Abstract
Background:
There has been a surge in high level studies investigating platelet rich plasma (PRP) for tendon and ligament injuries. A number of meta-analysis have been published, but few studies have focused exclusively on tendon and ligament pathology.
Purpose:
To perform a meta-analysis assessing the ability of PRP to reduce pain in patients with tendon and ligament injuries.
Study Design:
Systematic review and meta-analysis
Methods:
This study followed the PRISMA (Preferred Reporting Items and Systematic Reviews and Meta-Analyses) guidelines. A comprehensive search of the literature was carried out in April 2017 using electronic databases PubMed, MEDLINE, and the Cochrane Library. Only Level I studies were included. Platelet and leukocyte count, injection volume, kit used, participant age/gender, comparator, and activating agent used were recorded. The short-term and long-term efficacy of PRP was assessed using the visual analog scale (VAS), which measures pain intensity. Pathology subgroups (rotator cuff, tendinopathy, ACL, and lateral epicondylitis) were evaluated. Funnel plots and Egger’s test were used to screen for publication bias and sensitivity analysis was performed to evaluate the impact of potential outliers by removing studies one at a time.
Results:
Thirty-seven articles were included in this review, 21 (1031 participants) of which could be included in the quantitative analysis. The majority of studies published investigated rotator cuff (38.1%) or lateral epicondylitis (38.1%). 17 studies (844 participants) reported short-term VAS data and 14 studies (771 participants) reported long-term VAS data. Overall, long-term follow-up results showed significantly less pain in the PRP group compared to control (WMD: −0.84; 95% CI: −1.23, −0.44; p<0.01). Patients treated for rotator cuff injury (WMD: −0.53; 95% CI: −0.98, −0.09; p=0.02) and lateral epicondylitis (WMD: −1.39; 95% CI: −2.49, −0.29; p=0.01) both reported significantly less pain in the long-term. Substantial heterogeneity was reported at baseline (I2: 72.0%, p<0.01), short term follow-up (I2: 72.5%, p<0.01), long term follow-up (I2: 76.1%, p<0.01), and overall (I2: 75.8%, p<0.01). The funnel plot appeared to be asymmetric, with some missingness at the lower right portion of the plot suggesting possible publication bias.
Conclusion:
This review shows that PRP may reduce the pain associated with lateral epicondylitis and rotator cuff pathology.
Introduction
Tendon and ligament pathology causes marked morbidity and can have a major impact on work, recreational activities, and overall quality of life. Treatment modalities such as non-steroidal anti-inflammatory drugs and corticosteroids can be used to treat tendinopathy and other disorders, but only offer symptomatic relief.67 Acute injury and chronic pathology often require surgical intervention, but surgical outcomes are unpredictable and often associated with persistent pain and discomfort. The poor self-repair capability of these tissues and the limitations of current surgical and injection-based interventions have led to increased interest in platelet rich plasma (PRP).30,35,65,67
Platelet-rich plasma is an autologous mixture of highly concentrated platelets and associated growth factors produced by centrifugal separation of whole blood.33,40,43 The growth factors released by PRP have been shown to promote cell recruitment, proliferation, and angiogenesis.24 It has also been suggested that PRP may induce a transient inflammatory event that triggers a regenerative response.28 Other groups have hypothesized that PRP may have beneficial immunomodulatory effects on tenocytes.3
Multiple meta-analyses have been published on PRP usage for tendon or ligament injuries.2,68,75,84,86 The majority of these studies have focused exclusively on rotator cuff healing or include low powered studies, which limits their clinical utility. Previous groups have attempted similar reviews that focused exclusively on tendon/ligament injuries49,72,84, but these reviews were limited by a scarcity of high-level-evidence publications when they were published. Additionally, while previous reviews such as those by Cai et al.8 and Warth et al.79 analyzed similar datasets, they report contrasting results and conclusions. Within studies that analyzed data from multiple outcome measures, PRP has been shown to be efficacious by some outcome scales and not others. For instance, a meta-analysis on PRP for rotator cuff healing by Yang et al.83 found that as a whole, PRP significantly improved Constant and VAS scores over control, but there was no significant difference in UCLA shoulder scores or retear rates.
The comparative clinical efficacy of PRP, placebo (saline), autologous whole blood, dry needling, and corticosteroids for ligament and tendon injury is unclear and controversial. Studies using animal models, for example, have not conclusively shown that PRP affects tendon repair, which is consistent with the contradictory outcomes of the clinical use of PRP for managing tendon injury.52 However, despite a lack agreement within the literature regarding the beneficial effects of PRP, there has been a surge in both clinical use and randomized controlled studies.63 The recent increase in high-level randomized, controlled studies investigating the clinical efficacy of PRP prompted our systematic review and meta-analysis of the literature. This is the first study to synthesize data exclusively from Level I randomized, controlled clinical trials for ligament and tendon injuries. The goal of this study is to provide clinicians with an overview of the currently available data on PRP for tendon and ligament injuries. The clinical outcome being evaluated emphasizes PRP’s efficacy in reducing pain.
Materials/Methods
Search Methods for Identification of Studies
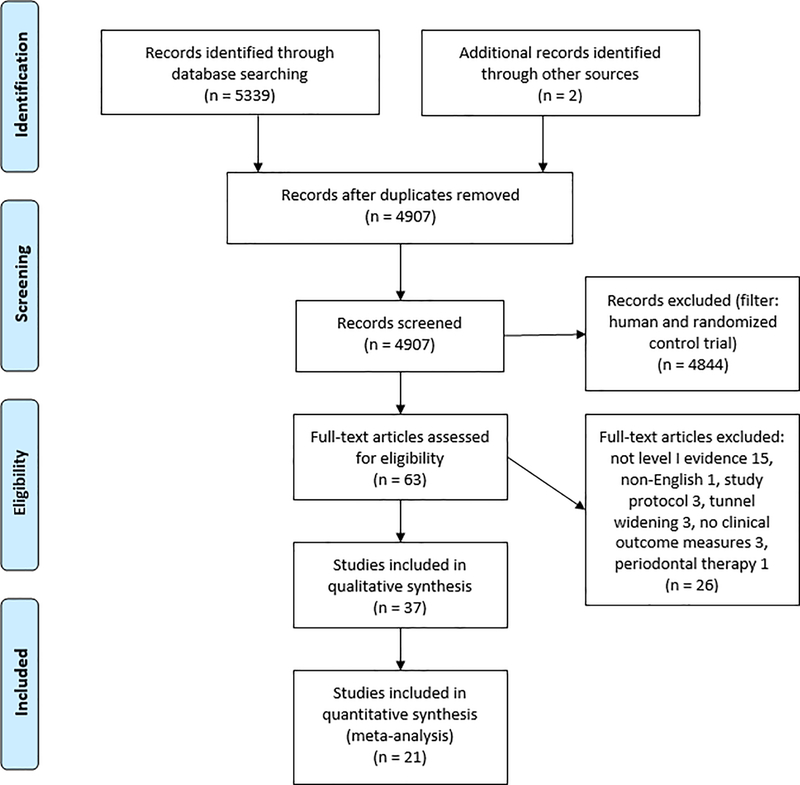
This study followed the PRISMA (Preferred Reporting Items and Systematic Reviews and Meta-Analyses) guidelines and the PRISMA-IPD Statement47,70 A comprehensive search of the literature was carried out in April 2017 using electronic databases PubMed, MEDLINE, and the Cochrane Library (Figure 1). Two authors (X.C. and I.J.) independently performed the initial screening and study selection. Any disagreements were resolved by discussion under the guidance of a third reviewer (C.T.V.). The search terms used were “PRP OR platelet rich plasma AND tendon OR ligament OR tendinopathy.” This yielded 4907 results after duplicates were removed. These results were filtered to only include “Randomized Control Trial” and “Human” species, leaving 63 results. Irrelevant and non-English articles were further excluded, leaving 53 full-text articles to be assessed for eligibility.
Figure 1.
Flow diagram outlining the process of literature search, selection, and review in this study.
Of the 53 articles reviewed, 18 articles were excluded because they did not meet the inclusion criteria (detailed below). Two articles were added from biographies, resulting in 37 articles for qualitative synthesis. Of the 37 articles available for qualitative analysis, 2 sets of articles were combined because they used the same study population. A total of 21 studies were available for meta-analysis. A repeat search was performed in June 2017 and did not yield any new studies for inclusion.
Inclusion Criteria
Level I evidence studies, as defined by the Oxford Center for Evidence-based Medicine (CEBM), that reported clinical outcomes for platelet-rich plasma injection (PRP) and platelet-rich fibrin matrix (PRFM) were considered for inclusion. Only full-length papers published in English were included. No studies were excluded based on follow-up time, although most studies followed patients clinically for at least 6 months. The number of injections, injection needle gauge, injection location, PRP concentration, PRP preparation method, PRP dosage, and usage of activating factors were recorded but not used as bases of inclusion/exclusion. Saline injection, dry needling, autologous whole blood injection, and corticosteroid injection were deemed appropriate controls. Basic science ex vivo and in vivo studies, animal studies, letters to the editor, editorials, personal correspondence, study protocols, levels II, III, IV, or V evidence, and studies investigating PRP usage in periodontal therapy were excluded.
Quantitative analysis
The outcome of VAS scores was categorized as baseline, short term (up to 6.5 months follow-up), and long term (1 year or more follow-up). When studies did not report the SD, they were calculated from the SE or 95% CI. The SD calculated from the 95% CI used critical values from the t distribution due to the small sample size. In cases where the SD was not reported, or a median (range/IQR) was reported, the authors were contacted via email to obtain the raw data. If the authors did not respond, the mean was calculated from the median and interquartile ranges as suggested by Wan et. al.77 and the SD was calculated using the Cochrane method. VAS scores that were reported as a 0–100 scale were converted to a 0–10 scale to be consistent across the studies.
The weighted mean differences (WMD) with 95% confidence intervals were calculated for the continuous outcome of VAS for each study. Since VAS was the only outcome of interest, and the unit of measurement was the same across studies, the mean difference was not standardized. A random effects model was used since it was expected that there would be some variability across studies, and under the assumption that the true effect would not be the same across all the studies.
Meta-analysis was performed by time subgroups (baseline, short, and long-term) as well as overall, to determine the efficacy of PRP versus control on VAS pain scores. Pathology subgroups (rotator cuff, tendinopathy, ACL, and lateral epicondylitis) were evaluated within each time point to determine if there was heterogeneity of PRP efficacy by pathology. The outputs from the forest plots were used to assess heterogeneity. The I2 test was used to determine heterogeneity based on the thresholds reported in the Cochrane Handbook for Systematic Reviews of Interventions: 0–40% might not be important, 30–60% may represent moderate heterogeneity, 50–90% may represent substantial heterogeneity, and 75–100% considerable heterogeneity. To evaluate possible reasons for heterogeneity, meta-regression was conducted using baseline difference in PRP versus control and pathology as possible explanatory factors; a permutation test approach was used to calculate the p-values for the meta-regression. Funnel plots were generated to visually assess asymmetry and potential publication bias, along with the Egger’s test. Sensitivity analysis was performed to evaluate the impact of potential outliers by removing studies one at a time. All analyses were performed using STATA version 14.2 (StataCorp LP, College Station, TX, USA).
Results
Overview
37 randomized, controlled studies reporting clinical efficacy data on PRP for tendon or ligament pathology/injury were included in this review. Five of these studies used the same dataset, leaving 34 unique study populations (Table 1, “Overview”). Six different pathology subgroups were identified: Rotator cuff injury (RC), lateral epicondylitis (LE), patellar tendinopathy (PT), achilles tendinopathy (AT), anterior cruciate ligament injury (ACL), and hamstring tendinopathy (HT). The majority of studies (72.97%) investigated RC or LE. Eleven different controls were used, with over half of studies (45.95%) using surgical repair without additional treatment as a control. No study reported severe adverse events (SAEs).
Table 1.
Overview of Studies
| Overview | Therapy | Demographics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Pathology | Comparator | SAEs | Kit | Activation | Leukocyes per ul | Platelets per ul | Volume (ml) | Country | Mean Age | Male | Female |
| Pandey et al. 88, 2016 | RC | Repair w/o tx | None Reported | None | CaCl2 | 4 | 474,000 | 8 | India | 54 | 74 | 28 |
| Zou et al.55, 2016 | AT | Repair w/o tx | 0 | WEGO Platelet-Rich Plasma Preparation Kits (WEGO Ltd., Shandong, China) | None | Not reported | Not reported | 3–4 | China | 29 | 35 | 1 |
| Seijas et al. 64, 2016 | ACL | Repair w/o tx | None Reported | None | CaCl2 | Not reported | Not reported | 4 | Spain | Not reported | 37 | 6 |
| Zumstein et al.89, 2016 | RC | Repair w/o tx | 0 | PRF Process (Nice, France) | None | Not reported | Not reported | Not reported | France | 66 | 18 | 17 |
| Carr et al.9, 2015 | RC | Repair w/o tx | None Reported | Magellan Autologous Platelet Separator System (Arteriocyte) | Thrombin | Not reported | Not reported | <5 | Britain | 54 | 27 | 33 |
| Lebiedziński et al.42, 2015 | LE | Betamethasone and lignocaine | None Reported | Arthrex Autologous Conditioned Plasma (ACP) system (Naples, FL, USA) | None | Not reported | Not reported | Not reported | Poland | 50 | 40 | 59 |
| Jo et al.32, 2015 | RC | Repair w/o tx | None Reported | COBE Spectra LRS Turbo; Caridian BCT | Calcium gluconate | 40 | 1,000,000 | 9* | South Korea | 61 | 27 | 57 |
| Wang et al. 78, 2015 | RC | Repair w/o tx | 0 | Arthrex Autologous Conditioned Plasma (ACP) system (Naples, FL, USA) | CaCl2 | Not reported | 470,000 | 2–4^ | Australia | 59 | 28 | 32 |
| Davenport et al.14, 2015 | HT | Whole blood | 0 | SmartPReP; Harvest Technologies | None | Not reported | Not reported | 3 | USA | 46 | 3 | 14 |
| Montalvan et al.48, 2015 | LE | Saline | None Reported | Arthrex Autologous Conditioned Plasma (ACP) system (Naples, FL, USA) | None | Not reported | Not reported | 2^ | France | 47 | 34 | 16 |
| Tetschke et al. 73, 2015 | LE | Laser | 0 | Arthrex Autologous Conditioned Plasma (ACP) system (Naples, FL, USA) | None | Not reported | Not reported | 3–5^ | Germany | 52 | 21 | 31 |
| Behera et al. 6, 2015 | LE | Bupivacaine | 0 | None | CaCl2 | 600–800 | 600,000–800,000 | 3 | India | 38 | 7 | 17 |
| Gautam et al25., 2015 | LE | Corticosteroid | None Reported | None | None | Not reported | Not reported | 2 | India | Not reported | N/A | NA |
| Malavolta et al.44, 2014 | RC | Repair w/o tx | 0 | MCS+ 9000 blood cell separator and 994-CFE apheresis set | Thrombin | Not reported | Not reported | 20 | Brazil | 55 | 17 | 37 |
| Dragoo et al. 23, 2014 | PT | Dry needling | 0 | GPS III Platelet Separation System (Biomet Biologics, Warsaw, Indiana) | None | Not reported | Not reported | 6 | USA | 33 | 20 | 1 |
| Raeissadat et al.57, 2014 | LE | Autologous whole blood | 0 | Rooyagen kit (Arya Mabna Tashkhis Corporation, RN: 312569) | None | 6740 | 1,227,000 | 2 | Iran | 44 | 14 | 47 |
| Raeissadat et al.58, 2014 | LE | Autologous whole blood | 0 | Rooyagen kit (Arya Mabna Tashkhis Corporation, RN: 312569) | None | Not reported | 990,000 | 2 | Iran | 46 | 8 | 32 |
| Kesikburun et al.34, 2013 | RC | Saline | 0 | GPS III Platelet Separation System (Biomet Biologics, Warsaw, Indiana) | None | Not reported | 1,014,900 | 5 | Turkey | 53 | 13 | 27 |
| Krogh et al.38, 2013 | LE | Saline or glucocorticoid | 0 | Recover GPS II system (Biomet Biologics Inc, Warsaw, Indiana) | None | Not reported | Not reported | 3–3.5 | Denmark | 45 | 31 | 29 |
| Weber et al.80, 2013 | RC | Repair w/o tx | None Reported | Cascade system (Musculoskeletal Transplant Foundation, Edison, New Jersey) | Calcium | Not reported | Not reported | Not reported | USA | 62 | 36 | 24 |
| Rha et al.60, 2013 | RC | Dry needling | 0 | Prosys PRP Platelet Concentration System | None | Not reported | Not reported | 3 | South Korea | 53 | 17 | 22 |
| Jo et al.31, 2013 | RC | Repair w/o tx | None Reported | COBE Spectra LRS Turbo; Caridian BCT | Calcium gluconate | 40 | 1,000,000 | 9* | South Korea | 63 | 24 | 24 |
| Vetrano et al.76, 2013 | PT | Extracorporeal shock wave therapy | 0 | MyCells Autologous Platelet Preparation System (Kaylight Ltd, RamatHasharon, Israel) | None | Not reported | 0.89–1.1 × 10^12 | 4~ | Italy | 27 | 37 | 9 |
| Ruiz-Moneo et al.61, 2013 | RC | Repair w/o tx | 0 | PRGF System1, B.T.I. Biotechnology Institute, Vitoria-Gasteiz, Spain | CaCl2 | 0 | 600,000 | Not reported | Spain | 56 | 30 | 39 |
| de Almeida et al.15, 2012 | ACL | Repair w/o tx | 0 | Haemonetics MCS1 9000 cell separato + specific kit for platelet apheresis 995-E (Haemonetics Corp, Braintree, Massachusetts) | CaCl2 | 0.91 | 1,185,166 | 20–40* | Brazil | 24 | 24 | 3 |
| Gumina et al., 2012 | RC | Repair w/o tx | None Reported | RegenKit; Regen Lab, Le MontSur-Lausanne, Switzerland) | None | 7000 | 400,000 | 0.4–0.5* | Italy | 61 | 41 | 35 |
| Cervellin et al., 2012 | ACL | Repair w/o tx | 0 | GPS II - Plasmax - Platelet Concentration System (Biomet Biologics, Warsaw, IN, USA) | CaCl2 and Thrombin | Not reported | Not reported | Not reported | Italy | 23 | 40 | 0 |
| Creaney et al.13, 2011 | LE | Whole blood | None Reported | None | None | Not reported | 652,000 | 2 | United Kingdom | 51 | 74 | 56 |
| Peerbooms et al.56, 2010 // Gosens et al.27, 2011 | LE | Corticosteroid | 0 | Recover™ Platelet Separation Kit (Biomet Biologics, Warsaw, Indiana) | None | Not reported | Not reported | 3 | Netherlands | 47 | 48 | 52 |
| de Jonge et al.16, 2011 // de Vos et al.17, 2011 // de Vos et al.18, 2010 | AT | Saline | 0 | GPS III Platelet Separation System (Biomet Biologics, Warsaw, Indiana) | Soluble type I collagen | Not reported | Not reported | 4 | Netherlands | 50 | 26 | 28 |
| Randelli et al.59, 2011 | RC | Repair w/o tx | 0 | GPS II - Plasmax - Platelet Concentration System (Biomet Biologics, Warsaw, IN, USA) | CaCl2 and Thrombin | Not reported | Not reported | 6 | Italy | 61 | 21 | 42 |
| Castricini et al., 2011 | RC | Repair w/o tx | 0 | None | CaCl2 | Not reported | Not reported | Not specified | Italy | 55 | 40 | 48 |
| Thanasas et al.10, 2011 | LE | Whole blood | 0 | GPS III Platelet Separation System (Biomet Biologics, Warsaw, Indiana) | None | 11,644 | 1,292,500 | 3 | Greece | 36 | 8 | 21 |
| Nin et al.51, 2009 | ACL | Repair w/o tx | None Reported | None | CaCl2 | Not reported | 837,000 | 4* | Spain | 26 | 78 | 22 |
multiple treatments
PRP gel
ACP
There was extensive variability in the way that PRP was prepared (Table 1, “Therapy”). PRP injection procedures also varied widely. For example, twenty different PRP kits were used and 6 studies used methods that did not utilize or describe the utilization of a specific kit. The most commonly used kits were the Biomet Biologics GPS II/III system (9 studies) and the Arthrex Autologous Conditioned Plasma (ACP) system (4 studies), which together make up 35% of included studies. Of the 37 studies included in this review, 17 did not activate PRP. All but 3 of the 18 studies that did include an activating agent used some form of calcium (CaCl2, calcium gluconate, or CaCl2 and thrombin) to activate PRP. Two studies used thrombin alone and one study used Type I collagen. Few studies attempted to quantify platelets (40.5%) or leukocytes (24.3%), but the majority reported the volume of PRP injection or gel (Table 2). The calculation of average platelet concentration excludes values from Vetrano et al., 2013 due to the reported platelet concentration being an extreme outlier (1,000,000-fold higher than the next highest reported concentration). The authors could not be reached for clarification.
Table 2.
Average PRP metrics
| Number of studies reporting value | Mean volume or concentration(±SD) | |
|---|---|---|
| PRP volume | 31 | 5.30 ± 5.76mL |
| Platelet concentration | 15 | 845,897 ± 298,033 platelets/uL |
| WBC concentration in leukocyte-poor CRP | 6 | 131 ± 279WBCs/uL |
| WBC concentration in leukocyte-rich CRP | 3 | 8,461 ± 2,759WBCs/uL |
There was extensive heterogeneity in the studies included in this review. Studies were conducted across 17 unique countries and included geographical regions of Europe (22 studies), Asia (9 studies), North America (3 studies), South America (2 studies), and Australia (1 study) (Table 1, “Demographics”). The mean patient age ranged from 23 to 66 years and the female-to-male ratio of study subjects ranged from 0 to 4.67. There was considerable heterogeneity in female-male ratios, which varied by country and by individual study. Average participant age also varied greatly across studies. The three highest mean ages amongst all studies were 66, 63, and 62, while the three lowest mean ages were 23, 24, and 26.
There was also a variety of clinical outcome measures used. A number of pathology-specific scores were reported, including the Shoulder Pain and Disability Index (SPADI), Patient-rated Tennis Elbow Evaluation (PRTEE) questionnaire, and International Knee Documentation Committee (IKDC) score. Even among studies investigating the same pathology, different pathology-specific clinical outcomes were reported. For example, of the 14 studies investigating PRP for rotator cuff pathology, 8 studies used the Constant score, 7 studies used the UCLA score, 6 studies used the Simple Shoulder Test (SST), 2 studies used the Oxford Shoulder Score (OSS), and 1 study used the Western Ontario Rotator Cuff (WORC) index.
Quantitative analysis of studies reporting VAS score
A total of 21 studies were included in the meta-analysis evaluating the efficacy of PRP versus various controls with respect to pain (VAS) for tendon and ligament injuries. VAS data from 21 of the 37 studies included in this review were available at baseline. 17 studies reported short-term VAS data and 14 studies reported long-term VAS data (Table 3). There was no significant difference in VAS between PRP and control at baseline. However, patients who received PRP reported having less pain at short-term follow-up (WMD: −0.72; 95% CI: −1.10, −0.34; p<0.01), long term follow-up (WMD: −0.84; 95% CI: −1.23, −0.44; p<0.01), and overall (WMD: −0.56; 95% CI: −0.76, −0.37; p<0.01; Supplement, Figure 1). Substantial heterogeneity was reported at baseline (I2: 72.0%, p<0.01), short term follow-up (I2: 72.5%, p<0.01), long term follow-up (I2: 76.1%, p<0.01), and overall (I2: 75.8%, p<0.01).
Table 3.
Meta-analysis comparisons by subgroups for VAS
| Subgroups | Number of studies | Number of participants | Statistical Method | Effect size |
|---|---|---|---|---|
| Baseline (Overall) | 21 | 1031 | Weighted Mean Difference (Random, 95% CI) | −0.29 (−0.58, 0.01) |
| Rotator Cuff | 8 | 469 | −0.21 (−0.60, 0.18) | |
| Tendinopathy | 2 | 68 | 0.56 (−0.35, 1.46) | |
| ACL | 3 | 104 | −1.84 (−2.51, −1.17) | |
| Lateral Epicondylitis | 8 | 390 | 0.01 (−0.30, 0.33) | |
| Short Term (Overall) | 17 | 844 | Weighted Mean Difference (Random, 95% CI) | −0.72 (−1.10, −0.34) |
| Rotator Cuff | 6 | 360 | −0.45 (−0.75, 0.15) | |
| Tendinopathy | 2 | 63 | −0.04 (−2.88, 2.81) | |
| ACL Lateral Epicondylitis |
1 8 |
37 384 |
−1.26 (−2.33, −0.19) −1.14 (−1.85, −0.43) |
|
| Long Term (Overall) | 14 | 771 | Weighted Mean Difference (Random, 95% CI) | −0.84 (−1.23, −0.44) |
| Rotator Cuff | 6 | 361 | −0.53 (−0.98, −0.09) | |
| Tendinopathy | 1 | 46 | −1.70 (−2.90, −0.50) | |
| ACL | 2 | 77 | −0.17 (−0.46, 0.12) | |
| Lateral Epicondylitis | 5 | 226 | −1.39 (−2.49, −0.29) |
To further explore if the heterogeneity could be explained by the tendon/ligament pathology, pathology subgroups (rotator cuff, tendinopathy, ACL, and lateral epicondylitis) were assessed within each time point. At baseline, the mean VAS pain score for rotator cuff, tendinopathy, and lateral epicondylitis subgroups did not differ between PRP treatment and control. However, patients with ACL injuries who received PRP treatment reported significantly less pain at baseline than controls (WMD: −1.84; 95% CI: −2.51, −1.17; p<0.01; Supplement, Figure 2). Further investigation will be needed to determine if the difference had to do with how the patients were randomized, or if there were other study-related factors leading to these results in studies treating ACL injury. Within each pathology subgroup, only rotator cuff showed significant heterogeneity (I2: 59.6%, p=0.02) while the other subgroups showed little to moderate heterogeneity that was not significant.
Short term follow-up:
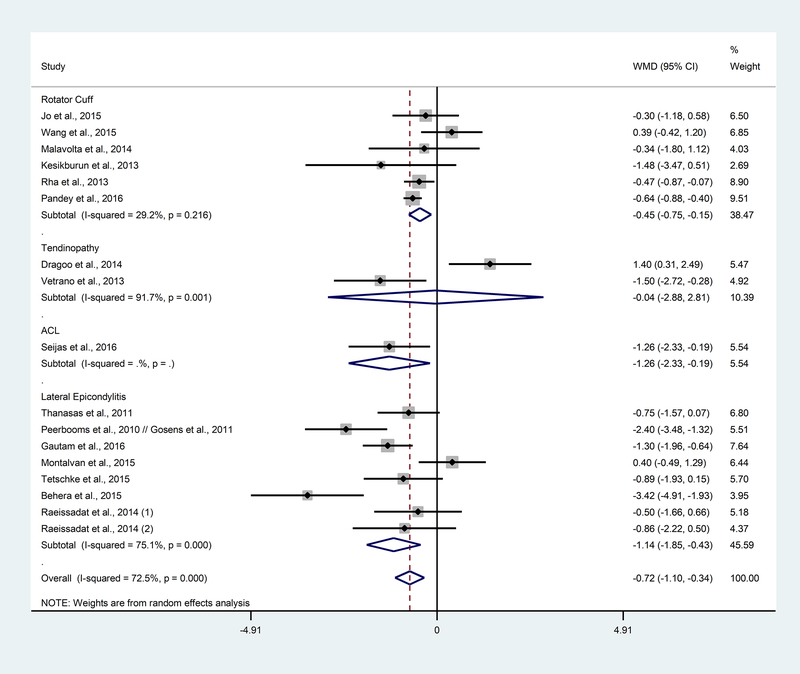
For short term (2–6.5 months) follow-up, there was no difference in pain between PRP-treated and control groups for patients with tendinopathy injury. Rotator cuff injury/pathology (WMD: −0.45; 95% CI: −0.75, −0.15; p<0.01), ACL injury (WMD: −1.26; 95% CI: −2.33, −0.19; p=0.02), and lateral epicondylitis (WMD: −1.14; 95% CI: −1.85, −0.43; p<0.01) showed a decrease in pain for the PRP group compared to control (Figure 2). Overall, the pooled results showed a significant difference in pain between PRP and control at short-term (WMD: −0.72; 95% CI: −1.10, −0.34; p<0.01). Since there were very few studies reported for tendinopathy and ACL, more data is needed to determine if the results from other similar studies are consistent with these findings. At short term follow-up, there was substantial heterogeneity (I2: 72.5%, p<0.01) between the studies overall. There was also substantial heterogeneity at the short-term follow-up in the tendinopathy (I2: 91.7%, p<0.01) and lateral epicondylitis (I2: 75.1%, p<0.01) pathology sub-groups.
Figure 2. Forest plot of the weighted mean difference between PRP and control by pathology subgroup at short term.
WMD=weighted mean difference; CI=confidence intervals.
Long term follow-up:
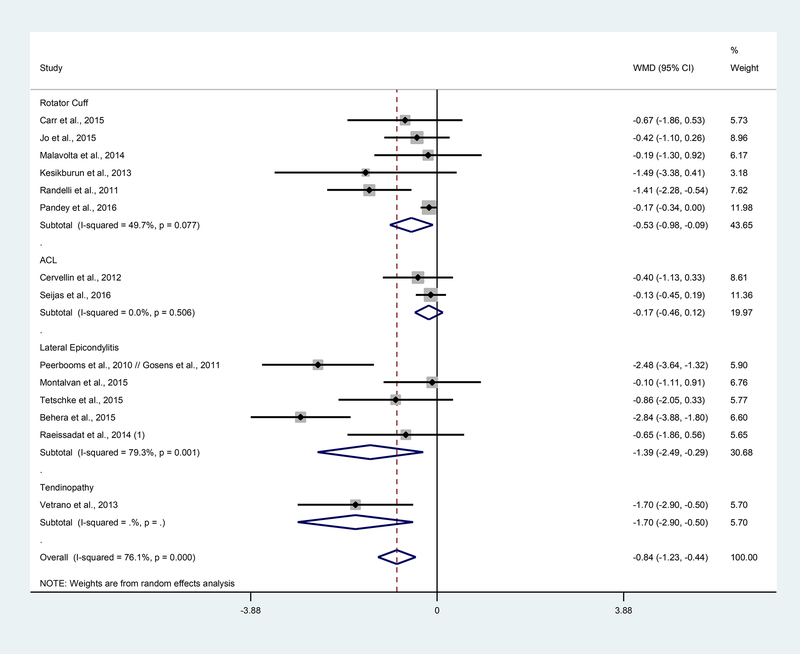
Overall, long term follow-up results showed less pain in the PRP group compared to control (WMD: −0.84; 95% CI: −1.23, −0.44; p<0.01). Looking at pathology subgroups, rotator cuff (WMD: −0.53; 95% CI: −0.98, −0.09; p=0.02), lateral epicondylitis (WMD: −1.39; 95% CI: −2.49, −0.29; p=0.01), and tendinopathy (WMD: −1.70; 95% CI: −2.90, −0.50; p<0.01) patients reported less pain when treated with PRP; PRP did not affect pain in the ACL pathology (Figure 3). There were only 1–2 ACL and tendinopathy studies available for meta-analysis at long term follow-up, so more data would need to be collected and analyzed to determine if these results remain consistent. There was considerable heterogeneity reported in lateral epicondylitis (I2: 79.3%, p<0.01) and for the overall studies in long term follow-up (I2: 76.1%, p<0.01).
Figure 3. Forest plot of the weighted mean difference between PRP and control by pathology subgroup at long term.
WMD=weighted mean difference; CI=confidence intervals.
To determine if the different pathologies were contributing to the heterogeneity, meta-regression using a permutation-based test approach was used. After adjusting for whether there was a baseline difference in PRP versus control, pathology did not appear to contribute to heterogeneity (short term: p=0.39, long term: p=0.29).
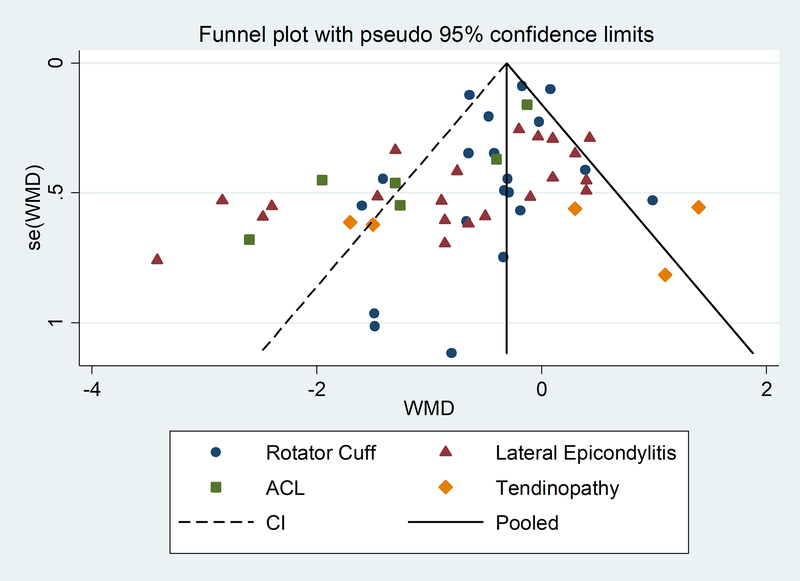
Publication bias was assessed including all the data collected using a funnel plot (Supplement, Figure 3). The funnel plot appeared to be asymmetric, with some missingness at the lower right portion of the plot suggesting possible publication bias, along with outliers, especially to the left. The outliers were predominantly from studies where the pathology of interest was ACL or lateral epicondylitis (Figure 4). When an Egger’s test was performed, there was indication of small-study effects (p<0.01), most likely due to heterogeneity. Separate funnel plots were produced for the time subgroups that showed that there were some data missing at the lower right portion of the funnel plot and some outliers (Supplement, Figure 5a, 6a, and 7a). The Egger’s test indicated that there was a small-study effect for the long-term results (p<0.01). Supplement Figure 4 shows the overall funnel plot with pathology subgroup indicators. When looking at each time subgroup by pathology, it was evident that outliers were predominately from ACL at baseline, and lateral epicondylitis at short and long term, suggesting possible heterogeneity due to pathology (Supplement, Figures 5b, 6b, and 7b).
Figure 4.
Funnel plot of meta-analysis by pathology subgroups
A sensitivity analysis was performed removing potential outliers observed from the funnel plots for all the data collected. The baseline time point became significant when the papers from Peerbooms et al. (WMD: −0.33; 95% CI: −0.64, −0.03; p=0.03), Vetrano et al. (WMD: −0.31; 95% CI: −0.61, −0.01; p=0.04), Dragoo et al. (WMD: −0.32; 95% CI: −0.62, −0.02; p=0.04), and Wang et al. (WMD: −0.34; 95% CI: −0.63, −0.04; p=0.03) were removed one at a time. For short-term follow-up, when the Dragoo et al. study was removed, the tendinopathy subgroup became significant (WMD: −1.50; 95% CI: −2.72, −0.28; p=0.02) although the overall short-term results did not change. For the long-term subgroup, lateral epicondylitis became non-significant when the Behera et al. or the Peerbooms et al. paper were removed. All other outliers did not change the results for either the pathology subgroups or overall results. None of these studies were removed from the final analysis as there was no indication of error in the data reported, and they were relevant to the study.
Discussion
Our review of the literature suggests that PRP for tendon and ligament pathology is safe; of the 1937 unique patients treated with PRP, no significant adverse events were reported. These results are in agreement with the existing literature, which concludes that PRP is a safe treatment option for injured musculoskeletal tissues.45
Our meta-analysis of VAS scores showed significant improvement in PRP-treated groups compared to control groups in patients with tendon and ligament injuries at both short-term and long-term follow-ups. These findings contrast those in Moraes et al., which reported no significant difference in pain reduction between patients treated with PRP versus alternative therapies. Moraes et al., however, only qualitatively analyzed a few studies, many of which were not of Level I evidence. Additionally, we found evidence that PRP significantly decreased pain compared to control treatments in patients with rotator cuff and lateral epicondylitis injuries. Our findings on rotator cuff healing contrast the results reported in a meta-analysis by Warth et al., which found no significant difference in VAS score improvement between PRP-treated patients and control population with rotator cuff tears. Our findings on lateral epicondylitis are in agreement with a recent meta-analysis by Arirachakaran et al.,5 which found that PRP treatment led to significantly improved VAS scores compared to alternative treatments in patients with lateral epicondylitis.
We choose to review the efficacy of PRP for both tendons and ligaments because the tissues are morphologically similar. This approach allowed us to include a large number of Level I studies in this review, but also has several limitations. While both tendons and ligaments are classified as dense regular connective tissue and the two tissue types share a number of similarities,22,26,74 there are inherent biological differences between tendons and ligaments that should be considered. One of the main differences between the tendons and ligaments is that ligaments are more metabolically active than tendons.1 Ligaments also have higher DNA content, more cellular nuclei, greater amounts of reducible cross-links, and are composed of more type III collagen by percentage. Even among different types of tendons there are clinically and biologically important differences36,52,66. For example, successful repair of short tendons (e.g. rotator cuff tendons) generally depends on tendon-to-bone integration, whereas effective repair of longer tendons (e.g. flexor tendons) usually depends more on prevention of repair-site gapping and maintenance of tendon gliding. 36 Nevertheless, we believe our approach is reasonable given the lack of standardization in both the literature and the treatment itself.
Common weaknesses of the studies included in this review were potential bias caused by small-study effects, reporting bias, and lack of blinding. Twelve studies included in the quantitative analysis were single-blind or not blinded at all, which may have contributed to bias. Data collected using a funnel plot showed asymmetry, which suggests publication bias, and an Egger’s test found evidence of small study effects. Overall, these biases are most likely due to heterogeneity in study populations such as average age, sex ratio and other study-related factors. Differences in pathology as a whole were not found to contribute significantly to heterogeneity, which might be a result of not having enough studies, especially for the ACL and tendinopathy groups. At baseline, outliers on the funnel plot were predominately from ACL studies. This suggest that heterogeneity may be caused by ACL studies, including the papers published by Seijas et al. and Cervellin et al. The VAS scores of PRP-treated patients in both of these studies were significantly (p<0.05) lower at baseline than those in the control group. Further analysis with more data is needed to determine if pathology or other study-related factors could have contributed to the heterogeneity in this study.
This review has several weaknesses. Firstly, heterogeneity in the way clinical outcomes were measured limited our ability to make quantitative comparisons. To accommodate for the heterogeneity, we focused exclusively on VAS scores when evaluating outcomes quantitatively. Additionally, sixteen of the studies that were included in this review only blinded patients or did not blind at all. Several studies experienced some patient loss to follow-up. However, the percentage lost to follow-up was small (<5% for most studies), so this factor is unlikely to have skewed the overall results. Another common weakness was the lack of clarification regarding the composition of PRP. No paper quantified the exact composition of the PRP ‘drug’. While growth factors (PDGF, VEGF, EGF, FGF85, TGF-B) within patients’ autologous whole blood do not vary significantly with age or gender, and the concentration of growth factors has not been strongly correlated with platelet count, the method of PRP preparation greatly impacts cytokine concentrations.43,53,81,82 Given that most studies prepare PRP using different PRP kits, there is unknown and unquantified variability in platelet count and growth factor concentration. In order to resolve the heterogeneity within PRP formulations, studies have attempted to establish classification systems for PRP.20,21,46 For example, DeLong et al.19 created the PAW system, which relies on absolute platelet count, manner of platelet activation, and presence or absence of white blood cells (WBCs). However, the lack of reported information on requisite factors, such as platelet count, precludes categorization of many studies. Without biochemical analysis, there is no way to determine how PRP composition affects clinical outcomes and makes comparing different studies less reliable.7 Finally, in addition to the controllable variability between studies, the inherent variability of PRP itself cannot be overlooked. Because PRP is a point-of-care autologous therapy, no two patients are receiving exactly the same treatment.82
In addition to platelets, PRP contains varying levels of leukocytes (monocytes, basophils, eosinophils, and neutrophils) that may either positively or negatively affect the repair process.41 The decision to include or exclude WBCs in PRP application is a major point of controversy. Leukocytes have been associated with inflammatory cytokines (IL-1, TNF-⍺), which could have potentially deleterious effects on tissue regeneration.71 However, a recent meta-analysis by Fitzpatrick et al. found that leukocyte-rich PRP, such as that in the Biomet GPS III, had a significantly greater positive effect on tendon healing than leukocyte-poor PRP. In this review, we observed various leukocyte concentrations in PRP formulations across studies, with 77% of studies not reporting leukocyte concentrations at all. This heterogeneity highlights a need to quantify and report WBC concentrations in future randomized, controlled studies in order to standardize PRP treatments and increase comparability between studies. Overall, the effect of leukocytes in PRP is not well-studied and warrants further investigation.
Platelet activation is another subject of extensive research, as the addition or exclusion of activation agents is likely to impact the efficacy of PRP. Activation has been suggested as a required process of PRP preparation11,39,87, but over half of the studies included in this review (57%) did not use any form of activation. A recent study comparing the different types of PRP activating agents found that PRP activated with CaCl2, thrombin, or CaCl2/thrombin combinations had significantly higher growth factor release compared to non-activated PRP and platelet-poor plasma. This suggests that the underlying therapeutic efficacy of many current studies may be limited by their lack of activation usage. Additionally, different activating agents release different concentrations of growth factors over time. For example, the CaCl2/thrombin combination produced higher growth factor release compared to CaCl2 alone or Type III collagen alone in the first hour, but CaCl2 activation alone produced the highest growth factor release over a 24 hours period. Thus, both the compound used to activate PRP and the time between activation and application affect the overall PRP formulation. More work is needed to standardize the use of activating agents in order to determine the most effective method of activation, if any is needed at all.
Because we did not focus on a specific pathology or anatomic region of the body, there is extensive heterogeneity in the studies. However, this review shows that even among studies investigating PRP for the same pathology, there is a great deal of variability in the literature itself. For example, rotator cuff was the only pathology subgroup that showed significant heterogeneity at baseline. This heterogeneity may be attributable to different degrees of injury in patient populations, as tear size ranged 10mm to 50mm, which includes the broad spectrum of partial to large tears.12 Another source of heterogeneity was the control treatment. For example, lateral epicondylitis studies used a variety of different control treatments, including betamethasone with lignocaine, saline, corticosteroids, bupivacaine, and whole blood. More data is needed to determine what factors may have contributed to the heterogeneity in the lateral epicondylitis group, as there was substantial heterogeneity reported in both short- and long-term VAS scores for this pathology.
The quantitative analysis conducted in this review has several limitations. In order to accommodate for the variability between studies and outcome measures, only studies that reported VAS scores were included in the quantitative analysis. While this allowed us to compare clinical efficacy across pathologies and include a large number of high quality studies, it also meant that only 21 of the 37 studies included in this review could be analyzed quantitatively. Another limitation of our quantitative analysis is VAS itself, which is generally less sensitive than pathology-specific outcome measures. Additionally, VAS scores were compared as if they were identical, despite the fact that there was some variability in the way that scores were obtained. For example, numeric, non-numeric, pathology-specific (e.g. VAS with neer test34), and 100-point VAS scores were considered equivalent and converted to a non-specific 10-point VAS score for quantitative analysis. Lastly, the quantitative analysis does not tell us whether PRP improves functional outcomes because VAS only measures pain severity. While a number of studies did report functional outcomes, the heterogeneity of the pathologies and outcome measures precluded meaningful comparison. Nevertheless, given the data available in the current literature, our analysis of VAS scores provides the most comprehensive evaluation of PRP’s efficacy in pain reduction to date.
The lack of comparability between studies highlights the need for standardization in the way outcomes are measured. The Patient-Reported Outcomes Measurement Information System (PROMIS®) is a recently developed tool that may help address this problem. PROMIS scores have correlated well with current measurement instruments used in orthopaedic evaluations and may help address study heterogeneity, reduce bias and increase comparability between studies.29 For instance, a recent study by Anthony et al.4 found that both the PROMIS Upper Extremity (UE) and PROMIS Physical Function (PF) instruments had good to excellent correlations with common shoulder instability patient-reported outcome instruments, including the American Shoulder and Elbow Surgeons (ASES) shoulder assessment form and Short Form-36 Health Survey Physical Function subscale (SF-36 PF). Overbeek et al.54 found that the PROMIS PF instrument moderately correlated with the Quick Disability of Arm, Shoulder and Hand (QuickDASH), suggesting that the new assessment tool may be used to measure upper extremity disability. The psychometrically validated, dynamic system measures patient-reported outcomes efficiently in study participants with a wide range of chronic diseases and demographic characteristics by administering survey questions based on the respondent’s previous answers. This computer adaptive testing provides precise measurements and eases survey burden on patients by screening out irrelevant questions.
This review highlights a critical need for PRP characterization and standardization, but more research is also needed on tendon and ligament biology itself. Tendon and ligament regeneration has proved an elusive goal for tissue engineering owing to the specialized nature of these tissues and the high mechanical demands placed on the extracellular matrix (ECM) of these structures in the human body.35 The mechanical environment impacts expression of extracellular matrix proteins, growth factors, transcription factors, and cytokines that can alter tendon structure and cell viability.36 The particular biological properties of the tendon extracellular matrix (ECM) have hindered attempts to promote regeneration35, and no agreement exists on the most optimal delivery method of in vivo growth factors.52 More work is needed to clarify the impact of PRP on scar formation and on how to maximize PRP utilization given the mechanical properties of tendons and ligaments. Future studies combining PRP and scaffold technology may help improve the structural performance of damaged and degenerated tissues, but have not been well-studied clinically. Studies have shown that, in tendon healing, scaffolds stimulate healing and protect the healing area from detrimental forces, thereby maintaining the integrity of the healing zone.69 Examples of previously investigated scaffolding materials include hydrogel, collagen, polyglycolic acid (PGLA), fibrin, chondrocytes, and combinations of these compounds.37,50,62 Overall, there is a need to investigate the biochemical properties of each material and how they impact the mechanical properties of tendons and ligaments in order to draw reliable conclusions and meaningful comparisons between PRP formulations.
Conclusion
This systematic review and meta-analysis has found evidence that suggests PRP may provide both short-term and long-term pain relief for tendon and ligament injuries and pathologies compared to alternative treatments. In particular, there was evidence that PRP is associated with pain in rotator cuff injuries and lateral epicondylitis, but there was insufficient evidence to draw conclusions for all other pathologies. While these findings are encouraging, the heterogeneous nature of the studies conducted to date and the failure to characterize the exact composition of the PRP ‘drug’ limits definitive conclusions. According to our review, PRP is safe and may be efficacious. However, we cannot issue recommendations for or against its usage until more homogenous, high-quality evidence on the optimal preparation, dosage, and efficacy is made available. The greatest limiting factor for PRP is the lack of standardization. More research needs to be conducted to understand how leukocyte inclusion, activation, and platelet concentration effect therapeutic efficacy.
Supplementary Material
What is known about this subject:
Platelet-rich plasma (PRP) is highly concentrated, autologously derived plasma that contains platelets and associated growth factors extracted from blood. Human and animal trials have shown mixed results regarding the efficacy of PRP to aid in healing and pain reduction in tendons and ligaments.
What this study adds to existing knowledge:
This is the first study to synthesize data exclusively from Level I randomized, controlled clinical trials for ligament and tendon injuries. Our meta-analysis provides the highest level and most current evidence on PRP’s ability to reduce pain in tendon and ligament injuries.
References
- 1.Amiel D, Frank C, Harwood F, Fronek J, Akeson W. Tendons and ligaments: a morphological and biochemical comparison. J Orthop Res. 1984;1(3):257–265. doi: 10.1002/jor.1100010305. [DOI] [PubMed] [Google Scholar]
- 2.Andia I, Latorre PM, Gomez MC, Burgos-Alonso N, Abate M, Maffulli N. Platelet-rich plasma in the conservative treatment of painful tendinopathy: a systematic review and meta-analysis of controlled studies. Br Med Bull. 2014;110(1):99–115. doi: 10.1093/bmb/ldu007. [DOI] [PubMed] [Google Scholar]
- 3.Andia I, Rubio-Azpeitia E, Maffulli N. Platelet-rich plasma modulates the secretion of inflammatory/angiogenic proteins by inflamed tenocytes. Clin Orthop Relat Res. 2015;473(5):1624–1634. doi: 10.1007/s11999-015-4179-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthony CA, Glass NA, Hancock K, Bollier M, Wolf BR, Hettrich CM. Performance of PROMIS Instruments in Patients With Shoulder Instability. The American Journal of Sports Medicine. 2017;45(2):449–453. doi: 10.1177/0363546516668304. [DOI] [PubMed] [Google Scholar]
- 5.Arirachakaran A, Sukthuayat A, Sisayanarane T, Laoratanavoraphong S, Kanchanatawan W, Kongtharvonskul J. Platelet-rich plasma versus autologous blood versus steroid injection in lateral epicondylitis: systematic review and network meta-analysis. J Orthop Traumatol. 2016;17(2):101–112. doi: 10.1007/s10195-015-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behera P, Dhillon M, Aggarwal S, Marwaha N, Prakash M. Leukocyte-poor platelet-rich plasma versus bupivacaine for recalcitrant lateral epicondylar tendinopathy. J Orthop Surg (Hong Kong). 2015;23(1):6–10. doi: 10.1177/230949901502300102. [DOI] [PubMed] [Google Scholar]
- 7.Breddin HK. Can platelet aggregometry be standardized? Platelets. 2005;16(3–4):151–158. doi: 10.1080/09537100400020161. [DOI] [PubMed] [Google Scholar]
- 8.Cai Y-Z, Zhang C, Lin X-J. Efficacy of platelet-rich plasma in arthroscopic repair of full-thickness rotator cuff tears: a meta-analysis. J Shoulder Elbow Surg. 2015;24(12):1852–1859. doi: 10.1016/j.jse.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Carr AJ, Murphy R, Dakin SG, et al. Platelet-Rich Plasma Injection With Arthroscopic Acromioplasty for Chronic Rotator Cuff Tendinopathy: A Randomized Controlled Trial. The American Journal of Sports Medicine. 2015;43(12):2891–2897. doi: 10.1177/0363546515608485. [DOI] [PubMed] [Google Scholar]
- 10.Castricini R, Longo UG, De Benedetto M, et al. Platelet-rich plasma augmentation for arthroscopic rotator cuff repair: a randomized controlled trial. The American Journal of Sports Medicine. 2011;39(2):258–265. doi: 10.1177/0363546510390780. [DOI] [PubMed] [Google Scholar]
- 11.Cavallo C, Roffi A, Grigolo B, et al. Platelet-Rich Plasma: The Choice of Activation Method Affects the Release of Bioactive Molecules. BioMed Research International. 2016;2016(1):6591717–6591717. doi: 10.1155/2016/6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cofield RH. Subscapular muscle transposition for repair of chronic rotator cuff tears. 1982;154(5). [PubMed] [Google Scholar]
- 13.Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, single-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. British journal of sports medicine. 2011;45(12):966–971. doi: 10.1136/bjsm.2010.082503. [DOI] [PubMed] [Google Scholar]
- 14.Davenport KL, Campos JS, Nguyen J, Saboeiro G, Adler RS, Moley PJ. Ultrasound-Guided Intratendinous Injections With Platelet-Rich Plasma or Autologous Whole Blood for Treatment of Proximal Hamstring Tendinopathy: A Double-Blind Randomized Controlled Trial. J Ultrasound Med. 2015;34(8):1455–1463. doi: 10.7863/ultra.34.8.1455. [DOI] [PubMed] [Google Scholar]
- 15.de Almeida AM, Demange MK, Sobrado MF, Rodrigues MB, Pedrinelli A, Hernandez AJ. Patellar tendon healing with platelet-rich plasma: a prospective randomized controlled trial. The American Journal of Sports Medicine. 2012;40(6):1282–1288. doi: 10.1177/0363546512441344. [DOI] [PubMed] [Google Scholar]
- 16.de Jonge S, de Vos RJ, Weir A, et al. One-year follow-up of platelet-rich plasma treatment in chronic Achilles tendinopathy: a double-blind randomized placebo-controlled trial. The American Journal of Sports Medicine. 2011;39(8):1623–1629. doi: 10.1177/0363546511404877. [DOI] [PubMed] [Google Scholar]
- 17.de Vos RJ, Weir A, Tol JL, Verhaar JAN, Weinans H, van Schie HTM. No effects of PRP on ultrasonographic tendon structure and neovascularisation in chronic midportion Achilles tendinopathy. British journal of sports medicine. 2011;45(5):387–392. doi: 10.1136/bjsm.2010.076398. [DOI] [PubMed] [Google Scholar]
- 18.de Vos RJ, Weir A, van Schie HTM, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144–149. doi: 10.1001/jama.2009.1986. [DOI] [PubMed] [Google Scholar]
- 19.DeLong JM, Russell RP, Mazzocca AD. Platelet-rich plasma: the PAW classification system. Arthroscopy. 2012;28(7):998–1009. doi: 10.1016/j.arthro.2012.04.148. [DOI] [PubMed] [Google Scholar]
- 20.Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang C-Q, Pinto NR, Bielecki T. Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 2014;4(1):3–9. [PMC free article] [PubMed] [Google Scholar]
- 21.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 22.Doroski DM, Brink KS, Temenoff JS. Techniques for biological characterization of tissue-engineered tendon and ligament. Biomaterials. 2007;28(2):187–202. doi: 10.1016/j.biomaterials.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 23.Dragoo JL, Wasterlain AS, Braun HJ, Nead KT. Platelet-rich plasma as a treatment for patellar tendinopathy: a double-blind, randomized controlled trial. The American Journal of Sports Medicine. 2014;42(3):610–618. doi: 10.1177/0363546513518416. [DOI] [PubMed] [Google Scholar]
- 24.Galatz LM, Gerstenfeld L, Heber-Katz E, Rodeo SA. Tendon regeneration and scar formation: The concept of scarless healing. Journal of Orthopaedic Research. 2015;33(6):823–831. doi: 10.1002/jor.22853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gautam VK, Verma S, Batra S, Bhatnagar N, Arora S. Platelet-rich plasma versus corticosteroid injection for recalcitrant lateral epicondylitis: clinical and ultrasonographic evaluation. J Orthop Surg (Hong Kong). 2015;23(1):1–5. doi: 10.1177/230949901502300101. [DOI] [PubMed] [Google Scholar]
- 26.Goh JC-H, Ouyang H-W, Teoh S-H, Chan CKC, Lee EH. Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng. 2003;9 Suppl 1(supplement 1):S31–S44. doi: 10.1089/10763270360696969. [DOI] [PubMed] [Google Scholar]
- 27.Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. The American Journal of Sports Medicine. 2011;39(6):1200–1208. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 28.Hudgens JL, Sugg KB, Grekin JA, Gumucio JP, Bedi A, Mendias CL. Platelet-Rich Plasma Activates Proinflammatory Signaling Pathways and Induces Oxidative Stress in Tendon Fibroblasts. The American Journal of Sports Medicine. 2016;44(8):1931–1940. doi: 10.1177/0363546516637176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hung M, Stuart AR, Higgins TF, Saltzman CL, Kubiak EN. Computerized Adaptive Testing Using the PROMIS Physical Function Item Bank Reduces Test Burden With Less Ceiling Effects Compared With the Short Musculoskeletal Function Assessment in Orthopaedic Trauma Patients. J Orthop Trauma. 2014;28(8):439–443. doi: 10.1097/BOT.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 30.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. The Journal of hand surgery. 2008;33(1):102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Jo CH, Shin JS, Lee YG, et al. Platelet-Rich Plasma for Arthroscopic Repair of Large to Massive Rotator Cuff Tears A Randomized, Single-Blind, Parallel-Group Trial. Am J Sports Med. 2013;41(10):0363546513497925–2248. doi: 10.1177/0363546513497925. [DOI] [PubMed] [Google Scholar]
- 32.Jo CH, Shin JS, Shin WH, Lee SY, Yoon KS, Shin S. Platelet-rich plasma for arthroscopic repair of medium to large rotator cuff tears: a randomized controlled trial. The American Journal of Sports Medicine. 2015;43(9):2102–2110. doi: 10.1177/0363546515587081. [DOI] [PubMed] [Google Scholar]
- 33.Kelly BA, Proffen BL, Haslauer CM, Murray MM. Platelets and plasma stimulate sheep rotator cuff tendon tenocytes when cultured in an extracellular matrix scaffold. J Orthop Res. 2016;34(4):623–629. doi: 10.1002/jor.23058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kesikburun S, Tan AK, Yilmaz B, Yaşar E, Yazicioğlu K. Platelet-rich plasma injections in the treatment of chronic rotator cuff tendinopathy: a randomized controlled trial with 1-year follow-up. The American Journal of Sports Medicine. 2013;41(11):2609–2616. doi: 10.1177/0363546513496542. [DOI] [PubMed] [Google Scholar]
- 35.Kew SJ, Gwynne JH, Enea D, et al. Regeneration and repair of tendon and ligament tissue using collagen fibre biomaterials. Acta Biomater. 2011;7(9):3237–3247. doi: 10.1016/j.actbio.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Killian ML, Cavinatto L, Galatz LM, Thomopoulos S. The role of mechanobiology in tendon healing. J Shoulder Elbow Surg. 2012;21(2):228–237. doi: 10.1016/j.jse.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim BS, Kim EJ, Choi JS, Jeong JH, Jo CH, Cho YW. Human collagen-based multilayer scaffolds for tendon-to-bone interface tissue engineering. J Biomed Mater Res A. 2014;102(11):4044–4054. doi: 10.1002/jbm.a.35057. [DOI] [PubMed] [Google Scholar]
- 38.Krogh TP, Fredberg U, Stengaard-Pedersen K, Christensen R, Jensen P, Ellingsen T. Treatment of lateral epicondylitis with platelet-rich plasma, glucocorticoid, or saline: a randomized, double-blind, placebo-controlled trial. The American Journal of Sports Medicine. 2013;41(3):625–635. doi: 10.1177/0363546512472975. [DOI] [PubMed] [Google Scholar]
- 39.Kutlu B, TiğlƖ AydƖn RS, Akman AC, Gümüşderelioglu M, Nohutcu RM. Platelet-rich plasma-loaded chitosan scaffolds: preparation and growth factor release kinetics. J Biomed Mater Res Part B Appl Biomater. 2013;101(1):28–35. doi: 10.1002/jbm.b.32806. [DOI] [PubMed] [Google Scholar]
- 40.Lana JF, Weglein A, Vicente E, et al. Platelet Rich Plasma and Its Growth Factors: The State of the Art. In: Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. doi: 10.1007/978-3-642-40117-6_1. [DOI] [Google Scholar]
- 41.LaPrade RF, Geeslin AG, Murray IR, et al. Biologic Treatments for Sports Injuries II Think Tank-Current Concepts, Future Research, and Barriers to Advancement, Part 1: Biologics Overview, Ligament Injury, Tendinopathy. The American Journal of Sports Medicine. 2016;44(12):3270–3283. doi: 10.1177/0363546516634674. [DOI] [PubMed] [Google Scholar]
- 42.Lebiedziński R, Synder M, Buchcic P, Polguj M, Grzegorzewski A, Sibiński M. A randomized study of autologous conditioned plasma and steroid injections in the treatment of lateral epicondylitis. Int Orthop. 2015;39(11):2199–2203. doi: 10.1007/s00264-015-2861-0. [DOI] [PubMed] [Google Scholar]
- 43.Liao H-T, Marra KG, Rubin JP. Application of platelet-rich plasma and platelet-rich fibrin in fat grafting: basic science and literature review. Tissue Eng Part B Rev. 2014;20(4):267–276. doi: 10.1089/ten.TEB.2013.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malavolta EA, Conforto Gracitelli ME, Ferreira Neto AA, Henrique Assuncao J, Bordalo-Rodrigues M, de Camargo OP. Platelet-Rich Plasma in Rotator Cuff Repair A Prospective Randomized Study. Am J Sports Med. 2014;42(10):2446–2454. doi: 10.1177/0363546514541777. [DOI] [PubMed] [Google Scholar]
- 45.McCarrel TM, Mall NA, Lee AS, Cole BJ, Butty DC, Fortier LA. Considerations for the use of platelet-rich plasma in orthopedics. Sports Med. 2014;44(8):1025–1036. doi: 10.1007/s40279-014-0195-5. [DOI] [PubMed] [Google Scholar]
- 46.Mishra A, Harmon K, Woodall J, Vieira A. Sports medicine applications of platelet rich plasma. 2012;13(7). [DOI] [PubMed] [Google Scholar]
- 47.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montalvan B, Le Goux P, Klouche S, Borgel D, Hardy P, Breban M. Inefficacy of ultrasound-guided local injections of autologous conditioned plasma for recent epicondylitis: results of a double-blind placebo-controlled randomized clinical trial with one-year follow-up. Rheumatology (Oxford). 2016;55(2):279–285. doi: 10.1093/rheumatology/kev326. [DOI] [PubMed] [Google Scholar]
- 49.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Moraes VY, ed. Cochrane Database Syst Rev. 2014;40(4):CD010071. doi: 10.1002/14651858.CD010071.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murray MM, Spindler KP, Devin C, et al. Use of a collagen-platelet rich plasma scaffold to stimulate healing of a central defect in the canine ACL. J Orthop Res. 2006;24(4):820–830. doi: 10.1002/jor.20073. [DOI] [PubMed] [Google Scholar]
- 51.Nin JRV, Gasque GM, Azcárate AV, Beola JDA, Gonzalez MH. Has platelet-rich plasma any role in anterior cruciate ligament allograft healing? Arthroscopy. 2009;25(11):1206–1213. doi: 10.1016/j.arthro.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Nourissat G, Berenbaum F, Duprez D. Tendon injury: from biology to tendon repair. Nature Reviews Rheumatology. 2015;11(4):223–233. doi: 10.1038/nrrheum.2015.26. [DOI] [PubMed] [Google Scholar]
- 53.Oh JH, Kim W, Park KU, Roh YH. Comparison of the Cellular Composition and Cytokine-Release Kinetics of Various Platelet-Rich Plasma Preparations. The American Journal of Sports Medicine. 2015;43(12):3062–3070. doi: 10.1177/0363546515608481. [DOI] [PubMed] [Google Scholar]
- 54.Overbeek CL, Nota SPFT, Jayakumar P, Hageman MG, Ring D. The PROMIS physical function correlates with the QuickDASH in patients with upper extremity illness. Clin Orthop Relat Res. 2015;473(1):311–317. doi: 10.1007/s11999-014-3840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandey V, Bandi A, Madi S, et al. Does application of moderately concentrated platelet-rich plasma improve clinical and structural outcome after arthroscopic repair of medium-sized to large rotator cuff tear? A randomized controlled trial. J Shoulder Elbow Surg. 2016;25(8):1312–1322. doi: 10.1016/j.jse.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 56.Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. The American Journal of Sports Medicine. 2010;38(2):255–262. doi: 10.1177/0363546509355445. [DOI] [PubMed] [Google Scholar]
- 57.Raeissadat SA, Rayegani SM, Hassanabadi H, Rahimi R, Sedighipour L, Rostami K. Is Platelet-rich plasma superior to whole blood in the management of chronic tennis elbow: one year randomized clinical trial. BMC Sports Science, Medicine and Rehabilitation 2015 7:1. 2014;6(1):12. doi: 10.1186/2052-1847-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raeissadat SA, Sedighipour L, Rayegani SM, Bahrami MH, Bayat M, Rahimi R. Effect of Platelet-Rich Plasma (PRP) versus Autologous Whole Blood on Pain and Function Improvement in Tennis Elbow: A Randomized Clinical Trial. Pain Research and Treatment. 2014;2014(2):191525–191528. doi: 10.1155/2014/191525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Randelli P, Arrigoni P, Ragone V, Aliprandi A, Cabitza P. Platelet rich plasma in arthroscopic rotator cuff repair: a prospective RCT study, 2-year follow-up. J Shoulder Elbow Surg. 2011;20(4):518–528. doi: 10.1016/j.jse.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Rha D-W, Park G-Y, Kim Y-K, Kim MT, Lee SC. Comparison of the therapeutic effects of ultrasound-guided platelet-rich plasma injection and dry needling in rotator cuff disease: a randomized controlled trial. Clin Rehabil. 2013;27(2):113–122. doi: 10.1177/0269215512448388. [DOI] [PubMed] [Google Scholar]
- 61.Ruiz-Moneo P, Molano-Muñoz J, Prieto E, Algorta J. Plasma rich in growth factors in arthroscopic rotator cuff repair: a randomized, double-blind, controlled clinical trial. Arthroscopy. 2013;29(1):2–9. doi: 10.1016/j.arthro.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 62.Salamanna F, Veronesi F, Maglio M, Bella Della E, Sartori M, Fini M. New and emerging strategies in platelet-rich plasma application in musculoskeletal regenerative procedures: general overview on still open questions and outlook. BioMed Research International. 2015;2015(9):846045–24. doi: 10.1155/2015/846045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3–4):165–174. doi: 10.1007/s12178-008-9032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seijas R, Cusco X, Sallent A, Serra I, Ares O, Cugat R. Pain in donor site after BTB-ACL reconstruction with PRGF: a randomized trial. Archives of Orthopaedic and Trauma Surgery. 2016;136(6):829–835. doi: 10.1007/s00402-016-2458-0. [DOI] [PubMed] [Google Scholar]
- 65.Sharma P, Maffulli N. Basic biology of tendon injury and healing. The surgeon. 2005;3(5):309–316. doi: 10.1016/S1479-666X(05)80109-X. [DOI] [PubMed] [Google Scholar]
- 66.Sharma P, Maffulli N. Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact. 2006;6(2):181–190. [PubMed] [Google Scholar]
- 67.Sharma P, Maffulli N. Tendinopathy and tendon injury: the future. Disabil Rehabil. 2008;30(20–22):1733–1745. doi: 10.1080/09638280701788274. [DOI] [PubMed] [Google Scholar]
- 68.Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 69.Smith BD, Grande DA. The current state of scaffolds for musculoskeletal regenerative applications. Nature Reviews Rheumatology. 2015;11(4):213–222. doi: 10.1038/nrrheum.2015.27. [DOI] [PubMed] [Google Scholar]
- 70.Stewart LA, Clarke M, Rovers M, et al. Preferred Reporting Items for Systematic Review and Meta-Analyses of individual participant data: the PRISMA-IPD Statement. JAMA. 2015;313(16):1657–1665. doi: 10.1001/jama.2015.3656. [DOI] [PubMed] [Google Scholar]
- 71.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. The American Journal of Sports Medicine. 2011;39(10):2135–2140. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 72.Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21(4):344–352. doi: 10.1097/JSM.0b013e31821d0f65. [DOI] [PubMed] [Google Scholar]
- 73.Tetschke E, Rudolf M, Lohmann CH, Stärke C. Autologous proliferative therapies in recalcitrant lateral epicondylitis. Am J Phys Med Rehabil. 2015;94(9):696–706. doi: 10.1097/PHM.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 74.Thomopoulos S, Williams GR, Gimbel JA, Favata M, Soslowsky LJ. Variation of biomechanical, structural, and compositional properties along the tendon to bone insertion site. J Orthop Res. 2003;21(3):413–419. doi: 10.1016/S0736-0266(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 75.Vavken P, Sadoghi P, Palmer M, et al. Platelet-Rich Plasma Reduces Retear Rates After Arthroscopic Repair of Small- and Medium-Sized Rotator Cuff Tears but Is Not Cost-Effective. The American Journal of Sports Medicine. 2015;43(12):3071–3076. doi: 10.1177/0363546515572777. [DOI] [PubMed] [Google Scholar]
- 76.Vetrano M, Castorina A, Vulpiani MC, Baldini R, Pavan A, Ferretti A. Platelet-rich plasma versus focused shock waves in the treatment of jumper’s knee in athletes. The American Journal of Sports Medicine. 2013;41(4):795–803. doi: 10.1177/0363546513475345. [DOI] [PubMed] [Google Scholar]
- 77.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang A, McCann P, Colliver J, et al. Do postoperative platelet-rich plasma injections accelerate early tendon healing and functional recovery after arthroscopic supraspinatus repair? A randomized controlled trial. The American Journal of Sports Medicine. 2015;43(6):1430–1437. doi: 10.1177/0363546515572602. [DOI] [PubMed] [Google Scholar]
- 79.Warth RJ, Dornan GJ, James EW, Horan MP, Millett PJ. Clinical and structural outcomes after arthroscopic repair of full-thickness rotator cuff tears with and without platelet-rich product supplementation: a meta-analysis and meta-regression. Arthroscopy. 2015;31(2):306–320. doi: 10.1016/j.arthro.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Weber SC, Kauffman JI, Parise C, Weber SJ, Katz SD. Platelet-rich fibrin matrix in the management of arthroscopic repair of the rotator cuff: a prospective, randomized, double-blinded study. The American Journal of Sports Medicine. 2013;41(2):263–270. doi: 10.1177/0363546512467621. [DOI] [PubMed] [Google Scholar]
- 81.Weibrich G, Kleis WK, Streckbein P, Moergel M, Hitzler WE, Hafner G. Comparison of point-of-care methods for preparation of platelet concentrate (platelet-rich plasma. 2012;27(4). [PubMed] [Google Scholar]
- 82.Weibrich G, Kleis WKG, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30(2):97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 83.Yang J, Sun Y, Xu P, Cheng B. Can patients get better clinical outcomes by using PRP in rotator cuff repair: a meta-analysis of randomized controlled trials. 2016;56(11). [PubMed] [Google Scholar]
- 84.Yuan T, Zhang CQ, Wang JH. Augmenting tendon and ligament repair with platelet-rich plasma (PRP. 2013;3(3). [PMC free article] [PubMed] [Google Scholar]
- 85.Yun Y-R, Won JE, Jeon E, et al. Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng. 2010;2010(1):218142–218142. doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhao J-G, Zhao L, Jiang Y-X, Wang Z-L, Wang J, Zhang P. Platelet-rich plasma in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Arthroscopy. 2015;31(1):125–135. doi: 10.1016/j.arthro.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 87.Zhu Y, Yuan M, Meng HY, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthr Cartil. 2013;21(11):1627–1637. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 88.Zou J, Mo X, Shi Z, et al. A Prospective Study of Platelet-Rich Plasma as Biological Augmentation for Acute Achilles Tendon Rupture Repair. BioMed Research International. 2016;2016(1):9364170–9364178. doi: 10.1155/2016/9364170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zumstein MA, Rumian A, Thélu CÉ, et al. SECEC Research Grant 2008 II: Use of platelet- and leucocyte-rich fibrin (L-PRF) does not affect late rotator cuff tendon healing: a prospective randomized controlled study. J Shoulder Elbow Surg. 2016;25(1):2–11. doi: 10.1016/j.jse.2015.09.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.