Abstract
Introduction
The objective of the present feasibility study was to investigate the use of a new treatment modality—percutaneous peripheral nerve stimulation (PNS)—in controlling the often severe and long‐lasting pain following total knee arthroplasty (TKA).
Methods
For patients undergoing a primary, unilateral TKA, both femoral and sciatic open‐coil percutaneous leads (SPR Therapeutics, Cleveland, OH) were placed up to seven days prior to surgery using ultrasound guidance. The leads were connected to external stimulators and used both at home and in the hospital for up to six weeks total.
Results
In six of seven subjects (86%), the average of daily pain scores across the first two weeks was <4 on the 0–10 Numeric Rating Scale for pain. A majority of subjects (four out of seven; 57%) had ceased opioid use within the first week (median time to opioid cessation for all subjects was six days). Gross sensory/motor function was maintained during stimulation, enabling stimulation during physical therapy and activities of daily living. At 12 weeks following surgery, six of seven subjects had improved by >10% on the Six‐Minute Walk Test compared to preoperative levels, and WOMAC scores improved by an average of 85% compared to before surgery. No falls, motor block, or lead infections were reported.
Conclusions
This feasibility study suggests that for TKA, ultrasound‐guided percutaneous PNS is feasible in the immediate perioperative period and may provide analgesia without the undesirable systemic effects of opioids or quadriceps weakness induced by local anesthetics‐based peripheral nerve blocks.
Keywords: Postoperative analgesia, total knee replacement, opioid cessation, percutaneous peripheral nerve stimulation
INTRODUCTION
Total knee arthroplasty (TKA) is among the most common surgical procedures 1, 2. Following TKA, patients commonly experience prolonged moderate‐to‐severe postoperative pain, extended opioid use, and delayed functional recovery 3, 4, 5. Postoperative pain is most often treated with opioid analgesics, however, these have a high risk of misuse and debilitating side effects (e.g., sedation, dizziness, nausea, constipation, urinary retention, and sleeping problems) that often interfere with physical rehabilitation and function. Recent studies show that opioid use continues at least four weeks following TKA in over 70% of patients, with the median time to opioid cessation approximately 45–60 days 4, 6, 7.
Peripheral nerve blocks provide effective postoperative analgesia during hospitalization 8. However, single‐injection blocks (e.g., femoral nerve block, adductor canal block) provide analgesia for less than a day; while continuous peripheral nerve blocks are rarely used for greater than a few days or following discharge due to the inconvenience of carrying a portable infusion pump and local anesthetic reservoir, the relatively rapid consumption of local anesthetic, as well as the risks of infection and catheter dislodgement 8. In addition, peripheral nerve blocks often induce sensory, motor, and proprioception deficits, which may potentially interfere with physical rehabilitation and possibly increase the risk of falls 9, 10.
Percutaneous peripheral nerve stimulation (PNS) is a non‐opioid pain treatment that delivers electrical stimulation to peripheral nerve fibers through a percutaneous lead connected to an external pulse generator. Previous studies have demonstrated the safety and effectiveness of percutaneous PNS for the treatment of various chronic pain conditions, including low back pain, neuropathic pain (e.g., phantom limb pain and residual limb pain), and shoulder pain 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22. Recent studies suggest that percutaneous PNS can produce immediate reductions in postoperative pain following TKA in a brief (single day) in‐office test more than a week following surgery: 9 out of 10 subjects (90%) experienced >50% pain relief, and the average pain relief was 75% with stimulation compared to baseline 23, 24. The primary aim of this prospective feasibility study was to determine if using percutaneous PNS is feasible in the immediate perioperative period following TKA 25. The secondary aims of the study were to investigate the analgesic, opioid sparing potential, and impact on functional recovery of percutaneous PNS following TKA relative to published averages, as well as to produce data to help power a subsequent randomized, controlled clinical trial.
METHODS
This prospective feasibility study was approved by the Food and Drug Administration (FDA) under an investigational device exemption (IDE) and the University of California San Diego Institutional Review Board (IRB). The study was prospectively registered with ClinicalTrials.gov (NCT02468934). Subjects were enrolled in the study after providing written, informed consent and meeting all inclusion/exclusion criteria. Inclusion criteria included being scheduled to undergo primary, unilateral TKA and at least 21 years of age. Exclusion criteria included body mass indexs greater than 40 kg/m2; increased risk of infection (e.g., compromised immune system, history of valvular heart disease, history of skin infections; evidence of joint or overlying skin infection of the affected limb); implanted cardiac pacemaker/defibrillator or deep brain stimulator; increased risk of excessive bleeding (i.e., bleeding disorder, INR ≥ 1.5 for patients on warfarin); comorbidity affecting the ipsilateral leg (e.g., radiculopathy, fibromyalgia, and central nervous system disorder); allergy to skin surface electrodes or medical grade adhesives; and pregnancy.
Subjects received percutaneous PNS systems (SPRINT® PNS System, SPR Therapeutics, Inc., Cleveland, OH, USA) that have since been FDA cleared for up to 60 days for the treatment of chronic pain and acute pain, including post‐traumatic and postoperative pain. The system includes a percutaneous lead (MicroLead; SPR Therapeutics, Cleveland, OH, USA) that is 0.2 mm in diameter and has an open‐coil design (Fig. 1) intended to resist infection (<0.1% infection rate when used for up to 60 days) 26. Each lead was preloaded in a 20‐gauge introducer needle and inserted percutaneously using ultrasound guidance up to seven days prior to TKA to deliver PNS to the femoral and sciatic nerves. One lead was inserted near the femoral crease of the leg ipsilateral to the knee undergoing TKA using an anterior, in‐plane ultrasound approach (approximately 5–10 mm away from the femoral nerve). A second lead was inserted using a subgluteal in‐plane approach to a distance of approximately 10–30 mm from the sciatic nerve. Each lead was connected to an external pulse generator to evoke comfortable sensations in the regions surrounding the knee. Stimulation was delivered at 100 Hz with amplitude up to 20 mA and pulse duration up to 200 µs. For subjects who underwent lead placement more than two days prior to TKA, stimulation was delivered continuously until the day of surgery with the exception of showering.
Figure 1.
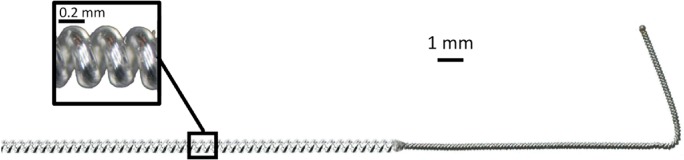
A small‐diameter (0.2 mm) open‐coiled, helical electrical lead with an anchoring wire (MicroLead; SPR Therapeutics, Inc., Cleveland, OH, USA; figure used with permission from SPR Therapeutics).
Immediately prior to surgery, the leads were disconnected from the external pulse generators and secured beneath sterile bandages. A single‐injection adductor canal block was administered under ultrasound guidance with ropivacaine 0.5% and epinephrine (20 mL) 27. Spinal or general anesthetic was used to provide surgical anesthesia. Within 20 h after TKA surgery, stimulators were reconnected to the leads and turned on. Subjects were instructed to use stimulation continuously during and following hospital discharge (i.e., 24 h per day except when showering and during battery changes), and subjects continued using stimulation for up to a total of six weeks, after which the leads were removed by an investigator using gentle traction.
Outcomes
Average pain at rest, while walking, and overall were each measured using the 0–10 numerical rating scale of the Brief Pain Inventory‐Short Form, Question 5 (BPI‐5). Pain scores over the previous 24 h were assessed daily during the percutaneous PNS therapy using a diary. Pain over the previous week was also assessed verbally during phone calls or visits weekly, and these pain scores were used to replace missing pain scores in the diary from the previous week. The diary was also used to record medication use, including opioids and non‐opioids. Opioid cessation was defined as no opioid usage for that day and through the end of therapy.
Functional recovery was assessed using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) 28, 29. Questions were scored using a 0–10 numeric rating scale, with lower numbers indicating improved pain, stiffness, or physical function. Functional recovery was also assessed using walking tests, including the Timed Up and Go (TUG) test and the Six‐Minute Walk Test (6MWT) 30, 31, 32, 33, 34.
RESULTS
Seven subjects scheduled to undergo primary, unilateral TKA to treat osteoarthritis enrolled in the study between March and October 2016 (Table 1). All subjects underwent lead placement, and electrical stimulation produced comfortable sensations in the distributions of the femoral and sciatic nerves without evoking motor responses in muscles innervated by the respective nerves. Gross sensory/motor function was maintained during stimulation, enabling stimulation during physical therapy and activities of daily living. The leads were left indwelling for a median (interquartile) duration of 38 (32–42) days (Table 2).
Table 1.
Patient characteristics.
| Subject # | Age (years) | Sex | Knee | Height (cm) | Weight (kg) |
|---|---|---|---|---|---|
| 1 | 63 | Male | Left | 188 | 109 |
| 2 | 77 | Male | Left | 175 | 84 |
| 3 | 72 | Male | Right | 178 | 82 |
| 4 | 66 | Female | Right | 152 | 94 |
| 5 | 71 | Female | Left | 165 | 83 |
| 6 | 74 | Female | Right | 165 | 73 |
| 7 | 51 | Female | Left | 165 | 88 |
Table 2.
Days of opioid discontinuation and lead removal.
| Subject # | Post‐operative day of opioid discontinuation | Post‐operative day of lead removal | Reason for lead removal |
|---|---|---|---|
| 1 | 48 | 8 | Adverse event unrelated to device; only mild pain at time of lead removal |
| 2 | 65 | 43 | End of treatment period |
| 3 | 4 | 8 | Discomfort during stimulation; only mild pain at time of lead removal |
| 4 | 29 | 47 | End of treatment period |
| 5 | 0 | 42 | End of treatment period |
| 6 | 4 | 32 | No pain or opioid use |
| 7 | 6 | 38 | End of treatment period |
A majority of subjects using percutaneous PNS had well‐controlled postoperative pain following TKA during the study. During the first week following TKA, the average of daily pain scores for pain at rest, while walking, and overall was mild (<4 out of 10 on BPI‐5) 35 in six of seven subjects (86%) (Fig. 2). Pain continued to be well controlled and mild for six of seven subjects at weeks 2, 3, and 4 as well.
Figure 2.
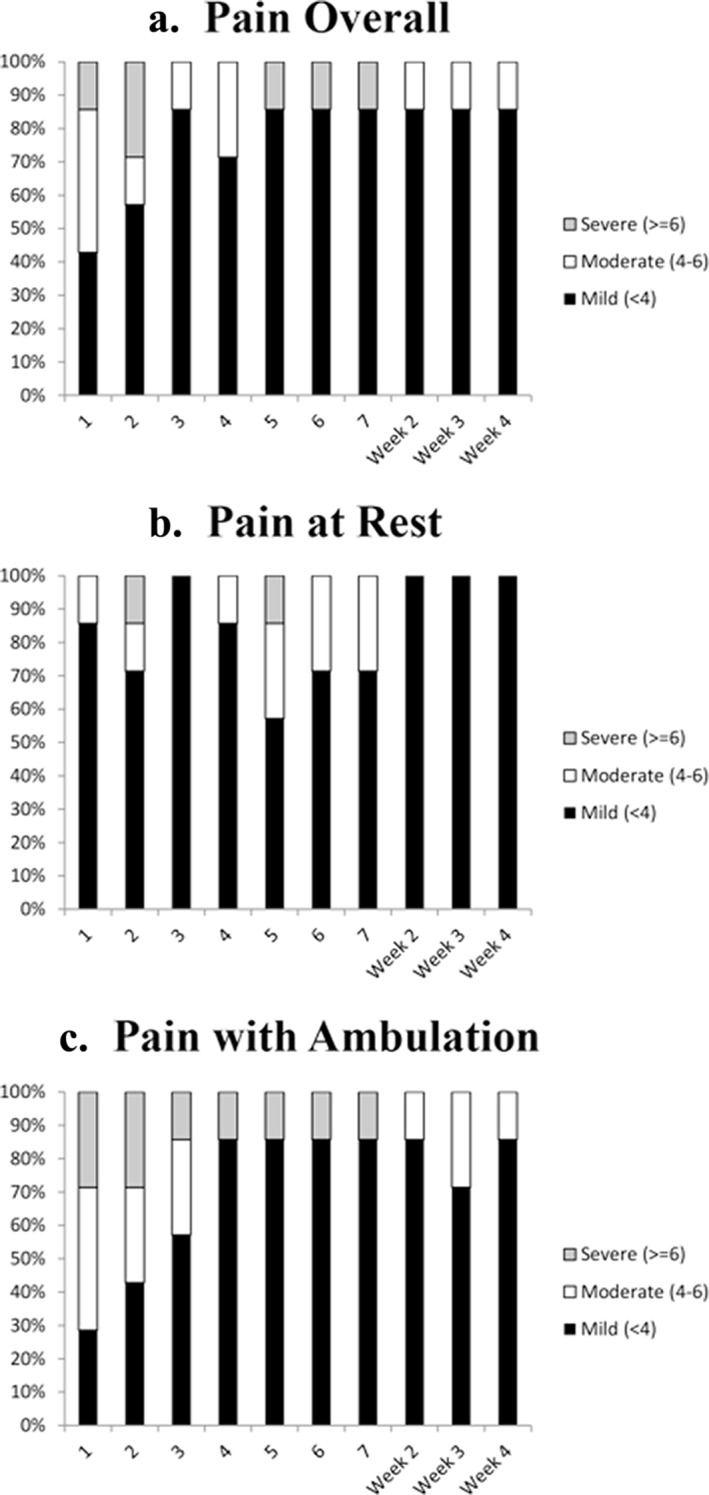
Percentage of subjects with mild, moderate, and severe post‐operative pain following TKA overall (Panel A), at rest (Panel B), and during ambulation (Panel C).
Four of the seven subjects (57%) had well controlled and mild pain and had discontinued opioid use within the first week (Figs. 3 and 4). One of the four subjects did not use opioids during the entire therapy, while the other three subjects discontinued opioid use on postoperative days 4, 4, and 6. The median time to opioid cessation across all seven subjects was six days (Table 2).
Figure 3.
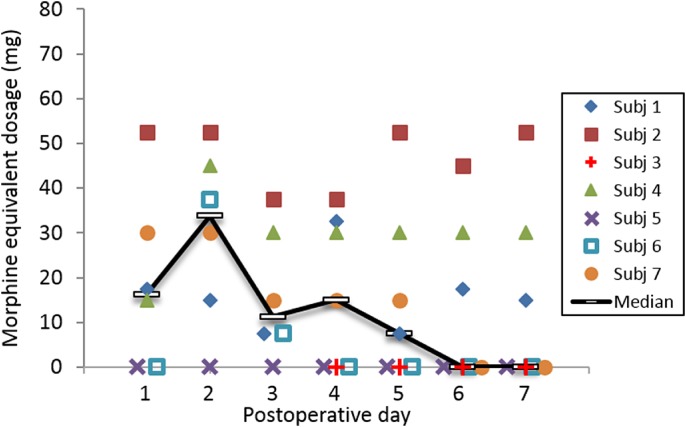
Opioid requirements (oral morphine equivalents). Data were unavailable for Subject 3 prior to day 5. [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4.
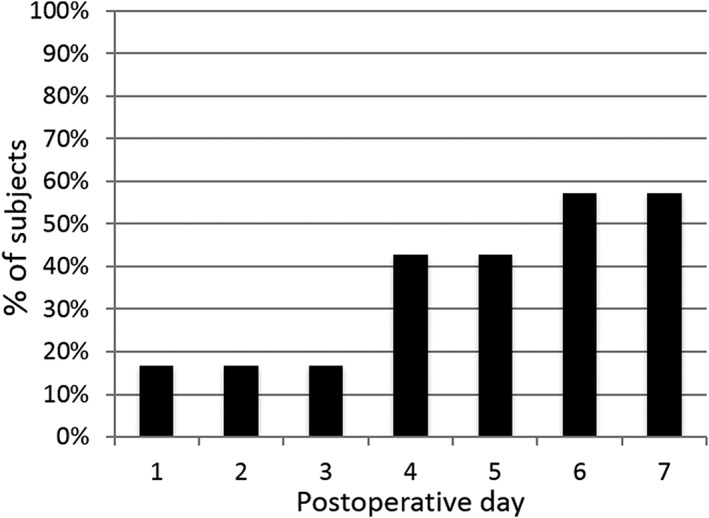
Percentage of subjects with well‐controlled pain (BPI‐5 score <4 out of 10) without opioids through post‐operative day 7 (n = 7).
Subjects had returned approximately to preoperative walking levels on both the TUG test and 6MWT by two weeks following TKA (Table 3). All seven subjects completed the TUG test preoperatively (average = 13.9 ± 1.5 s). Six of the seven subjects were administered the TUG test on the day of discharge from the hospital following TKA (subject 3 was discharged early on POD 1 before the TUG test could be administered). Of these six subjects, all 6 (100%) were able to complete the test (average = 34.9 ± 10.7 s). Two weeks following surgery, five of seven subjects (71%) had returned approximately to preoperative levels or better on the TUG test (within 5% of preoperative times) (average = 13.6 ± 4.3 s). Also, all seven subjects completed the 6MWT test preoperatively (average = 323 ± 46 m); and by two weeks following surgery, six of seven subjects (86%) had returned approximately to preoperative levels (≥ 95% of preoperative distance) (average distance = 311 ± 88 m). By 12 weeks following surgery, six of seven subjects had improved on the 6MWT by at least 10% compared to preoperative distances (average distance = 402 ± 55 m), with an average improvement of 26%.
Table 3.
Mobility tests.
| Subject # | TUG Test (s) | 6MWT (m) | |||||
|---|---|---|---|---|---|---|---|
| Prior to surgery | Day of discharge | w Weeks after surgery | Prior to surgery | w Weeks after surgery | w Weeks after surgery | 1w Weeks after surgery | |
| 1 | 13 | 22 | 15 | 300 | 293 | 307 | 333 |
| 2 | 16 | 52 | 16 | 303 | 290 | 366 | 402 |
| 3 | 14 | a | 12 | 408 | 417 | 468 | 468 |
| 4 | 14 | 30 | 14 | 303 | 295 | 341 | 347 |
| 5 | 11 | 40 | 21 | 343 | 156 | 240 | 366 |
| 6 | 14 | 39 | 7 | 343 | 409 | 463 | 439 |
| 7 | 15 | 28 | 10 | 266 | 324 | 402 | 459 |
| Mean | 14 | 35 | 14 | 324 | 312 | 370 | 402 |
| SD | 2 | 11 | 4 | 46 | 88 | 83 | 55 |
aNot assessed: subject was discharged early from hospital before TUG could be assessed.
Functional outcomes improved following surgery compared to before surgery as measured by the WOMAC questionnaire (Table 4). At two weeks following TKA, WOMAC scores improved by an average of 46% compared to before surgery, with five of seven subjects (71%) achieving a clinically significant improvement of ≥33%. By 12 weeks following surgery, the average improvement relative to before surgery was 85%, and all seven subjects (100%) had achieved clinically significant improvements in WOMAC 36, 37, 38.
Table 4.
Raw average scores and percentage improvement from baseline as measured with the WOMAC.
| Subject # | Prior to surgery | 2 weeks after surgery | 6 weeks after surgery | 12 week after surgery | |||
|---|---|---|---|---|---|---|---|
| 1 | 1.8 | 2.3 | −27% | 0.5 | 70% | 0.0 | 100% |
| 2 | 2.3 | 2.0 | 11% | 0.8 | 65% | 0.4 | 81% |
| 3 | 2.6 | 1.1 | 59% | 0.2 | 94% | 0.1 | 97% |
| 4 | 3.5 | 2.1 | 41% | 0.7 | 80% | 1.9 | 47% |
| 5 | 4.9 | 2.0 | 59% | 1.5 | 69% | 1.2 | 76% |
| 6 | 4.6 | 0.3 | 94% | 0.2 | 95% | 0.3 | 95% |
| 7 | 7.1 | 1.2 | 83% | 0.0 | 100% | 0.0 | 100% |
| Mean | 3.8 | 1.6 | 46% | 0.7 | 82% | 0.5 | 85% |
| SD | 1.9 | 0.7 | 39% | 0.5 | 13% | 0.7 | 19% |
Each question was assessed on a 0–10 numeric rating scale, with lower scores indicating improved function. Positive values of percentage change indicate improvement from pre‐operative baseline.
No falls, motor block, lead infections, or other serious device‐related adverse events were reported. One subject experienced discomfort at the site of the surface return electrode (a non‐serious, anticipated adverse event), which was resolved by moving the surface return electrode to a new location. Another subject experienced bruising around a lead insertion site (a non‐serious, anticipated adverse event), which resolved following lead removal without additional treatment. One subject experienced headaches, the cause of which could not be determined. One of 14 leads (7%) was dislodged inadvertently during therapy. Four of the seven subjects (Subjects 2, 4, 5, and 7) had leads removed as planned at approximately six weeks following TKA. The other three subjects (subjects 1, 3, and 6) underwent lead removal early on postoperative day 8, 8, and 32, respectively; and, the three subjects all had mild pain (BPI‐5 = 2, 3, and 0, respectively) at time of lead removal (Table 2). Across the seven subjects, 3 of 14 leads (21%) fractured during intentional extraction. The fragments were left in situ and did not produce subsequent complications.
DISCUSSION
The present study investigated the feasibility of using perioperative percutaneous PNS, a novel treatment for postoperative pain that enables prolonged non‐opioid therapy both during and many weeks following hospital discharge. The results suggest that PNS may provide well‐controlled postoperative pain and enable early opioid cessation, as well as an accelerated return to function following TKA.
Previous studies on outcomes following TKA using traditional approaches to postoperative analgesia have reported extended opioid use and delayed functional recovery. In recent studies of patients receiving various postoperative treatments (e.g., oral medications, local anesthetic‐based nerve blocks, transcutaneous electrical nerve stimulation, intravenous acetaminophen), average opioid consumption is often greatest during the immediate postoperative period (e.g., average daily morphine equivalent dosage [MED] of approximately 30–170 mg during postoperative days 0–3)7, 39, 40, 41, 42, 43; fewer than 30% of patients had ceased opioid use by four weeks following TKA, and the median time to opioid cessation was approximately 45–60 days 4, 6, 7. In the present study, average daily MED during postoperative days 0–3 was approximately 22 mg, a majority (4 of 7; 57%) of subjects reported opioid cessation within the first week following surgery, and the median time to opioid cessation was six days. Also, a recent study examining opioid use in over 24,000 patients that had undergone TKA showed that the median total MED during the first 90 days following surgery was 370 mg, and less than 30% of patients used <200 mg MED during this period 44. In the present study, the median total MED during the first 90 days following surgery was 120 mg, and 4 of the 7 subjects (57%) used <200 mg MED during this period. Additionally, published data suggest that patients typically do not return to preoperative levels on the 6MWT by four weeks following surgery (average distance = 81% of preoperative distance; range = 63–99%; <45% of subjects had returned to at least 95% of preoperative distances), and improved on average to 116% of preoperative distances (range = 99–130%) by 1 year following surgery 45, 46, 47, 48, 49, 50, 51. In contrast, subjects in the present study returned to preoperative levels by two weeks following surgery (average distance = 97% of preoperative distance; 6 of 7 subjects had returned to at least 95% of preoperative distances), and improved on average to 126% of preoperative distances by 12 weeks following surgery. While direct comparisons of previous studies to the present results must be considered cautiously, the outcomes of the present study are nonetheless promising and demonstrate the potential of percutaneous PNS to reduce opioid use and accelerate functional recovery following TKA.
Traditional neurostimulation systems have been used primarily for the treatment of chronic pain. Their use in treating non‐chronic (i.e., acute, subacute) postoperative pain has been greatly limited due to the need for permanently implanted devices (e.g., pulse generator/stimulator, electrode/lead) requiring invasive surgery for implantation, removal, and revision if necessary (e.g., to correct lead migration or failure) 52, 53, 54, 55, 56, 57, 58, 59. Percutaneous PNS avoids many of the drawbacks of traditional neurostimulation systems, requiring only a minimally invasive procedure (no incisions) to place the lead that makes it practical for perioperative use.
The coiled, fine‐wire lead has a unique design enabling percutaneous PNS for a prolonged duration. The leads were composed of a small‐diameter (0.2 mm) fluoropolymer‐insulated 7‐strand, type 316L stainless steel wire wound into an open helical coil with a single anchor at the tip (Fig. 1). Its small size enables percutaneous insertion of the lead using a 20‐gauge needle and non‐surgical removal with traction. The helical design helps keep the lead from moving by bending and stretching when subjected to force and encourages tissue ingrowth between the coils to secure the lead in place. These features theoretically decrease lead migration (which can cause discomfort and/or reduced analgesia) and reduce the risk of infection to 0.03 per 1000 indwelling days. No infections have been reported to date in over 330 lead placements when used to treat pain and left indwelling for up to 60 days 26. No complications were reported due to lead fragments retained upon lead removal (3 of 14 leads). The lack of infectious and neurologic complications is consistent with previous studies of percutaneous PNS for the treatment of pain, where a lower rate of lead fracture has been reported 11, 12, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24. Also, a recent study evaluating potential MRI related issues of retained fragments of the open‐coil lead demonstrated that retained lead fragments are MR Conditional at 1.5 Tesla with whole body averaged specific absorption rate (SAR) of 2 W/kg (i.e., with the MR system operating in the Normal Operating Mode) for 15 min of scanning per pulse sequence; at 3 Tesla, lead fragments passed safety testing for artifacts and magnetic field interactions (i.e., translation and torque), and all tested fragments exhibited physiologically inconsequential heating. While additional testing is needed to evaluate heating for fragments >11.2 cm in length, lead fractures that have occurred with this system have been shorter in length and most lead fractures that have been observed have occurred at or near the distal tip (<1.5 cm) 60.
Relatively recent developments may enable percutaneous PNS to become widely adopted for the treatment of post‐surgical pain. The broad availability of ultrasound machines and anesthesiologists trained in ultrasound‐guided regional anesthesia suggests that the proper equipment and training are already in place in most centers to implement this therapy. Also, the percutaneous PNS system used in the present study received U.S. FDA 510(k) clearance for the treatment of chronic pain and acute pain, including postoperative and post‐traumatic pain.
An important limitation of the current prospective feasibility study design is a lack of a control group as well as the small size of the study (n = 7). Using the data of the current series, future studies will be designed and conducted to further investigate the relative risks and benefits of percutaneous PNS for the treatment of postoperative pain.
Conclusions
This prospective feasibility study suggests that perioperative percutaneous PNS may enable reduced postoperative pain, earlier opioid cessation, and accelerated functional recovery following TKA.
Authorship Statements
Dr. Ilfeld was responsible for the study design, data analysis, conducting and providing guidance on study procedures, substantial contributions to first draft of manuscript and review of subsequent drafts.
Drs. Ball, Gabriel, Sztain, Monahan, Abramson, Khatibi, Said and Parekh were responsible for conducting study procedures and reviewing the manuscript.
Dr. Grant was responsible for the study design, data analysis and review of the manuscript.
Dr. Wongsarnpigoon was responsible for the study design, data analysis, investigator/coordinator training on protocol and use of stimulation system, substantial contributions to first draft of manuscript and review of subsequent drafts
Dr. Boggs was responsible for the study design, data analysis and review of the manuscript.
COMMENTS
When compared to the typical opioid consumption in TKA, the significant decrease in daily MED is remarkable. As this device IPG independent, the cost should not be prohibitive. The coil design to prevent dislocation is also novel, and begs me to consider other designs in neuromodulation that might be addressed.
Richard Paicius, MD
Newport Beach, CA, USA
***
This article describes a feasibility study of percutaneous peripheral nerve stimulation (PNS) for postoperative pain following knee replacement surgery. Although numbers so far are small, the results in terms of pain relief are encouraging. The treatment certainly deserves evaluation in a larger trial as the authors intend. Lead displacement rate is low but there is a relatively high rate of lead fracture at removal when compared to the other studies of PNS that are cited. Although most MRI imaging appears to be possible, the long term consequences of retained lead fragments (if any) at the sites that are being implanted are not known and patients with retained fragments should be followed up as part of the larger trial to ensure there are no associated adverse effects. Neuromodulation has in the great majority of cases been used in the setting of chronic pain and its extension into the acute pain arena has great potential. Knee replacement surgery is a clear example where it may prove useful and I await the results of the planned trial with interest.
James Fitzgerald, MA BM BCh
Oxford, United Kingdom
***
Total knee arthroscopy (TKA) is second only to colectomy with 17% of patients continuing to take opioids long term after surgery, more than double the risk of surgeries such as herniorrhaphy a surgery with a well‐known elevated risk for chronic neuropathic pain. Surgery and the resultant writing of post‐operative opioids results in 3.3 billion unused narcotic pills each year. For patients who were opioid naïve prior to surgery, 8.2% of TKA and 4.3% of total hip replacement (THA) patients were using opioids at 6 months. Additionally, 53.3% of TKA and 34.7% of THA patients who reported opioid use the day of surgery continued to use opioids at 6 months. Patients leave the hospital with an abundance of narcotics: the average total prescribed morphine equivalent daily dosage was 1405 ± 616 mg (range, 273‐3250 mg). The primary barriers to short stay TKA is pain and blood loss, and epidural anesthesia can lead to a high incidence of post‐op nausea and orthostasis. For all the miracle that a total knee replacement is, the above results in enormous societal burden, and morbidity that exists long after the surgery, recovery and discharge from care. At year following TKA about 13% of patients continue to report disabling pain. The above abstract is exciting for so many reasons, and while a nascent toe in the water for neuromodulation for acute peri‐and postsurgical pain, the implications beyond are profound. Any reduction in opioid use, any increase in perioperative mobility results in enormous downstream risk reductions. Clearly the next steps must be for an RCT. In the meantime, the fact that the FDA has 510K cleared the device for use now, likely underscores the federal government's understanding that surgical opioid use is a gateway to our existing epidemic.
William Porter McRoberts, MD
Ft. Lauderdale, FL, USA
References
1. QuintilesIMS Institute. An Analysis of the Impact of Opioid Overprescribing in America, September, 2017.
2. Goesling J, Moser SE, Zaidi B, Hassett AL, Hilliard P, Hallstrom B, Clauw DJ, Brummett CM. Trends and Predictors of Opioid Use Following Total Knee and Total Hip Arthroplasty, Pain. 2016 Jun;157 (6):1259–1265. doi: 10.1097/j.pain.0000000000000516
3. Hernandez N, Parry J, Taunton M. Patients at Risk: Large Opioid Prescriptions After Total Knee Arthroplasty, The Journal of Arthroplasty. 2017 August; 32(8):2395–2398.
4. Ayalon O, Liu S, Flics S, Cahill J, Juliano K, Cornell CN. A Multimodal Clinical Pathway Can Reduce Length of Stay After Total Knee Arthroplasty, HSS J. 2011 Feb;7(1):9–15. Published online 2010 May 22. doi: 10.1007/s11420-010-9164-1
Comments not included in the Early View version of this paper.
Acknowledgements
The authors would like to thank the staff of the Clinical and Translational Research Institute at the University California San Diego (San Diego, CA).
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: Funding for this project provided by the National Institute on Aging of the National Institutes of Health [Grant number R44AG052196] and SPR Therapeutics (Cleveland, OH). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the funding entities.
Conflict of Interest: Dr. Ilfeld's institution has received funding from SPR Therapeutics for this and other research. In addition, Dr. Ilfeld's institution has received funding for other research from Infutronics (Natick, MA), Baxter Healthcare (New Providence, NJ), Smiths Medical (St. Paul, MN), and Summit Medical (Salt Lake City, UT), Teleflex Medical (Research Triangle Park, NC), Ferrosan Medical (Szczecin, Poland), Myoscience (Fremont, CA), Epimed (Farmers Branch, TX), Heron Pharmaceuticals (San Diego, CA), and Pacira Pharmaceuticals (San Diego, CA). In addition, Dr. Ilfeld has previously acted as a consultant to Pacira Pharmaceuticals. Dr. Ball's institution has received funding from SPR Therapeutics for this and other research. Dr. Gabriel's institution has received funding from SPR Therapeutics for this and other research. In addition, this author's institution has received funding for other research from Infutronics (Natick, MA), Ferrosan Medical (Szczecin, Poland), and Myoscience (Fremont, CA). Dr. Sztain's institution has received funding from SPR Therapeutics for this and other research. In addition, this author's institution has received funding for other research from Infutronics (Natick, MA), Teleflex Medical (Research Triangle Park, NC), and Ferrosan Medical (Szczecin, Poland). Dr. Monahan's institution has received funding from SPR Therapeutics for this and other research. In addition, this author's institution has received funding for other research from Infutronics (Natick, MA), Teleflex Medical (Research Triangle Park, NC), and Smiths Medical (St. Paul, MN). Dr. Abramson's institution has received funding from SPR Therapeutics for this and other research. In addition, this author's institution has received funding for other research from Teleflex Medical (Research Triangle Park, NC). Dr. Khatibi's institution has received funding from SPR Therapeutics for this and other research. In addition, this author's institution has received funding for other research from Infutronics (Natick, MA), Teleflex Medical (Research Triangle Park, NC), and Ferrosan Medical (Szczecin, Poland). Dr. Said's institution has received funding from SPR Therapeutics for this and other research. In addition, this author's institution has received funding for other research from Infutronics (Natick, MA), Teleflex Medical (Research Triangle Park, NC), and Ferrosan Medical (Szczecin, Poland). Dr. Parekh's institution has received funding from SPR Therapeutics for this and other research. Dr. Grant's institution has received funding from SPR Therapeutics for this and other research. In addition, this author's institution has received funding for other research from Cara Therapeutics and Durrect. Dr. Grant also acts as a consultant to BBraun Medical. Dr. Wongsarnpigoon is an employee of SPR Therapeutics and has an ownership interest in the company. Dr. Boggs is an employee of SPR Therapeutics and has an ownership interest in the company.
REFERENCES
- 1. Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States—an alternative projection model. Osteoarthr Cartil 2017;25:1797–1803. [DOI] [PubMed] [Google Scholar]
- 2. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am 2014;96:624–630. [DOI] [PubMed] [Google Scholar]
- 3. Chan EY, Blyth FM, Nairn L, Fransen M. Acute postoperative pain following hospital discharge after total knee arthroplasty. Osteoarthr Cartil 2013;21:1257–1263. [DOI] [PubMed] [Google Scholar]
- 4. Goesling J, Moser SE, Zaidi B et al. Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain 2016;157:1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alzahrani K, Gandhi R, Debeer J, Petruccelli D, Mahomed N. Prevalence of clinically significant improvement following total knee replacement. J Rheumatol 2011;38:753–759. [DOI] [PubMed] [Google Scholar]
- 6. Carroll I, Barelka P, Wang CK et al. A pilot cohort study of the determinants of longitudinal opioid use after surgery. Anesth Analg 2012;115:694–702. [DOI] [PubMed] [Google Scholar]
- 7. Hah JM, Mackey S, Barelka PL et al. Self‐loathing aspects of depression reduce postoperative opioid cessation rate. Pain Med 2014;15:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ilfeld BM. Continuous peripheral nerve blocks: an update of the published evidence and comparison with novel, alternative analgesic modalities. Anesth Analg 2017;124:308–335. [DOI] [PubMed] [Google Scholar]
- 9. Ilfeld BM, Duke KB, Donohue MC. The association between lower extremity continuous peripheral nerve blocks and patient falls after knee and hip arthroplasty. Anesth Analg 2010;111:1552–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ilfeld BM. Single-injection and continuous femoral nerve blocks are associated with different risks of falling. Anesthesiology 2014;121:662–669. [DOI] [PubMed] [Google Scholar]
- 11. Kapural L, Gilmore CA, Chae J et al. Percutaneous peripheral nerve stimulation for the treatment of chronic low back pain: two clinical case reports of sustained pain relief. Pain Pract 2017. 18:94–103. [DOI] [PubMed] [Google Scholar]
- 12. Rauck RL, Cohen SP, Gilmore CA et al. Treatment of post‐amputation pain with peripheral nerve stimulation. Neuromodulation 2014;17:188–197. [DOI] [PubMed] [Google Scholar]
- 13. Rauck RL, Kapural L, Cohen SP et al. Peripheral nerve stimulation for the treatment of postamputation pain – a case report. Pain Pract 2012;12:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Renzenbrink GJ, Ijzerman MJ. Percutaneous neuromuscular electrical stimulation (P‐NMES) for treating shoulder pain in chronic hemiplegia. Effects on shoulder pain and quality of life. Clin Rehabil 2004;18:359–365. [DOI] [PubMed] [Google Scholar]
- 15. Yu DT, Chae J, Walker ME, Fang ZP. Percutaneous intramuscular neuromuscular electric stimulation for the treatment of shoulder subluxation and pain in patients with chronic hemiplegia: a pilot study. Arch Phys Med Rehabil 2001;82:20–25. [DOI] [PubMed] [Google Scholar]
- 16. Yu DT, Chae J, Walker ME et al. Intramuscular neuromuscular electric stimulation for poststroke shoulder pain: a multicenter randomized clinical trial. Arch Phys Med Rehabil 2004;85:695–704. [DOI] [PubMed] [Google Scholar]
- 17. Chae J, Wilson RD, Bennett ME, Lechman TE, Stager KW. Single‐lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case series. Pain Pract 2013;13:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chae J, Yu DT, Walker ME et al. Intramuscular electrical stimulation for hemiplegic shoulder pain: a 12‐month follow‐up of a multiple‐center, randomized clinical trial. Am J Phys Med Rehabil 2005;84:832–842. [DOI] [PubMed] [Google Scholar]
- 19. Wilson RD, Bennett ME, Lechman TE, Stager KW, Chae J. Single‐lead percutaneous peripheral nerve stimulation for the treatment of hemiplegic shoulder pain: a case report. Arch Phys Med Rehabil 2011;92:837–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilson RD, Harris MA, Bennett ME, Chae J. Single‐lead percutaneous peripheral nerve stimulation for the treatment of shoulder pain from subacromial impingement syndrome. Pm R 2012;4:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson RD, Gunzler DD, Bennett ME, Chae J. Peripheral nerve stimulation compared with usual care for pain relief of hemiplegic shoulder pain: a randomized controlled trial. Am J Phys Med Rehabil 2014;93:17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilson RD, Harris MA, Gunzler DD, Bennett ME, Chae J. Percutaneous peripheral nerve stimulation for chronic pain in subacromial impingement syndrome: a case series. Neuromodulation 2014;11:12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ilfeld BM, Grant SA, Gilmore CA et al. Neurostimulation for post‐surgical analgesia: a novel system enabling ultrasound‐guided percutaneous peripheral nerve stimulation. Pain Pract 2017;17:892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ilfeld BM, Gilmore CA, Grant SA et al. Ultrasound‐guided percutaneous peripheral nerve stimulation for analgesia following total knee arthroplasty: a prospective feasibility study. J Orthop Surg Res 2017;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ilfeld BM, Ball ST, Gabriel RA, Sztain JF, Monahan AM, Abramson WB et al. Perioperative percutaneous peripheral nerve stimulation utilizing preoperative lead placement for the treatment of postoperative pain [abstract]. Reg Anesth Pain Med 2017;2603. [Google Scholar]
- 26. Ilfeld BM, Gabriel RA, Saulino MF et al. Infection rates of electrical leads used for percutaneous neurostimulation of the peripheral nervous system. Pain Pract 2017;17:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jaeger P, Grevstad U, Henningsen M, Gottschau B, Mathiesen O, Dahl J. Effect of adductor‐canal‐blockade on established, severe postoperative pain after total knee arthroplasty: a randomised study. Acta Anaesthesiol Scand 2012;56:1013–1019. [DOI] [PubMed] [Google Scholar]
- 28. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–1840. [PubMed] [Google Scholar]
- 29. Bourne R. Measuring tools for functional outcomes in total knee arthroplasty. Clin Orthop Relat Res 2008/11/01 2008;466:2634–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39:142–148. [DOI] [PubMed] [Google Scholar]
- 31. Ko V, Naylor JM, Harris IA, Crosbie J, Yeo AE. The six‐minute walk test is an excellent predictor of functional ambulation after total knee arthroplasty. BMC Musculoskelet Disord 2013;14:1:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holm B, Kristensen MT, Myhrmann L et al. The role of pain for early rehabilitation in fast track total knee arthroplasty. Disabil Rehabil 2010;32:300–306. [DOI] [PubMed] [Google Scholar]
- 33. Fetherston CM, Ward S. Relationships between post operative pain management and short term functional mobility in total knee arthroplasty patients with a femoral nerve catheter: a preliminary study. J Orthop Surg Res 2011;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parent E, Moffet H. Comparative responsiveness of locomotor tests and questionnaires used to follow early recovery after total knee arthroplasty. Arch Phys Med Rehabil 2002;83:70–80. [DOI] [PubMed] [Google Scholar]
- 35. Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate‐to‐severe postoperative pain on the numeric rating scale: a cut‐off point analysis applying four different methods. Br J Anaesth 2011;107:619–626. [DOI] [PubMed] [Google Scholar]
- 36. Hmamouchi I, Allali F, Tahiri L et al. Clinically important improvement in the WOMAC and predictor factors for response to non‐specific non‐steroidal anti‐inflammatory drugs in osteoarthritic patients: a prospective study. BMC Res Notes 2012;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF‐36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Care Res 2001;45:384–391. [DOI] [PubMed] [Google Scholar]
- 38. Weigl M, Angst F, Aeschlimann A, Lehmann S, Stucki G. Predictors for response to rehabilitation in patients with hip or knee osteoarthritis: a comparison of logistic regression models with three different definitions of responder. Osteoarthr Cartil 2006;14:641–651. [DOI] [PubMed] [Google Scholar]
- 39. Nader A, Kendall MC, Wixson RL, Chung B, Polakow LM, McCarthy RJ. A randomized trial of epidural analgesia followed by continuous femoral analgesia compared with oral opioid analgesia on short‐ and long‐term functional recovery after total knee replacement. Pain Med 2012;13:937–947. [DOI] [PubMed] [Google Scholar]
- 40. Rakel B, Zimmerman MB, Geasland K et al. Transcutaneous electrical nerve stimulation for the control of pain during rehabilitation after total knee arthroplasty: A randomized, blinded, placebo-controlled trial. Pain 2014;155;2599–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raiff D, Vaughan C, McGee A. Impact of intraoperative acetaminophen administration on postoperative opioid consumption in patients undergoing hip or knee replacement. Hosp Pharm 2014;49:1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jæger P, Koscielniak‐Nielsen ZJ, Schrøder HM, et al. Adductor canal block for postoperative pain treatment after revision knee arthroplasty: a blinded, randomized, placebo‐controlled study. 2014. [DOI] [PMC free article] [PubMed]
- 43. Gallipani A, Mathis AS, Lee Ghin H, Fahim G. Adverse effect profile comparison of pain regimens with and without intravenous acetaminophen in total hip and knee arthroplasty patients. SAGE Open Med 2017;5:205031211769914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Namba RS, Inacio MCS, Pratt NL, Graves SE, Roughead EE, Paxton EW. Persistent opioid use following total knee arthroplasty: a signal for close surveillance. J Arthroplast 2018;33:331–336. [DOI] [PubMed] [Google Scholar]
- 45. Kennedy DM, Stratford PW, Wessel J, Gollish JD, Penney D. Assessing stability and change of four performance measures: a longitudinal study evaluating outcome following total hip and knee arthroplasty. BMC Musculoskelet Disord 2005;6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Carli F, Clemente A, Asenjo JF et al. Analgesia and functional outcome after total knee arthroplasty: periarticular infiltration vs continuous femoral nerve block. Br J Anaesth 2010;105:185–195. [DOI] [PubMed] [Google Scholar]
- 47. Bade MJ, Kohrt WM, Stevens‐Lapsley JE. Outcomes before and after total knee arthroplasty compared to healthy adults. J Orthop Sports Phys Ther 2010;40:559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bade MJ, Stevens‐Lapsley JE. Early high‐intensity rehabilitation following total knee arthroplasty improves outcomes. J Orthop Sports Phys Ther 2011;41:932–941. [DOI] [PubMed] [Google Scholar]
- 49. Stevens‐Lapsley JE, Balter JE, Wolfe P, Eckhoff DG, Kohrt WM. Early neuromuscular electrical stimulation to improve quadriceps muscle strength after total knee arthroplasty: a randomized controlled trial. Phys Ther, 2012 2012;92:210–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stevens‐Lapsley JE, Petterson SC, Mizner RL, Snyder‐Mackler L. Impact of body mass index on functional performance after total knee arthroplasty. J Arthroplast 2010;25:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mizner RL, Petterson SC, Clements KE, Zeni JA, Jr. , Irrgang JJ, Snyder‐Mackler L. Measuring functional improvement after total knee arthroplasty requires both performance‐based and patient‐report assessments. A longitudinal analysis of outcomes. J Arthroplast 2011;26:728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Long DM. Electrical stimulation for relief of pain from chronic nerve injury. J Neurosurg 1973;39:718–722. [DOI] [PubMed] [Google Scholar]
- 53. Nashold BS, Jr. , Goldner JL, Mullen JB, Bright DS. Long‐term pain control by direct peripheral‐nerve stimulation. J Bone Joint Surg Am 1982;64:1–10. [PubMed] [Google Scholar]
- 54. Picaza JA, Cannon BW, Hunter SE, Boyd AS, Guma J, Maurer D. Pain suppression by peripheral nerve stimulation. Part II. Observations with implanted devices. Surg Neurol 1975;4:115–126. [PubMed] [Google Scholar]
- 55. Campbell JN, Long DM. Peripheral nerve stimulation in the treatment of intractable pain. J Neurosurg 1976;45:692–699. [DOI] [PubMed] [Google Scholar]
- 56. Long DM, Erickson D, Campbell J, North R. Electrical stimulation of the spinal cord and peripheral nerves for pain control. A 10‐year experience. Appl Neurophysiol 1981;44:207–217. [DOI] [PubMed] [Google Scholar]
- 57. Mobbs RJ, Nair S, Blum P. Peripheral nerve stimulation for the treatment of chronic pain. J Clin Neurosci 2007;14:216–221. [DOI] [PubMed] [Google Scholar]
- 58. Nashold BS, Jr. , Mullen JB, Avery R. Peripheral nerve stimulation for pain relief using a multicontact electrode system. Tech Note. J Neurosurg 1979;51:872–873. [DOI] [PubMed] [Google Scholar]
- 59. Picaza JA, Hunter SE, Cannon BW. Pain suppression by peripheral nerve stimulation. Chronic effects of implanted devices. Appl Neurophysiol 1977;40:223–234. [DOI] [PubMed] [Google Scholar]
- 60. Shellock FG, Zare A, Ilfeld BM, Chae J, Strother RB. In vitro magnetic resonance imaging evaluation of fragmented, open‐coil, percutaneous peripheral nerve stimulation leads. Neuromodulation. 2018;21:276–283. [DOI] [PubMed] [Google Scholar]


