Abstract
New treatment modalities for glioblastoma multiforme (GBM) are urgently needed. Proton therapy is considered one of the most effective forms of radiation therapy for GBM. DNA alkylating agents such as temozolomide (TMZ) are known to increase the radiosensitivity of GBM to photon radiation. TMZ is a fairly impotent agent, while duocarmycin SA (DSA) is an extremely potent cytotoxic agent capable of inducing a sequence-selective alkylation of duplex DNA. Here, the effects of sub-nM concentrations of DSA on the radiosensitivity of a human GBM cell line (U-138) to proton irradiation were examined. Radiation sensitivity was determined by viability, apoptosis, necrosis and clonogenic assays. DSA concentrations as low as 0.001 nM significantly sensitized U-138 cells to proton irradiation. DSA demonstrates synergistic cytotoxicity against GBM cells treated with proton radiation in vitro, which may represent a novel therapeutic alternative for the treatment of GBM.
Glioblastoma multiforme (GBM) is the most common and the most deadly primary brain tumor in adults. Median survival for patients diagnosed with glioblastoma is only 15–18 months due to consistent recurrence following tumor resection and adjuvant therapy.1 GBM is aggressive, drug- and radioresistant, and characterized by an elevated cell proliferation rate, genetic instability, diffuse infiltration at diagnosis and high angiogenesis induction.2 The current standard of care for treatment of GBM consists of surgery followed by concurrent photon radiation and chemotherapy with the DNA alkylating drug temozolomide (TMZ).3 Unfortunately, because GBM tumor cells are highly infiltrative throughout the brain at diagnosis, complete removal of all tumor cells by surgery is not possible and disease recurrence following tumor resection results. Subsequent to surgery, radiation is the major component of treatment for patients.4 Ionizing radiation such as photons and protons damages the DNA of cancer cells, resulting in cell death. The effectiveness of photon irradiation is compromised by the physical and biological characteristics of the photon beam itself, resulting in a need to balance local control of tumor growth with the incidence of side effects resulting from damage to healthy tissue surrounding the tumor due to lateral scattering of the photon beam.5
Proton radiation has become a preferred option for treating CNS malignancies, including GBM, because proton beams can be deposited at the site of the tumor more precisely than is possible with photon radiation and with minimal lateral scattering, minimizing damage to healthy tissue surrounding the tumor.6 Multiple clinical trials using proton beams have been conducted for GBM patients. Of particular interest is the work of Mizumoto and coworkers demonstrating in a phase I/II clinical trial that median survival rates increased to 21.6 months after employing proton therapy.7 The higher efficacy of proton irradiation arises from an observed increase in single and double stranded DNA breaks, increased Chk2 phosphorylation, and reduced cell cycle recovery from G2 arrest leading to caspase-3 activation, PARP cleavage and apoptosis.8 These findings demonstrate that proton irradiation is more effective in treating GBM than more traditional photon treatment.
The use of TMZ as an adjuvant chemotherapy in the treatment of GBM is believed to augment the effectiveness of the primary radiation therapy by sensitizing tumor cells to the toxic effects of radiation.9 Several studies have demonstrated an improvement for GBM patients who received TMZ compared with those who did not, in both the median survival time (14.6 vs 12 months) and in the 2-year survival rate (27% vs 10%).10 TMZ is selected as much because of its relatively low systemic toxicity at the high concentrations necessary to treat GBM as for its efficacy in killing tumor cells. While clinically useful, TMZ is a fairly impotent compound, with a reported IC50 of >500 μM (U138 GBM) and a value of 598 μM in our assay conditions.11 More recently, the DNA alkylating drug carmustine has been loaded onto biodegradable controlled-release polymeric wafers (Gliadel® wafers) that are implanted after surgery. This allows for local delivery of the chemotherapeutic agent in the three-week period between surgery and the initiation of radiation and systemic chemotherapy with TMZ. Furthermore, use of carmustine wafers has virtually no systemic toxicity due to extremely limited systemic exposure. Gliadel® has been reported to extend median survival by 2–4 months for newly diagnosed and recurrent GBM patients following tumor resection.12
Despite these advancements in treatment, patient survival remains poor for several reasons. First, GBM tumor cells are highly resistant to both radiation and chemotherapy. Second, dose-limiting toxicities arise from radiation therapy resulting from damage to healthy tissues surrounding the site of the tumor. Third, the effectiveness of chemotherapeutic agents is challenged by the difficulty involved in crossing the blood-brain barrier in order to reach the tumor, resulting in low concentrations of drug at the tumor site even at toxic systemic concentrations.
Therefore, strategies that permit lowering of the dose of radiation through the use of potent DNA alkylating chemotherapeutic agents that can sensitize tumor cells to radiation (radiosensitizers) are of significant interest in the treatment of GBM. The duocarmycin class of antitumor antibiotics, exemplified by duocarmycin SA (DSA, Fig. 1), are among the most powerful anticancer agents known, often possessing low pM IC50 values across several cancer cell lines. These small-molecule DNA minor groove binding agents that selectively alkylate adenine at the N3 position have been the subject of intense research in both academia and the pharmaceutical industry for 30 years.13 A comparison of the published potencies of DSA and carmustine indicates a roughly one million-fold difference in potency (L1210 cells, IC50 = 6–10 pM for DSA and 4.5 μM for carmustine).14 The irreversible alkylation of DNA by this class of agents results in over stabilization of the DNA double helix resulting in impaired repair, transcription and replication of DNA and ultimately resulting in G2-M phase cell cycle arrest.15 Convincing evidence has shown that combinatorial treatment with drugs that induce G2-M arrest may enhance the radiosensitivity of tumor cells.8 Additionally, these agents have shown activity in a variety of multi-drug resistant tumors,15 which is of particular interest in the treatment of GBM.
Fig. 1.
Duocarmycin SA (DSA).
Multitudes of derivatives of the duocarmycins have been prepared over the years to elaborate their properties, and several analogs including adozelesin, carzelesin and bizelesin progressed into clinical trials for the treatment of cancer.16 More recently, derivatives conjugated to antibodies have been the focus of intense interest. Synthon Biopharmaceuticals SYD985 targets HER2-positive metastatic breast cancer and is currently in Phase 3 clinical trials.17
As part of an effort to increase the radiosensitivity of GBM cells to proton radiation, and eventually to improve patient therapeutic outcomes, we investigated the effects of sub-nM concentrations of DSA on a human glioblastoma cell line. We postulated that the combination of duocarmycin SA and proton radiation may be complementary and potentially synergistic in their capacity to kill GBM cells.
The below experiments were performed using the human glioblastoma cancer cell line U-138 MG. In vitro cytotoxicity was measured using three different assays: 1) cell viability, 2) apoptosis/necrosis monitored using flow cytometry, and 3) colony formation (cell survival and proliferation). In order to determine the short- and long-term toxicity on U-138 cells induced by DSA alone, proton radiation alone, or a combination of DSA and proton irradiation, the experiments described below were undertaken.
To determine the effects of DSA, protons, or TMZ alone in GBM cells, we treated U-138 GBM cells with the two chemotherapeutic agents or proton doses and assessed the number of viable cells after 72 h (Fig. 2). DSA, TMZ and proton irradiation all had a growth inhibitory effect on U-138 cells in a concentration/dose dependent manner. DSA produced a significant concentration-dependent decrease in cell viability, with 65% cell survival observed at 0.1 nM, and plateauing at a minimum of 25% cell survival at 0.5 nM, with no increase in cytotoxicity observed at higher doses. The IC50 of DSA for U-138 MG cells in this cell viability assay was measured as 0.4 nM (Fig. 2A). This is in comparison to 598 μM IC50 for TMZ, a difference of more than a million-fold for the two agents (Fig. 2C). Proton irradiation also reduced cell viability in U-138 cells in a dose dependent manner, with a maximal effect at 12 Gray (Gy, Fig. 2B). Proton irradiation in the range of 1–4 Gy did not affect cell growth. The calculated IC50 from proton irradiation was 15.3 Gy, though this value corresponds with the plateau in the maximal effectiveness for proton irradiation. These results are illustrative of the poor radio- and chemosensitivity observed with U-138 GBM cells. Due to the enormous difference in activities between DSA and TMZ, further experiments were performed using DSA only.
Fig. 2.
Sensitivity of U-138 cells to DSA, proton irradiation and TMZ at 72 h. post-treatment. (A) Number of viable cells exposed to DSA, (B) proton irradiation and (C) TMZ. (*p < 0.05, **p < 0.01, ***p < 0.001).
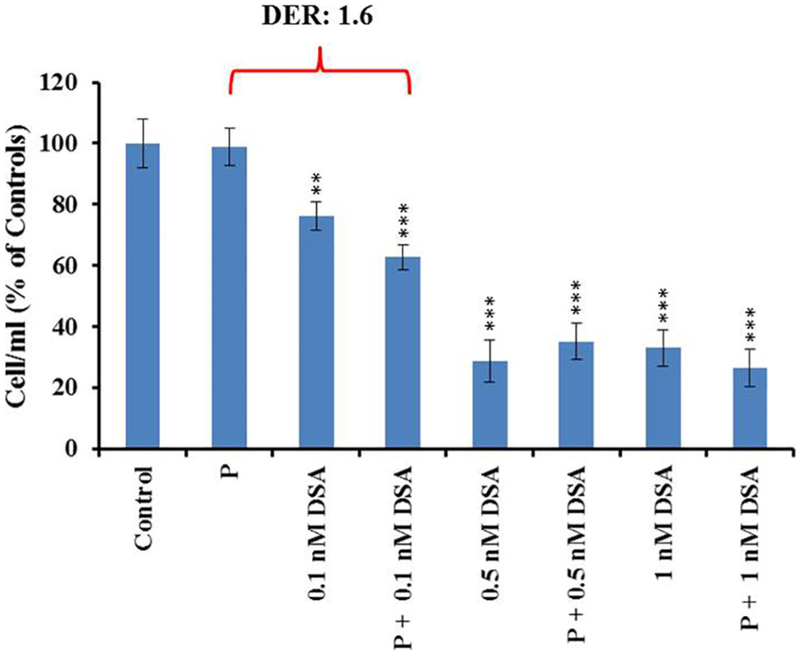
The effects of DSA alone at concentrations of 0.1, 0.5 and 1.0 nM or in combination with proton exposures of 2 Gy were investigated (Fig. 3). While no measureable cell death was observed at a proton radiation dose of 2 Gy, a modest additive effect from a 2 Gy dose of protons in combination with 0.1 nM DSA was observed (DER: 1.6). Calculated DER for DSA at high concentrations (0.5 and 1 nM) combined with protons were elevated. However, this phenomenon must be attributed to the high rate of cell killing induced by DSA and not due to an additive effect between both toxic agents.
Fig. 3.
U-138 cell viability after proton irradiation alone, DSA alone or combined DSA and proton at 72 h. post treatment.
To investigate the mechanism of cell death resulting from DSA exposure and/or proton irradiation, apoptosis and necrosis assays employing flow cytometry were performed. Apoptosis was monitored using ANNEXIN V affinity assay, while necrosis was measured using 7-aminoactinomycin D (7-AAD) assay. Cells treated with 0.1 nM DSA or 2 Gy protons exhibited a statistically significant increase of apoptosis and necrosis (Fig. 4).
Fig. 4.
Incidence of apoptosis (A) or necrosis (B) in U-138 cell at 72 h after treatment with protons alone or combined with DSA.
Increasing the dose of radiation from 2 Gy to 6 Gy did not produce an increase in either apoptosis or necrosis. Most importantly, treatment with 0.1 nM DSA and 2 Gy of protons was demonstrated to be additive for both apoptosis and necrosis (DER: 2.5 and 4, respectively). These data suggested that a DSA-mediated increase in radiosensitivity of U-138 GBM cells may result from the activation of apoptotic and necrotic pathways likely triggered by DNA damage and/or repair impairments.
The clonogenic assay is considered the “gold standard” in radio-biological toxicology studies as an indication of the long-term survival and self-renewal of tumor cells after treatment. GBM cells were exposed to DSA, proton irradiation or a combination of the two and assessed using the clonogenic assay. Fourteen days post exposure colonies of 50 or more surviving cells were counted and survival curves calculated (Fig. 5).
Fig. 5.
Cell survival curves for U-138 cells treated with DSA (panel A), protons (panel B) and protons and 0.001 nM DSA (panel C).
DSA demonstrated a steep concentration-response relationship in this more long-term proliferation assay, more in line with that expected given the potency of DSA and indicative of a strong cytotoxic effect on the tumor cells (Fig. 5A). Proton irradiation in the absence of DSA, on the other hand, produced a much weaker cytotoxic effect on the GBM cells, again demonstrating the radioresistant nature of GBM cells (Fig. 5B). The IC50 value of protons for U-138 cells was 0.964 Gy. DSA demonstrated strong cytotoxicity, with an IC50 of 0.0018 nM (1.8 pM). For this reason a concentration of 0.001 nM (1 pM) of DSA was chosen for the clonogenic assay testing DSA and proton irradiation in combination.
To investigate if DSA enhances the radiosensitivity of U-138 cells to proton irradiation, cells were exposed to 0.001 nM DSA before irradiation and then analyzed using the clonogenic assay (Fig. 5C). Survival fractions at different proton radiation doses (1–8 Gy) were greatly reduced in U-138 cells after they were exposed to DSA. The radiation survival curves obtained by the clonogenic assay showed that DSA pretreatment greatly sensitized human glioma cells to proton irradiation (proton IC50: 0.964, proton +DSA IC50: 0.443). The survival fractions measured for the combination of DSA and proton irradiation was 54% smaller than that expected on the basis of the treatment effects of each modality separately, demonstrating a significant synergistic interaction in the U-138 glioma cells (DER: 2.17).
In this report, we show that the combined effect of DSA with proton irradiation is superior to treatment with either the drug or radiation alone and demonstrates additive effects in cell viability assay, as well as apoptosis and necrosis assays. In the clonogenic cell survival and proliferation assay a true synergistic effect was observed at a minuscule 1 pM concentration of DSA. These data suggest the potential of DSA as a sensitizer to proton radiation in GBM cancer cells.
Chemotherapy-induced radiation sensitization occurs through several different modes of cell death including apoptosis, mitotic catastrophe, senescence and autophagy.18–21 Members of the duocarmycin class of agents are known to produce inter-nucleosomal DNA fragmentation and morphological changes characteristic of programmed cell death.22 These responses were associated with strong inhibition of cell growth and clonogenic survival suggesting that at pM concentrations the agent induced cell death by a target cell activated pathway.13 The correlation between modalities of cell death mediated by DSA alone and that resulting from DSA-induced radiosensitivity needs to be further investigated.
A potential benefit in the development of chemo-radiation therapies is the ability to induce cancer cell toxicity using lower doses of one or both of the chemotherapeutic drug and radiation. GBM patients generally receive high doses of radiation during treatment, with significant attendant toxicity to the patient. The additive and synergistic cytotoxic effects observed in this study suggest that very low concentrations of DSA or another duocarmycin derivative may allow for GBM-specific cell death at lower doses of radiation with less damage to surrounding healthy tissues. Duocarmycin derivatives have been shown to be very toxic in a number of clinical trials, and have never been approved as chemotherapeutic drugs. Of particular interest is the possibility of developing deriva tives of DSA conjugated to specific GBM antibodies, as the high cytotoxicity observed for DSA with proton radiation in these studies indicates that the small dose of cytotoxic drug delivered by an antibody-drug conjugates (ADC) may be sufficient to treat GBM if employed in combination with proton radiation.23 Currently, an anti-EGFR-based antibody (ABT-414) conjugated to the tubulin polymerization inhibitor monomethyl auristatin F (MMAF) is in phase II/III trials for patients with glioblastoma.24
It is important to note that the polarity and size of DSA may prevent its crossing of the blood brain barrier (BBB). In high-grade gliomas disruption of the BBB is often detected, and can result in increased access of drug to the tumor. The most promising potential for the development of a DSA-based treatment for GBM is through the use of an ADC, as with the anti-EGFR ADC mentioned above. Local delivery devices have also been employed in the treatment of glioblastoma to deliver investigational ADCs directly to the tumor using intra-cerebral catheters.25 Additionally, DSA or one of its derivatives could be loaded onto a biodegradable wafer in a manner similar to carmustine on Gliadel®.26
In summary, DSA demonstrates synergistic cytotoxicity against GBM cells treated with proton radiation in vitro, which may represent a novel therapeutic alternative for the treatment of GBM. The radiosensitizing effects of DSA observed in this study are encouraging because drugs showing efficacy against malignant glioma are still uncommon.
Supplementary Material
Acknowledgements
We thank Dr. Jerry Slater for his support, Cinthia Ramirez, Jonathan Sackett, Katherine Winters and Elyssa Mejia, for their technical assistance. This work was supported by the Loma Linda University Medical Center, Radiation Medicine Department and School of Pharmacy, California, United States.
Footnotes
A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bmcl.2018.04.008.
References
- 1.Louis D, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen P, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. [DOI] [PubMed] [Google Scholar]
- 3.Sulman E, Ismaila N, Armstrong TS, Tsien C, Batchelor TT, Cloughesy T, et al. Radiation therapy for glioblastoma: american society of clinical oncology clinical practice guideline endorsement of the american society for radiation oncology guideline. Clin Oncol. 2016;35:361–369. [DOI] [PubMed] [Google Scholar]
- 4.Combs S, Schmid T, Vaupel P, Multhoff G. Stress response leading to resistance in glioblastoma – the need for innovative Radiotherapy (iRT) Concepts. Cancers. 2016;8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzek M, Thornton A, Rabinov J, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91:251–260. [DOI] [PubMed] [Google Scholar]
- 6.Allen AM, Pawlicki T, Dong L, et al. An evidence based review of proton beam therapy: the report of astro’s emerging technology committee. Radiother Oncol. 2012;103:8–11. [DOI] [PubMed] [Google Scholar]
- 7.Mizumoto M, Tsuboi K, Igaki H, et al. Phase I/II trial of hyperfractionated concomitant boost proton radiotherapy for supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2010;77:98–105. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Steven L, Fang B, Gillin M, Mohan R, Chang J. Therapy-resistant cancer stem cells have differing sensitivity to photon versus proton beam radiation. Thorac Oncol. 2013;8:1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Meir E, Hadjipanayis C, Norden A, Shu H, Wen P, Olson J. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stupp R, Mason W, van den Bent M, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England J Med. 2005;352:987–996. [DOI] [PubMed] [Google Scholar]
- 11.Sang Y, Lee S. Temozolomide resistance in glioblastoma Multiforme. Genes Diseases. 2016;3:198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashby L, Smith K, Stea B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol. 2016;14:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boger D, Johnson D. CC-1065 and the duocarmycins: understanding their biological function through mechanistic studies. Angew Chem, Int Ed Engl. 1996;35:1438–1474. [Google Scholar]
- 14.MacMillan K, Boger D. Fundamental relationships between structure, reactivity, and biological activity for the duocarmycins and CC-1065. J Med Chem. 2009;52:5771–5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tietze L, Krewer B, von Hof Marian, Frauendorf H, Schuberth I. Determination of the biological activity and structure activity relationships of drugs based on the highly cytotoxic duocarmycins and CC-1065. Toxins. 2009;1:134–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz G, Patnaik A, Hammond LA, et al. A phase I study of bizelesin, a highly potent and selective DNA-interactive agent, in patients with advanced solid malignancies. Ann Oncol. 2003. May;14:775–782. [DOI] [PubMed] [Google Scholar]
- 17.Dokter W, Rubink R, van der Lee M, et al. Preclinical Profile of the HER2-Targeting ADC SYD983/SYD985: Introduction of a New Duocarmycin-Based Linker-Drug Platform. Mol Cancer Ther. 2014;13:2618–2629. [DOI] [PubMed] [Google Scholar]
- 18.Zou H, Zhao S, Zhang J, Lv G, Zhang X, Yu H, Wang H, Wang L. Enhanced radiation-induced cytotoxic effect by 2-ME in glioma cells is mediated by induction of cell cycle arrest and DNA damage via activation of ATM pathways. Brain Res. 2007;1185:231–238. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell J, Choudhuri R, Fabre K, et al. In vitro and in vivo radiation sensitization of human tumor cells by a novel checkpoint kinase inhibitor, AZD7762. Clin Cancer Res. 2010;16:2076–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lehmann B, McCubrey J, Terrian D. Radiosensitization of prostate cancer by priming the Wild-Type p53-Dependent cellular senescence pathway. Cancer Biol Ther. 2007;6:1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsuboi Y, Kurimoto M, Nagai S, et al. Induction of autophagic cell death and radiosensitization by the pharmacological inhibition of nuclear factor kappa B activation in human glioma cell lines. J Neurosurg. 2009;110:594–604. [DOI] [PubMed] [Google Scholar]
- 22.Wrasidlo D, Johnson D, Boger D. Induction of endonucleolytic DNA fragmentation and apoptosis by the duocarmycins. Bioorg Med Chem Lett. 1994;4:631–636. [Google Scholar]
- 23.Gan HK, van den Bent M, Lassman AB, Reardon DA, Scott AM. Antibody-drug conjugates in glioblastoma therapy: the right drugs to the right cells. Nat Rev Clin Oncol. 2017;14:695–707. [DOI] [PubMed] [Google Scholar]
- 24.Gan HK, Burgess AW, Clayton AH, Scott AM. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72:2924–2930. [DOI] [PubMed] [Google Scholar]
- 25.Sampson JH et al. Intracerebral infusion of anEGFR-targeted toxin in recurrent malignant brain tumors. Neuro Oncol. 2008;10:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashby LS, Smith KA, Baldassarre S. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol. 2016;14:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.