Abstract
Background & objective:
The current study aimed at assessing the relationship between gastritis and peptic ulcer susceptibility and inflammation-related gene polymorphisms in Iranian patients.Gastritis and peptic ulcer are common medical complications with serious outcomes on the quality of life. Inflammatory responses of gastric mucosa are associated with helicobacter pylori, but most infected patients remain asymptomatic. There is strong evidence that inflammatory response is a major part of its etiology.
Methods:
The current case-control study aimed atexamining genetic polymorphisms in inflammatory cytokines interleukin (IL)-4, and IL-10 using polymerase chain reaction-variable number tandem reaped (PCR-VNTR) and PCR-restriction fragment length polymorphism(RFLP) methods, respectively in 603 genotyped patients admitted to Mohammadi Hospital in Bandar Abbas, Iran(198 patients with gastritis, 84 with peptic ulcer, and 321 patients as controls).
Results:
No significant associations were detected in genotype and allele frequencies of IL-10 and IL-4 between the case (with gastritis and peptic ulcer) and control groups.
Conclusion:
In conclusion, the results of the analyses suggested that these polymorphisms may not predispose the carriers to gastritis and peptic ulcer development.
Key Words: Interleukin, Gastritis, Polymorphism, Peptic Ulcer
Introduction
A large body of epidemiological evidence display a relationship between Helicobacter pylori infection, gastritis, and peptic ulcer (1). H. pylori infection can activate inflammatory cytokines, inflammatory cells, and arouse free radical species following malignant transformation. According to genetic variations, people responses to inflammation may influence the degree of inflammation and then the risk for cancer (2).
H. pylori has a global distribution with a prevalence of 25% in the developed countries and 90% in the developing countries (3). However, clinical evidence is only observed in a small population, which suggests
involvement of many other factors such as host factors in its pathogenesis (4). Chronic gastric inflam-( mation, activated neutrophils, and mononuclear cells produce a variety of cytokines vital to regulating inflammation (5). Moreover, inflammation is mediated by proand anti-inflammatory cytokines suggesting that genetic polymorphisms of cytokine genes directly affect diversity of people and cytokine responses, which may affect clinical outcomes in different people. The pleiotropic cytokine interleukin (IL)-10 has a dual capability to suppress cancer immunity (6). IL10 restricts production of pro-inflammatory cytokines by limiting Th1 and stimulating Th2 and B-lymphocytes, and thus downregulates inflammatory responses (7, 8). Human IL-10 gene is located on the long arm of chromosome 1q31-32, comprising five exons and three introns, with three polymorphisms identified in its promoter region-592 (C>A), -1082 (G>A) and -819 (C>T) affect expression of IL-10, and are associated with increased risk of gastric cancer (7,9). The anti-inflammatory cytokine IL-4 has a key role in activation and differentiation of mast cells, erythrocytes, B-cells, and development of Th2, which is a subgroup of lymphocytes. Similar to IL-4, IL-2 and IL-10; Th2 causes production of antibody in human (10). A polymorphism is situated in the intron 3 of the gene, which contains a 70-bp variable number tandem repeat (VNTR). Frequency of VNTR repeats varies from two to four times, triple repeat is more common, and is observed in 76% of people.
The double repeat is less common, and is observed in 23% of people. Also, quadruple repeat is very rare, and is reported only in specific populations and races. The triple repeat causes greater production of IL-4 (11, 12). Therefore, the current study aimed at measuring the association of IL-10 and IL-4 polymorphisms with increased risk of gastritis and peptic ulcer.
Materials and Methods
People presenting with stomachache and indigestion diagnosed with gastritis or peptic ulcer accompany
H. pylori infection according to endoscopy and histopathology findings were enrolled as the patient group. Patients that used non-steroidal anti-inflammatory drugs (NSAIDs) and the ones under the treatment for
H. pylori were excluded. People with the history of exposure to H. pylori or active or chronic H. pylori infection (according to their serology tests) with no gastric problems or history of gastrointestinal diseases were selected as the control group. The current study protocol was approved by the Ethics Committee of the institute, and written informed consent was obtained from both control and case groups.
Endoscopy and Gastric Histology
Endoscopy was conducted using a video processor and video gastroscopy for every patient (Fujinon, Wayne, NJ, USA). The researcher took two biopsies from the corpus, antrum, or ulcer edge of every patient; one specimen was immediately fixed in the formalin for histological testing, and the other was placed in distilled water for diagnosis stage. The biopsies of detection were kept at -20°C until processing stage. The pathological sections were stained with Hematoxylin & Eosin, and assessed by a pathologist using Sydney System criteria (13). Therefore, endoscopic observation and histopathological confirmations were used to characterize patients’ pathology.
Serology
Blood samples (5 mL) were taken from all control subjects of the study. The serum was tested for IgG by the enzyme-linked immunosorbent assay (ELISA) kit, according to the manufacturer’s instruction. The specificity and sensitivity of the method is 97%. A subject is considered positive, if antibody is detected in her/his serum.
Analysis of IL-4 and IL-10 Genes
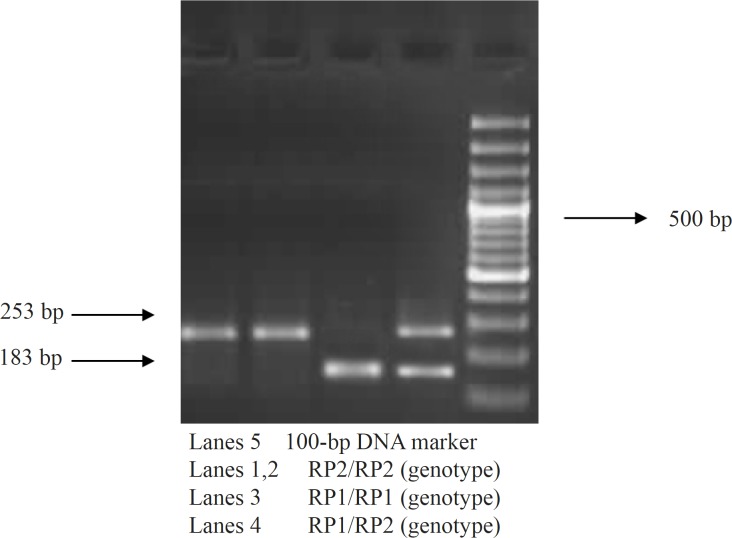
In the current study, genomic DNA was extracted from the entire blood using a QIAamp DNA Mini Kit (QIAGEN, USA). A polymerase chain reaction (PCR)-VNTR assay was amplified to detect IL-4 (intron 3) 70-bp VNTR polymorphism in the primer sequence as described by Wu et al. (14). The PCR re-e action mixture contained 100 ng of genomic DNA, 20 pmol of each primer, and 0.3 mM of each dNTP, 10X KCL, 1.5 mM MgCl2, and 1.5 units of Taq polymerase. First, the sequence of cycling was conducted at 95°C for four minutes, and then, 35 cycles were run at 95°C for 30 seconds followed by 59°C for 40 seconds, which specified at 73°C for 40 seconds, and finally at 73°C for six minutes. PCR of IL-4 (VNTR) was directly visualized on 2% agarose gel stained with ethidium bromide, and finally, alleles were identified based on the sizes of amplified fragments. The Rp2 and Rp1 alleles were 253and 183-bp fragments, respectively (Figure 1).
Figure 1.
PCR-VNTR genotype analysis of IL-4 gene in the case group
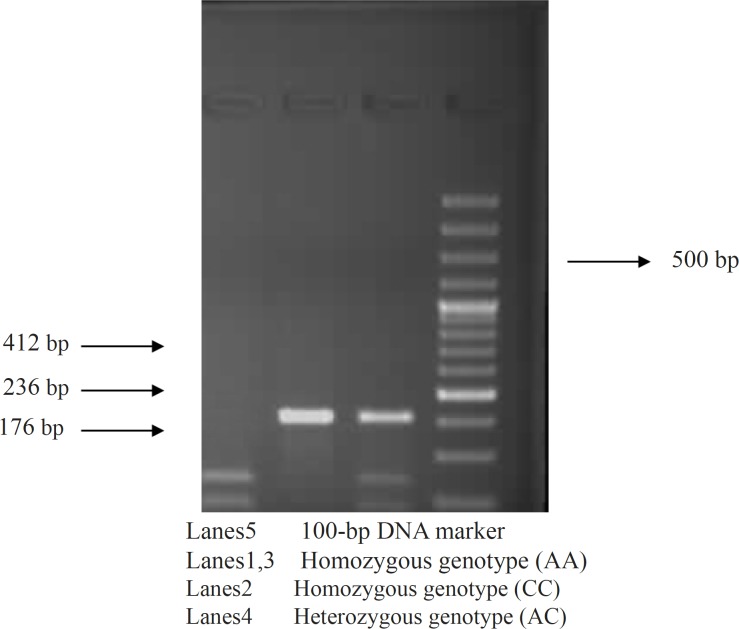
The IL-10 promoter polymorphism (PCR-restriction fragment length polymorphism) as described by Turner et al., (15) was used to detect the -592 SNP by introducing two alleles, A and C (A-C). A 100-ng of genomic DNA was amplified in a 25 mL final volume containing 1.5mM MgCL2, 10X KCL buffer, 25 pmol of each primer, 0.2 mM of each dNTP, and 1 unit of Taq polymerase was used. The cycling conditions were as follows: 95°C for five minutes followed by 35 cycles at 94°C for 40 seconds, 61°C for one minute, 72°C for 40 seconds, and finally, 72°C for 10 minutes. The PCR product was a 412-bp fragment and the restriction fragment length polymorphism assay was performed in a 15-mL reaction mixture containing PCR product (10 mL), buffer (1.8 mL), enzyme (Rsa), and distilled water (3 mL). The reaction mixture was incubated at 37°C for 16 hours. However, at position -592, 412-bp fragments were amplified and digested A allele with RSaI site demonstrated a two fragments, 236and 176-bp, whereas the –592 C allele without RSa site produced no splitting (Figure 2).
Figure 2.
PCR-VNTR (Rsa1) genotye analysis of IL-10 gene in the case groups
Statistical Analysis
In the current study, Hardy–Weinberg equilibrium for alleles at individual loci was assessed by χ2 test. Comparison of IL-10 (-592) and IL-4 genotype frequencies between the case and control groups were performed by χ 2 test. Crude odds ratios (ORs) with 95% confidence interval (CI) were calculated by logistic regression analysis. Statistical analysis was conducted with SPSS (SPSS16.0, SPSS Co., Seoul, Korea) and the P-value<0.05 was considered statistically significant.
Results
A total of 603 people including 198 patients with gastritis, 84 with peptic ulcer, and 321 healthy controls were studied. The mean age of the patients with gastritis was 41 years, ranged 17-85; the patients with peptic ulcer had the mean age of 42 years, ranged 23-Subjects in the control group had the mean age of 41 years, ranged 21-74. There were significant differences between the three groups in terms of family history of ulcer and smoking habits. Demographic characteristics are shown in Table 1.
Table 1.
Demographic Characteristics of the Cases and Controls
| Gastritis (N=198) | Peptic Ulcer (N=84) | Control (N=321) | |
|---|---|---|---|
| Age, yr | 41.2±16.1 | 42.8 ±15.5 | 41.8 ±15.9 |
| Male | 103±52 | 40±47.6 | 162±50.4 |
| Female | 95±48 | 44±52.4 | 159±49.6 |
| Smokers | 67±33.8 | 34±40.5 | 51±15.9 |
| Nonsmokers | 131±66.2 | 50±59.5 | 270±84.1 |
Data are expressed as mean±SD.
The genotype frequency distribution for IL-4 RP1/ RP2 was 99.5% among patients with chronic gastritis, 96.4% among patients with peptic ulcer, and 94.4% among the controls. Genotype AC in IL-10 was the most frequent in patients with gastritis 45.5%, 46.4% in peptic ulcer, and 51.4% in the control group.
No significant relationship was observed between genotype and allele frequencies in IL-10 and none in patients with gastritis and peptic ulcer in IL-4 either. Genotypes IL-4 and IL-10 are presented in tables 2 and 3.
Table 2.
OR and 95%CI for IL-4 and IL-10 Genotypes in Gastritis Cases and Controls
| Polymorphism | Genotype | Gastritis (N=198) | Control (N=321) | OR | P value |
|---|---|---|---|---|---|
| IL-4 | RP1-RP1 | 9 (4.5) | 18 (5.6) | 1.0 | - |
| RP1-RP2 | 48 (24.2) | 63 (19.6) | 1.52 (0.58-4.05) | 0.47 | |
| RP2-RP2 | 141 (71.3) | 240 (74.8) | 1.17(0.48-2.91) | 0.86 | |
| RP1-RP2+RP2-RP2 | 189 (95.5) | 303(94.4) | 1.25(0.52-3.07) | 0.74 | |
| IL-10 | AA | 104 (52.5) | 153 (47.7) | 1.0 | - |
| AC | 90 (45.5) | 165 (51.4) | 0.80(0.55-1.17) | 0.26 | |
| CC | 4 (2.0) | 3(0.9) | 1.96(0.36-11.30) | 0.30 | |
| AC+CC | 94 (47.5) | 168 (52.3) | 0.82(0.57-1.19) | 0.32 | |
OR, odds ratio
Table 3.
OR and 95%CI for IL-4 and IL-10 Genotypes in Peptic Ulcer Cases and Controls
| Polymorphism | Genotype | Peptic Ulcer(N=84) | Control (N=321) | OR | P-value |
|---|---|---|---|---|---|
| IL-4 | RP1-RP1 | 3 (3.6) | 18 (5.6) | 1.0 | - |
| RP1-RP2 | 18 (21.4) | 63 (19.6) | 1.74 (0.41-8.26) | 0.31 | |
| RP2-RP2 | 63 (75) | 240 (74.8) | 1.58(0.42-6.95) | 0.34 | |
| R | P1-RP2+RP2-RP2 | 81(96.4) | 303(94.4) | 1.60(0.43-7.03) | 0.33 |
| IL-10 | AA | 42 (50) | 153 (47.7) | 1.0 | - |
| AC | 39 (46.4) | 165 (51.4) | 0.86(0.51-1.44) | 0.63 | |
| CC | 3 (3.6) | 3(0.9) | 3.64(0.56-23.68) | 0.12 | |
| AC+CC | 42 (50) | 168 (52.3) | 0.91(0.55-1.51) | 0.79 |
OR, odds ratio
Discussion
The responses of the host›s immune system including cytokine activity play an important role in causing gastritis due to infections such as H. pylori, and ultimately in the progress of the disease and more advanced clinical complications such as gastrointestinal ulcers, and specifically gastric and duodenal ulcers, and also stomach and gastric cancer. Many genetic studies proposed a relationship between cytokines and diseases associated with the immune system. Yet, despite studies on cytokines and their genetic role and different genotypes and polymorphisms, the relationship between different polymorphisms of the host›s cytokines and severity or nature of the disease remains unclear (16).The current study aimed at ex-t amining IL-4 and IL-10 because they act in two different systems of the cytokine immune response. The current study specifically focused on the significance of two polymorphisms of two anti-inflammatory cytokines, IL-4 and IL-10, in a population of normal controls and H. pylori-related gastric disease groups, but since no clear data were observed on the role of IL-4 in bacterial infections, its role in the systemic infections requires further investigation (17).
It is found that IL-4 has an inhibitory role in the expression, production, and release of inflammatory cytokines and can suppress activity, production, or release of cytokines derived from monocytes such as tumor necrosis factor (TNF), IL-1, IL-6, IL-8, and macrophage inflammatory protein (MIP)-1α. Other activities attributed to IL-4 include its effect on different body cells including increased proliferation of vascular endothelium and skin fibroblasts, and decreased proliferation of spherocytes and vascular smooth muscle cells (17). IL-4 acts through inhibition of IFN-γ, which is in fact inhibition of macrophages, and inhibits cell-dependent immune reactions (18). Since IL-4 has a wide variety of activities, perhaps this is the reason why no specific role is identified for this cytokine. Therefore, the diversity in IL-4 activities may also be a reason for contradictory results in different studies. Besides, IL-10 is an anti-inflammatory cytokine responsible for the development and differentiation of B-cells, inhibition of immunological responses, and prevention of B-cells activities.
IL-10 is also the main cytokine against anti-inflam- matory cytokines TNF-α (9,19). In the current study, no significant relationship was observed between being a carrier of any alleles or combination of alleles and the development of inflammation, gastritis, and thus gastric cancer. There may be different conditions underlying the genetics of H.
Pylori-related gastritis and gastric cancer. Although no specifically similar study is conducted, these polymorphisms are studied separately or in other diseases or cancers.
In agreement with the current study results, Rad et al., reported no significant relationship between being carrier of low-frequency or risk alleles in IL-10-592 and atrophic gastritis and intestinal metaplasia (20). Moreover, another study in China observed no association between intestinal metaplasia and IL-10 polymorphisms, which also confirmed the current study results on IL-10-592 polymorphism (21). Hunt PJ and Yam-Yao found a significant relationship between IL10-592 and VNTR intron 3 of IL-4 and other diseases such as rheumatism, arthritis, asthma, rhinitis, Atopic dermatitis, gravies disease, multiple sclerosis, and bladder cancer, which may be due to the role of immune system and immune responses in such diseases (22, 23).
According to the current study results, it might be concluded that regardless of other confounding and environmental factors and regarding only gender and age, these polymorphisms in the two sites in these two cytokines do not pose any increased or decreased risk for gastritis and peptic ulcer following H. pylori infection. As discussed earlier, the role of these cytokines in the inflammatory diseases is not yet fully understood, and thus these results cannot be considered definitive. Another reason for uncertainty is the role of haplotypes in these cytokines. Studies revealed that different haplotypes affect secretion of cytokines, which was not investigated in the current study, and requires further studies.
Acknowledge
I would like to express my deepest appreciation to staffs of Molecular Medicine Research Center of HormozganUniversity of Medical Sciences who provided me the possibility to complete this article.
Conflict of interest
The authors declared no conflict of interests.
References
- 1.Vannarath S, Vilaichone RK, Rasachak B, Mairiang P, Yamaoka Y, Shiota S, et al. Virulence genes of helicobacter pylori in gastritis, peptic ulcer and gastric cancer in Laos. Asian Pac J Cancer Prev. 2014;15(20):9027–31. doi: 10.7314/apjcp.2014.15.20.9027. [DOI] [PubMed] [Google Scholar]
- 2.QadriQ , Rasool R, Afroze D, Naqash S, Gulzar G, Yousuf A, et al. Study of TLR4 and IL-8 Gene Polymorphisms in H pylori-Induced Inflammation in Gastric Cancer in an Ethnic Kashmiri Population. Immunol Invest. 2014;43(4):324–36. doi: 10.3109/08820139.2013.854378. [DOI] [PubMed] [Google Scholar]
- 3.El-Omar EM, Carrington M, Chow W-H, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404(6776):398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 4.Kim J, Cho YA, Choi IJ, Lee Y-S, Kim S-Y, Shin A, et al. Effects of interleukin-10 polymorphisms, Helicobacter pylori infection, and smoking on the risk of noncardia gastric cancer. PloS one. 2012;7:e29643. doi: 10.1371/journal.pone.0029643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78(5):1043–51. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 6.Xue H, Lin B, An J, Zhu Y, Huang G. Interleukin-10-819 promoter polymorphism in association with gastric cancer risk. BMC Cancer. 2012;12(1):102. doi: 10.1186/1471-2407-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burada F, Angelescu C, Ioana M, Mitrut P, Moraru E, Riza A, et al. IL-10-1082 A/G POLYMORPHISM AND RISK OF THE GASTRIC CANCER. Annals of RSCB. 2010;15(1):93–7. [Google Scholar]
- 8.El-Omar EM, Rabkin CS, Gammon MD, Vaughan TL, Risch HA, Schoenberg JB, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124(5):1193–201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 9.Sosroseno W, Herminajeng E, Goeno S. The interleukin network in the immunopathogenesis of oral diseases. Asian Pac J Allergy Immunol. 1994;12(2):161–8. [PubMed] [Google Scholar]
- 10.Nakashima H, Miyake K, Inoue Y, Shimizu S, Akahoshi M, Tanaka Y, et al. Association between IL-4 genotype and IL-4 production in the Japanese population. Genes Immun. 2002;3(2):107–9. doi: 10.1038/sj.gene.6363830. [DOI] [PubMed] [Google Scholar]
- 11.Kesarwani P, Ahirwar D, Singh R, Manchanda PK, Mittal RD. Do IL-4 intron 3 VNTR and IL-6 (-174) G/C variants reflect ethnic variation? A comparative study between the global and North Indian populations. Asian Pac J Cancer Prev. 2008;9(1):76–80. [PubMed] [Google Scholar]
- 12.Stolte M, Meining A. The updated Sydney system: classification and grading of gastritis as the basis of diagnosis and treatment. Can J Gastroenterol. 2001;15(9):591–8. doi: 10.1155/2001/367832. [DOI] [PubMed] [Google Scholar]
- 13.Wu M, Huang C, Tsai JJ, Chen H, Tsai F. Polymorphisms of the interleukin-4 gene in Chinese patients with systemic lupus erythematosus in Taiwan. Lupus. 2003;12(1):21–5. doi: 10.1191/0961203303lu249oa. [DOI] [PubMed] [Google Scholar]
- 14.Turner D, Williams D, Sankaran D, Lazarus M, Sinnott P, Hutchinson I. An investigation of polymorphism in the interleukin‐10 gene promoter. Eur JImmunogenet. 1997;24(1):1–8. doi: 10.1111/j.1365-2370.1997.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 15.Murphy G, Thornton J, McManus R, Swan N, Ryan B, O’Morain C, et al. Association of gastric disease with polymorphisms in the inflammatory related genes IL-1B, IL-1RN, IL-10, TNF and TLR4. Eur J Gastroenterol Hepatol. 2009;21(6):630–5. doi: 10.1097/MEG.0b013e3283140eea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MA, Hural J. Functions of IL-4 and control of its expression. Crit Rev Immunol. 1997;17(1):31–2. doi: 10.1615/critrevimmunol.v17.i1.10. [DOI] [PubMed] [Google Scholar]
- 17.Shekari M, Kordi-Tamandani DM, MalekZadeh K, Sobti RC, Karimi S, Suri V. Effect of anti-inflammatory (IL-4, IL-10) cytokine genes in relation to risk of cervical carcinoma. Am J Clin Oncol. 2012;35(6):514–9. doi: 10.1097/COC.0b013e31822d9c12. [DOI] [PubMed] [Google Scholar]
- 18.Gibson AW, Edberg JC, Wu J, Westendorp RG, Huizinga TW, Kimberly RP. Novel single nucleotide polymorphisms in the distal IL-10 promoter affect IL-10 production and enhance the risk of systemic lupus erythematosus. J Immunol. 2001;166(6):3915–22. doi: 10.4049/jimmunol.166.6.3915. [DOI] [PubMed] [Google Scholar]
- 19.Rad R, Dossumbekova A, Neu B, Lang R, Bauer S, Saur D, et al. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004;53(8):1082–9. doi: 10.1136/gut.2003.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leung WK, Chan MC, To K-F, Man EP, Ng EK, Chu ES, et al. H pylori genotypes and cytokine gene polymorphisms influence the development of gastric intestinal metaplasia in a Chinese population. J Gastroenterol Hepatol. 2006;101(4):714–20. doi: 10.1111/j.1572-0241.2006.00560.x. [DOI] [PubMed] [Google Scholar]
- 21.Seno H, Satoh K, Tsuji S, Shiratsuchi T, Harada Y, Hamajima N, et al. Novel interleukin‐4 and interleukin‐1 receptor antagonist gene variations associated with non‐cardia gastric cancer in Japan: comprehensive analysis of 207 polymorphisms of 11 cytokine genes. J Gastroenterol Hepatol. 2007;22(5):729–37. doi: 10.1111/j.1440-1746.2007.04934.x. [DOI] [PubMed] [Google Scholar]
- 22.Hunt P, Marshall S, Weetman A, Bell J, Wass J, Welsh K. Cytokine gene polymorphisms in autoimmune thyroid disease. J Clin Endocrinol Metab. 2000;85(5):1984–8. doi: 10.1210/jcem.85.5.6588. [DOI] [PubMed] [Google Scholar]
- 23.Zhu S, Chan-Yeung M, Becker AB, Dimich-Ward H, Ferguson AC, Manfreda J, et al. Polymorphisms of the IL-4, TNF-α, and Fc α RI β genes and the risk of allergic disorders in At-risk infants. Am J Respir Crit Care Med. 2000;161(5):1655–9. doi: 10.1164/ajrccm.161.5.9906086. [DOI] [PubMed] [Google Scholar]