Abstract
Initial subjective response to the rewarding properties of alcohol predicts voluntary consumption and the risk for alcohol use disorders. We assessed the initial subjective reward to alcohol in rats using a single exposure conditioned place preference (SE-CPP) paradigm. Sprague-Dawley rats demonstrate preference for a context paired with a single systemic injection of ethanol (1.0 g/kg, delivered intraperitoneally). However, expression of SE-CPP in males depended on pairing ethanol with the first exposure of two (ethanol; saline) to the conditioning apparatus and procedures, while conditioning day did not appre-ciably affect SE-CPP in females, consistent with the view that females experience heightened addiction vulnerability. This model offers researchers a high throughput assay for investigating factors that in-fluence alcohol reward and may point the way toward more effective prevention and treatment efforts.
Keywords: Conditioned place preference, Alcohol use disorder, Ethanol, Sex differences, Susceptibility, Novelty
1. Introduction
The response to one’s initial drug experience has long been studied in the clinic as a predictor of abuse liability (Haertzen, Kocher, & Miyasato, 1983; Schuckit, 1984; de Wit, 1998). The sub-jective response to alcohol has been defined as an endophenotype in the etiology of alcoholism reflecting its capacity to predict future alcohol use and misuse (King, de Wit, McNamara, & Cao, 2011; Ray, Mackillop, & Monti, 2010). Better understanding of the underlying neurobiology of the initial subjective response to alcohol will aid researchers in detecting relevant biomarkers and further the development of more effective intervention and prevention strategies. However, there are few animal models for assessing initial subjective reward, impeding progress in identifying the underlying mechanisms involved (Lynch, Nicholson, Dance, Morgan, & Foley, 2010).
We developed a method for assessing initial subjective reward to alcohol (ethanol) using a single exposure conditioned place preference paradigm (SE-CPP). We found reliable place preference in three strains of mice to a context paired just one time with a moderate dose of ethanol (Grisel et al., 2014). Although assessing genetic influences using inbred mice in the SE-CPP is useful, there are a wide array of selected lines for modeling facets of human alcoholism in rats, which help make this species especially appealing to basic researchers (Ciccocioppo, 2013). Moreover, rats have long been appreciated by those investigating social and developmental influences on complex behaviors, such as addiction (Iannaccone & Jacob, 2009; Nylander & Roman, 2013; Varlinskaya & Spear, 2015). Therefore, we sought to extend the model to male and female Sprague-Dawley (outbred) rats. Historically, obtaining CPP to ethanol in rats has proven difficult (Cunningham, 1981) with few positive results involving multiple ethanol exposures (Bozarth, 1990; Morales, Varlinskaya, & Spear, 2012). Here, we demonstrate SE-CPP to ethanol in Sprague-Dawley rats and provide evidence for a simple and generalizable model capable of evaluating innate liability to ethanol reward in rats and mice.
2. Methods
2.1. Subjects
Adult (65e90 days old) male and female Sprague-Dawley rats (Charles-River; Kingston, NY, USA) were group housed by sex two to three per polycarbonate cage under standard conditions with free access to food and water and maintained on a 12:12 light-dark cycle (lights off at 06:00 pm). All subjects were conditioned and tested 3e6 h into their dark (active) cycle. All procedures were approved by the Bucknell University Animal Care and Use Committee and met the National Institutes of Health guidelines for ethical and humane animal research.
2.2. Conditioned place preference procedure
We employed an unbiased 3-compartment conditioned place preference apparatus, which is identical to that previously described (Grisel et al., 2014) except enlarged for use in rats (127 30 46 cm). A neutral center compartment separates chambers that have distinct tactile floor cues. The protocol uses a 3-day procedure, including conditioning on days 1 and 3, and testing on day 5, conducted on alternate days across a 5-day period. On days 1 & 3 subjects were weighed and transported to the testing area across the hall from the colony room, and half immediately received intraperitoneal (i.p.) injections of ethanol (1.0 g/kg, 20% by volume in saline) and the other half received equivolume saline before being relegated to one compartment of the apparatus for a 30-min conditioning session; each animal received ethanol on one conditioning day and saline on the other (or vice versa). To avoid potential confounds of social interaction following conditioning, animals housed together received the same treatment on each day but otherwise day of ethanol administration and compartment type were counterbalanced. On day 5 all subjects received saline injections, and were immediately placed into the neutral center compartment, which allowed free access to move throughout the apparatus. Behavior was recorded for 30 min by a ceiling-mounted camera and scored later by blind observers with an inter-rater reliability of 0.995, measured by Pearson’s r. The apparatus was cleaned with a dilute, low-residue solution and dried between each subject’s conditioning and test sessions.
2.3. Statistical analyses
We wanted to characterize the response to SE-CPP in male and females rats in our 5-day paradigm, so first we conducted a two-way ANOVA with sex and conditioning day as between-subjects factors and preference scores [(Total time on ethanol-side)/(Total time on ethanol-side + Total time on saline-side)*(100)] as the dependent factor. Then, to address our initial hypothesis that male and female rats would exhibit a SE-CPP to ethanol we compared preference scores to the null hypothesis value of 50% using t-tests. Statistical significance was set at P < 0.05.
3. Results
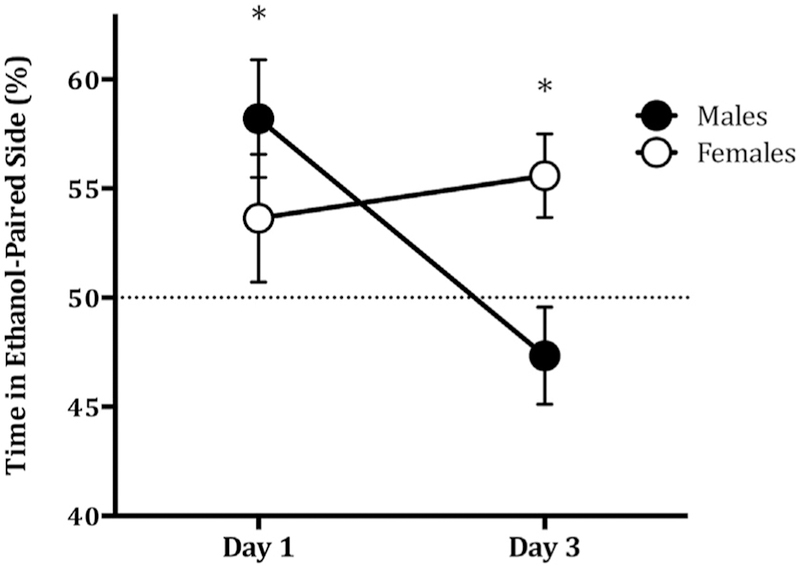
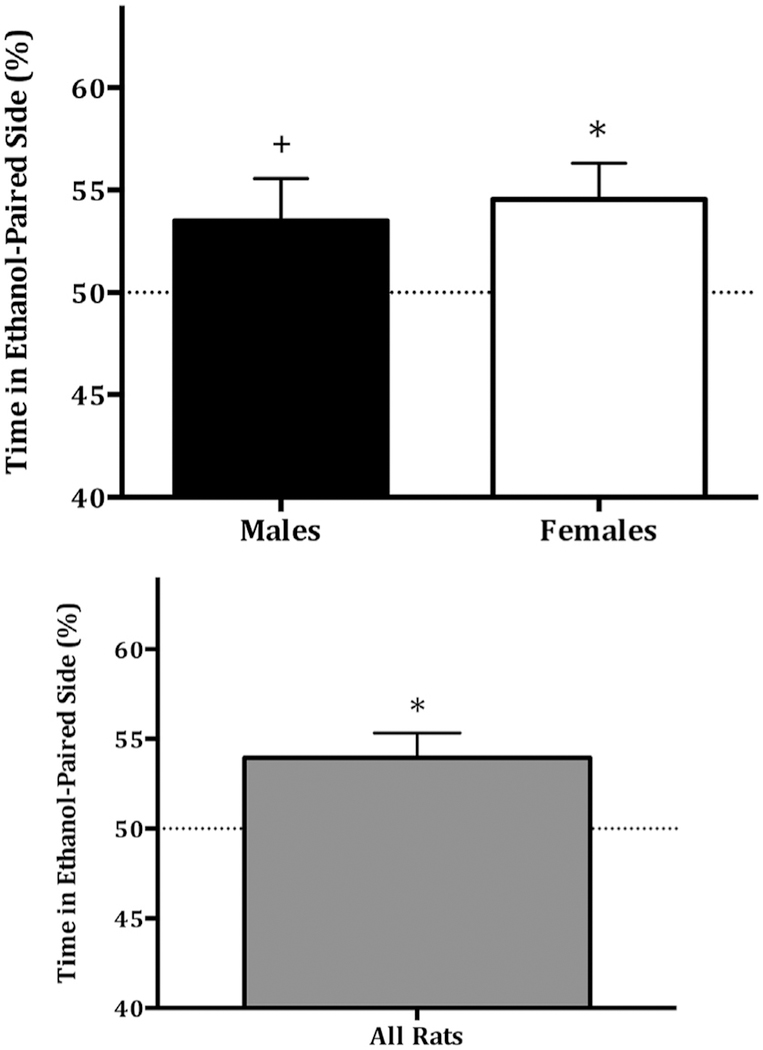
The two-way ANOVA revealed no main effect of sex (F (1,35)= 0.293, P = 0.592), no main effect of day of ethanol adminstration (F (1,35) = 2.48 μt 6, P = 0.125), but there was a significant interaction between sex and day of ethanol administration (F (1,35) = 5.494, P < 0.05; Fig. 1). To determine which factors influenced SE-CPP, we followed this up with four separate t-tests comparing preferences scores in the following groups: females day 1, females day 3, males day 1, and males day 3, to the null hypothesis value of 50% (equal time in ethanol-paired and saline-paired contexts). We adjusted for multiple tests using a Bonferroni correction and alpha levels of 0.0125. Males that received ethanol on conditioning day 1 showed a significant place preference (Mean ± SEM = 58.20 ± 2.70, t(9) = 3.036, P < 0.0125; Fig. 1), but did not if they received ethanol on conditioning day 3 (Mean ± SEM = 48.29 ± 2.18, t(8) = 0.785, P = 0.23; Fig. 1). Conversely, females showed a place preference if they received ethanol on conditioning day 3 (Mean ± SEM = 55.58 ± 1.93, t(7) = 2.888, P < 0.0125; Fig. 1), but not if they received ethanol on day 1 (Mean ± SEM = 53.64 ± 2.93, t(8) = 1.241, P = 0.125; Fig. 1). Overall Sprague-Dawley rats demonstrated conditioned place preference to a single i.p. injection of ethanol, as indicated by a preference score significantly above 50%, i.e., no preference (t(35) = 2.946, P < 0.01; Fig. 2). Furthermore, preference scores assessed separately by sex, but collapsing across day, revealed that the ethanol place preference was evident in females (t(16) = 2.583, < 0.05; Fig. 2) and marginally significant in males (t(18) = 1.692, p = 0.054; Fig. 2).
Fig. 1. Mean ± SEM % place preference of a Sex x Ethanol administration day interaction.
Percent preference for male (closed circles; day 1 n = 10, day 3 n = 9) and female (open circles; day 1 n = 9, day 3 n = 8) rats shown separated by day of ethanol administration. A two-way ANOVA revealed no main effect of sex and no main effect of day, but there was a significant interaction between sex and day (F (1,35) = 5.494, P < 0.05). *P < 0.05 relative to no preference at 50%.
Fig. 2. Mean ± SEM % place preference to 1.0 g/kg ethanol expressed as [time in ethanol context/(time in ethanol context + time in Saline Context)].
Upper panel shows % preference for male (n = 19) and female (n = 17) rats, respectively. Lower panel shows % preference for all rats (N = 36). *P < 0.05 relative to no preference at 50% as determined by one-tailed t-tests. +P = 0.054.
4. Discussion
We found that adult male and female Sprague-Dawley rats exhibit a significant conditioned place preference to a single, moderate dose (1.0 g/kg) of ethanol. Our results extend previous findings of SE-CPP to rats, and suggest SE-CPP as a generalizable model for assessing and investigating initial subjective reward to ethanol in rodents. Subjects received the systemic injection of ethanol on either day 1 or 3 of the study, and equivolume saline on the other, immediately before a 30 min conditioning session (and remain undisturbed on days 2 & 4). On the test day (5) both male and female rats preferred the ethanol paired context. However, researchers interested in using this model in rats should consider the potential influence of sex differences in conditioning, as male rats only showed a significant place preference when receiving ethanol on the first conditioning day. Females were less affected by conditioning day, though if anything, preference was stronger when ethanol was administered on day 3 (see Fig. 1). We did not see any evidence for sex differences using this paradigm in any of the three strains of mice tested (Grisel et al., 2014).
Male and female rodents exhibit subtle but significant differences on various measures of addictive-like behavior suggesting that females may experience heightened vulnerability (Carroll & Lynch, 2016). One well-documented difference is that female rodents tend to drink more alcohol than males (e.g., Lancaster & Spiegel, 1992). Similarly, females might be more prone to developing an ethanol place preference for some of the same reasons they show faster acquisition of self-administration than males for low doses of various drugs of abuse (Carroll, Lynch, Roth, Morgan, & Cosgrove, 2004), and develop greater preference for cocaine over food compared to males (Perry, Westenbroek, & Becker, 2013). Gonadal hormones might also influence the rewarding properties of ethanol differently in male and female rats. Using a biased, multiple exposure paradigm, Torres, Walker, Beas, and O’Dell (2014) reported that adolescent and adult female rats showed a preference only at 1.0 g/kg (the same dose used in the present study), which did not depend on estrous cycle, and males and ovariectomized females did not show a preference at any dose tested (0–2.5 g/kg).
Female rats display greater striatal dopamine (DA) release to various drugs of abuse, including ethanol, relative to males (Blanchard et al., 1993; for review see; Becker, Perry, & Westenbroek, 2012). Increased DA signaling might function to enhance the salience of the ethanol-paired context to a greater extent in females than males, as in the present study expression of preference in males was contingent on a combination of ethanol and exposure to a novel apparatus and testing procedures (Fig. 2, day 1 males). Similarly, in a single trial paradigm, Bevins (2001) reported that a sub-threshold dose of cocaine, insufficient to support a CPP alone, facilitated a CPP when paired with a novel object, which involves a DA-dependent mechanism (Besheer, Jensen, & Bevins, 1999). Consistent with these findings, Morales et al. (2012) found that male Sprague Dawley rats demonstrated an ethanol CPP, following multiple drug pairings, only if the rats were not pre-exposed to the testing apparatus prior to conditioning, suggesting males might habituate more readily than females. Thus, male rats in our study might have habituated to the testing apparatus by day 3, accounting for the absence of a CPP and suggesting a combination of novelty to the apparatus and ethanol might be necessary to facilitate ethanol-CPP induction in male rats, but not in females.
We observe an ethanol CPP when rats receive a single moderate dose of ethanol using a single exposure, high throughput protocol. Place conditioning procedures have become increasing popular tools for assessing the rewarding effects of drugs and non-drug reward (see Tzschentke, 2007 for a comprehensive review) and the SE-CPP model offers researchers an expedient tool for studying molecular and cellular mechanisms underlying initial ethanol experiences (Franklin et al., 2009; Melis, Camarini, Ungless, & Bonci, 2002) as well as genetic and environmental influences of ethanol reward in rats and mice (Ciccocioppo, 2013).
Acknowledgements
This work was supported by the National Institute on Alcohol Abuse and Alcoholism, United States (R15 AA022506). We thank Samuel Baum for his help analyzing behavioral videotapes.
References
- Becker JB, Perry AN, & Westenbroek C (2012). Sex differences in the neural mechanisms mediating addiction: A new synthesis and hypothesis. Biology of Sex Differences, 3, 14 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, Jensen HC, & Bevins RA (1999). Dopamine antagonism in a novel-object recognition and a novel-object place conditioning preparation with rats. Behavioural Brain Research, 103, 35e44. 10.1016/S0166-4328(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Bevins RA (2001). Novelty seeking and reward: Implications for the study of high-risk behaviors. Current Directions in Psychological Science, 10, 189e193. [Google Scholar]
- Blanchard BA, Steindorf S, Wang S, LeFevre R, Mankes RF, & Glick SD (1993). Prenatal Ethanol exposure alters ethanol-induced dopamine release in nucleus accumbens and striatum in male and female rats. Alcoholism: Clinical and Experimental Research, 17, 974e981. 10.1111/j.1530-0277.1993.tb05651.x. [DOI] [PubMed] [Google Scholar]
- Bozarth MA (1990). Evidence for the rewarding effects of ethanol using the conditioned place preference method. Pharmacology Biochemistry and Behavior, 35, 485e487. 10.1016/0091-3057(90)90191-J. [DOI] [PubMed] [Google Scholar]
- Carroll ME, & Lynch WJ (2016). How to study sex differences in addiction using animal models. Addiction Biology, 21, 1007e1029. 10.1111/adb.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll ME, Lynch WJ, Roth ME, Morgan AD, & Cosgrove KP (2004). Sex and estrogen influence drug abuse. Trends in Pharmacological Sciences, 25, 273e279. 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R (2013). Genetically selected alcohol preferring rats to model human alcoholism. Current Topics in Behavioral Neurosciences, 13, 251e269. 10.1007/7854_2012_199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL (1981). Spatial aversion conditioning with ethanol. Pharmacology Biochemistry and Behavior, 14, 263e264. 10.1016/0091-3057(81)90255-0. [DOI] [PubMed] [Google Scholar]
- Franklin KM, Engleman EA, Ingraham CM, McClaren JA, Keith CM, McBride WJ, et al. (2009). A single, moderate ethanol exposure alters extra-cellular dopamine levels and dopamine d receptor function in the nucleus accumbens of wistar rats. Alcoholism: Clinical and Experimental Research, 33(10), 1721e1730. 10.1111/j.1530-0277.2009.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Beasley JB, Bertram EC, Decker BE, Duan CA, Etuma M, et al. (2014). Initial subjective reward: Single-exposure conditioned place preference to alcohol in mice. Frontiers in Neuroscience, 8, 345 10.3389/fnins.2014.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertzen CA, Kocher TR, & Miyasato K (1983). Reinforcements from the first drug experience can predict later drug habits and/or addiction: Results with coffee, cigarettes, alcohol, barbiturates, minor and major tranquilizers, stimu-lants, marijuana, hallucinogens, heroin, opiates and cocaine. Drug and Alcohol Dependence, 11, 147e165. 10.1016/0376-8716(83)90076-5. [DOI] [PubMed] [Google Scholar]
- Iannaccone PM, & Jacob HJ (2009). Rats! Disease Models & Mechanisms, 2, 206e210. 10.1242/dmm.002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, de Wit H, McNamara PJ, & Cao D (2011). Rewarding, stimulant, and sedative alcohol responses and relationship to future binge drinking. Archives of General Psychiatry, 68, 389e399. 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, & Spiegel KS (1992). Sex differences in pattern of drinking. Alcohol, 9, 415e420. 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Nicholson KL, Dance ME, Morgan RW, & Foley PL (2010). Animal models of substance abuse and addiction: Implications for science, animal welfare, and society. Comparative Medicine, 60, 177e188. [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, & Bonci A (2002). Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. Journal of Neuroscience, 22, 2074e2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Varlinskaya EI, & Spear LP (2012). Evidence for conditioned place preference to a moderate dose of ethanol in adult male Sprague-Dawley rats. Alcohol, 46, 643e648. 10.1016/j.alcohol.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylander I, & Roman E (2013). Is the rodent maternal separation model a valid and effective model for studies on the early-life impact on ethanol consump-tion? Psychopharmacology (Berlin), 229, 555e569. 10.1007/s00213-013-3217-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry AN, Westenbroek C, & Becker JB (2013). The development of a preference for cocaine over food identifies individual rats with addiction-like behaviors. PLoS One, 8, e79465 10.1371/journal.pone.0079465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Mackillop J, & Monti PM (2010). Subjective responses to alcohol consumption as endophenotypes: Advancing behavioral genetics in etiological and treatment models of alcoholism. Substance Use & Misuse, 45, 1742e1765. 10.3109/10826084.2010.482427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA (1984). Subjective responses to alcohol in sons of alcoholics and control subjects. Archives of General Psychiatry, 41, 879e884. 10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Torres OV, Walker EM, Beas BS, & O’Dell LE (2014). Female rats display enhanced rewarding effects of ethanol that are hormone dependent. Alco-holism: Clinical and Experimental Research, 38, 108e115. 10.1111/acer.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM (2007). Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addiction Biology, 12, 227e462. 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, & Spear LP (2015). Social consequences of ethanol: Impact of age, stress, and prior history of ethanol exposure. Physiology & Behavior, 148, 145e150. 10.1016/j.physbeh.2014.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H (1998). Individual differences in acute effects of drugs in humans: Their relevance to risk for abuse. NIDA Research Monograph, 169, 176e187. Retrieved from: http://www.ncbi.nlm.nih.gov/pubmed/9686416. [PubMed] [Google Scholar]