Abstract
Background & objective:
Pneumocystis pneumonia (PCP) is responsible for pulmonary infection in immunocompromised patients. The current study aimed at investigating the frequency of Pneumocystis colonization in patients hospitalized in the intensive care unit (ICU) and evaluating the relationship between PCP and Pneumocystis colonization.
Methods:
The current cross sectional study was conducted on bronchoalveolar lavage (BAL) fluids of 100patients collected from surgery and neurosurgery ICUs with different underlying corticosteroid therapy conditions. Patients were divided into 2 groups (patients receiving and not receiving corticosteroids). Direct examination on BAL fluids was performed by the Gomori methenamine silver and Giemsa staining techniques. Additionally, 2 filtered air samples of the 2 above mentioned units were collected. A nested-PCR targeted mtLSUrRNA gene and sequencing were used to identify Pneumocystis spp.
Results:
In direct microscopy, 31 out of 100 hospitalized patients (31%) showed positive results. Twenty-three (46%) of smear positive patients were from the group of patients receiving corticosteroid, the other 8(16%) were from the group of patients not receiving corticosteroids (P= 0.001). Pneumocystis jirovecii DNA was detected in 77 out of 100 BAL samples by nested-PCR (77%) in which 40 (52%) and 37 (48%) samples were obtained from the patients receiving and not receiving corticosteroids, respectively. Pneumocystis genome was observed in 1 of the 2 filtered air samples.
Conclusion:
A significant number of patients receiving corticosteroids were also colonized by P. jirovecii that may be predisposed to PCP or be transmitted to susceptible patients. A significant relationship was observed between the mean hospital stay and detection rate.
Key Words: Pneumocystis jiroveci, Corticosteroids, Immunosuppression, PCR, Iran
Introduction
Pneumocystis spp. are eukaryotic, dimorphic, yeastlike fungi. Among five Pneumocystis species, Pneumocystis jirovecii is responsible for Pneumocystis pneumonia (PCP) in patients with immune system impairments (1).
Prevalence of human immunodeficiency virus (HIV) increased the rate of PCP worldwide. The use of routine prophylaxis in patients infected with HIV led to reduced rates of PCP in the population infected with HIV. Despite this decrease, it remains one of the leading causes of pneumonia in patients with other types of immunodeficiency (2-4). Solid tumor, organ transplantation, and cystic fibrosis are also observed in patients with cancers (particularly hematologic malignancies). Corticosteroids, chemotherapeutic agents, and other immunosuppressive medications are considered as important risk factors to develop PCP (57). In patients hospitalized in the intensive care units (ICUs), delay in intubation and increased duration of mechanical ventilation are decisive factors that elevate the rate of mortality by PCP (8-10). The respiratory tract of healthy people is also colonized with the microorganism (11). Pneumocystis spp. carriers serve as the reservoirs for the pathogen and predispose the host to subsequent opportunistic infections (8). Person-to-person transmission due to air pollution and previous colonization of pneumocystis in patients admitted to ICU can play important roles to spread infection between them. Development of PCP can intensify the underlying disease and complicate the therapeutic process for the patients. Therefore, the diagnosis of PCP should be considered in patients who are at risk for PCP. Quick assessment is very critical in immunocompromised patients or the ones treated with immunosuppressive drugs (12,13). The current study aimed at investigating the frequency of pneumocystis spp. colonization in patients admitted to surgery and neurosurgery ICUs of a teaching university hospital, Ardabil, Iran.
Materials and Methods Clinical specimens
In the current cross sectional study, a total of 100 BAL specimens were obtained during 2011 to 2014 from patients with pulmonary discharge without any evidence of PCP and 50 oral washing samples from healthy individuals in ICU. The clinical specimens were collected from patients admitted to surgery and neurosurgery ICUs of Fatemi Hospital, affiliated to Ardabil University of Medical Sciences, Ardabil, Iran.
Fifty out of 100 patients received corticosteroids (dexamethasone), while the others (50 ones) received no corticosteroid. All of the patients underwent suction and BAL sampling as part of their ordinary clinical and diagnostic assessments. Oral washing samples were obtained by gargling with 20 mL sterile physiologic serum (0.9% NaCl) for 1 minute from 50 healthy volunteers. Additionally, to examine the presence of Pneumocystis spp. in air, 2 filtered air samples of surgery and neurosurgery ICUs of the hospital were prepared. The study was approved by the Ethical Committee of Ardabil University of Medical Sciences (ID:IR.ARUMS REC.1396162) and informed consent was obtained from all study volunteers.
Approximately 3-4 mL of each BAL sample was transferred into sterile tubes, thoroughly vortexmixed, and divided into 2 aliquots. Two smears were prepared from 1 aliquot of each specimen for the Giemsa and Gomori methenamine silver (GMS) staining for the microscopic examinations and other aliquotswere stored at -80ºC for polymerase chain reaction (PCR).
Microscopic detection of pneumocystis by staining methods
The specimens were thoroughly mixed and centrifuged at 13000 g for 20 minutes; 10% NaCl solution was applied to loosen the mucoid materials in specimens if needed. The resultant pellets were used for direct microscopic examination and nested-PCR. For direct microscopic examination, smears were prepared, fixed, and stained by the Gomori methenamin silver (GMS) and Giemsa methods. Stained smears were observed by 2 experienced microscopists to enhance the sensitivity of the method (14).
A total of 2 air samples were collected from surgery and neurosurgery ICUs via the filters embedded in the main rooms of the units.
A0.2-µm Millipore filter and air pump were used for air filtration. The filter cassette was placed about
1.5 m from the floor, in the center of patients room. Nearly 3 m3 air was passed through the filter within 2 hours, while the filters were kept humid with sterile distilled water during filtration. Filters were put into sterile falcon and transferred to laboratory and used for DNA extraction.
Nested-PCR
DNA was extracted using QIAamp® DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer`s protocol. Extracted DNAs were stored at -20ºC until analysis.
Nested-PCR protocol targeting a conserved region of mtLSUrRNA was used to detect Pneumocystis spp. in specimens (7,15). The primer sequences for the initial amplification were pAZ102-H: 5´-GTGTACGTTGCAAAGTACTC-3´ as forward and pA Z102E:5´-GATGGCTGTTTCCAAGCCCA-3´ as revers which resulted in a 346-bp product.
The primers nested within the first step AZ102- X:5′-GTGAAATACAAATCGGACTAGG-3′as for- ward and pAZ102-Y: 5´-TCACTTAATATTAATTGGGGAGC -3´ as revers were used for the second amplification reaction and yielded a 260-bp product (1). Each reaction mixture for early PCR (final volume: 50 μL) consisted of 10µL 10X PCR buffer, 3 µM MgCl2, 100 µM deoxynucleoside triphosphates, 0.5μM primers, 5 U Tag DNA polymerase, 5 μL DNA template solution, and enough ultrapure distilled water (DNase and RNase free). Controls consisted of reaction mixture alone as negative and positive controls containing 2 μL DNA samples obtained from a patient with histologically confirmed pneumocystis (gifted from Prof. SabineSchmoldt, Max von Pettenkofer-Institutfür Hygiene und Medizinische, Mikrobiologie, Germany). Initial amplification reaction was carried out under the fallowing condition: 1 cycle at 94°C for 4 minutes, 35 cycles of 94˚C for 45 seconds, 54˚C for 60 seconds and 72˚C for 60 seconds, and a final extension for 7 minutes at 72˚C. For the nested-PCR, the following protocol was used with 2 μL of the initial amplification product as the template, 2 µM MgCl2 and 200µM deoxynucleoside triphosphates in 50 μL of reaction mixture. For the second amplification: 1 cycle at 94˚C for 4 minutes and 30 cycles at 94˚C for 45 seconds, 59˚C for 45 seconds, and 72˚C for 60 seconds, followed by a final extension cycle at 72˚C for 7 minutes.
The amplification products were electrophoresed on 1.5% agarose gel, and then, stained with ethidium bromide to visualize the amplicon. All the positive bands revealed in electrophoresis were extracted (Silica Bead DNA Gel Extraction Kit, Fermentas, Lithuania) using low melting point agarose (Fermentas, Lithuania) and then, purified and sequenced (MWG DNA Sequencing Services, Germany) to identify Pneumocystis spp.
Statistical analysis
Data were analyzed by Chi-square and t test with SPSS software version 15.0. In all statistical analyses P <0.05 was considered significant.
Results
A total of 100 patients, 38 (38%) from neurosurgery ICU and 62 (62%) from surgery ICU (67 males and 33 females) and 50 in healthy immunocompetent group were included in the current study. The patients were at the age range of 13-87 years (mean: 43.1±21.5); they were divided into 2 groups of patients who received corticosteroids (50%) and not received corticosteroids (50%). Dexamethasone systemically was administered to group of patients who received corticosteroids within a week before specimen collection. Corticosteroid perception ranged 8 to 336 mg daily with an average accumulative dose of 86.22 mg. The mean of hospital stay was 22.7±1.54 days (ranged 7 to 110). Hospitalization average for patients with direct positive microscopy was 17.4±2.2 and for patients with direct negative microscopy was 10.6±1.1 days (P=0.04), but the average of hospitalization in aforementioned groups was not significant regardingPCR results (13.7±1.6 vs. 9.5±1.1, respectively).
Although 72% of the patients received 1 or more antibiotics,none of them had trimethoprim/sulfamethoxazole (TMP-SMX) in their regimen. There was no significant difference between the patients who received and not receivedantibiotics basedon the results of direct microscopy and nested-PCR. The results of nested-PCR and direct microscopy tests are summarized in Table 1.
Table 1.
Frequency of Colonization of Pneumocystis jirovecii Among the Study Groups and Healthy Individuals
| Test | Patients |
Healthy Individuals;N (%) | |
|---|---|---|---|
| Patients who Received Corticosteroids; N (%) | Patients who did not Receive Corticosteroids; N (%) | ||
| Direct microscopy | 23 (46) | 8 (16) | ND |
| Total | 31 (31) | 0 (0) | |
| Nested-PCR | 40 (52) | 37 (48) | 8(16) |
| Total | 77 (77) | 11 (22) | |
| Each group comprised of 50 cases. | |||
ND, not detected
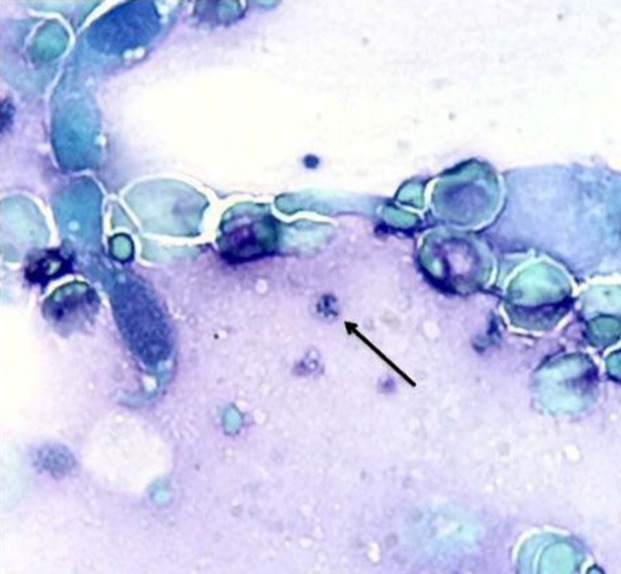
In direct microscopy testing, a total of 31 out of 100 hospitalized patients (31%) showed positive results (Figure 1). Twenty-three (46%) of smear positive patients were from the group of patients who received corticosteroids, the other 8 (16%) were from the group of patients who did not receive corticosteroids (P= 0.001).The direct microscopy did not show positive results in oral washing specimens for healthy individuals.
Figure 1.
Cyst of Pneumocystis spp. in BAL sample, stained with Giemsa
Atotal of 77 (77%) out of 100 subjects showed positive results using nested-PCR (Figure 2). Forty-one (52%) of positive specimens were from the group of patients who received corticosteroids and 37 (48%) from the group of patients who did not receive corticosteroids (P= 0.33). There were significant correlation between the patients who received corticosteroids with the accumulative dose of ≥88 mg and PCR positive results (P=0.02); on the other hand, 100% of the patients had positive result in PCR. Eight (16%) out of 50 oral wash specimens, obtained from healthy individuals, showed positive nested-PCR results. There was no correlation between the colonization rate and gender or age of the patients (P >0.05). The air filtered sample collected from 1 of the ICUs gave positive result in nested-PCR. Sequence analysis of the nested-PCR products of partial mtLSUrRNA gene revealed that all positive specimens, except 1 (Pneumocystis carinii), belonged to the same Pneumocystis species, Pneumocystis jirovecii.
Figure 2.
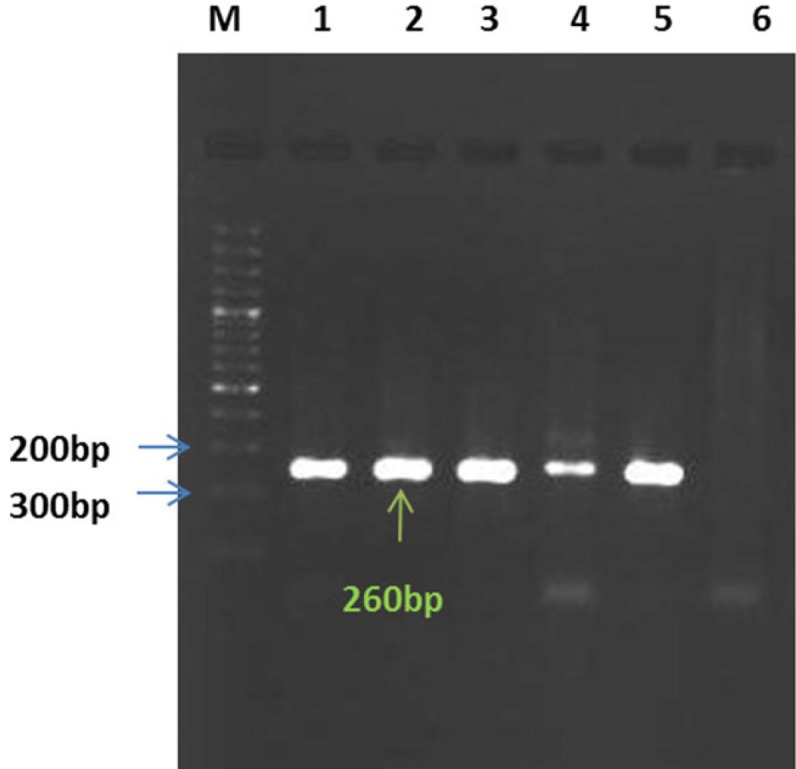
Nested-PCR results: Lanes 1, 2, 3, and 4 are positive samples; Lane5: positive control; Lane6: negative control; Lane M: the 100-bp Ladder (Fermentase, Germany)
Discussion
PCP is a seriouspneumonia that generally affects immunocompromised individuals. The incidence of PCP increases among patients receiving immunosuppressive medications in recent years (4,8). Upon large epidemiological studies, the mortality rates decreased in Europe in the last 2 decades, thanks to the systematic use of sulfa prophylaxis, adjunctive steroids (16). To the authors` best knowledge, the current study was the first on the frequency of PCP on intubated patients in ICU in Ardabil, Iran. The frequency of Pneumocystis jirovecii colonization in healthy immunocompetent adults was reported 0% to 20% in different studies (17). In the current study, using nested-PCR, 8 (16%) healthy individuals were colonized with Pneumocystis jirovecii.
The results of nested-PCR in the current study showed that the colonization rate of Pneumocystis jirovecii in intubated patients admitted to ICU (77%) was significantly higher than that of the healthy individuals(n=8; 16%). Nested-PCR and direct microscopy showed 77% and 31% positive results for Pneumocystis jirovecii, respectively. The frequency of Pneumocystis jirovecii colonization in immunocompromised HIV-positive patients was reported 10% to 69% and for non-HIVimmunosuppressed individuals with underlying diseases was 15.5% to 59% in different studies (17,18). The difference in colonization rates in different studies is largely attributed to the type of detection methods.
The sensitivity of direct microscopy depends on the organism burden in the specimen. Previous studies indicated that the diagnostic yield of smear stainingof BAL andmicroscopic examinationis more than 90% in individuals with comorbid HIV-PCP, while it is very low in non HIV-PCP patients (3,19). The diagnostic yield of direct microscopy was significantly higher in the group of patients who received corticosteroids(n=23; 46%) in comparison with the ones who did not receive corticosteroids (n=8; 16%). This could be due to the higher burden of organism in the specimens obtained from the patients who received corticosteroids.
However, there were no significant differences regarding colonization between the patients who received and not receivedcorticosteroids (P=0.33). This finding showedno significant possible association between receiving corticosteroids and Pneumocystis jirovecii colonization. Regardless of receiving corticosteroids, patients with underlying diseases were more susceptible to be colonized with Pneumocystis jirovecii than the healthy individuals. Despite this finding, there was a positive correlation between corticosteroid doses taken by patients and the results of Nested-PCR. It was revealed that all the patients that received 88 mgor more of cumulative doses of corticosteroids had positive results in PCR (P=0.002). This finding postulated that this amount of cumulative doses of corticosteroid may initiate the colonization and development of Pneumocystis spp. infection. Higher doses of oral corticosteroids in hospitalized patients may increase the colonization risk of P.jirovecii as a potential agent of pneumonia in such individuals (8). Based on the current reports, P. jirovecii DNA copy numbers were significantly lower in the colonized patients compared with the ones with PCP (1.3×107vs.3.4×103copy/µL, P<0.05).
To diagnose PCP, a lower (1.6×103copy/µL) and upper 2×104copy/µL) cutoff value to achieve 100% sensitivity and specificity were determined (20). Other reports on different genes showed different cutoff values (21-23).
Another approach of the current study was to examine the air samples of the ICUs for the presence of Pneumocystis spp. In the nested-PCR, P.jirovecii was detected in a sample obtained from one of the ICUs. Hence, the positive PCR result of filtered air sample postulated the possible role of spreading Pneumocystis spp. from a human reservoir to the surrounding environment and transmitting nosocomial infections to other inpatients. Different populations such as patients with acute or chronic pulmonary diseases, pregnant females, and health care workers are at risk for colonization with Pneumocystis spp. In most of the cases, there was no sign of infection; however, different studies supported the idea that Pneumocystis spp. may participate in progression of underlying lung diseases or development of PCP (8). However, considering previous reports and recent animal models, transmission experiments indicated that 12 hours of contact between seeder and recipient was sufficient for the cystic forms to be aerially transmitted (24, 25). The results confirmedthe study hypothesis about pneumocystisnosocomial transmission among inpatients via inhalation of Pneumocystis cysts.
In summary, the findings demonstrated that Pneumocystis spp. are the prevalent microorganisms and PCP is a deadly infection, especially in the absence of specific treatment, where as pulmonary colonization is a less-severe presentation of Pneumocystis spp. infection. Therefore, it is critical to discriminate these 2 clinical manifestations of Pneumocystis spp. infection. In order to increase the specificity and sensitivity of quantitative PCR assays targeting different genes by genomic copy number consideration, Pneumocystis jirovecii burdens evaluation and diagnosis of Pneumocystis colonization can be useful along with prophylactic therapy of PCP in immunocompromised individuals.
Conclusion
Results of the current study demonstrated a high presence of P. jirovecii DNA sources in BALspecimens acquired from surgery and neurosurgery ICUs. Also,the current study supported the concept of developing sensitive methods to detect and isolate patients colonized with Pneumocystis spp. to prevent organism transmission in hospitals. Use of prophylactic treatmentfor immunosuppressed patients or people with underlying diseases and decrease in hospital stay declined the incidence rate of PCP and mortality caused by these microorganisms. Long-term hospitalization and medication with high doses of corticosteroid arevery critical in Pneumocystis spp. colonization. It was suggested that isolation of patients under treatment with corticosteroid in ICU can play an essential role in to prevent air born transmission of pneumocystis.
Acknowledgments
The current study was granted (No: 85261) byArdabil University of Medical Sciences, Ardabil, Iran.
Conflicts of interest
The authors declared no conflict of interest.
References
- 1.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults Clinical infectious diseases. Clin Infect Dis. 2007;44(S2):S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montes-Cano MA, De la Horra C, martin-Juan J, varela JM, Torronteras R, Respaldiza N, et al. Pneumocystis jirovecii genotypes in the spanish population. Clin Infect Dis. 2004;39(1):123–8. doi: 10.1086/421778. [DOI] [PubMed] [Google Scholar]
- 3.Morris A, Wei K, Afshar K, Huang L. Epidemiology and clinical significance of pneumocystis colonization. J Infect Dis. 2008;197(1):10–7. doi: 10.1086/523814. [DOI] [PubMed] [Google Scholar]
- 4.Aliouat-Denis C-M, Chabé M, Demanche C, Viscogliosi E, Guillot J, Delhaes L, et al. Pneumocystis species, co-evolution and pathogenic power. Infect Genet Evol. 2008;8(5):708–26. doi: 10.1016/j.meegid.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Azoulay E, Bergeron A, Chevret S, Bele N, Schlemmer B, Menotti J. Polymerase chain reaction for diagnosing pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2009;135(3):655–61. doi: 10.1378/chest.08-1309. [DOI] [PubMed] [Google Scholar]
- 6.Bollée G, Sarfati C, Thiéry G, Bergeron A, de Miranda S, Menotti J, et al. Clinical picture of Pneumocystis jiroveci pneumonia in cancer patients. Chest. 2007;132(4):1305–10. doi: 10.1378/chest.07-0223. [DOI] [PubMed] [Google Scholar]
- 7.Respaldiza N, Montes-Cano MA, Dapena FJ, de la Horra C, Mateos I, Medrano FJ, et al. Prevalence of colonisation and genotypic characterisation of Pneumocystis jirovecii among cystic fibrosis patients in Spain. Clin Microbiol Infect. 2005;11(12):1012–5. doi: 10.1111/j.1469-0691.2005.01276.x. [DOI] [PubMed] [Google Scholar]
- 8.Morris A, Norris KA. Colonization by Pneumocystis jirovecii and its role in disease. Clin Microbiol Rev. 2012;25(2):297–317. doi: 10.1128/CMR.00013-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Festic E, gajic O, Limper AH, Aksamit TR. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection. Chest. 2005;128(2):573–9. doi: 10.1378/chest.128.2.573. [DOI] [PubMed] [Google Scholar]
- 10.Nevez G, Chabé M, Rabodonirina M, Virmaux M, Dei-Cas E, Hauser P, et al. Nosocomial Pneumocystis jirovecii infections. Parasite. 2008;15(3):359–65. doi: 10.1051/parasite/2008153359. [DOI] [PubMed] [Google Scholar]
- 11.Medrano FJ, Montes-Cano M, Conde M, de la Horra C, Respaldiza N, Gasch A, et al. Pneumocystis jirovecii in general population. Emerg Infect Dis. 2005;11(2):245–50. doi: 10.3201/eid1102.040487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez M, Fishman JA. Prevention of Infection Due to Pneumocystis spp in Human Immunodeficiency Virus-Negative Immunocompromised Patients. Clin Microbiol Rev. 2004;17(4):770–82. doi: 10.1128/CMR.17.4.770-782.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Damiani C, Choukri F, Le Gal S, Menotti J, Sarfati C, Nevez G, et al. Possible nosocomial transmission of Pneumocystis jirovecii. Emerg Infect Dis. 2012;18(5):877–8. doi: 10.3201/eid1805.111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swisher BL, Chandler FW. Grocott-Gomori Methenamine Silver Method for Detecting Fungi: Practical Considerations. Laboratory Medicine. 2016;13(9):568–70. [Google Scholar]
- 15.Beard CB, Roux P, Nevez G, Hauser PM, Kovacs JA, Unnasch TR, et al. Strain typing methods and molecular epidemiology of Pneumocystis pneumonia. Emerg Infect Dis. 2004;10(10):1729–35. doi: 10.3201/eid1010.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvarez-Martinez MJ, Moreno A, Miro JM, Valls ME, Rivas PV, de Lazzari E, et al. Pneumocystis jirovecii pneumonia in Spanish HIV-infected patients in the combined antiretroviral therapy era: prevalence of dihydropteroate synthase mutations and prognostic factors of mortality. Diagn Microbiol Infect Dis. 2008;62(1):34–43. doi: 10.1016/j.diagmicrobio.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhoff LV, Sheagren JN. Epidemiology and clinical significance of blood cultures positive for coagulase-negative staphylococcus. Infect Control Hosp Epidemiol. 1985;6(12):479–86. doi: 10.1017/s0195941700063591. [DOI] [PubMed] [Google Scholar]
- 18.Huang L, Crothers K, Morris A, Groner G, Fox M, Turner JR, Merrifield C, Eiser S, Zucchi P, Beard CB. Pneumocystis Colonization in HIV‐Infected Patients. Journal of Eukaryotic Microbiology. 2003;50(s1):616–7. doi: 10.1111/j.1550-7408.2003.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 19.Helweg-Larsen J. Pneumocystis jiroveci Applied molecular microbiology, epidemiology and diagnosis. Dan Med Bull. 2004;51(3):251–73. [PubMed] [Google Scholar]
- 20.Damiani C, Le Gal S, Da Costa C, Virmaux M, Nevez G, Totet A. Combined quantification of pulmonary Pneumocystis jirovecii DNA and serum (1->3)-beta-D-glucan for differential diagnosis of pneumocystis pneumonia and Pneumocystis colonization. J Clin Microbiol. 2013;51(10):3380–8. doi: 10.1128/JCM.01554-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maillet M, Maubon D, Brion JP, Francois P, Molina L, Stahl JP, et al. Pneumocystis jirovecii (Pj) quantitative PCR to differentiate Pj pneumonia from Pj colonization in immunocompromised patients. Eur J Clin Microbiol Infect Dis. 2014;33(3):331–6. doi: 10.1007/s10096-013-1960-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flori P, Bellete B, Durand F, Raberin H, Cazorla C, Hafid J, et al. Comparison between real-time PCR, conventional PCR and different staining techniques for diagnosing Pneumocystis jiroveci pneumonia from bronchoalveolar lavage specimens. J Med Microbiol. 2004;53(Pt 7):603–7. doi: 10.1099/jmm.0.45528-0. [DOI] [PubMed] [Google Scholar]
- 23.Matsumura Y, Ito Y, Iinuma Y, Yasuma K, Yamamoto M, Matsushima A, et al. Quantitative real-time PCR and the (1-->3)-beta-D-glucan assay for differentiation between Pneumocystis jirovecii pneumonia and colonization. Clin Microbiol Infect. 2012;18(6):591–7. doi: 10.1111/j.1469-0691.2011.03605.x. [DOI] [PubMed] [Google Scholar]
- 24.Martinez A, Halliez MC, Aliouat el M, Chabe M, Standaert-Vitse A, Frealle E, et al. Growth and airborne transmission of cell-sorted life cycle stages of Pneumocystis carinii. PLoS One. 2013;8(11):e79958. doi: 10.1371/journal.pone.0079958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rivero L, de la Horra C, Montes-Cano MA, Rodriguez-Herrera A, Respaldiza N, Friaza V, et al. Pneumocystis jirovecii transmission from immunocompetent carriers to infant. Emerg Infect Dis. 2008;14(7):1116–8. doi: 10.3201/eid1407.071431. [DOI] [PMC free article] [PubMed] [Google Scholar]