Abstract
Background:
Congenital Hyperinsulinism (HI) causes severe hypoglycemia in neonates and children. We reviewed our experience with pancreatectomy for the various types of HI.
Methods:
From 1998–2018, 500 patients with HI underwent pancreatectomy: 246 for focal HI, 202 for diffuse HI, 37 for atypical HI (16 for Localized Islet Nuclear Enlargement [LINE], 21 for Beckwith-Wiedemann Syndrome), and 15 for insulinoma. Focal HI neonates were treated with partial pancreatectomy. Patients with diffuse HI who failed medical management underwent near-total (98%) pancreatectomy. Atypical HI patients had pancreatectomies tailored to the PET scan and biopsy findings.
Results:
The vast majority of pancreatectomies for focal HI were <50%, and many were 2–10%. 97% of focal HI patients are cured. For diffuse disease patients, 31% were euglycemic, 20% were hyperglycemic, and 49% required treatment for hypoglycemia; the incidence of diabetes increased with long-term follow-up. All 15 insulinoma patients were cured.
Conclusions:
Our approach to patients with focal HI can distinguish focal from diffuse HI, localize focal lesions, and permit partial pancreatectomy with cure in almost all focal patients. Surgery does not cure diffuse disease but can help prevent severe hypoglycemia and brain damage. Surgery can be curative for insulinoma and for some cases of atypical HI.
Keywords: Congenital hyperinsulinism, Pancreatectomy, Localized Islet Nuclear Enlargement, Beckwith-Wiedemann Syndrome, Insulinoma, Multiple Endocrine Neoplasia-type 1 (MEN1)
Level of Evidence: Level IV
Congenital hyperinsulinism (HI) is the most common cause of persistent hypoglycemia in neonates and can lead to irreversible brain damage (1). Inappropriate oversecretion of insulin is the hallmark of HI. Neonates with HI may have diffuse involvement of the pancreatic β-cells (diffuse HI) or focal adenomatous islet cell hyperplasia (focal HI). The focal and diffuse forms of HI are predominantly caused by inactivating mutations of ABCC8 or KCNJ11, the two genes that encode the β-cell ATP-dependent potassium channel (KATP). Biallelic recessive, or less commonly, dominant mutations cause diffuse HI, whereas loss of heterozygosity together with inheritance of a paternal recessive mutation cause focal HI (2–4). These two forms of HI are clinically identical. Patients with diffuse disease on this genetic basis often require near-total pancreatectomy, which has the long-term risk of diabetes mellitus (5–7). Conversely, babies with focal disease can be cured with a selective partial pancreatectomy with little risk of subsequent diabetes (8,9). The 18-fluoroDOPA PET/CT imaging study can help localize focal lesions and permit partial pancreatectomy with cure in almost all focal HI patients (10–14). In contrast, surgery is not usually necessary in other genetic forms of HI that result from mutations of glutamate dehydrogenase or other genes that are responsive to diazoxide treatment (1).
In addition to the focal and diffuse forms of HI, there are rare cases that we categorize as atypical HI. Among those are patients with features of diffuse HI restricted to a region of the pancreas which we call Localized Islet Nuclear Enlargement (LINE)(5), and unusual cases of Beckwith-Wiedemann Syndrome (BWS) and HI that show dramatic pancreatic endocrine hyperplasia (15,16). Babies with severe HI associated with LINE or BWS can be treated with pancreatectomy, the extent of which is guided by the PET scan, the intraoperative pancreatic biopsy findings, and the disease severity.
Insulinomas are very rare in children and have not been reported before 2 years of age. Although most insulinomas are solitary and sporadic, 10–20% occur in the setting of Multiple Endocrine Neoplasia-type 1 (MEN-1) in which multiple tumors may be present. Insulinomas can be successfully treated by insulinoma enucleation or partial pancreatectomy (17,18).
We reviewed our experience with a multidisciplinary approach (pediatric endocrinology, genetics, radiology, pathology, and surgery) to diagnose and treat appropriately selected patients with the various forms of HI by pancreatectomy with a focus on surgical technical details and patient outcomes.
1. Methods
1.1. Study Population
Between October 1998 and April 2018, 500 patients underwent pancreatectectomy to treat HI in its various forms (focal HI, diffuse HI, atypical HI, and insulinoma) at the Children’s Hospital of Philadelphia (CHOP). The surgical technical details and patient outcomes were analyzed. This retrospective review was approved by the CHOP Institutional Review Board.
1.2. Diagnosis and Medical Management by Pediatric Endocrinology
The diagnosis of congenital HI was established if fasting hypoglycemia (glucose <50 mg/dL) occurred simultaneously with an inappropriately detectable plasma insulin (>2.0 μU/mL), low plasma beta-hydroxybutyrate (<1.8 mmol/L) and free fatty acids (<1.7 mmol/L), and an inappropriate glycemic response to intravenous glucagon (≥30 mg/dL rise in serum glucose level) (19). Medical therapy to maintain euglycemia was standardized and involved high continuous intravenous infusions of glucose as measured by the Glucose Infusion Rate (which is the amount of glucose infused in mg/kg/minute), frequent oral feedings, and administration of diazoxide, glucagon, and octreotide as appropriate. Genetic testing is crucial (20). For those patients who fail medical therapy or likely had the focal form of HI as defined by genetics and imaging studies, surgery was performed. A summary of the diagnostic tests and imaging studies for older children with insulinoma has been previously described by our group (17).
1.3. Imaging Studies Performed by Radiology
Conventional non-invasive imaging studies such as ultrasound, computerized tomography and magnetic resonance have been used to try to distinguish between focal and diffuse HI and to localize genetically suspected focal lesions without success (9). Invasive interventional tests (Arterial Stimulation with Venous Sampling [ASVS], and Transhepatic Portal Venous Sampling [TPHVS]) were developed in the late 1980s and were used at CHOP until 2004 (21,22). These imaging studies take several hours to perform, are technically very demanding, and their sensitivity and specificity for distinguishing between focal and diffuse HI are limited (22). They have been replaced by what is now considered the gold-standard imaging study: 18Fluoro-L-3–4 dihydroxyphenylalanine positron emission tomography merged with a low-radiation computerized tomography (18-fluoroDOPA PET/CT). The study was originally developed in the late 1990s for the detection of tumors of neuroendocrine origin in adults, and has been used in HI patients at CHOP since 2004 (10–14). The 18-fluoroDOPA PET/CT is also often helpful in delineating the diseased pancreatic areas in cases of LINE and BWS, and we have found that it is also sensitive in the detection of the extremely rare ectopic focal lesions (23,24).
1.4. Surgical Technique
All operations were approached in a similar manner via a transverse supraumbilical laparotomy (9). If a focal lesion has been localized by PET scan, then that portion of the pancreas is exposed first. If diffuse disease is likely or if a suspected focal lesion has not been localized preoperatively, then the pancreas is completely exposed by an extended Kocher maneuver, entry into the lesser sac, and mobilization of the inferior pancreatic border. The pancreas is inspected under 4X loupe magnification and carefully palpated in an attempt to identify a focal lesion which can be identified in more than two-thirds of focal HI cases in our experience. Focal lesions often have subtle differences in appearance (ranging from a slightly reddish color to a marble-like appearance) and/or texture compared to normal tissue (focal lesions are often slightly firmer). If no focal lesion is identified, 2–3mm biopsies are taken sharply with tenotomy scissors from the pancreatic head, body, and tail for intraoperative frozen section analysis. The use of cautery for either biopsy or focal resection is avoided because cautery causes artifact that affects the interpretation of the pathology and the surgical margins.
Patients with intraoperative confirmation of diffuse HI undergo near-total (98%) pancreatectomy. A near-total pancreatectomy involves the resection of the entire pancreas leaving only a tiny residual piece of pancreatic tissue between the common bile duct (CBD) and the duodenal wall. The splenic vein and artery are skeletonized and preserved. The intrapancreatic segment of the CBD is identified and skeletonized for a true near-total pancreatectomy to be performed. To help with the identification and dissection of the CBD within the pancreatic head, a vessel loop or two are placed around the extrapancreatic section of the CBD posterior to the duodenum and then swung within the duodenal C-loop. At the completion of this dissection, the gallbladder is milked to ensure that there is no bile leak from the CBD. CBD complications (intraoperative injury or postoperative stricture) have been reported to occur in up to 16% of pancreatectomies involving the pancreatic head (25, 26). We manage an injury to the common bile duct with a choledochoduodenostomy in which a 4Fr 2-cm-long silastic stent is placed across the biliary-duodenal anastomosis and secured with a single absorbable 6–0 stitch, which permits the stent to pass through the gastrointestinal tract weeks later once the absorbable retaining suture dissolves. In babies with diffuse disease, a gastrostomy tube is also placed for possible long-term enteral access if needed.
When the intraoperative biopsies demonstrate normal pancreatic histology, a further search for the focal lesion is conducted. The preoperative PET/CT study greatly facilitates the search. Intraoperative high-resolution ultrasound can sometimes help in localizing a focal lesion particularly if the lesion has a pseudocapsule, but most focal lesions have similar echogenicity to normal pancreas. We routinely arrange for intraoperative ultrasound if the genetics suggest a focal lesion but the PET/CT does not show a focal lesion which is true for 15% of our focal lesion cases (14). Ultrasound helps delineate the course of the pancreatic duct which is only 0.30.4 mm in diameter. Focal lesions that are buried within the pancreatic tissue can be impossible to see or feel, so it is necessary to patiently take additional biopsies of suspicious areas for frozen section analysis until the lesion is found.
Focal lesions are usually 10 mm or less in diameter, but can be much larger. They are irregularly shaped and frequently have octopus-like tentacles, which makes the intraoperative frozen section confirmation of clear margins imperative. Once the focal lesion is identified, a partial pancreatectomy is performed and free-of-disease surgical margins are confirmed before concluding the surgery. Small and superficial lesions in the body or tail are treated by simple resection using tenotomy scissors, not cautery. Deep periductal lesions in the body and tail are treated by distal pancreatectomy. Intraoperative ultrasound can identify the tiny pancreatic duct and facilitate operative planning since injury to the pancreatic duct should be avoided.
Superficial and small lesions in the head of the pancreas can also be treated by simple resection. On the other hand, deep lesions of the pancreatic head can be tricky to excise with clear margins without causing damage to the CBD and pancreatic duct. To ensure a complete resection of the lesion in these challenging cases, almost all of the pancreatic head is removed while preserving duodenal blood supply, and a Roux-en-Y pancreaticojejunostomy is constructed to drain the remaining pancreatic body and tail, thereby preserving the endocrine and exocrine functions of the pancreas. In our experience, this approach has been required in about 30% of focal lesions located in the pancreatic head (27). The end of a retrocolic, 25 cm long Roux-en-Y jejunal limb is meticulously anastomosed to the capsule of the pancreatic body (just beyond the cut surface of the pancreas) with fine interrupted monofilament suture to tuck the cut end of the pancreas into the jejunal lumen. The omentum is then wrapped around the anastomosis to help seal potential leaks. In cases of near-total and pancreatic head resections it is crucial to preserve the gastroduodenal artery as well as the vessels supplying the third and fourth portion of the duodenum (superior/inferior posterior/anterior pancreaticoduodenal arteries) if possible to avoid duodenal ischemia. Rarely, a focal lesion in the head will extend into the duodenal wall in which case a Whipple procedure may be needed. Drains are not used since pancreatic fistulae have not been a problem with neonatal pancreatectomy.
Laparoscopic surgery can be used in HI patients with focal disease of the pancreatic body or tail. The surgery is done via three or four 3–5 mm ports and to facilitate pancreatic exposure the stomach is tacked up to the anterior abdominal wall with 2–3 transabdominal-transgastric stitches near the greater curvature. However, a major drawback to the laparoscopic approach is the limited tactile feedback to help palpate a non-visible focal lesion, and the inability to apply an ultrasound probe directly on the pancreas to identify the pancreatic duct.
Patients with atypical forms of HI such as LINE and BWS are clinically heterogeneous and require a medical and surgical treatment plan that is individually crafted according to the severity of disease. For those patients with atypical HI who require surgery, intraoperative frozen sections are crucial to determining the extent of pancreatectomy (28). BWS patients undergo genetic analysis of both the pancreatectomy specimen and the skin biopsies harvested from each side of the laparotomy wound (16).
Insulinoma patients undergo laparotomy once a variety of imaging studies localize the lesion, and are treated with either enucleation of the insulinoma, or partial pancreatectomy if the insulinoma is close to the pancreatic duct as identified by intraoperative ultrasound. Patients who have Multiple Endocrine Neoplasia type 1 (MEN1) had intraoperative ultrasound examination of the entire pancreas to search for additional neuroendocrine tumors.
1.5. Histopathology
Expert pediatric anatomic pathology interpretation is essential. The biopsies are placed in freezing embedding medium (Cryomatrix, Thermo Shandon, Pittsburgh, PA) and snap frozen. Cryostat sections are fixed in methanol and stained with Hematoxylin and Eosin. After the surgery, all frozen samples are processed for routine histology and confirmation of findings based on paraffin-embedded sections.
The histologic criteria of Rahier (29) were used for the diagnosis of the focal lesion. Briefly, a focal lesion is characterized by a tumor-like proliferation of islet cells that push exocrine elements aside or haphazardly incorporate them (30,31). Unlike insulinomas, the focal lesion retains the lobular architecture of the normal pancreas, and exocrine elements usually remain within the lesion. The lesions often have irregular borders and the endocrine cells frequently have enlarged nuclei. Islets outside the lesion appear normal. Insulinomas also differ from focal lesions because they are usually straightforward to identify intraoperatively and occur in older children. Patients with diffuse disease have abnormal islets containing 5–10% of cells with enlarged (3 times or greater than normal size) nuclei present throughout the pancreas (30), whereas patients with LINE have the same histologic abnormality confined to a portion of pancreas (28). Patients with BWS show a remarkable increase in endocrine tissue relative to the amount of exocrine tissue, with variable degrees of loss of the normal lobular architecture of the pancreatic parenchyma (15,16).
Histologically, insulinomas are characterized by a monotonous proliferation of small to medium-sized insulin-staining cells with abundant pale to eosinophilic cytoplasm and round to oval nuclei with minimal nuclear pleomorphism. Tumors are usually well-circumscribed and at least partially, if not fully, encapsulated (18).
1.6. Postoperative Management and Follow-up
Postoperative pain after neonatal pancreatectomy is managed by an epidural catheter or intravenous narcotics. Patients are kept NPO until bowel function resumes. The intravenous glucose infusion is re-started immediately after the operation at a very low GIR (2 mg/kg/min) in part because the stress of the surgery induces hepatic glycogenolysis. The GIR is advanced to 5 mg/kg/min 12–18 hours after the surgery and to 8 mg/kg/min (equivalent to the physiological hepatic glucose release during fasting periods) 24–36 hours after the surgery. Plasma glucose levels are measured hourly in the beginning and spaced out as they become stable. If the plasma glucose levels are persistently high (>250 mg/dl for 6–8 hours) an intravenous insulin infusion is started. The immediate postoperative oscillations in the plasma glucose levels are not reflective of the eventual long-term outcome, because factors like surgical stress and pain can alter glucose homeostasis. When bowel function is evident, enteral feedings are started and advanced gradually, and simultaneously the intravenous GIR is gradually weaned off. When patients are exclusively on enteral feeds, a “cure” fasting test is performed. If patients are able to maintain euglycemia for 18 hours and/or demonstrate appropriate ketogenesis (betahydroxybutyrate > 2 mmol/L), they are considered completely cured. If the time to hypoglycemia is less than 18 hours, the next step is to determine a regimen of frequent feeds and short fasting periods that will allow the patient to be managed safely at home. Patients who cannot be weaned from the intravenous GIR are obviously not cured and will need further assessment to determine if additional surgery is required. After hospital discharge, a complete response at follow-up is defined as no requirement for glycemic medications, no continuous tube feedings, and no diabetes mellitus.
2. Results
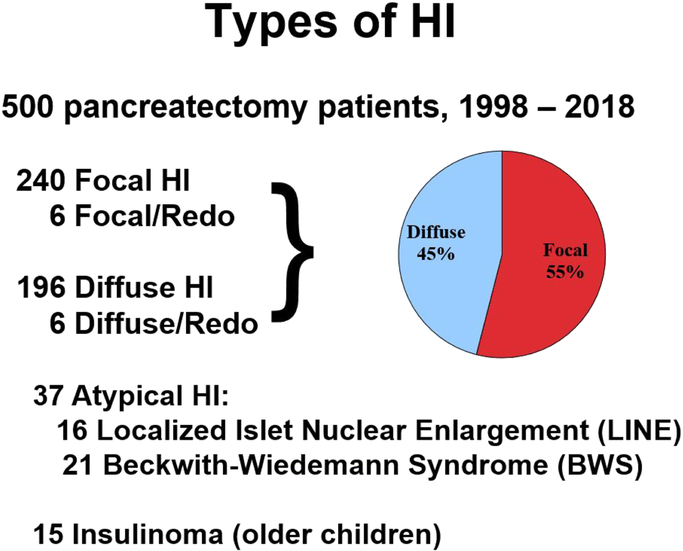
During the two decade study period, 500 neonates and children underwent pancreatectomy at CHOP: 246 for focal HI, 202 for diffuse HI, 37 for atypical HI (16 for LINE, 21 for BWS), and 15 for insulinoma (Figure 1).
Figure 1.
Types of Hyperinsulinism.
The percentages of focal vs diffuse HI cases in this series were 55% and 45%, respectively.“Redo” cases had an initial pancreatectomy performed at another institution.
2.1. Focal Hyperinsulinism
There were 246 focal HI patients treated by partial pancreatectomy, including our previously reported 38 patients treated between 1998 and 2003 (9), which was prior to the use of the 18-fluoroDOPA PET/CT scan imaging which began at CHOP in 2004. There were 124 girls and 122 boys, and the age of operation ranged from one week to 14 months, with the median age of 7 weeks. Virtually all of the pancreatectomies for focal HI were less than 50% (range 2% - 98%), and many were local excisions of 2–10%. 55% of patients had involvement of the pancreatic head or neck with the focal lesion. Forty lesions required pancreatic head resection with Roux-en-Y pancreaticojejunostomy (including two Whipple procedures) to preserve the normal body and tail; 23 of these patients were previously reported (27). Laparoscopic surgery was performed in five patients with lesions in the pancreatic body or tail. One incomplete laparoscopic resection required reexploration and a curative distal pancreatectomy using an open approach, and the other four patients had laparoscopic distal pancreatectomies with cure.
97% of focal HI patients had a complete response to surgery and are cured. Nine patients required an additional resection for residual disease, 3 of these patients had their initial surgery at other hospitals, and 8 of nine patients are cured. Seven patients have required glycemic medications, six of whom had pancreatectomies early in our experience, all had disease in the pancreatic head, and early on we were reluctant to do a pancreatic head resection and Roux-en-Y pancreaticojejunostomy, which led to inadequate resection. There was one injury to the common bile duct during a pancreatic head resection treated with choledochoduodenostomy. Due to our splenic vessel-sparing pancreatectomy approach, the spleen was preserved in all cases. There were eight cases of post-operative small bowel obstruction requiring laparotomy, five for reduction of small bowel-to-small bowel intussusception (32) and three for lysis of adhesions. There were no pancreatic fistulae.
Only one HI focal patient is diabetic excluding two referrals for additional resection after a 95% pancreatectomy had been performed at another hospital; in those two patients, the focal lesion was present in the residual pancreatic head tissue, and the lesion was resected. One of these patients had additional focal lesions in the small intestine (23). A third referral patient after 95% pancreatectomy in 1999 has persistent hypoglycemia requiring medical therapy despite two additional operations at CHOP leading to a complete pancreatectomy with choledochoduodenostomy; we suspect that there is additional unresected ectopic focal lesion within the duodenal wall (24).
2.2. Diffuse Hyperinsulinism
There were 202 diffuse HI patients, 102 girls and 100 boys. The age of operation ranged from one week to 26 months, with the median age of 6 weeks. 189 diffuse HI patients who failed medical management underwent biopsies to confirm the diagnosis then near-total (98%) pancreatectomy, including five patients who were referred after subtotal pancreatectomy (two were performed laparoscopically) at other hospitals but the patients had severe postoperative hypoglycemia necessitating a near-total pancreatectomy at CHOP. Thirteen patients were explored for possible focal disease, but biopsies showed diffuse disease which could be managed medically, so only a gastrostomy was placed. For diffuse disease patients at the time of hospital discharge, near-total pancreatectomy resulted in 31% having well-controlled plasma glucoses, 20% were hyperglycemic, and 49% required treatment for persistent hypoglycemia such as supportive management with frequent or continuous feedings or continuous intragastric dextrose. The incidence of diabetes has increased with long-term follow-up. A previous report from our group showed that 47% of patients who were 10 – 20 years of age at follow-up had diabetes (5).
Complications included three patients who required completion pancreatectomy due to persistent severe postoperative hypoglycemia, three CBD injuries that required choledochoduodenostomy, and one late CBD stricture that required a Roux-en-Y choledochojejunostomy. There was one distal duodenal resection due to ischemia, one interventional radiology drainage of an intraabdominal collection that was compressing the duodenal C-loop, and four cases of small bowel obstruction requiring laparotomy, three for reduction of small bowel-to-small bowel intussusception (32) and one for lysis of adhesions. With our splenic vessel-sparing pancreatectomy approach, no splenectomies were required. There were no pancreatic fistulae.
All complications after pancreatectomy for focal and diffuse disease occurred during the initial hospitalization except for the CBD stricture and the four cases of adhesive intestinal obstruction, all of which happened within the first postoperative year.
2.3. Atypical Hyperinsulinism (LINE and BWS)
There were 16 LINE patients. None were diagnosed with LINE preoperatively, molecular genetic testing in peripheral blood was negative in all patients (no KATP or other mutations were identified), and all patients were unresponsive to diazoxide treatment. Compared to focal and diffuse HI patients, the clinical presentation was much later in infancy with a median age of four months. The outcomes were promising after pancreatectomy (range 15 – 95%): ten patients passed a cure fast, and six are well-controlled with supplemental medical therapy.
There were 21 BWS patients who underwent pancreatectomy (range 2 – 99%). Patients displayed a wide range of clinical features from classical BWS to only mild hemihypertrophy (11p overgrowth spectrum). PET scan findings such as increased uptake and bulbar enlargement of a portion of the pancreas combined with intraoperative pancreatic biopsies showing endocrine hyperplasia guided the extent of pancreatectomy. We have previously reported our surgical approach to the rare BWS patients who require pancreatectomy, and have shown that pancreatectomy can enhance HI management of these patients (15,16).
2.4. Insulinoma
There were 15 insulinoma patients, and the first 8 insulinoma patients have been reported previously (17). There were 11 boys and 4 girls. The age at operation ranged from 4 to 26 years, with a median age of 11 years. The insulinoma was successfully localized preoperatively in 87% (13/15) of cases after multiple imaging techniques were applied. Abdominal MRI localized the insulinoma in 7/13 patients (54% successful), but abdominal ultrasound (20%), endoscopic ultrasound (22%), CT scan (31%), and octreotide scan (0%) were much less successful. If all of these imaging modalities failed to localize the insulinoma, then 18-fluoroDOPA PET/CT (67% successful), ASVS (50%) or THPVS (25%) were used. All lesions were excised in their entirety either by enucleation (eleven cases) or distal pancreatectomy (four cases). Complications included 3 pancreatic fistulae all of which were initially managed by ERCP and stent placement, but 2 patients eventually required a second more extensive distal pancreatectomy. All 15 patients passed a cure fast and are asymptomatic on follow-up. The three MEN1 patients require lifelong follow-up.
3. Discussion
Congenital hyperinsulinism is a rare derangement of glucose metabolism, which carries an estimated incidence of 1 to 1.4 in 50,000 live births, leading to an estimated incidence of about 80 new medically-unresponsive cases in the United States each year (33). Subtotal pancreatectomy for management of persistent infantile hypoglycemia was first performed at CHOP in 1950 (34). In 1999, we reported a 35 year experience with subtotal (<95%) and neartotal (95–98%) pancreatectomy in 53 patients with HI (35). We found that 21% of the patients had histologic evidence of a focal lesion in the pancreatectomy specimen which led us to consider a pancreas-sparing strategy for babies with focal HI. Brunelle and the group from Paris described a method of transhepatic portal and pancreatic venous sampling of insulin to distinguish focal from diffuse HI (21), which permitted limited pancreatectomy that was curative in patients with focal disease and this approach prevented the development of postoperative diabetes (8). We adopted the pancreas-sparing strategy promulgated by the Paris group, and modified it by developing two additional preoperative diagnostic methods in the infants – acute insulin response testing (36) and an angiographic localization procedure using selective arterial calcium injection (ASVS) (37). From 1998 until 2003, there were 62 pancreatectomies for HI performed at CHOP and 38 (61%) of these cases proved to be focal HI treated by partial pancreatectomy with a cure rate of 92% (35/38) (9).
Laparoscopic surgery can be used for pancreatectomy but we have found that laparoscopy does not permit tactile feedback to help palpate a non-visible focal lesion, and a suitable ultrasound probe has not been developed to identify the tiny neonatal pancreatic duct. Accordingly, laparoscopic resections for tail or body lesions by necessity often lead to distal pancreatectomy whereas open resections can often remove much less pancreas. The dissection and resection of the pancreatic head is significantly more technically demanding than for the distal pancreas. A high rate of CBD injury has been observed in cases of true laparoscopic neartotal pancreatectomies in which the CBD is identified and dissected laparoscopically. Recent reports claim a lower rate of CBD injuries, but a detailed analysis reveals that in those cases the CBD is neither identified nor dissected and the pancreatectomy ends just beyond the superior mesenteric vessels, which means that those cases are not near-total but rather distal pancreatectomies (38, 39). CBD injury is very rare in our experience. We have had to reoperate on babies with diffuse disease who had an inadequate laparoscopic pancreatectomy performed at other hospitals.
In terms of molecular genetics, there are 11 different genes associated with various forms of HI. Our group has shown that the presence of the monoallelic recessive KATP mutation predicted focal HI with 97% sensitivity and 90% specificity (19). However, nine percent of diffuse HI cases and 2% of focal HI cases lack identified genetic causes, highlighting the importance of continued research to identify new genetic defects. Severe HI in BWS is exclusively a feature of mosaic pUPD11 and is not seen in other BWS patients. Notably, BWS patients who had pUPD11 in conjunction with a paternally transmitted inactivating KATP mutation had the most severe hypoglycemia (16). The clinical recognition of UPD-BWS HI patients is complicated by the fact that physical features of BWS may not be readily apparent since pUPD11 may be present in pancreas, but not be detectable in peripheral tissues, such as blood or skin. LINE likely represents another clinically, histologically, and potentially genetically distinct group of patients with HI. We speculate that these patients may have postzygotic somatic dominant ABCC8 and GCK mutations in β-cells in the abnormal regions. Aneuploidy of chromosome 11 and other chromosomes is common in both MEN1 and nonMEN1 insulinomas. Our novel observation of a paternal parent-of-origin effect in all MEN1 and most non-MEN1 tumors suggests a critical role for imprinted growth-regulatory genes in the 11p region in the genesis of β-cell endocrine tumors in children (17).
We have reported that 47% of patients with surgically treated HI who are 10 – 20 years of age at follow-up have diabetes (5), and all patients who developed diabetes had a ≥ 95% pancreatectomy. In their study of 105 patients with HI who underwent pancreatectomy, Beltrand from the Paris group found that no patients with focal HI required antidiabetic treatment, but 91% of patients with diffuse HI who underwent near-total pancreatectomy required insulin by 14 years of age (6). Arya from the London group reported that the incidence of insulin-dependent diabetes was 96% at 11 years after near-total pancreatectomy for diffuse HI (7). New biologic therapeutic strategies are needed for patients with diffuse HI. Octreotide therapy has the risk of causing necrotizing enterocolitis in neonates (40). Work from our group has shown that the Glucagon-Like-Peptide-1 receptor antagonist exendin-(9–39) elevates blood glucose in congenital hyperinsulinism owing to inactivating mutations in the KATP channel (41). In addition, exendin-(9–39) significantly inhibited amino acid-stimulated insulin secretion in pancreatic islets isolated from neonates with KATP HI. The GLP-1 receptor may be a therapeutic target for the treatment of children with KATP HI. The use of continuous glucose monitoring is a new modality to enhance the management of post-pancreatectomy diabetic patients. CHOP investigators are also leading a pilot study designed to determine if the bihormonal bionic pancreas provides improved blood glucose control, compared to the current standard of care, in individuals with HI who developed diabetes after having a pancreatectomy (42).
We have learned that a multidisciplinary approach involving specialists in pediatric endocrinology, genetics, radiology, pathology and surgery can effectively diagnose and treat cases of congenital HI (Figure 2). Preoperative localization with 18-fluoroDOPA PET/CT facilitates the intraoperative search for focal lesions using loupe-magnification and frozen section analysis of pancreatic biopsies. This approach to patients with focal HI can distinguish focal from diffuse disease, localize focal lesions, and permit partial pancreatectomy with cure in almost all patients. Surgery can be curative for insulinoma and for some cases of atypical HI. Surgery does not cure diffuse disease but can help prevent severe hypoglycemia and brain damage.
Figure 2.
Congenital Hyperinsulinism Paradigm 2018
How this paper will improve care:
A multidisciplinary approach to focal HI patients can localize focal lesions and permit partial pancreatectomy with cure in almost all focal patients. Surgery facilitates management of diffuse disease patients. Surgery can be curative for insulinoma and some atypical HI cases.
Acknowledgments
Funding: NIH R37 DK056268 (C.A.S.), NIH R01 DK098517 (D.D.D.L.), the Goldsmith Foundation, and Congenital Hyperinsulinism International
Footnotes
Conflict of interest: The authors have no conflict of interest to declare.
All authors participated in study design; data acquisition, analysis, and interpretation; and manuscript drafting and critical review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palladino AA, Stanley CA. A specialized team approach to diagnosis and medical versus surgical treatment of infants with congenital hyperinsulinism. Semin Pediatr Surg 2011;20:32–7. [DOI] [PubMed] [Google Scholar]
- 2.Thomas PM, Cote GJ, Wohlk N et al. : Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinism of infancy. Science 1995;268:426–429 [DOI] [PubMed] [Google Scholar]
- 3.Kane C, Shepherd RM, Squires PE, et al. : Loss of functional KATP channels in pancreatic betacells causes persistent hyperinsulinemic hypoglycemia of infancy. Nature Medicine 1996;2:1344–47. [DOI] [PubMed] [Google Scholar]
- 4.Verkarre V, Fournet JC, De Lonlay P, et al. : Paternal mutation of the sulfonylurea receptor (SUR1) gene and maternal loss of 11p15 imprinted genes lead to persistent hyperinsulinism in focal adenomatous hyperplasia. J Clin Invest 98;102:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lord K, Radcliffe J, Gallagher PR, et al. : High risk of diabetes and neurobehavioral deficits in individuals with surgically treated hyperinsulinism. J Clin Endocrinol Metab 2015;100:41334139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beltrand J, Caquard M, Arnoux JB, et al. : Glucose metabolism in 105 children and adolescents after pancreatectomy for congenital hyperinsulinism. Diabetes Care 2012;35:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arya VB, Senniappan S, Demirbilek H, Alam S, et al. : Pediatric endocrine and exocrine function in children following near-total pancreatectomy for diffuse congenital hyperinsulinism. PLoS ONE 2014;9:e98054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cretolle C, Nihoul Fekete C, Jan D, et al. : Partial pancreatectomy is curative in focal form of permanent hyperinsulinemic hypoglycaemia of infancy: A report of 45 cases from 1983 to 2000. J Pediatr Surg 2002;37:155–158. [DOI] [PubMed] [Google Scholar]
- 9.Adzick NS, Thornton PS, Stanley CA, et al. : A multidisciplinary approach to the focal form of congenital hyperinsulinism leads to successful treatment by partial pancreatectomy. J Pediatr Surg 2004;39:270–5. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro MJ, De Lonlay P, Delzescaux T, et al. : Characterization of hyperinsulinism in infancy assessed with PET and 18F-fluoro-L-DOPA. J Nucl Med 2005;46:560–6. [PubMed] [Google Scholar]
- 11.Otonkoski T, Näntö-Salonen K, Seppänen M, et al. : Noninvasive diagnosis of focal hyperinsulinism of infancy with [18F]-DOPA positron emission tomography. Diabetes 2006;55:13–8. [PubMed] [Google Scholar]
- 12.Hardy O, Suchi M, Adzick NS, Alavi A, et al. : Diagnosis and localization of focal congenital hyperinsulinism by 18F-fluorodopa PET scan. J Pediatrics 2007;150:140–145. [DOI] [PubMed] [Google Scholar]
- 13.Hardy OT, Hernandez-Pampaloni M, Saffer JR, et al. : Accuracy of [18F]-fluorodopa PET for diagnosing and localizing focal congenital hyperinsulinism. J Clin Endocrinol Metab 2007;92:4706–4711. [DOI] [PubMed] [Google Scholar]
- 14.Laje P, States LJ, Zhuang H, et al. : Accuracy of PET/CT Scan in the diagnosis of the focal form of congenital hyperinsulinism. J Pediatr Surg. 2013;48:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laje P, Palladino AA, Bhatti TR, et al. : Pancreatic surgery in infants with BeckwithWiedemann Syndrome and hyperinsulinism. J Pediatric Surg 2013;48:2511–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalish JM, Boodhansingh KE, Bhatti TR, et al. : Congenital hyperinsulinism in children with paternal 11p uniparental isodisomy and Beckwith-Wiedemann syndrome. J Medical Genetics 2016;53:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peranteau WP, Palladino AA, Bhatti TR, et al. : The surgical management of insulinomas in children. J Pediatr Surg 2013;48:2517–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatti TR, Ganapathy K, Huppman AR, et al. : Histologic and molecular profile of pediatric insulinomas: Evidence of a paternal parent-of-origin effect. J Clin Endocrinol Metab 2016;101:914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferrara C, Patel P, Becker S, et al. : Biomarkers of insulin for the diagnosis of hyperinsulinemic hypoglycemia in infants and children. J Pediatr 2016;168:212–9. [DOI] [PubMed] [Google Scholar]
- 20.Snider KE, Becker S, Boyajian L, et al. : Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab 2013;98:E355–E363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brunelle F, Negre V, Barth MO, et al. : Pancreatic venous samplings in infants and children with primary hyperinsulinism. Pediatr Radiol 1989;19:100–113. [DOI] [PubMed] [Google Scholar]
- 22.Ferry RJ, Kelly A, Grimberg A, et al. : Calcium-stimulated insulin increase in diffuse and focal forms of congenital hyperinsulinism. J Pediatr 2000;137:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peranteau WH, Bathaii SM, Pawel B, et al. : Multiple ectopic lesions of focal islet adenomatosis identified by positron emission tomography scan in an infant with congenital hyperinsulinism. J Pediatr Surg 2007;42:188–92. [DOI] [PubMed] [Google Scholar]
- 24.Hussain K, Seppänen M, Näntö-Salonen K, et al. : The diagnosis of ectopic focal hyperinsulinism of infancy with [18F]-dopa positron emission tomography. J Clin Endocrinol Metab. 2006;91:2839–42. [DOI] [PubMed] [Google Scholar]
- 25.McAndrew HF, Smith V, Spitz L. Surgical complications of pancreatectomy for persistent hyperinsulinaemic hypoglycaemia of infancy. J Pediatr Surg 2003;38:13–6. [DOI] [PubMed] [Google Scholar]
- 26.Pierro A, Nah SA. Surgical management of congenital hyperinsulinism of infancy. Semin Pediatr Surg. 2011;20:50–3. [DOI] [PubMed] [Google Scholar]
- 27.Laje P, Stanley CA, Palladino AA, et al. Pancreatic head resection and Roux-en-Y pancreaticojejunostomy for the treatment of the focal form of congenital hyperinsulinism. J Pediatr Surg. 2012;47:130–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sempoux C, Capito C, Bellanne-Chantelot C, et al. : Morphological mosaicism of the pancreatic islets: A novel anatomopathological form of persistent hyperinsulemic hypoglycemia of infancy. J Clin Endocrinol Metab 2011;96:3785–3793. [DOI] [PubMed] [Google Scholar]
- 29.Rahier J, Guiot Y, Sempoux C. Morphologic analysis of focal and diffuse forms of congenital hyperinsulinism. Semin Pediatr Surg 2011;20:3–12. [DOI] [PubMed] [Google Scholar]
- 30.Suchi M, MacMullen C, Thornton PS, et al. : Histopathology of congenital hyperinsulinism: retrospective study with genotype correlations. Pediatr Dev Pathol 2003;6:322–33. [DOI] [PubMed] [Google Scholar]
- 31.Suchi M, MacMullen CM, Thornton PS, et al. : Molecular and immunohistochemical analyses of the focal form of congenital hyperinsulinism. Mod Pathol 2006;19:122–9. [DOI] [PubMed] [Google Scholar]
- 32.Laje P, Stanley CA, Adzick NS. Intussusception after pancreatic surgery in children: A case series. J Pediatr Surg 2010;45:1496–9. [DOI] [PubMed] [Google Scholar]
- 33.Glaser B, Blech I, Krakinovsky Y, et al. : ABCC8 mutation allele frequency in the Ashkenzazi Jewish population and risk of focal hyperinsulinemic hypoglycemia. Genet Med 2011;13:891–4. [DOI] [PubMed] [Google Scholar]
- 34.Hamilton JP, Baker L, Kaye R, et al. : Subtotal pancreatectomy in the management of severe persistent idiopathic hypoglycemia in children. Pediatrics 1967;39:49–58. [PubMed] [Google Scholar]
- 35.Lovvorn HN 3rd, Nance ML, Ferry RJ Jr, et al. : Congenital hyperinsulinism and the surgeon: lessons learned over 35 years. J Pediatr Surg 1999;34:786–92. [DOI] [PubMed] [Google Scholar]
- 36.Grimberg A, Ferry RJ, Kelly A, et al. : Dysregulation of insulin secretion in children with congenital hyperinsulinism due to sulfonylurea receptor mutation. Diabetes 2001;50:322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferry RJ, Kelly A, Grimberg A, et al. : Calcium-stimulated insulin increase in diffuse and focal forms of congenital hyperinsulinism. J Pediatr 2000;137:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Shanafey S Laparoscopic vs open pancreatectomy for persistent hyperinsulinemic hypoglycemia of infancy. J Pediatr Surg 2009;44:957–61. [DOI] [PubMed] [Google Scholar]
- 39.Esposito C, De Lagausie P, Escolino M, et al. : Laparoscopic resection of pancreatic tumors in children: Results of a multicentric survey. J Laparoendoscopic Advanced Surgical Techniques 2017;27:533–538. [DOI] [PubMed] [Google Scholar]
- 40.Laje P, Halaby L, Adzick NS, et al. : Necrotizing enterocolitis in neonates receiving octreotide for the management of congenital hyperinsulinism. Pediatric Diabetes 2010;11:142–7. [DOI] [PubMed] [Google Scholar]
- 41.Calabria AC, Li C, Gallagher PR, et al. : The Glucagon-Like-Peptide-1 Receptor Antagonist Exendin-(9–39) elevates blood glucose in congenital hyperinsulinism owing to inactivating mutations in the ATP-sensitive K+ channel. Diabetes 2012:61:2585–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bionic Pancreas in Children with Hyperinsulinism and Post-Pancreatectomy Diabetes. Sponsor: Children’s Hospital of Philadelphia. Principal Investigator: Diva De Leon . ClinicalTrials.gov Identifier: NCT03303196. https://clinicaltrials.gov/ct2/show/NCT03303196