Abstract
Background and Aims
The molecular events that determine intestinal cell differentiation are poorly understood and it is unclear whether it is primarily a passive event or an active process. It is clinically important to gain a greater understanding of the process, since in colorectal cancer (CRC), the degree of differentiation of a tumour is associated with patient survival. SGK1 has previously been identified as a gene that is principally expressed in differentiated intestinal cells. In colorectal cancer (CRC), there is marked downregulation of SGK1 compared to normal tissue.
Methods
An inducible SGK1 viral overexpression system was utilised to induce re-expression of SGK1 in CRC cell lines. Transcriptomic and phenotypic analyses of these CRC lines was performed and validation in mouse and human cohorts was performed.
Results
We demonstrate that SGK1 is upregulated in response to, and an important controller of, intestinal cell differentiation. Re-expression of SGK1 in CRC cell lines results in features of differentiation, decreased migration rates, and inhibition of metastasis in an orthotopic xenograft model. These effects may be mediated in part by SGK1-induced PKP3 expression and increased degradation of MYC.
Conclusions
Our results suggest that SGK1 is an important mediator of differentiation of colorectal cells and may inhibit CRC metastasis.
Introduction
The unit structure of the normal large bowel is the crypt. Stem cells at the crypt base produce progeny that expand in number and differentiate as they pass to the lumen into which they are shed. Although the biology of the stem cell niche is relatively well understood, much less is known about the process of differentiation. The involvement of signalling pathways such as Wnt, TGFβ/BMP/Hedgehog, Notch and Ephrins is required for production of the main differentiated cell types (colonocyte, neuroendocrine, goblet, paneth). However, especially for colonocytes, it is unclear to what extent differentiation is a fundamentally passive process – for example, resulting from absence of microenvironmental Wnt activity, which declines as cells move up the crypt – or an active process, in which unknown pathways are switched on in order to drive the expression of genes involved in the functioning of the differentiated cells.
The degree of differentiation (usually termed “grade”) of colorectal cancers (CRC) is a well-established tumour feature that is associated with patient prognosis, and its assessment forms part of the American Joint Committee on Cancer (AJCC) recommendations [1]. The five year cumulative survival for patients with well-differentiated CRCs is 80.9%, compared with 76.9% and 45.5% for moderately and poorly differentiated lesions respectively [2]. Since CRC patients rarely die from effects of the primary cancer, poor differentiation must ultimately increase the risk of developing metastatic disease.
The molecular pathways that determine CRC differentiation are incompletely described. There are two main theories to account for how an undifferentiated tumour might arise: (i) cancer cells are selected for their ability to down-regulate differentiation gene(s), the so-called “de-differentiation” hypothesis; or (ii) differentiation reflects the cell of origin of the cancer in the crypt [3] [4]. However, whilst it is evident that CRCs demonstrate histological and growth characteristics similar to undifferentiated or stem cells in normal tissue, neither hypothesis fully explains why CRCs vary in their degree of differentiation.
Serum/glucocorticoid-regulated kinase 1 (SGK1) is a highly conserved member of the AGC family of serine/threonine kinases. It was originally identified as being transcriptionally activated in response to glucocorticoid stimuli in a mammary epithelial cell line [5]. SGK1 is also induced by various cell stresses and is involved in the cell survival response. Its transcription is influenced by different types of hormones, growth factors, cytokines and conditions of cellular stress [6]. SGK1 also regulates a number of other ion channels, including K+, Ca2+ and Cl- channels, and glucose transporters such as GLUT1 and SGLT1, thus controlling cell volume and osmolality [6]. In the colorectum, such proteins are expected to be important in the functioning of differentiated colonocytes, since these cells primarily have a water-absorbing role. In keeping with this notion, we have previously demonstrated that SGK1 expression in normal intestinal epithelium is highest at the top of the crypt, corresponding to the positions of differentiated colonocytes, with reduced expression in intestinal adenomas [7].
Much remains unknown about SGK1’s function, and it appears to have a number of tissue-specific effects in both normal cells and cancers. SGK1 has been proposed to form an alternative arm of Pi3 kinase (PI3K) signalling to the canonical AKT arm. AKT and SGK1 have very similar catalytic domains, recognising similar substrate motifs in vitro, and are both activated in a PI3K-dependent manner. This means that SGK1 potentially provides a parallel pathway to PI3K/AKT signalling and an alternative player in tumorigenesis [8][9]. SGK1 effects PI3K signalling through mTORC2 [10]. SGK1 is phosphorylated, and therefore activated, by PDKs. SGK1 expression is also regulated at the mRNA level, for example by Hippo pathway members Yap and Taz [11], and by glucocorticoid and mineralocorticoid receptors. Downstream, SGK1 phosphorylates the E3 ubiquitin ligase NEDD4-2, thus tagging it for degradation and allowing sodium channel expression. Other SGK1 targets include Foxo3, NRDG1 and MNK1. Nuclear SGK1 may also play a role in the epigenetic control of gene expression [12]. SGK1 activation has been proposed as a mechanism of cancer resistance to PI3K inhibitors [13]. There have been few studies of SGK1 in CRC. The kinase inhibitors SI113 and EMD638683 have anti-SGK1 activity and have been demonstrated to inhibit the growth of RKO and CACO2 CRC cells respectively [14], often in combination with genotoxic therapies [15]. Sgk1 knockout has also been reported to reduce intestinal tumour numbers in mice with germline Apc mutations [16].
In this study, we examine the role of SGK1 in intestinal cell differentiation, both in the normal crypt and in colorectal cancers. We determine the consequences of SGK1 re-expression for CRC behaviour in model systems in vitro and in vivo, and identify c-Myc inhibition and plakophilin 3 expression as important downstream mechanisms. Finally, we test for associations between SGK1 levels and the clinico-pathological features of a set of sporadic CRCs. Our findings suggest that the level of SGK1 expression is a strong determinant of CRC differentiation status, supporting the cancer “de-differentiation” hypothesis. We also demonstrate that the differentiation status of a cancer is strongly associated with its metastatic potential. Our findings potentially open new avenues for research into CRC metastasis.
Materials and methods
Cell lines and cell culture
Human CRC cell lines were obtained from collaborators, and are commercially available from the American Type Culture Collection (ATCC): HT29 (ATCC® HTB-38™), LS174T (ATCC® CL-188™), SW480 (ATCC® CCL-228™). Murine MC-38 cell line is commercially available from Kerafast (ENH204). All CRC cell lines were grown in DMEM. Media were supplemented with 10% foetal calf serum and 1% penicillin/streptomycin, and incubated at 37°C and 5% CO2. For sodium butyrate treatment, cells at 80% confluence were treated with 5mM NaBut diluted in medium. Mycoplasma status was tested prior to each experiment using the Lonza Mycoalert mycoplasma detection kit (LT07-218).
NaBut and expression of luciferase
A 1.7kb region upstream of the start codon of rat Sgk1 was cloned into the pGL3-enhancer vector. Cells were transiently transfected with either of the Sgk1 promoter-pGL3 constructs and the Renilla luciferase pGL4.75 vector (Promega), as a control for transfection efficiency. Twenty-four hours after transfection, experimental cells were treated with 5mM NaBut and luciferase activity was measured 48 hours later with the Dual-GloTM Luciferase Assay System (Promega).
Generation of SGK1-expressing construct
For lentivirus production, a 3X-FLAG tag was added to the 3’ end of the full coding sequence of human SGK1. Transcription of SGK1 sequences was initiated by the CMV promoter, regulated by the Tet operon in a Tet-Off regulated manner.
Western Blotting
Cells were harvested in lysis buffer supplemented with protease and phosphatase inhibitors (Roche). Protein content was measured by BCA assay (Pierce) and equal amounts were run onto pre-cast 4-12% NuPage gels (Invitrogen). Blotted membranes were blocked in 5% skimmed milk in PBS and incubated with the indicated primary and secondary antibodies.
Immunocytofluorescence
Cells were grown in confocal dishes with cover glass bottom (PAA), fixed in 3.7% paraformaldehyde for 10 minutes at room temperature and permeabilised with 0.1% TritonX-100 in PBS for 5 minutes at room temperature. Fixed cells were incubated either with serum obtained from the species in which the secondary was raised or with 1% BSA to prevent non-specific binding. Cells were then incubated with primary antibodies for 1 hour, rinsed, incubated with fluorescently-labelled secondary antibodies for 1 hour in the dark, and finally washed and counterstained with DAPI (Sigma). Samples were analysed with the ZEISS 510 MetaHead confocal microscope.
Tissue Microarrays
TMAs (CO484a, CO485) were obtained from Insight Biotechnology Ltd, the UK suppliers of US Biomax. They comprised histological sections of colon cancer specimens with information on TNM and clinical stage, differentiation and pathological grade. Scoring for SGK1 protein expression was performed following IHC on a scale of 1-5 for each specimen.
Microarrays and SGK1 expression from villi and crypts
Endoscopic biopsies were taken from the descending colon of five normal control patients and were microdissected into crypt tops and bottoms. RNA was extracted, amplified and quantified using Illumina beadchip microarrays. Analysis was performed using the R package “limma”.
RNA sequencing
RNA was isolated from pelleted cells using the Qiagen Rneasy MinElute clean-up kit and analysed using Tapestation. RNA sequencing libraries were prepared using the QuantSeq 3’ mRNA library prep kits (Lexogen) and sequencing performed on an Illumina Hiseq 4000 with 75bp paired end reads. RNA sequencing for the CRC cell lines were performed in duplicate and for animal experiments in triplicate. Sequencing was performed to an average depth of 5 million reads. Reads were de-multiplexed and mapped using Hisat2 to the reference human genome GRCh37. Feature counting was performed using Htseq version 0.6.1 and read normalisation and differential expression, using DESeq version 1.10.1. JavaGSEA was used using the Molecular Signatures Database on pre-ranked lists to perform gene set enrichment analysis. Curated signatures for cellular senescence, apoptosis and proliferation were obtained from published genesets [17].
Proliferation and apoptosis
Cell proliferation was measured with the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (Promega). Apoptosis was measured with the Caspase-Glo 3/7 Assay (Promega) under basal conditions and upon osmotic stress stimulus, achieved by treating the cells with 500mM sorbitol (Sigma) for 24 hours.
Anchorage-independent growth assay
Anchorage-independent growth was measured as the ability of cells to form colonies in soft agar. Briefly, a bottom layer consisting of 0.6% agar (Sigma, A9414) diluted in tissue culture medium was plated out in 6-well plates. Equal numbers of cells were diluted in 0.3% agar in tissue culture medium and plated onto the bottom layer at a concentration of 1000 cells per well, in triplicates. Cells were incubated for three weeks and fed with 200μl of medium twice weekly. Images were acquired with a scanner and the number of colonies formed was counted for each well.
Boyden transwell assay
8uM transwell assays were obtained from Corning and 300,000 cells were trypsinised and suspended in DMEM with 0% FBS and placed in the upper chamber. DMEM with 10% FBS was placed in the lower chamber. 16 hours later, cells were removed from the upper chamber and cells on the lower surface of the transwell assay were stained using Kwik-Diff stains (ThermoScientific). Automated cell counting was performed using the Nikon NIS Elements software package.
Stable isotope labelling of amino acids in culture (SILAC)
SILAC was performed with the SILAC Protein Quantitation Kit (ThermoScientific/Pierce) according to instructions. HT29-SGK1 cells were cultured in ‘heavy’ medium (13C6Lys + 13C6/15N4Arg) and HT29 parental cells in ‘light’ medium in the presence of doxycyline for 5 passages, after which incorporation of the heavy label was measured by tandem mass spectrometry. Resulting peptides were analysed by LC-MS/MS using an Ultimate3000™ HPLC system coupled on-line to a 3D high-capacity ion trap tandem mass spectrometer via a pneumatically assisted nano-electrospray source as described previously [18].
Mouse models
Animals were a mix of 5-10 week old male and female JAX™ NSG mice (NOD.Cg-Prkdcscid Il2rgtm1WjI/SzJ) obtained from Charles River Laboratories, UK. Animals were maintained under specific pathogen-free conditions, and food and water were supplied ad libitum. Housing and all procedures involving animals were performed according to protocols approved by the United Kingdom Home Office, in compliance with Animals (Scientific Procedures) Act and home office guidelines on animal welfare. Orthotopic implantation of tumours was performed on 5-10 week old mice under anaesthesia by isofluorane inhalation, using a 30 gauge custom-made needle under direct endoscopy vision with the Coloview system (Karl Storz) [19].
TCGA human cohort data
The human cohort data was obtained from The Cancer Genome Atlas (TCGA) which is publically available for the scientific research community. Ethical approval for the data was obtained from the National Institute of Health (NIH).
Results
SGK1 is preferentially expressed in intestinal tissue compartments comprised of differentiated cells
In order to confirm that expression of SGK1 alters along the crypt axis of the colon and small intestine, endoscopic biopsies from the terminal ileum and colon from 5 patients were obtained and microdissected into crypt tops and bottoms. RNA was extracted and global gene expression was quantified using microarrays. SGK1 expression was 9.6-fold higher in small bowel villi than crypts (p=0.003) and 10.8-fold higher in colonic crypt tops than bottoms (p=0.1), consistent with previous data showing that SGK1 is expressed predominantly in differentiated enterocytes and colonocytes. Increased expression of SGK1 was associated with increasing expression of SHBG, CIDEC, DUSP5 and MAF, known drivers of differentiation in cell lines. DUSP5 was especially interesting, as low expression is known to be associated with poor tumour differentiation, lymph node metastasis, distant metastasis and poorer overall survival [20]. Expression of SGK1 was negatively associated with expression of ASCL2, OLFM4, EPHB2, RETNLB, MSI1 and SOX9, genes associated with stem cell maintenance and function (Supp. Table 1).
Since CRCs are typified by reduced differentiation, we compared the levels of SGK1 mRNA in CRCs with those in normal intestine (data from The Cancer Genome Atlas). SGK1 expression was >10-fold lower in the former (Supp. Figure 1). In keeping with this, SGK1 was recently identified as a “hub gene”, the reduced expression of which is central to a set of gene expression changes in colorectal carcinogenesis (24).
Agents that cause differentiation of colorectal cells also induce SGK1 mRNA expression
Sodium butyrate (NaBut) is a short-chain fatty acid normally found in the colorectal lumen. NaBut promotes cellular differentiation in vitro in a number of CRC cell lines (26). Since our data showed SGK1 to be upregulated in differentiated cells of the intestine, at the crypt tops, we investigated whether SGK1 expression was induced by NaBut-driven differentiation in CRC lines. Two cell lines were selected, LS174T which expresses almost no endogenous SGK1, and HT29 which expresses modest levels [23]. Upon NaBut treatment, both HT29 and LS174T cells showed a significant increase in SGK1 transcript levels over time, as measured by qRT-PCR (p<0.01) (Figure 1A). This increase was confirmed in luciferase reporter assays (3.9-fold for HT29, 1.7-fold for LS174T; data not shown).
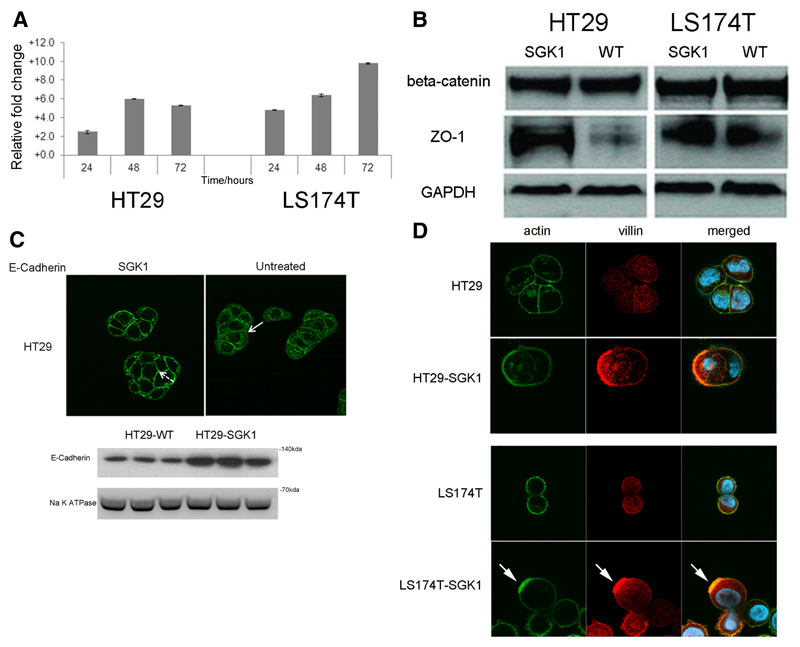
Figure 1. SGK1 causes colorectal cancer cell differentiation.
A. SGK1 transcription is increased upon sodium butyrate (NaBut) treatment in colorectal cancer cells. The column chart plots fold-change increases in SGK1 transcript levels, as measured by qRT-PCR, in HT29 and LS174T colorectal cancer cells upon treatment with NaBut, normalized to untreated control and relative to levels at time=0 hrs. Both cell lines showed a significant increase in SGK1 between 0 and 24 hours, and between 24 and 48 hours (t-test, p<0.01 in all cases, experiments in triplicate). SGK1 levels plateaued after 48 hours in HT29, whereas a further significant increase was found in LS174T.
B. Representative western blots of junctional proteins in HT29-SGK1 and LS174T-SGK1 cells and untreated wild-type controls. There are essentially unchanged levels of beta-catenin and an increase in ZO-1 abundance upon SGK1 re-expression. A similar ZO-1 increase was also found for cell line HCT116 (not shown).
C. Representative immunofluorescence images suggest re-localisation of E-cadherin from the cytoplasm in parenteral controls (solid arrows) to the membrane in SGK1 re-expressing HT29 (dashed arrows). The cellular fractionation blot confirms increased expression of E-cadherin in the membrane. Note that LS174T cells carry bi-allelic mutations of the CDH1 gene, resulting in protein absence.
D. Immunofluorescence analysis on HT29-SGK1 and LS174T-SGK1 and parental controls illustrates the formation of actin ‘caps’ (green, arrowed), co-localised with the apical marker villin (red), in SGK1-transduced cells only. This indicates the establishment of cell polarity owing to SGK1 re-expression.
SGK1 expression induces differentiation of CRC cells
The NaBut experiments demonstrated that SGK1 is induced by differentiating agents. Based on these data and the SGK1 expression pattern in the crypt, we pursued the hypothesis that SGK1 is a key player in signalling pathways that induce and directly control differentiation of colorectal cells. To determine experimentally if this could be the case, we developed a system to establish stable, inducible expression of SGK1 using a lentivirus-based system. HT29 and LS174T CRC cell lines were used as models and comparisons made to their control counterparts that had been transduced with an empty lentivirus (HT29-SGK1 vs. HT29-WT, LS174T-SGK1 vs. LS174T-WT). This system resulted in levels of SGK1 mRNA expression that were comparable to those in normal intestinal tissues (Supp. Figure 2) and in the production of SGK1 protein (Supp. Figure 3). Taking this approach, it was possible to demonstrate that functionally active SGK1 protein was expressed, based on reduced levels of the known SGK1 phosphorylation target, NEDD4-2 (Supp. Figure 4).
The process of differentiation in colonocytes is characterised by several histological features, including the formation of junctional complexes that maintain cell shape and the establishment of basal-apical cell polarity, with the formation of actin filament caps that co-localize with apical markers in differentiated colonocytes [24]. Upon NaBut-induced differentiation, LS174T cells are known to undergo specific morphological changes, which include the appearance of mucin-like granules [21]. We found that similar morphological changes were induced by re-expression of SGK1 in the absence of NaBut, and there was an increased tendency for cells to grow as colonies. HT29 cells treated with NaBut or in which expression of SGK1 was induced, showed comparable morphological changes (Supp. Figure 5).
We investigated whether other features of differentiation could be identified in our CRC cell lines following re-expression of SGK1, specifically investigating the levels and distribution of proteins important for the formation of key junctional proteins, beta-catenin, ZO-1 and E-cadherin. SGK1 caused an increase in total ZO-1 but not beta-catenin (Figure 1B, Supp. Figure 6). Immunofluoresence analysis of E-cadherin demonstrated increased membrane expression following SGK1 re-expression, and this was confirmed following cell fractionation western blotting (Figure 1C). We then assessed markers of cell polarity, which is a feature of differentiated intestinal epithelial cells. We found that LS174T and HT29 displayed actin caps upon re-expression of SGK1, and these were not seen in control cells. Moreover, the apical marker villin, which showed diffuse cytoplasmic expression in control cells, co-localised with the actin caps (Figure 1D). Together these findings demonstrated the ability of SGK1 re-expression to induce colonocyte differentiation.
We also transiently expressed SGK1 in the HCT116 CRC cell line, which has very low endogenous SGK1 and does not spontaneously differentiate in culture, perhaps having irreversibly lost the ability to do so. Following transient high-level SGK1 expression, HCT116 cells ruptured, with no evidence of differentiation.
SGK1 re-expressing CRC lines demonstrate reduced tumorigenicity, characterised by decreased anchorage-independent growth and cellular migration
To understand the phenotypic changes in cells that are induced by SGK1, we performed a number of functional assays using the re-expression cell lines, HT29-SGK1 and LS174T-SGK1. Twenty-four hours after re-expression of SGK1, we observed a significant reduction of ~40% in apoptosis rates under basal conditions, and a more modest but significant reduction upon induction of osmotic stress with sorbitol (Figure 2A). Apoptosis rates subsequently increased over the time course of the experiment to reach levels comparable to or exceeding those of control cells (data not shown), suggesting that the SGK1-induced protection was only temporary, or that resistant clones had grown out. SGK1 re-expression also led to modest, but significant, reductions in proliferation in the SGK1 re-expressing cells compared to controls (Figure 2A), and this was maintained over time.
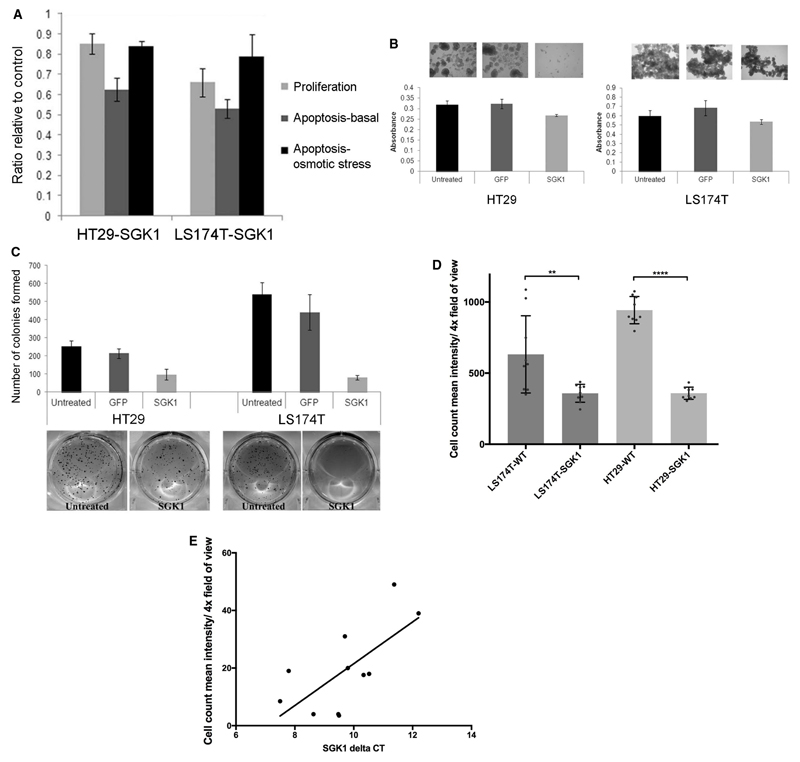
Figure 2. Effects of SGK1-induced differentiation on colon cancer cell proliferation and tumorigenicity.
A. The column chart displays ratios of HT29-SGK1 and LS174T-SGK1 proliferation (measured by tetrazolium compound assay) and apoptosis (basal and upon osmotic stress stimulation) 24 hours after removal of doxycycline, relative to controls with doxycycline maintained. Proliferation and apoptosis were significantly reduced in SGK1-expressing cells (all t-test p-values <0.05, experiments done in triplicate).
B. Representative images show colonospheres formed by untransduced, GFP-transduced (GFP) and SGK1-transduced (SGK1) cells for HT29 and LS174T colorectal cancer cell lines. Images were acquired following 10 days of cell culturing conditions that favour stem cell growth and colonosphere formation. The column charts represent absorbance measurements from an endpoint tetrazolium compound assay (day 10), showing decreased proliferation rates of the stem cell populations upon re-expression of SGK1 (t-test p-values<0.01 in all cases; experiments done in triplicate).
C. The column charts show numbers of colonies formed in soft agar by untreated, GFP-transduced (GFP) or SGK1-transduced (SGK1) cells. Results are an average of three experiments (t-test p-values<0.005 in all cases). Below the chart are representative images displaying macroscopic differences between untreated controls or SGK1-transduced cells.
D. Cellular migration is reduced on SGK1 re-expression (reduction in migration rate 43% for LS174T and 62% for HT29; t-test, p<0.01 and p<0.0001 respectively.
E. SGK1 mRNA expressions and CRC baseline migration rate. There is a significant negative correlation (Pearson correlation=0.68, p=0.02) between native SGK1 expression levels and migration in a panel of CRC lines (COLO205, GP2D, HCT116, HT29, LS174T, LS180, SNU-C4, SW1222, SW48, SW480, T84). Note that higher deltaCt corresponds to lower SGK1 expression.
To probe the effects of SGK1 on stem-like cell properties, colonosphere formation assays were performed. Upon re-expression of SGK1, both HT29 and LS174T cell lines showed dramatically impaired formation of colonospheres (Figure 2B), in keeping with SGK1-mediated reversion of the CRC cell lines to a more differentiated phenotype. To investigate the effects on anchorage-independent growth, soft agar assays were performed to assess characteristics observed in differentiated cells, anoikis (cell death) and the inability to proliferate, when there is loss of adhesion from the extracellular matrix. SGK1 re-expression results in reduced colony formation (Figure 2C).
Finally, we performed a Boyden migration assay that assesses cellular motility across a transwell membrane towards a growth factor gradient. At 24 hours, re-expression of SGK1 resulted in a significant reduction in rates of migration compared with cells that had been transduced with an empty lentiviral construct (Figure 2D). This observation was confirmed using a different approach, where a migration assay was performed on 11 CRC cell lines that naturally expressed different amounts of SGK1. This assay identified that lines with low SGK1 mRNA expression, such as LS174T and SW480, exhibited the highest migration rate whereas cell line with higher SGK1 (COLO205, SNU-C4, SW1222) demonstrated low cellular migration (Figure 2E).
The Cancer Cell Line Encyclopaedia (CCLE) was then utilised to obtain SGK1 expression for a total of 43 metastatic and primary colorectal cancer cell lines. Of the 42 cell lines, 9 were derived from metastatic sites. Primary CRC lines expressed significantly higher SGK1 than lines derived from metastases (2.7 Transcripts Per Kilobase Million (TPM) vs 6.7 TPM, p<0.05), but expression was both low and variable (Supp. Table 2).
In summary, these findings point towards a link between increased SGK1 expression and reduced proliferation, apoptosis, stem cell phenotype and motility.
SGK1 re-expression results in enrichment of gene networks involved in cell differentiation and reduction of MYC signalling
SGK1 could in principle influence numerous gene networks through protein phosphorylation, thus amplifying environmental or other triggers for differentiation. To gain clues about the gene expression changes induced by SGK1, it was re-expressed in 7 CRC cell lines (GP2D, HT29, HCT116, LS174T, SW1222, SW480 and T84). Whole-transcriptome profiling was performed using 3’-RNA sequencing.
Three genes were found to be differentially expressed: SGK1, NEFM, and RNA18S5 (p.adj all <0.05). To determine if there were more subtle changes in expression of genesets, GSEA using signatures from the MsigDB databases and curated genesets were performed. Re-expression of SGK1 was associated with enrichment for gene networks involved not only in the “protein kinase cascade” and “stress-activated protein kinase signalling” as expected from existing data, but also for “cell polarity”, “differentiation” and “cell matrix adhesion”. A focussed analysis of cancer signalling pathways demonstrated that expression levels of genes in several pro-oncogenic pathways were significantly decreased following SGK1 re-expression. This effect was most pronounced for the gene sets “MYC targets” and “retinoblastoma/E2F signalling” (NES=-26.1 and -1.56 respectively, p<0.001 for both) (Figure 3A, Table 1). Following re-expression of SGK1, expression of MYC mRNA was reduced by 23.6% (p=0.03) and expression of c-Myc target PGK1 was reduced by 23.1% (p=0.03).
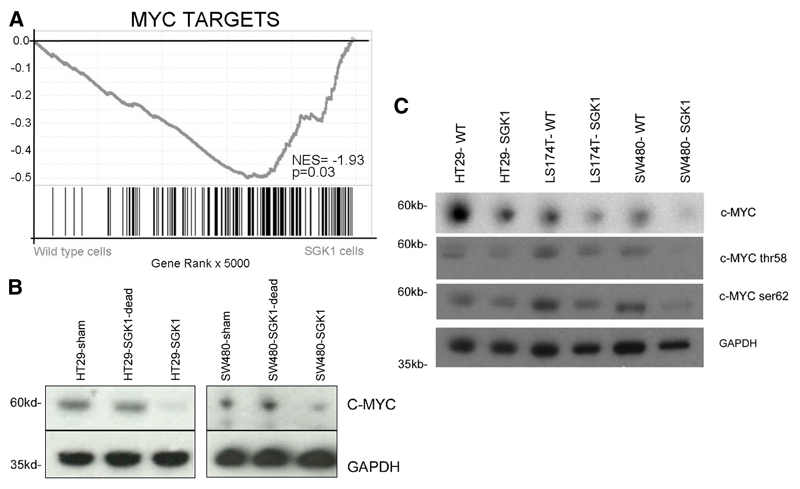
Figure 3. Reduced expression of MYC and its targets after SGK1 re-expression.
A. Gene set enrichment analysis of RNA sequencing data shows greatly reduced expression of c-Myc target genes following SGK1 re-expression.
B. Representative western blots demonstrating reduced MYC protein expression following SGK1 re-expression in HT29 and SW480, but not after re-expression of a kinase-dead SGK1.
C. Representative western blots demonstrating decreased total MYC and total phospho-MYC (Ser62 and Thr58) following SGK1 re-expression in HT29, LS174T and SW480 cell lines.
Table 1. Top dysregulated pathways in cell lines re-expressing SGK1 compared with parental controls.
The normalised enrichment core (NES) shows that SGK1 re-expressing cell lines have significantly less expression of gene sets such as Rb/E2F signalling, MYC targets and EMT, with increased expression of gene sets including differentiation, cell polarity and adhesion.
| Gene Set | NES | p-value |
|---|---|---|
| Protein kinase cascade | 1.34 | 0.004 |
| Cell polarity | 1.70 | 0.010 |
| Regulation of cell differentiation | 1.54 | 0.014 |
| Cell substrate adhesion | 1.45 | 0.031 |
| Stress activated protein kinase signalling pathway | 1.39 | 0.040 |
| Cell matrix adhesion | 1.43 | 0.044 |
| Regulation of cell adhesion | 1.47 | 0.047 |
| Epithelial morphogenesis | -1.38 | 0.005 |
| Epithelial-mesenchymal transition | -1.69 | 0.013 |
| MYC targets | -2.61 | <0.001 |
| mTORC1 signalling | -2.61 | <0.001 |
| Rb/E2F signalling | -1.56 | <0.001 |
In CRC, c-Myc lies at the crossroads of many growth-promoting signal transduction pathways and plays a key role as a pro-metastatic transcription factor, regulating epithelial-to-mesenchymal transition, cell-cell matrix interactions and cellular invasion in cell and murine models. In order to confirm reduction in c-Myc protein, we transduced CRC lines HT29 and SW480 with SGK1 re-expression lentivirus, a kinase dead SGK1 re-expression lentivirus or an empty lentiviral vector. Upon re-expression of SGK1, there was a consistent and significant decrease in c-Myc protein expression only in the cell lines carrying the SGK1 re-expression vector (mean reduction=56% at day 4 (Figure 3B, Supp. Figure 7). Using antibodies specific to phospho-Ser62 and phospho-Thr58, we demonstrated that there was a corresponding decrease in phosphorylation at both c-Myc Ser62 and Thr58 in the SGK1 re-expressing cells (Figure 3C).
SGK1-induced differentiation is mediated by plakophilin 3
We searched for proteins that were differentially expressed as a consequence of SGK1 re-expression and hence were potential downstream effectors of SGK1-induced differentiation. We utilized stable isotope labelling of amino acids in culture (SILAC) on our HT29-SGK1 and parental cells, followed by mass spectrometry differential analysis. The experiment identified a small number of proteins showing differential expression upon SGK1 re-expression. Mass spectrometry results consistently indicated an increased abundance (~20-fold) of plakophilin 3 (PKP3) (Figure 4A), an essential component of desmosomes that has been linked to metastatic potential and differentiation in tumours [25] [26]. Western blotting (Supp. Figure 8A) confirmed higher levels of PKP3 expression in both HT29-SGK1 and LS174T-SGK1 cells compared to parental cells. Investigation of PKP3 expression by immunohistochemistry in normal human colon showed increased expression in differentiated cells, paralleling SGK1 expression (Figure 4B).
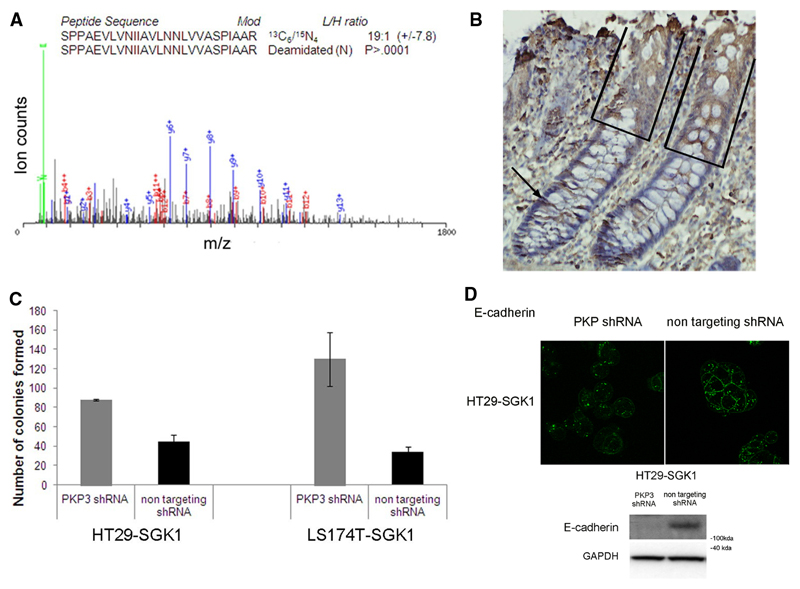
Figure 4. Plakophilin 3 is induced by SGK1 and in turn mediates features of differentiation.
A. Quantitative SILAC mass spectrometry reveals elevated PKP3 in SGK1 re-expressing cells. MS/MS spectrum shows PKP3-derived peptide 727-752, [M+3H]+ 884.2 (calculated MW 2649.5Da), where the fragment ions are indicated (green: immonium ions; blue: y-ions; red: b-ions). The light (SGK1 expressing cells) versus heavy (control cells) ratio as well as the significance (P) are indicated.
B. Immunohistochemistry on paraffin sections derived from normal human colonic tissue shows that expression of PKP3 is stronger in the differentiated compartment of the colonic crypt (boxed), where SGK1 is also expressed, that in the proliferative compartment (arrowed).
C. The bar chart represents the number of colonies formed in soft agar for HT29-SGK1 and LS174T-SGK1 cells with PKP3 knock-down (grey bars) or non-targeting shRNA (black bars), showing that SGK1-induced impaired anchorage-independent growth is reversed upon PKP3 knock-down (t-test p-values<0.005 in all cases).
D. Immunofluorescence shows that the SGK1-induced re-localisation of E-cadherin to the cell membrane is impaired upon PKP3 knockdown by shRNA compared with non-targeting shRNA controls. Representative western blots demonstrating decreased total E-Cadherin when SGK1 re-expressing cells are subjected to PKP3 knockdown by shRNA compared with non-targeting shRNA controls (results for LS174T cells not shown due to E-cadherin deficiency).
To assess whether PKP3 mediates the effects of SGK1 on cell differentiation, we engineered lentiviral-based shRNA knockdown of PKP3 in HT29-SGK1 and LS174T-SGK1 cells. When compared to control cells re-expressing SGK1 and transduced with a non-targeting shRNA virus, abolition of PKP3 expression (Supp. Figure 8B) lowered membranous E-cadherin expression, and also restored colony-forming ability (Figure 4 C, D).
SGK1 suppresses cancer cell metastasis in an orthotopic xenograft model through inhibition of metastatic outgrowth
Our experiments demonstrated that SGK1 exerted diverse effects on the cancer cell phenotype. Many of the processes affected – differentiation, migration, anchorage-independent growth, inter-cellular adhesion and c-Myc expression – are important in metastasis. We therefore developed an orthotopic xenograft mouse model of CRC metastasis, by endosocopically injecting genetically-modified CRC cells into the sub-mucosa of the mouse descending colon. We initially tested Sgk1 knockout in MC38 CRC cells, using a CRISPR/Cas9 strategy as part of a whole genome screen in 8 mice. Sgk1 knockout did not detectably change metastasis rates, as might be expected given the very low Sgk1 levels in CRCs (details not shown). We therefore tested the effects on metastasis of over-expressing SGK1. LS174T-SGK1 and LS174T-WT cells were labelled using a luciferase-expressing lentivirus (pHIV-Zsgreen-luc2) to enable in vivo imaging, and transplanted into Nod/Scid Gamma mice under endoscopic guidance. We found SGK1 mRNA expression in the LS174T-SGK1 cell lines and primary xenografts to be at levels comparable to normal epithelium (data not shown).
All animals (n=16) developed tumours in the colonic lumen following successful implantation. By day 21, mice became symptomatic principally due to intestinal obstruction but also metastatic outgrowth. All mice developed lung metastases, but secondary spread to the liver was uncommon (4/16 mice) and lesions in bone and other distant sites were not detected. At day 21, mice were culled, lungs were resected and ex vivo analysis performed.
Ex vivo imaging demonstrated that there was a striking 25.8-fold decrease in lung metastasis burden (p<0.008) in the mice that received LS174T-SGK1 compared to those that received LS174T parental cells. In a replication cohort (n=15), there was a similar (20.4-fold) decrease in lung metastasis burden (p<0.001; Figure 5A, B). By contrast, there was no significant difference in primary tumour size between the LS174T-SGK1 and LS174T-WT groups (Supp. Figure 9A).
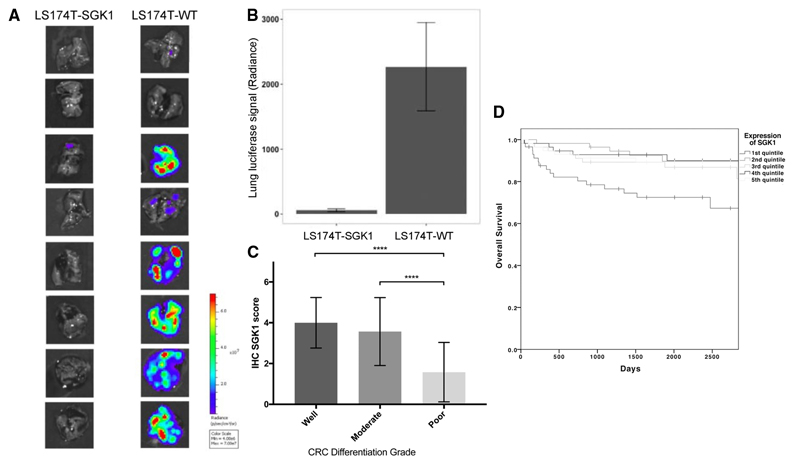
Figure 5. SGK1 re-expression reduces lung metastases in an orthotopic xenograft model of CRC and is associated with poor differentiation and inferior prognosis in human CRCs.
A. Ex vivo analysis of luminescence from lung metastases of LS174T-SGK1 and parental cells, demonstrates only very small lesions in the former.
B. Lung metastasis burden after orthotopic delivery of LS174T-SGK1 is much lower than in parental cells.
C. Immunohistochemical detection of SGK1 in a human CRC tissue microarray shows significantly less expression in poorly differentiated CRCs (p<0.01).
D. Data from TCGA demonstrate that patients with the lowest expression of SGK1 have significantly poorer overall survival (p<0.001).
These findings are in keeping with SGK1-mediated differentiation in vitro not limiting primary tumour growth, broadly consistent with the modest decreases in proliferation found in vitro (see above), but acting potently as a suppressor of metastasis.
To determine the stage at which SGK1 exerted its effect on metastasis, whether inhibiting the ability of cells to migrate away from the primary tumour, or inhibiting later stages in metastasis such as the ability of cells to survive in the circulation and enter metastatic tissues, a tail vein assay was performed. LS174T-SGK1 and LS174T-WT cells were injected into the tail veins of 8 Nod/Scid Gamma mice. Bioluminescence ex vivo analysis confirmed a marked reduction in metastasis in the LS174T-SGK1 cells, comparable in magnitude to the reduction seen in the orthotopic xenografts (p<0.001; Supp. Figure 9B).
SGK1 expression levels are reduced in poorly differentiated human CRCs, but not in lymphatic metastases
Having established that SGK1 expression could induce features of differentiation in CRC cell lines, we examined this association in human CRCs. A tissue microarray of sporadic CRC resection specimens (n=86) was obtained comprising of cancers of principally stage II/III CRC and from patients with a mean age of 51.7 years (Supp. Table 3). Differentiation grading had been performed by accredited histopathologists according to AJCC guidelines. We examined SGK1 expression using immunohistochemistry (Supp. Figure 10). This demonstrated that there was a decrease in SGK1 expression in poorly differentiated than well or moderately differentiated cancers (t-test, p<0.01; Figure 5C). This result validated our experimental findings suggesting an association between low SGK1 expression and poor colorectal cancer differentiation in both CRC lines and tumour resections specimens.
Low primary SGK1 expression is a biomarker of poor prognosis in different stages of human CRC
Poor differentiation (high grade) is a well-known poor prognostic feature of CRC. Undifferentiated CRC are associated with increased cellular motility and features such as tumour and epithelial-mesenchymal transition (EMT) [27]. Patients with poorly differentiated CRCs tend to be younger, have larger, right-sided tumours with lymphovascular invasion, a higher frequency of MSI, a significantly poorer 4 year overall survival [28] and a higher propensity to develop metastasis [29]. As we have demonstrated the expression of SGK1 to be closely associated with CRC differentiation/grade, we sought to determine whether SGK1 expression could be utilised as a similar marker of poor prognosis.
The Cancer Genome Atlas sample set comprised an annotated set of US-based patient genomes with CRC of various stages [30]. A set of cohort of 284 patients has detailed follow-up information and RNAseq had been performed on all specimens, enabling an analysis of the effect of SGK1 expression. SGK1 expression was expressed as quintiles as it was non-normally distributed and quintiles used as a quantitative variable. This analysis found a significant association between lower SGK1 expression and poorer survival (p=0.023) (Figure 5D). There was no correlation between SGK1 expression and tumour stage and the association of low expression of SGK1 with poorer prognosis was observed across all stages of CRC except the small number of patients with stage 1 disease (Supp. Figure 11).
Discussion
SGK1 is a serine/threonine kinase that shares homology with proteins of the AKT family. To date, the role of SGK1 has been most clearly elucidated in regulation of ion channels [31], neuronal function [32] and T helper cell differentiation [33]. More recently, there has been a growing realization that SGK1 lies at the junction of, and could mediate the effects of, several oncogenic signalling networks. The effects of SGK1 are variable, depending on the tissues or cell lines analysed and the model systems used. A number of studies have highlighted the pro-proliferative effects of SGK1: in hepatocyte regeneration, SGK1 mediated the phosphorylation of ERK2 and the activation of the Ras-MAPK kinase pathway [34]; and in breast cancer, activation of SGK1 sustained mTOR activation in the context of AKT inhibition through direct phosphorylation of TSC2 [35]. SGK1 is also thought to act in conjunction with PHLPP1 to enable Ras-mutant cancers survive anoikis during metastasis [36]. By contrast, in HEK293 cells, SGK1 inhibited the Ras-MAPK kinase pathway through phosphorylation of B-Raf, which is likely to exert an anti-proliferative effect [37].
In terms of regulation of SGK1 expression, in CRC, mutations in SGK1 may be observed in up to 2% of cases, although the mutations are usually missense and not isolated to the kinase domain [38]. Our previous work has identified that down-regulation in CRCs appears to be independent of promoter hypermethylation [39] and these observations suggest that control is likely to be transcriptionally regulated.
Our focus in this work was on the potential role of SGK1 in actively promoting differentiation, based on our validated finding that in the colorectum, SGK1 is expressed predominantly in the terminally differentiated epithelial cell compartment, in which cells have little proliferative potential, but are required to survive and function in a potentially hostile osmolar environment. We initially showed that SGK1 is induced by the extrinsic differentiating agent NaBut, consistent with NaBut acting, at least in part, through SGK1. Based on this preliminary evidence, we directly pursued the hypothesis that SGK1 re-expression induces features of differentiation. For this, we used both cellular and molecular read-outs in CRC cell lines that retain some differentiation capability. We found that SGK1 induces not only a gene expression profile of differentiation, but also several specific features of differentiation, including cell polarity, increased tight junction protein expression, and relocation of adherens junction proteins to the membrane. Stem cell-like features, proliferation, apoptosis, growth in soft agar and migration were correspondingly decreased. Our data showed that mechanisms of SGK1 action plausibly include reduction of c-Myc signalling, and increased expression of the desmosome protein plakophilin 3. In a CRC cell line orthotopic xenograft model, we found SGK1 re-expression to reduce the growth of lung metastases. The results of this assay suggested that SGK1-mediated differentiation of CRC lines inhibits the later stages of metastasis, perhaps functioning by limiting the ability of cells to migrate into the lung parenchyma from the endothelial cells.
Some caveats must be applied to our data. First, SGK1 expression can be hard to assess using western blots/IHC, at least in part because the protein has multiple isoforms and there are few antibodies with good specificities. We therefore relied on mRNA expression using a pan-isoform probe or total isoform expression by sequencing as a read-out for many of our assays. We did, however, perform western blot analysis of tagged SGK1, a limited analysis of native SGK1, and also showed that functional SGK1 protein was produced based on assessment of NEDD4-2 levels. In combination, these experiments strongly supported the existence of active SGK1 in our experiments. Second, given the low SGK1 levels in CRC cell lines (including those with differentiation capacity) and the potential redundancy between SGK1 and related SGKs, our experiments necessarily relied on SGK1 re-expression rather than knock-down. Whilst we appreciate that over-expression of kinases can lead to non-physiological effects, SGK1 re-expression in our systems led to near-physiological levels of mRNA. Finally, we have utilised an endoscopic transplantation model to determine effect on metastasis. The limitation of this approach is that as the lumen of the descending colon is narrow and mice become symptomatic relatively early. By contrast, the alternative, a caecal transplantation model allows mice to survive for a relatively longer period and often leads to increased levels of liver metastasis. We utilised the endoscopic transplantation model as it does not involve the creation of a track made from the external surface of the bowel and believe that, anatomically, the caecum of the mouse is markedly different from human.
One largely unanswered question in the field is the differences and similarities between the AKT and SGK1 arms of the PI3K pathway. We speculate that whilst both arms promote cell survival, which may explain some of the data showing that loss of SGK1 is deleterious to cells (see above), SGK1 does this in the context of its additional function of promoting differentiation, particularly in the intestine. In human CRCs, features of differentiation are not necessarily lost, with well and moderately differentiated cancers retaining some ability to form glandular units. Tumour differentiation is an important histological feature that is associated with poor cancer prognosis. The mechanisms through which the differentiation status of a tumour occurs and affects its behaviour and patient prognosis have, however, been somewhat overlooked. Our findings are consistent with the reduced expression of SGK1 being one important means by which tumours may “de-differentiate”, and acquire other malignant phenotypes such as migration and invasion. We conclude that a renewed focus on therapeutic agents to differentiate tumours, rather than simply to target cell survival, is justified.
Supplementary Material
Statement of Translational Relevance.
The degree of differentiation (usually termed “grade”) of colorectal cancers (CRC) is a well-established tumour feature that is associated with patient prognosis, and its assessment forms part of the American Joint Committee on Cancer (AJCC) recommendations. Serum/glucocorticoid-regulated kinase 1 (SGK1) is a highly conserved member of the AGC family of serine/threonine kinases. We have previously demonstrated that SGK1 expression in normal intestinal epithelium is highest at the top of the crypt, corresponding to the positions of differentiated colonocytes. In this study, we examine the role of SGK1 in intestinal cell differentiation, both in the normal crypt and in colorectal cancers. Our findings suggest that the level of SGK1 expression is a strong determinant of CRC differentiation status, identify c-Myc inhibition and plakophilin 3 expression as important downstream mechanisms and demonstrate that increased expression of SGK1 in an orthotopic xenograft model reduces metastases. In human CRCs, lower SGK1 expression is associated with poorer differentiation and worse prognosis. Strategies to increase SGK1 pathway activity have potential use as anti-metastatic agents, perhaps in the neo-adjuvant setting.
Acknowledgements
We would like to thank members of the Tomlinson lab for their help and advice. L.L is supported by a Guts UK charity trainee award, a Doctoral training program (DTP) award and the Medical Research Council (MRC). I.T. is supported by a Cancer Research UK (CR-UK) Programme Grant.
References
- 1.Amin Mahul B., editor. AJCC Cancer Staging Manual. Springer; [cited 2016 Dec 9]. [Internet] Available from: http://www.springer.com/us/book/9783319406176. [Google Scholar]
- 2.Takeuchi K, Kuwano H, Tsuzuki Y, Ando T, Sekihara M, Hara T, et al. Clinicopathological characteristics of poorly differentiated adenocarcinoma of the colon and rectum. Hepatogastroenterology. 2004 Dec;51(60):1698–702. [PubMed] [Google Scholar]
- 3.Tenen DG. Disruption of differentiation in human cancer: AML shows the way. Nat Rev Cancer. 2003 Feb;3(2):89–101. doi: 10.1038/nrc989. [DOI] [PubMed] [Google Scholar]
- 4.Sell S. Stem cell origin of cancer and differentiation therapy. Crit Rev Oncol Hematol. 2004 Jul;51(1):1–28. doi: 10.1016/j.critrevonc.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Webster MK, Goya L, Ge Y, Maiyar AC, Firestone GL. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993 Apr;13(4):2031–40. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang F, Böhmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006 Oct;86(4):1151–78. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 7.Segditsas S, Sieber O, Deheragoda M, East P, Rowan A, Jeffery R, et al. Putative direct and indirect Wnt targets identified through consistent gene expression changes in APC-mutant intestinal adenomas from humans and mice. Hum Mol Genet. 2008 Dec 15;17(24):3864–75. doi: 10.1093/hmg/ddn286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lien EC, Dibble CC, Toker A. PI3K signaling in cancer: beyond AKT. Curr Opin Cell Biol. 2017 Apr;45:62–71. doi: 10.1016/j.ceb.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Cristofano A. SGK1: The Dark Side of PI3K Signaling. Curr Top Dev Biol. 2017;123:49–71. doi: 10.1016/bs.ctdb.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt KM, Hellerbrand C, Ruemmele P, Michalski CW, Kong B, Kroemer A, et al. Inhibition of mTORC2 component RICTOR impairs tumor growth in pancreatic cancer models. Oncotarget. 2017 Apr 11;8(15):24491–505. doi: 10.18632/oncotarget.15524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoo G, Kim T, Chung C, Hwang D-S, Lim D-S. The novel YAP target gene, SGK1, upregulates TAZ activity by blocking GSK3β-mediated TAZ destabilization. Biochem Biophys Res Commun. 2017;490(3):650–6. doi: 10.1016/j.bbrc.2017.06.092. [DOI] [PubMed] [Google Scholar]
- 12.Dattilo V, D’Antona L, Talarico C, Capula M, Catalogna G, Iuliano R, et al. SGK1 affects RAN/RANBP1/RANGAP1 via SP1 to play a critical role in pre-miRNA nuclear export: a new route of epigenomic regulation. Sci Rep. 2017 Mar 30;7 doi: 10.1038/srep45361. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talarico C, Dattilo V, D’Antona L, Menniti M, Bianco C, Ortuso F, et al. SGK1, the New Player in the Game of Resistance: Chemo-Radio Molecular Target and Strategy for Inhibition. Cell Physiol Biochem. 2016;39(5):1863–76. doi: 10.1159/000447885. [DOI] [PubMed] [Google Scholar]
- 14.D’Antona L, Amato R, Talarico C, Ortuso F, Menniti M, Dattilo V, et al. SI113, a specific inhibitor of the Sgk1 kinase activity that counteracts cancer cell proliferation. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2015;35(5):2006–18. doi: 10.1159/000374008. [DOI] [PubMed] [Google Scholar]
- 15.Liang X, Lan C, Jiao G, Fu W, Long X, An Y, et al. Therapeutic inhibition of SGK1 suppresses colorectal cancer. Exp Mol Med. 2017 24;49(11):e399. doi: 10.1038/emm.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang K, Gu S, Nasir O, Föller M, Ackermann TF, Klingel K, et al. SGK1-dependent intestinal tumor growth in APC-deficient mice. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol. 2010;25(2–3):271–8. doi: 10.1159/000276561. [DOI] [PubMed] [Google Scholar]
- 17.Davis H, Irshad S, Bansal M, Rafferty H, Boitsova T, Bardella C, et al. Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside the stem cell niche. Nat Med. 2015 Jan;21(1):62–70. doi: 10.1038/nm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batycka M, Inglis NF, Cook K, Adam A, Fraser-Pitt D, Smith DGE, et al. Ultra-fast tandem mass spectrometry scanning combined with monolithic column liquid chromatography increases throughput in proteomic analysis. Rapid Commun Mass Spectrom RCM. 2006;20(14):2074–80. doi: 10.1002/rcm.2563. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond E, Halpern Z, Elinav E, Brazowski E, Jung S, Varol C. Utilization of murine colonoscopy for orthotopic implantation of colorectal cancer. PloS One. 2011;6(12):e28858. doi: 10.1371/journal.pone.0028858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan X, Liu L, Li H, Huang L, Yin M, Pan C, et al. Dual specificity phosphatase 5 is a novel prognostic indicator for patients with advanced colorectal cancer. Am J Cancer Res. 2016 Oct 1;6(10):2323–33. [PMC free article] [PubMed] [Google Scholar]
- 21.Identification of hub genes, key miRNAs and potential molecular mechanisms of colorectal cancer. [cited 2017 Dec 18]; doi: 10.3892/or.2017.5930. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho SB, Yan PS, Dahiya R, Neuschwander-Tetri BA, Basbaum C, Kim YS. Stable differentiation of a human colon adenocarcinoma cell line by sodium butyrate is associated with multidrug resistance. J Cell Physiol. 1994 Aug;160(2):213–26. doi: 10.1002/jcp.1041600202. [DOI] [PubMed] [Google Scholar]
- 23.Medico E, Russo M, Picco G, Cancelliere C, Valtorta E, Corti G, et al. The molecular landscape of colorectal cancer cell lines unveils clinically actionable kinase targets. Nat Commun. 2015 Apr 30;6 doi: 10.1038/ncomms8002. 7002. [DOI] [PubMed] [Google Scholar]
- 24.Baas AF, Kuipers J, van der Wel NN, Batlle E, Koerten HK, Peters PJ, et al. Complete polarization of single intestinal epithelial cells upon activation of LKB1 by STRAD. Cell. 2004 Feb 6;116(3):457–66. doi: 10.1016/s0092-8674(04)00114-x. [DOI] [PubMed] [Google Scholar]
- 25.Khapare N, Kundu ST, Sehgal L, Sawant M, Priya R, Gosavi P, et al. Plakophilin3 loss leads to an increase in PRL3 levels promoting K8 dephosphorylation, which is required for transformation and metastasis. PloS One. 2012;7(6):e38561. doi: 10.1371/journal.pone.0038561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kundu ST, Gosavi P, Khapare N, Patel R, Hosing AS, Maru GB, et al. Plakophilin3 downregulation leads to a decrease in cell adhesion and promotes metastasis. Int J Cancer. 2008 Nov 15;123(10):2303–14. doi: 10.1002/ijc.23797. [DOI] [PubMed] [Google Scholar]
- 27.Zlobec I, Lugli A. Epithelial mesenchymal transition and tumor budding in aggressive colorectal cancer: Tumor budding as oncotarget. Oncotarget. 2010 Nov 28;1(7):651–61. doi: 10.18632/oncotarget.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao H, Yoon YS, Hong S-M, Roh SA, Cho D-H, Yu CS, et al. Poorly differentiated colorectal cancers: correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol. 2013 Sep;140(3):341–7. doi: 10.1309/AJCP8P2DYNKGRBVI. [DOI] [PubMed] [Google Scholar]
- 29.Derwinger K, Kodeda K, Bexe-Lindskog E, Taflin H. Tumour differentiation grade is associated with TNM staging and the risk of node metastasis in colorectal cancer. Acta Oncol. 2010 Jan 1;49(1):57–62. doi: 10.3109/02841860903334411. [DOI] [PubMed] [Google Scholar]
- 30.Network TCGA. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012 Jul 19;487(7407):330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lang F, Stournaras C, Alesutan I. Regulation of transport across cell membranes by the serum- and glucocorticoid-inducible kinase SGK1. Mol Membr Biol. 2014 Feb 1;31(1):29–36. doi: 10.3109/09687688.2013.874598. [DOI] [PubMed] [Google Scholar]
- 32.Lang F, Strutz-Seebohm N, Seebohm G, Lang UE. Significance of SGK1 in the regulation of neuronal function. J Physiol. 2010 Sep 15;588(18):3349–54. doi: 10.1113/jphysiol.2010.190926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heikamp EB, Patel CH, Collins S, Waickman A, Oh M-H, Sun I-H, et al. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat Immunol. 2014 May;15(5):457–64. doi: 10.1038/ni.2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Won M, Park KA, Byun HS, Kim Y-R, Choi BL, Hong JH, et al. Protein kinase SGK1 enhances MEK/ERK complex formation through the phosphorylation of ERK2: implication for the positive regulatory role of SGK1 on the ERK function during liver regeneration. J Hepatol. 2009 Jul;51(1):67–76. doi: 10.1016/j.jhep.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 35.Castel P, Ellis H, Bago R, Toska E, Razavi P, Carmona FJ, et al. PDK1-SGK1 Signaling Sustains AKT-Independent mTORC1 Activation and Confers Resistance to PI3Kα Inhibition. Cancer Cell. 2016 Aug 8;30(2):229–42. doi: 10.1016/j.ccell.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mason JA, Schafer ZT. Ras-ling with new therapeutic targets for metastasis. Small GTPases. 2017 May 4;:1–5. doi: 10.1080/21541248.2017.1310650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang B-H, Tang ED, Zhu T, Greenberg ME, Vojtek AB, Guan K-L. Serum- and Glucocorticoid-inducible Kinase SGK Phosphorylates and Negatively Regulates B-Raf. J Biol Chem. 2001 Aug 24;276(34):31620–6. doi: 10.1074/jbc.M102808200. [DOI] [PubMed] [Google Scholar]
- 38.cBioPortal for Cancer Genomics:Results- SGK1. [cited 2018 Jul 17]; [Internet] Available from: http://www.cbioportal.org/index.do?cancer_study_id=all&Z_SCORE_THRESHOLD=2.0&RPPA_SCORE_THRESHOLD=2.0&data_priority=0&case_set_id=all&gene_list=SGK1&geneset_list=%20&tab_index=tab_visualize&Action=Submit&cancer_study_list=coad_tcga_pan_can_atlas_2018%2Ccoadread_dfci_2016%2Ccoadread_genentech%2Ccoadread_tcga_pub%2Ccoadread_tcga%2Ccoadread_mskcc%2Cread_tcga_pan_can_atlas_2018%2Ccrc_msk_2018&show_samples=false&clinicallist=CANCER_STUDY%2CPROFILED_IN_COPY_NUMBER_ALTERATION%2CPROFILED_IN_MUTATION_EXTENDED.
- 39.Lessi F, Beggs A, de Palo M, Anti M, Macarone Palmieri R, Francesconi S, et al. Down-regulation of serum/glucocorticoid regulated kinase 1 in colorectal tumours is largely independent of promoter hypermethylation. PloS One. 2010 Nov 5;5(11):e13840. doi: 10.1371/journal.pone.0013840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.