Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP; AdcyaP1) and its cognate PAC1 receptor (Adcyap1r1), have tissue-specific distributions in the lower urinary tract (LUT). The afferent limb of the micturition reflex is often compromised following bladder injury, disease and inflammatory conditions. We have previously demonstrated that PACAP signaling contributes to increased voiding frequency and decreased bladder capacity with cystitis. Thus, the present studies investigated the sensory components (e.g., urothelial cells, bladder afferent nerves) of the urinary bladder that may underlie the pathophysiology of aberrant PACAP activation. We utilized bladder-pelvic nerve preparations and urothelial sheet preparations to characterize PACAP-induced bladder afferent nerve discharge with distention and PACAP- induced Ca2+ activity, respectively. We determined that PACAP38 (100 nM) significantly (p ≤ 0.01) increased bladder afferent nerve activity with distention that was blocked with a PAC1/VPAC2 receptor antagonist PACAP6–38 (300 nM). PACAP38 (100 nM) also increased Ca2+ activity in urothelial cells over that observed in control preparations. Taken together, these results establish a role for PACAP signaling in bladder sensory components (e.g., urothelial cells, bladder afferent nerves) that may ultimately facilitate increased voiding frequency.
Keywords: neuropeptides, urinary bladder distension, nerve activity, micturition
Introduction
The storage and elimination functions of the micturition reflex involve the coordination of the structural features of the urinary bladder and complex neural pathways organized in the central nervous system (CNS) and peripheral nervous system (PNS) (Andersson and Arner,2004; Merrill et al., 2016). The mature micturition reflex is a spinobulbospinal reflex pathway activated by mechanoreceptors in the urinary bladder wall (Beckel and Holstege, 2011). The lower urinary tract (LUT) reflex mechanisms, organized at the level of the lumbosacral spinal cord, are modulated predominantly by supraspinal control (de Groat, 1990; 1993). The switch between the storage and voiding phases of the micturition reflex occurs when mechanoreceptor activity in the urinary bladder exceeds a threshold. As the urinary bladder fills, slowly adapting mechanoreceptors in the bladder wall increase their activity, signaling to initiate elimination through activation of sensory afferents (e.g., Αδ and C-fibers) (Fowler et al., 2008).
Bladder afferents contain a variety of neuroactive compounds including neuropeptides: calcitonin-gene related peptide (CGRP), substance P (Sub P), vasoactive intestinal polypeptide (VIP), pituitary adenylate cyclase-activating polypeptide (PACAP), cholecystokinin and enkephalins (Donovan et al., 1983; De Groat, 1986; Keast and De Groat, 1992; Vizzard et al., 1994; Vizzard, 2000; 2001). All of these neuropeptides, except CGRP, are predominantly expressed in small diameter (presumably C-fiber) afferents in dorsal root ganglia (DRG) (Donovan et al., 1983; De Groat, 1986; Keast and De Groat, 1992; Vizzard et al., 1994; Vizzard, 2000; 2001). PACAP is expressed in peripheral autonomic and sensory neurons and can exert differential downstream effects depending on the receptor subtype expression in the target tissue. PACAP (Adcyap1) and its cognate receptor, PAC1 (Adcyap1r1), have tissue-specific distributions in the LUT (Girard et al., 2017). Dense PACAP expression is present in LUT pathways in the CNS and PNS including expression in the urinary bladder (Braas et al., 2006; Girard et al., 2008; Girard et al., 2016; Gonzalez et al., 2016a; Girard et al., 2017). PACAP- immunoreactivity (IR) and PAC1 receptor-IR is exhibited throughout the urinary bladder in nerve fibers in the urinary bladder smooth muscle, urothelium, suburothelial nerve plexus, DRG, and surrounding blood vessels (Fahrenkrug and Hannibal, 1998a; b; Braas et al., 2006; Girard et al., 2008; Girard et al., 2016; Gonzalez et al., 2016a; Girard et al., 2017).
Increases in PACAP expression in lumbosacral DRG are observed after nerve injury, inflammation, spinal cord injury or bladder inflammation induced by cyclophosphamide (Zhang et al., 1995; Zhang et al., 1996; Larsen et al., 1997; Moller et al., 1997b; Vizzard, 2000). Chronic pathological conditions inducing tissue irritation or inflammation can alter the properties of sensory pathways leading to a reduction in pain threshold (allodynia) and an amplification of painful sensations (hyperalgesia) (Raja et al., 1988). Urinary bladder inflammation can also increase afferent nerve activity to noxious and non-noxious stimuli (Habler et al., 1990; Sengupta and Gebhart, 1994) elicit painful sensations and result in altered urinary bladder function. Previous studies have demonstrated PACAP modulation of ionic conductances that underlie neuronal excitability or PACAP facilitation of spinal reflexes (Xu and Wiesenfeld-Hallin, 1996). Activation of the PACAP-selective PAC1 receptor produces different plasma membrane and endosomal signals that can integrate to produce changes in neuronal excitability (May and Parsons, 2017). In this brief communication, we hypothesized that PACAP signaling contributes to bladder afferent nerve excitability and Ca2+ signaling in the urothelium that may ultimately affect urinary bladder function (e.g., bladder hyperreflexia).
Materials and Methods
Animals and Ethical Approval
Male C57BI/6 mice (4–6 months old) purchased from Jackson Laboratories (Bar Harbor, ME) were housed with littermates and maintained in standard laboratory conditions with food and water available ad libitum. The University of Vermont Institutional Animal Care and Use Committee approved all experimental protocols involving animal use. Animal care was under the supervision of the University of Vermont’s Office of Animal Care Management in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edn). All efforts were made to minimize the potential for animal pain, stress or distress.
Bladder-pelvic nerve electrophysiology
Male C57BI/6J mice (n=12) were euthanized with isoflurane (4–5% in O2) followed by decapitation. The urinary bladder and surrounding tissues were isolated as previously described (Gonzalez et al., 2016b). Briefly, the urinary bladder, urethra, ureters, postganglionic nerves, major pelvic ganglia, and pelvic nerves were excised and transferred to ice-cold HEPES dissection solution consisting of (mM): 134 NaCl, 6 KCI, 10 glucose, 10 HEPES, 1 MgCl2, 2 CaCl2, 10 glucose and adjusted to pH 7.3 with NaOH. The ureters were tied close to the bladder wall and the pelvic nerves were isolated and cleaned of connective tissue before placing the preparation into a recording chamber. All experiments were conducted in physiological saline solution (PSS) (35° C - 37° C) consisting of (mM): 119 NaCI, 4.7 KCI, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 7 glucose and constantly bubbled with Biological Gas (95% O2, 5% CO2) to maintain pH at 7.4. One arm of a triple lumen cannula was inserted through the urethra into the bladder, ligated and the cannula attached to a remote-controlled syringe pump and pressure transducer to monitor intravesical pressure. The remaining arm of the cannula was used to empty the bladder. One of the pelvic nerves was attached to a suction electrode to record distention-evoked afferent nerve activity.
The bladder was filled with PSS at a rate of 30 μl/min up to 26 mmHg and then manually emptied. There was a 10 min rest period between the emptying phase and the start of the next filling phase. For the co-administration studies, bladders were first pretreated with the PAC1 receptor antagonist followed by PACAP38. Administration of drugs began once bladder afferent nerve activity to the vehicle plateaued for two consecutive filling phases (usually after 5–6 filling cycles). Bladder afferent nerve activity was collected and amplified with a Neurolog head stage (NL104, Digitimer), filtered (band pass 200–4000 Hz) using a Digitimer NL125/NI126 filter and digitized with a Power 1401 analog to digital interface (Cambridge Electronic Design, Cambridge, UK). The acquisition rates for nerve activity and bladder pressure were 25,000 Hz and 100 Hz, respectively. Data were analyzed offline via Spike 2 software (version 5.11, Cambridge Electronic Design, Cambridge, UK).
Analysis of afferent nerve activity
The threshold for action potential detection was set at twice the root mean square of the recorded signal in the absence of action potentials. Baseline afferent activity was measured by binning afferent activity in 1-s bins and taking the average of the lowest bins of afferent activity within 10 s of either side of the selected pressure. A two-way repeated measures ANOVA followed by Sidak’s multiple comparisons test was then performed to compare these frequency group means. Percentage of control PACAP38 baseline afferent nerve activity was graphed but statistical analyses were performed on the mean data as described above. Linear regression analyses of control and PACAP38 afferent frequency were performed to determine the relationship between low pressure (< 8 mm Hg) distention, high pressure (≥ 8 mm Hg) distention and afferent nerve activity (Hz). We performed linear analyses around a bladder distention pressure of 8 mm Hg because PACAP38 significantly increased baseline afferent nerve activity at ≥ 8 mm Hg.
Urothelial sheet preparation and Ca2+ imaging
Urinary bladders were removed from adult male mice (n = 5) and placed in cold HEPES solution. The urothelium was removed from the detrusor and carefully cleaned of lamina propria with sharpened forceps until all visible lamina propria was removed. The urothelial sheets were loaded for 90 minutes (37° C) with the Ca2+ sensitive fluorescent dye, Cal 520 (AAT Bioquest, Inc.,) + pluronic acid (2.5 mg/ml) in HEPES solution and placed in a specialized chamber for imaging. The urothelium was superfused with PSS and visualized with a 60X water immersion (NA 1.2) fluorescent objective. Images were collected with a Noran Oz laser scanning confocal microscope at a rate of 16 images/s for 88–125 s. Cal 520 was excited at 488 nm, and the emitted fluorescence collected at >500 nm. Imaging fields were 133 × 133 pm (512 × 512 pixels). Ca2+ events were initially visualized offline using software developed in our laboratory by Dr. Adrian Bonev.
Ca2+ event analysis
Detailed analysis of Ca2+ events in urothelial cells was made using custom-written software (Volumetry G9: Grant Hennig) as previously described (Heppner et al., 2017). To localize cells that had resolvable Ca2+ activity, movies were differentiated (Δt = ± 1.6s), then frame averaging (± 0.19 s) and Gaussian smoothing (3×3 pixels SD = 1.0 [0.8 × 0.8 μm]) were applied. Particles less than 30 pixels (2μm2) in total area were filtered out and calcium transient particle (PTCL) files were created. Ca2+ transient PTCL areas were integrated to show the degree of activity in active cells both spatially and temporally (see Fig. 3).
Figure 3: Integrated Ca2+ activity in urothelial cells before and after the addition of PACAP38 (100 nM) during a continuous recording.
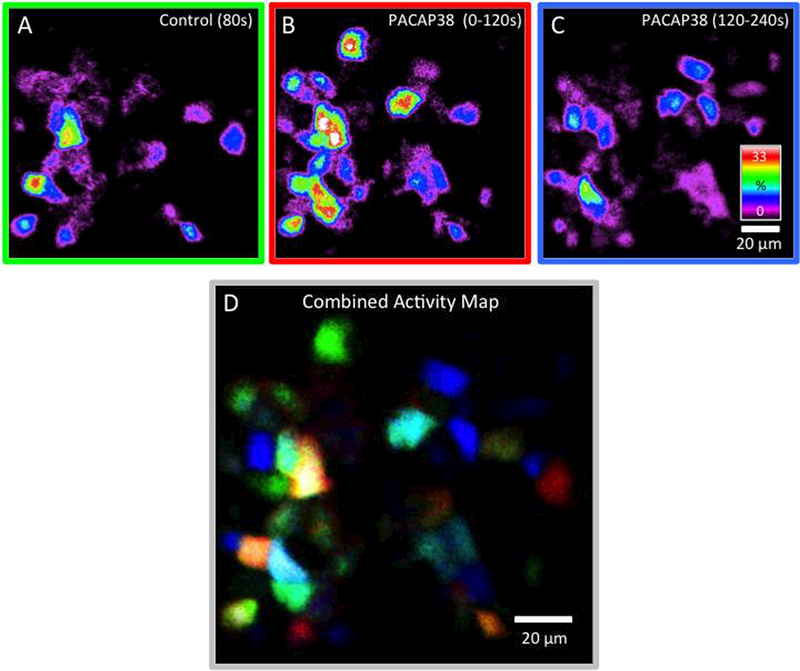
A) Integrated Ca2+ activity in urothelial cells during the first 80s under control conditions (no drugs) reveals Ca2+ activity in approximately half of the urothelial cells. B) After the addition of PACAP38 (100 nM), a substantial number of previously quiescent cells became active (0–120s). C) The response to PACAP38 waned over time (120–240s). D) To visualize the populations of cells that were active during control conditions and after the addition of PACAP38, each of the top 3 panels was converted to grayscale and assigned a color channel (A = red, B = green & C = blue) and combined to create an RGB image. Calibration bar in C and D represents 20 μm. Integrated Ca2+ activity is presented on a spectrum scale with white representing Ca2+ transients in cells that were active for the equivalent of 1/3rd of duration of the movie segment. Chi-square analyses demonstrated a statistical difference (p ≤ 0.001) in the incidence of quiescent cells increasing activity after PACAP38 addition and of cells that ceased activity after PACAP38 addition in urothelial preparations.
Figure preparation
Images were imported into Photoshop 7.0 (Adobe Systems, San Jose, CA) or PowerPoint (Microsoft PowerPoint for Mac 2011, Version 14.7.3, Microsoft Corporation) where groups of images were assembled and labeled.
Materials
All standard chemicals were obtained from Sigma-Aldrich or Fisher and were either analytical or laboratory grade. PACAP38 and the PAC1 receptor antagonist, PACAP(6–38) were purchased from Bachem, Torrance, CA. Before its use, stock solutions of PACAP38 and PACAP(6–38) were diluted with PSS to a working concentration of 100 nM and 300 nM, respectively.
Statistics
All values represent means ± SEM. For the of events, The Gaussian filter used in Ca2+ analyses required the standard deviation (SD) to set the broadness of the filter. Data were compared on GraphPad Prism (v. 6.07, La Jolla, CA) with one-way or two-way repeated measures ANOVA and Student’s unpaired or paired t test where appropriate. For categorical variables, distribution of frequency of cell populations with different activity patterns in urothelial sheets was compared using the chi-square test. All statistical analyses were 2-tailed, used an a priori alpha level of 0.05 to determine statistical significance, and were performed using SAS statistical software (SAS Institute Inc., Cary, NC).
Results
PACAP38 increased distention-evoked bladder afferent nerve discharge
In ex vivo preparations, slow filling with physiological saline resulted in an increase in bladder pressure consistent with a thin-walled elastic sphere that can be simplified into two relatively linear phases (Fig. 1A); the first phase was gradual during the initial filling (low pressure), but as the bladder became increasingly distended (≥ 8 mm Hg, high pressure), there was a rapid rise to peak bladder pressure (~ 24 mm Hg). Afferent nerve activity, as measured in the same preparation, appeared to follow a similar two-phase pattern. The activity appeared relatively quiescent during the initial filling but became increasingly active, especially upon bladder high pressure distention. Under baseline control conditions, afferent nerve activity during the slower initial low pressure fill to ~ 8 mm Hg increased gradually from 0 to ~ 100 Hz, whereas activity in the rapid high pressure second phase increased rapidly to ~ 190 Hz.
Figure 1: PACAP38 increased distention-evoked bladder afferent nerve discharge.
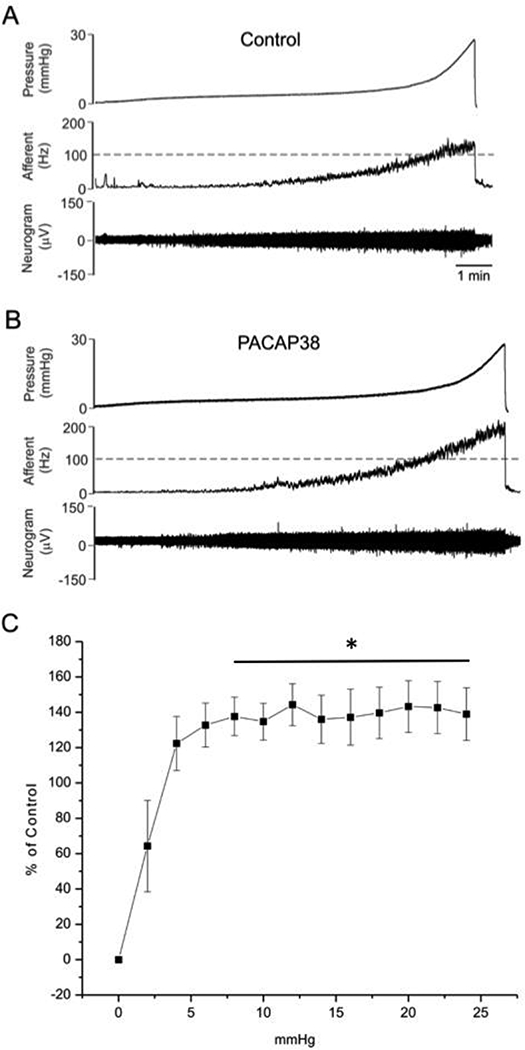
Representative traces of control (vehicle)(A) and PACAP38 (100 nM)(B) instillation from the same preparation. (B) PACAP38 significantly increased mean bladder afferent nerve frequency (impulse/sec) (n=7); values are mean ± SEM. PACAP38 infusions significantly increased nerve firing compared to baseline responses approximately 1.5-fold during the high pressure phase (8 – 24 mmHg) (baseline, 100 – 190 Hz versus PACAP38,150 – 250 Hz, n = 7, p ≤ 0.01)(C). Data are graphed as the PACAP-induced increase in bladder afferent nerve activity over control (% control)(C). Statistical analyses were performed on mean data (mean ± SEM); *, p ≤ 0.01. Group data were compared using a repeated measures ANOVA followed by Sidak’s multiple comparisons test.
The application of PACAP38 had no apparent effects on the pressure profiles during bladder filling compared to vehicle instillations alone (compare pressure profiles, Fig. 1A and 1B). In twelve (n=12) preparations that were evaluated, PACAP38 (100 nM) increased mean baseline afferent nerve frequency in 7 of 12 preparations from 0–24 mm Hg (Fig. 1B, C). Notably, the continuous superfusion of PACAP38 (100 nM) had a significant (p = 0.034) treatment effect on mean bladder afferent nerve frequency (Hz). Whereas nerve activity during baseline and PACAP38 treatment did not appear different in the initial low pressure fill phase, PACAP38 infusions significantly increased nerve firing compared to baseline responses approximately 1.5-fold during the high pressure phase (8 – 24 mmHg) (baseline, 100 – 190 Hz versus PACAP38, 150 – 250 Hz, n = 7, p ≤ 0.01). The changes in activity are expressed as percent increase from control in Fig. 1C. Given that bladder pressure profiles were unchanged with PACAP38 compared to baseline controls (Fig. 2A, C), then an increase in nerve activity as a function of pressure implicated an increase in frequency over the same temporal window. Accordingly, afferent frequency was plotted as a function of time (Fig. 2A-D). Although there was a small apparent increase in afferent frequency from regression analyses during the low pressure phase after PACAP38 treatment, the changes did not appear statistically different (Fig. 2B; red regression line, p = 0.27) in agreement with activity/pressure data above. However PACAP38 (100 nM) superfusions produced a 1.6-fold increase in afferent nerve activity frequency during the high urinary bladder distention phase (Fig. 2D; ≥ 8 mm Hg, blue regression line, p ≤ 0.04; Fig. 2E). The superfusion of the preparation with the PAC1/VPAC2 receptor antagonist PACAP6–38 (300 nM) for 20 minutes followed by PACAP38 (100 nM) addition, blocked the increase in afferent nerve excitability; superfusion of VIP (100 nM) had no effect on bladder afferent nerve activity (284.3 ± 56 to 274.1 ± 44 impulse/s, control and VIP, respectively; data not shown).
Figure 2: Bladder afferent nerve frequency plotted as a function of time with PACAP38 application.
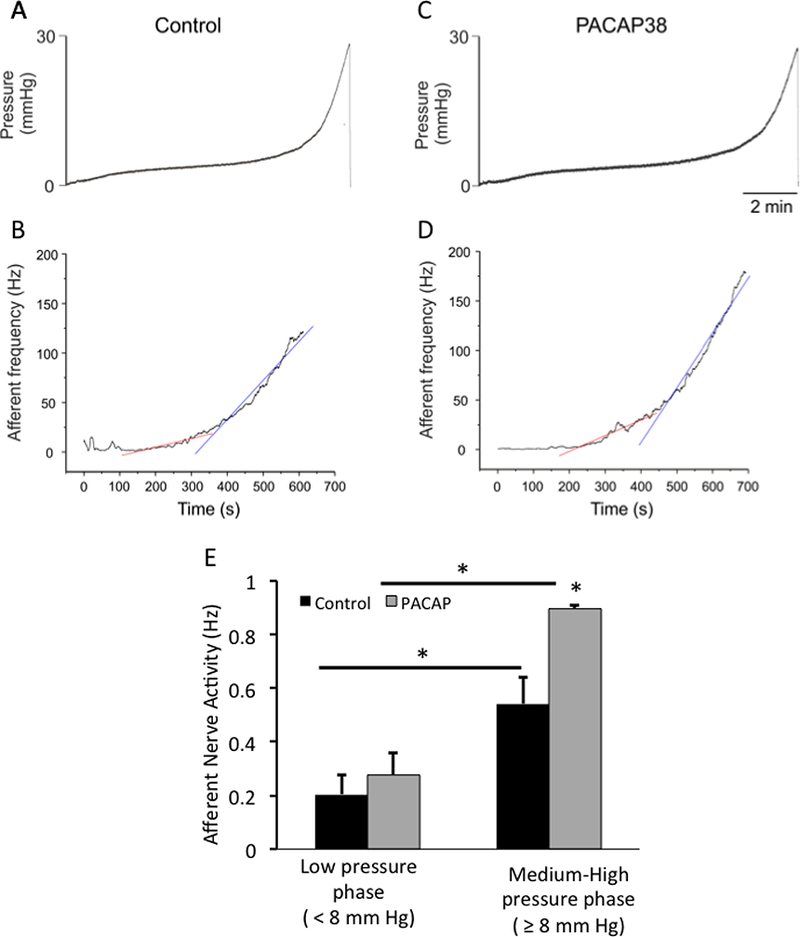
Although there was a small apparent increase in afferent frequency from regression analyses during the low pressure phase after PACAP38 treatment, the changes were not statistically different (B; red regression line, p = 0.27) in agreement with activity/pressure data (see Fig. 1). PACAP38 (100 nM) superfusions produced a 1.6-fold increase in bladder afferent nerve activity frequency during the high urinary bladder distention phase (D; ≥ 8 mm Hg, blue regression line, p ≤ 0.04; E). Group data were compared using ANOVA followed by Sidak’s multiple comparisons test.
PACAP38 effects on Ca2+ events in urothelial cells
Previous studies in rats demonstrated PAC1R-IR in urothelial cells and PACAP38 application to urothelial sheets resulted in increased ATP secretion (Girard et al., 2008). We evaluated the effects of PACAP38 (100 nM) superfusion on Ca2+ activity in cells within urothelial sheets isolated from the murine bladder. Continuous recordings (6 min) of Ca2+ activity in cells in urothelial sheets (n=4 preparations, cells=81, range 11–34 cells per field of view) revealed three subpopulations of urothelial cells with differing Ca2+ responses to PACAP (Fig. 3A-D). The largest subpopulation of urothelial cells (50.52 ± 3.21%) was quiescent during control (no drug) conditions but demonstrated heightened Ca2+ signals upon PACAP38 infusions. The PACAP- mediated Ca2+ responses were maximal within 2 min of PACAP application and diminished to a lower but elevated plateau by 4 – 6 min (Fig. 3A-D). These temporal parameters were comparable to responses seen previously in HEK cells stably expressing PAC1Hop1 receptors (May et al., 2014). Another urothelial cell population (31.08 ± 5.00%) demonstrated basal Ca2+ activity in control (no drug) conditions and was unresponsive to PACAP. The last and smallest population (18.40 ± 5.68%) demonstrated basal Ca2+ activity but became quiescent upon PACAP exposure. The damping effects of PACAP are not understood but may reflect preferential PAC1 receptor conformations in these cells that promote receptor internalization rather than G protein signaling (May and Parsons, 2017). Chi-square analyses demonstrated a statistical difference (p ≤ 0.001) in the incidence of quiescent cells increasing activity after PACAP38 addition and of cells that ceased activity after PACAP38 addition in urothelial preparations assuming a null distribution of cells in three subpopulations.
Discussion
The current studies show that PACAP signaling contributes to bladder afferent nerve hyperexcitability with bladder distention; the PACAP responses were blocked by the PAC1/VPAC2 receptor antagonist, PACAP(6–38) and not stimulated by VIP, suggesting that the effects were mediated by PAC1 receptor signaling. Further PACAP38 also increased Ca2+ activity in a large population of urothelial cells. Hence in aggregate, these studies demonstrate that the sensory components (e.g., bladder afferent nerves, urothelial cells) of the urinary bladder can respond to PACAP38 application and may contribute to micturition reflex function.
PACAP peptides exhibit many diverse functions in endocrine, nervous, gastrointestinal, and cardiovascular systems and the lower urinary tract (LUT) and are expressed in many CNS neurons and sensory and autonomic ganglia (Koves et al., 1990; Arimura et al., 1991; Koves et al., 1991; Ghatei et al., 1993; Masuo et al., 1993; Tatsuno et al., 1994; May and Braas, 1995; Portbury et al., 1995; Shiotani et al., 1995; Braas and May, 1996; Holgert et al., 1996; Klimaschewski et al., 1996; Sundler et al., 1996; Brandenburg et al., 1997; Moller et al.,1997a; Moller et al., 1997b; Nogi et al., 1997; Arimura, 1998; Beaudet et al., 1998; Braas et al., 1998; May et al., 1998; Braas and May, 1999; Beaudet et al., 2000; Cheppudira et al., 2009). PACAP facilitates neuronal calcium influx, induces depolarization of the membrane, activates AC and PLC, and stimulates neurotransmitter secretion (Murase et al., 1993; Tatsuno et al., 1994; May and Braas, 1995; Braas and May, 1996; Beaudet et al., 1998; May et al., 1998; Braas and May, 1999; Beaudet et al., 2000). In the LUT, widespread PACAP-IR has been demonstrated in nerve fibers within the urinary bladder smooth muscle, suburothelial plexus and surrounding blood vessels (Fahrenkrug and Hannibal, 1998a; b; Braas et al., 2006; Girard et al., 2008; Girard et al., 2016; Gonzalez et al., 2016a; Girard et al., 2017). Neonatal capsaicin treatment significantly reduced PACAP suggesting these fibers are derived from sensory neurons (Fahrenkrug and Hannibal, 1998b). These results are consistent with the expression of PACAP in DRG and its neurochemical plasticity following nerve injury or inflammation (Zhang et al., 1995; Zhang et al., 1996; Larsen et al., 1997; Moller et al., 1997a; Vizzard, 2000).
Bladder dysfunction and altered somatic sensation have previously been demonstrated in mice with a genetic disruption or deletion to PACAP or VIP (May and Vizzard, 2010). Both PACAP−/− mice and VIP−/− mice exhibited an increase in bladder mass with hypertrophy specific to the lamina propria and detrusor smooth muscle in PACAP−/− mice or only the detrusor smooth muscle in VIP−/− mice (Jensen et al., 2008; May and Vizzard, 2010). Functionally, PACAP−/− and VIP−/− mice have increased bladder capacity, void volumes, and longer intercontraction intervals (Studeny et al., 2008; May and Vizzard, 2010). Pharmacological studies targeting PACAP/receptor signaling wildtype mice or rats have demonstrated its role in bladder dysfunction with inflammation. Intrathecal (L6-S1) or intravesical administration of a PAC1 receptor antagonist, PACAP(6–38), increased bladder capacity but not intravesical pressure with intermediate (48 hr) CYP-induced cystitis (Braas et al., 2006; Girard et al., 2016). The different routes of administration with similar functional effects suggest PACAP(6–38) may have multiple sites of action. No effects on in vivo bladder function were observed with intrathecal or intravesical administration of PACAP(6–38) in control (no inflammation) conditions suggesting a role(s) for PACAP/PAC1 signaling in micturition reflexes only following inflammation (Braas et al., 2006). However, intravesical PACAP(6–38) effects on bladder afferent nerve activity should be evaluated in the ex vivo bladder-nerve preparation in future studies to complement in vivo studies (Braas et al., 2006). Although the specific site(s) of action of PAC1 receptor antagonist, PACAP(6–38) is unknown, the inhibition of aberrant PACAP signaling may be a promising target to reduce voiding frequency with cystitis.
In the present study, we determined that PACAP38, identified previously as one of the inflammatory mediators of cystitis (Vizzard, 2000; Braas et al., 2006), was able to stimulate an increase in distention-evoked bladder afferent nerve activity. The PACAP38-induced increase in distention-evoked afferent nerve activity was specific to ligand/receptor activation because PACAP38 co-administration with a PAC1/VPAC2 receptor antagonist, PACAP(6–38), attenuated the increase in bladder afferent nerve activity. As described above, many components of the LUT express PACAP or PAC1R (Fahrenkrug and Hannibal, 1998a; b; Braas et al., 2006; Girard et al., 2008; Girard et al., 2016; Gonzalez et al., 2016a; Girard et al., 2017); how PACAP infusions in the current studies activate the afferent fibers remains to be further investigated. PACAP38 may have direct actions on afferent terminals to increase activity following bladder distention. However, PCR transcript analyses suggest that PAC1 receptor expression levels in sensory neurons are relatively low with uncertain functional attributes (Braas et al., 2006). The urothelial cell layer that lines the bladder wall express significant levels of PAC1 receptor transcripts and immunoreactivity (Braas et al., 2006), and hence, the observed increase in bladder afferent nerve activity may represent indirect PACAP/PAC1 receptor-mediated activation via ATP release from the urothelium (Girard et al., 2008). The current studies also demonstrate Ca2+ activity in different populations of cells in urothelial sheet preparations. Approximately fifty-percent of urothelial cells exhibited no Ca2+ activity during control conditions (no drugs) but became active after the addition of PACAP38. Following stimulation, the urothelium can release signaling mediators to produce localized vascular changes (Birder and de Groat, 2007; Fowler et al., 2008) and to influence adjacent tissues and cells, including: detrusor smooth muscle, afferent nerve fibers in the suburothelial nerve plexus, inflammatory cells and interstitial cells within the bladder (Birder and de Groat, 2007; Fowler et al., 2008; Birder and Andersson, 2013; Merrill et al., 2016). Upon stimulation, the urothelium can release ATP to activate purinergic receptors on underlying sensory nerve fibers (Girard et al., 2008). We previously demonstrated that ATP release was evoked by PACAP27, PACAP38 and VIP application to cultured urothelial cells with PACAP27, and that PAC1 receptor antagonism blocked ATP release (Girard et al., 2008). These previous results suggest PACAP and PAC1 signaling may regulate micturition reflex function at the level of the urothelium (Girard et al., 2008). However, the effects of PACAP38 co-administration with a PAC1/VPAC2 receptor antagonist, PACAP(6–38), on Ca2+ activity should be evaluated in urothelial sheet preparations to confirm this finding (Girard et al., 2008). Future studies should examine if PACAP38 signaling contributes to bladder afferent nerve excitability through purinergic mechanisms that may ultimately facilitate increased voiding frequency. The release of signaling molecules from the urothelium can be altered with injury, inflammation and disease (Birder, 2005; Birder and de Groat, 2007; Arms and Vizzard, 2011; Birder and Andersson, 2013; Merrill et al., 2013; Gonzalez et al., 2014a; Gonzalez et al., 2014b; Merrill et al., 2016) and affect overall bladder afferent nerve activity. Thus, a comprehensive understanding of the downstream signaling effectors that interact in the afferent limb of the micturition reflex in control situations and following bladder dysfunction induced by injury, inflammation or disease may provide insights into novel therapeutic approaches.
Acknowledgments
Grants
Research described herein was funded by the National Institutes of Health (NIH) grants to RO1- DK051369 (MAV), RO1-DK060481 (MAV) and R37-DK053832 (MTN). This publication was also made possible by NIH Grants: 5 P30 RR032135 from the COBRE Program of the National Center for Research Resources and 8 P30 GM103498 from the National Institute of General Medical Sciences.
Footnotes
Conflicts of Interest
The authors declare that the research described from the Vizzard laboratory were conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funding entity, NIH, had no role in the studies described including: design, data collection and analysis of studies, decision to publish or preparation of the review. The contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Authors and Contributions
Conceived, discussed and outlined the study: TJH, GWH, VM, MTN, MAV. Performed experiments: TH, GWH. Drafted and revised paper: TJH, GWH, VM, MTN, MAV.
References
- Andersson KE, and Arner A (2004). Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84(3), 935–986. doi: 10.1152/physrev.00038.2003. [DOI] [PubMed] [Google Scholar]
- Arimura A (1998). Perspectives on pituitary adenylate cyclase activating polypeptide (PACAP) in the neuroendocrine, endocrine, and nervous systems. Jpn J Physiol 48(5), 301–331. [DOI] [PubMed] [Google Scholar]
- Arimura A, Somogyvari-Vigh A, Miyata A, Mizuno K, Coy DH, and Kitada C (1991). Tissue distribution of PACAP as determined by RIA: highly abundant in the rat brain and testes. Endocrinology 129(5), 2787–2789. doi: 10.1210/endo-129-5-2787. [DOI] [PubMed] [Google Scholar]
- Arms L, and Vizzard MA (2011). Neuropeptides in lower urinary tract function. Handb Exp Pharmacol (202), 395–423. doi: 10.1007/978-3-642-16499-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudet MM, Braas KM, and May V (1998). Pituitary adenylate cyclase activating polypeptide (PACAP) expression in sympathetic preganglionic projection neurons to the superior cervical ganglion. J Neurobiol 36(3), 325–336. [PubMed] [Google Scholar]
- Beaudet MM, Parsons RL, Braas KM, and May V (2000). Mechanisms mediating pituitary adenylate cyclase-activating polypeptide depolarization of rat sympathetic neurons. J Neurosci 20(19), 7353–7361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckel JM, and Holstege G (2011). Neuroanatomy of the lower urinary tract. Handb Exp Pharmacol (202), 99–116. doi: 10.1007/978-3-642-16499-6_6. [DOI] [PubMed] [Google Scholar]
- Birder L, and Andersson KE (2013). Urothelial signaling. Physiol Rev 93(2), 653–680. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA (2005). More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol 289(3), F489–495. doi: 10.1152/ajprenal.00467.2004. [DOI] [PubMed] [Google Scholar]
- Birder LA, and de Groat WC (2007). Mechanisms of disease: involvement of the urothelium in bladder dysfunction. Nat Clin Pract Urol 4(1), 46–54. doi: 10.1038/ncpuro0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, and May V (1996). Pituitary adenylate cyclase-activating polypeptides, PACAP-38 and PACAP-27, regulation of sympathetic neuron catecholamine, and neuropeptide Y expression through activation of type I PACAP/VIP receptor isoforms. Ann N Y Acad Sci 805, 204–216; discussion 217–208. [DOI] [PubMed] [Google Scholar]
- Braas KM, and May V (1999). Pituitary adenylate cyclase-activating polypeptides directly stimulate sympathetic neuron neuropeptide Y release through PAC(1) receptor isoform activation of specific intracellular signaling pathways. J Biol Chem 274(39), 27702–27710. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Harakall SA, Hardwick JC, and Parsons RL (1998). Pituitary adenylate cyclase-activating polypeptide expression and modulation of neuronal excitability in guinea pig cardiac ganglia. J Neurosci 18(23), 9766–9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, et al. (2006). Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol 290(4), R951–962. doi: 10.1152/ajpregu.00734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandenburg CA, May V, and Braas KM (1997). Identification of endogenous sympathetic neuron pituitary adenylate cyclase-activating polypeptide (PACAP): depolarization regulates production and secretion through induction of multiple propeptide transcripts. J Neurosci 17(11), 4045–4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheppudira BP, Girard BM, Malley SE, Dattilio A, Schutz KC, May V, et al. (2009). Involvement of JAK-STAT signaling/function after cyclophosphamide- induced bladder inflammation in female rats. Am J Physiol Renal Physiol 297(4), F1038–1044. doi: 10.1152/ajprenal.00110.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC (1986). Spinal cord projections and neuropeptides in visceral afferent neurons. Prog Brain Res 67, 165–187. [DOI] [PubMed] [Google Scholar]
- de Groat WC (1990). Central neural control of the lower urinary tract. Ciba Found Symp 151, 27–44; discussion 44–56. [DOI] [PubMed] [Google Scholar]
- de Groat WC (1993). Anatomy and physiology of the lower urinary tract. Urol Clin North Am 20(3), 383–401. [PubMed] [Google Scholar]
- Donovan MK, Winternitz SR, and Wyss JM (1983). An analysis of the sensory innervation of the urinary system in the rat. Brain Res Bull 11(3), 321–324. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, and Hannibal J (1998a). PACAP in visceral afferent nerves supplying the rat digestive and urinary tracts. Ann N Y Acad Sci 865, 542–546. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J, and Hannibal J (1998b). Pituitary adenylate cyclase activating polypeptide immunoreactivity in capsaicin-sensitive nerve fibres supplying the rat urinary tract. Neuroscience 83(4), 1261–1272. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, and de Groat WC (2008). The neural control of micturition. Nat Rev Neurosci 9(6), 453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatei MA, Takahashi K, Suzuki Y, Gardiner J, Jones PM, and Bloom SR (1993). Distribution, molecular characterization of pituitary adenylate cyclase-activating polypeptide and its precursor encoding messenger RNA in human and rat tissues. J Endocrinol 136(1), 159–166. [DOI] [PubMed] [Google Scholar]
- Girard BM, Malley SE, Mathews MM, May V, and Vizzard MA (2016). Intravesical PAC1 Receptor Antagonist, PACAP(6–38), Reduces Urinary Bladder Frequency and Pelvic Sensitivity in NGF-OE Mice. J Mol Neurosci 59(2), 290–299. doi: 10.1007/s12031-016-0764-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Tooke K, and Vizzard MA (2017). PACAP/Receptor System in Urinary Bladder Dysfunction and Pelvic Pain Following Urinary Bladder Inflammation or Stress. Front Syst Neurosci 11, 90. doi: 10.3389/fnsys.2017.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Wolf-Johnston A, Braas KM, Birder LA, May V, and Vizzard MA (2008). PACAP-mediated ATP release from rat urothelium and regulation of PACAP/VIP and receptor mRNA in micturition pathways after cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36(1–3), 310–320. doi: 10.1007/s12031-008-9104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EJ, Arms L, and Vizzard MA (2014a). The role(s) of cytokines/chemokines in urinary bladder inflammation and dysfunction. Biomed Res Int 2014, 120525. doi: 10.1155/2014/120525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EJ, Girard B, Braas KM, May V, and Vizzard MA (2016a). “Neuroplasticity of PACAP Expression and Function in Micturition Reflex Pathways,” in Pituitary Adenylate Cyclase Activating Polypeptide — PACAP, eds. Reglodi D & Tamas A. (Cham: Springer International Publishing; ), 313–334. [Google Scholar]
- Gonzalez EJ, Heppner TJ, Nelson MT, and Vizzard MA (2016b). Purinergic signalling underlies transforming growth factor-beta-mediated bladder afferent nerve hyperexcitability. J Physiol 594(13), 3575–3588. doi: 10.1113/JP272148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EJ, Merrill L, and Vizzard MA (2014b). Bladder sensory physiology: neuroactive compounds and receptors, sensory transducers, and target-derived growth factors as targets to improve function. Am J Physiol Regul Integr Comp Physiol 306(12), R869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habler HJ, Janig W, and Koltzenburg M (1990). Activation of unmyelinated afferent fibres by mechanical stimuli and inflammation of the urinary bladder in the cat. J Physiol 425, 545–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner TJ, Hennig GW, Nelson MT, and Vizzard MA (2017). Rhythmic Calcium Events in the Lamina Propria Network of the Urinary Bladder of Rat Pups. Front Syst Neurosci 11, 87. doi: 10.3389/fnsys.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgert H, Holmberg K, Hannibal J, Fahrenkrug J, Brimijoin S, Hartman BK, et al. (1996). PACAP in the adrenal gland--relationship with choline acetyltransferase, enkephalin and chromaffin cells and effects of immunological sympathectomy. Neuroreport 8(1), 297–301. [DOI] [PubMed] [Google Scholar]
- Jensen DG, Studeny S, May V, Waschek J, and Vizzard MA (2008). Expression of phosphorylated cAMP response element binding protein (p-CREB) in bladder afferent pathways in VIP−/− mice with cyclophosphamide (CYP)-induced cystitis. J Mol Neurosci 36(1–3), 299–309. doi: 10.1007/sl2031-008-9045-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast JR, and De Groat WC (1992). Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol 319(4), 615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Klimaschewski L, Hauser C, and Heym C (1996). PACAP immunoreactivity in the rat superior cervical ganglion in comparison to VIP. Neuroreport 7(15–17), 2797–2801. [DOI] [PubMed] [Google Scholar]
- Koves K, Arimura A, Gorcs TG, and Somogyvari-Vigh A (1991). Comparative distribution of immunoreactive pituitary adenylate cyclase activating polypeptide and vasoactive intestinal polypeptide in rat forebrain. Neuroendocrinology 54(2), 159–169. [DOI] [PubMed] [Google Scholar]
- Koves K, Arimura A, Somogyvari-Vigh A, Vigh S, and Miller J (1990). Immunohistochemical demonstration of a novel hypothalamic peptide, pituitary adenylate cyclase-activating polypeptide, in the ovine hypothalamus. Endocrinology 127(1), 264–271. doi: 10.1210/endo-127-l-264. [DOI] [PubMed] [Google Scholar]
- Larsen JO, Hannibal J, Knudsen SM, and Fahrenkrug J (1997). Expression of pituitary adenylate cyclase-activating polypeptide (PACAP) in the mesencephalic trigeminal nucleus of the rat after transsection of the masseteric nerve. Brain Res Mol Brain Res 46(1–2), 109–117. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Suzuki N, Matsumoto H, Tokito F, Matsumoto Y, Tsuda M, et al. (1993). Regional distribution of pituitary adenylate cyclase activating polypeptide (PACAP) in the rat central nervous system as determined by sandwich-enzyme immunoassay. Brain Res 602(1), 57–63. [DOI] [PubMed] [Google Scholar]
- May V, Beaudet MM, Parsons RL, Hardwick JC, Gauthier EA, Durda JP, et al. (1998). Mechanisms of pituitary adenylate cyclase activating polypeptide (PACAP)-induced depolarization of sympathetic superior cervical ganglion (SCG) neurons. Ann N Y Acad Sci 865, 164–175. [DOI] [PubMed] [Google Scholar]
- May V, and Braas KM (1995). Pituitary adenylate cyclase-activating polypeptide (PACAP) regulation of sympathetic neuron neuropeptide Y and catecholamine expression. J Neurochem 65(3), 978–987. [DOI] [PubMed] [Google Scholar]
- May V, Clason TA, Buttolph TR, Girard BM, and Parsons RL (2014). Calcium influx, but not intracellular calcium release, supports PACAP-mediated ERK activation in HEK PAC1 receptor cells. J Mol Neurosci 54(3), 342–350. doi: 10.1007/s12031-014-0300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V, and Parsons RL (2017). G Protein-Coupled Receptor Endosomal Signaling and Regulation of Neuronal Excitability and Stress Responses: Signaling Options and Lessons From the PAC1 Receptor. J Cell Physiol 232(4), 698–706. doi: 10.1002/jcp.25615. [DOI] [PubMed] [Google Scholar]
- May V, and Vizzard MA (2010). Bladder dysfunction and altered somatic sensitivity in PACAP−/− mice. J Urol 183(2), 772–779. doi: 10.1016/j.juro.2009.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Girard B, Arms L, Guertin P, and Vizzard MA (2013). Neuropeptide/Receptor expression and plasticity in micturition pathways. Curr Pharm Des 19(24), 4411–4422. [DOI] [PubMed] [Google Scholar]
- Merrill L, Gonzalez EJ, Girard BM, and Vizzard MA (2016). Receptors, channels, and signalling in the urothelial sensory system in the bladder. Nat Rev Urol 13(4), 193–204. doi: 10.1038/nrurol.2016.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller K, Reimer M, Ekblad E, Hannibal J, Fahrenkrug J, Kanje M, et al. (1997a). The effects of axotomy and preganglionic denervation on the expression of pituitary adenylate cyclase activating peptide (PACAP), galanin and PACAP type 1 receptors in the rat superior cervical ganglion. Brain Res 775(1–2), 166–182. [DOI] [PubMed] [Google Scholar]
- Moller K, Reimer M, Hannibal J, Fahrenkrug J, Sundler F, and Kanje M (1997b). Pituitary adenylate cyclase-activating peptide (PACAP) and PACAP type 1 receptor expression in regenerating adult mouse and rat superior cervical ganglia in vitro. Brain Res 775(1–2), 156–165. [DOI] [PubMed] [Google Scholar]
- Murase T, Kondo K, Otake K, and Oiso Y (1993). Pituitary adenylate cyclase-activating polypeptide stimulates arginine vasopressin release in conscious rats. Neuroendocrinology 57(6), 1092–1096. [DOI] [PubMed] [Google Scholar]
- Nogi H, Hashimoto H, Hagihara N, Shimada S, Yamamoto K, Matsuda T, et al. (1997). Distribution of mRNAs for pituitary adenylate cyclase-activating polypeptide (PACAP), PACAP receptor, vasoactive intestinal polypeptide (VIP), and VIP receptors in the rat superior cervical ganglion. Neurosci Lett 227(1), 37–40. [DOI] [PubMed] [Google Scholar]
- Portbury AL, McConalogue K, Furness JB, and Young HM (1995). Distribution of pituitary adenylyl cyclase activating peptide (PACAP) immunoreactivity in neurons of the guinea-pig digestive tract and their projections in the ileum and colon. Cell Tissue Res 279(2), 385–392. [DOI] [PubMed] [Google Scholar]
- Raja SN, Meyer RA, and Campbell JN (1988). Peripheral mechanisms of somatic pain. Anesthesiology 68(4), 571–590. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, and Gebhart GF (1994). Mechanosensitive properties of pelvic nerve afferent fibers innervating the urinary bladder of the rat. J Neurophysiol 72(5), 2420–2430. doi: 10.1152/jn.1994.72.5.2420. [DOI] [PubMed] [Google Scholar]
- Shiotani Y, Kimura S, Ohshige Y, Yanaihara C, and Yanaihara N (1995). Immunohistochemical localization of pituitary adenylate cyclase-activating polypeptide (PACAP) in the adrenal medulla of the rat. Peptides 16(6), 1045–1050. [DOI] [PubMed] [Google Scholar]
- Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, et al. (2008). Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. J Mol Neurosci 36(1–3), 175–187. doi: 10.1007/s12031-008-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler F, Ekblad E, Hannibal J, Moller K, Zhang YZ, Mulder H, et al. (1996). Pituitary adenylate cyclase-activating peptide in sensory and autonomic ganglia: localization and regulation. Ann N Y Acad Sci 805, 410–426; discussion 427–418. [DOI] [PubMed] [Google Scholar]
- Tatsuno I, Somogyvari-Vigh A, and Arimura A (1994). Developmental changes of pituitary adenylate cyclase activating polypeptide (PACAP) and its receptor in the rat brain. Peptides 15(1), 55–60. [DOI] [PubMed] [Google Scholar]
- Vizzard MA (2000). Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol 420(3), 335–348. [PubMed] [Google Scholar]
- Vizzard MA (2001). Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat 21(2), 125–138. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erdman SL, Forstermann U, and de Groat WC (1994). Ontogeny of nitric oxide synthase in the lumbosacral spinal cord of the neonatal rat. Brain Res Dev Brain Res 81(2), 201–217. [DOI] [PubMed] [Google Scholar]
- Xu XJ, and Wiesenfeld-Hallin Z (1996). Intrathecal pituitary adenylate cyclase activating polypeptide facilitates the spinal nociceptive flexor reflex in the rat. Neuroscience 72(3), 801–804. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Shi TJ, Ji RR, Zhang YZ, Sundler F, Hannibal J, et al. (1995). Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res 705(1–2), 149–158. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Hannibal J, Zhao Q, Moller K, Danielsen N, Fahrenkrug J, et al. (1996). Pituitary adenylate cyclase activating peptide expression in the rat dorsal root ganglia: up-regulation after peripheral nerve injury. Neuroscience 74(4), 1099–1110. [DOI] [PubMed] [Google Scholar]


