Abstract
Purpose:
The purpose of this study was to determine whether seeding density of placental mesenchymal stromal cells (PMSCs) on extracellular matrix (ECM) during in utero repair of myelomeningocele (MMC) affects motor function and neuronal preservation in the ovine model.
Methods:
MMC defects were surgically created in 33 fetuses and repaired following randomization into four treatment groups: ECM only (n=10), PMSC-ECM (42K cells/cm2) (n=8), PMSC-ECM (167K cells/cm2) (n=7), or PMSC-ECM (250–300K cells/cm2) (n=8). Motor function was evaluated using the Sheep Locomotor Rating Scale (SLR). Serial sections of the lumbar spinal cord were analyzed by measuring their cross-sectional area which were then normalized to normal lambs. Large neurons (LN, diameter 30–70μm) were counted manually and density calculated per mm2 gray matter.
Results:
Lambs treated with PMSCs at any density had a higher median SLR score (15 [IQR 13.5–15]) than ECM alone (6.5 [IQR 4–12.75], p=0.036). Cross-sectional areas of spinal cord and gray matter were highest in the PMSC-ECM (167K/cm2) group (p=0.002 and 0.006, respectively). LN density was highest in the greatest density PMSC-ECM (250–300K/cm2) group (p=0.045) which positively correlated with SLR score (r=0.807, p<0.0001).
Conclusions:
Fetal repair of myelomeningocele with high density PMSC-ECM resulted in increased large neuron density, which strongly correlated with improved motor function.
Keywords: Myelomeningocele, Fetal surgery, Tissue engineering, Placenta, Mesenchymal stromal cell, Neuroprotection
1. INTRODUCTION
Myelomeningocele (MMC), commonly known as spina bifida, is a congenital birth defect that affects one out of every 2,700 live births in the United States [1]. MMC results from failure of neurulation during the 4th week of embryonic development and can lead to lifelong paralysis, incontinence, musculoskeletal deformities, and severe cognitive disabilities [2]. The landmark Management of Myelomeningocele Study (MOMS) demonstrated that in utero surgical repair of the MMC defect improves lower limb motor function, suggesting a capacity for improved neurologic outcomes with early intervention [3]. However, functional recovery was incomplete, and 58% of prenatally treated children were still unable to walk independently at 30 months of age. Translational studies investigating the potential of mesenchymal stromal cells (MSCs) to augment prenatal repair aim to further improve functional outcomes for these patients [4].
While the mechanism of action of MSCs has not been definitely established, it is widely believed that paracrine secretion plays a key role in their therapeutic effect [5, 6]. MSCs have been isolated from a variety of human tissues, including bone marrow, adipose, dermis, placenta, and amniotic fluid [7, 8]. Placental mesenchymal stromal cells (PMSCs) are particularly appealing for neurologic disorders and injury, such as MMC, due to their increased immunomodulatory and neuroprotective capabilities [9, 10]. PMSCs can be isolated from chorionic villi, which can be collected by chorionic villus sampling early in gestation, and present an opportunity for autologous use in prenatally diagnosed congenital disorders [11].
Application of early gestation PMSCs during prenatal repair of MMC consistently improves postnatal hindlimb function in the well-established ovine model [12–14]. In addition to improved motor function, Kabagambe et al showed a trend towards increased large neuron density within the gray matter of animals treated with PMSCs seeded on small intestine submucosa-derived extracellular matrix (ECM) [13]. Large neurons, defined as neurons with a diameter of 30–70 μm, a discernable nucleolus, and location within the ventral gray matter of the spinal cord, are considered to be motor neurons [15]. Increased large neuron density may be the result of decreased neuronal death during gestation and the underlying etiology of the observed functional improvements. Using a retinoic acid-induced rodent model of MMC, Chen et al demonstrated a trend towards decreased apoptotic cell density within the spinal cords of pups treated with higher PMSC seeding densities during prenatal patch repair [16]. Therefore, we hypothesized that higher PMSC seeding density would improve neuronal preservation compared to low PMSC seeding density following in utero repair in the ovine model of MMC.
2. MATERIALS & METHODS
2.1. PMSCs
PMSCs were isolated from chorionic villi of one second trimester human placenta and fully characterized as previously described [17]. Briefly, the cells were cultured in Dulbecco’s Modified Eagle Medium/High Glucose with 5% fetal bovine serum (Hyclone, Thermo Fisher Scientific), 100 U/ml penicillin/100 μg/ml streptomycin (Thermo Fisher Scientific), 20 ng/ml recombinant human basic fibroblast growth factor (R and D Systems), and 20 ng/ml recombinant human epidermal growth factor (R and D Systems). PMSCs were transduced with green fluorescent protein (GFP)-containing lentiviral vector (University of California Davis, Institute for Regenerative Cures, Sacramento, CA) at passage 4 with a multiplicity of infection of 5.
Twenty-four hours prior to the scheduled MMC repair, PMSCs at passage 6 were seeded onto a 6 cm by 2 cm sheet of four-ply small intestine submucosa-derived extracellular matrix (PMSC-ECM) (Cook Biotech, West Lafayette, IN) at three different densities: Low (42,000 cells/cm2); Medium (167,000 cells/cm2); or High (250–300,000 cells/cm2). These three densities were chosen based on our previous studies in ovine and rodent models [12, 13, 16]. Seeded ECMs were incubated in culture medium overnight in a humidified 37 °C, 5% CO2 incubator. Control ECMs without cells were incubated in culture medium under the same conditions. On the day of MMC repair, ECMs seeded with GFP-tagged cells were viewed by fluorescent microscopy and imaged to confirm adherence of PMSCs to the ECM, as previously described [11].
2.2. MMC Defect Creation and Repair
Animal work was approved by the Institutional Animal Care and Use Committee and care was in compliance with the Guide for the Care and Use of Laboratory Animals. The facilities used to conduct this study were accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care, International. Time-mated pregnant Dorper ewes were purchased from an institution-approved breeder and delivered to the housing facility one week before MMC defect creation. Some animals included in this study (6 animals treated with ECM only and 8 treated with PMSC-ECM [42K cells/cm2]) were reported in a previous publication regarding motor function outcomes. They have been included here to complete evaluation of the effect of PMSC seeding density on motor function, spinal cord morphology, and large neuron density. Additional histologic analysis has been performed on these animals to ensure consistency between treatment groups. Defect creation and repair were performed in the same manner for all animals.
Fetal MMC defects were surgically created at mean gestational age 77 ± 3 days as previously described [12, 18, 19]. Briefly, after induction of general anesthesia, each ewe underwent a survival laparotomy and hysterotomy, followed by fetal MMC defect creation. The fetal lumbosacral area was exposed, followed by removal of the skin, paraspinal muscles, L1-L6 vertebral laminae, and the dorsal portion of the dura matter overlying the exposed spinal cord. Lost amniotic fluid was replaced with normal saline, antibiotics (penicillin 1 million units, gentamicin 100 mg) added to the amniotic cavity, followed by closure of the hysterotomy and the maternal laparotomy.
Fetuses were each assigned to repair with one of the following treatments: ECM only (n=10), Low density PMSC-ECM (n=8), Medium density PMSC-ECM (n=7), or High density PMSC-ECM (n=8). Twins were assigned to different treatment groups. MMC defects were repaired at a mean of 25 ± 4 days following defect creation through a second survival laparotomy and hysterotomy, as previously described [12, 13]. Briefly, the spinal cord was re-exposed from L1-L6 by removing overlying fibrinous material. The ECM was placed over the defect with the cell side facing the spinal cord in the PMSC treated groups and secured at each corner. Skin flaps were raised, and the fetal skin was closed over the ECM patch. Lost amniotic fluid was replaced with normal saline, intra-amniotic antibiotics (penicillin 1 million units, gentamicin 100 mg) added, followed by maternal hysterotomy and laparotomy closure.
2.3. Postnatal Hindlimb Motor Function
Lambs were delivered by spontaneous vaginal delivery or by cesarean section if spontaneous vaginal delivery had not occurred by the estimated delivery date.
Locomotor function was assessed using the Sheep Locomotor Rating scale (SLR), as previously described [20]. Briefly, hindlimb motor function was scored on a scale of 0–15 based on joint movement, weight support, locomotion, coordination, and an obstacle clearance test. A score of 0 represented complete paraplegia, while a score of 15 represented normal spontaneous ambulation with demonstrated ability to step over an obstacle.
Lamb motor function testing was video recorded two hours after birth and 24–48 hours later and analyzed by two examiners. The best performance score for each lamb was used for subsequent statistical analysis. To ensure the welfare of animals with severe spinal cord injury, IACUC protocol required that all animals be euthanized within 48 hours of delivery.
2.4. Lumbar Spine Imaging and Histologic Analysis
Lambs were euthanized after completing the second motor function evaluation and immediately perfused with 1 liter of 1X phosphate buffered saline and 2 liters of 10% formalin. A block of tissue encompassing the entire region of the repaired surgical defect, the spinal cord, and at least L1-L6 vertebral bodies was dissected. Magnetic resonance imaging (MRI) of this tissue block was performed to assess the level and degree of spinal angulation [21]. Lines were drawn through the midportion of the lumbar vertebral bodies on midsagittal imaging, crossing at the point of maximal angulation. The supplementary angle of the two intersecting lines was measured and represented the degree of spinal angulation. Lambs with spinal angulation of greater than 60 degrees were excluded from further analysis due to the potential for confounding spinal cord compression.
The spinal cord was then dissected by lumbar segment, dehydrated in 30% sucrose, cryopreserved, and sectioned as previously described [12]. Briefly, a series of 20-μm sections were obtained from each lumbar spinal segment. Nine sections from each lumbar segment (L1-L6) were stained with Cresyl Violet and imaged at 10X with a Zeiss Observer D1 microscope (Carl Zeiss AS, Norway).
A minimum of 6 of the 9 stained images were analyzed with ImageJ software to determine the height and width for each lumbar segment. For each lamb, the lumbar segment with the greatest deformity, determined by dividing the height over the width of the spinal cord, was defined the MMC epicenter. Only the lumbar segment determined to be the MMC epicenter from each lamb was used for all further analysis. Epicenter analysis consisted of measuring the cross-sectional area of both the overall spinal cord and the gray matter, which were then normalized to the average cross-sectional area for the corresponding lumbar level of normal newborn lambs (n=3). Large neurons (LN), defined as cells 30–70 μm in diameter within the ventral gray matter, were manually counted by a blinded reviewer using Zeiss Zen One software (Carl Zeiss, Inc., Thornwood, NY). Large neuron density per mm2 of gray matter was calculated for each lamb and normalized to the average large neuron density for the corresponding lumbar level of normal newborn lambs.
2.5. Statistical analysis
Median SLR scores for lambs that received PMSCs at any density were compared to lambs repaired with ECM only with the Mann Whitney U test. Median SLR scores for each treatment group were compared with the Kruskal-Wallis test. Mean total spinal cord and gray matter cross-sectional areas and LN density of each treatment group were compared using one-way ANOVA and Tukey’s test for post-hoc analysis. Spearman’s rank-order correlation was used to analyze the relationship between SLR scores and each histologic parameter. All analyses were completed using GraphPad Prism (GraphPad Software, La Jolla, CA).
3. RESULTS
3.1. Evaluation of Motor Function
Thirty-three lambs underwent MMC defect creation and repair. Three were found to have spinal angulation greater than 60 degrees – two ECM only (86.7 and 70.4 degrees) and one High density PMSC-ECM (61 degrees). These 3 lambs were excluded from any further analysis. Additionally, three other lambs died during delivery due to dystocia – one Medium density PMSCECM and two High density PMSC-ECM. Postnatal motor function could not be evaluated for these 3 lambs; however, their spinal cord tissue was included in subsequent histologic analyses.
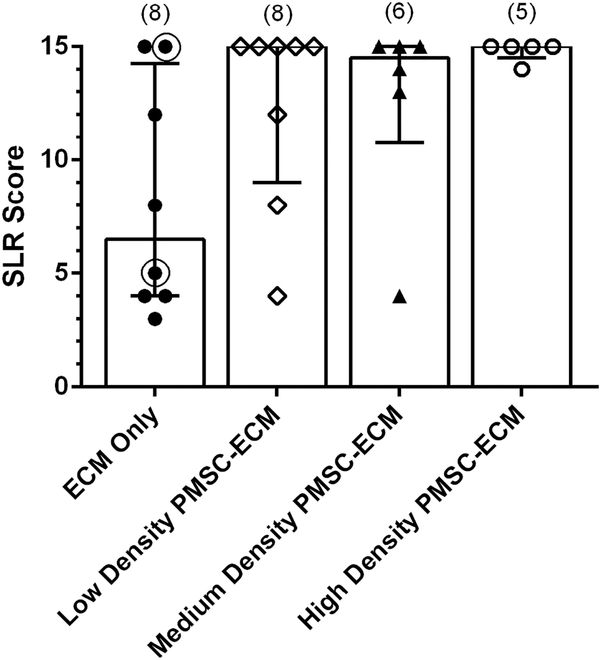
Lambs treated with PMSCs at any density had a higher median SLR score (15 [IQR 13.5–15]) than lambs repaired with ECM only (6.5 [IQR 4–12.75], p = 0.036). When subdivided by PMSC seeding density, there was no significant difference in SLR score among the PMSC treatment groups (p = 0.055) (Figure 1).
Figure 1.
Median Sheep Locomotor Rating Scale Score by Prenatal Treatment.
Of the fetal lambs that underwent MMC creation and repair, 27 were appropriate for evaluation of postnatal motor function using the Sheep Locomotor Rating Scale (SLR). An SLR score of 0 represents complete hindlimb paralysis, while a score of 15 indicates normal spontaneous ambulation with ability to clear an obstacle. There was no significant difference in SLR score among PMSC seeding density groups (p=0.055). SLR scores for six of the eight lambs in the ECM only group and all of the lambs in the Low density PMSC-ECM (42K/cm2) group have been previously published [13]. The two lambs added to the ECM only group since publication are circled.
3.2. Histologic analysis
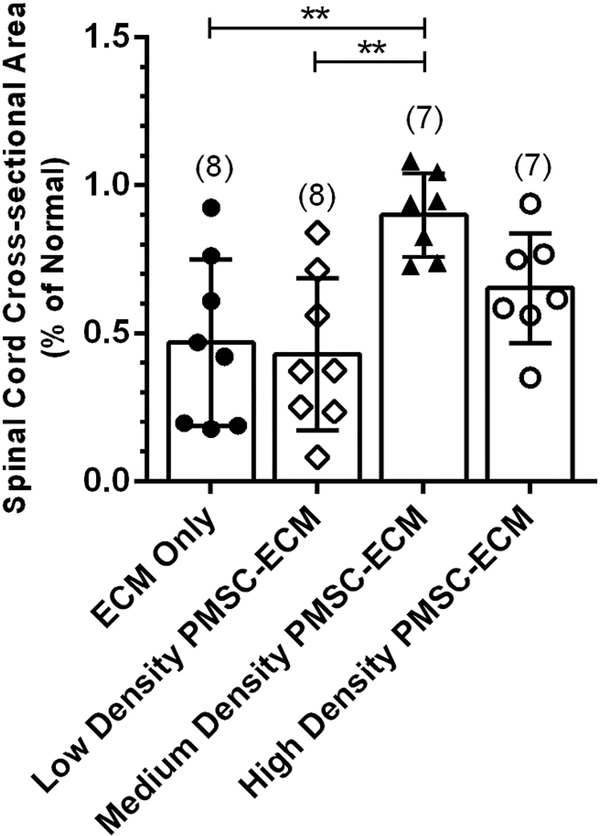
Spinal cord cross-sectional area was significantly different among treatment groups (F(3,26) = 6.60, p = 0.002) (Figure 2). Lambs repaired with Medium density PMSC-ECM had the highest mean normalized spinal cord cross-sectional area (0.90 ± 0.14% of normal), which was significantly higher than lambs repaired with Low density PMSC-ECM (0.43 ± 0.26% of normal) or ECM only (0.47 ± 0.28% of normal). There was no significant difference between the Medium density PMSC-ECM and High density PMSC-ECM (0.65 ± 0.19% of normal) treatment groups.
Figure 2.
Normalized Spinal Cord Cross-sectional Area by Prenatal Treatment.
Serial sections at the MMC defect epicenter for each lamb were imaged and the cross-sectional spinal cord area measured and normalized to the cross-sectional area for the corresponding lumbar level of normal lambs. Lambs that were repaired with Medium density PMSC-ECM had significantly higher spinal cord cross-sectional areas (0.90 ± 0.14% of normal) when compared to lambs that received Low density PMSC-ECM (0.43 ± 0.26% of normal) or the ECM vehicle alone (0.47 ± 0.28% of normal).
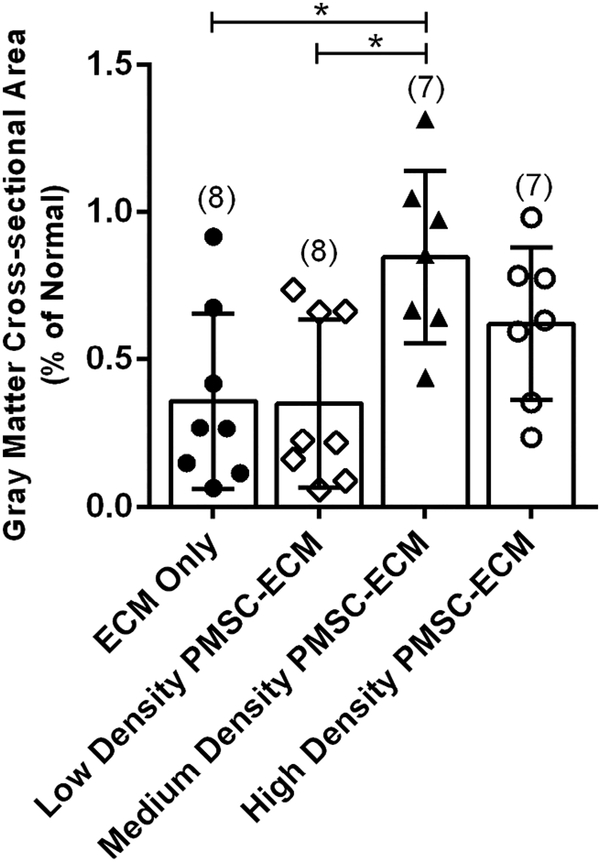
Similarly, there was a significant difference amongst treatment groups for the normalized cross-sectional area of the gray matter (F(3,26) = 5.19, p = 0.006) (Figure 3). The mean gray matter cross-sectional area was significantly higher in the Medium density PMSC-ECM group (0.85 ± 0.29% of normal) compared to the Low density PMSC-ECM (0.35 ± 0.28% of normal) or the ECM only (0.36 ± 0.30% of normal) treatment groups. There was no significant difference between the Medium density PMSC-ECM and High density PMSC-ECM (0.62 ± 0.26% of normal) treatment groups.
Figure 3.
Normalized Gray Matter Cross-sectional Area by Prenatal Treatment.
Serial sections at the MMC defect epicenter for each lamb were imaged and the cross-sectional area of the gray matter measured and normalized to the cross-sectional area for the corresponding lumbar level of normal lambs. Lambs that were repaired with Medium density PMSC-ECM had a significantly higher mean gray matter cross-sectional area (0.85 ± 0.29% of normal) when compared to lambs that received Low density PMSC-ECM (0.35 ± 0.28% of normal) or the ECM vehicle alone (0.36 ± 0.30% of normal).
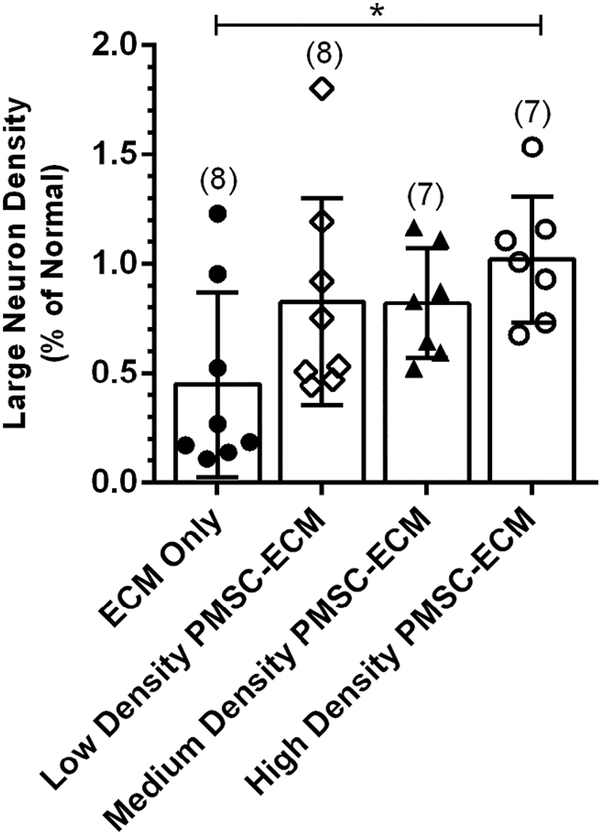
Normalized large neuron density within the gray matter was also significantly different between treatment groups (F(3,26) = 3.08, p = 0.045) (Figure 4). Large neuron density was greatest in the High density PMSC-ECM group (1.02 ± 0.29% of normal), which was significantly higher than the ECM only group (0.45 ± 0.42% of normal). There was no significant difference between lambs treated with Low density PMSC-ECM (0.83 ± 0.47% of normal) or Medium density PMSC-ECM (0.82 ± 0.25% of normal) compared to lambs treated with High density PMSC-ECM.
Figure 4.
Normalized Large Neuron Density by Prenatal Treatment.
Serial cross-sections at the MMC defect epicenter for each lamb were imaged and large neurons (LN), defined as cells within the gray matter with a diameter of 30–70 μm, were manually counted. LN density per mm2 of gray matter was calculated for each lamb and normalized to average LN density for the corresponding lumbar level of normal lambs. Lambs that were repaired with the High density PMSC-ECM had significantly higher LN density (1.02 ± 0.29% of normal) than those repaired with the ECM vehicle alone (0.45 ± 0.42% of normal).
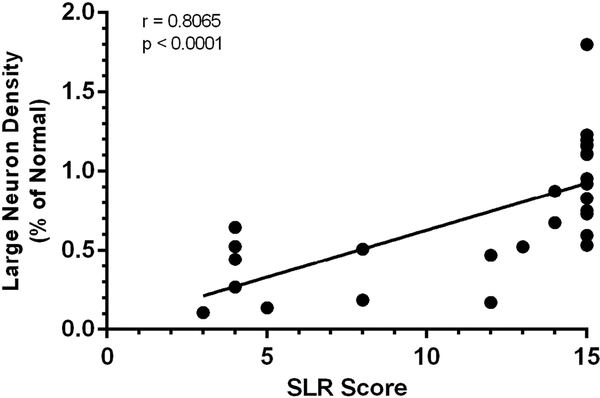
Spinal cord cross-sectional area was positively correlated with SLR score (r = 0.415, p = 0.032) and gray matter cross-sectional area was also positively correlated with SLR score (r = 0.461, p = 0.016). There was a strong positive association between the normalized LN density and SLR score (r = 0.807, p<0.0001) (Figure 5).
Figure 5.
Correlation between Large Neuron Density and Sheep Locomotor Rating Score
Results of Spearman’s correlation indicated a significant positive association between large neuron density per mm2 of gray matter and Sheep Locomotor Rating (SLR) score (r = 0.8065, p<0.0001). An SLR score of 0 represents complete hindlimb paralysis, while a score of 15 indicates a normal spontaneous ambulation with ability to clear an obstacle.
4. DISCUSSION
We have demonstrated that PMSC seeding density on ECM used for in utero patch repair of ovine MMC affects the preservation of spinal cord, gray matter, and large neurons. Higher seeding densities resulted in increased cross-sectional spinal cord and gray matter area, as well as increased LN density at the point of maximal cord deformation. The greatest spinal cord and gray matter cross-sectional areas were observed in the Medium density PMSC-ECM group, while the greatest LN density was found in lambs treated with High density PMSC-ECM. The reason for these differences is unclear, and indeed there was no statistical difference between the Medium and High density PMSC-ECM treatment groups by any metric. However, to determine the optimal seeding density for translation to clinical use, the correlation between each histologic parameter and postnatal functional outcomes was assessed. Spinal cord or gray matter cross-sectional area was only weakly associated with postnatal motor function, while a strong association was found between LN density and SLR scores. Therefore, it appears that spinal cord volume may be less clinical relevant than neuronal density.
While we were unable to detect a significant difference in hindlimb motor function with increasing PMSC seeding density, there was a significant improvement in functional outcomes for lambs that received any PMSCs compared to those repaired with ECM only. Amongst all lambs treated with PMSCs at any seeding density, nearly two thirds (12/19, 63%) exhibited normal ambulation and ability to clear an obstacle compared to only a quarter of the lambs treated with the ECM vehicle alone (2/8, 25%). The proportion of lambs with normal postnatal locomotor function increased to 80% for those treated with the highest seeding density of PMSCs (4/5). The remaining lamb in this treatment group demonstrated near normal ambulation, but was unable to clear an obstacle resulting in an SLR score of 14. There was a strong positive correlation between LN density and SLR score, suggesting that neuronal preservation within the spinal cord may be the mechanism by which PMSCs exert their therapeutic effect.
These findings are consistent with our previous studies in both the ovine and rodent models, and additionally suggest that a higher seeding density may exert a more robust effect when used to augment fetal repair of MMC [12, 13, 16]. While this effect difference is not captured in a difference in SLR score in sheep, the underlying neuronal preservation may translate to improved or more sustained motor function in humans. As reported by Wang et al and Kabagambe et al, augmentation of in utero MMC repair with PMSCs seeded on ECM at the lowest seeding density (42K/cm2) consistently improved postnatal hindlimb motor function compared to the ECM vehicle alone [12, 13]. The three seeding densities evaluated in this study were chosen based on previous in vitro work reported by Lankford et al, which determined the optimal loading density of PMSCs on ECM to be 300K-500K cells/cm2, as well as an in vivo study performed by Chen et al, which showed improved spinal cord architecture and reduced apoptotic density with higher PMSC seeding density following fetal repair in a rodent model of MMC [16, 17]. Unlike the rodent model of MMC, lambs are able to be evaluated for clinically relevant postnatal functional outcomes, which can then be correlated to the observed spinal cord morphology and histology [20, 22].
The spinal cord injury observed in patients with MMC is widely believed to be the result of both exposure to amniotic fluid and ongoing intrauterine trauma throughout gestation, known as the “two-hit hypothesis” [22]. This suggested that some spinal cord damage could be prevented or possibly even reversed by prenatal defect repair, which was subsequently confirmed in both small and large animal models [23, 24]. We have demonstrated improved spinal cord preservation and increased neuron density with our PMSC therapy compared to prenatal repair alone. In vitro studies of PMSCs derived from early gestation chorionic villus have previously shown unique immunomodulatory and neuroprotective capabilities [9, 10]. We now have provided evidence of in vivo neuronal preservation.
The results of this study, combined with the previous studies by Kabagambe et al, Chen et al, and Lankford et al, provide support for translation of this PMSC-based therapy to clinical application for prenatal repair of MMC. The exact mechanism of action of these cells has not been fully elucidated, but spinal cord neuronal preservation appears to play a key role. While the Medium density PMSC-ECM treatment group had the highest spinal cord and gray matter cross-sectional areas, lambs treated with the highest seeding density had the greatest LN density, as well as a larger proportion capable of normal ambulation. While it is not yet known whether these functional improvements will persist long-term,the optimal seeding density for clinical use should maximize both motor function and tissue preservation.
5. CONCLUSION
In summary, augmentation of in utero repair of myelomeningocele with a high seeding density of placental mesenchymal stromal cells resulted in increased neuronal density within the spinal cord, which was associated with improved hindlimb motor function in the ovine model.
How this paper advances the field:
High seeding density of placental stem cells on extracellular matrix demonstrated better functional and histologic outcomes after prenatal repair of ovine spina bifida when compared to lower seeding densities and has therefore been selected for translation to human clinical trials.
Acknowledgements:
We thank Cook Biotech Inc. for their generous gifting of ECM material.
Funding: This work was supported by funding from the California Institute of Regenerative Medicine (Grant #: PC1–08103), Shriners Hospital for Children research grant #85120-NCA-16, NIH (#5R01NS100761–02), and March of Dimes Foundation grant (#5FY1682). The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Type of Study: Basic Science
Level of Evidence: N/A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Major Birth Defects from Population-based Birth Defects Surveillance Programs in the United States, 2009–2013. Birth Defects Research Part A: Clinical and Molecular Teratology 2016;106:S1–S177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Adzick NS. Fetal myelomeningocele: Natural history, pathophysiology, and in-utero intervention. Seminars in Fetal and Neonatal Medicine;15(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Adzick NS, Thom EA, Spong CY, Brock JWI, Burrows PK, Johnson MP, et al. A Randomized Trial of Prenatal versus Postnatal Repair of Myelomeningocele. New England Journal of Medicine 2011;364(11):993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Vanover M, Wang A, Farmer D. Potential clinical applications of placental stem cells for use in fetal therapy of birth defects. Placenta 2017. [DOI] [PubMed] [Google Scholar]
- [5].Xiaoting L, Yue D, Yuelin Z, Hung-Fat T, Qizhou L. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transplantation 2014;23(9):1045–59. [DOI] [PubMed] [Google Scholar]
- [6].Horwitz EM, Prather WR. Cytokines as the major mechanism of mesenchymal stem cell clinical activity: expanding the spectrum of cell therapy. The Israel Medical Association journal : IMAJ 2009;11(4):209–11. [PubMed] [Google Scholar]
- [7].Bačenková D, Rosocha J, Tóthová T, Rosocha L, Šarissk M. Isolation and basic characterization of human term amnion and chorion mesenchymal stromal cells. Cytotherapy 2011;13(9):1047–56. [DOI] [PubMed] [Google Scholar]
- [8].Hsiao STF, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, et al. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells and Development 2012;21(12):2189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Barlow S, Brooke G, Chatterjee K, Price G, Pelekanos R, Rossetti T, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells and Development 2008;17(6):1095–107. [DOI] [PubMed] [Google Scholar]
- [10].Kumar PBJ, Lankford L, Keller B, Farmer DL, Wang A. Role of human placenta-derived mesenchymal stem cells in neuroprotection and neurogenesis. International Society for Stem Cell Research 14th Annual Meeting San Francisco, CA; 2016. [Google Scholar]
- [11].Lankford L, Selby T, Becker J, Ryzhuk V, Long C, Farmer D, et al. Early gestation chorionic villi-derived stromal cells for fetal tissue engineering. World journal of stem cells 2015;7(1):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang A, Brown EG, Lankford L, Keller BA, Pivetti CD, Sitkin NA, et al. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl Med 2015;4(6):659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kabagambe S, Keller B, Becker J, Goodman L, Pivetti C, Lankford L, et al. Placental mesenchymal stromal cells seeded on clinical grade extracellular matrix improve ambulation in ovine myelomeningocele. Journal of pediatric surgery 2018;53(1):178–82. [DOI] [PubMed] [Google Scholar]
- [14].Brown EG, Keller BA, Lankford L, Pivetti CD, Hirose S, Farmer DL, et al. Age Does Matter: A Pilot Comparison of Placenta-Derived Stromal Cells for in utero Repair of Myelomeningocele Using a Lamb Model. Fetal Diagnosis and Therapy 2016;39(3):179–85. [DOI] [PubMed] [Google Scholar]
- [15].Gensel JC, Tovar CA, Hamers FP, Deibert RJ, Beattie MS, Bresnahan JC. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. Journal of neurotrauma 2006;23(1):36–54. [DOI] [PubMed] [Google Scholar]
- [16].Chen YJ, Chung K, Pivetti C, Lankford L, Kabagambe SK, Vanover M, et al. Fetal surgical repair with placenta-derived mesenchymal stromal cell engineered patch in a rodent model of myelomeningocele. Journal of pediatric surgery 2018;53(1):183–8. [DOI] [PubMed] [Google Scholar]
- [17].Lankford L, Chen YJ, Saenz Z, Kumar P, Long C, Farmer D, et al. Manufacture and preparation of human placenta-derived mesenchymal stromal cells for local tissue delivery. Cytotherapy 2017;19(6):680–8. [DOI] [PubMed] [Google Scholar]
- [18].Meuli M, Meuli-Simmen C, Yingling CD, Hutchins GM, Hoffman KM, Harrison MR, et al. Creation of myelomeningocele in utero: A model of functional damage from spinal cord exposure in fetal sheep. Journal of pediatric surgery 1995;30(7):1028–33. [DOI] [PubMed] [Google Scholar]
- [19].von Koch CS, Compagnone N, Hirose S, Yoder S, Harrison MR, Farmer DL. Myelomeningocele: Characterization of a surgically induced sheep model and its central nervous system similarities and differences to the human disease. American journal of obstetrics and gynecology 2005;193(4):1456–62. [DOI] [PubMed] [Google Scholar]
- [20].Brown EG, Keller BA, Pivetti CD, Sitkin NA, Wang A, Farmer DL, et al. Development of a locomotor rating scale for testing motor function in sheep. Journal of pediatric surgery 2015;50(4):617–21. [DOI] [PubMed] [Google Scholar]
- [21].Vanover MA PC, Galganksi LA, Saadai P, Wang A, Farmer DL. Spinal Angulation: A Limitation of the Fetal Lamb Model of Myelomeningocele. International Fetal Medicine and Surgery Society 36th Annual Meeting Jackson Hole, Wyoming; 2017. [Google Scholar]
- [22].Heffez DS, Aryanpur J, Hutchins GM, Freeman JM. The paralysis associated with myelomeningocele: clinical and experimental data implicating a preventable spinal cord injury. Neurosurgery 1990;26(6):987–92. [PubMed] [Google Scholar]
- [23].Heffez DS, Aryanpur J, Rotellini NA, Hutchins GM, Freeman JM. Intrauterine repair of experimental surgically created dysraphism. Neurosurgery 1993;32(6):1005–10. [DOI] [PubMed] [Google Scholar]
- [24].Meuli M, Meuli-Simmen C, Yingling CD, Hutchins GM, Timmel GB, Harrison MR, et al. In utero repair of experimental myelomeningocele saves neurological function at birth. Journal of pediatric surgery 1996;31(3):397–402. [DOI] [PubMed] [Google Scholar]