five patients had MPS of multiple vascular beds. There were nearly 3 times fewer non-therapeutic IR procedures (only diagnostic IR procedures, no arterial obstruction found) in the second decade compared to the first decade (8 % vs. 22%, p=0.055) (Table 1).
Overall in-hospital mortality
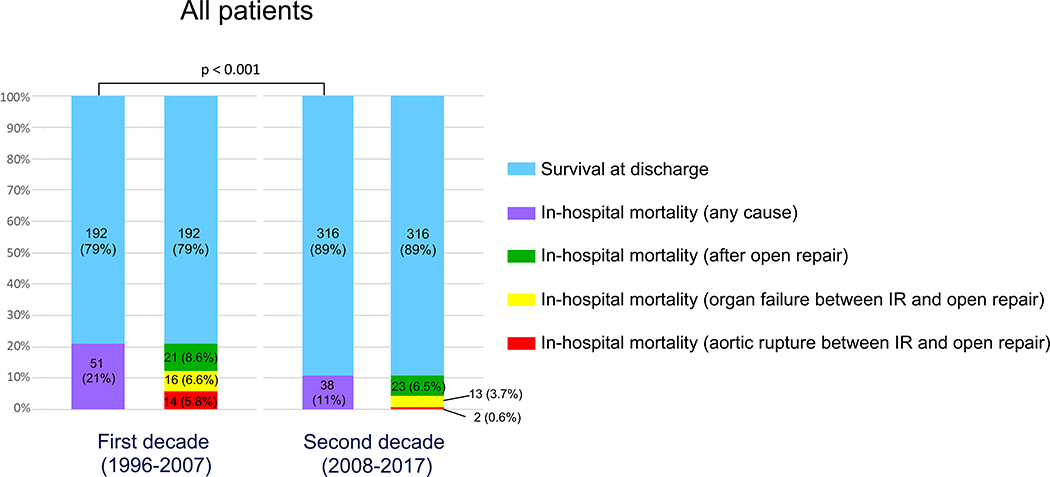
Patient demographics, comorbidities, and clinical condition on admission were similar between the two decades (Table 1, S2). However, in-hospital mortality (all comers treated with IR and/or OR) improved by approximately 50% (first decade: 21.0%, second decade: 10.7%; p < 0.001) (Figure 2).
Figure 2. Comparison of in-hospital outcomes of all patients with acute type A aortic dissection between the first decade (1996–2007) and second decade (2008–2017).
The overall in-hospital mortality decreased by 50% from first decade to second decade (p < 0.001).
IR = endovascular treatment of malperfusion syndrome by interventional radiology.
In-hospital mortality of patients with MPS treated with upfront IR in the second decade
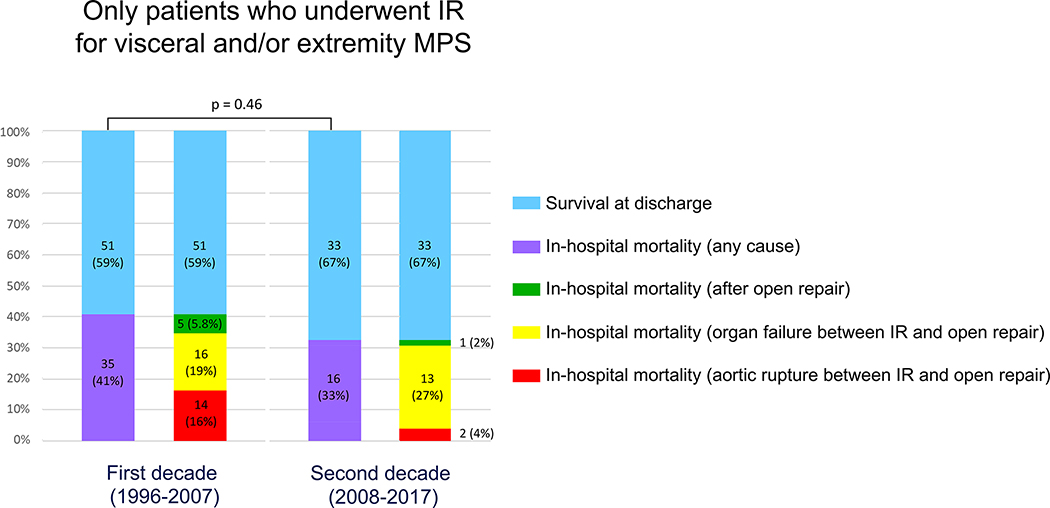
Compared to the first decade, there was an absolute but non-significant 8% reduction in in-hospital mortality of MPS patients treated with IR first (Figure 3). However, in the second decade, the mortality from aortic rupture significantly decreased from 16% to 4% (p=0.05) (Figure 3), and the risk of aortic rupture was approximately 7 times lower than the risk of death from organ failure (Figure 4). In the second decade, in patients suspected to have MPS who were treated with upfront IR, 87% of the mortality was attributable to organ failure due to complications from MPS after the arterial obstruction was resolved with fenestration/stenting (Figures 3, 4). The operative mortality was 43% for unstable ATAAD patients with MPS (n=7) and 3.8% for patients undergoing IR + delayed OR (n=26). The in-hospital mortality of all MPS patients in the second decade (n=69) was 29%, including patients treated with IR first or OR first (unstable patients, patients with cerebral, spinal or coronary MPS). There were no complications from IR procedure except for one patient whose aorta ruptured and died during access for the IR procedure.
Figure 3: Perioperative outcomes of patients with acute type A aortic dissection and malperfusion syndrome (MPS) after upfront endovascular treatment by interventional radiology (IR) for visceral and/or extremity MPS.
There is a trend of decreased overall in-hospital mortality (black) between the two decades, but not significantly different (p=0.46). The in-hospital mortality due to aortic rupture (dark gray) was significantly lower in the second decade (4% vs 16%, p=0.05). The in-hospital mortality due to organ failure between IR and open aortic repair was not significantly different between the two decades.
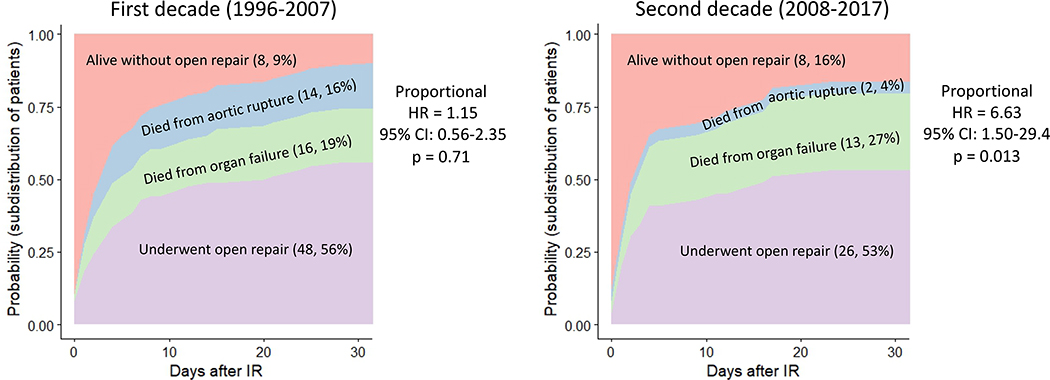
Figure 4: Thirty-day outcomes after endovascular treatment by interventional radiology (IR).
The risk of death between IR and open aortic repair from organ failure vs. from aortic rupture was similar in the first decade with proportional hazard ratio (HR) of 1.15; however, significantly increased in the second decade with proportional HR of 6.63, p = 0.013. Proportional hazard assumption satisfied in both decades (Schoenfeld residuals test: p = 0.52 in the first and p = 0.12 in the second decade, respectively). Data expressed as (number, percentage).
HR = cause-specific hazard ratio of death from organ failure/death from aortic rupture.
We divided all patients with MPS treated with IR first over two decades into a high-risk group (celiac/mesenteric MPS, n=84) and a low-risk group (non-celiac/mesenteric MPS, n=51). Compared to the first decade (n=54), in-hospital mortality decreased from 44% to 33% in the second decade (n=30) for the high-risk group (p=0.32) and from 34% (n=32) to 32% (n=19) for the low-risk group (p=0.84). There was also no significant difference in in-hospital mortality between the high- and low-risk groups for the whole cohort over two decades (40% vs. 33%, p= 0.41) or in the second decade (33% vs. 32%, p=0.90).
Operative outcomes after open repair
For all patients who underwent OR, there was no significant difference in the incidence of peri-operative myocardial infarction, stroke, post-operative acute kidney injury, or 30-day mortality (8.0% vs. 5.4%) between the two decades (Table S3, S4).
Short-term outcomes: Fenestration/stenting + delayed OR vs. upfront OR
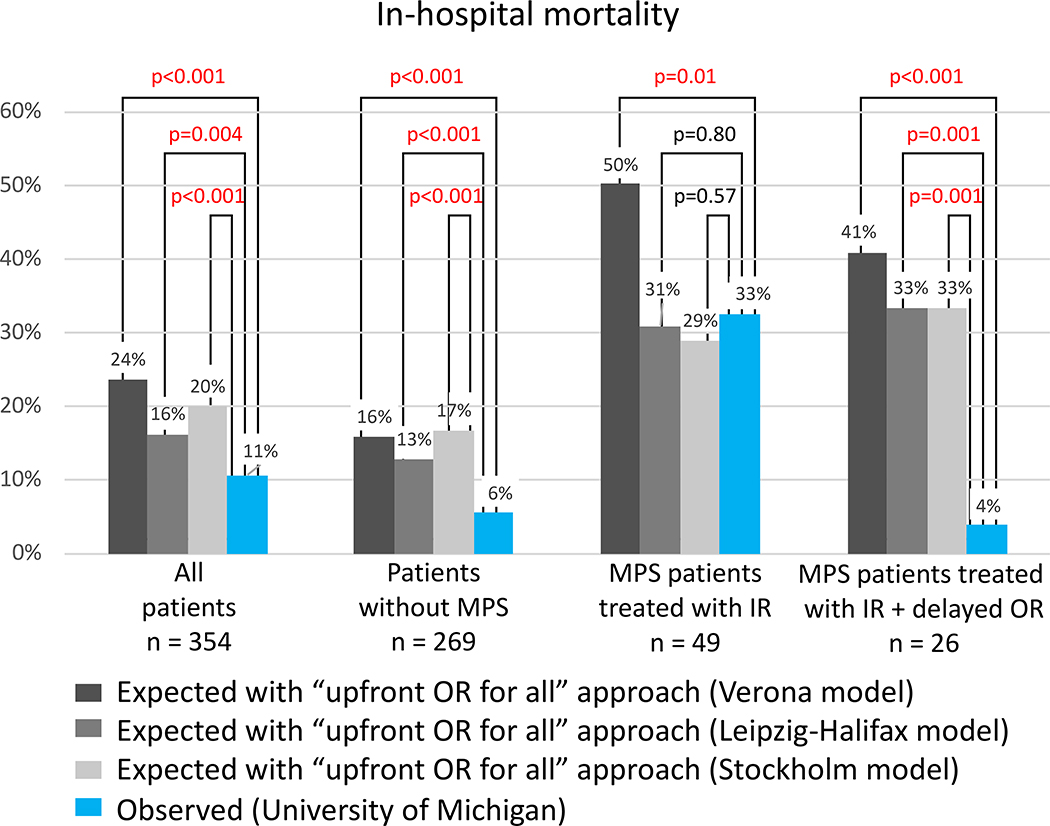
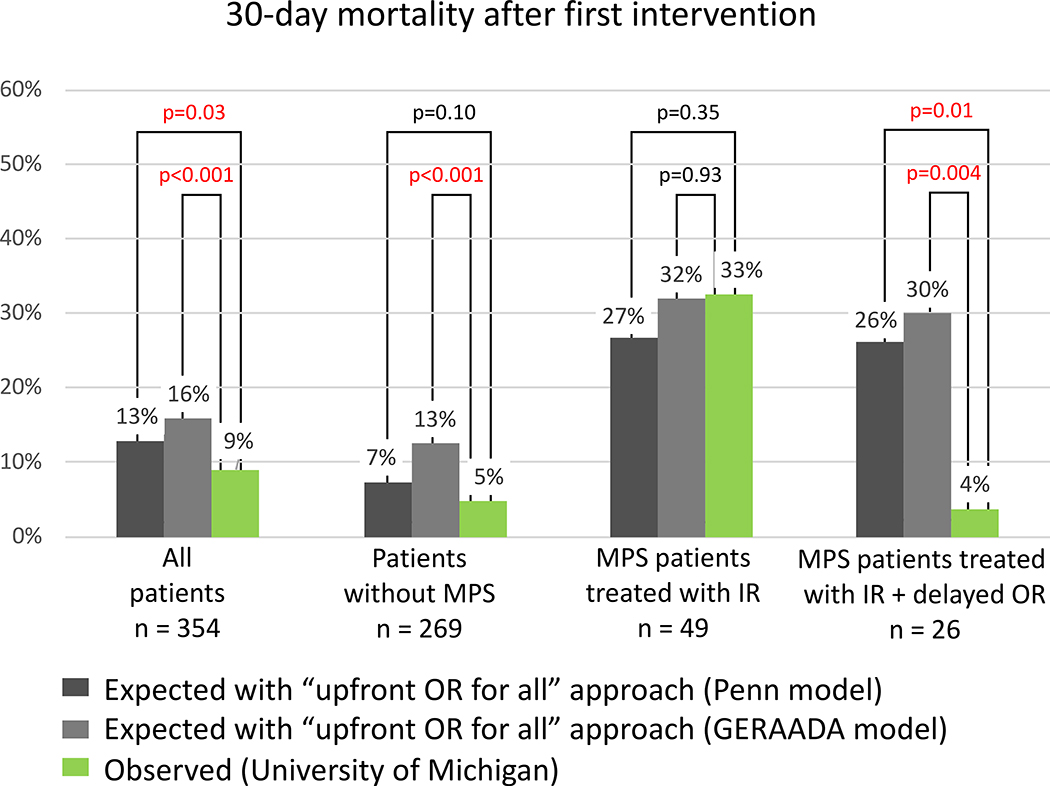
Our observed (O) in-hospital mortality of all ATAAD patients and patients without MPS was significantly lower than the expected (E) mortality calculated from the Verona, Leipzig-Halifax, and Stockholm models (O/E ratio: 0.33–0.69) (Figure 5). The same observation regarding 30-day mortality was found using the Penn and GERAADA models (O/E ratio: 0.38–0.69) (Figure 6). The observed in-hospital mortality of patients with MPS treated with IR and delayed OR was significantly lower than the expected mortality calculated by the Verona model (O/E ratio = 0.60) (Figure 5). The observed in-hospital mortality (3.8%) or 30-day mortality (3.8%) of patients who had IR and delayed OR was significantly lower than the expected mortality of all models (Figure 5, 6).
Figure 5. Comparison of observed “in-hospital mortality” at the University of Michigan with that expected following an “upfront open repair for every patient” approach, according to Verona, Leipzig-Halifax, and Stockholm prognostic models, during the second decade (2008–2017).
All patients (n=354) includes patients without any MPS (n=269), with MPS but unstable (tamponade or rupture) (n=7), with non-IR-amenable MPS (ie, isolated cerebral, coronary, or spinal MPS) (n=29), and with IR-amenable MPS (ie, visceral or extremity) (n=49). IR indicates endovascular treatment (interventional radiology); OR, open repair; and MPS, malperfusion syndrome.
Figure 6. Comparison of observed “30-day mortality after first intervention” at the University of Michigan with that expected following an “upfront open repair for every patient” approach, according to Penn and GERAADA prognostic models, during the second decade (2008–2017).
First intervention is defined as either endovascular treatment of malperfusion syndrome or open repair of acute type A aortic dissection, whichever occurred first. All patients (n=354) include patients without any MPS (n=269), with MPS but unstable (tamponade or rupture) (n=7), with non-IR-amenable MPS (ie, isolated cerebral, coronary, or spinal MPS) (n=29), and with IR-amenable MPS (ie, visceral or extremity) (n=49). GERAADA indicates German Registry for Acute Aortic Dissection type A; IR, endovascular treatment (interventional radiology); MPS, malperfusion syndrome; and OR, open repair.
Long-term survival of ATAAD with or without MPS
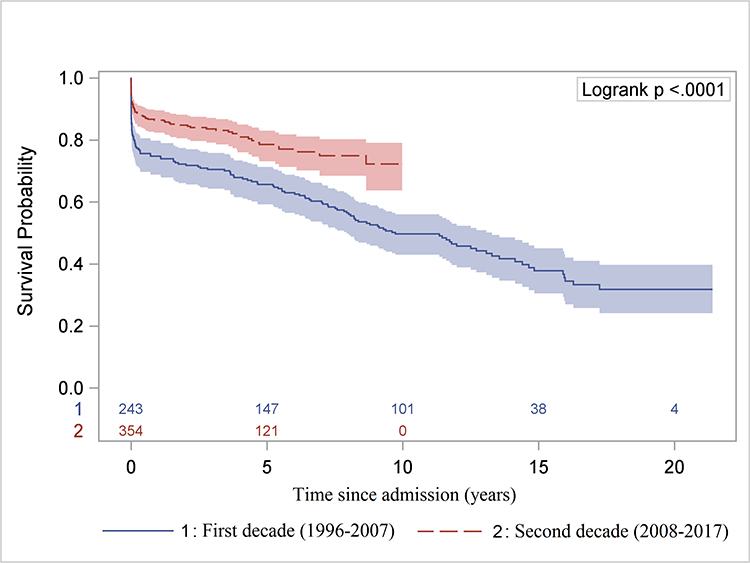
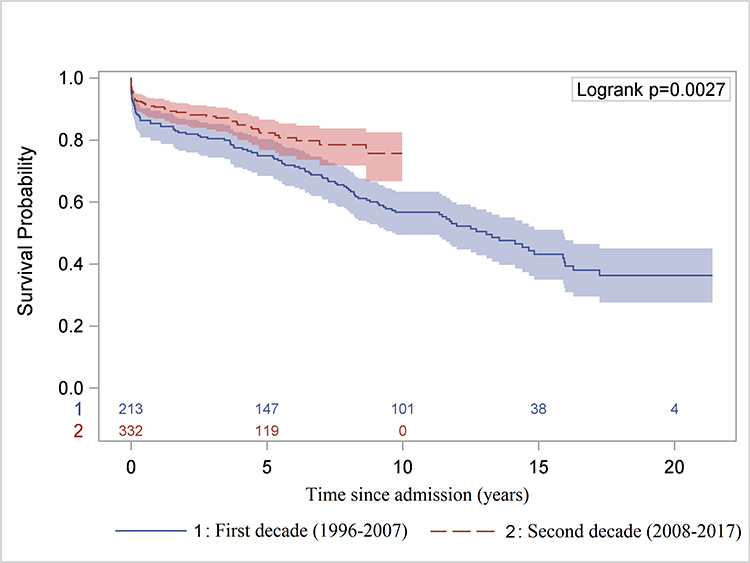
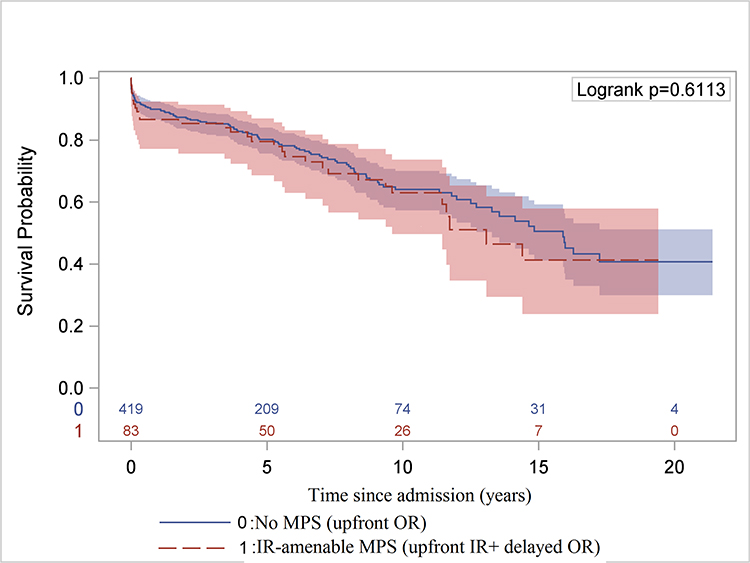
The long-term survival of all ATAAD patients significantly improved in the second decade (Figure 7A, 7B). Compared to patients without MPS, the long-term survival of patients with MPS was not significantly different after endovascular fenestration/stenting and delayed OR (Figure 7C, Supplemental Figure S3).
Figure 7. Long-term Kaplan-Meier survival analysis.
A, Comparison of overall survival since hospital admission in the first versus second decade for all patients. B, Comparison of overall survival after hospital discharge (landmark survival analysis) in the first decade versus the second decade only for patients who underwent open repair. C, Overall survival since hospital admission for patients of ATAAD without any MPS who underwent upfront OR versus those with MPS treated with upfront endovascular reperfusion followed by OR of ATAAD, including 9 patients who had delayed OR >30 days after IR. ATAAD indicates acute type A aortic dissection; IR, interventional radiology; and MPS, malperfusion syndrome.
DISCUSSION
We present a twenty-year experience managing ATAAD patients with MPS by performing upfront endovascular fenestration/stenting followed by urgent open aortic repair at the time of resolution of critical organ failure33,34 with a focus on the second decade (2008–2017). Our approach was based on the following hypotheses for a selected subset of patients with MPS: (1) the immediate risk of dying from organ failure due to MPS is higher than that from aortic rupture; and (2) endovascular reperfusion (through fenestration/stenting) as an upfront intervention is more beneficial than an upfront open aortic repair21.
Compared to the first decade, the principal improvement in in-hospital mortality was the decrease in mortality from aortic rupture from 16% to 4% in the second decade (Figures 3, 4). Ninety-six percent of patients either survived to discharge or underwent delayed open repair (69.5%) or died from end-organ failure (26.5%) before delayed OR (Figure 3, 4). Even after the branch arterial obstruction was resolved with fenestration/stenting, the risk (hazard ratio) of dying from end-organ failure was approximately seven times higher than the risk of aortic rupture. These findings support our first hypothesis that for ATAAD patients with MPS but without aortic rupture or cardiac tamponade, the risk of dying from organ failure due to MPS is higher than the risk of dying from aortic rupture. The premise of our approach is that not every untreated ATAAD will rupture, but every untreated MPS will result in death. For isolated renal MPS, we recommend upfront open aortic repair instead of fenestration/stenting by IR since renal MPS is less life-threatening. However, very frequently, renal MPS occurs in combination with other sites of malperfusion. We recommend IR evaluation if patients continue to show evidence of malperfusion after open repair. It should also be noted that unsuspected SMA obstruction was frequently found in conjunction with evaluation of suspected renal or leg malperfusion. In addition, the efficacy of hypothermia for hypoxic relief of a statically or even dynamically obstructed SMA or iliac artery has not been established.
Three primary modifications in our management strategy in the second decade contributed to a lower incidence of aortic rupture between IR and OR. First, we were more selective in routing patients to IR. Over time we learned to clearly differentiate between malperfusion and MPS. We consider malperfusion to be inadequate blood flow to an end organ resulting in ischemia but not necessarily necrosis, as might follow from branch arterial obstruction as a result of the dissection, severe AI, pump failure from coronary artery dissection or tamponade. In the case of branch artery obstruction, the end organ could be ischemic but not necrotic, especially at an early stage. MPS, however, is late stage malperfusion characterized by cell/tissue/organ death and malfunction, such as necrotic bowel from mesenteric malperfusion or necrotic muscle from extremity malperfusion. In the second decade, we sent only patients with suspected MPS due to branch artery obstruction to IR first, and 45 out of 49 patients (92%) had confirmed malperfusion based on the BP gradient and had fenestration/stenting of aorta and/or branch vessels. Four of 49 (8%) had a non-therapeutic arteriography due to resolution of dynamic malperfusion from new re-entry tears and/or BP control 19,20,22. (Table 1, Figure S2) The dynamic malperfusion still caused organ damage (MPS) based on the clinical assessment before its resolution. Furthermore, only one ATAAD patient has undergone non-therapeutic IR since 2012 (Figure S2).
Malperfusion with dynamic obstruction that is medically corrected with no end-organ damage should be treated with immediate OR to prevent aortic rupture. Static obstruction prevents delivery of effective blood flow to the affected arterial bed and is not reliably relieved by proximal aortic repair or TEVAR. Subclinical malperfusion, defined as imaging evidence only of peripheral malperfusion without resultant severe organ failure, is not an indication for delayed OR. Only when critical organ necrosis and failure are both present and due to dissection-related malperfusion with concomitant relative hemodynamic stability (no aortic rupture, no tamponade) can an “IR first” approach be beneficial.
Second, there was improvement in patient care in the intensive care unit (ICU) between the IR procedure and delayed OR. We aggressively pursued anti-impulse therapy for ATAAD patients, with meticulous systolic blood pressure (SBP) control (goal SBP 90–110 mmHg) and adequate analgesia and sedation. We recommend against lightening sedation for a neurological exam before the open aortic repair. We kept patients intubated and sedated until OR if we anticipated that the patient would recover from MPS complications and be ready for OR within a week.
Third, we took patients for OR sooner following IR in the second decade. The median wait time from IR to OR decreased from 4 days to 2 days in the second decade (Table 1), including from 7 days to 4 days in the high-risk MPS group (i.e. celiac/mesenteric MPS), and 2 days to 1 day in the non-high-risk MPS group (i.e. no celiac/mesenteric MPS). We waited for absence of ongoing organ (such as bowel, liver, skeletal muscle) necrosis; resolution of metabolic acidosis, shock, and ARDS; and whether the patient could clinically tolerate cardiopulmonary bypass and circulatory arrest. We did not wait for complete recovery of organ function, such as renal function, to perform OR.
Is our approach (IR + delayed OR) for ATAAD patients with MPS better than the traditional approach (upfront OR for every patient)? There are several advantages to resolving critical malperfusion first with the IR approach (fenestration/stenting).
1) Our approach resolves the arterial obstruction immediately and completely with a percutaneous procedure. The average time to resolve dynamic obstruction is 30 minutes. At the end of the procedure, the blood pressure in each visceral branch (celiac, SMA, and renal arteries) and in the iliac arteries is measured to confirm that arterial obstruction has been relieved. Open aortic repair or thoracic endovascular aortic repair (TEVAR) can help resolve dynamic malperfusion but not static malperfusion due to the occlusion of aortic branch vessels from dissection and false lumen thrombosis of the branch vessels. Static malperfusion can be completely resolved by an IR approach (fenestration/stenting of the branch vessels). In 135 patients suspected with MPS, 106 (79%) patients had fenestration/stenting of aortic branch vessels, (Table S1), and 17 (13%) patients had “additional” procedures for true lumen thrombosis of aortic branch vessels, such as thrombolysis and thrombectomy/embolectomy, none of which could be achieved by upfront OR or TEVAR. Once the arterial obstruction was corrected and patient recovered from MPS, the operative mortality of open aortic repair was very low (3.7%) (Figure 3, 4).
2) Our approach gives borderline operative candidates with MPS time to recover and avoids futile open aortic repair in those who are not salvageable. Patients with MPS are critically ill and operative mortality has been reported as high as 45% 1–16 if upfront OR is performed with cardiopulmonary bypass and hypothermic circulatory arrest. The IR approach is percutaneous and has less of a hemodynamic insult to the patient than OR but achieves a similar goal, to resolve malperfusion, thus giving patients the chance to recover from organ failure, bowel resection, or amputation; and decrease the risk of open aortic repair. In patients with SMA obstruction and suspected bowel infarction needing laparotomy, up-front angiographic correction of SMA obstruction provides a more reliable intra-operative assessment of margins of bowel viability. For those who do not recover from organ necrosis and failure, an open aortic repair would likely be futile, may consume more resources than a percutaneous procedure alone, and may have a more negative impact on the patient and the family both physically and emotionally.
3) Our approach provides favorable short- and long-term survival. Compared to expected mortality as calculated by the Verona model28, which is the only model that includes all comers of ATAAD with MPS treated with upfront OR and non-operatively, the observed in-hospital mortality in all patients and patients with MPS treated with upfront IR was significantly lower than expected (Figure 5, 6). Our observed in-hospital mortality and 30-day mortality in all ATAAD patients with or without MPS (n= 354) were significantly lower than expected mortalities calculated from all five models (Figure 5, 6). The observed in-hospital and 30-day mortality in patients with MPS (n=49) treated with upfront IR were not significantly lower than the expected mortalities calculated from the other four models (Leipzig-Halifax model15, Stockholm model16, Penn model29, and GERAADA model30). A possible explanation is that those four studies excluded patients who were not surgical candidates, such as those who had extensive necrotic bowel or those who refused open surgery. In our cohort, we had 19 patients who might be considered “not surgical candidates”: 3 had completely dead bowel, 1 refused amputation, 3 had brain death, and for 12 patients the family withdrew care after the IR procedure (see supplemental table S5). These patients were still included in our in-hospital mortality using our IR first approach. After patients were treated with upfront IR and recovered from MPS, the operative mortality of OR was 6 to 8 times lower than the expected mortality calculated from all 5 models (Figures 5 and 6), and the long-term survival (5-year survival: 80%; 10-year survival: 63%) was similar to patients with ATAAD without MPS (Figure 7C). In other reports9,10, MPS patients treated with upfront OR had worse long-term survival (5-year survival: 42–54%; 10-year survival: 43%) compared to patients without MPS (5-year survival: 56–66%; 10-year survival: 46%).
The combination of cerebral malperfusion with simultaneous visceral or limb malperfusion is a very difficult to treat. When patients present with a neurologic deficit but without visceral or limb necrosis (MPS), we recommend immediate open aortic repair. If a patient has visceral or limb MPS in addition to a neurologic deficit, we recommend IR fenestration and stenting first, and open aortic repair as soon as patients can tolerate the procedure.
Any approach to treat ATAAD patients with MPS comes with a very high risk of complications, including mortality for various reasons. Two (4%) patients with MPS died from aortic rupture which could have potentially been prevented by upfront OR. However, based on the Penn and GERAADA models, the 30-day operative mortality would have been 26–31% for those two patients based on their preoperative condition. Surgeons have to balance the 30% risk of operative mortality and the 4% risk of aortic rupture. We would recommend accepting the latter and performing fenestration/stenting first. If patients recover from MPS, the operative mortality was 4% in our series in the second decade. Taken together, our results support our second hypothesis that endovascular reperfusion through fenestration/stenting as the first intervention prior to definitive operative repair is more beneficial than upfront open aortic repair in stable ATAAD patients with MPS.
Our study is limited by being a single-center and retrospective experience. There is a learning curve with the IR approach and not every institution has the resources and IR expertise available. In the case of pure dynamic obstruction, fenestration with an aortic true lumen stent is within the toolbox of most interventional radiologists. If an institution cares for aortic dissection patients on a regular basis, then the interventions should be done with the assistance of IVUS. More complicated cases (dynamic plus static obstruction of visceral and iliac vessels, false lumen thrombosis in the SMA, renal arteries, and iliac arteries, etc.) should probably be transferred to a higher volume institution. It was not ideal to compare the outcome of our approach to the models in the literature since we did not know the detailed inclusion and exclusion criteria of other studies. A prospective randomized trial would be ideal to compare those two methods. However, given the life-or-death circumstance and critical clinical presentation that accompanies ATAAD with MPS, it would be almost impossible to conduct a randomized prospective trial to compare upfront IR + delayed OR vs. upfront OR. The MPS cases undergoing IR were hemodynamically relatively stable. It is difficult to compare these patients to the literature with MPS in other series (mortality up to 45%) because of unspecified inclusion and exclusion criteria. However, our in-hospital mortality of all comers with MPS (stable or unstable) in the second decade was 29%. We believe our findings could potentially change the “standard of care” approach for patients with ATAAD and MPS.
In conclusion, for most patients with acute type A aortic dissection immediate open repair remains the recommended approach to prevent death. However, for patients who are relatively stable (no rupture or tamponade) presenting with MPS and significant end-organ dysfunction, a staged approach (upfront endovascular reperfusion by fenestration/stenting followed by delayed open aortic repair at the time of resolution of severe organ failure) may be more beneficial.
Supplementary Material
Clinical Perspective
1). What is new?
All acute type A aortic dissection (ATAAD) patients are traditionally managed with emergent open aortic repair, including those with malperfusion syndrome (MPS) – end organ necrosis and failure due to malperfusion.
For two decades, we have treated relatively stable patients with visceral and/or extremity MPS with endovascular reperfusion through fenestration/stenting first followed by delayed open aortic repair.
In the second decade, the in-hospital mortality of all comers was 11%.
The 30-day operative mortality utilizing this approach for MPS patients was 3.7%, which was significantly lower than the expected mortalities with “upfront open repair for every patient” models in the literature.
2). What are the clinical implications?
Patients with an ATAAD complicated by visceral and/or extremity MPS but without aortic rupture or cardiac tamponade have a greater risk of dying from organ failure than from aortic rupture.
Percutaneous endovascular fenestration/stenting of the aorta and branch vessels can quickly and completely revascularize the obstructed arteries with less trauma and a low risk of aortic rupture.
This approach provides “borderline” patients the opportunity to recover and improve short- and long-term survival, and prevents a futile open aortic repair in the presence of unsalvageable organ damage and failure.
This approach could change the algorithm for treating ATAAD patients with MPS.
ACKNOWLEDGEMENTS:
The authors would like to thank the support from the Data Warehouse in the Department of Cardiac Surgery led by Dr. Donald Likosky, including Jeremy Wolverton, Amy Geltz, Mary Barry, Brett Cross, Mary Ryzak and other team members; the support from the Department of Cardiac Surgery and the Frankel Cardiovascular Center at the University of Michigan.
FUNDING SOURCES:
Dr. Yang is supported by the NHLBI of NIH K08HL130614, R01HL141891, Phil Jenkins and Steve and Darlene Szatmari Funds. Dr. Patel is supported by the Joe D. Morris Collegiate Professorship, the David Hamilton Fund, and the Phil Jenkins Breakthrough Fund in Cardiac Surgery. Dr. Deeb is supported by the Herbert Sloan Collegiate Professorship, Jamie Buhr Fund, and Richard Nerod Fund.
Footnotes
DISCLOSURES:
No conflicts of interest related to this study to disclose.
REFERENCES
- 1.Fann JI, Sarris GE, Mitchell RS, Shumway NE, Stinson EB, Oyer PE, Miller DC. Treatment of patients with aortic dissection presenting with peripheral vascular complications. Ann Surg. 1990;212:705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toda R, Moriyama Y, Masuda H, Iguro Y, Yamaoka A, Taira A. Organ malperfusion in acute aortic dissection. Jpn J Thorac Cardiovasc Surg. 2000;48:545–550. [DOI] [PubMed] [Google Scholar]
- 3.Mehta RH, Suzuki T, Hagan PG, Bossone E, Gilon D, Llovet A, Maroto LC, Cooper JV, Smith DE, Armstrong WF, Nienaber CA, Eagle KA; International Registry of Acute Aortic Dissection (IRAD) Investigators. Predicting death in patients with acute type A aortic dissection. Circulation. 2002;105:200–206. [DOI] [PubMed] [Google Scholar]
- 4.Girardi LN, Krieger KH, Lee LY, Mack CA, Tortolani AJ, Isom OW. Management strategies for type A dissection complicated by peripheral vascular malperfusion. Ann Thorac Surg. 2004;77:1309–1314; discussion 1314. [DOI] [PubMed] [Google Scholar]
- 5.Trimarchi S, Nienaber CA, Rampoldi V, Myrmel T, Suzuki T, Mehta RH, Bossone E, Cooper JV, Smith DE, Menicanti L, Frigiola A, Oh JK, Deeb MG, Isselbacher EM, Eagle KA, International Registry of Acute Aortic Dissection Investigators. Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection experience. J Thorac Cardiovasc Surg. 2005:129:112–122. [DOI] [PubMed] [Google Scholar]
- 6.Yagdi T, Atay Y, Engin C, Mahmudov R, Tetik O, Iyem H, Posacioglu H, Apaydin AZ, Buket S. Impact of organ malperfusion on mortality and morbidity in acute type A aortic dissections. J Card Surg. 2006;21:363–369. [DOI] [PubMed] [Google Scholar]
- 7.Immer FF, Grobety V, Lauten A, Carrel TP. Does malperfusion syndrome affect early and mid-term outcome in patients suffering from acute type A aortic dissection? Interact Cardiovasc Thorac Surg. 2006;5:187–190. [DOI] [PubMed] [Google Scholar]
- 8.Rampoldi V, Trimarchi S, Eagle KA, Nienaber CA, Oh JK, Bossone E, Myrmel T, Sangiorgi GM, De Vincentiis C, Cooper JV, Fang J, Smith D, Tsai T, Raghupathy A, Fattori R, Sechtem U, Deeb MG, Sundt TM 3rd, Isselbacher EM, International Registry of Acute Aortic Dissection (IRAD) Investigators. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection score. Ann Thorac Surg. 2007;83:55–61. [DOI] [PubMed] [Google Scholar]
- 9.Geirsson A, Szeto WY, Pochettino A, McGarvey ML, Keane MG, Woo YJ, Augoustides JG, Bavaria JE. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg. 2007;32:255–262. [DOI] [PubMed] [Google Scholar]
- 10.Girdauskas E, Kuntze T, Borger MA, Falk V, Mohr FW. Surgical risk of preoperative malperfusion in acute type A aortic dissection. J Thorac Cardiovasc Surg. 2009;138:1363–1369. [DOI] [PubMed] [Google Scholar]
- 11.Di Eusanio M, Trimarchi S, Patel HJ, Hutchison S, Suzuki T, Peterson MD, Di Bartolomeo R, Folesani G, Pyeritz RE, Braverman AC, Montgomery DG, Isselbacher EM, Nienaber CA, Eagle KA, Fattori R. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. 2013;145:385–390. [DOI] [PubMed] [Google Scholar]
- 12.Pacini D, Leone A, Belotti LM, Fortuna D, Gabbieri D, Zussa C, Contini A, Di Bartolomeo R, RERIC (Emilia Romagna Cardiac Surgery Registry) Investigators. Acute type A aortic dissection: significance of multiorgan malperfusion. Eur J Cardiothorac Surg. 2013;43:820–826. [DOI] [PubMed] [Google Scholar]
- 13.Conzelmann LO, Weigang E, Mehlhorn U, Abugameh A, Hoffmann I, Blettner M, Etz CD, Czerny M, Vahl CF, GERAADA Investigators. Mortality in patients with acute aortic dissection type A: analysis of pre- and intraoperative risk factors from the German Registry for Acute Aortic Dissection Type A (GERAADA). Eur J Cardiothorac Surg. 2016;49:e44–52. [DOI] [PubMed] [Google Scholar]
- 14.Berretta P, Patel HJ, Gleason TG, Sundt TM, Myrmel T, Desai N, Korach A, Panza A, Bavaria J, Khoynezhad A, Woznicki E, Montgomery D, Isselbacher EM, Di Bartolomeo R, Fattori R, Nienaber CA, Eagle KA, Trimarchi S, Di Eusanio M. IRAD experience on surgical type A acute dissection patients: results and predictors of mortality. Ann Cardiothorac Surg. 2016;5:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leontyev S, Légaré JF, Borger MA, Buth KJ, Funkat AK, Gerhard J, Mohr FW. Creation of a scorecard to predict in-hospital death in patients undergoing operations for acute type A aortic dissection. Ann Thorac Surg. 2016;101:1700–1706. [DOI] [PubMed] [Google Scholar]
- 16.Mejàre-Berggren H, Olsson C. Validation and adjustment of the Leipzig-Halifax acute aortic dissection type A scorecard. Ann Thorac Surg. 2017;104:1577–1582. [DOI] [PubMed] [Google Scholar]
- 17.Ehrlich MP, Ergin MA, McCullough JN, Lansman SL, Galla JD, Bodian CA, Apaydin A, Griepp RB. Results of immediate surgical treatment of all acute type A dissections. Circulation. 2000;102:III 248–252. [DOI] [PubMed] [Google Scholar]
- 18.Preece R, Srivastava V, Akowuah E, Kendall S. Should limb revascularization take priority over dissection repair in type A aortic dissection presenting as isolated acute limb ischaemia. Interact Cardiovasc Thorac Surg. 2017;25:643–646. [DOI] [PubMed] [Google Scholar]
- 19.Williams DM, Lee DY, Hamilton BH, Marx MV, Narasimham DL, Kazanjian SN, Prince MR, Andrews JC, Cho KJ, Deeb GM. The dissected aorta: percutaneous treatment of ischemic complications--principles and results. J Vasc Interv Radiol. 1997;8:605–625. [DOI] [PubMed] [Google Scholar]
- 20.Williams DM, Lee DY, Hamilton BH, Marx MV, Narasimham DL, Kazanjian SN, Prince MR, Andrews JC, Cho KJ, Deeb GM. The dissected aorta: part III. Anatomy and radiologic diagnosis of branch-vessel compromise. Radiology. 1997;203:37–44. [DOI] [PubMed] [Google Scholar]
- 21.Deeb GM, Williams DM, Bolling SF, Quint LE, Monaghan H, Sievers J, Karavite D, Shea M. Surgical delay for acute type A dissection with malperfusion. Ann Thorac Surg. 1997;64:1669–1675; discussion 1675–1677. [DOI] [PubMed] [Google Scholar]
- 22.Patel HJ, Williams DM, Dasika NL, Suzuki Y, Deeb GM. Operative delay for peripheral malperfusion syndrome in acute type A aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg. 2008;135:1288–1295; discussion 1295–1296. [DOI] [PubMed] [Google Scholar]
- 23.Lauterbach SR, Cambria RP, Brewster DC, Gertler JP, Lamuraglia GM, Isselbacher EM, Hilgenberg AD, Moncure AC. Contemporary management of aortic branch compromise resulting from acute aortic dissection. J Vasc Surg. 2001;33:1185–1192. [DOI] [PubMed] [Google Scholar]
- 24.Midulla M, Renaud A, Martinelli T, Koussa M, Mounier-Vehier C, Prat A, Beregi JP. Endovascular fenestration in aortic dissection with acute malperfusion syndrome: immediate and late follow-up. J Thorac Cardiovasc Surg. 2011;142:66–72. [DOI] [PubMed] [Google Scholar]
- 25.Tsagakis K, Konorza T, Dohle DS, Kottenberg E, Buck T, Thielmann M, Erbel R, Jakob H. Hybrid operating room concept for combined diagnostics, intervention and surgery in acute type A dissection. Eur J Cardiothorac Surg. 2013;43:397–404. [DOI] [PubMed] [Google Scholar]
- 26.Yamashiro S, Arakaki R, Kise Y, Inafuku H, Kuniyoshi Y. Management of visceral malperfusion complicated with acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2015;21:346–351. [DOI] [PubMed] [Google Scholar]
- 27.Goldberg JB, Lansman SL, Kai M, Tang GHL, Malekan R, Spielvogel D. Malperfusion in type A dissection: consider reperfusion first. Semin Thorac Cardiovasc Surg. 2017;29:181–185. [DOI] [PubMed] [Google Scholar]
- 28.Santini F, Montalbano G, Casali G, Messina A, Iafrancesco M, Luciani GB, Rossi A, Mazzucco A. Clinical presentation is the main predictor of in-hospital death for patients with acute type A aortic dissection admitted for surgical treatment: a 25 years experience. Int J Cardiol. 2007;115:305–311. [DOI] [PubMed] [Google Scholar]
- 29.Augoustides JG, Geirsson A, Szeto WY, Walsh EK, Cornelius B, Pochettino A, Bavaria JE. Observational study of mortality risk stratification by ischemic presentation in patients with acute type A aortic dissection: the Penn classification. Nat Clin Pract Cardiovasc Med. 2009;6:140–146. [DOI] [PubMed] [Google Scholar]
- 30.Czerny M, Schoenhoff F, Etz C, Englberger L, Khaladj N, Zierer A, Weigang E, Hoffmann I, Blettner M, Carrel TP. The impact of pre-operative malperfusion on outcome in acute type A aortic dissection: Results from the GERAADA Registry. J Am Coll Cardiol. 2015;65:2628–2635. [DOI] [PubMed] [Google Scholar]
- 31.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. [DOI] [PubMed] [Google Scholar]
- 32.Therneau T, Crowson C, Atkinson E. Multi-state models and competing risks. CRAN-R. (https://cran.r-project.org/web/packages/survival/vignettes/compete.pdf).
- 33.Yang B, Patel HJ, Williams DM, Dasika NL, Deeb GM. Management of type A dissection with malperfusion. Ann Cardiothorac Surg. 2016;5:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamman AV, Yang B, Kim KM, Williams DM, Deeb GM, Patel HJ. Visceral malperfusion in aortic dissection: the Michigan experience. Semin Thorac Cardiovasc Surg. 2017;29:173–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.