Abstract
PURPOSE
Transcatheter arterial embolization (TAE) is increasingly used as the first-line treatment for hemorrhage complicating pancreatitis and post-pancreatectomy. However, the optimal therapeutic strategy remains unclear.
METHODS
Among 1924 consecutive patients, 40 patients with severe pancreatic hemorrhage in Xuanwu Hospital were enrolled between 2005 and 2017. Patients underwent angiography and direct TAE for primary diagnosis and treatment of bleeding. Repeat TAE, watch and wait, and laparotomy were used as the other therapeutic options. Patient data, technical success, and 90-day survival were identified.
RESULTS
Pancreatic diseases underlying hemorrhage included acute pancreatitis (n=19, 47.5%), chronic pancreatitis (n=12, 30%), and pancreatic cancer (n=9, 22.5%). A history of percutaneous catheter drainage or pancreatic surgery was seen in 29 patients (72.5%). There were 48 angiographies, 31 embolizations, and 5 laparotomies performed. Rebleeding occurred in 8 patients (20%); 4 of whom underwent re-embolization, 3 had laparotomy, and 1 had conservative treatment. Successful clinical hemostasis was achieved in 37 patients. Complications were observed in only 2 patients with renal failure and 1 patient with hepatic insufficiency. In total, 25 patients (62.5%) were alive at the 90-day follow-up.
CONCLUSION
Endovascular management is effective for achieving hemostasis in severe pancreatic hemorrhage with a high success rate and low recurrence, and laparotomy is not suitable for rebleeding cases.
Pancreas-associated hemorrhage is an uncommon but severe complication of pancreatic diseases. It includes acute pancreatitis (AP), chronic pancreatitis (CP), and iatrogenic bleeding, such as post-pancreatectomy hemorrhage (PPH) and pancreatic necrosectomy. Since pancreatic enzymes are released during pancreatitis or post-pancreatectomy, vascular structures, particularly the visceral arteries, are corroded by the proteolytic activity of these enzymes. The resulting acute hemorrhage has been recognized as a rapidly lethal condition, and the reported mortality rates range from 40% to 50% in patients with pancreatitis (1, 2) and from 30% to 50% in patients with PPH (3, 4). Recently, owing to rapid diagnoses using angiography and prompt retrieval using endovascular intervention, the mortality rate has been reduced considerably. However, the initial step and overall therapeutic strategy for pancreatic intra-abdominal hemorrhage or bleeding pseudoaneurysm remain controversial, particularly because of the failure to detect the bleeding site and to control the bleeding. Moreover, studies regarding massive hemorrhage are limited to review articles and short case series with the hemostasis methods, which still have no best therapeutic strategy.
The aim of the present study was to report a center-specific experience with angiography and transcatheter arterial embolization (TAE) as the first-step choice for the treatment of massive pancreatic hemorrhage, including severe bleeding pseudoaneurysm with AP, CP and late PPH, to illustrate the effectiveness of this strategy and to compare the outcomes between the TAE procedures and surgical intervention in the management of pancreatic hemorrhage. Moreover, in the present study, the pre-hemorrhage clinical characteristics and other recent 10-year studies investigating pancreatic hemorrhage were reviewed.
Methods
This retrospective, observational study was conducted at the Department of General Surgery, Xuanwu Hospital, Capital Medical University. The local ethics committee approved the study according to the Declaration of Helsinki. Informed consent was obtained from each participant.
Patient selection
A retrospective review of the administrative diagnostic database was performed to identify patients with pancreatitis (International Classification of Disease (ICD)-10 codes K85 and K86) and pancreatic cancer (ICD-10 code C25) who were treated at Xuanwu Hospital of the Capital Medical University between January 2005 and December 2017. The medical records of the patients who underwent an endovascular procedure for acute hemorrhage, including gastrointestinal bleeding (intraluminal) and intraperitoneal bleeding/bleeding in the drainage (extraluminal), were obtained. Patients were enrolled in the study if they met the following criteria: 1) severe extra- or intraluminal hemorrhage in AP and CP associated with clinical shock and a rapid decrease in hemoglobin concentration >30 g/L requiring blood transfusion and 2) late PPH, defined as any hemorrhagic event that occurred >24 h after the end of pancreatic surgery and graded B or C according to the International Study Group of Pancreatic Surgery (ISGPS) definition (5). Exclusion criteria were as follows: 1) early PPH (<24 h after the end of surgery) caused by insufficient hemostasis; 2) mucosal hemorrhage caused by peptic ulcer, anastomotic ulcer, or anastomotic dehiscence after pancreatic surgery; 3) stress gastrointestinal bleeding, hemorrhage in the abdominal wall vessel or muscle from the sinus tract after surgery or drainage intervention; and 4) direct surgical treatment as the primary treatment for hemorrhage without angiography diagnosis. Medical data regarding the patients’ prehemorrhage clinical characteristics, management, and outcomes were retrospectively analyzed.
Angiography and TAE procedure
Endovascular treatment was performed if the hemodynamic stability of the patients was successfully maintained by fluids, blood transfusion (packed red blood cells and fresh frozen plasma), vasoconstrictor pumping, and ice saline stomach washing for intraluminal bleeding or uterine cavity gauze abdominal packing for extraluminal bleeding. All patients underwent angiography performed by four board-certified radiologists with 10 to 20 years of clinical experience in endovascular therapy. Digital subtraction angiography (DSA) was performed in all patients by accessing the femoral artery using a 5 F Simmons 1 catheter (Cook Medical). Superselective catheterization was achieved using a coaxially introduced 2.8 F microcatheter (Transcend; Boston Scientific). The TAE procedure was performed using pushable steel coils/microcoils (MWCE-35 3-3/5-8 or MWCE-18S 3-2; Cook Medical) and N-butyl cyanoacrylate (NBCA) (Glubran 2; GEM Srl) mixed with contrast at ratios ranging from 1:1 to 1:3 and/or gelfoam. The coaxial technique was used in all patients to prevent back bleeding, and the standard “two-point” technique was used to treat embolism and to control the bleeding artery both proximal and distal to the vascular lesion employing coils and/or NBCA; the sac was also packed with materials to treat embolism. After embolization, angiography was immediately repeated to confirm that the bleeding had ceased. The vascular sheath was removed after 24 h in case of recurrent bleeding.
Surgical intervention
The indication for urgent laparotomy was based on the following findings: 1) obvious bleeding that drained percutaneously via the abdominal drainage or nasogastric tube that was considered inaccessible to endovascular treatment; 2) serious ischemic complications caused by the TAE procedure, including ischemia of the intestinal artery and distal colic artery; and (3) failure of the TAE or repeat TAE procedures.
Outcomes
Patients were retrospectively followed up for 90 days after the treatments. The primary end point was technical success, which was defined as the absence of presentation for further hemorrhage. The secondary end points were as follows: 1) rebleeding, which was defined as the requirement for an additional procedure (angiography, repeat TAE, or laparotomy) due to hemorrhage; 2) 90-day survival, which was defined as patients who lived for 90 days after the hemorrhage and interventional treatment during follow-up; and 3) major complications (renal failure, hepatic insufficiency, and bowel ischemia), which was defined according to international reporting standards (6).
Statistical analysis
Patient characteristics, clinical presentations, and outcomes are expressed as median, interquartile range (IQR), or number (%), unless otherwise specified. The SPSS version 17.0 software (SPSS Inc.) was used for statistical analysis. Chi-square and Fisher’s exact test were used to determine the advantage of the technique of hemostasis and the association between hemorrhage and patient clinical characteristics. A P value <0.05 was considered statistically significant.
Results
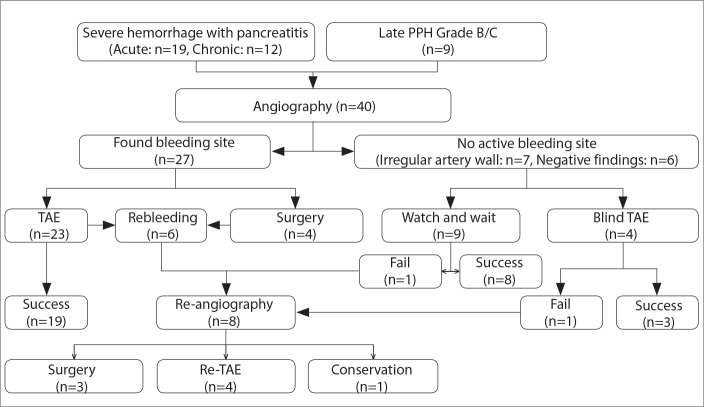
A total of 1924 patients were identified between January 2005 and December 2017, with 306 patients with pancreatic tumor and 1618 patients with AP or CP. Of the patients, 658 (34.2%) underwent surgical or interventional treatment. Of the 1924 patients, 40 (2.1%) who experienced severe hemorrhage were included in the study. Nine patients experienced PPH, while severe hemorrhage was attributed to AP in 19 patients and CP in 12 patients. Patient data included the endovascular and surgical management of hemorrhage (Fig. 1).
Figure 1.
Algorithm for the management of severe hemorrhage complicating pancreatitis and post-pancreatectomy. PPH, post-pancreatectomy hemorrhage; TAE, transarterial embolization.
The most common severe hemorrhage symptoms were bleeding from the abdominal drainage (n=28, 70%) and gastrointestinal tract (n=15, 37.5%). Other symptoms included severe abdominal pain and high fever (>39°C). In total, 22 patients (55%) presented with hemorrhagic shock with a heart rate >100 beats/min and systolic blood pressure <100 mm Hg. The amylase levels before the endovascular procedure, particularly those in the drainage, were extremely high as shown in Table 1 (median, 600 U/L; IQR, 310–15 609 U/L), and these data were available for 23 patients. Table 1 shows the demographic and medical history of the study patients.
Table 1.
Patient baseline characteristics and medical history
| Patient characteristics (n=40) | |
|---|---|
| Gender (male/female) | 29/11 |
|
| |
| Age (years), median (IQR) | 51 (41.0–62.0) |
|
| |
| BMI (kg/m2), median (IQR) | 26.8 (24.9–28.2) |
|
| |
| Disease cause, n (%) | |
| Acute pancreatitis | 19 (47.5) |
| Chronic pancreatitis | 12 (30) |
| Pancreatic tumor | 9 (22.5) |
|
| |
| Signal bleeding symptom, n (%) | |
| Bleeding in drainage | 28 (70) |
| GI bleeding | 15 (37.5) |
| Both bleeding GI and drainage | 3 (7.5) |
|
| |
| Hemorrhagic shock, n (%) | 22 (55) |
| Severe abdominal pain | 10 (25) |
| High fever (>39 °C) | 4 (10) |
|
| |
| Laboratory index, median (IQR) | |
| Amylase of blood before DSA (U/L) | 359 (69–1103) |
| Lipase of blood before DSA (U/L) | 233 (65–811) |
| Amylase of drainage before DSA (U/L) | 600 (310–15 609) |
| Hemoglobin before DSA (g/L) | 65 (36–80) |
| Platelet before DSA (×1010/L) | 8.6 (21–215) |
| APTT before DSA (s) | 75 (52–135) |
|
| |
| Blood transfusion, units of packed RBCs, median (IQR) | 30 (15–45) |
|
| |
| Duration in ICU (days), median (IQR) | 22 (12–52) |
|
| |
| Intervention or surgery before bleeding, n (%) | 29 (72.5) |
| Debridement | 16 (55.2) |
| PCD | 4 (13.8) |
| Whipple | 9 (34.5) |
|
| |
| Bleeding time after surgery (days), median (IQR) | 9 (5.8–17.5) |
IQR, interquartile range; BMI, body mass index; GI, gastrointestinal tract; DSA, digital subtraction angiography; APTT, activated partial thromboplastin time; RBC, red blood cells; ICU, intensive care unit; PCD, percutaneous catheter drainage.
Concurrently, 29 patients (72.5%) had a history of interventional therapy or surgery, and the hemorrhage rate was 4.4% in 658 patients who underwent iatrogenic procedure. However, the hemorrhage rate with no surgical intervention was only 0.87% (χ2=21.812, P = 0.000). Of the 29 patients who underwent surgical interventions, 16 (55.2%) underwent pancreatic necrosectomy debridement, including laparotomy and laparoscopy assisted, 4 (13.8%) underwent percutaneous catheter drainage (PCD), and 9 (34.5%) underwent pancreatoduodenectomy (Whipple procedure). The median bleeding time after surgical intervention was 9 days (IQR, 5.8–17.5 days).
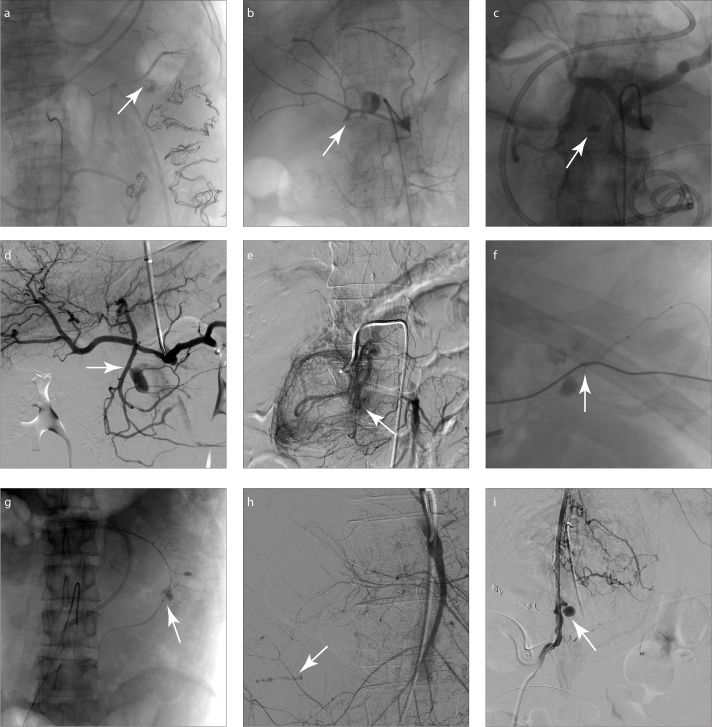
Forty patients underwent a total of 48 DSA procedures (8 repeat DSAs for rebleeding). Active bleeding was observed in 27 patients with contrast extravasation, and bleeding pseudoaneurysm was observed in 10 patients at the first DSA diagnosis. Seventeen patients had an irregular arterial wall, including 10 patients with active bleeding. Six patients had a negative DSA presentation, while 34 arteries had a positive DSA presentation, which mainly included the splenic artery (SpA), gastroduodenal artery (GDA), pancreaticoduodenal artery (PDA) arcade, and branches of the superior mesenteric artery (SMA) (Fig. 2). Table 2 summarizes the details.
Figure 2. a–i.
DSA images show the bleeding site and bleeding pseudoaneurysm (arrow), in (a) splenic artery, (b, c) gastroduodenal artery, (d, e) pancreaticoduodenal artery, (f) right gastric artery, (g) inferior mesenteric artery, (h) jejunal artery, and (i) left phrenic artery.
Table 2.
Details of angiographic findings, treatments, and outcomes
| Treatment details | n (%) |
|---|---|
| Angiographic presentation (n=40) | |
| Contrast extravasation | 27 (55.0) |
| Bleeding pseudoaneurysm | 10 (20.0) |
| Irregular artery wall | 17 (37.5) |
| Negative findings | 6 (15.0) |
|
| |
| Involved artery (n=34) | |
| SpA | 8 (23.5) |
| GDA | 7 (20.6) |
| SMA | 1 (2.9) |
| PDA (superior/inferior) | 4 (11.8) |
| RCA | 3 (8.8) |
| LCA | 1 (2.9) |
| ICA | 1 (2.9) |
| Jejunal artery | 2 (5.9) |
| IMA | 2 (5.9) |
| Gastric artery (right/left) | 2 (5.9) |
| Gastroepiploic artery (right/left) | 2 (5.9) |
| Phrenic artery (left) | 1 (2.9) |
|
| |
| First technique (n=40) | |
| TAE | 23 (57.5) |
| Blind (empirical) TAE | 4 (10.0) |
| Surgery | 4 (12.5) |
| Watch and wait | 9 (22.5) |
|
| |
| First TAE materials | |
| Coil trapping alone | 18 (52.9) |
| NBCA alone | 5 (23.5) |
| Combine coiling and NBCA | 4 (11.8) |
| Combine coiling and gelfoam | 4 (11.8) |
|
| |
| Complications | 3 |
| Renal failure | 2 (5.0) |
| Hepatic insufficiency | 1 (2.5) |
|
| |
| Outcome | |
| First technical success | 32/40 (80.0) |
| Re-intervention | 7/40 (17.5) |
| Overall technical success | 37/40 (92.5) |
| 90-day survival | 25/40 (62.5) |
SpA, splenic artery; GDA, gastroduodenal artery; SMA, superior mesenteric artery; PDA, pancreaticoduodenal artery; RCA, right colic artery; LCA, left colic artery; ICA, internal carotid artery; IMA, inferior mesenteric artery; TAE, transarterial embolization; NBCA, N-butyl cyanoacrylate.
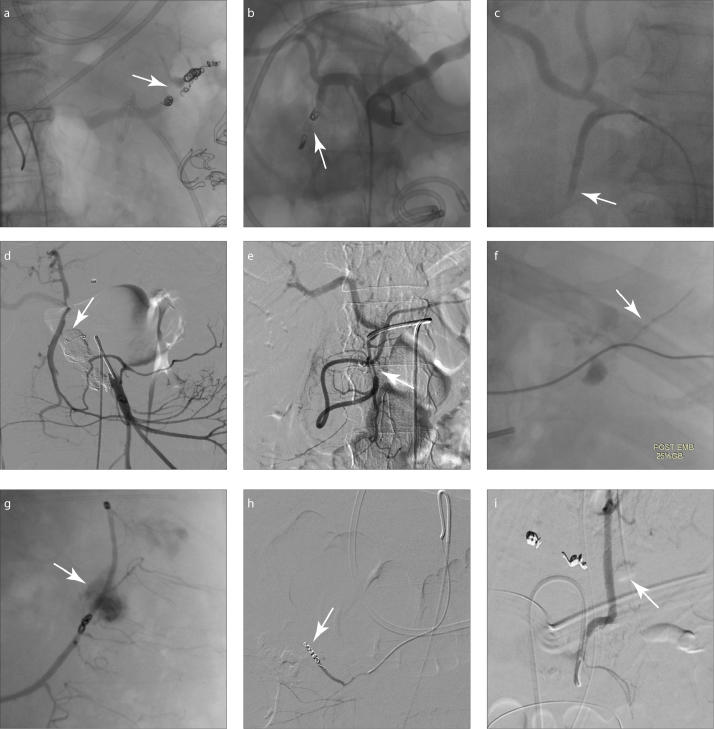
There were 31 procedures performed at the first DSA diagnosis; 27 cases were treated with TAE or blind (empirical) TAE (Fig. 3), while 4 cases underwent surgery for laparotomy. Nine cases followed the watch and wait (WAW) policy for observation due to suspected variceal hemorrhage of left-sided portal hypertension. With regard to the TAE materials, coils were used in 18 arteries (mean, 3.8 coils per artery) with the “two-point” technique, mainly for the SpA and GDA; NBCA was used in 9 arteries (1:1 to 1:3 dilution with lipiodol), mainly for the GDA and PDA arcade; and gelfoam was used in 4 arteries with coils using the “sandwich” technique. In 8 arteries, two different embolic materials were used in a single procedure, including coils and NBCA in 4 arteries and coils and gelfoam in 4 arteries. No misplaced coils were observed in the nontarget arteries on the final angiography. In 4 laparotomies and one repeat laparotomy, the choice was made according to the blood supply of the small intestine and colon, in case of ischemic complications. The branches of the SMA and inferior mesenteric artery (IMA) were suspected by DSA presentation, and during the operation, the bleeding sites were confirmed with one SMA, one right colic artery, one internal carotid artery (ICA), and two bleeding jejunal arteries.
Figure 3. a–i.
Post-embolization DSA demonstrated no contrast leak in the abdomen with embolization procedure (“sandwich” or “two-point” technique) of (a) splenic artery with coils and N-butyl cyanoacrylate (NBCA), (b) gastroduodenal artery with coils and gelfoam, (c) gastroduodenal artery with NBCA, (d, e) pancreaticoduodenal artery with coils and/or NBCA, (f) right gastric artery with NBCA, (g) inferior mesenteric artery with coils, (h) jejunal artery with coils, and (i) left phrenic artery with NBCA.
Eight patients experienced rebleeding and underwent second DSA. Of the eight patients, one patient had no positive DSA presentation at the time of initial angiography and underwent conservative treatment, five patients suffered from rebleeding following TAE, and two patients suffered from rebleeding of the SMA branches following laparotomy. Among these patients, the rebleeding site was found in six patients as follows: two patients exhibited in situ rebleeding in the SpA, one patient exhibited rebleeding in the artery of the Drummond arcade (ICA–left colic artery), and three patients exhibited rebleeding in other arteries. The rebleeding site was not identified in one patient during repeat DSA. The repeat TAE procedure was performed in four patients with a positive DSA presentation, three patients underwent laparotomy for suspected rebleeding in the SMA, jejunal artery, and distal ICA, and one patient was followed using the WAW policy due to the lack of positive findings (Table 3).
Table 3.
Case details and outcomes in patients with rebleeding after initial management
| No. | Disease | Previous surgery | Pancreatic fistula | Hg | Bleeding day after surgery | Location | First treatment | First TAE material | Rebleeding day after first treatment | Location | Second treatment | Second TAE material | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AP | Debridement (lap-assisted) | Yes | 62 | 17 | SpA | TAE | Coils+ NBCA | 7 | In situ | Re-TAE | Coils | Died |
| 2 | CP | PCD | No | 62 | 12 | GDA | TAE | NBCA | 12 | SpA | Re-TAE | Coils | Died |
| 3 | CP | Whipple | Yes | 50 | 26 | GDA | TAE | Coils+ gelfoam | 7 | – | WAW | – | Alive |
| 4 | AP | Debridement | Yes | 60 | 7 | ICA | TAE | Coils | 9 | In situ (LCA) | Re-TAE | Coils | Alive |
| 5 | CP | Debridement (lap-assisted) | No | 44 | 15 | LGA | TAE | Coils (blind) | 8 | Phrenic A. | Re-TAE | NBCA | Alive |
| 6 | AP | Debridement | Yes | 72 | 22 | SMA | Laparotomy | – | 6 | Jejunal A. | Laparotomy | – | Died |
| 7 | AP | Debridement (lap-assisted) | Yes | 40 | 5 | RCA | Laparotomy | – | 0 | – | Laparotomy | – | Died |
| 8 | AP | PCD | Yes | 80 | 6 | – | WAW | – | 15 | ICA | Laparotomy | – | Died |
Hg, hemoglobin; TAE, transarterial embolization; AP, acute pancreatitis; lap-assisted, laparoscopy-assisted; SpA, splenic artery; NBCA, N-butyl cyanoacrylate; Re-TAE, repeat TAE; CP, chronic pancreatitis; PCD, percutaneous catheter drainage; GDA, gastroduodenal artery; WAW, watch and wait; ICA, internal carotid artery; LCA, left colic artery; LGA, left gastroepiploic artery; SMA, superior mesenteric artery; RCA, right colic artery; A., artery.
Table 1 shows the procedure outcomes. Successful hemostasis without rebleeding was achieved in 32 patients (80%) during the initial decision for treatment or WAW, and overall technical success was obtained in 37 patients (92.5%). Only three patients died as a consequence of intra-abdominal bleeding, with one repeat TAE failure and two surgical failures (Table 3). Complications were observed in only three patients with AP, including two patients with renal failure and one patient with hepatic insufficiency after the TAE procedure due to NBCA reflux into the common hepatic artery. There were no occurrences of bowel ischemia. Splenic infarction was excluded in the present study. In total, 25 patients (62.5%) were alive for 90 days after hemorrhage, and 15 patients (37.5%) died within the 90-day follow-up with rebound of serious abdominal infection and sepsis (n=7, 17.5%), multiple organ dysfunction syndrome (n=4, 10.0%), hemostasis failure (n=3, 7.5%), and lung infection (n=1, 2.5%). There was no rebleeding within the 90-day follow-up period.
We compared the outcomes between the TAE procedure and surgery for laparotomy following DSA diagnosis (Table 4). Although there was no significant difference in the first technical success (24/27 vs. 2/5; P = 0.085) between the patients who underwent the TAE procedure and those who underwent laparotomy, the TAE group clearly achieved a significantly higher overall technical success rate than the surgery group (26/27 vs. 2/5; P = 0.008). Indeed, the TAE group exhibited significantly higher survival rate than the surgery group during the 90-day follow-up (20/27 vs. 1/5; P = 0.037).
Table 4.
Comparison of technical success and outcomes between the TAE and surgery groups
| Group | n | First technical success | Overall technical success | 90-day survival |
|---|---|---|---|---|
| TAE | 27 | 24 | 26 | 20 |
| Surgery | 5 | 2 | 2 | 1 |
| Fisher’s P value | 0.085 | 0.008* | 0.037* |
TAE, transarterial embolization.
P < 0.05.
Discussion
In the present study, the characteristics of pancreatitis-related hemorrhage and PPH were described, and the endovascular management of these patients was evaluated as the first rescue procedure. To date, most published studies have focused on evaluating hemorrhage associated with a single disease, such as bleeding pseudoaneurysm of AP and CP or PPH. Only a few series have reported the outcomes of TAE in both diseases, but these studies did not show the standard for severe abdominal hemorrhage or the clinical characteristics of the hemorrhage cases (7, 8). In our study, the standard mainly depended on the PPH of the ISGPS definition with Grade B/C, as well as the pancreatitis-related hemorrhage. The signs of severe hemorrhage in patients suffering from pancreatitis and post-pancreatectomy include bleeding from the gastrointestinal tract or abdominal drainage, hemorrhagic shock, and rapid decrease in hemoglobin.
Remarkably, most patients had a pancreatic fistula before the bleeding occurred, with evidence of elevated amylase in the drainage. Pancreatic fistula and severe inflammation result in the local spread of exocrine proteolytic and lipolytic enzyme-rich fluids that weaken tissue and lead to elastolytic erosions of the vessel wall (9). Thus, it is firmly believed that the most important factor associated with the risk of bleeding is pancreatic fistula after pancreaticoduodenectomy (10) and pancreatic necrosectomy (11). Furthermore, vessel injury may occur due to iatrogenic causes after pancreatic surgery and percutaneous drains (8, 12). Surgical and draining procedures that are applied for long-term lavage and treatment of pancreatic fluid collections also increase the risk of injuring the visceral artery. Even a slight injury around the pancreas could damage the weakened arterial wall or form pseudoaneurysms. In our study, 29 patients (72.5%) had PCD or surgical history. Indeed, the most important factors associated with the risk of pancreatic bleeding are pancreatic fistula and iatrogenic injury.
During the previous decade, reviews have revealed that endovascular procedures, including angiography and TAE, were more frequently used to detect and to control bleeding because TAE can be applied in patients who cannot tolerate laparotomy or anesthesia (1, 13). However, surgical intervention was less frequently used without any comparison with the endovascular procedures. It is particularly important that our results revealed a significant advantage of the TAE procedure over laparotomy with respect to the technical success and 90-day survival rates. Moreover, most previous studies reported that the success rates of endovascular procedures are high with multiple embolizations and prolonged supportive treatment. Taina et al. (14) study, involving 58 patients with bleeding pancreatic pseudoaneurysms, achieved a success rate of 96.6% and a mortality rate of 13.8% without hemorrhage classification. However, Nicole et al. (15) reported a 30-day survival rate of 70% in the embolization group, which included patients with Grade B/C PPH. Other studies also revealed a higher success rate, but more recent studies demonstrated varying mortality rates (16–18). According to our data, the first technical success rate was 80% in 32 patients who underwent interventional treatment or observation. The overall technical success rate was 92.5%, and the 90-day survival rate was 62.5%.
The materials used in the TAE procedures may vary (e.g., embolic agents such as coils, NBCA, gelfoam, and particles or stent grafts). First, coils are the most widely used embolic materials in recent studies. In our series, coils and microcoils were used in approximately 75% of the patients with the “two-point” or “sandwich” technique to occlude the efferent and afferent arteries, and minimize the potential for rebleeding. Importantly, the arcade, including the GDA-SMA (PDA arcade), SMA-IMA (Riolan arcade or Drummond arcade), and SPA-left gastroepiploic artery, should be carefully attended to during the procedure. Coiling feeders from both ends of the arcade are usually required; in one patient, the rebleeding, which was due to an embolism, occurred only at one end of the arcade. With regard to the pseudoaneurysm sac, we packed the entire sac when bleeding of the pseudoaneurysm occurred. One study indicated that packing the sac with coils increases the risk of rupture (19), but these ruptures did not occur in our study. In addition, NBCA has been increasingly reported in recent studies, the liquid nature of this embolic agent allows its delivery through the pathologic or tortuous vessels via the microcatheter and filling the bleeding sac without the risk of new vessel injury or ruptured sac (7, 20). In addition, NBCA can be used individually or in combination with other embolic materials, such as coils and gelfoam. However, NBCA is more difficult to control than coils because an assessment of the flow rate across the pseudoaneurysm or bleeding position is required to calculate the NBCA/contrast ratio, and this affects the viscosity and polymerization rates. An NBCA concentration of 20%–50% is recommended to allow filling and complete exclusion of the bleeding or pseudoaneurysm. We used NBCA in 10 cases at a 25%–50% concentration. However, in one case, hepatic insufficiency occurred during the process of GDA embolism due to occlusion of the hepatic artery; we believe that in this case NBCA overflowed into the GDA with reflux. Gelfoam is a temporary embolic material that may be rapidly resorbed by pancreatic proteolytic enzymes and has the potential to migrate into the small arteries, potentiating tissue ischemia; thus, it is usually used between two coils in a “sandwich” technique (19).
Notably, the rebleeding rate was as high as 20%, which required an additional endovascular procedure or laparotomy; this rate was as high as that reported in most published studies (ranging between 20% and 30%) (21, 22). Current studies indicate that the mortality rates reach 40% to 50% following direct surgery for the treatment of initial pancreatic bleeding, and no related studies revealed the mortality rate in rebleeding cases (23, 24). In our study, three patients with rebleeding eventually died following repeat laparotomy, demonstrating that repeat TAE might be a better strategy for the treatment of rebleeding than repeat laparotomy. Studies of negative angiography with no detectable bleeding site vary in the literature; the negative rate has increased in recent years from 15% to 44% in several large-scale studies (11, 14, 25–27). In our studies, no bleeding site was detected in 13 patients using angiography. A high success rate was achieved in patients who received empirical TAE in our study. Recently, interventional radiologists have become more active in performing empirical TAE of arteries such as GDA and SpA and this technique is increasingly important in treating hemorrhage in patients with negative angiographies.
Our policy was to perform angiography in all patients with suspected severe pancreatic hemorrhage after maintaining basic hemodynamic stability. Angiography can clearly identify the rupture position in an artery, whereas computed tomography scan can only locate hematoma. Moreover, hemostasis with TAE could be continued after angiography without delay due to transferring the patient, which is superior to any other examination. Based on these advantages and our results, we propose an algorithm for managing severe hemorrhage occurring in the setting of pancreatitis or PPH as shown in Fig. 1. First, TAE should be performed in patients with explicitly active bleeding with contrast extravasation. Blind empirical TAE also should be attempted if the patient has a negative DSA presentation for active bleeding, particularly in patients with an abnormal vascular wall, such as a pseudoaneurysm-related irregular vascular wall. Laparotomy should be reserved for patients with active bleeding that is not suitable for TAE according to the blood supply to the organ, such as the SMA or celiac trunk, common hepatic artery, and patients with unstable hemodynamics, or rebleeding cases. The WAW policy could be adopted only in hemodynamically stable negative DSA patients who are suspected to have massive variceal hemorrhage due to left-sided portal hypertension. In the present study, 9 cases were suspected with massive variceal hemorrhage, and 8 cases achieved hemostasis with conservative treatment. Rebleeding cases require angiography to be performed again; repeat TAE should be performed for in situ rebleeding or a new rupture; a conservative treatment may be an alternative for stable patients with rebleeding, but surgery appears to be of no benefit in rebleeding cases.
The limitations of the current study are its retrospective nature in a single center and the length of the retrospective time, which was >10 years in some cases. Although the inclusion criteria were limited to Grade B to C according to the ISGPS definition, heterogeneity existed, and the study included patients undergoing pancreatic surgery and those suffering from AP or CP because severe hemorrhage is a rare but potentially lethal complication of pancreatic diseases. Additionally, the patients’ characteristics could have been biased because only patients who underwent angiography first were selected; patients who were treated directly via surgical intervention or endoscopy without angiography were excluded from the study.
In conclusion, endovascular techniques could be the optimal choice for diagnosis and management of pancreatic bleeding. TAE is the main component in the proposed algorithm with other measures, such as observation or surgery, used in cases where TAE is not suitable. Laparotomy is not suitable for rebleeding cases, as it results in low technical success rate and survival rate. Further investigation is required to identify the factors predictive of rebleeding risk and develop methods to keep the mortality rate in an acceptable range.
Main points.
The important factors associated with the risk of pancreatic bleeding are pancreatic fistula and iatrogenic injury.
Endovascular techniques could be the optimal choice for determining the location of the bleeding diagnosis, and TAE is the main management for hemostasis.
The “two-point” or “sandwich” technique was required to occlude both sides of the arterial arcades, which could minimize the rebleeding risk in the TAE procedure.
Laparotomy is not suitable for rebleeding cases, as it has low technical success rate and survival rate compared with the TAE procedure.
Footnotes
Financial disclosure
Capital Major Special Program for Clinical Characteristic Application of the Beijing Science and Technology Commission Foundation of PR China (Grant: Z171100001017077) to Fei Li and National Natural Science Foundation of PR China (Grant: 81470587) to Tao Luo.
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Carr JA, Cho JS, Shepard AD, Nypaver TJ, Reddy DJ. Visceral pseudoaneurysms due to pancreatic pseudocysts: rare but lethal complications of pancreatitis. J Vasc Surg. 2000;32:722–730. doi: 10.1067/mva.2000.110055. [DOI] [PubMed] [Google Scholar]
- 2.Balachandra S, Siriwardena AK. Systematic appraisal of the management of the major vascular complications of pancreatitis. Am J Surg. 2005;190:489–495. doi: 10.1016/j.amjsurg.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Limongelli P, Khorsandi SE, Pai M, et al. Management of delayed postoperative hemorrhage after pancreatico-duodenectomy: a meta-analysis. Arch Surg. 2008;143:1001–1007. doi: 10.1001/archsurg.143.10.1001. [DOI] [PubMed] [Google Scholar]
- 4.Miura F, Asano T, Amano H, et al. Management of postoperative arterial hemorrhage after pancreato-biliary surgery according to the site of bleeding: re-laparotomy or interventional radiology. J Hepatobiliary Pancreat Surg. 2009;16:56–63. doi: 10.1007/s00534-008-0012-3. [DOI] [PubMed] [Google Scholar]
- 5.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Cardella JF, Kundu S, Miller DL, Millward SF, Sacks D. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189–191. doi: 10.1016/j.jvir.2009.04.035. [DOI] [PubMed] [Google Scholar]
- 7.Izaki K, Yamaguchi M, Kawasaki R, Okada T, Sugimura K, Sugimoto K. N-butyl cyanoacrylate embolization for pseudoaneurysms complicating pancreatitis or pancreatectomy. J Vasc Interv Radiol. 2011;22:302–308. doi: 10.1016/j.jvir.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Yekebas EF, Wolfram L, Cataldegirmen G, et al. Postpancreatectomy hemorrhage: diagnosis and treatment: an analysis in 1669 consecutive pancreatic resections. Ann Surg. 2007;246:269–280. doi: 10.1097/01.sla.0000262953.77735.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flati G, Andren-Sandberg A, La Pinta M, Porowska B, Carboni M. Potentially fatal bleeding in acute pancreatitis: pathophysiology, prevention, and treatment. Pancreas. 2003;26:8–14. doi: 10.1097/00006676-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Sanjay P, Kellner M, Tait IS. The role of interventional radiology in the management of surgical complications after pancreatoduodenectomy. HPB (Oxford) 2012;14:812–817. doi: 10.1111/j.1477-2574.2012.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pottier E, Ronot M, Gaujoux S, et al. Endovascular management of delayed post-pancreatectomy haemorrhage. Eur Radiol. 2016;26:3456–3465. doi: 10.1007/s00330-016-4213-x. [DOI] [PubMed] [Google Scholar]
- 12.Li A, Cao F, Li J, et al. Step-up mini-invasive surgery for infected pancreatic necrosis: Results from prospective cohort study. Pancreatology. 2016;16:508–514. doi: 10.1016/j.pan.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Barge JU, Lopera JE. Vascular complications of pancreatitis: role of interventional therapy. Korean J Radiol. 2012;13:S45–55. doi: 10.3348/kjr.2012.13.S1.S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nykanen T, Udd M, Peltola EK, Leppaniemi A, Kylanpaa L. Bleeding pancreatic pseudoaneurysms: management by angioembolization combined with therapeutic endoscopy. Surg Endosc. 2017;31:692–703. doi: 10.1007/s00464-016-5023-6. [DOI] [PubMed] [Google Scholar]
- 15.Hassold N, Wolfschmidt F, Dierks A, Klein I, Bley T, Kickuth R. Effectiveness and outcome of endovascular therapy for late-onset postpancreatectomy hemorrhage using covered stents and embolization. J Vasc Surg. 2016;64:1373–1383. doi: 10.1016/j.jvs.2016.05.071. [DOI] [PubMed] [Google Scholar]
- 16.Fitzpatrick J, Bhat R, Young JA. Angiographic embolization is an effective treatment of severe hemorrhage in pancreatitis. Pancreas. 2014;43:436–439. doi: 10.1097/MPA.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 17.Gluszek S, Nawacki L, Matykiewicz J, Kot M, Kuchinka J. Severe vascular complications of acute pancreatitis. Pol Przegl Chir. 2015;87:485–490. doi: 10.1515/pjs-2015-0093. [DOI] [PubMed] [Google Scholar]
- 18.Asari S, Matsumoto I, Toyama H, et al. Recommendation of treatment strategy for postpancreatectomy hemorrhage: Lessons from a single-center experience in 35 patients. Pancreatology. 2016;16:454–463. doi: 10.1016/j.pan.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Kirby JM, Vora P, Midia M, Rawlinson J. Vascular complications of pancreatitis: imaging and intervention. Cardiovasc Intervent Radiol. 2008;31:957–970. doi: 10.1007/s00270-007-9138-y. [DOI] [PubMed] [Google Scholar]
- 20.Kim J, Shin JH, Yoon HK, et al. Endovascular intervention for management of pancreatitis-related bleeding: a retrospective analysis of thirty-seven patients at a single institution. Diagn Interv Radiol. 2015;21:140–147. doi: 10.5152/dir.2014.14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi SH, Moon HJ, Heo JS, Joh JW, Kim YI. Delayed hemorrhage after pancreaticoduodenectomy. J Am Coll Surg. 2004;199:186–191. doi: 10.1016/j.jamcollsurg.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Beyer L, Bonmardion R, Marciano S, et al. Results of non-operative therapy for delayed hemorrhage after pancreaticoduodenectomy. J Gastrointest Surg. 2009;13:922–928. doi: 10.1007/s11605-009-0818-6. [DOI] [PubMed] [Google Scholar]
- 23.Lee HG, Heo JS, Choi SH, Choi DW. Management of bleeding from pseudoaneurysms following pancreaticoduodenectomy. World J Gastroenterol. 2010;16:1239–1244. doi: 10.3748/wjg.v16.i10.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding X, Zhu J, Zhu M, et al. Therapeutic management of hemorrhage from visceral artery pseudoaneurysms after pancreatic surgery. J Gastrointest Surg. 2011;15:1417–1425. doi: 10.1007/s11605-011-1561-3. [DOI] [PubMed] [Google Scholar]
- 25.Hyare H, Desigan S, Brookes JA, Guiney MJ, Lees WR. Endovascular management of major arterial hemorrhage as a complication of inflammatory pancreatic disease. J Vasc Interv Radiol. 2007;18:591–596. doi: 10.1016/j.jvir.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 26.Blanc T, Cortes A, Goere D, et al. Hemorrhage after pancreaticoduodenectomy: when is surgery still indicated? Am J Surg. 2007;194:3–9. doi: 10.1016/j.amjsurg.2006.08.088. [DOI] [PubMed] [Google Scholar]
- 27.Phillip V, Rasch S, Gaa J, Schmid RM, Algul H. Spontaneous bleeding in pancreatitis treated by transcatheter arterial coil embolization: a retrospective study. PLoS One. 2013;8:e72903. doi: 10.1371/journal.pone.0072903. [DOI] [PMC free article] [PubMed] [Google Scholar]