Abstract
Thyroid cancer is a frequent endocrine-related malignancy with continuously increasing incidence, and recently the development in understanding its molecular pathogenesis is mainly through the explanation of the original role of several key signaling pathways and related molecular distributors. Central to these mechanisms are the genetic and epigenetic alterations in these pathways such as mutation and DNA rearrangements. However, it does not mean that all the somatic abnormalities in a cancer genome are involved in cancer development and just driver mutations are concerned in tumor initiation. By way of illustrations, MAPK pathway motivated by BRAFV600E and RAS and RET / PTC rearrangements are suggesting driver genetic alterations in follicular derived thyroid cancers considered in the current review.
Key Words: Thyroid Cancer, Proto Oncogene Protein B raf, MAP kinase signaling system, Proto-Oncogene Proteins p21(ras)
Introduction
Thyroid cancer is the most common endocrine-related cancer that its incidence has continuously increased in the last three decades all over the world (1-5). Thyroid carcinomas are heterogeneous groups of neoplasm with typical histopathological features similar to other tumors (6).
The thyroid gland is composed of two main types of epithelial cells: the follicular cells, which convert iodine into thyroxine, also known as T4, and Triiodothyronine, also known as T3. The thyroid hormones, triiodothyronine (T3) and its prohormone, thyroxine (T4), are tyrosine-based hormones produced by the thyroid gland primarily responsible for regulation of metabolism. Another type of epithelial cells is paralfollicular or C-cells, which secrete calcitonin. Primary thyroid cancers mostly initiate from thyroid follicular cells (epithelial tumors) and develop three main pathological types of carcinomas: papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC) and anaplastic thyroid carcinoma (ATC) contrary to medullary thyroid carcinoma (MTC) that arises from thyroid parafollicular (C) cells (7-9). Due to well differentiation and indolent tumor growth, PTC and FTC are classified as differentiated thyroid cancer (DTC). PTC consists of 85%-90% of all thyroid cancer cases, followed by FTC (5%-10%) and MTC (about 2%), while ATC accounts for a smaller amount than 2% of thyroid cancers and usually happens in the aged people (10).
The classic treatment for thyroid cancer is thyroidectomy and adjuvant radioiodine ablation that most patients can be cured, but still surgically inoperative recurrence, refractoriness to radioiodine in DTC, poorly differentiated thyroid carcinoma and ATC are unsolved. Similar to other solid cancers, thyroid cancer is commenced by genetic alterations and epigenetic changes in driver oncogenes or tumor suppressor genes (11-14). Recent advancement of molecular technologies brought a new insight to the thyroid tumors diagnosis and prognosis. The current review mainly focused on the follicular thyroid cell derived cancers genetics in order to shed light on driver genetic alterations and their importance in thyroid tumor genesis.
Molecular genetics of thyroid cancer
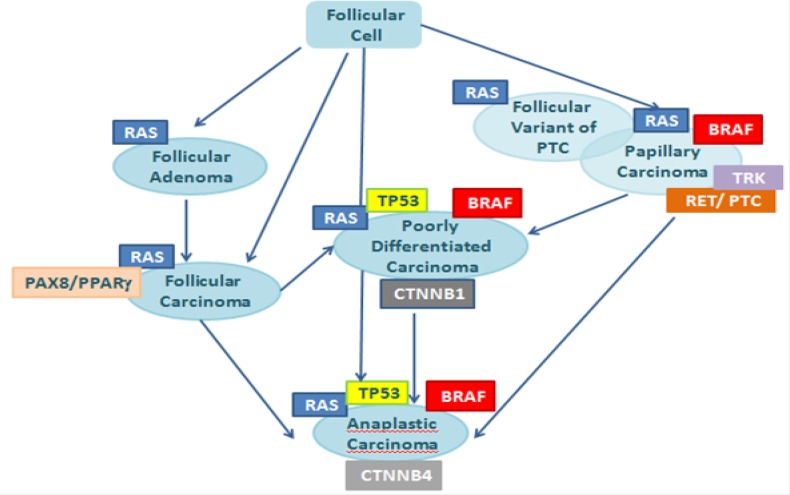
Thyroid cancer comes up as a result of multiple genetic and epigenetic alterations in the DNA of cancer cells. There are numerous somatic point mutations and chromosomal rearrangements in different steps of follicular cell-derived thyroid cancer (Figure 1) (15,16) that mainly belong to the MAPK signaling pathway and RET/PTC rearrangements (17).
Figure 1.
Stepwise dedifferentiation of follicular cell-derived thyroid cancer.
It should be kept in mind that not all the somatic abnormalities of a cancer genome are involved in initiation of the cancer since some are the consequences of carcinogenesis; hence, the terms “driver” and “passenger” mutation are made up. A driver mutation is an oncogenesis implication in cancer stem cells and is positively selected in the microenvironment of the tissue where the cancer begins and is not needed for maintenance of the final cancer (although it often is) (18,19).
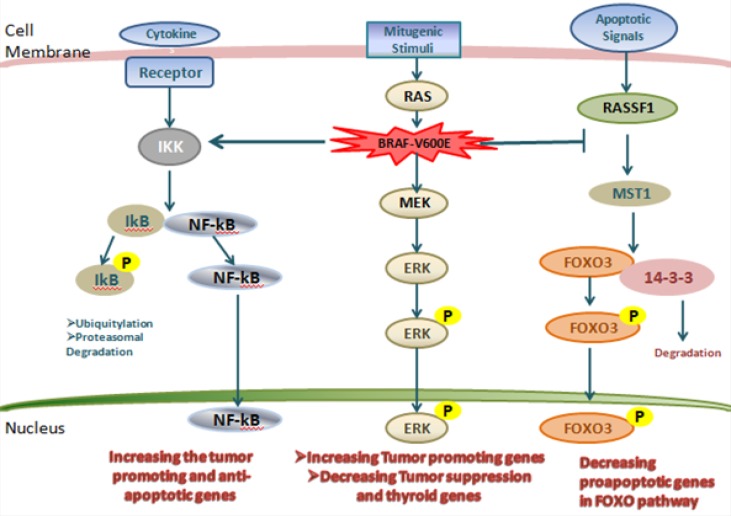
A passenger mutation is not selected, is not given clonal increase and therefore does not contribute to cancer development. Since somatic mutations without functional consequences often happen during cell division, passenger mutations are initiated within cancer genomes (20). One of the problematic issues is the discrimination of driver from passenger mutations. Whole-genome sequencing, however, incorporating analysis of more than 20,000 proteincoding genes and unknown numbers of functional elements in intronic and intergenic DNA, presents a greater challenge. Investigation of the biological consequences of putative driver mutations often consolidate the evidence implicating them in oncogenesis and provide insight into the subverted biological processes by which they contribute to cancer development. Thyroid cancer is a genetically simple disease with a relatively low number of mutations in each tumor. Driver mutations and gene fusions are identified in most of thyroid cancers suggesting that two main cell signaling pathways, MAPK and PI3K-AKT, are involved in the development of thyroid tumors (17,21). The MAPK/ERK pathway, also known as the Ras-Raf-MEK-ERK pathway, is a transporter of a signal from a receptor on the cell surface to the nucleus (DNA) (Figure 2). After binding a signaling molecule to its target receptor on the cell surface, this signaling pathway initiates and when the DNA in the nucleus expresses a protein in order to make some changes in the cell, it is terminated (22). This pathway has lots of proteins, including MAPK (mitogen-activated protein kinases, originally called ERK, extracellular signal-regulated kinases) and is connected with the cell proliferation, differentiation, migration, and senescence; and apoptosis components of the MAPK/ ERK pathway were discovered when they were found in cancer cells (6, 22-24).
Figure 2.
The MAPK and related pathways in thyroid cancer.
Nuclear factor-κB (NF-κB) pathway that is leading to activation of the inhibitor of κB (IκB) kinase (IKK), resulting in the phosphorylation of IκB and dissociation from NF-κB. Free NF-κB then enters the nucleus to promote the expression of tumor-promoting genes. On the right side of the figure is the RASSF1–mammalian STE20-like protein kinase 1 (MST1)– fork head box O3 (FOXO3) pathway and activated MST1 then phosphorylates FOXO3 on Ser207. Phosphorylated FOXO3 enters the nucleus to promote the expression of pro-apoptotic genes in the FOXO pathway. In the middle of the figure is a unique and powerful mechanism of thyroid tumor genesis driven by BRAF-V600E. DAPK1, death-associated protein kinase 1; HIF1A, hypoxia-inducible factor 1α; MMP, matrix metalloproteinase; NIS, sodium–iodide symporter; TGFB1, transforming growth factor β1; TIMP3, tissue inhibitor of metallo proteinases 3; TPO, thyroid peroxidase; TSHR, thyroid-stimulating hormone receptor; TSP1, thrombospondin 1; UPA, urokinase plasminogen activator; UPAR, urokinase plasminogen activator receptor; VEGFA, vascular endothelial growth factor A (25).
In early thyroid cancer, MAPK pathway is motivated by mutations in BRAF and RAS or by RET / PTC rearrangements. A key driver mutation upsetting MAPK pathway is the point mutation of BRAF, which makes the expression of BRAFV600E mutant protein resulting in constitutive activation of the serine/threonine kinase (26-31). In fact, amino acid substitution at posi-, tion 600 in BRAF, from a Valine (V) to a glutamic acid (E) is the result of V600E mutation. This mu-i tation occurs within the activation segment of the kinase domain (Figure 3). BRAF mutations are also frequently found in tumors with no driver mutations in NRAS, KIT, and other genes. BRAFV600E mutation is found in about 45% of PTCs (32, 33). However, some human PTC tumors show intra-tumors heterogeneity in the BRAF genotype — a minority of cells have BRAFV600E while the majority contain wild-type BRAF (34).
Figure 3.
Schematic of BRAF V600E mutation. Functional domains of BRAF are depicted. CR1: conserved regions 1. CR2: conserved region 2.
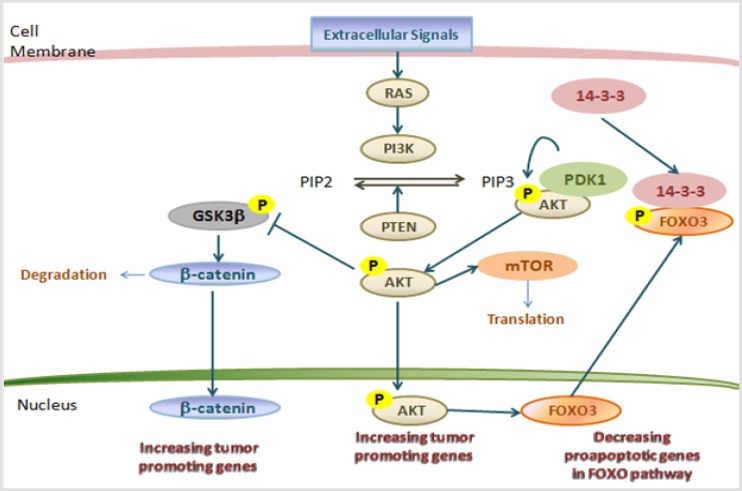
After BRAF mutations in thyroid cancer, RAS mutations are the most important driver genetic alteration (35, 36). RAS is in bound with GTP and when intrinsic GTPase of RAS hydrolyses GTP and converts RAS into an inactive GDP-bound state the RAS signaling is terminated (Figure 4) (37). There are three isoforms of RAS: HRAS, KRAS, and NRAS, and NRAS is predominantly mutated in thyroid tumors, mostly involving codons 12 and 61(30,38). The RAS mutations in follicular thyroid adenoma (FTA), a supposed premalignant lesion, suggests that activated RAS may have a role in early follicular thyroid cell tumor genesis and higher aggressive tumor behaviors (38,39). The expression of mutant HRAS was induced and resulted in differentiated colonies (39-41). Moreover, in the thyroid gland of transgenic mouse studies with conditional physiological expression of a KRAS had no transformation, but simultaneous KRAS mutant expression and PTEN deletion induced a rapid occurrence of aggressive FTC (42-44).
Figure 4.
The PI3K–AKT and related pathways in thyroid cancer (37).
Another main driver genetic alteration in thyroid cancer is the rearranged during transfusion (RET) proto-oncogene. RET (rearranged during transfecntion), is localized on chromosome 10 (10q11.2) and has 21 exons (45). The natural alternative splicing of the RET gene consequences in making three different isoforms of the protein RET; RET51, RET43, and RET9, which have 51, 43, and 9 amino acids in their C-terminal tail respectively (46). Each protein is divided into three domains: an N-terminal extra-i cellular domain with four cadherin-like repeats and a cysteine-rich region, a hydrophobic transmembrane domain and a cytoplasmic tyrosine kinase domain, which is split by an insertion of 27 amino acids (47). As a result of its capability to transform NIH/3T3 cells by DNA rearrangement, the RET proto-oncogene was first recognized in 1985 (48). The proteins that RET encodes is a cellular tyrosine kinase transmembrane receptor that is separated into the three main domains: an N-terminal extracellular domain containing fourcadherin-like regions; a cysteine-rich region with a transmembrane domain; and a cytoplasmic domain with tyrosine kinase activity (47, 49-51). Four diverse ligands are described: Glial Derived Neurotrophic (GDN) factors, Neurturin (NRTN), Artimin (ARTN), and Persepin (PSPN), respectively (47, 52, 53). DNA rearrangements are a result of homologous recombination, gene conversion, and illegitimate recombination. During homologous recombination in a cell containing more than one copy of a given chromosome, one copy can combine with corresponding segments of the other. This kind of recombination is ultimately dependent upon the DNA sequence homology between the two copies. Several types of RET/PTC rearrangements are reported (Table 1) (54,55). The presence of RET/PTC rearrangement in microcarcinoma powerfully supports the hypothesis of a driving role of this oncogene in the tumor transformation (56).
Table 1.
Different types of RET/PTC rearrangements in thyroid tumors according to Nikiforov YE (57)
| Oncogene | Donor gene | Chromosomal location |
|---|---|---|
| RET/PTC1 | CCD6(formerly H4) | 10q21 |
| RET/PTC2 | PRKAR1A | 17q23 |
| RET/PTC3 | NCO4 (formerly Ele 1) | 10q11.2 |
| RET/PTC4 | NCO4 (formerly Ele1) | 10q11.2 |
| RET/PTC5 | Golgas | 14q |
| RET/PTC6 | TRIM24 | 7q32-34 |
| RET/PTC7 | TRIM33 | 1p13 |
| RET/PTC8 | KTN1 | 14q22.1 |
| RET/PTC9 | RFG9 | 18q21-22 |
| ELKS-RET | ELKS | 12p13.3 |
| PCM1-RET | PCM1 | 8p21-22 |
| RFP-RET | TRIM27 | 6p21 |
| HOOK3-RET | HOOK3 | 8p11.21 |
The described RET/PTC prevalence in thyroid tumors varies greatly in different studies (58-65). However, this difference can be the consequence of tumor heterogeneity, ethnical and geographic variations, and dissimilar sensitivities of detection methods (66-68). RET/PTC rearrangements are more often in thyroid cancers after radiation exposure (50-80%) (69-72). The biological mechanisms of radiation carcinogenesis related to RET/PTC rearrangements are studied several times. It is observed that damage to cellular DNA is responsible for mutagenesis and carcinogenesis and those double-strand breaks are the most important event for the direct generation of gene translocations and rearrangements (21, 73-77). Thanks to the recent advanced next generation sequencing and whole genome sequencing, the number of candidate genetic changes in thyroid cancer has increased (78). But it is really important to discriminate between driver and passenger ones. Other genetic changes considered as passenger mutations include: PI3K (phosphatidylinositol-3 kinase), β-catenin (CTNNB1), TP53, is citrate dehydrogenase 1 (IDH1), anaplastic lymphoma kinase (ALK), and epidermal growth factor receptor (EGFR) (79-89). The preferential occurrences of these mutations in PDTC and ATC, which are the most aggressive thyroid cancers, indicate the fact that they may have a role in the progression and aggressiveness of thyroid cancer.
Conclusions
While diverse oncogenes are involved in thyroid tumor genesis, BRAF and RAS mutations, and RET/ PTC rearrangements are most frequently involved as a driver changes. Notwithstanding all these observations, no strong supporting data still show a classic prognostic role for BRAF and RAS mutations, and RET/PTC rearrangements. But it is clear that RET/ PTC rearrangements are correlated with radiation exposure and are more recurrent in patients with radio induced PTC.
Acknowledgment
Authors acknowledge their gratitude to Endocrinology and Metabolism Population Sciences Institute, Tehran University of Medical Sciences. This article was a part of a superior project granted by the National Institute for Medical Research Development (NIMAD, Grant number: 957222).
Conflict of Interest
The authors declared no conflict of interest.
References
- 1.Curado M-P, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer incidence in five continents. Vol 4. Lyon: IARC Press, International Agency for Research on Cancer; 2007. [Google Scholar]
- 2.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: update on epidemiology and risk factors. J Cancer Epidemiol. 2013:2013. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larijani B, Shirzad M, Mohagheghi M, Haghpanah V, Mosavi-Jarrahi A, Tavangar S, et al. Epidemiologic analysis of the Tehran cancer institute data system registry (TCIDSR) Asian Pac J Cancer Prev. 2004;5(1):36–9. [PubMed] [Google Scholar]
- 4.Haghpanah V, Soliemanpour B, Heshmat R, Mosavi-Jarrahi A, Tavangar S, Malekzadeh R, et al. Endocrine cancer in Iran: based on cancer registry system. Indian J Cancer. 2006;43(2):80–5. doi: 10.4103/0019-509x.25889. [DOI] [PubMed] [Google Scholar]
- 5.Larijani B, Mohagheghi MA, Bastanhagh MH, Mosavi-Jarrahi AR, Haghpanah V, Tavangar SM, et al. Primary thyroid malignancies in Tehran, Iran. Med Princ Pract. 2005;14(6):396–400. doi: 10.1159/000088112. [DOI] [PubMed] [Google Scholar]
- 6.Nasseri-Moghaddam S, Malekzadeh R, Sotoudeh M, Tavangar M, Azimi K, Sohrabpour AA, et al. Lower esophagus in dyspeptic Iranian patients: a prospective study. J Gastroenterol Hepatol. 2003;18(3):315–21. doi: 10.1046/j.1440-1746.2003.02969.x. [DOI] [PubMed] [Google Scholar]
- 7.DeLellis RA. Pathology and genetics of tumours of endocrine organs. Lyon: IARC; 2004. [Google Scholar]
- 8.Nikiforov YE, Biddinger PW, Thompson LD. Diagnostic pathology and molecular genetics of the thyroid: a comprehensive guide for practicing thyroid pathology. Philadelphia: Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 9.Sanii S, Saffar H, Tabriz HM, Qorbani M, Haghpanah V, Tavangar SM. Expression of matrix metalloproteinase-2, but not caspase-3, facilitates distinction between benign and malignant thyroid follicular neoplasms. Asian Pac J Cancer Prev. 2012;13(5):2175–8. doi: 10.7314/apjcp.2012.13.5.2175. [DOI] [PubMed] [Google Scholar]
- 10.Larijani B, Shirzad M, Mohagheghi M, Haghpanah V, Jarahi AM, Tavangar S, et al. Epidemiologic feature of thyroid cancer based on cancer registry data system. Iran J Public Health. 2005;34(4):1–7. [Google Scholar]
- 11.Rashid S. Hallmarks of Cancer Cell. Cancer and Chemoprevention: An Overview. Vol. 13. New York: Springer; 2017. p. 3. [Google Scholar]
- 12.Hanahan D, Weinberg RA. The hallmarks of cancer. cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 13.Amoli MM, Yazdani N, Amiri P, Sayahzadeh F, Haghpanah V, Tavangar SM, et al. HLA-DR association in papillary thyroid carcinoma. Dis Markers. 2010;28(1):49–53. doi: 10.3233/DMA-2010-0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khatami F, Tavangar SM. Current Diagnostic Status of Pheochromocytomaand Future Perspective: A Mini Review. Iran J Pathol. 2017;12(3):313–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Rossing M. Classification of follicular cell-derived thyroid cancer by global RNA profiling. J Mol Endocrinol. 2013;50(2):R39–51. doi: 10.1530/JME-12-0170. [DOI] [PubMed] [Google Scholar]
- 16.Katoh H, Yamashita K, Enomoto T, Watanabe M. Classification and general considerations of thyroid cancer. Ann Clin Pathol. 2015;3(1):1045–54. [Google Scholar]
- 17.Nikiforov YE. Molecular analysis of thyroid tumors. Mod Pathol. 2011;24(S2):S34–43. doi: 10.1038/modpathol.2010.167. [DOI] [PubMed] [Google Scholar]
- 18.Pon JR, Marra MA. Driver and passenger mutations in cancer. Annu Rev Pathol. 2015;10:25–50. doi: 10.1146/annurev-pathol-012414-040312. [DOI] [PubMed] [Google Scholar]
- 19.Bozic I, Antal T, Ohtsuki H, Carter H, Kim D, Chen S, Karchin R, Kinzler KW, Vogelstein B, Nowak MA. Accumulation of driver and passenger mutations during tumor progression. Proc Natl Acad Sci U S A. 2010;107(43):18545–50. doi: 10.1073/pnas.1010978107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsumoto T, Shimizu T, Takai A, Marusawa H. Exploring the mechanisms of gastrointestinal cancer development using deep sequencing analysis. Cancers. 2015;7(2):1037–51. doi: 10.3390/cancers7020823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kajbafzadeh A-M, Payabvash S, Salmasi AH, Monajemzadeh M, Tavangar SM. Smooth muscle cell apoptosis and defective neural development in congenital ureteropelvic junction obstruction. J Urol. 2006;176(2):718–23. doi: 10.1016/j.juro.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 22.Sun Y, Liu W-Z, Liu T, Feng X, Yang N, Zhou H-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600–4. doi: 10.3109/10799893.2015.1030412. [DOI] [PubMed] [Google Scholar]
- 23.Haddadi-Nezhad S, Larijani B, Tavangar SM, Nouraei SM. Comparison of fine-needle-nonaspiration with fine-needle-aspiration technique in the cytologic studies of thyroid nodules. Endocr Pathol. 2003;14(4):369–73. doi: 10.1385/ep:14:4:369. [DOI] [PubMed] [Google Scholar]
- 24.Tavangar S, Monajemzadeh M, Larijani B, Haghpanah V. Immunohistochemical study of oestrogen receptors in 351 human thyroid glands. Singapore Med J. 2007;48(8):744–7. [PubMed] [Google Scholar]
- 25.Orton RJ, Sturm OE, Vyshemirsky V, Calder M, Gilbert DR, Kolch W. Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem J. 2005;392(2):249–61. doi: 10.1042/BJ20050908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003;95(8):625–7. doi: 10.1093/jnci/95.8.625. [DOI] [PubMed] [Google Scholar]
- 28.Soares P, Trovisco V, Rocha AS, Lima J, Castro P, Preto A, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22(29):4578. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 29.Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88(9):4393–7. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 30.Kimura ET, Nikiforova MN, Zhu Z, Knauf JA, Nikiforov YE, Fagin JA. High prevalence of BRAF mutations in thyroid cancer. Cancer Res. 2003;63(7):1454–7. [PubMed] [Google Scholar]
- 31.Khatami F, Larijani B, Tavangar S. Circulating Tumor BRAF Mutation and Personalized Thyroid Cancer Treatment. Asian Pac J Cancer Prev. 2017;18(2):293–4. doi: 10.22034/APJCP.2017.18.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarchoan M, LiVolsi VA, Brose MS. BRAF Mutation and Thyroid Cancer Recurrence. J Clin Oncol. 2015;33(1):7–8. doi: 10.1200/JCO.2014.59.3657. [DOI] [PubMed] [Google Scholar]
- 33.Larijani B, Khorgami Z, Tavangar S, Haghpanah V, Mehdipour P. Prevalence of BRAFV600E mutation in Iranian patients with papillary thyroid carcinoma: a single-center study. J Appl Sci. 2009;9(19):3593–7. [Google Scholar]
- 34.Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–62. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 35.Howell GM, Hodak SP, Yip L. RAS mutations in thyroid cancer. Oncologist. 2013;18(8):926–32. doi: 10.1634/theoncologist.2013-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohammadi-asl J, Larijani B, Khorgami Z, Tavangar SM, Haghpanah V, Kheirollahi M, et al. Qualitative and quantitative promoter hypermethylation patterns of the P16, TSHR, RASSF1A and RARβ2 genes in papillary thyroid carcinoma. Med Oncol. 2011;28(4):1123–8. doi: 10.1007/s12032-010-9587-z. [DOI] [PubMed] [Google Scholar]
- 37.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13(3):184–99. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing M. Clinical utility of RAS mutations in thyroid cancer: a blurred picture now emerging clearer. BMC Med. 2016;14(1):12. doi: 10.1186/s12916-016-0559-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puzziello A, Guerra A, Murino A, Izzo G, Carrano M, Angrisani E, et al. Benign thyroid nodules with RAS mutation grow faster. Clin Endocrinol (Oxf) 2016;84(5):736–40. doi: 10.1111/cen.12875. [DOI] [PubMed] [Google Scholar]
- 40.Gire V, Wynford-Thomas D. RAS oncogene activation induces proliferation in normal human thyroid epithelial cells without loss of differentiation. Oncogene. 2000;19(6):737. doi: 10.1038/sj.onc.1203399. [DOI] [PubMed] [Google Scholar]
- 41.Bond J, Wyllie F, Rowson J, Radulescu A, Wynford-Thomas D. In vitro reconstruction of tumour initiation in a human epithelium. Oncogene. 1994;9(1):281–90. [PubMed] [Google Scholar]
- 42.Miller KA, Yeager N, Baker K, Liao X-H, Refetoff S, Di Cristofano A. Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res. 2009;69(8):3689–94. doi: 10.1158/0008-5472.CAN-09-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sarmadi S, Izadi-Mood N, Sotoudeh K, Tavangar SM. Altered PTEN expression; a diagnostic marker for differentiating normal, hyperplastic and neoplastic endometrium. Diagn Pathol. 2009;4(1):41. doi: 10.1186/1746-1596-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Razavi SA, Modarressi MH, Yaghmaei P, Tavangar SM, Hedayati M. Circulating levels of PTEN and KLLN in papillary thyroid carcinoma: can they be considered as novel diagnostic biomarkers? Endocrine. 2017:57(3):428–35. doi: 10.1007/s12020-017-1368-4. [DOI] [PubMed] [Google Scholar]
- 45.Ceccherini I, Bocciardi R, Luo Y, Pasini B, Hofstra R, Takahashi M, et al. Exon Structure and Flanking Intronic Sequences of the Human RET Proto-oncogene. Biochem Biophys Res Commun. 1993;196(3):1288–95. doi: 10.1006/bbrc.1993.2392. [DOI] [PubMed] [Google Scholar]
- 46.Myers SM, Eng C, Ponder BA, Mulligan LM. Characterization of RET proto-oncogene 3' splicing variants and polyadenylation sites: a novel C-terminus for RET. Oncogene. 1995;11(10):2039–45. [PubMed] [Google Scholar]
- 47.Arighi E, Borrello MG, Sariola H. RET tyrosine kinase signaling in development and cancer. Cytokine Growth Factor Rev. 2005;16(4):441–67. doi: 10.1016/j.cytogfr.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42(2):581–8. doi: 10.1016/0092-8674(85)90115-1. [DOI] [PubMed] [Google Scholar]
- 49.Anders J, Kjær S, Ibá-ez CF. Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calcium-binding site. J Biol Chem. 2001;276(38):35808–17. doi: 10.1074/jbc.M104968200. [DOI] [PubMed] [Google Scholar]
- 50.Ibá-ez CF. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb Perspect Biol. 2013;5(2):a009134. doi: 10.1101/cshperspect.a009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goodman KM, Kjær S, Beuron F, Knowles PP, Nawrotek A, Burns EM, et al. RET recognition of GDNF-GFRα1 ligand by a composite binding site promotes membrane-proximal self-association. Cell Rep. 2014;8(6):1894–904. doi: 10.1016/j.celrep.2014.08.040. [DOI] [PubMed] [Google Scholar]
- 52.Santoro M, Rosati R, Grieco M, Berlingieri M, D'amato G, De Franciscis V, et al. The ret proto-oncogene is consistently expressed in human pheochromocytomas and thyroid medullary carcinomas. Oncogene. 1990;5(10):1595–8. [PubMed] [Google Scholar]
- 53.Menicali E, Moretti S, Voce P, Romagnoli S, Avenia N, Puxeddu E. Intracellular Signal Transduction and Modification of the Tumor Microenvironment Induced by RET/PTCs in Papillary Thyroid Carcinoma. Front Endocrinol (Lausanne) 2012;3:67. doi: 10.3389/fendo.2012.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greco A, Borrello M, Miranda C, Degl'Innocenti D, Pierotti M. Molecular pathology of differentiated thyroid cancer. Q J Nucl Med Mol Imaging. 2009;53(5):440–53. [PubMed] [Google Scholar]
- 55.Ciampi R, Giordano TJ, Wikenheiser-Brokamp K, Koenig RJ, Nikiforov YE. HOOK3-RET: a novel type of RET/PTC rearrangement in papillary thyroid carcinoma. Endocr Relat Cancer. 2007;14(2):445–52. doi: 10.1677/ERC-07-0039. [DOI] [PubMed] [Google Scholar]
- 56.Viglietto G, Chiappetta G, Martinez-Tello FJ, Fukunaga FH, Tallini G, Rigopoulou D, et al. RET/PTC oncogene activation is an early event in thyroid carcinogenesis. Oncogene. 1995;11(6):1207–10. [PubMed] [Google Scholar]
- 57.Nikiforov YE. RET/PTC rearrangement in thyroid tumors. Endocr Pathol. 2002;13(1):3–16. doi: 10.1385/ep:13:1:03. [DOI] [PubMed] [Google Scholar]
- 58.Zou M, Shi Y, Farid NR. Low rate of ret proto‐oncogene activation (PTC/retTPC) in papillary thyroid carcinomas from saudi arabia. Cancer. 1994;73(1):176–80. doi: 10.1002/1097-0142(19940101)73:1<176::aid-cncr2820730130>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 59.Tallini G, Asa SL, Fuller GN. RET oncogene activation in papillary thyroid carcinoma. Adv Anat Pathol. 2001;8(6):345–54. doi: 10.1097/00125480-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Chua EL, Wu WM, Tran KT, McCarthy SW, Lauer CS, Dubourdieu D, et al. Prevalence and distribution of ret/ptc 1, 2, and 3 in papillary thyroid carcinoma in New Caledonia and Australia. J Clin Endocrinol Metab. 2000;85(8):2733–9. doi: 10.1210/jcem.85.8.6722. [DOI] [PubMed] [Google Scholar]
- 61.Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM. The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab. 2000;85(3):1170–5. doi: 10.1210/jcem.85.3.6472. [DOI] [PubMed] [Google Scholar]
- 62.Sheils O, O'Leary J, Uhlmann V, Lüttich K, Sweeney E. ret/PTC-1 activation in Hashimoto thyroiditis. Int J Surg Pathol. 2000;8(3):185–9. doi: 10.1177/106689690000800305. [DOI] [PubMed] [Google Scholar]
- 63.Puxeddu E, Moretti S, Giannico A, Martinelli M, Marino C, Avenia N, et al. Ret/PTC activation does not influence clinical and pathological features of adult papillary thyroid carcinomas. Eur J Endocrinol. 2003;148(5):505–13. doi: 10.1530/eje.0.1480505. [DOI] [PubMed] [Google Scholar]
- 64.Rhoden KJ, Unger K, Salvatore G, Yilmaz Y, Vovk V, Chiappetta G, et al. RET/papillary thyroid cancer rearrangement in nonneoplastic thyrocytes: follicular cells of Hashimoto's thyroiditis share low-level recombination events with a subset of papillary carcinoma. J Clin Endocrinol Metab. 2006;91(6):2414–23. doi: 10.1210/jc.2006-0240. [DOI] [PubMed] [Google Scholar]
- 65.Rhoden KJ, Johnson C, Brandao G, Howe JG, Smith BR, Tallini G. Real-time quantitative RT-PCR identifies distinct c-RET, RET/PTC1 and RET/PTC3 expression patterns in papillary thyroid carcinoma. Lab Invest. 2004;84(12) doi: 10.1038/labinvest.3700198. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Z, Ciampi R, Nikiforova MN, Gandhi M, Nikiforov YE. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab. 2006;91(9):3603–10. doi: 10.1210/jc.2006-1006. [DOI] [PubMed] [Google Scholar]
- 67.Marotta V, Guerra A, Sapio MR, Vitale M. RET/PTC rearrangement in benign and malignant thyroid diseases: a clinical standpoint. Eur J Endocrinol. 2011;165(4):499–507. doi: 10.1530/EJE-11-0499. [DOI] [PubMed] [Google Scholar]
- 68.Unger K, Zitzelsberger H, Salvatore G, Santoro M, Bogdanova T, Braselmann H, et al. Heterogeneity in the distribution of RET/PTC rearrangements within individual post-Chernobyl papillary thyroid carcinomas. J Clin Endocrinol Metab. 2004;89(9):4272–9. doi: 10.1210/jc.2003-031870. [DOI] [PubMed] [Google Scholar]
- 69.Hieber L, Huber R, Bauer V, Schäffner Q, Braselmann H, Thomas G, et al. Chromosomal rearrangements in post-Chernobyl papillary thyroid carcinomas: evaluation by spectral karyotyping and automated interphase FISH. J Biomed Biotechnol. 2011;2011:693691. doi: 10.1155/2011/693691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamatani K, Eguchi H, Ito R, Mukai M, Takahashi K, Taga M, et al. RET/PTC rearrangements preferentially occurred in papillary thyroid cancer among atomic bomb survivors exposed to high radiation dose. Cancer Res. 2008;68(17):7176–82. doi: 10.1158/0008-5472.CAN-08-0293. [DOI] [PubMed] [Google Scholar]
- 71.Di Cristofaro J, Vasko V, Savchenko V, Cherenko S, Larin A, Ringel M, et al. ret/PTC1 and ret/PTC3 in thyroid tumors from Chernobyl liquidators: comparison with sporadic tumors from Ukrainian and French patients. Endocr Relat Cancer. 2005;12(1):173–83. doi: 10.1677/erc.1.00884. [DOI] [PubMed] [Google Scholar]
- 72.Thomas G, Bunnell H, Cook H, Williams E, Nerovnya A, Cherstvoy E, et al. High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab. 1999;84(11):4232–8. doi: 10.1210/jcem.84.11.6129. [DOI] [PubMed] [Google Scholar]
- 73.Goodhead Dt. Initial events in the cellular effects of ionizing radiations: clustered damage in DNA. Int J Radiat Biol. 1994;65(1):7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- 74.Caudill CM, Zhu Z, Ciampi R, Stringer JR, Nikiforov YE. Dose-dependent generation of RET/PTC in human thyroid cells after in vitro exposure to γ-radiation: a model of carcinogenic chromosomal rearrangement induced by ionizing radiation. J Clin Endocrinol Metab. 2005;90(4):2364–9. doi: 10.1210/jc.2004-1811. [DOI] [PubMed] [Google Scholar]
- 75.Leeman‐Neill RJ, Brenner AV, Little MP, Bogdanova TI, Hatch M, Zurnadzy LY, et al. RET/PTC and PAX8/PPARγ chromosomal rearrangements in post‐Chernobyl thyroid cancer and their association with iodine‐131 radiation dose and other characteristics. Cancer. 2013;119(10):1792–9. doi: 10.1002/cncr.27893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Su X, Li Z, He C, Chen W, Fu X, Yang A. Radiation exposure, young age, and female gender are associated with high prevalence of RET/PTC1 and RET/PTC3 in papillary thyroid cancer: a meta-analysis. Oncotarget. 2016;7(13):16716. doi: 10.18632/oncotarget.7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang YY, Liu ZB, Ye XG, Ren WM. Iodine regulates G2/M progression induced by CCL21/CCR7 interaction in primary cultures of papillary thyroid cancer cells with RET/PTC expression. Mol Med Rep. 2016;14(4):3941–6. doi: 10.3892/mmr.2016.5686. [DOI] [PubMed] [Google Scholar]
- 78.Zheng B, Liu J, Gu J, Lu Y, Zhang W, Li M, et al. A three‐gene panel that distinguishes benign from malignant thyroid nodules. Int J Cancer. 2015;136(7):1646–54. doi: 10.1002/ijc.29172. [DOI] [PubMed] [Google Scholar]
- 79.Perrone F, Bertolotti A, Montemurro G, Paolini B, Pierotti MA, Colecchia M. Frequent mutation and nuclear localization of β-catenin in Sertoli cell tumors of the testis. Am J Surg Pathol. 2014;38(1):66–71. doi: 10.1097/PAS.0b013e31829cdbc6. [DOI] [PubMed] [Google Scholar]
- 80.Garcia-Rostan G, Camp RL, Herrero A, Carcangiu ML, Rimm DL, Tallini G. β-catenin dysregulation in thyroid neoplasms: down-regulation, aberrant nuclear expression, and CTNNB1 exon 3 mutations are markers for aggressive tumor phenotypes and poor prognosis. Am J Surg Pathol. 2001;158(3):987–96. doi: 10.1016/s0002-9440(10)64045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fagin JA, Matsuo K, Karmakar A, Chen DL, Tang S-h, Koeffler HP. High prevalence of mutations of the p53 gene in poorly differentiated human thyroid carcinomas. J Clin Invest. 1993;91(1):179. doi: 10.1172/JCI116168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Donghi R, Longoni A, Pilotti S, Michieli P, Della Porta G, Pierotti MA. Gene p53 mutations are restricted to poorly differentiated and undifferentiated carcinomas of the thyroid gland. J Clin Invest. 1993;91(4):1753. doi: 10.1172/JCI116385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Murugan AK, Bojdani E, Xing M. Identification and functional characterization of isocitrate dehydrogenase 1 (IDH1) mutations in thyroid cancer. Biochem Biophys Res Commun. 2010;393(3):555–9. doi: 10.1016/j.bbrc.2010.02.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hemerly JP, Bastos AU, Cerutti JM. Identification of several novel non-p R132 IDH1 variants in thyroid carcinomas. Eur J Endocrinol. 2010;163(5):747–55. doi: 10.1530/EJE-10-0473. [DOI] [PubMed] [Google Scholar]
- 85.Omidfar K, Moinfar Z, Sohi AN, Tavangar SM, Haghpanah V, Heshmat R, et al. Expression of EGFRvIII in thyroid carcinoma: immunohistochemical study by camel antibodies. Immunol Invest. 2009;38(2):165–80. doi: 10.1080/08820130902735998. [DOI] [PubMed] [Google Scholar]
- 86.Haghpanah V, Shooshtarizadeh P, Heshmat R, Larijani B, Tavangar SM. Immunohistochemical analysis of survivin expression in thyroid follicular adenoma and carcinoma. Appl Immunohistochem Mol Morphol. 2006;14(4):422–5. doi: 10.1097/01.pai.0000213100.88074.b8. [DOI] [PubMed] [Google Scholar]
- 87.Tabriz HM, Adabi K, Lashkari A, Heshmat R, Haghpanah V, Larijani B, et al. Immunohistochemical analysis of nm23 protein expression in thyroid papillary carcinoma and follicular neoplasm. Pathol Res Pract. 2009;205(2):83–7. doi: 10.1016/j.prp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 88.Haghpanah V, Ghaffari SH, Rahimpour P, Abbasi A, Saeedi M, Pak H, et al. Vitamin D receptor gene polymorphisms in patients with thyroid cancer. Gene Ther Mol Biol B. 2007;11(2):299–304. [Google Scholar]