Abstract
MicroRNA miR-192-5p is one of the most abundant microRNAs in the kidney and targets the mRNA for ATP1B1 (β1 subunit of Na+/K+-ATPase). Na+/K+-ATPase drives renal tubular reabsorption. We hypothesized that miR-192-5p in the kidney would protect against the development of hypertension. We found miR-192-5p levels were significantly lower in kidney biopsy specimens from patients with hypertension (n=8) or hypertensive nephrosclerosis (n=32) compared to levels in controls (n=10). Similarly, Dahl salt-sensitive rats showed less miR-192-5p in the renal cortex compared with congenic SS.13BN26 rats that had reduced salt sensitivity (n=9, p<0.05). Treatment with anti-miR-192-5p delivered through renal artery injection in uninephrectomized SS.13BN26 rats exacerbated hypertension significantly. Mean arterial pressure on a 4% NaCl high-salt diet at day 14 post anti-miR-192-5p treatment was 16 mmHg higher than in rats treated with scrambled anti-miR (n=8 and 6, p<0.05). Similarly, Mir192 knockout mice on the high-salt diet treated with angiotensin II for 14 days exhibited a mean arterial pressure 22 mmHg higher than wild-type mice (n=9 and 5, p<0.05). Furthermore, protein levels of ATP1B1 were higher in Dahl rats than in SS.13BN26 rats. Na+/K+-ATPase activity increased in the renal cortex of SS.13BN26 rats nine days post-treatment with anti-miR-192-5p compared to that of control anti-miR treated rats. Intra-renal knockdown of ATP1B1 attenuated hypertension in SS.13BN26 rats with intra-renal knockdown of miR-192-5p. In conclusion, miR-192-5p in the kidney protects against the development of hypertension, which is mediated, at least in part, by targeting Atp1b1.
Keywords: Hypertension, microRNA, kidney, Na+/K+-ATPase, salt
Introduction
Hypertension is estimated to affect 46% of the U.S. adult population.1 It increases the risk of developing heart disease, stroke, and kidney disease, which are leading causes of death.2 A large number of hypertensive patients, especially African-Americans, exhibits increased blood pressure salt-sensitivity.3–6
MicroRNAs are small, highly conserved, non-coding RNAs that regulate the level of their target proteins by translational repression or mRNA degradation,7–10 and the effects of several microRNAs in kidney disease have been reported.11–16 The role of miR-192 in particular has been studied extensively in the renal glomeruli, where it contributes to the glomerular sclerosis found in diabetic nephropathy.17–20 However, much less is known about the role renal microRNAs play in hypertension. It has been reported that hsa-miR-181a and hsa-miR-663 levels are lower in hypertensive kidneys and could contribute to upregulation of renin expression.21 Renal miR-214–3p contributes to hypertension in rats and possibly in humans, which is mediated, at least in part, by targeting endothelial nitric oxide synthase (eNOS) directly.22
We showed previously that miR-192-5p was one of the most abundant microRNAs in the kidney.23 In addition, we have shown that the level of miR-192-5p is 20-fold higher in the proximal tubules than in the glomeruli, and miR-192-5p targets the mRNA of the β1 subunit of the Na+/K+-ATPase (ATP1B1) through its unexpected complementarity with a sequence in the 5’ instead of the 3’ untranslated region (UTR).24 The Na+/K+-ATPase drives renal tubular reabsorption of sodium and, secondarily, many other substances. Reduction of miR-192-5p blunts the adaptive increase of urine output in mice fed a high-salt diet.24 Small microRNA deep sequencing analysis of human kidney biopsy specimens has demonstrated lower levels of miR-192-5p in patients with hypertensive nephrosclerosis.22 However, the functional role of miR-192 in the development of hypertension remains unknown.
In the present study, we identified lower levels of miR-192-5p in kidney biopsy specimens from patients with hypertension in addition to patients with hypertensive nephrosclerosis. Subsequently, we performed studies in a rat model of hypertension and a Mir192 knockout (KO) mouse model with the goal of elucidating the functional role of miR-192 in the development of hypertension.
Methods
The data that support the findings of the present study are available from the corresponding author upon reasonable request.
Human Kidney Biopsy Samples
Protocols involving the use of human materials and information were approved by and carried out at Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. Records in the pathological archive at the hospital were searched to identify subjects diagnosed with hypertensive nephrosclerosis without any other type of renal injury, hypertensive subjects diagnosed with minimal or no renal injury, and normotensive subjects with minimal or no renal injury, as described previously.22 Kidney biopsy blocks were cut into 10μm sections, and total RNA was extracted from three sections of each subject using the miRNeasy FFPE kit (Qiagen, Germantown, MD, US) as described previously.22, 25
Animal Models
The Institutional Animal Care and Use Committee at the Medical College of Wisconsin approved all animal protocols used in the present study. All animals were housed in an American Association for Accreditation of Laboratory Animal Care-accredited animal care facility at the Medical College of Wisconsin with free access to food and water. Male Dahl salt-sensitive (SS) and congenic SS.13BN26 (L26) rats were generated as described previously.26, 27 All animals were fed a custom AIN-76A purified rodent chow (0.4% NaCl, Purified AIN-76; Dyets, Bethlehem, PA, US) after weaning. Expression experiments were performed in 18 rats per strain placed on a diet containing 0.4% NaCl (LS) at weaning and kept on this diet until they were 6-weeks-old. At that time, half of the rats from each strain were switched to a high salt diet of 4.0% NaCl (HS) for a week. At the end of the seventh week of life, tissues were collected from all rats. The male rats in experiments that used renal artery injections were 10-weeks-old. Mir192 KO mice of the 129S1/SvlmJ background were bred in-house from homozygous breeder pairs obtained initially from Beckman Research Institute of City of Hope.19 Mice of the background 129S1/SvlmJ strain were obtained from Jackson laboratories (Bar Harbor, ME, US) and used as wild-type (WT) controls. The KO and WT mice were on the same diet and maintained in the same environment at MCW for at least one week before surgical preparation and two weeks before baseline measurements were started.
Blood Pressure Monitoring
Blood pressure monitoring was performed using DSI PA-C40, HD-S10, or DSI TA11PA-C10 transmitter devices implanted in the rats or mice as per the manufacturer’s instructions. Telemeters were implanted into the carotid artery of both rats and mice. After a seven-day recovery period, blood pressure data were collected and analyzed using Dataquest A.R.T. 4.31 software (Data Sciences International, St. Paul, MN, US).28–30
Right Nephrectomy
Animals were anesthetized. An incision was made in the right flank to expose the right kidney. A suture was tied at the hilum to prevent bleeding, the kidney was removed, and the incision sutured.31–34
Renal Artery Injection
Animals were anesthetized. An incision was made in the left inguinal area to expose and isolate the left femoral artery. A stretched P10 catheter prefilled with saline was inserted and advanced into the aorta to the level of the left renal artery. A midline incision was made to reveal the left kidney and renal artery. The catheter was guided into the left renal artery with the aid of forceps and advanced from the femoral end. The renal vein was clamped with an aneurysm clip at the renal hilum immediately before injection of LNA. Ultrasound was applied to the kidney at 1MHz and 10% power for 60 seconds after the injection. The clamp was removed, and the catheter pulled out from the femoral end where the femoral artery was tied, after which the femoral and the abdominal incisions were sutured.
Locked Nucleic Acid-modified anti-miR
A scrambled anti-miR or anti-miR-192-5p oligonucleotides from Exiqon (Woburn, MA, US) were injected through the left renal artery of 10- to 11-week-old SS.13BN26 rats at a dose of 2mg/Kg body weight. We reported previously that anti-miRs were effective when administered systemically (intravenously or intraperitoneally) at the dose of 10mg/Kg.24,34 We used 20% of the systemic dose for intra-renal artery administration in the present study. Tissues were collected 3–5 days later for qPCR quantification of miR-192-5p or two weeks after the injection when blood pressure measurements via radiofrequency telemetry were completed.
Locked Nucleic Acid-modified GapmeR
GapmeR that targeted Atp1b1 or a control scrambled GapmeR from Exiqon was injected through the left renal artery of 10- to 11-week-old SS.13BN26 rats at a dose of 5mg/Kg body weight. Preliminary experiments performed in 17 SD rats and 6 SS rats indicated the GapmeR was efficient for knocking down ATP1B1 in the renal cortex at the dose of 5mg/Kg, but not 1 or 2 mg/Kg.
Real Time RT-PCR
RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA).35 TaqMan MicroRNA Assays and CYBR Green Chemistry were used in an Applied Biosystems real-time PCR instrument as described previously.34–36
Western Blot
Proteins were extracted from renal cortex samples using the Mem-PER Plus Membrane Protein Extraction Kit from Thermo Fisher Scientific (Waltham, MA, US). A Monoclonal Antibody for ATP1B1 from Thermo Fisher Scientific (MA1–16732) was used at a 1:2500 dilution. Coomassie Blue staining was used to normalize protein loading as described previously.34, 35 A Bio-Rad Criterion 10% Tris-HCl gel was used to analyze the 50KDa target protein.37
Na+/K+-ATPase Activity Assay
Protein was extracted as described above, and an ATPase/GTPase activity Assay kit from Sigma-Aldrich (St. Louis, MO, US) was used following the manufacturer’s instructions. Na+/K+-ATPase activity was calculated as the ouabain-sensitive fraction of the detected ATPase activity.
Statistical Analysis
Data were expressed as means ± SEM and analyzed for significance using the Students t-test for comparisons between two groups. A two-way ANOVA followed by multiple comparisons with the Holm-Sidak test were used for comparisons between multiple groups. Two-way repeated measures ANOVA was used to analyze data obtained on consecutive days. p<0.05 was considered significant.
Results
miR-192-5p levels are lower in kidney biopsies from patients with hypertension or hypertensive nephrosclerosis
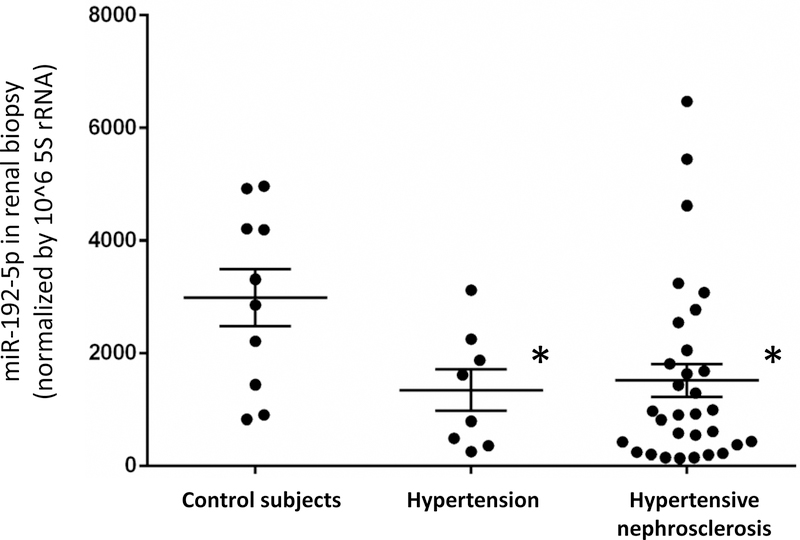
The level of miR-192-5p in kidney biopsy specimens from patients with hypertension but no overt renal histological abnormalities (n=8) was 45% of the level in control subjects (n=10, p<0.05). The level of miR-192-5p in hypertensive nephrosclerosis without any other type of kidney disease (n=32) was 52% of the control (p<0.05, Figure 1). Clinical characteristics of these patients were described previously,22 and reproduced in Supplemental Table S1 with permission. The three groups of patients had similar age and sex distributions. Estimated GFR tended to be lower in the hypertensive nephrosclerosis group than in the other two groups, but the difference was not statistically significant. The hypertension and hypertensive nephrosclerosis groups had higher blood pressure than did the controls, were treated with one or more anti-hypertensive medications, and included subjects with diabetes. miR-192-5p remained significantly lower in the hypertension and hypertensive nephrosclerosis groups compared to the controls after the diabetic subjects were removed from the analysis. We did not have sufficient data to test the effect of the medications.
Figure 1. MiR-192-5p levels are lower in kidney biopsies from patients with hypertension or hypertensive nephrosclerosis.
miR-192-5p was measured with real-time PCR and normalized by 5s rRNA. See Supplemental Table S4 in Liu et al22 and the text for the characteristics of the patients and biopsy specimens. N=10 control subjects, 8 hypertension, and 32 hypertensive nephrosclerosis; *, p<0.05 vs. control subjects.
Intrarenal knockdown of miR-192-5p exacerbates hypertension in SS.13BN26 rats
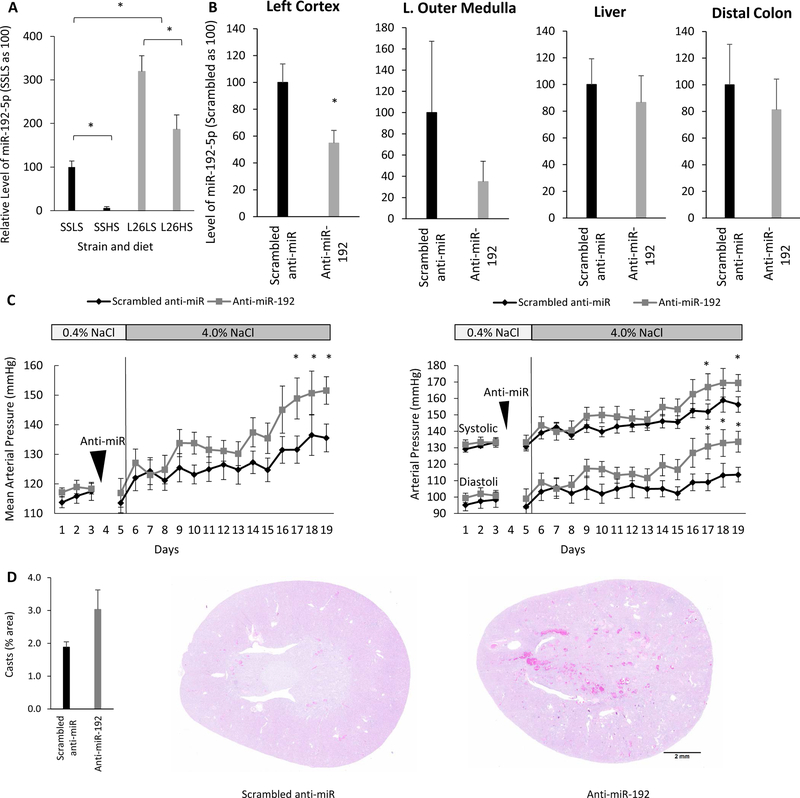
The Dahl salt-sensitive (SS) rat is the polygenic, hereditary model of human salt-sensitive hypertension used most commonly.38, 39 The SS.13BN26 (L26) rat is a congenic strain derived from the SS rat that develops substantially milder salt-sensitive hypertension and renal injury.26, 27, 40 MiR-192-5p levels were significantly lower in the renal cortex of SS rats on the LS diet compared with those in the less salt-sensitive L26 strain (Figure 2A). MiR-192-5p decreased significantly in both strains in response to the HS diet. However, the level of miR-192-5p remained significantly lower in SS rats than in L26 rats on the HS diet.
Figure 2. Intrarenal knockdown of miR-192-5p exacerbates salt-induced hypertension in SS.13BN26 rats.
A. The level of miR-192-5p was lower in the renal cortex of SS rats than in that of SS.13BN26 (L26) rats. LS, 0.4% NaCl diet; HS, 4% NaCl diet for 7days; n=9, *, p< 0.05. B. Renal artery delivery of anti-miR-192-5p, at a dose of 2mg/Kg of body weight, decreased detectable miR-192-5p, specifically in the kidney. n=3, *, p< 0.05. C. Intrarenal knockdown of miR-192-5p exacerbated salt-induced hypertension in SS.13BN26 rats. Mean arterial pressure, systolic blood pressure, and diastolic blood pressure are shown. n=6 anti-miR-Scrambled and 8 anti-miR-192-5p, *, p< 0.05. D. Intrarenal knockdown of miR-192-5p tended to increase the formation of protein casts in the kidney. Hematoxylin and eosin (HE) stained kidney sections are shown. n=4, p=0.055.
Intra-renal artery injection was used subsequently to deliver either LNA anti-miR-192-5p or scrambled anti-miR specifically into the left kidney of L26 rats that had undergone a right nephrectomy procedure. Right nephrectomy was performed to prevent compensation from the right kidney as it was not feasible to inject anti-miR into both kidneys. The level of miR-192-5p detectable by qPCR was significantly lower in the renal cortex five days after the intra-renal artery delivery of anti-miR-192-5p (Figure 2B). The level of miR-192-5p tended to decrease in the renal outer medulla, but the difference was not statistically significant. It is possible that the anti-miR delivered through the renal artery injection did not reach the medulla efficiently. The anti-miR treatment did not affect the level of miR-192-5p in the liver and colon (Figure 2B). The liver and colon were chosen for testing because they are among the major sites of miR-192-5p expression outside the kidney. Kidney-specific knockdown is important to test the functional role of renal miR-192-5p, particularly because miR-192-5p is abundant in the gastrointestinal tract where sodium absorption occurs.
Mean arterial blood pressure (MAP) of L26 rats treated with anti-miR-192-5p reached 152+/−5 mmHg at day 14 after the anti-miR treatment and the initiation of the HS diet, which was significantly higher than that of L26 rats treated with a control anti-miR and HS (136+/−5 mmHg, n=8 and 6, p<0.05). The HS-induced increases in MAP in the anti-miR-192-5p-treated L26 rats and the scrambled anti-miR-treated rats were 35 mmHg and 22 mmHg, respectively. The effect on BP was observed for both systolic (SBP) and diastolic (DBP) pressures (Figure 2C).
Kidneys were collected at the end of the study for histological analysis. Trichrome staining did not show any significant difference in glomerulosclerosis or tubulointerstitial fibrosis, although there was a trend for greater injury in the anti-miR-192-5p treated group (Supplemental Figure S1). Tubular protein casts analysis on slides with hematoxylin and eosin (HE) staining showed that the rats treated with anti-miR-192-5p tended to have an increased amount of tubular protein casts (p=0.055), suggesting increased kidney damage (Figure 2D).
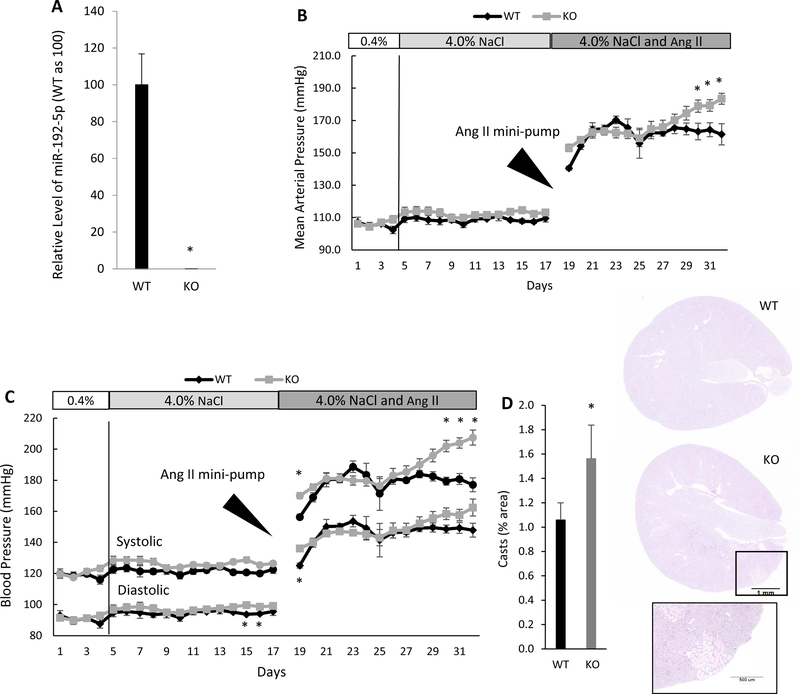
Mir192 knockout exacerbates angiotensin II-induced hypertension in mice
Mir192 KO mice were created on the 129S1/SvlmJ mouse background.19 Wild type (WT) 129S1/SvlmJ mice obtained from Jackson Laboratories were used as controls. The KO was confirmed by qPCR analysis of miR-192-5p (Figure 3A). MAP tended to increase slightly in both WT and KO mice in response to the HS diet (Figure 3B). DBP was significantly higher in the KO mice compared to the WT mice by the end of the 14-day HS treatment (Figure 3C). The mice were challenged further for another 14 days with angiotensin II (1µg/Kg/min given via osmotic mini-pump) in addition to the HS diet. The angiotensin II treatment increased the SBP and DBP immediately in both strains. The increase was significantly greater in the KO mice on the first day that recording was resumed (Figure 3C); however, there was no difference between the two strains by the second day. The KO mice experienced a further elevation of MAP and SBP during the final week of recording that resulted in a significantly higher MAP compared with WT mice at the end of the study (183+/−3 mmHg vs 161+/−7 mmHg; n=5 WT and 9 KO, p<0.05: Figure 3B). The SBP also was significantly higher in the KO mice than in the WT in the last 3 days of treatment, while the difference in DBP between the two strains was not statistically significant (Figure 3C).
Figure 3. Mir192 knockout (KO) exacerbates hypertension in mice induced by angiotensin II and a high-salt diet.
A. Mir192 KO mice did not express miR-192-5p in the renal cortex. n=5 wild-type (WT) and 6 KO, *, p< 0.05. B and C. Mir192 knockout exacerbated hypertension in mice treated with a high-salt diet and angiotensin II. Mean arterial pressure (B) and systolic and diastolic blood pressures (C) are shown. n=5 WT and 9 KO, *, p< 0.05. D. Mir192 KO exacerbates protein cast formation in mice treated with the high-salt diet and angiotensin II. Hematoxylin and eosin (HE) stained kidney sections are shown. n=4 WT and 6 KO, *, p< 0.05.
Kidneys collected at the end of the study did not show significant differences in fibrosis. However, protein casts in the Mir192 KO were increased significantly compared with those in the WT (Figure 3D). These results were consistent with the results of L26 rats treated with anti-miR-192-5p and suggested that reduced levels of miR-192 exacerbate hypertension and renal damage.
Targeting Atp1b1 contributes to miR-192-5p’s effect on blood pressure
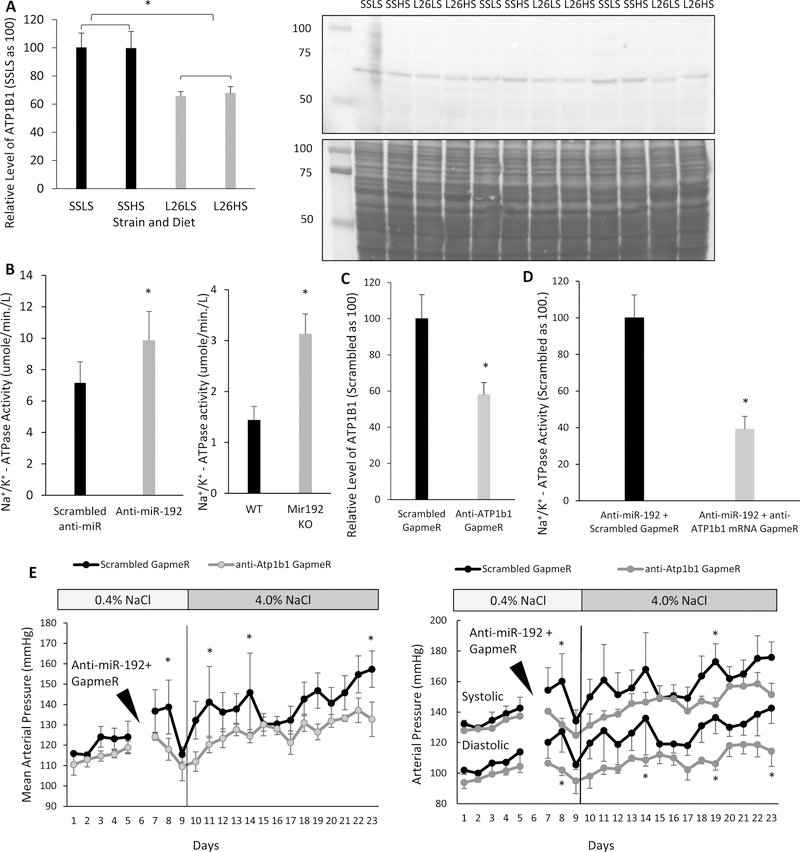
Atp1b1 mRNA is a confirmed target gene for miR-192-5p.24 We have reported above that SS rats have reduced levels of miR-192-5p in the renal cortex compared to L26 rats (Figure 2A). Western blot analysis of the same tissues showed a significantly higher level of ATP1B1 in SS rats compared with that in L26 rats (Figure 4A).
Figure 4. Atp1b1 targeting contributes to the protective effect of miR-192-5p against hypertension.
A. The level of ATP1B1 in the renal cortex was higher in SS than that in SS.13BN26 (L26) rats. Western blot signals of ATP1B1 were normalized by Coomassie blue staining of the membrane shown below the Western blot. n=3, *, p< 0.05. B. Na+/K+-ATPase activity in the renal cortex was increased in SS.13BN26 rats with intrarenal knockdown of miR-192-5p and in Mir192 KO mice at the end of the blood pressure experiment shown in Figure 3. n=5 Scrambled anti-miR and 6 anti-miR-192-5p; n=5 WT and 6 KO; *, p< 0.05. C. Anti-Atp1b1 GapmeR reduced the level of ATP1B1 based on Western blot analysis of the renal cortex. n=3*, p< 0.05. D. Anti-Atp1b1 GapmeR treatment reduced the activity of Na+/K+-ATPase. n=3, *, p< 0.05. E. Knockdown of ATP1B1 attenuated hypertension in L26 rats treated simultaneously with anti-miR-192-5p. Anti-miR-192 and anti-Atp1b1 GapmeR or scrambled GapmeR were administered by intrarenal artery injection at the time indicated. n=4, *, p< 0.05 between rats treated with anti-miR-192-5p and anti-Atp1b1 GapmeR and those treated with anti-miR-192-5p and scrambled GapmeR.
ATP1B1 is required for Na+/K+-ATPase activity, which provides the driving force for renal tubular reabsorption. Na+/K+-ATPase activity was measured in the renal cortex of L26 rats treated with intrarenal injection of either anti-miR-192-5p or scrambled anti-miR and in Mir192 KO and WT mice at the end of the blood pressure experiment shown in Figure 3. Na+/K+-ATPase activity was significantly higher in L26 rats with miR-192-5p knockdown and in Mir192 KO mice (Figure 4B).
Finally, we performed a restoration experiment in which we treated L26 rats with a double intra-renal artery injection of anti-miR-192-5p and either anti-Atp1b1 GapmeR or control GapmeR. We tested the efficacy of the GapmeR alone without anti-miR-192-5p first. Anti-Atp1b1 GapmeR reduced the protein level of ATP1B1 in the renal cortex (Figure 4C). The restoration experiment with the double intra-renal artery injection showed that rats treated with anti-Atp1b1 GapmeR had reduced Na+/K+-ATPase activity (Figure 4D) and exhibited a blunted BP elevation (Figure 4E) compared with those treated with a control GapmeR, all of which were treated with anti-miR-192-5p. After 14 days of HS, the MAP was 157+/−9 mmHg in the group treated with anti-miR-192-5p and the control GapmeR compared with 132+/−8mmHg in the group treated with anti-miR-192-5p and anti-Atp1b1 GapmeR. Significant differences in MAP also were observed on the second day post injection. The BP effect was observed for both SBP and DBP (Figure 4E). Changes of body weight during the treatment period and tubular cast area at the end of the experiment were not significantly different between the two groups (Supplemental Figure S2, S3).
Discussion
The findings of the present study suggest that miR-192, particularly miR-192-5p in the kidney, protects against the development of hypertension, which is mediated, at least in part, by suppressing ATP1B1.
MiR-192 is most abundant in the gastrointestinal tract, with the greatest expression in the colon. Other components of the digestive system, including the liver, also express significant levels of miR-192. Outside the digestive system, the kidney is the only organ with substantial expression of miR-192.41 MiR-192 is pro-fibrotic in glomeruli and acts as a downstream mediator of TGF-beta/Smad3 signaling,17, 19, 42 and a reduction in miR-192 leads to decreased proteinuria.18 In severe diabetic nephropathy, the loss of miR-192 is associated with increased tubulointerstitial fibrosis and decreased estimated GFR.43 A potassium load, sodium depletion, or aldosterone infusion each reduces miR-192 expression in the kidney significantly, although it is unclear whether miR-192 plays a functional role in the physiological response to these stimuli.44 We found previously that knocking down miR-192-5p in mice by intraperitoneal injection of LNA-modified anti-miR oligonucleotides resulted in an increased expression of ATP1B1 and a blunted adaptive increase of urine output in response to HS diet.24
The findings of the present study advance this area of research significantly by demonstrating an anti-hypertensive effect of miR-192 in two models of hypertension. The beneficial effect of miR-192-5p in attenuating hypertension and the injurious effect of miR-192-5p in promoting glomerular sclerosis in diabetes suggest the role of miR-192-5p in the kidney might be tissue-specific. In renal tubules, where miR-192-5p is particularly abundant, its beneficial effect might outweigh any injurious effect it might have. We do not have time course data to know whether tubular cast or hypertension occurs first following miR-192 inhibition or genetic deletion in the present study. However, it is well-established that hypertension, and high renal perfusion pressure specifically, exacerbates renal injury substantially in the SS rat, from which the SS.13BN26 rat was derived. In addition, our data indicate the hypertensive effect of miR-192-5p inhibition is mediated at least in part by elevation of ATP1B1. Therefore, it appears that miR-192-5p prevents hypertension with consequent protection against renal tubular injury, although we cannot rule out the possibility that the protection against renal tubular injury contributes to the attenuation of hypertension.
Clinical data support the role of miR-192 in human kidney disease and hypertension. A study of patients with chronic kidney diseases showed an inverse relation between proteinuria and urinary levels of miR-192 and a positive correlation between miR-192 and glomerular filtration rate.45 Previously, the same group had reported that miR-192 levels were higher in patients with hypertensive nephrosclerosis. However, the kidney biopsies used in that study included complex pathological findings that could confound the interpretation of the data.46 In our human studies, which excluded kidney specimens with other pathological changes, miR-192-5p was lower in the kidneys of hypertensive nephrosclerosis patients according to agnostic deep sequencing analysis22 and targeted qPCR analysis in a separate cohort of hypertensive or hypertensive nephrosclerosis patients shown in the present study. Moreover, the gene that encodes miR-192 is one of only six miRNA genes that show differential expression in human kidney biopsies with hypertensive nephrosclerosis and are located in genomic proximity (within 1 Mb) with BP-associated single nucleotide polymorphisms.22
The kidney-specific double knockdown experiment presented in the present study demonstrated an important role of Atp1b1 targeting in mediating the effect of renal miR-192-5p on hypertension. The Na+/K+-ATPase is a transmembrane ion transporter found in all cells and is composed of a catalytic α subunit, a β subunit that helps deliver and stabilize the α subunit in the cell membrane, and a tissue-specific auxiliary regulatory subunit. ATP1B1, the β1 subunit of the Na+/K+-ATPase, is necessary not only for the function of the α subunit, but is the limiting factor in the formation of the active heterodimer.47, 48 Reduced expression of miR-192-5p leads to an increase in Na+/K+-ATPase function, which could, in turn, contribute to hypertension and kidney injury.
We observed an inverse relation between the levels of miR-192-5p and ATP1B1 or Na+/K+-ATPase activities in multiple settings in the present study, consistent with the targeting and suppression of Atp1b1 by miR-192-5p. However, this inverse relation was not observed in all conditions. For example, a high salt diet decreased miR-192-5p, but ATP1B1 was not elevated under high salt conditions. Renal Na+/K+-ATPase is essential to drive tubular transport and is regulated strongly by many mechanisms, including those of angiotensin, aldosterone, atrial natriuretic peptide, and dopamine.49 The way miR-192 is integrated with these other mechanisms to regulate and fine-tune Na+/K+-ATPase activities remains to be studied. The presence of such integrated and potentially redundant mechanisms for Na+/K+-ATPase regulation might explain why miR-192-5p and Na+/K+-ATPase are often, but not always, correlated inversely.
Perspectives
Of the hundreds of microRNAs expressed in the kidney, miR-192-5p is one of the first microRNAs that have been shown to play a functional role in the development of hypertension. It will be valuable to explore mechanistic roles of other microRNAs in hypertension and their therapeutic potential. Several microRNAs, including miR-192-5p, are differentially expressed in patients with hypertension and located in genomic proximity with DNA sequence variants associated with blood pressure variation in humans.22 Another two of these microRNAs, miR-214–3p and miR-29, have recently been shown to be involved in the development of hypertension.22,50
Supplementary Material
Novelty and Significance.
What is new?
miR-192-5p is one of the most abundant microRNAs in the kidney. However, prior to the present study, whether miR-192-5p was involved in the development of hypertension was unknown.
What is relevant?
We analyzed the level of miR-192-5p in scarcely available kidney biopsy specimens from patients with hypertension or hypertensive nephrosclerosis. We examined the role and mechanism of miR-192-5p in the development of hypertension using rat and mouse models.
Summary
miR-192-5p in the kidney protects against the development of hypertension in animal models and possibly in humans. The effect was mediated, at least in part, by targeting Atp1b1.
Acknowledgments
Sources of Funding
The present study was supported by National Institutes of Health grants HL121233, HL125409, HL082798 and GM066730 (to ML), American Heart Association grant 14PRE20120006 (to MAB), and National Natural Science Foundation of China grants 81570603 and 81770741 (to FW).
Footnotes
Statement of competing financial interests
The authors declare no conflicts of financial interest.
References
- 1.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, Wright JT Jr.: 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension, 71: e13–e115, 2018. [DOI] [PubMed] [Google Scholar]
- 2.Heron M: Deaths: Leading causes for 2014. National Vital Statistics Reports: From the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System, 65: 1–96, 2016. [PubMed] [Google Scholar]
- 3.Peters RM, Flack JM: Salt sensitivity and hypertension in African Americans: Implications for cardiovascular nurses. Progress in Cardiovascular Nursing, 15: 138–144, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Bakir AA, Dunea G: Renal disease in the inner city. Seminars in Nephrology, 21: 334–345, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Lopes AA: Hypertension in black people: Pathophysiology and therapeutic aspects. Journal of Human Hypertension, 16 Suppl 1: S11–12, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Schmidlin O, Forman A, Leone A, Sebastian A, Morris RC Jr.: Salt sensitivity in blacks: Evidence that the initial pressor effect of NaCl involves inhibition of vasodilatation by asymmetrical dimethylarginine. Hypertension, 58: 380–385, 2011. [DOI] [PubMed] [Google Scholar]
- 7.Couzin J: Breakthrough of the year. Small RNAs make big splash. Science, 298: 2296–2297, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Meister G: miRNAs get an early start on translational silencing. Cell, 131: 25–28, 2007. [DOI] [PubMed] [Google Scholar]
- 9.Couzin J: MicroRNAs make big impression in disease after disease. Science, 319: 1782–1784, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Liang M, Liu Y, Mladinov D, Cowley AW Jr., Trivedi H, Fang Y, Xu X, Ding X, Tian Z: MicroRNA: A new frontier in kidney and blood pressure research. American Journal of Physiology Renal Physiology, 297: F553–558, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato M, Arce L, Natarajan R: MicroRNAs and their role in progressive kidney diseases. Clinical Journal of the American Society of Nephrology:CJASN, 4: 1255–1266, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato M, Park JT, Natarajan R: MicroRNAs and the glomerulus. Experimental Cell Research, 318: 993–1000, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudnicki M, Beckers A, Neuwirt H, Vandesompele J: RNA expression signatures and posttranscriptional regulation in diabetic nephropathy. Nephrology, Dialysis, Transplantation:Official Publication of the European Dialysis and Transplant Association-European Renal Association, 30 Suppl 4: iv35–42, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Trionfini P, Benigni A, Remuzzi G: MicroRNAs in kidney physiology and disease. Nature Reviews Nephrology, 11: 23–33, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Bhatt K, Kato M, Natarajan R: Mini-review: Emerging roles of microRNAs in the pathophysiology of renal diseases. American Journal of Physiology Renal Physiology, 310: F109–118, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudnicki M, Perco P, B DH, Leierer J, Heinzel A, Muhlberger I, Schweibert N, Sunzenauer J, Regele H, Kronbichler A, Mestdagh P, Vandesompele J, Mayer B, Mayer G: Renal microRNA- and RNA-profiles in progressive chronic kidney disease. European Journal of Clinical Investigation, 46: 213–226, 2016. [DOI] [PubMed] [Google Scholar]
- 17.Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R: MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proceedings of the National Academy of Sciences of the United States of America, 104: 3432–3437, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putta S, Lanting L, Sun G, Lawson G, Kato M, Natarajan R: Inhibiting microRNA-192 ameliorates renal fibrosis in diabetic nephropathy. Journal of the American Society of Nephrology:JASN, 23: 458–469, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deshpande SD, Putta S, Wang M, Lai JY, Bitzer M, Nelson RG, Lanting LL, Kato M, Natarajan R: Transforming growth factor-beta-induced cross talk between p53 and a microRNA in the pathogenesis of diabetic nephropathy. Diabetes, 62: 3151–3162, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato M, Dang V, Wang M, Park JT, Deshpande S, Kadam S, Mardiros A, Zhan Y, Oettgen P, Putta S, Yuan H, Lanting L, Natarajan R: TGF-beta induces acetylation of chromatin and of Ets-1 to alleviate repression of miR-192 in diabetic nephropathy. Science Signaling, 6: ra43, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YH, Charchar FJ, Morris BJ: Gene expression profiling reveals renin mRNA overexpression in human hypertensive kidneys and a role for microRNAs. Hypertension, 58: 1093–1098, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Usa K, Wang F, Liu P, Geurts AM, Li J, Williams AM, Regner KR, Kong Y, Liu H, Nie J, Liang M: miR-214–3p in the kidney contributes to the development of hypertension. J. Am. Soc. Nephrol, 29: 2518–2528, 2018.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tian Z, Greene AS, Pietrusz JL, Matus IR, Liang M: MicroRNA-target pairs in the rat kidney identified by microRNA microarray, proteomic, and bioinformatic analysis. Genome Research, 18: 404–411, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mladinov D, Liu Y, Mattson DL, Liang M: MicroRNAs contribute to the maintenance of cell-type-specific physiological characteristics: miR-192 targets Na+/K+-ATPase beta1. Nucleic Acids Research, 41: 1273–1283, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker MA, Davis SJ, Liu P, Pan X, Williams AM, Iczkowski KA, Gallagher ST, Bishop K, Regner KR, Liu Y, Liang M: Tissue-specific microRNA expression patterns in four types of kidney disease. Journal of the American Society of Nephrology:JASN, 28: 2985–2992, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng D, Yang C, Geurts AM, Kurth T, Liang M, Lazar J, Mattson DL, O’Connor PM, Cowley AW Jr.: Increased expression of NAD(P)H oxidase subunit p67(phox) in the renal medulla contributes to excess oxidative stress and salt-sensitive hypertension. Cell Metabolism, 15: 201–208, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cowley AW Jr., Moreno C, Jacob HJ, Peterson CB, Stingo FC, Ahn KW, Liu P, Vannucci M, Laud PW, Reddy P, Lazar J, Evans L, Yang C, Kurth T, Liang M: Characterization of biological pathways associated with a 1.37 Mbp genomic region protective of hypertension in Dahl S rats. Physiological Genomics, 46: 398–410, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cowley AW Jr., Ryan RP, Kurth T, Skelton MM, Schock-Kusch D, Gretz N: Progression of glomerular filtration rate reduction determined in conscious Dahl salt-sensitive hypertensive rats. Hypertension, 62: 85–90, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kriegel AJ, Baker MA, Liu Y, Liu P, Cowley AW Jr., Liang M: Endogenous microRNAs in human microvascular endothelial cells regulate mRNAs encoded by hypertension-related genes. Hypertension, 66: 793–799, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang B, Cheng Y, Usa K, Liu Y, Baker MA, Mattson DL, He Y, Wang N, Liang M: Renal tumor necrosis factor alpha contributes to hypertension in Dahl salt-sensitive rats. Scientific Reports, 6: 21960, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Usa K, Singh RJ, Netzel BC, Liu Y, Raff H, Liang M: Renal interstitial corticosterone and 11-dehydrocorticosterone in conscious rats. American Journal of Physiology Renal Physiology, 293: F186–192, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Singh RJ, Usa K, Netzel BC, Liang M: Renal medullary 11 beta-hydroxysteroid dehydrogenase type 1 in Dahl salt-sensitive hypertension. Physiological Genomics, 36: 52–58, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Z, Liu Y, Usa K, Mladinov D, Fang Y, Ding X, Greene AS, Cowley AW Jr., Liang M: Novel role of fumarate metabolism in dahl-salt sensitive hypertension. Hypertension, 54: 255–260, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Taylor NE, Lu L, Usa K, Cowley AW Jr., Ferreri NR, Yeo NC, Liang M: Renal medullary microRNAs in Dahl salt-sensitive rats: miR-29b regulates several collagens and related genes. Hypertension, 55: 974–982, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriegel AJ, Fang Y, Liu Y, Tian Z, Mladinov D, Matus IR, Ding X, Greene AS, Liang M: MicroRNA-target pairs in human renal epithelial cells treated with transforming growth factor beta 1: A novel role of miR-382. Nucleic Acids Research, 38: 8338–8347, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liang M, Pietrusz JL: Thiol-related genes in diabetic complications: A novel protective role for endogenous thioredoxin 2. Arteriosclerosis, Thrombosis, and Vascular Biology, 27: 77–83, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Curthoys NP, Taylor L, Hoffert JD, Knepper MA: Proteomic analysis of the adaptive response of rat renal proximal tubules to metabolic acidosis. American Journal of Physiology Renal Physiology, 292: F140–147, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Cowley AW Jr., Roman RJ: The role of the kidney in hypertension. JAMA, 275: 1581–1589, 1996. [PubMed] [Google Scholar]
- 39.Rapp JP: Genetic analysis of inherited hypertension in the rat. Physiological Reviews, 80: 135–172, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Lu L, Li P, Yang C, Kurth T, Misale M, Skelton M, Moreno C, Roman RJ, Greene AS, Jacob HJ, Lazar J, Liang M, Cowley AW Jr.: Dynamic convergence and divergence of renal genomic and biological pathways in protection from Dahl salt-sensitive hypertension. Physiological Genomics, 41: 63–70, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang Y, Ridzon D, Wong L, Chen C: Characterization of microRNA expression profiles in normal human tissues. BMC Genomics, 8: 166, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung AC, Huang XR, Meng X, Lan HY: miR-192 mediates TGF-beta/Smad3-driven renal fibrosis. Journal of the American Society of Nephrology:JASN, 21: 1317–1325, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krupa A, Jenkins R, Luo DD, Lewis A, Phillips A, Fraser D: Loss of microRNA-192 promotes fibrogenesis in diabetic nephropathy. Journal of the American Society of Nephrology:JASN, 21: 438–447, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elvira-Matelot E, Zhou XO, Farman N, Beaurain G, Henrion-Caude A, Hadchouel J, Jeunemaitre X: Regulation of WNK1 expression by miR-192 and aldosterone. Journal of the American Society of Nephrology:JASN, 21: 1724–1731, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szeto CC, Ching-Ha KB, Ka-Bik L, Mac-Moune LF, Cheung-Lung CP, Gang W, Kai-Ming C, Kam-Tao LP: Micro-RNA expression in the urinary sediment of patients with chronic kidney diseases. Disease Markers, 33: 137–144, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang G, Kwan BC, Lai FM, Choi PC, Chow KM, Li PK, Szeto CC: Intrarenal expression of miRNAs in patients with hypertensive nephrosclerosis. American Journal of Hypertension, 23: 78–84, 2010. [DOI] [PubMed] [Google Scholar]
- 47.Gorokhova S, Bibert S, Geering K, Heintz N: A novel family of transmembrane proteins interacting with beta subunits of the Na, K-ATPase. Human Molecular Genetics, 16: 2394–2410, 2007. [DOI] [PubMed] [Google Scholar]
- 48.Toyoshima C, Kanai R, Cornelius F: First crystal structures of Na+, K+-ATPase: New light on the oldest ion pump. Structure, 19: 1732–1738, 2011. [DOI] [PubMed] [Google Scholar]
- 49.Taub M, Garimella S, Kim D, Rajkhowa T, Cutuli F: Renal proximal tubule Na, K-ATPase is controlled by CREB-regulated transcriptional coactivators as well as salt-inducible kinase 1. Cellular Signalling, 27: 2568–2578, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Widlansky ME, Jensen DM, Wang J, Liu Y, Geurts AM, Kriegel AJ, Liu P, Ying R, Zhang G, Casati M, Chu C, Malik M, Branum A, Tanner MJ, Tyagi S, Usa K, Liang M. miR-29 contributes to normal endothelial function and can restore it in cardiometabolic disorders. EMBO Mol Med 2018; 10: e8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.