Abstract
Background:
The last decade has seen several advances in radical prostatectomy (RP) technique and post-RP care that are relevant to erectile function (EF) recovery.
Objective:
We examined whether these practice changes have led to observed improvements in EF rates over time.
Design, setting, and participants:
We identified 2364 patients treated with either open or minimally-invasive RP at a single academic center in 2008–2015. To mitigate confounding by the surgical learning curve, only patients treated by surgeons who performed at least 100 procedures were considered.
Intervention:
EF before and after RP was assessed by the International Index of Erectile Function 6 (IIEF-6), with recovery defined as IIEF-6 ≥24.
Outcome measurements and statistical analysis:
We analyzed EF recovery rates of patients treated with bilateral nerve-sparing surgery and free from adjuvant/salvage treatment at the time of EF assessment. Local polynomial regression analyses explored changes in the outcomes over time. Linear and logistic regression analyses were used to estimate the influence of year of surgery on baseline variables and EF recovery.
Results and limitations:
We observed a significant decrease over time of the EF recovery rates at both 12 and 24 mo post-RP (all p = 0.01). However, patient’s age at surgery increased over time (mean increase of 0.5 per year; p < 0.01), with a resultant increase in risk of comorbidity (odds ratio [OR] = 1.1, 95% confidence interval [CI]: 1.02–1.15; p=0.008) and thus decrease in baseline IIEF-6 score (0.35 points per year; p = 0.0003). After accounting for baseline and pathological characteristics, urinary function, and type of surgery in a multivariable analysis, year of surgery was not associated with EF recovery (12 mo: OR = 0.97, 95% CI: 0.91–1.03, p = 0.4; 24 mo: OR = 0.97, 95% CI: 0.91–1.03, p = 0.3).
Conclusions:
Findings from a high-volume center suggest that, despite the advancements in surgical and postoperative care, EF outcomes after RP have not improved over the last decade. Additional strategies are required to improve EF recovery after RP.
Patient summary:
The probability of regaining potency after surgery for prostate cancer did not improve over the last decade; more efforts are needed to improve patient’s care after radical prostatectomy.
Keywords: Erectile function, International Index of Erectile Function, Penile rehabilitation, Prostate cancer, Radical prostatectomy
1. Introduction
Erectile dysfunction (ED) represents a troublesome issue for patients surgically treated for prostate cancer. The probability that a patient will have adequate erectile function (EF) after radical prostatectomy (RP) has been reported to be 35% at 2 yr in a large, long-term prospective trial [1].
Since the introduction of the open RP technique, several efforts have been made to improve functional outcomes after surgery, while ensuring oncological safety. The anatomic nerve-sparing (NS) approach proposed by Walsh [2] in the 1980s led to a dramatic improvement in the post-RP EF recovery rates. Since then, several other advances have been made to reduce the risk of postoperative ED [3]. Detailed study of prostate anatomy, for instance, has led to a refinement of the NS technique over the last two decades, with the aim to reduce injury to the neurovascular bundles [4,5]. The introduction of robotic surgery led to a further evolution of the RP technique: the magnified three-dimensional view and the seven-degree motion provided by the robotic instruments may lead to a more precise identification of the multiple periprostatic fascia layers. The concept of the incremental NS approach has been developed, which allows up to four to five different degrees of conservation of the periprostatic neurovascular tissue [5–7]. Furthermore, other authors have proposed that even minor details in the surgical technique, such as the use of electrocautery in the proximity of the neurovascular bundles or mechanical stretch of neural tissue occurring during the procedure, could influence the chance of EF recovery after surgery [8–10]. Finally, the results of randomized trials on the use of phosphodiesterase type 5 inhibitors (PDE5is) to enhance the recovery of EF after surgery [11] encouraged the adoption of penile rehabilitation protocols aimed to preserve an adequate functional oxygenation of the erectile tissue in the early phase after surgery, thus potentially preventing the onset of permanent ED [12].
It remains unknown whether these incremental changes in surgical and postoperative management have led to an observed overall improvement in the EF outcomes after surgery over time. Here, we examine changes in the EF recovery rates of patients treated with RP at a single, high-volume center over the last decade.
2. Patients and methods
After obtaining institutional review board approval, we retrospectively identified 3665 patients who had available EF data and were treated with either open or minimally-invasive RP at our institution between 2008 and 2015. Patient-reported EF was electronically collected through our web-based platform [13] using the International Index of Erectile Function 6 (IIEF-6) questionnaire as part of routine clinical care. The surveys are administered at baseline (before RP) and postoperatively at 3, 6, 9, 12, 18, and 24 mo or during a follow-up. According to the postoperative care strategy of our institution, patients are recommended to start daily or ondemand PDE5is as soon as possible following surgery. The postoperative survey allows patients to report the use of PDE5is to enhance potency after surgery and to provide the answers to the IIEF-6 questionnaire on the basis of experience with the medication. Data on the use of intracavernous injection (ICI) therapy are also recorded. Similarly, urinary function (UF) is assessed with a validated survey at the same time points and scored from 0 to 21 (good UF is defined as a score ≥17) [13].
Preoperative data including patients’ age at surgery and relevant comorbidities (as scored with the Charlson comorbidity index [CCI]) as well as pathological findings were recorded. Similarly, data on the occurrence and timing of any adjuvant or salvage treatment over the follow-up period were considered.
To mitigate confounding by the surgical learning curve, only patients treated by surgeons who performed at least 100 procedures with a specific surgical technique (eg open [ORP], laparoscopic [LRP], or robotic RP [RARP]) were considered for the analyses (n = 2948); moreover, 268 patients who received neo-adjuvant treatment were excluded. Patients with missing EF or pathological data were also excluded (n = 316), leading to a final cohort of 2364 patients.
2.1. Statistical analyses
Our goal was to assess changes in EF recovery after RP over time. EF recovery was defined as an IIEF-6 score ≥24 [14]. We assessed EF recovery at 1 and 2 yr after surgery as binary outcomes: patients were considered to have recovered at 1 yr if they reported an IIEF-6 score ≥24 within 9–15 mo from surgery (ie, 12 mo with a 3-mo window); a total of 318 patients reporting EF data at 3–6 months but missing data within the 9–15-mo window were not included in the 1-yr outcome analyses. Similarly, patients were considered to have recovered at 2 yr if they reported an IIEF-6 score ≥24 at the 24 mo assessment; therefore, 373 patients reporting EF data at the 18 mo assessment but missing data at 24 mo were excluded from the 2-yr outcome analyses. As a sensitivity analysis, we included all patients with follow-up data using the assessment closest to 1 and 2 yr after surgery. Moreover, the same analyses were performed using different definitions of EF recovery (eg, IIEF-6 ≥22; IIEF-6 ≥26).
Given an expected increase over time in the proportion of cancers categorized as being at high risk [15], a phenomenon that could have resulted in a lower number of patients receiving a bilateral NS (bNS) surgery and to a higher rate of adjuvant or salvage therapy, we analyzed EF recovery rates of patients treated with bNS surgery and free from adjuvant/salvage treatment at the time of the last EF assessment. Therefore, the 1- and 2-yr EF assessment cohorts included a final number of 1779 and 1095 patients, respectively. The quality of NS has been routinely reported by the surgeon at the time of RP and graded as: 1 (resected), 2 (definite damage), 3 (possible damage), and 4 (complete preservation). As such, patients receiving an NS surgery (score 2–4) may represent a heterogenous cohort which could have influenced the results of EF recovery. Therefore, we have performed a further analysis to assess the association between year of surgery and the chance of EF recovery in the subgroup of patients receiving a complete preservation of nerve bundles and among those treated with a lower grade of NS as reported by the surgeon. Furthermore, although we have considered only surgeons who have performed at least 100 procedures with a specific technique, we performed a supplementary analysis predicting EF recovery and adjusting for the different surgeon.
Local polynomial regression models were used to explore the relationship between 1- and 2-yr EF recovery rates with the year of surgery. Similarly, changes in the use of PDE5is, ICI, and in baseline characteristics (eg, age, CCI score, baseline IIEF-6 score) over time were also assessed. Univariate linear and logistic regression analyses were used to estimate the association between the investigated outcomes and the year of treatment. Multivariable logistic regression analyses (including year of surgery, age, baseline IIEF-6 score, CCI score, use of PDE5is, UF status, and pathological characteristics) were conducted to predict post-RP EF recovery at 1 and 2 yr. The adjusted rates of EF recovery were then plotted over the year of treatment.
Statistical analyses were conducted using Stata version 13.0 (StataCorp, College Station, TX, USA), with a two-sided significance level set at p < 0.05.
3. Results
The clinical and pathological characteristics of the study cohort are reported in Table 1. Overall, 95.3% of patients underwent a bNS procedure; moreover, 11% patients had salvage or adjuvant treatments within 2 yr of surgery. Among patients treated with bNS and free from adjuvant/salvage treatment at the time of follow-up assessment, the overall rate of EF recovery was 27% and 34% at 1- and 2-yr post-RP, respectively (Table 2).
Table 1 –
Characteristics of the overall cohort of patients (n = 2364)
| Age, median (IQR) | 61 (56–66) |
| Charlson comorbidity score, n (%) | |
| 0 | 1910 (81) |
| 1 | 234 (10) |
| ≥2 | 220 (9) |
| PSA, median (IQR) | 5.6 (4.1–8.1) |
| Nerve-sparing, n (%) | |
| None | 33 (1.4) |
| Unilateral | 78 (3.3) |
| Bilateral | 2253 (95.3) |
| Gleason grade, n (%) | |
| 1 | 336 (14.2) |
| 2 | 1297 (54.9) |
| 3 | 503 (21.3) |
| 4 | 111 (4.7) |
| 5 | 117 (4.9) |
| pT stage, n (%) | |
| pT2 | 1360 (57.5) |
| pT3 | 968 (41) |
| pT4 | 36 (1.5) |
| Positive surgical margins, n (%) | 408 (17) |
| Surgical approach, n (%) | |
| Open | 614 (26) |
| Laparoscopic | 633 (27) |
| Robotic | 1117 (47) |
| Rates of adjuvant/salvage treatment (%) a | |
| 1 yr | 8 |
| 2 yr | 11 |
IQR = interquartile range; PSA = prostate-specific antigen.
Estimated with Kaplan-Meier analysis
Table 2 –
Preoperative and postoperative erectile function of patients treated with bilateral nerve-sparing surgery and being free from adjuvant or salvage treatment at the time of erectile function assessment
| Baseline IIEF-6 score, median (IQR) | 27 (19–30) |
| EF recovery at 12 mo post-RP, n (%) | 483 (27) a |
| EF recovery at 24 mo post-RP, n (%) | 377 (34) a |
| Regular use of PDE5is, n (%) | |
| 12-mo assessment | 716 (40) a |
| 24-mo assessment | 158 (14) a |
| Use of ICI over the follow-up period, n (%) | 741 (31) |
EF = erectile function; ICI = intracavernous injection; IIEF = International Index of Erectile Function; IQR = interquartile range; PDE5is = phosphodiesterase type 5 inhibitors; RP = radical prostatectomy.
A total of 1779 and 1095 patients treated with bilateral nerve-sparing surgery were free from adjuvant/salvage treatment at the 12- and 24-mo assessment, respectively.
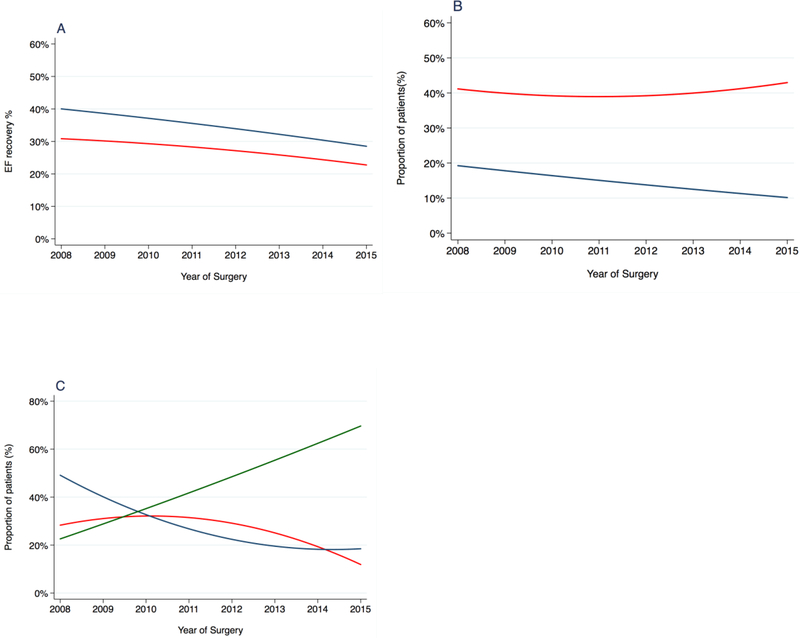
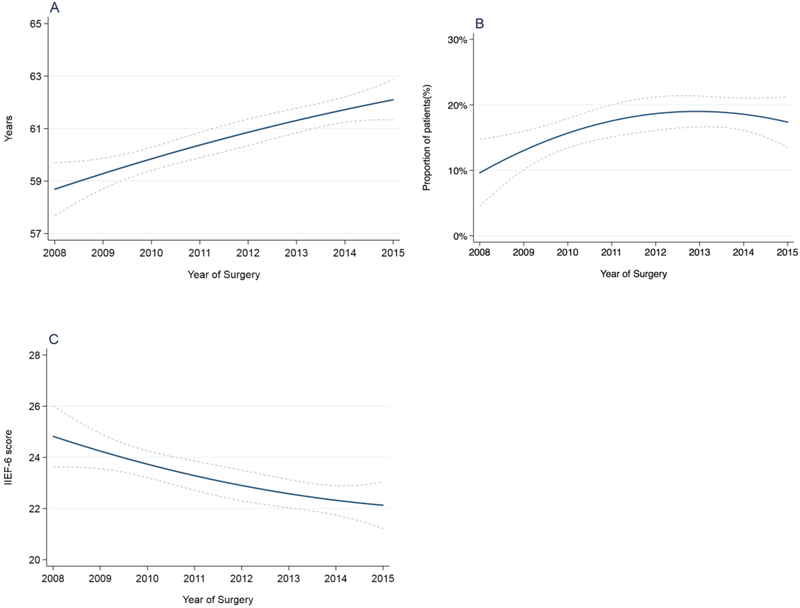
Figure 1A reports the changes in the EF outcomes over time, showing a significant decrease of both 1-yr (odds ratio [OR] = 0.94; 95% confidence interval [CI]: 0.90–0.99; p = 0.01) and 2-yr (OR = 0.93; 95% CI: 0.88–0.98; p = 0.01) EF (Table 3). However, over the same time period, we observed a significant increase in patient age at surgery of 0.45 per year (95% CI: 0.29–0.61; p < 0.0001), with a concomitant increase in the risk of comorbidities (CCI ≥1 OR = 1.08; 95% CI: 1.02–1,15; p = 0.008) and a decrease of 0.35 point per year (95% CI: –0.54 to – 0.16; p = 0.0003) in preoperative IIEF-6 (Fig. 2A–C; Table 3). Likewise, the use of PDE5is at 2 yr after surgery significantly decreased over time (OR = 0.90; 95% CI: 0.83–0.97; p = 0.008; Fig. 1B; Table 3). Conversely, we did not find a statistically significant change over time in the rate of patients reporting to have used ICIs over the postoperative follow-up (OR = 0.96; 95% CI: 0.92–1.01; p = 0.1; Supplementary Fig. 1). Finally, we observed significant changes in the surgical approach throughout the investigated time period, with an increase in the number of patients treated with RARP (OR = 1.33; 95% CI: 1.22–1.40; p < 0.001) and a decrease of LRP (OR = 0.81; 95% CI: 0.77–0.85; p < 0.0001) and ORP (OR = 0.87; 95% CI: 0.82–0.91; p < 0.0001) procedures (Fig. 1C; Table 3).
Fig. 1 –
(A) Unadjusted rate of patients reporting erectile function recovery at 12 (red line) and 24 (blue line) mo after radical prostatectomy (RP). (B) Rate of patients reporting regular use of phosphodiesterase type 5 inhibitors at 12 (red line) and 24 (blue line) mo assessment. (C) Rate of patients treated with different surgical approach: open RP (red line); laparoscopic RP (blue); robot-assisted RP (green).
EF = erectile function.
Table 3 –
Univariate logistic and linear regression analyses estimating the association between year of surgery and outcomes
| Variable | OR | 95% CI | p value |
|---|---|---|---|
| Outcome | |||
| EF recovery at 12 mo | 0.94 | 0.90–0.99 | 0.01 |
| EF recovery at 24 mo | 0.93 | 0.88–0.98 | 0.01 |
| Use of PDE5is at 12 mo | 1.02 | 0.97–1.06 | 0.5 |
| Use of PDE5is at 24 mo | 0.90 | 0.83–0.97 | 0.008 |
| Baseline characteristics | |||
| CCI score (0 vs >=1) | 1.08 | 1.02–1.15 | 0.008 |
| ORP | 0.87 | 0.82–0.91 | <0.0001 |
| LRP | 0.81 | 0.77–0.85 | <0.0001 |
| RARP | 1.33 | 1.27–1.40 | <0.0001 |
| Coefficient | |||
| Baseline IIEF-6 score | –0.35 | –0.54 to –0.16 | 0.0003 |
| Age | 0.45 | 0.29–0.61 | <0.0001 |
CI = confidence interval; CCI = Charlson comorbidity index; EF = erectile function; IIEF = International Index of Erectile Function; IQR = interquartile range; LRP = laparoscopic radical prostatectomy; OR = odds ratio; ORP = open radical prostatectomy; PCa = prostate cancer; PDE5is = phosphodiesterase type 5 inhibitors; RARP = robot-assisted radical prostatectomy.
Fig. 2 –
(A) Patients’ age at surgery, (B) rate of patients with a Charlson comorbidity index score ≥1 at surgery, and (C) baseline IIEF-6 score over the investigated time period. The dashed lines represent 95% confidence interval.
IIEF-6 = International Index of Erectile Function-6.
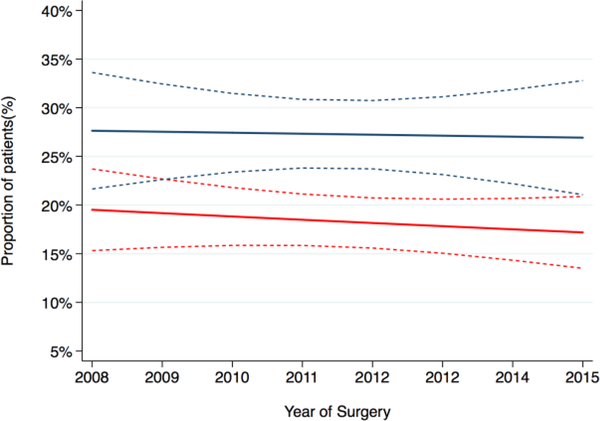
At multivariable analysis, the year of treatment was not significantly associated with the probability of EF recovery at 1- and 2-yr post-RP (Table 4) and the adjusted probability of EF recovery after surgery appeared stable over time (Fig. 3). This suggests that the apparent increase in ED is likely a result of changing case mix over time.
Table 4 –
Multivariable logistic regression analyses testing predictors of erectile function recovery at 12 and 24 mo from radical prostatectomy
| EF recovery at 12 mo | EF recovery at 24 mo | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Year of surgery | 0.98 | 0.92–1.04 | 0.4 | 0.99 | 0.93–1.07 | 0.9 |
| Baseline IIEF-6 | 1.16 | 1.13–1.20 | <0.0001 | 1.14 | 1.10–1.17 | <0.0001 |
| Age | 0.95 | 0.93–0.97 | <0.0001 | 0.94 | 0.92–0.96 | <0.0001 |
| Surgical approach | ||||||
| ORP vs LRP | 0.75 | 0.54–1.04 | 0.08 | 0.76 | 0.51–1.11 | 0.2 |
| ORP vs RARP | 1.11 | 0.83–1.48 | 0.5 | 1.06 | 0.75–1.48 | 0.8 |
| CCI score | 0.71 | 0.59–0.86 | 0.001 | 0.78 | 0.64–0.96 | 0.02 |
| Use of PDE5is | 1.05 | 0.93–1.19 | 0.4 | 1.17 | 0.97–1.40 | 0.1 |
| Urinary function a | 1.43 | 1.08–1.89 | 0.01 | 1.66 | 1.16–2.39 | 0.006 |
| pT stage | ||||||
| pT2 vs ≥ pT3 | 0.95 | 0.73–1.25 | 0.7 | 0.87 | 0.63–1.21 | 0.4 |
| Gleason grade group | ||||||
| 1 vs 2 | 0.92 | 0.67–1.27 | 0.6 | 0.73 | 0.49–1.09 | 0.1 |
| 1 vs 3 | 0.85 | 0.55–1.29 | 0.4 | 0.60 | 0.36–1.01 | 0.052 |
| 1 vs 4 | 0.82 | 0.37–1.82 | 0.6 | 0.77 | 0.29–2.03 | 0.6 |
| 1 vs 5 | 0.65 | 0.25–1.68 | 0.4 | 0.52 | 0.16–1.72 | 0.3 |
CI = confidence interval; CCI = Charlson comorbidity index; EF = erectile function; IIEF = International Index of Erectile Function; LRP = laparoscopic radical prostatectomy; OR = odds ratio; ORP = open radical prostatectomy; PDE5is= phosphodiesterase type 5 inhibitors; RARP = robot-assisted radical prostatectomy.
Urinary function status at the time of erectile function assessment.
Fig. 3 –
Adjusted rate of erectile function recovery at 12 (red line) and 24 (blue line) mo after surgery over the investigated time period. The dashed lines represent the 95% confidence interval. There is no significant change over time (p = 0.2 and 0.3, respectively).
Sensitivity analyses using different EF recovery definitions showed comparable results at the multivariable logistic regression predicting EF outcomes (Supplementary Table 1); similarly, when we expanded the cohort to include all patients with at least one EF follow-up assessment but who were not evaluated within the eligible time frame, we did not observe any significant association between year of surgery and the adjusted probability of EF recovery (1-yr EF recovery: OR = 0.97; 95% CI: 0.91–1.02; p = 0.2; 2-yr EF recovery: OR = 0.97; 95% CI: 0.91– 1.03; p = 0.3). Moreover, the same findings were confirmed after adjusting the multivariable analysis for different surgeons (Supplementary Table 2).
Overall, 46% of patients received complete bilateral nerve preservation. At the logistic regression analysis including only patients treated with perfect NS, year of surgery was not associated with the chance of EF recovery after adjusting for other baseline and postoperative factors (Supplementary Table 3; Supplementary Fig. 3). Similar results were observed in the subgroup of patients receiving a lower grade of NS (Supplementary Table 3; Supplementary Fig. 3).
4. Discussion
We examined changes in the post-RP EF outcomes occurring over the last decade in a high-volume center. After adjusting for patient baseline and pathological characteristics, EF recovery rates after RP appeared to be stable through time.
Given refinements in surgical technique over time and increased use of postoperative penile rehabilitation protocols, we expected to see temporal changes in the probability of EF recovery after RP [15]. Our results suggest that those changes in surgical and post-RP management did not result in an overall observable increase of the chance of EF recovery after surgery. In light of this evidence, future research efforts should be focused on developing further strategies aimed at improving the EF outcomes after RP.
We observed an overall rate of 27% and 34% of EF recovery at 1 and 2 yr post-RP; these rates could seem significantly lower than those in previously published series. In meta-analyses of 15 RP series, for instance, the overall recovery rate ranged from 26% to 90% and from 47% to 94% at 1 and 2 yr after surgery, respectively [16]. However, most of the included studies were single-center series with highly selected patients that were more likely to recover EF after surgery: Menon et al [17], for instance, reported a 94% EF recovery rate in a selected small cohort of 84 young men with a mean age of 55 yr and a normal preoperative EF [17]. Conversely, large randomized trials comparing outcomes of RP with surveillance strategies showed lower EF recovery rates of 14–26% and 19–35% at 1 and 2 yr after RP, respectively [18–20]. These data are in line with our findings and reflect the larger heterogeneity in the baseline characteristics of the included patients, influencing the overall probability of EF recovery after surgery.
The use of the robotic approach for the surgical treatment of prostate cancer has constantly increased over the last decades in the USA [21,22]; at the same time, the knowledge of prostate anatomy has improved [5], leading to a refinement of the surgical technique. The concept of incremental NS, for instance, has been recently proposed [6,7] and retrospective studies have shown an improvement in the rate of EF recovery as the degree of preservation of the neurovascular tissue increase on a scale from 1 to 4 [6]. Further advances in the robotic surgical technique have been introduced, thus including the athermal NS approach—avoiding the use of electrocautery during prostate dissection showed improved potency rate at 1 yr after surgery [10]. Our data confirm the significant increase in the rate of RARP procedures over the last decade; however, the spread of robotic surgery did not affect the EF outcomes after treatment. Whether the new developed surgical techniques are able to significantly improve the probability of EF recovery after RP, should be investigated in multicenter randomized trials comparing the outcomes of different surgical techniques and their consistency among different surgeons.
The concept of penile rehabilitation post-RP emerged more than a decade ago. A survey conducted in 2009 showed that 87% of physicians had prescribed some form of erectile aids after surgery, and 95% of them suggested PDE5is [23]. In our series, about 40% of patients were using PDE5is at 1 yr after surgery, and this rate was stable over the years; conversely, we observed a decrease in the use of PDE5is at the 2-yr assessment. This factor could have an influence on the probability of EF recovery. However, randomized trials have been inconclusive regarding the improvement of unassisted EF associated with PDE5is therapy after RP [24]. Moreover, PDE5is were available throughout the entire time period covered by our study; some improvements in the post-RP functional erections may be observed when comparing patients treated in the last decade with those treated in the pre-PDE5is era.
We reported an increase in patient age at surgery over time; this finding confirms a previous study showing a 1–4 yr increase in the median age of patients submitted to RP over the last two decades [25]. It is likely that the spread of active surveillance programs for low-risk diseases may have resulted in an increasing proportion of patients with higher-risk disease submitted to RP, which in turn is associated with older age [26]. As a consequence of ageing, patients treated in more recent years at our center had worse baseline EF and health status. These factors could be partially responsible for the unchanged EF recovery rates observed over the last decade. Indeed, considering the strong association of advanced age [27], decreased baseline EF [28], and comorbidities [29], with the risk of postoperative ED, it is possible that patients treated more recently were unlikely to benefit from a more precise surgical technique or a refined postoperative rehabilitation protocol in terms of EF outcomes.
Our study has some limitations. First, as a single-center series, these results may not be generalized to a larger population; indeed, it could be the case that the advances in surgical technique and postoperative management occurred in the last decade may have not been implemented in the everyday clinical practice at our institute. However, as an academic highvolume center, the surgical care has been up-to-date over time; this is also suggested by the evidence of the increased use of the robotic approach for RP. Studies involving different institutions are certainly needed to validate our findings. Second, while our web-based survey is able to capture the use of PDE5is, we lacked data on the use of other erectile aids such as ICIs and vacuum device for the entire cohort of patients. Similarly, we have analyzed only data concerning patients EF: it has been shown that the way of dealing with sexual functioning after RP goes well beyond the evaluation of functional erections. Indeed, patients submitted to RP could experience several bothersome sexual difficulties, thus involving impairments of sexual desire, anejaculation, orgasmic troubles, as well as cosmetic alterations, such as penile shortening or shape alterations [11]. All these issues deserve to be further investigated. Finally, although we provided data at a reasonable follow-up of 2 yr after surgery, a further improvement of EF may have occurred at later timings, as recently demonstrated in other studies [30].
5. Conclusions
Despite the advancements in surgical technique and postoperative management of patients treated with RP, we did not observe a significant change in the probability of EF recovery after surgery over the last decade in a high-volume center. Additional strategies are required to improve EF outcomes after RP.
Supplementary Material
Despite the widespread use of robotic surgery and advances in surgical technique and patient postoperative management, we did not observe an improvement in the rate of post-radical prostatectomy (RP) erectile function (EF) recovery over the last decade. These findings encourage research for additional strategies to improve EF after RP.
Acknowledgments:
This work was supported in part by the Sidney Kimmel Center for Prostate and Urologic Cancers, P50-CA92629 SPORE grant from the US National Cancer Institute, and the P30-CA008748 Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center.
Funding/Support and role of the sponsor: None.
Footnotes
Financial disclosures: Paolo Capogrosso certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med 2013;368:436–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Walsh PC. Anatomic radical prostatectomy: evolution of the surgical technique. J Urol 1998;160:2418–24. [DOI] [PubMed] [Google Scholar]
- [3].Salonia A, Adaikan G, Buvat J, et al. Sexual rehabilitation after treatment for prostate cancerpart 1: recommendations from the fourth International Consultation for Sexual Medicine (ICSM 2015). J Sex Med 2017;14:285–96. [DOI] [PubMed] [Google Scholar]
- [4].Montorsi F, Wilson TG, Rosen RC, et al. Best practices in robot-assisted radical prostatectomy: recommendations of the Pasadena Consensus Panel. Eur Urol 2012;62:368–81. [DOI] [PubMed] [Google Scholar]
- [5].Walz J, Epstein JI, Ganzer R, et al. A critical analysis of the current knowledge of surgical anatomy of the prostate related to optimisation of cancer control and preservation of continence and erection in candidates for radical prostatectomy: an update. Eur Urol 2016;70:301–11. [DOI] [PubMed] [Google Scholar]
- [6].Tewari AK, Srivastava A, Huang MW, et al. Anatomical grades of nerve sparing: a riskstratified approach to neural-hammock sparing during robot-assisted radical prostatectomy (RARP). BJU Int 2011;108:984–92. [DOI] [PubMed] [Google Scholar]
- [7].Schatloff O, Chauhan S, Sivaraman A, Kameh D, Palmer KJ, Patel VR. Anatomic grading of nerve sparing during robot-assisted radical prostatectomy. Eur Urol 2012;61:796–802. [DOI] [PubMed] [Google Scholar]
- [8].Mulhall JP, Slovick R, Hotaling J, et al. Erectile dysfunction after radical prostatectomy: hemodynamic profiles and their correlation with the recovery of erectile function. J Urol 2002;167:1371–5. [DOI] [PubMed] [Google Scholar]
- [9].Kowalczyk KJ, Huang AC, Hevelone ND, et al. Stepwise approach for nerve sparing without countertraction during robot-assisted radical prostatectomy: technique and outcomes. Eur Urol 2011;60:536–47. [DOI] [PubMed] [Google Scholar]
- [10].Tewari A, Rao S, Martinez-Salamanca JI, et al. Cancer control and the preservation of neurovascular tissue: how to meet competing goals during robotic radical prostatectomy. BJU Int 2008;101:1013–8. [DOI] [PubMed] [Google Scholar]
- [11].Salonia A, Adaikan G, Buvat J, et al. Sexual rehabilitation after treatment for prostate cancer-part 2: recommendations from the fourth International Consultation for Sexual Medicine (ICSM 2015). J Sex Med 2017;14:297–315. [DOI] [PubMed] [Google Scholar]
- [12].Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol 2009;55:334–47. [DOI] [PubMed] [Google Scholar]
- [13].Vickers AJ, Savage CJ, Shouery M, Eastham JA, Scardino PT, Basch EM. Validation study of a web-based assessment of functional recovery after radical prostatectomy. Health Qual Life Outcomes. 2010;8:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Terrier JE, Mulhall JP, Nelson CJ. Exploring the optimal erectile function domain score cutoff that defines sexual satisfaction after radical prostatectomy. J Sex Med 2017;14:804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lee DJ, Mallin K, Graves AJ, et al. Recent changes in prostate cancer screening practices and epidemiology. J Urol 2017;198:1230–40. [DOI] [PubMed] [Google Scholar]
- [16].Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol 2012;62:418–30. [DOI] [PubMed] [Google Scholar]
- [17].Menon M, Shrivastava A, Bhandari M, Satyanarayana R, Siva S, Agarwal PK. Vattikuti Institute prostatectomy: technical modifications in 2009. Eur Urol 2009;56:89–96. [DOI] [PubMed] [Google Scholar]
- [18].Donovan JL, Hamdy FC, Lane JA, et al. Patient-reported outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med 2016;375:1425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012;367:203–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Barocas DA, Alvarez J, Resnick MJ, et al. Association between radiation therapy, surgery, or observation for localized prostate cancer and patient-reported outcomes after 3 years. JAMA 2017;317:1126–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hu JC, Hevelone ND, Ferreira MD, et al. Patterns of care for radical prostatectomy in the United States from 2003 to 2005. J Urol 2008;180:1969–74. [DOI] [PubMed] [Google Scholar]
- [22].Jacobs EF, Boris R, Masterson TA. Advances in robotic-assisted radical prostatectomy over time. Prostate Cancer 2013;2013:902686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Teloken P, Mesquita G, Montorsi F, Mulhall PJ. Post-radical prostatectomy pharmacological penile rehabilitation: practice patterns among the international society for sexual medicine practitioners. J Sex Med 2009;6:2032–8. [DOI] [PubMed] [Google Scholar]
- [24].Liu C, Lopez DS, Chen M, Wang R. Penile rehabilitation therapy following radical prostatectomy: a meta-analysis. J Sex Med 2017;14:1496–503. [DOI] [PubMed] [Google Scholar]
- [25].Loft MD, Berg KD, Kjaer A, et al. Temporal trends in clinical and pathological characteristics for men undergoing radical prostatectomy between 1995 and 2013 at Rigshospitalet, Copenhagen, Denmark, and Stanford University Hospital, United States. Clin Genitourin Cancer. In press. doi: 10.1016/j.clgc.2017.08.014 [DOI] [PubMed] [Google Scholar]
- [26].Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol 2010;28:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rabbani F, Stapleton AM, Kattan MW, Wheeler TM. Factors predicting recovery of erections after radical prostatectomy. J Urol 2000;164:1929–34. [PubMed] [Google Scholar]
- [28].Salomon G, Isbarn H, Budaeus L, et al. Importance of baseline potency rate assessment of men diagnosed with clinically localized prostate cancer prior to radical prostatectomy. J Sex Med 2009;6:498–504. [DOI] [PubMed] [Google Scholar]
- [29].Novara G, Ficarra V, D’Elia C, et al. Preoperative criteria to select patients for bilateral nerve-sparing robotic-assisted radical prostatectomy. J Sex Med 2010;7:839–45. [DOI] [PubMed] [Google Scholar]
- [30].Lee JK, Assel M, Thong AE, et al. Unexpected long-term improvements in urinary and erectile function in a large cohort of men with self-reported outcomes following radical prostatectomy. Eur Urol 2015;68:899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.