Abstract
Blood-brain barrier (BBB) damage is a characteristic feature of diabetes mellitus pathology, and plays significant roles in diabetes-associated neurological disorders. However, effective treatments for diabetes targeting BBB damage are yet to be developed. Fibroblast growth factor 21 (FGF21) is a potent regulator of lipid and glucose metabolism. In this study we tested the hypothesis that recombinant FGF21 (rFGF21) administration may reduce type 2 diabetes (T2D)-induced BBB disruption via NF-E2 related factor-2 (Nrf2) upregulation. Our experimental results show that rFGF21 treatment significantly ameliorated BBB permeability, and preserved junction protein expression in db/db mice in vivo. This protective effect was further confirmed by ameliorated transendothelial permeability and junction protein loss by rFGF21 under hyperglycemia and IL1β (HG-IL1β) condition in cultured human brain microvascular endothelial cells (HBMEC) in vitro. We further reveal that rFGF21 can activate FGF receptor 1 (FGFR1) that increases its binding with Kelch ECH associating protein 1 (Keap1), a repressor of Nrf2, thereby reducing Keap1-Nrf2 interaction leading to Nrf2 release. These data suggest that rFGF21 administration may decrease T2D-induced BBB permeability, which is in part via FGFR1-Keap1-Nrf2 activation pathway. This study may provide an impetus for development of therapeutics targeting BBB damage in diabetes.
Keywords: Fibroblast growth factor 21 (FGF21), diabetes, Blood brain barrier (BBB), hyperglycemia, inflammation, FGFR1, Nrf2, Keap1
Introduction:
Diabetes mellitus represents a pathological condition caused by insufficient insulin production or insulin resistance leading to metabolism dysfunctions of lipids and proteins. Type 2 diabetes (T2D) is the most common form of diabetes in which the cells are not able to absorb and use insulin (insulin resistance) [1]. Diabetes causes structural and functional alterations of multiple organ systems including brain. Multiple neurological disorders including dementia, stroke and anxiety/depression have been extensively documented in diabetes mellitus [2]. Indeed diabetes has been regarded as a risk factor of stroke, and negatively impacts the outcomes of stroke as demonstrated in T2D mouse models [3].
Blood-brain barrier (BBB) is mainly composed of endothelial cells that are connected by tight junction and adherens junction proteins. BBB acts as a gate keeper allowing nutrient transportation while preventing harmful substances from entering the brain [4]. It has been demonstrated that diabetic conditions can impair BBB function [5,6]. Particularly, BBB permeability is increased in hyperglycemia or experimental diabetic animal models [7,8]. Accumulating evidence indicate that BBB disruption in T2D is one of the crucial risk factors leading to neurological deficits. Preclinical studies in mice indeed show that vascular injury following stroke is significantly exacerbated in diabetic subjects [9], hence development of new anti-diabetes compounds targeting both metabolic dysfunction and neurological/cognitive complications are considered clinically significant [2].
Fibroblast growth factor 21 (FGF21) is a 181-amino acid circulating protein that has been demonstrated to be a robust regulator of metabolism [10]. FGF21 exerts its physiological function mainly through interaction with FGF receptor 1c (FGFR1) and co-factor β-Klotho. FGF21 knockout mice show significantly increased adipose mass [11], whereas FGF21 overexpression in transgenic mice extends lifespan without reducing food intake, and increases insulin sensitivity [12]. Moreover, administration of rFGF21 to obese diabetic rodents markedly alleviated hyperglycemia, lowered triglycerides and body weight [13,14]. Studies using type 2 diabetes animal models show that recombinant FGF21 (rFGF21) significantly improves insulin sensitivity and corrects dyslipidemia [15]. Our very recent study using high fat diet-induced obese diabetic mice demonstrates that rFGF21 attenuated diabetes-associated cognitive dysfunction through metabolic regulation and anti-inflammation [16]. These findings imply that FGF21 may play critical roles in diabetic pathophysiology. However, whether FGF21 has protective effect against diabetes-associated BBB injury remains elusive. In this study we therefore aim to investigate the putative roles of rFGF21 administration in BBB dysfunction in diabetes mouse models.
NF-E2 related factor-2 (Nrf2) is a transcriptional regulator of multiple cytoprotective genes including anti-oxidant and glutathione generating enzymes [17]. Early brain inflammation and oxidative stress in the T2D brain disturb metabolic and cellular homeostasis[18,19], which later provokes defective Nrf2-dependent signaling functions [20] that are linked to brain vascular dysfunction in T2D [21]. Experimental studies show that BBB disruption is associated with defective Nrf2 signaling in diabetic mice [22] and under hyperglycemic condition in vitro [23]. Nrf2 is a rapidly turned-over protein that is normally sequestered in the cytoplasm by Kelch ECH associating protein 1 (Keap1), a repressor of Nrf2. Keap1 binds to Nrf2 and promotes its degradation by the ubiquitin pathway [24]. Interestingly, emerging evidence suggested FGF21 might engage in upregulation or activation of Nrf2 [25,26]. Thus in this study we tested the hypothesis that rFGF21 administration may reduce T2D-induced BBB permeability via Nrf2 upregulation, using T2D db/db mice and cultured human brain microvascular endothelial cells (HBMECs) monolayer.
Material and methods:
All animal experiments were performed following protocols approved by the Massachusetts General Hospital Animal Care and Use Committee in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experimenters were blinded to data acquisition.
Animals and drug treatments:
Male leptin receptor deficient mice (db/db) mouse and control mice (db/+) were purchased from Jackson Laboratory (Bar Harbor, Maine) at ages of 16 weeks. The animals were randomly assigned to vehicle-treated or rFGF21-treated groups. rFGF21 was administered at the dose of 3mg/kg/day for 10 days using IP-implanted Alzet osmotic pumps (DURECT Corporation, CA) before being sacrificed. The dose was based on previously established dose regimen of rFGF21 treatment in rodents [13].
Mice BBB permeability:
For assessment of BBB permeability, mice were introperitoneally injected with sodium fluorescein (NaFl, M.W 376) at the dose of 200 mg/kg (n= 8). One hour after NaFl injection, the mice were sacrificed by transcardial perfusion with saline under isoflurane anesthesia. The hippocampus and cortex were isolated and NaFl was extracted using 30% trichloroacetic acid (TCA), and the fluorescence (excitation 440 nm and emission 525 nm) was quantified using fluorescence microplate reader (SpectraMax M5, Molecular Device). The extraction and quantification procedures were performed in a blinded manner. Fluorescence emission values from duplicate wells containing brain extracts were fit to a standard curve, averaged, and expressed relative to total protein as determined by Bradford assay.
Human brain microvascular endothelial cell (HBMEC) culture and viability:
Primary HBMEC was obtained from Cell Systems Corporation (ACBRI376, Kirkland, WA), and cultured in complete growth media EBM-2 containing supplements growth factors (Lonza, Walkersville, MD). The viability of cultured HBMEC was assessed by WST assay in 24-well plates. Briefly, after treatment, WST-1 reagent (50 μl) (Donjindo Molecular Technologies) was added to each well (containing 450-μl medium) and the cells were incubated for 2 h at 37 °C. The absorbency at 450 nm was measured against a background blank control using microplate reader (SpectraMax M5, Molecular Devices).
Endothelial monolayer permeability:
Endothelial monolayer permeability was assessed following our previously published methods [27] except that the cells were treated with hyperglycemia and IL-1β. Briefly, primary HBMEC was cultured on the inner surface of collagen-coated Transwell inserts (6.5-mm diameter, 0.4-μm pore size; Corning, NY) in complete EBM-2 containing 25 mM glucose (hyperglycemia). The cell monolayer normally reaches confluency after 2 days. Then the cells were switched to hyperglycemia EBM without FBS or growth factors but with IL-1β (20ng/ml) for 16 hrs. For rFGF21-treated group, rFGF21 was added to the medium (final concentration 50nM) during IL-1β treatment. The media in both upper and lower chambers were then removed and replaced with fresh media without supplement. Permeability was measured by adding 0.1 mg/ml of fluorescein isothiocyanate (FITC)-labeled dextran (Sigma) to the upper chamber, with lower compartment containing 500 μl fresh serum-free media. After incubation for 20 min, 100 μl sample from the lower compartment was measured for fluorescence at excitation 490 nm and emission 520 nm. All independent experiments were performed in duplicate or triplicate.
Western blot:
For mouse brain tissue, the mouse was cardio-perfused with saline after 10-day treatment with rFGF21 (for junction protein Western blot), or after 7-day rFGF21 treatment (for Nrf2, Keap1 Western blot and co-IP etc.), then the cortex was dissected and protein was extracted using lysis buffer. For total protein isolation of cultured HBMEC, the cells were washed twice with PBS, then lysed with lysis buffer. For junction proteins examination, plasma membrane protein was isolated using the “Plasma Membrane protein Extraction Kit” (Abcam). Equal amount of protein were separated in a 4–20% NuPage gel and transferred to nitrocellulose membranes. Actin was used as the loading control. The membranes were incubated with primary anti-FGFR1 (1:1000, abcam), pFGFR1 (1:1000, abcam), β-Klotho (1:2000, R&D systems), Nrf2, Keap1, ZO-1 (1:1000, cell signaling), Occludin (1:1000, abcam), Claudin-5 (1:1000, ThermoFisher), VE-cadherin (1:1000, Enzo Life Sciences), IL-1 β (1:1000, cell signaling), Na,K-ATPase (1:1000, Abcam), Nrf2 (1:1000, cell signaling), p-Nrf2 (1:1000, abcam), Keap1 (1:400, Santa Cruz), Smad2 (1:1000, Abcam), p-Smad2 (1:1000, Cell signaling), Snail (1:1000, cell signaling), Histone-H3 (1:2000, cell signaling), and anti-β-actin antibody (1:10,000; Sigma) at 4 °C overnight. After washing with PBS containing 0.1% Tween 20, the membranes were then incubated with horseradish peroxidase-linked secondary antibody (1:2000) for 1 h at room temperature, developed by enhanced chemiluminescence (Pierce, Rockford). The optical density of protein bands from repeated experiments was quantified with ImageJ, normalized by actin, and then statistically analyzed.
Immunohistochemistry for junction protein in brain vessel:
Coronal brain sections (16 μm) were fixed in 100% cold aceton and blocked with 3% bovine serum albumin (BSA) for 1 h and incubated at 4 °C overnight with anti-VE-cadherin (rabbit monoclonal, 1:200, Abcam) and occludin (rabbit monoclonal, 1:200, abcam) together with rat anti-CD31 antibody (mouse monocolona1:100; BD Bioscience). The sections were then washed and incubated for 1 h with fluorescence-conjugated secondary antibodies (Jackson ImmunoResearch). Vectashield mounting medium containing DAPI (Vector Laboratory, Burlingame, CA) was used to coverslip the slides. Fluorescent signals were examined using Nikon Eclipse T300 fluorescence microscope.
Immunocytochemistry:
Immunostaining for junction proteins in HBMEC was performed following our previously published methods [27]. Briefly, cultured HBMEC in 24-well plates were washed with PBS and fixed with 4% paraformaldehyde (PFA) for 30 min, then washed with PBS containing 0.1% Tween and further incubated with 5% FBS for 1 h. Next the cells were incubated with primary antibodies against VE-cadherin (rabbit monoclonal; 1:200; Abcam) and ZO-1 (rabbit polyclonal; 1:200; Abcam) at 4 °C overnight. After PBS washing, the cells were incubated with fluorescence-conjugated secondary antibodies (Jackson ImmunoResearch), for 1 h at room temperature. Vectashield mounting medium containing DAPI was used to cover the wells. Fluorescent signals were examined using Nikon Eclipse T300 fluorescence microscope.
Co-immunoprecipitation (Co-IP):
Proteins were extracted from mouse brain or cultured HBMEC, and immunoprecipitation was performed using 2 μg polyclonal antibodies against mouse Keap1 (Santa Cruz Biotechnology) and FGFR1 (Abcam). After 3 hr incubation, protein G sepharose was added and incubated overnight at 4°C, and then centrifuged for 1 min at 12 000 g. The precipitates were rinsed with immuno-precipitation buffer (0.5% NP-40, Tris-Cl pH 8.0, 0.15 M NaCl) four times to remove non-specific binding molecules. IgG was used as a negative control for precipitation. The protein levels of Nrf2, pNrf2 and Keap1 in precipitates were then assessed by Western blot.
Statistical analysis:
Results were expressed as mean ± SD. The number of samples was 6 per group for mice BBB permeability and endothelial monolayer permeability assays, and 4 for western blot experiments. Multiple comparisons were evaluated by one-way ANOVA followed by Tukey- Kramer’s tests between all groups. p< 0.05 was considered statistically significant.
Results:
rFGF-21 treatment protects against diabetes-induced BBB permeability and junction protein loss in db/db mice
(1). rFGF-21 treatment ameliorated BBB leakage of NaFl in db/db mice
NaFl is a fluorescent tracer (M.W 376) that has been used for measuring BBB permeability in animal models of brain injury [28–30]. We first examined the BBB integrity of db/db mice by testing the permeability of NaFl. The db/+ mice was used as negative control. We show that NaFl permeability was significantly increased in db/db compared to db/+ mice (Figure 1A). We also tested the BBB leakage using bigger tracers FITC-Dextran (4k, 10k), but found no detectable leakage of these tracers (data not shown). These data suggest an early and moderate BBB damage at this age (~16 weeks) of db/db mice, which is consistent with previous report [29]. For the rFGF21 treatment group (3mg/kg/day for 10 days), BBB permeability assay shows that rFGF21 significantly decreased NaFl permeability compared to non-treated group (Figure 1A). Moreover, we show that rFGF21 treatment significantly decreased blood glucose level in db/db mice (Figure S1), which is consistent with previous studies [13], whereas the body weight of db/db mice was slightly reduced by rFGF21.
Figure 1: rFGF-21 reduces diabetes-induced BBB permeability and rescues junction protein expression in db/db mice.
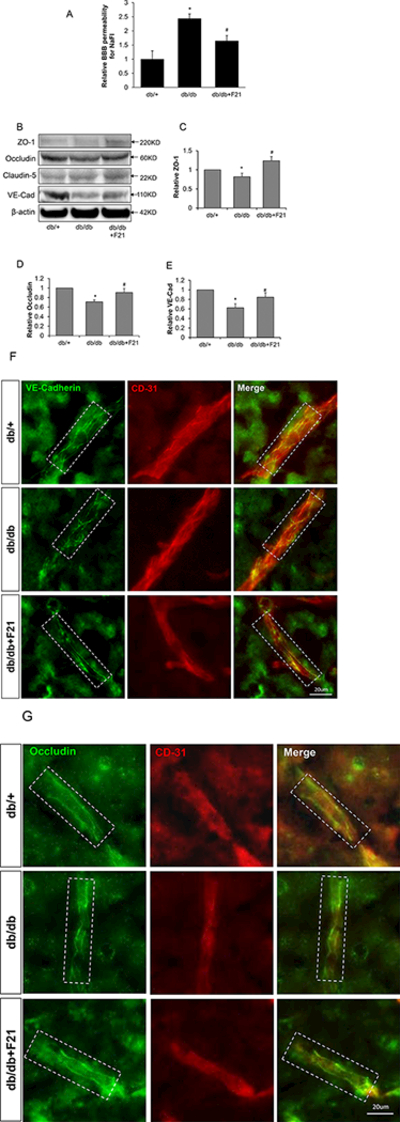
db/db mice at age of 16 weeks were treated with rFGF21 for 10 days. BBB leakage was measured by permeability to sodium fluorescein (NaFl), and junction protein expression was measured by Western blot and immunohistochemistry. (A) BBB permeability for NaFl after rFGF21 treatment in db/db mice. (B) Representative Western blot images of junction proteins ZO-1, Occludin, Claudin-5 and VE-cadherin. (C-E) Quantification of western blot results for ZO-1, Occuldin and VE-cadherin. (F) Immunohistochemistry for VE-cadherin in db/db brain vessels after rFGF21 treatment. (G) Immunohistochemistry for occludin in db/db brain vessels after rFGF21 treatment, dashed white box show the vessels. (* p<0.05 vs. db/+; # p<0.05 vs. db/db; n=6).
(2). rFGF21 treatment rescued junction protein expression in db/db mice.
Tight junction and adherens junction proteins play crucial roles in maintaining BBB integrity [31,32]. We next examined the junction protein expression in the brain cortex of db/db mice compared to db/+ by Western blot. We show that ZO-1, Occludin and VE-Cadherin protein levels were significantly decreased in db/db mice compared to db/+, while rFGF21 treatment significantly rescued the expression of ZO-1, occludin and VE-Cadherin (Figure 1B-E).
(3). Immunostaining for junction proteins in brain vessels of db/db mice after rFGF21 treatment
We further performed immunostaining for occludin and VE-cadherin in brain vessels by co-staining with CD31. The results show that occludin and VE-cadherin staining is integral and continuous along brain vessels in db/+ mice, whereas in db/db mice the staining is fragmented, losing continuity. rFGF21 treatment reversed this process, making occludin and VE-cadherin staining more integral and continuous compared to untreated db/db group (Figure 1F, E).
rFGF21 treatment reduced endothelial monolayer permeability after Hyperglycemia-IL1β (HG-IL1β) injury
Inflammation has been demonstrated to be a key mechanism of diabetes pathogenesis [33,34]. We first detected IL-1β protein level, an inflammation marker, in the brain cortex of db/db mice using Western blot. Our results show that IL-1β protein level was significantly increased in db/db mice brain compared to db/+ mice, suggesting increased inflammation in db/db mice brain at 16 weeks of age (Figure 2A).
Figure 2: rFGF21 reduced the permeability of cultured HBMEC monolayer after Hyperglycemia-IL1β (HG-IL1β) injury.
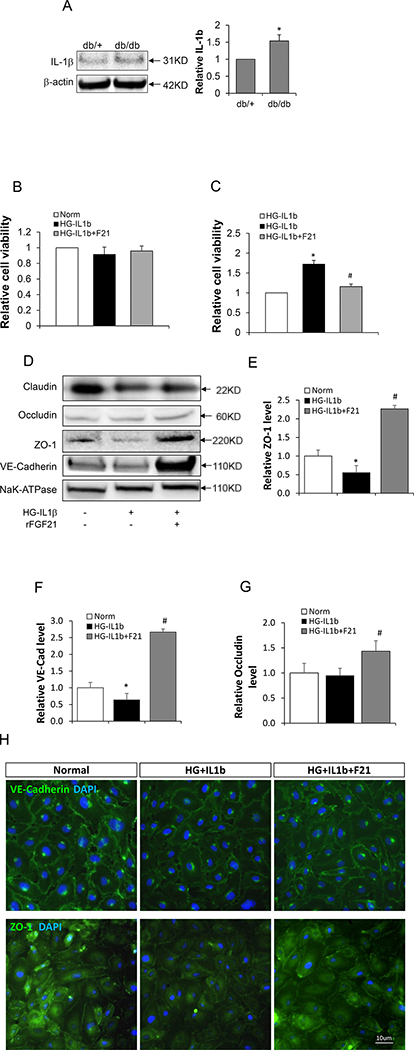
Cultured HBMEC monolayer treated with HG-IL1β was used to mimic BBB injury under diabetic conditions. The monolayer was then treated with rFGF21 and the permeability was measured. (A) IL-1β protein level in diabetic mice brain measured by Western blot (* p<0.05 vs. db/+, n=4); (B) Viability of cultured HBMEC after treatment with HG-IL1β and rFGF21; (C) Relative HBMEC monolayer permeability after treatment with HG-IL1β and rFGF21 (* p<0.05 vs. norm; # p<0.05 vs. HG-IL1β; n=6); (D) Representative western blot images of junction protein expression in cultured HBMEC after HG-IL1β and rFGF21 treatment; (E, F, G) Quantification of junction protein ZO-1, VE-cadherin and Occludin in cultured HBMEC (*p<0.05 vs. norm; # p<0.05 vs. HG-IL1β; n=6); (H) Immunocytochemistry of ZO-1 and VE-cadherin in cultured HBMEC after HG-IL1β and rFGF21 treatment.
Based on the increased IL-1β level in db/db mice brain, we used hyperglycemia (25mM Glucose) plus IL-1β (20ng/ml) (HG-IL1β) in HBMEC monolayer culture as an in vitro model of diabetic BBB injury. We first show that treatment with HG-IL1β or HG-IL1β+rFGF21 (50nM) did not significantly change the cell viability of HBMEC (Figure 2B). Next we examined the permeability of HBMEC monolayer using the methods as we previously described [27]. We show that HG-IL1β injury significantly increased the permeability of HBMEC monolayer, whereas this increase was significantly reversed by rFGF21 treatment (Figure 2C).
rFGF21 treatment rescued junction protein loss in cultured HBMEC after HG-IL1β injury
To investigate the putative mechanisms of compromised HBMEC monolayer integrity, we next isolated the plasma membrane fractions of HBMEC and examined the junction protein expression by Western blot. We show that ZO-1 and VE-cadherin protein levels were significantly reduced by HG-IL1β injury (Figure 2D-F), whereas this reduction was rescued by rFGF21 treatment. Occludin protein level was also significantly upregulated by rFGF21 treatment (Figure 2G).
Moreover, we performed immunocytochemistry for ZO-1 and VE-cadherin in cultured HBMEC. We show that the protein levels of ZO-1 and VE-cadherin on plasma membrane were significantly decreased by HG-IL1β injury, and this decrease was rescued by rFGF21 treatment (Figure 2H).
rFGF21 protection against HG-IL1β-induced HBMEC monolayer permeability is mediated by FGFR1
We next investigated whether the protection effect of rFGF21 on HBMEC monolayer permeability is mediated through its receptor FGFR1 and β-Klotho. We used a specific inhibitor of FGFR1, PD173074 (50 nM) [35], to co-treat HBMEC with rFGF21. PD173074 significantly abolishes the protection effect of rFGF21 on endothelial monolayer integrity (Figure 3A). Moreover, Western blot confirmed that rFGF21 treatment increases FGFR1 activation marked by FGFR1 phosphorylation (Pfgfr1), and this effect is reversed by PD173074 (Figure 3B, C). rFGF21 did not change the protein level of β-Klotho (Figure 3B). These results suggest that rFGF21 may protect HBMEC monolayer integrity at least in part through FGFR1-dependent mechanism.
Figure 3. FGFR1 is required for the protection effect of FGF21 against HG-IL1β induced HBMEC monolayer permeability.
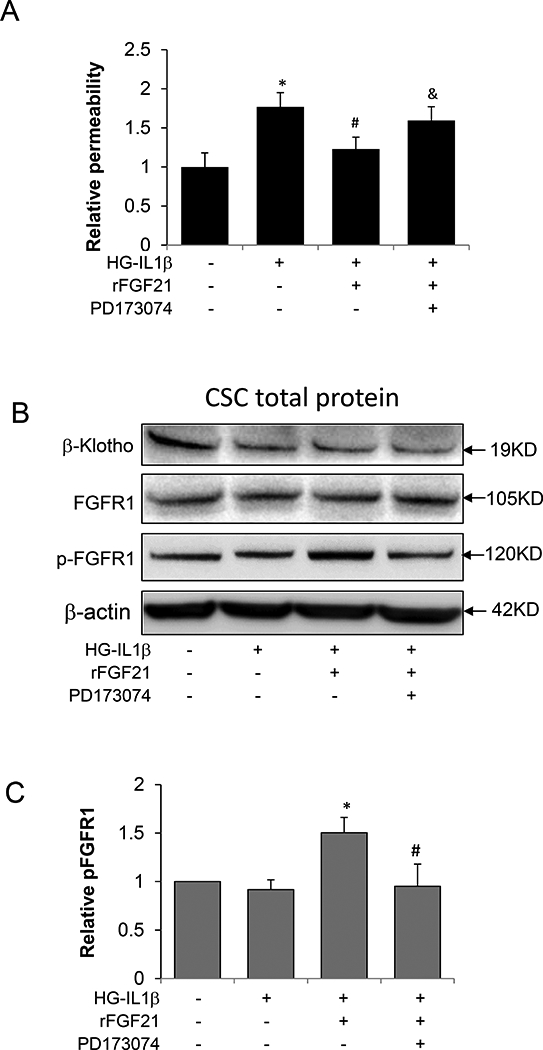
The FGFR1 inhibitor, PD173074, was used to examine the roles of FGFR1 in BBB protection effect by FGF21 using cultured HBMEC monolayer. (A) Relative permeability of HBMEC monolayer after HG-IL1β and FGF21 treatment with or without PD173074; (B) Representative Western Blot images to measure FGFR1, pFGFR1 and β-Klotho in cultured HBMEC after HG-IL1β and FGF21 treatment with or without PD173074; (C) Quantification of pFGFR1 in cultured HBMEC. (* p<0.05 vs. HG-IL1β; #p<0.05 vs. HG-IL1β+F21; n=4).
Nrf2-Keap1 interaction is involved in the protection effect of FGF21 against diabetes-induced BBB leakage
(1). pNrf2 (phospho-Nrf2) level was increased by rFGF21 in db/db mice and cultured HBMEC after HG-IL1β injury.
Nrf2 has protective roles against BBB damage in CNS pathologies [23,36]. We next investigated whether Nrf2 is involved in the protective effect of FGF21 on BBB by examining the protein levels of total Nrf2 and phosphorylated Nrf2 (pNrf2), the activated form of Nrf2 [37], in the nuclear extract of db/db mice brain after rFGF21 treatment. Histone-H3 was used as internal nuclear protein marker. We show that both Nrf2 and pNrf2 level was decreased in db/db mice compared to db/+, whereas this decrease was significantly rescued by rFGF21 (Figure 4A). Moreover, we examined the protein levels of Nrf2 and pNrf2 in nuclear fraction of cultured HBMEC. Nrf2 and pNrf2 protein levels were significantly decreased by HG-IL1β injury, and this decrease was significantly rescued by rFGF21 treatment (Figure 4B).
Figure 4. Nrf2-Keap1 pathway is involved in the protection effect of FGF21 against HG-IL1β induced endothelial monolayer permeability.
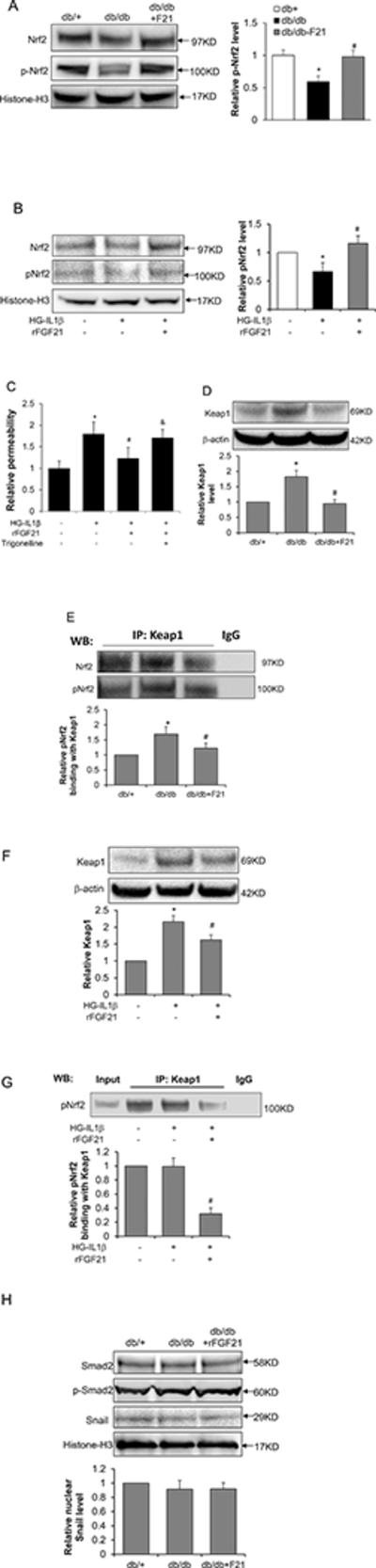
The involvement of Nrf2 in FGF21 protection for BBB was examined using db/db mice and cultured HBMEC monolayer. (A) Nrf2 and pNrf2 protein levels measured by Western blot in nuclear extract of db/db mice brain after rFGF21 treatment (* p<0.05 vs. db/+; # p<0.05 vs. db/db; n=4); (B) Nrf2 and pNrf2 protein levels measured by Western blot in cultured HBMEC after HG-IL1β and rFGF21 treatment (* p<0.05 vs. norm; # p<0.05 vs. HG-IL1β; n=3). (C) Relative HBMEC monolayer permeability after treatment with HG-IL1β and rFGF21, with or without Trigonelline, the Nrf2 inhibitor (* p<0.05 vs. norm; # p<0.05 vs. HG-IL1β; & p<0.05 vs. HG-IL1β+F21; n=4). The roles of Nrf2-Keap1 interaction in BBB protection by FGF21 was further investigated using db/db mice and cultured HBMEC. (D) Representative Western blot images and quantification for Keap1 protein in db/db mice brain after FGF21 treatment; (E) Representative Western blot images and quantification for Nrf2 and pNrf2 detection after Co-IP with Keap1 antibody using the brain lysate of db/db mice after rFGF21 treatment (* p<0.05 vs. db/+; # p<0.05 vs. db/db; n=4). Keap1 and Nrf2 interaction was also measured in cultured HBMEC after HG-ΙL1β and rFGF21 treatment. (F) Keap1 Western blot and quantification in cultured HBMCE; (G) Co-IP was performed using Keap1 antibody with HBMCE lysate, and pNrf2 in the precipitate was assessed using Western blot (* p<0.05 vs. norm; # p<0.05 vs. HG- IL1β; n=3). (H) Representative Western blot images of Smad2, p-Smad2 and Snail in db/db mice after rFGF21 treatment.
(2). Nrf2 inhibitor blocked the protection effect of rFGF21 on HG-IL1β-induced endothelial monolayer permeability.
We further investigated whether Nrf2 is required for the protection effect of rFGF21 on HG- IL1β-induced endothelial monolayer permeability. During HG-IL1β injury HBMEC monolayer was treated with rFGF21 alone or rFGF21 combined with Nrf2 inhibitor, trigonelline [38], followed by measurement of permeability. We show that trigonelline (1μM) significantly blocked the protection effect of rFGF21 on HG-IL1β-induced endothelial monolayer permeability (Figure 4C), suggesting that Nrf2 is at least partially required for rFGF21 protection of BBB integrity.
(3). Keap1-Nrf2 interaction in db/db mice after rFGF21 treatment
Nrf2 is normally sequestered in the cytoplasm by its repressor Keap1 [24]. We further investigated whether Keap1 is involved in the Nrf2 upregulation by FGF21. We first examined Keap1 protein level in mice brain lysate by Western blot. Keap1 protein level was significantly increased in db/db compared to db/+ mice, and this increase was significantly ameliorated by rFGF21 treatment (Figure 4D).
We next performed co-IP to examine the Keap1-Nrf2 interaction by using Keap1 antibody to do immunoprecipitation, followed by Western blot using Nrf2 and pNrf2 antibodies. We observed significantly increased interaction of Keap1 with both Nrf2 and pNrf2 in db/db mice compared to db/+, whereas this increase was significantly blocked by rFGF21 treatment (Figure 4E). These results imply that rFGF21 might upregulate nuclear Nrf2 and pNrf2 levels through reducing Keap1 protein level and Keap1-Nrf2 interaction as well.
(4). Keap1-Nrf2 interaction in cultured HBMEC after HG-IL1β injury
To further investigate the roles of Keap1 and Nrf2 in rFGF21 protection of BBB integrity, we examined Keap1 protein level in cultured HBMEC by Western blot. Keap1 protein level in HBMEC cell lysate was significantly increased after HG-IL1β injury, which was significantly blocked by rFGF21 treatment (Figure 4F). We next performed Co-IP in HBMEC using anti- Keap1 antibody followed by Western blot for pNrf2. We show that rFGF21 treatment after HG- IL1β injury significantly reduced pNrf2 binding to Keap1 compared to non-treated control group (Figure 4G).
(5). Smad2-Snail pathway is not involved in the protection effect of rFGF21 against diabetes- induced BBB leakage.
We next asked whether other regulators might be involved in the protection effect of rFGF21 against diabetes-induced BBB leakage. Snail is a negative regulator of VE-Cadherin gene expression by suppressing the promoter activity [39]. Moreover, Snail can be up-regulated by Smad2 [40]. We examined the protein levels of Smad2, phophos-Smad2 (the activated form of Smad2), and Snail in the nuclear extract of db/db mice brain after rFGF21 treatment. Our results show that Smad2, p-Smad2 and Snail were not changed among db/+, db/db and db/db-FGF21 groups (Figure 4H), implying that Smad2-Snail pathway may not be involved in the protection effect of rFGF21 against diabetes-induced BBB leakage.
Keap1 interaction with FGFR1 in HBMEC cells
We further asked whether there is direct interaction between Keap1 and FGFR1. Co-IP was performed using Keap1 antibody for HBEMC cell lysate, FGFR1 and pFGFR1 in the precipitate were examined by Western blot. We show that Keap1 interacts with FGFR1 and pFGFR1, and the pFGFR1-Keap1 binding was significantly decreased by HG-IL1β injury, while rFGF21 treatment significantly increased pFGFR1-Keap1 binding (Figure 5A, B).
Figure 5. Keap1 interaction with FGFR1.
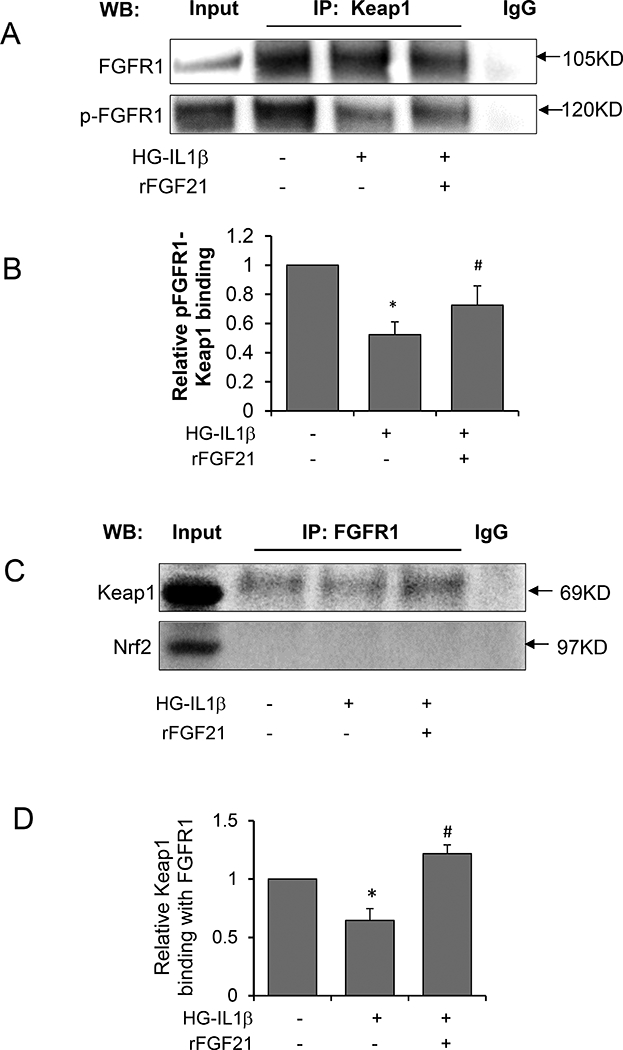
The interaction between Keap1 and FGFR1 was investigated by Co-IP using HBMEC cell lysate. (A) Representative Western blot image for FGFR1 and Pfgfr1 in the precipitate after Co-IP using Keap1 antibody; (B) Quantification of pFGFR1 protein levels; (C) Representative Western blot image for Keap1 and Nrf2 detection in the precipitate after Co-IP using FGFR1 antibody; (D) Quantification of Keap1 protein levels (* p<0.05 vs. norm; # p<0.05 vs. HG-IL1β; n=4).
We also used FGFR1 antibody to perform Co-IP and measured Keap1 protein levels in the precipitate. Keap1 interaction with FGFR1 was reduced after HG-IL1β insult, and rFGF21 treatment significantly rescued this interaction. Nrf2 was not detectable in the precipitate, suggesting that FGFR1 did not directly interact with Nrf2 (Figure 5C, D).
Overall, these results suggest that rFGF21 treatment may lead to increased binding of FGFR1 with Keap1, and subsequently decreased binding of Keap1 with Nrf2, therefore decreasing the Nrf2 ubiquitination and degradation in the cytoplasm, and eventually increasing the nuclear translocation of Nrf2 from cytoplasm.
Based on the above findings, the signaling pathways for rFGF21 protection against diabetes-induced BBB injury involving Nrf2 and Keap1 are depicted in the diagram (Figure 6).
Figure 6. Potential signaling pathways in the FGF21-FGFR1 protection against T2D-associated BBB permeability involving Keap1-Nrf2.
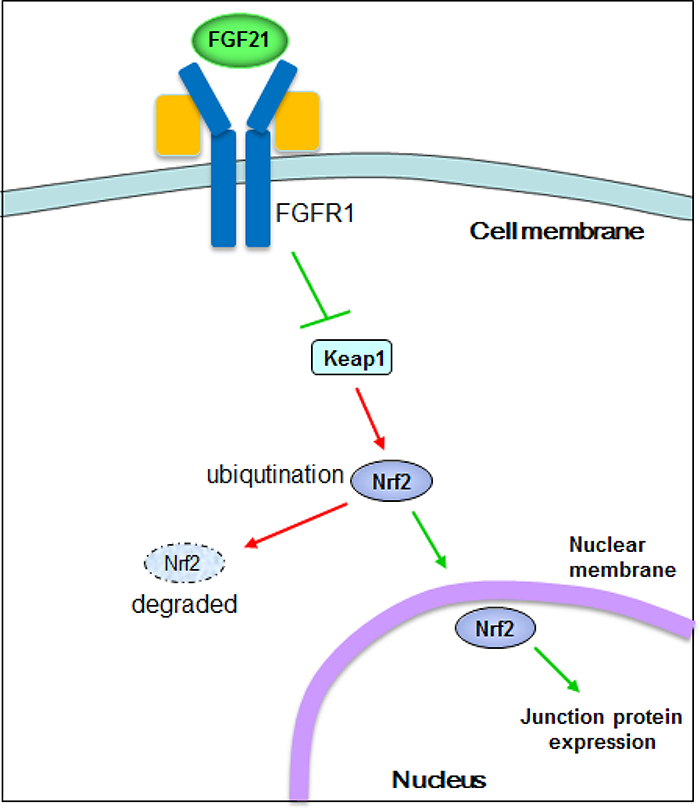
Under diabetic conditions, Nrf2 is negatively regulated by Keap1 binding, which leads to ubiquitination and degradation of Nrf2 by the proteasomes. Upon rFGF21 administration, FGFR1 binds to Keap1, which causes Nrf2 dissociation from Keap1 and increased translocation of Nrf2 into the nucleus, eventually leading to activation of cytoprotective genes.
Discussion:
BBB permeability is a characteristic feature of diabetes-associated CNS disorders including vascular dementia, Alzheimer’s disease and stroke, therefore understanding the mechanisms of T2D-induced BBB permeability is of high clinical priority. In this study we for the first time investigated the effect of rFGF21 administration on BBB permeability using both diabetes mice model and cultured HBMEC monolayer. We found that: (1) rFGF21 treatment ameliorated BBB permeability in db/db mice and HBMEC monolayer permeability after HG-IL1β insult; (2) rFGF21 treatment rescued junction proteins loss induced by diabetic condition; (3) the protection effect of rFGF21 on BBB permeability is dependent on FGFR1 activation; (4) the protection effect of rFGF21 on BBB permeability relies on Nrf2 activation; (5) rFGF21 treatment decreased the binding of Nrf2 to keap1, the negative regulator of Nrf2; (6) FGFR1 interacts with Keap1, but not Nrf2, and this interaction was increased by rFGF21 treatment. These data suggest a FGF21-FGFR1-Keap1-Nrf2 upregulation pathway in modulating BBB permeability in diabetes.
FGF21 has been recognized as a potent regulator of glucose and lipid metabolism thus playing crucial roles in diabetes-related pathology in db/db mice [13]. Accordingly, recombinant FGF21 has been applied in a big array of studies to treat db/db mice or diet-induced obesity (DIO) mice, which unanimously showed beneficial effects in reducing blood glucose and triglycerides (TGs) levels, reducing body weight, and improving insulin sensitivity [41,42]. Our recent study further demonstrated that rFGF21 improved obesity-induced cognitive dysfunction and anxiety-like behavior, potentially through reducing glucose tolerance, insulin resistance and hyperlipidemia [16]. It has been demonstrated that diabetic conditions significantly impair BBB function including glucose and amino acid transportation, permeability to harmful substances and immune regulation [6], however, whether FGF21 has protection effect against diabetes-associated BBB damage has not been investigated. In this study we for the first time found that rFGF21 administration ameliorated diabetes-induced BBB leakage, which extends our understanding of the functional spectrum of FGF21, and may also be part of the mechanisms underlying the protection effect of rFGF21 in ameliorating obesity-induced cognitive dysfunction [16].
Nrf2 is a master transcriptional regulator of cytoprotective genes including anti-oxidant and glutathione generating enzymes through binding to the antioxidant/electrophilic response element (ARE/EpRE) located in the promoter regions of target genes [17]. Previous studies using Nrf2 activators demonstrated that Nrf2 protects against BBB dysfunction in CNS disorders [23]. Nrf2 is normally sequestered in the cytoplasm through binding to Keap1, an actin cytoskeleton- associated adaptor protein [43]. Keap1 tags Nrf2 for ubiquitination leading to rapid degradation of Nrf2 by proteosome system. This Nrf2-Keap1 signaling pathway has been established as the major mechanism of cellular defense against oxidative stress in a wide array of pathological conditions, including type 2 diabetes [43]. Nrf2 upregulation by Keap1 knockout preserved the pancreatic β-cell mass and function in diabetic mice. Moreover, Nrf2 overexpression has been demonstrated to increase insulin sensitivity in diabetic mice, and decrease body weight and blood glucose level as well, potentially through enhanced energy consumption [44]. These findings reveal that Nrf2 has beneficial effect in overcoming the metabolic dysfunction in diabetes condition.
Recent experimental studies documented that Nrf2 protects against BBB damage in CNS pathologies [36]. In this study we revealed that FGF21 may protect against diabetes-induced BBB damage through upregulating Nrf2 levels. We also find that this Nrf2 upregulating effect might be mediated by FGFR1 binding to Keap1, which led to decreased binding of Keap1 with Nrf2. This is a novel mechanism that links FGF21 protection for BBB with Nrf2-Keap1 pathway under diabetic conditions, which broadens our understanding of the functions of Nrf2-Keap1 in diabetes. However, one limitation is that we did not investigate how Nrf2 is translocated from cytoplasm to nuclei, and how rFGF21 treatment affects this process. These questions warrant further investigation in the future.
There are a few more caveats in this study. First, the C57BLKS-Leprdb T2D mice containing leptin receptor deficiency may not reflect the diabetes etiology in humans, although FGF21 research in metabolic regulation has been translated from db/db T2D mice to human T2D [45]. There are variable pathological mechanisms among different T2D animal models [46], thus future studies testing rFGF21 in other diabetes models should be pursued. Second, we only tested Nrf2 mechanism in rFGF21 protection for BBB in T2D mice. However, other signaling pathways related to metabolic regulation might be also involved in T2D BBB permeability, which requires further investigations. Third, although the permeability assay using in vitro cultured endothelial monolayer has been commonly used, it may not reflect the physiological features of cerebrovascular endothelium, thus endothelium-astrocytes co-culture may be a better in vitro model for future studies [47].
Overall, in this study we for the first time discovered the beneficial effects of rFGF21 on diabetes-induced BBB permeability, and established FGFR1-Keap-Nrf2 pathway as a potential mechanism for this protection effect. These findings provide new insights into the mechanisms of diabetes-induced BBB damage, and may help in development of therapeutics targeting BBB permeability in T2D.
Supplementary Material
Acknowledgement:
This study was in part supported by AHA Scientist Development Grant 15SDG25550035 (Yu Z), National Natural Science Foundation of China 81771284 (Lin L), and National Institute of Health (NIH) 5R01NS099539 (Wang X).
List of abbreviations:
- FGF21
fibroblast growth factor 21
- BBB
blood-brain-barrier
- HBMEC
human brain microvascular endothelial cells
- FGFR1
FGF receptor 1
- IL-1β
interleukin 1β
- Nrf2, NF-E2
related factor-2
- NaFl
sodium fluorescein
- Co-IP
co-immunoprecipitation
Footnotes
Ethics Approval: All animal experiments were performed following protocols approved by the Massachusetts General Hospital Animal Care and Use Committee in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Availability of data and materials: The dataset supporting the conclusions of this article is included within the article.
Authors’ contributions: Z.Y, L.L, I.C, and Y.J performed the study and analyzed the data. Z.Y and XY.W designed the experiment, analyzed the data and wrote the paper. X.L, XJ.W and E.H.L helped in data analysis and paper writing. All authors have read and approved the manuscript.
Conflict of interest: The authors declare no competing financial interests in the manuscript.
References:
- 1.Wang DD, Hu FB (2018) Precision nutrition for prevention and management of type 2 diabetes. Lancet Diabetes Endocrinol. doi: 10.1016/S2213-8587(18)30037-8 [DOI] [PubMed] [Google Scholar]
- 2.Bogush M, Heldt NA, Persidsky Y (2017) Blood Brain Barrier Injury in Diabetes: Unrecognized Effects on Brain and Cognition. J Neuroimmune Pharmacol. doi: 10.1007/s11481-017-9752-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saczynski JS, Siggurdsson S, Jonsson PV, Eiriksdottir G, Olafsdottir E, Kjartansson O, Harris TB, van Buchem MA, Gudnason V, Launer LJ (2009) Glycemic status and brain injury in older individuals: the age gene/environment susceptibility-Reykjavik study. Diabetes Care 32 (9):1608–1613. doi: 10.2337/dc08-2300dc08-2300[pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haddad-Tovolli R, Dragano NRV, Ramalho AFS, Velloso LA (2017) Development and Function of the Blood-Brain Barrier in the Context of Metabolic Control. Front Neurosci 11:224. doi: 10.3389/fnins.2017.00224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duelli R, Maurer MH, Staudt R, Heiland S, Duembgen L, Kuschinsky W (2000) Increased cerebral glucose utilization and decreased glucose transporter Glut1 during chronic hyperglycemia in rat brain. Brain Res 858 (2):338–347. doi:S0006-8993(00)01942-9[pii] [DOI] [PubMed] [Google Scholar]
- 6.Prasad S, Sajja RK, Naik P, Cucullo L (2014) Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J Pharmacovigil 2 (2):125. doi: 10.4172/2329-6887.1000125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD (2007) Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia 50 (1):202–211. doi: 10.1007/s00125-006-0485-z [DOI] [PubMed] [Google Scholar]
- 8.Acharya NK, Levin EC, Clifford PM, Han M, Tourtellotte R, Chamberlain D, Pollaro M, Coretti NJ, Kosciuk MC, Nagele EP, Demarshall C, Freeman T, Shi Y, Guan C, Macphee CH, Wilensky RL, Nagele RG (2013) Diabetes and hypercholesterolemia increase blood-brain barrier permeability and brain amyloid deposition: beneficial effects of the LpPLA2 inhibitor darapladib. J Alzheimers Dis 35 (1):179–198. doi: 10.3233/JAD-122254 J078318R04323245 [pii] [DOI] [PubMed] [Google Scholar]
- 9.Sakata A, Mogi M, Iwanami J, Tsukuda K, Min LJ, Jing F, Ohshima K, Ito M, Horiuchi M (2011) Female type 2 diabetes mellitus mice exhibit severe ischemic brain damage. J Am Soc Hypertens 5 (1):7–11. doi: 10.1016/j.jash.2010.12.003 S1933–1711(10)00274–3 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Straub L, Wolfrum C (2015) FGF21, energy expenditure and weight loss - How much brown fat do you need? Mol Metab 4 (9):605–609. doi: 10.1016/j.molmet.2015.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badman MK, Koester A, Flier JS, Kharitonenkov A, Maratos-Flier E (2009) Fibroblast growth factor 21- deficient mice demonstrate impaired adaptation to ketosis. Endocrinology 150 (11):4931–4940. doi: 10.1210/en.2009-0532 en.2009–0532 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, Yu RT, Evans RM, Kliewer SA, Mangelsdorf DJ (2012) The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife 1:e00065. doi: 10.7554/eLife.00065 00065 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky GE, Hammond LJ, Moyers JS, Owens RA, Gromada J, Brozinick JT, Hawkins ED, Wroblewski VJ, Li DS, Mehrbod F, Jaskunas SR, Shanafelt AB (2005) FGF-21 as a novel metabolic regulator. J Clin Invest 115 (6):1627–1635. doi: 10.1172/JCI23606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J (2006) Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes 55 (9):2470–2478. doi:55/9/2470 10.2337/db05-1435 [DOI] [PubMed] [Google Scholar]
- 15.Kim HW, Lee JE, Cha JJ, Hyun YY, Kim JE, Lee MH, Song HK, Nam DH, Han JY, Han SY, Han KH, Kang YS, Cha DR (2013) Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology 154 (9):3366–3376. doi: 10.1210/en.2012-2276 [DOI] [PubMed] [Google Scholar]
- 16.Wang Q, Yuan J, Yu Z, Lin L, Jiang Y, Cao Z, Zhuang P, Whalen MJ, Song B, Wang XJ, Li X, Lo EH, Xu Y, Wang X (2017) FGF21 Attenuates High-Fat Diet-Induced Cognitive Impairment via Metabolic Regulation and Anti-inflammation of Obese Mice. Mol Neurobiol. doi: 10.1007/s12035-017-0663-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JM, Calkins MJ, Chan K, Kan YW, Johnson JA (2003) Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. J Biol Chem 278 (14):12029–12038. doi: 10.1074/jbc.M211558200 [DOI] [PubMed] [Google Scholar]
- 18.Bocci V, Valacchi G (2015) Nrf2 activation as target to implement therapeutic treatments. Front Chem 3:4. doi: 10.3389/fchem.2015.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurochkin AV, Chernov BK, Kirpichnikov MP, Kutyshenko VP, Bruskov VI (1989) [Complete assignment of signals in 1D and 2D H-NMR spectra of a 17-member oligonucleotide, a model symmetrical analog of lambda operators]. Mol Biol (Mosk) 23 (1):135–152 [PubMed] [Google Scholar]
- 20.Cheng X, Siow RC, Mann GE (2011) Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch- like ECH-associated protein 1 defense pathway. Antioxid Redox Signal 14 (3):469–487. doi: 10.1089/ars.2010.3283 [DOI] [PubMed] [Google Scholar]
- 21.Liu YJ, Chern Y (2015) AMPK-mediated regulation of neuronal metabolism and function in brain diseases. J Neurogenet 29 (2–3):50–58. doi: 10.3109/01677063.2015.1067203 [DOI] [PubMed] [Google Scholar]
- 22.Sajja RK, Prasad S, Tang S, Kaisar MA, Cucullo L (2017) Blood-brain barrier disruption in diabetic mice is linked to Nrf2 signaling deficits: Role of ABCB10? Neurosci Lett 653:152–158.S0304–3940(17)30457–3 [pii] doi: 10.1016/j.neulet.2017.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sajja RK, Green KN, Cucullo L (2015) Altered Nrf2 signaling mediates hypoglycemia-induced blood- brain barrier endothelial dysfunction in vitro. PLoS One 10 (3):e0122358. doi: 10.1371/journal.pone.0122358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang DD, Lo SC, Cross JV, Templeton DJ, Hannink M (2004) Keap1 is a redox-regulated substrate adaptor protein for a Cul3-dependent ubiquitin ligase complex. Mol Cell Biol 24 (24):10941–10953. doi: 10.1128/MCB.24.24.10941-10953.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang H, Feng A, Lin S, Yu L, Lin X, Yan X, Lu X, Zhang C (2018) Fibroblast growth factor-21 prevents diabetic cardiomyopathy via AMPK-mediated antioxidation and lipid-lowering effects in the heart. Cell Death Dis 9 (2):227. doi: 10.1038/s41419-018-0307-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y, Zhang J, Guo W, Li F, Sun W, Chen J, Zhang C, Lu X, Tan Y, Feng W, Fu Y, Liu GC, Xu Z, Cai L (2016) Up-regulation of Nrf2 is involved in FGF21-mediated fenofibrate protection against type 1 diabetic nephropathy. Free Radic Biol Med 93:94–109. doi: 10.1016/j.freeradbiomed.2016.02.002 S0891–5849(16)00045–9 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin L, Wang Q, Qian K, Cao Z, Xiao J, Wang X, Li X, Yu Z (2017) bFGF Protects Against Oxygen Glucose Deprivation/Reoxygenation-Induced Endothelial Monolayer Permeability via S1PR1-Dependent Mechanisms. Mol Neurobiol. doi: 10.1007/s12035-017-0544-0 [DOI] [PubMed] [Google Scholar]
- 28.Kaya M, Ahishali B (2011) Assessment of permeability in barrier type of endothelium in brain using tracers: Evans blue, sodium fluorescein, and horseradish peroxidase. Methods Mol Biol 763:369–382. doi: 10.1007/978-1-61779-191-8_25 [DOI] [PubMed] [Google Scholar]
- 29.Stranahan AM, Hao S, Dey A, Yu X, Baban B (2016) Blood-brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice. J Cereb Blood Flow Metab 36 (12):2108–2121. doi: 10.1177/0271678X16642233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatashita S, Hoff JT (1990) Brain edema and cerebrovascular permeability during cerebral ischemia in rats. Stroke 21 (4):582–588 [DOI] [PubMed] [Google Scholar]
- 31.Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV (2016) Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 4 (1):e1154641. doi: 10.1080/21688370.2016.1154641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu WY, Wang ZB, Zhang LC, Wei X, Li L (2012) Tight junction in blood-brain barrier: an overview of structure, regulation, and regulator substances. CNS Neurosci Ther 18 (8):609–615. doi: 10.1111/j.1755-5949.2012.00340.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wellen KE, Hotamisligil GS (2005) Inflammation, stress, and diabetes. J Clin Invest 115 (5):1111–1119. doi: 10.1172/JCI25102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Donath MY (2014) Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 13 (6):465–476. doi: 10.1038/nrd4275 [DOI] [PubMed] [Google Scholar]
- 35.Nguyen PT, Tsunematsu T, Yanagisawa S, Kudo Y, Miyauchi M, Kamata N, Takata T (2013) The FGFR1 inhibitor PD173074 induces mesenchymal-epithelial transition through the transcription factor AP-1. Br J Cancer 109 (8):2248–2258. doi: 10.1038/bjc.2013.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Moore AN, Redell JB, Dash PK (2007) Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci 27 (38):10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Yu Y, Ji T, Ma R, Chen M, Li G, Li F, Ding Q, Kang Q, Huang D, Liang X, Lin H, Cai X (2016) Clinical implication of Keap1 and phosphorylated Nrf2 expression in hepatocellular carcinoma. Cancer Med 5 (10):2678–2687. doi: 10.1002/cam4.788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arlt A, Sebens S, Krebs S, Geismann C, Grossmann M, Kruse ML, Schreiber S, Schafer H (2013) Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene 32 (40):4825–4835. doi: 10.1038/onc.2012.493 onc2012493 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Lopez D, Niu G, Huber P, Carter WB (2009) Tumor-induced upregulation of Twist, Snail, and Slug represses the activity of the human VE-cadherin promoter. Arch Biochem Biophys 482 (1–2):77–82. doi: 10.1016/j.abb.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 40.Cheng JC, Chang HM, Leung PC (2013) Transforming growth factor-beta1 inhibits trophoblast cell invasion by inducing Snail-mediated down-regulation of vascular endothelial-cadherin protein. J Biol Chem 288 (46):33181–33192. doi: 10.1074/jbc.M113.488866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, Hansen BC, Shanafelt AB, Etgen GJ (2007) The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology 148 (2):774–781. doi:en.2006–1168 [pii] 10.1210/en.2006-1168 [DOI] [PubMed] [Google Scholar]
- 42.Coskun T, Bina HA, Schneider MA, Dunbar JD, Hu CC, Chen Y, Moller DE, Kharitonenkov A (2008) Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 149 (12):6018–6027. doi: 10.1210/en.2008-0816 en.2008–0816 [pii] [DOI] [PubMed] [Google Scholar]
- 43.Tu J, Zhang X, Zhu Y, Dai Y, Li N, Yang F, Zhang Q, Brann DW, Wang R (2015) Cell-Permeable Peptide Targeting the Nrf2-Keap1 Interaction: A Potential Novel Therapy for Global Cerebral Ischemia. J Neurosci 35 (44):14727–14739. doi: 10.1523/JNEUROSCI.1304-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, Sugawara A, Kensler TW, Yamamoto M (2013) The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol 33 (15):2996–3010. doi: 10.1128/MCB.00225-13 MCB.00225–13 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Li Y (2015) Fibroblast Growth Factor 21 Analogs for Treating Metabolic Disorders. Front Endocrinol (Lausanne) 6:168. doi: 10.3389/fendo.2015.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baribault H (2016) Mouse Models of Type 2 Diabetes Mellitus in Drug Discovery. Methods Mol Biol 1438:153–175. doi: 10.1007/978-1-4939-3661-8_10 [DOI] [PubMed] [Google Scholar]
- 47.Li G, Simon MJ, Cancel LM, Shi ZD, Ji X, Tarbell JM, Morrison B 3rd, Fu BM (2010) Permeability of endothelial and astrocyte cocultures: in vitro blood-brain barrier models for drug delivery studies. Ann Biomed Eng 38 (8):2499–2511. doi: 10.1007/s10439-010-0023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


