Abstract
PURPOSE
Percutaneous tissue biopsy is a mainstay of diagnostic and interventional radiology, providing a minimally invasive method for diagnosing malignant and benign disease. The purpose of this review was to collect and summarize the best available evidence regarding the risk factors associated with bleeding complications in image-guided liver biopsy.
METHODS
A literature review was performed, searching Medline, EMBASE, CINAHL, the Cochrane Library, the National Institute for Health and Care Excellence (NICE) and Canadian Agency for Drugs and Technology in Health (CADTH) databases for any studies evaluating bleeding complications in image-guided liver biopsy. A total of 68 articles, published between January 1994 and April 2015, were reviewed in full, with 34 ultimately eligible for inclusion in the review.
RESULTS
Bleeding of any kind occurred in up to 10.9% of image-guided liver biopsies, with major bleeding episodes ranging from 0.1% to 4.6% and minor bleeding events occurring in up to 10.9% of biopsies. The overall rate of bleeding was, however, found to be less than 2%. Several risk factors (patient, operator, and procedure-related) were identified as potentially indicative of an increased risk of post-biopsy bleeding. Patient-related risk factors included patient age (>50 years or <2 years), inpatient status (8/12 vs. 4/12, P < 0.001), comorbidities and/or concurrent diagnoses and coagulation status (rate of bleeding was 3.3% for international normalized ratio [INR] 1.2–1.5 vs. 7.1% for INR >1.5, P < 0.001). There was no consensus on impact of operator experience (>200 biopsies/year vs. <50/year) on post-biopsy bleeding rate. Procedure-related risk factors included needle size (cutting biopsy vs. fine needle aspiration, P < 0.001) and the presence of a patent track on post-biopsy ultrasound (P < 0.001). Lastly there was no difference found between targeted vs. nontargeted biopsies and number of needle passes.
CONCLUSION
Reported rate of post-biopsy bleeding ranges between 0% and 10.9%, although the vast majority of studies reported bleeding rates under 2%. Several patient, operator, and procedure-related risk factors are associated with a higher risk of bleeding following liver biopsy.
Percutaneous tissue biopsy is a mainstay of diagnostic and interventional radiology, providing a minimally invasive method for diagnosing malignant and benign disease commonly in an out-patient setting (1–3). In particular, percutaneous liver biopsies constitute a large portion of all biopsies and are being performed with an ever-increasing rate (4). The use of imaging guidance has improved biopsy accuracy and decreased the rate of overall complications (1). Despite these advances, complications do occur and could result in significant morbidity and mortality for the patient (1, 5, 6).
Post-biopsy hemorrhage (PBH) is the main source of mortality following liver biopsy and occurs in up to 10.9% of cases (7). The majority of PBH occur within the first 2–4 hours following the procedure (8), necessitating the need for close monitoring of the patient during this period. Several risk factors have been identified that contribute to the likelihood of bleeding complications following liver biopsy. These risk factors are categorized as either patient-related, operator-related, or procedure-related and range from coagulation status, comorbidities and operator experience to needle gauge and biopsy method. The purpose of this literature review is to compile the best available evidence for PBH and to evaluate the current consensus on methods to minimize risk for liver biopsy.
Methods
Research ethics board
Since this study did not directly involve patients or patients’ clinical records, a Research Ethics Board approval was not required.
Terms and definitions
Image-guided biopsy was defined as obtaining tissue sample from a specific organ for diagnostic purposes using various needles under image guidance (5). Major bleeding complications were defined as a post-intervention event that were clinically relevant and required therapeutic intervention (e.g., blood transfusion, drainage, hospitalization). Minor bleeding complications were defined as post-intervention events that were not clinically relevant and did not require further medical attention (5).
Search strategy
Major medical databases were searched for studies summarizing risk factors for hemorrhage following percutaneous image-guided liver biopsy between January 1994 and April 2015. Databases included Medline, EMBASE, CINAHL and the Cochrane Library. We also searched for clinical guidelines and reviews in the UK National Health Service NICE and Canadian Agency for Drugs and Technology in Health (CADTH) databases. Searches were restricted to articles published in English, using the following subject headings and/or text words: hemorrhage, bleeding, complication, risk factor, liver biopsy, image-guided liver biopsy, and percutaneous. The reference lists from each article identified were cross-referenced and searched for additional eligible articles. All abstracts identified in the initial searches were reviewed for eligibility.
Selection of studies for inclusion
All abstracts were independently reviewed for relevance to the search criteria. Full-text article reviews were undertaken for any articles deemed to appropriately meet the essential inclusion criteria. Articles ultimately included in this review met the listed inclusion and exclusion criteria.
Inclusion and exclusion criteria
Eligible studies included clinical studies investigating complications associated with image-guided liver biopsy. Studies were limited to between Level Ib (randomized clinical trial) and Level III (retrospective observational study). Studies were eligible for inclusion, if they investigated or evaluated risk factors and/or complication rates for bleeding/hemorrhage in patients following image-guided percutaneous liver biopsy. No limitations were placed on the specific indication for biopsy, the nature of the biopsy (fine needle vs. tissue biopsy), the diagnosis, the patient age, concurrent conditions, diameter of biopsy needle or the method of biopsy. Studies were excluded if they met the following criteria: review studies, biopsy of other organs, blind liver biopsy or liver biopsy using a technique other than percutaneous techniques. Studies reporting bleeding complication rates without investigating potential risk factors were also excluded from the review.
Results
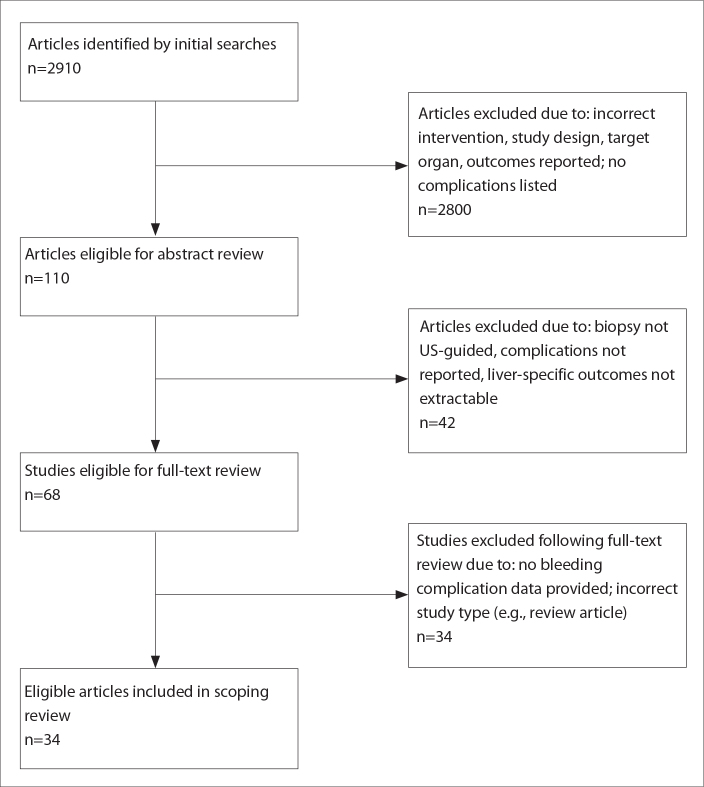
From an initial pool of 2910 of potentially eligible studies, 110 were selected for abstract review. Following exclusion of 42 of studies, 68 were reviewed as full-text articles, providing a total of 34 studies (3, 5, 9–40) for inclusion in this review (Fig.).
Figure.
PRISMA flowchart summarizing study eligibility.
As image-guided biopsy is an established diagnostic method, there are very few randomized trials evaluating its effectiveness. Instead, there are a large number of retrospective analyses evaluating both the diagnostic accuracy and value of biopsy, plus a large number cataloguing the various risk factors associated with the procedure. Of 34 studies eligible for inclusion in this review, only one study (40) was a randomized controlled trial, while five were prospective, single-arm, uncontrolled studies (13, 17, 37–39). The remaining studies were all retrospective chart reviews, apart from two that were retrospective audits of healthcare systems (9, 15). A summary of the included studies is presented in Table 1. Ten studies evaluated a pediatric population while 24 studies evaluated an adult population. Bleeding complications were reported in 19 of 34 studies, ranging from 0% to 10.9%. Minor bleeding (i.e., requiring no clinical intervention) was reported in 11 of 34 studies and ranged from 0.3% to 10.9%. Major bleeding (i.e., requiring medical intervention) was reported in 14 of 34 studies and ranged from 0.1% to 4.6% (Table 2).
Table 1.
Eligible study characteristics
| Lead author | Pub date | Study type/LOE | Patient population (age range) | Number of patients | Number of biopsies | Key findings |
|---|---|---|---|---|---|---|
| Lindor | 1996 | Randomized, controlled trial, Level Ib | Adult (44.9–48.5 y) | 836 | 836 | Use of US-guidance associated with a lower rate of bleeding complications (2.2% vs. 1.1%) |
| Beddy | 2007 | Prospective, Level II | Adult (8–76 y) | 500 | 500 | Overall bleeding complication rate: 0.2% |
| Kim | 2007 | Prospective, Level II | Adult (16–83 y) | 352 | 361 | Presence of a “patent track” on post-biopsy US was highly indicative of bleeding complication (P = 0.0008) |
| Thanos | 2005 | Prospective, Level II | Adult (57±12 y) | 767 | 767 | 18-gauge needles are safe and effective |
| Gunnesson | 2002 | Prospective, Level II | Adult (51.2±0.35 y) | 708 | 1086 | Hemothorax occurred in 0.1% of patients |
| Rossi | 2001 | Prospective, Level II | Adult (mean, 44.7 y; 18–85 y) | 140 | 142 | US-guidance recommended for percutaneous liver biopsy |
| Govender | 2013 | Retrospective, Level III | Pediatric (1 m to 21 y) | 470 | 597 | Major bleeding rate after biopsy: 1.3% |
| Short | 2013 | Retrospective, Level III | Pediatric (1 w to 22 y) | 213 | 328 | Younger age and lower preprocedural hematocrit predicted bleeding complications |
| Westheim | 2013 | Retrospective, Level III | Pediatric (1 m to 18 y) | 214 | 311 | Bleeding rates higher when operator was less experienced |
| Howlett | 2012 | Retrospective, Level III | Pediatric and adult | 3473 | 3496 | Rate of major bleeding complications: 0.11% (minor: 0.4%) |
| Matos | 2012 | Retrospective, Level III | Pediatric (1 w to 22 y) | 213 | 328 | Minor bleeding rate after biopsy: 5.5% |
| Mueller | 2012 | Retrospective, Level III | Adult (18–96 y) | 1961 | 2229 | Collected data on patient, operator, and procedure-related factors |
| Westheim | 2012 | Retrospective, Level III | Pediatric (5.8±5.2 y) | 190 | 275 | Focal lesions were associated with a significantly higher bleeding complication rate than other lesion categories |
| Potter | 2011 | Retrospective, Level III | Pediatric (8 d to 20 y) | 249 | 294 | Five patients (2%) required blood transfusions due to post-biopsy bleeding complications |
| Vijayaraghavan | 2011 | Retrospective, Level III | Adult (30–44 y) | n/s | 776 | Left-sided epigastric biopsy approach was associated with bleeding complications in 0.8% of cases |
| Atwell | 2010 | Retrospective, Level III | Pediatric and adult (15 d to 96 y) | 3636 | 3636 | Data collected on procedural (needle size, number of passes) and patient-related (INR, platelet count) factors |
| Seeff | 2010 | Retrospective, Level III | n/s | 1385 | 2740 | Low albumin, platelet count and INR associated with significantly greater bleeding rate |
| Sornsarkin | 2010 | Retrospective, Level III | Pediatric (6 m to 18 y) | 67 | 120 | Overall bleeding complication rate: 0.83% |
| West | 2010 | Retrospective, Level III | Adult (40–59 y) | 61187 | 61187 | Rate of major bleeding complication: 0.65% |
| Padia | 2009 | Retrospective, Level III | Adult (20–84 y) | 350 | 539 | Overall bleeding complication rate: 0.6% |
| Weigand | 2009 | Retrospective, Level III | Adult (47±15 y) | 715 | 715 | Major bleeding rate: 0.15%; minor bleeding rate: 0.3% |
| Hatfield | 2008 | Retrospective, Level III | Adult (range not specified) | n/s | 784 | Compared coaxial biopsy technique with non-coaxial techniques with and without gelatin sponge: no significant differences in bleeding rate |
| Myers | 2008 | Retrospective, Level III | Adult (41–64 y) | 3627 | 4275 | Bleeding rate was associated with abnormal platelet counts and INR |
| Amaral | 2006 | Retrospective, Level III | Pediatric (7–348 d) | 61 | 65 | Major and minor bleeding rates were equivalent (4.6%) |
| van der Poorten | 2006 | Retrospective, Level III | Adult (18–88 y) | 1398 | 1398 | Collected data on patient, operator, and procedure-related factors |
| Azzam | 2005 | Retrospective, Level III | Pediatric (13–90 d) | 63 | 66 | Bleeding occurred in 4.5% of patients post-biopsy |
| Firpi | 2005 | Retrospective, Level III | n/s | n/s | 3214 | Hemothorax rate lower with US-guidance (P = 0.1) |
| Chevalier | 2004 | Retrospective, Level III | Adult (46±12 y) | 600 | 600 | Bleeding occurred in 0.16% of patients |
| Nobili | 2003 | Retrospective, Level III | Pediatric (2.5 m to 18 y) | 140 | 144 | Compared US-guided biopsy with blind biopsy and found no bleeding complications with US-guided (4.7% when blinded) |
| Terjung | 2003 | Retrospective, Level III | Pediatric and adult (12–88 y) | 574 | 629 | Collected data on various concurrent conditions and their effect on bleeding rate and complications |
| Reimann | 2000 | Retrospective, Level III | Pediatric and adult (6–81 y) | n/s | 258 | Major bleeding rate: 1.6%; minor bleeding rate: 0.4% |
| Scheimann | 2000 | Retrospective, Level III | Pediatric and adult (1 m to 39 y) | 123 | 249 | Compared needle diameter and found no significant difference in bleeding rates |
| Thampanitchawong | 1999 | Retrospective, Level III | Pediatric and adult (15–85 y) | 459 | 484 | Lower platelet counts associated with significantly higher rate of post-biopsy bleeding (P = 0.014) |
| Gilmore | 1995 | Retrospective, Level III | Adult (60–69 y) | 1890 | 1500 | Higher INR (>1.5) and lower platelet counts associated with higher bleeding rates |
Pub, publication; LOE, level of evidence (Introducing Levels of Evidence to The Journal, The Journal of Bone and Joint Surgery - jbjs.org, Volume 85A, Number 1, January 2003); US, ultrasonography; y, years; m, months; w, weeks; d, days; n/s, not specified; INR, international normalized ratio.
Table 2.
Comparison of overall and bleeding complication rates in adult and pediatric patients
| All studies | Pediatric patients only (10 studies) | Adult patients only (24 studies) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No. of studies | Low (%) | High (%) | No. of studies | Low (%) | High (%) | No. of studies | Low (%) | High (%) | |
| Any complication | |||||||||
| Overall | 8 | 1.2 | 18 | 2 | 5 | 18 | 6 | 1.2 | 6.83 |
| Minor | 8 | 0 | 4.6 | 3 | 1 | 4.6 | 5 | 0.3 | 2.4 |
| Major | 7 | 2.4 | 25 | 3 | 4.6 | 25 | 4 | 2.4 | 22 |
|
| |||||||||
| Bleeding complication | |||||||||
| Overall | 19 | 0 | 10.9 | 5 | 0 | 4.5 | 14 | 0.1 | 1.7 |
| Major | 14 | 0.1 | 4.6 | 3 | 1.1 | 4.6 | 11 | 0.1 | 4.5 |
| Minor | 11 | 0.3 | 10.9 | 4 | 4.6 | 10.9 | 7 | 0.3 | 1.0 |
There was considerable heterogeneity in the outcomes reported in the eligible studies. We stratified the risk factors for PBH into one of three categories: risk factors related to i) the patient, ii) the procedure, or iii) the operator.
Patient-related risk factors include age, gender, status of coagulation factors and current coagulopathy status, inpatient versus outpatient status, concurrent disease (e.g., liver failure), and current medications (e.g., acetylsalicylic acid, low molecular weight heparin). Several patient-related risk factors were identified that increase the risk of a bleeding complication following liver biopsy.
Five studies (5, 25, 27, 30, 31) evaluated the impact of age on bleeding risk. Two of five studies demonstrated that a higher bleeding rate was associated with older patients. Specifically, patient age >50 years (31) and >70 years (5) were found to be associated with a significantly higher likelihood of post-biopsy bleeding (age >50 years, P = 0.02; age >70 years, P = 0.04). No significant change in hemoglobin levels pre- and post-biopsy were noted in these patient groups (25). In a pediatric population, younger age (1.8 months vs. 84 months) was found to be associated with a higher rate of bleeding complication (P = 0.05) (27).
The effect of gender on the rate of post-biopsy bleeding was evaluated in four studies (5, 25, 26, 30). No studies were able to identify a statistically significant difference in bleeding rate based on patient gender. Two studies (5, 26) observed slight trends towards increased bleeding rates in female patients, but these differences were not statistically significant.
Coagulation status as a risk factor for post-biopsy bleeding was evaluated in eight studies (5, 11, 15, 20, 26, 30, 31, 35). Several factors were evaluated in the eligible studies, including INR status, platelet count and overall coagulation status. An increased INR (>1.5) pre-biopsy was associated with a statistically significant increase in bleeding rate in four studies (11, 15, 20, 26). Four studies (11, 20, 26, 30) observed that decreased platelet counts were associated with a statistically significant higher rate of post-biopsy bleeding. There was considerable range in the platelet counts that were measured for each of these studies. Atwell et al. (11) noted a serum platelet count of 194×109/L in patients suffering a post-biopsy bleed versus 257×109/L in patients who did not (P < 0.001). Conversely, Thampanitchawong and Piratvisuth (30) noted a 25% bleed rate among patients with a platelet count below 70×109/L, compared to 4% in patients above this threshold (P = 0.014). Seeff et al. (26) found that a platelet count of <60 000 mm3 was associated with a statistically significant increase in bleeding (P < 0.0001). Finally, Gilmore (15) noted that 2.9% of patients with a platelet count below 150×109/L experienced a postprocedure bleed, while only 1.6% with a platelet count greater than 150×109/L bled post-biopsy.
Coagulation parameters were measured in three studies (5, 30, 31). An incomplete coagulation criteria was associated with a higher bleed rate in one study (2/47 bleeds vs. 16/710, P = 0.391) (5), while van der Poorten et al. (31) found a 9.5% bleed rate in patients with abnormal coagulation. Thampanitchawong and Piratvisuth (30) evaluated prothrombin time (PTT) and found that a PTT of >3 s was associated with a 10.5% bleed rate. In the same study, they noted that a PTT >10 s was associated with a greater bleed rate (10.3%) than a PTT <10 s (3.8%), although this difference was not statistically significant (P = 0.079).
Albumin levels were evaluated in one study (26) and it was observed that a lower albumin level was associated with a significantly higher bleed rate (albumin level, 3.7 g/L vs. 3.9 g/L, P = 0.02). Bilirubin levels were evaluated by Gilmore et al. (15) who found that a raised level above “normal” was associated with a higher rate of post-biopsy bleeding (2.7% when above normal vs. 1.1% when below normal).
Two studies (11, 31) compared inpatient status versus outpatient status for patients undergoing liver biopsy. While van der Poorten et al. (31) found a higher rate of hemorrhage in inpatients who suffered bleeding complications than in outpatients (8/12 vs. 4/12, P < 0.001; 42.9% risk of hemorrhage), Scheimann et al. (25) noted no significant change in hemoglobin between inpatients and outpatients (P = 0.69).
The effect of concurrent conditions on post-biopsy bleeding rates was evaluated in six studies (3, 20, 27, 29, 30, 35). Of the six studies, only one (27) performed statistical analysis. It was observed that a lower hematocrit level (29.3 vs. 34.3, P = 0.04) and hypoxia caused by sedation during biopsy were associated with a significantly increased risk of bleeding (11.1% vs. 0.5%, P=0.001). The relative effects of concurrent conditions on the risk of bleeding post-biopsy is summarized in Table 3.
Table 3.
Post-biopsy bleeding rates associated with concurrent conditions and/or diagnoses
| Post-biopsy bleeding rate (%) | Concurrent condition/diagnosis | Author |
|---|---|---|
| Diagnosis | ||
| 50 | Liver failure | Terjung |
| 41.7 | Liver failure | Westheim, 2012 |
| 34.4 | Cirrhosis | Terjung |
| 26.9 | Cancer/malignancy | Terjung |
| 25 | Cirrhosis | Short |
| 18.2 | Cholestasis | Short |
| 18.2 | Metabolic disease | Short |
| 12.5 | Leukemia/lymphoma | Weigand |
| 12.5 | AIDS | Terjung |
| 12.5 | Ascites | Myers |
| 9.7 | Cancer/malignancy | Terjung |
| 8.3 | Mycobacteriosis | Terjung |
| 1.6 | Cancer/malignancy | Terjung |
| 0.58 | Liver disease | Weigand |
| 0.45 | Other | Weigand |
|
| ||
| Prebiopsy testing | ||
| 34.3 | Hematocrit | Short |
| 11.1 | General tests | Short |
| 6.9 | Anti-fibrinolytics | Terjung |
| 0.22 | Abnormal liver function tests | Weigand |
|
| ||
| Coagulation | ||
| 26.4 | Bilirubin >2 mg/dL | Terjung |
| 18.1 | Platelet count <100 g/L | Terjung |
| 6.9 | Prophylactic prebiopsy platelet submission | Terjung |
| 5.8 | Hereditary coagulopathies | Terjung |
|
| ||
| Patient-related | ||
| 11.1 | Hypoxia due to sedation | Short |
| 9.7 | Hemodialysis | Terjung |
| 9.7 | History of previous bleed | Terjung |
|
| ||
| Pharmacological | ||
| 26.4 | Corticosteroids | Terjung |
| 13.9 | Metamizole | Terjung |
| 9.7 | Beta-lactam antibiotics | Terjung |
| 6.9 | Cytostatic drugs | Terjung |
Two studies (5, 18) evaluated the relationship between procedure type and risk of bleeding. Mueller et al. (5) compared the cutting biopsy (CB), using a 1.2 mm needle, with both fine needle aspiration (FNAC, 0.7 mm needle) and the Menghini technique (ABM, 1.4 mm needle). They observed that, when compared to FNAC, CB was associated with a greater proportion of bleeding postprocedure (8/368, 2.7% vs. 0/585, 0%, P < 0.001). ABM was associated with a lower rate of bleeding when compared with CB (2/717, 0.3% vs. 5/279, 1.8%, P = 0.026). Hatfield et al. (18) compared coaxial techniques versus non-coaxial techniques, both with and without gelatin sponges. They found no statistically significant differences in bleeding rate for any of the comparisons.
Three studies (11, 25, 37) compared needle size and bleeding rates. Each found no statistically significant difference in bleeding rates related to needle gauge. Scheimann et al. (25) compared 16-gauge Jamshidi needles with 18-gauge Monopty, 15-gauge ASAP and 18-gauge ASAP needles. Using hematocrit as an outcome, they found no significant differences (P > 0.05 for all comparisons). Atwell et al. (11) compared various needles sizes in over 15 000 biopsies and found no significant difference in bleeding rate associated with needle gauge (P = 0.88). Thanos et al. (37) noted only 3 incidences of minor complications in 767 patients undergoing CT-guided liver biopsy with an 18-gauge needle. Only one of these complications was related to bleeding (0.13%).
Three studies (5, 11, 35) investigated the relationship between lesion type and bleeding complications. All studies compared parenchymal lesions with focal lesions; only one study noted a significant difference between the bleeding rates in patients with these lesion types. Westheim et al. (35) found that the rate of bleeding in focal lesions was significantly lower than that of other categories (focal: 25/275, 9.1% vs. other: 11/25, 44%, P = 0.047). Neither Mueller et al. (5) nor Atwell et al. (11) (focal: 10/1836, 0.5% vs. parenchymal: 7/1836, 0.4%) noted a significant difference between lesion type and bleeding rate.
Two studies (11, 31) evaluated the effect of multiple passes on bleeding complications, with inconclusive results. While Atwell et al. (11) found that fewer passes were associated with significantly fewer bleeding complications (2.4 vs. 3.0, P < 0.001), van der Poorten et al. (31) found no significant difference in the rate of major complications based on number of needle punctures (P = 0.31).
Four studies (36, 38–40) evaluated the overall impact of image-guidance during biopsy on bleeding complication rates. The use of ultrasonography (US) guidance was generally associated with a lower rate of bleeding complications. In the lone randomized, controlled trial eligible for this review, Lindor et al. (40) noted that the bleeding complication rate was cut by 50% after the introduction of US guidance (2.2% without US guidance vs. 1.1% with US guidance, P = 0.08). Another study found that the specific risk of hemothorax was decreased with US guidance (P = 0.1) (36). Kim et al. (39) investigated the predictive value of a so-called “patent track”, detectable by post-biopsy US. The patent track refers to the linear colorflow signal that may be seen along the needle track immediately after withdrawal of the needle. The authors found that there was a statistically significant increase in the rate of post-biopsy bleeding in patients demonstrating the patent track on post-biopsy US (P < 0.001).
Two studies (11, 27) compared US versus computed tomography (CT) guidance in liver biopsy. Atwell et al. (11) found that the vast majority (95.7%) of biopsies were guided by US, with only 157 of 3636 liver biopsies guided by CT. Two bleeding complications occurred among the 157 CT-guided procedures (1.3%), a rate higher than that of the US-guided group, where 15 bleeding complications occurred (15/3479, 0.4%). Short et al. (27) found that US was chosen as the guidance imagery for a similarly high proportion of patients (>90%), although did not note a significant difference in the use of US-guidance in patients with (90.9%) or without (93.6%, P = 0.53) a bleeding complication.
Five studies (5, 15, 26, 31, 34) investigated the impact of operator experience on bleeding complications. The results of these comparisons are inconsistent, with only one study (5) illustrating a statistically significant difference in bleeding rate based on operator experience. Mueller et al. (5) found that experienced operators (those having performed greater than 150 liver biopsies) were more likely to have bleeding complications than less experienced operators (<150 biopsies) (bleed rate 0.7% for inexperienced vs. 2.0% for experienced, P = 0.01). Gilmore et al. (15) compared operators who had performed fewer than 20 biopsies with those who had performed >100 biopsies and found that, in contrast to Mueller, more experienced operators were less likely to be associated with bleeding complications (3.2% vs. 1.1%), although they did not perform any statistical analysis on these findings. Westheim et al. (34) compared bleeding rates in operators, using 10 biopsies as the threshold between experienced and inexperienced. While experienced operators had a generally higher rate of bleeding complications, there was no statistically significant difference when compared with less experienced operators (Table 4). Finally, van der Poorten et al. (31) and Seeff et al. (26) each found no significant differences between experienced and inexperienced operators but did not provide specific data. Furthermore, as outlined above, the definition of experienced versus inexperienced operators varied greatly between different studies, further adding to the heterogeneity of the studies.
Table 4.
Rate of bleeding complications as a function of operator experience (data from Westheim et al., 2013)
| Period | Experience level of operator | Major bleed rate, n (%) | Minor bleed rate, n (%) | Results |
|---|---|---|---|---|
| Prior to study | ≤10 biopsies | 1 (2.2) | 7 (15.2) | No statistically significant differences in bleeding rate |
| >10 biopsies | 2 (0.8) | 27 (10.2) | ||
|
| ||||
| During study period | ≤ 10 biopsies | 0 (0) | 0 (0) | No statistically significant differences in bleeding rate |
| >10 biopsies | 2 (0.9) | 21 (9.7) | ||
There was a trend towards an increased rate of bleeding complications in centers where more biopsies were performed on a per-year basis. In the Mueller report (5), which reported that bleeding complications were more common in experienced clinicians, the large center studied performed an average of over 200 biopsies each year. In contrast, in centers where fewer than 100 biopsies are performed each year, there was either no significant difference between experienced and inexperienced operators (15, 26, 31), or inexperienced operators were associated with more bleeding complications (3.2% for inexperienced operators [<20 biopsies] vs. 1.1% for experienced operators [>100 biopsies]) (34).
Discussion
Adverse events following image-guided liver biopsy are not common but can pose substantial challenges for physicians and patients. While major bleeding risk is known to increase in patients who are unable to cooperate (i.e., maintain specific position, respond to commands) (41), several other risk factors play important roles in the risk of bleeding complications following biopsy. In this review, we compiled the best available evidence to identify risk factors associated with hemorrhage with image-guided liver biopsy. The risk of bleeding complication was directly assessed in 19 articles, with 17 estimating the risk at less than 2%. This rate compares favorably to the rate of bleeding complications in transjugular liver biopsy, used most often in patients with ascites or potential coagulopathy, which has been estimated at 1%–3% (42, 43), and as low as 0.59% (44).
Risk factors were categorized as either patient-related, operator-related, or procedure-related. The preponderance of evidence is in the form of retrospective studies and the findings are somewhat heterogeneous; however, several risk factors were identified and should be considered by clinicians performing this procedure.
The current literature indicates several patient-related factors that contribute to the risk of bleeding complications, including needle size, use of cutting needles, and biopsy in highly vascular organs/lesions (i.e., renal and liver biopsies) (41). In our review, several patient-related factors were associated with bleeding following biopsy. While some factors such as gender showed no effect, others such as age or inpatient status were associated with bleeding risk. Several studies demonstrated that increasing age was associated with an increased risk of bleeding. There was also evidence to indicate that in a pediatric population, younger patients were generally at a higher risk of bleeding. Likewise, inpatient status was associated with a higher risk of biopsy-related bleeding than patients who underwent the procedure on an outpatient basis. Advanced age and inpatient status were associated with concurrent diagnoses such as hypertension or malignancy, as well as with an increased likelihood of prescription medication use, perhaps accounting for the higher rate of bleeding noted with these risk factors (Table 3). Patients presenting for biopsy with risk factors that may affect their ability to tolerate the procedure are more likely to experience bleeding complications than those with no such risk factors. This is supported by the evidence that indicates that coagulation profile is also a risk factor for biopsy-related bleeding. Our review found that an elevated INR (>1.5) was associated with an increased risk in bleeding, as was an incomplete coagulation profile and a decreased platelet count. The SIR and CIRSE guidelines currently classify biopsy procedures into low, medium and high risk for bleeding and recommend different preprocedure screening based on this risk stratification (45, 46). INR screening is recommended in all patients undergoing moderate or high-risk procedures and patients receiving warfarin or with suspected liver disease undergoing low-risk procedures. In all patients, PTT is only routinely required in patients receiving intravenous unfractionated heparin. Preprocedurally, platelet and hematocrit levels are only recommended in patients undergoing high-risk procedures (45). The evidence from our review suggests that clinicians should consider patients with elevated INR and/or decreased platelet counts to be at higher risk of biopsy-related bleeding and should take preprocedure precautions. Likewise, inpatients, those with concurrent diagnoses that may affect bleeding and/or coagulation, and those over 50 years of age (or infants) should be identified as potentially higher risk for bleeding and should be monitored accordingly.
Operator-related risk factors center largely on experience. The pooled findings are somewhat inconsistent, with some reports suggesting that experienced interventionists are associated with a higher risk of bleeding while others indicate that increased experience actually decreases the likelihood of bleeding. Biopsy, as with any physical procedure, is a learned skill and, as such, it would be expected that inexperienced physicians would be associated with more cases of adverse events and bleeding than experienced physicians. However, Mueller et al. (5) suggest that experienced physicians are often assigned the more difficult and/or potentially difficult cases, resulting in more bleeding complications due to the inherent obstacles presented by the patient or case, rather than the experience level of the operator. In a pediatric population especially, more experienced physicians are associated with greater rate of bleeding (34), although this is likely due to the difficulty associated with the procedure in an infant population, rather than the abilities of the operator.
Experienced physicians are likely to continue to be assigned challenging or potentially challenging cases. All clinicians, including the less experienced, should take as much care as possible when performing these procedures, mindful of the potential for biopsy-related adverse effects, taking whatever steps are available to minimize the inherent risk.
Several procedure-related risk factors were evaluated in the eligible studies. Previous studies have suggested that needle size and procedure type (cutting) are associated with higher risk of bleeding (41). Our findings support this observation, as we noted that, in a compilation of over 5000 biopsies, fewer needle passes was associated with a statistically significant decrease in bleeding rates. Similarly, while no statistical analysis was performed, cutting biopsy was associated with a greater percentage of bleeding complications when compared with fine needle aspiration (2.7% vs. 0%) and with the Menghini technique (1.8% vs. 0.3%). This is expected as cutting biopsies traverse more vascular solid tissue compared to fine needle aspiration of cystic fluid. Where our findings contradict those of previous studies is the effect of needle size on bleeding rate. Needle gauge was evaluated in a very limited number of studies as subset analyses. While logical, no significant difference in bleeding rate was noted with increased needle size. This counteracts previous studies that have indicated that larger needle gauge is associated with an increase in bleeding (41).
Lesion type was evaluated in over 6000 biopsies and, in the vast majority of these cases, was found not to be relevant to bleeding risk. Westheim et al. (35), in a relatively small study of 275 biopsies in a pediatric population, found that focal lesions were associated with a significantly lower rate of bleeding than other lesion types; however, bleeding complications in large adult populations were found not to differ based on lesion type (e.g., focal, parenchymal). Lesion size was evaluated in only one study of 2229 biopsies, with nearly identical rates of bleeding for all sizes of lesion. The combined evidence suggest that lesion characteristics play little role in the risk of bleeding following biopsy. Regarding procedure-related factors in general, the type of procedure employed appears to be the most important factor in determining the likelihood of bleeding complications and should be considered by clinicians in the preprocedure decision-making process.
Several recommendations were made by the various authors; these recommendations are summarized in Table 5. The most common recommendations center on proper prescreening of patients. Those with confirmed or suspected coagulopathy or comorbidities are the most at-risk for bleeding complications. A general recommendation was to use image-guidance for all biopsies, as it increases the accuracy and decreases the likelihood of complications. The use of 16- or 18-gauge needles was also recommended. The majority of these recommendations are supported by moderate-to-weak evidence. Retrospective reviews dominate the evidence available for this review, with only two prospective studies available for inclusion. The addition of prospective, observational studies to the literature would help to increase the strength of these recommendations and provide improved clinical guidance. With the increasing focus on healthcare costs in North America, there is a definite need to generate evidence through structured reports and quality assurance initiatives in order to support the efficacy and safety of interventional radiology procedures including biopsies (47).
Table 5.
Clinical recommendations for the safe performance of image-guided liver biopsy
| Recommendation | Author(s) | Strength of evidence | Comment |
|---|---|---|---|
| Always use image guidance | Lindor, Beddy, Gunnesson, Kim, Rossi, Firpi, Padia, Nobili | Moderate | Recommendation supported by Level Ib (RCT), II (prospective) and Level III (retrospective) evidence |
| Screen for pre-biopsy coagulopathy | Matos, Potter, Seeff, van der Poorten, Riemann, Thampanitchawong | Weak | Recommendation supported by Level III evidence (retrospective reviews only) |
| Be aware of potential risk factors, screen potential patients carefully | Westheim, West, Short, Terjung, | Weak | Recommendation supported by Level III evidence (retrospective reviews only) |
| Use 16- or 18-gauge needles | Matos, Vijayaraghavan, Chevalier, Thanos | Weak | Recommendation supported by Level III evidence (retrospective reviews only) |
| Minimize the period between INR assessment and biopsy | Howlett | Weak | Recommendation supported by only 1 Level III (retrospective) study |
| Schedule biopsy at least 10 days after last aspirin dose | Atwell | Weak | Recommendation supported by only 1 Level III (retrospective) study |
| In infants, mitigate risk with sedation with benzodiazepines | Azzam | Weak | Recommendation supported by only 1 Level III (retrospective) study |
| Consider transjugular biopsy in patients where the bleeding risk may outweigh the potential benefits | Terjung | Weak | Recommendation supported by only 1 Level III (retrospective) study |
Our study has some limitations. The vast majority of evidence detailing risk factors for post-biopsy bleeding, or complications in general, is retrospective and observational in nature. Only one eligible randomized controlled trial could be identified in our searches. We were only able to identify five prospective studies that evaluated patient safety and adverse effects of associated with liver biopsy. As a result, the vast majority of our evidence is from retrospective chart reviews. Although this type of evidence is of generally low methodological quality, the cohort nature of the studies provides the best available evidence outlining risk factors and adverse effects. As biopsy is a generally accepted method of diagnosis, it is not subject to randomized trials and adverse effects are generally captured as secondary outcomes in larger studies.
As with many systematic or scoping reviews, the heterogeneity of outcomes in the eligible studies is a limitation. Across the 34 eligible studies, no single risk factor was evaluated in more than 8 studies. Some risk factors were evaluated in only 2 of 34 studies. This lack of consistency through the studies limits the veracity with which conclusions can be drawn from the data. Similarly, studies reporting on a similar risk factor often defined that factor differently, adding to the heterogeneity of sample. “Experienced operator” was variably defined as a physician having completed anywhere from over 10 biopsies to over 100 biopsies (15). One study provided two separate definitions of “experienced operator” within the same study, depending on whether the operator’s skill was evaluated prior to the study period or during the stud period (34). Similarly, platelet counts were identified as an important risk factor for consideration in five studies but each study reported varying ranges and units. Studies variably set thresholds for platelet counts at 70×109 platelets/L, 150×109 platelets/L and 200×109 platelets/L, with another study providing platelet counts as density (platelets/mm3). Furthermore, the biopsies were not further categorized based on fine needle aspiration or tissue biopsy as most of the source studies did not specify this. This heterogeneity increases the difficulty in pooling data and compiling conclusions. Nonetheless, as with other heterogeneous outcomes, the available evidence provides sufficient evidence to support conclusions in a pragmatic and clinically-relevant sense and thus is of substantial value to clinicians.
In conclusion, this review found that image-guided liver biopsy is a safe and effective procedure, with bleeding complications occurring at a rate of between 0% and 25%, although the vast majority of studies reported rates for bleeding under 2%. Risk factors identified as potentially important when attempting to minimize the risk of bleeding during biopsy include patient age (either >50 years or <2 years), inpatient status, comorbidities or concurrent diagnosis, and coagulation status. Operator experience should be considered as a risk factor for bleeding although, paradoxically, the increased risk is associated with experienced clinicians being assigned to more challenging cases, a fact of the art of medicine that is inescapable.
Main points.
Post-biopsy hemorrhage (PBH) occurs in less than 2% of patients undergoing liver biopsy (0%–10.9%).
Several patient, procedure, and operator related factors could impact the rate of PBH.
There is moderate level of evidence that utilization of image guidance has impact on the rate of PBH.
There is weak evidence to support that age, coagulation status, patient comorbidity, needle size, and presence of a patent track correlate with PBH rate.
Footnotes
Conflict of interest disclosure
The authors declared no conflicts of interest.
References
- 1.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 2.Al Knawy B, Shiffman M. Percutaneous liver biopsy in clinical practice. Liver Int. 2007;27:1166–1173. doi: 10.1111/j.1478-3231.2007.01592.x. [DOI] [PubMed] [Google Scholar]
- 3.Weigand K, Weigand K. Percutaneous liver biopsy: retrospective study over 15 years comparing 287 inpatients with 428 outpatients. J Gastroenterol Hepatol. 2009;24:792–799. doi: 10.1111/j.1440-1746.2008.05718.x. [DOI] [PubMed] [Google Scholar]
- 4.Kose S, Ersan G, Tatar B, Adar P, Sengel BE. Evaluation of percutaneous liver biopsy complications in patients with chronic viral hepatitis. Eurasian J Med. 2015;47:161–164. doi: 10.5152/eurasianjmed.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mueller M, Kratzer W, Oeztuerk S, et al. Percutaneous ultrasonographically guided liver punctures: an analysis of 1961 patients over a period of ten years. BMC Gastroenterol. 2012;12:173. doi: 10.1186/1471-230X-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nazarian LN, Feld RI, Herrine SK, et al. Safety and efficacy of sonographically guided random core biopsy for diffuse liver disease. J Ultrasound Med. 2000;19:537–541. doi: 10.7863/jum.2000.19.8.537. [DOI] [PubMed] [Google Scholar]
- 7.Sparchez Z. Complications after percutaneous liver biopsy in diffuse hepatopathies. Rom J Gastroenterol. 2005;14:379–384. [PubMed] [Google Scholar]
- 8.Neuberger J, Grant A, Day C, Saxseena S. Guidelines on the use of liver biopsy in clinical practice. BSG Guidelines in Gastroenterology. 2004 [Google Scholar]
- 9.Howlett DC, Drinkwater KJ, Lawrence D, Barter S, Nicholson T. Findings of the UK national audit evaluating image-guided or image-assisted liver biopsy. Part II. Minor and major complications and procedure-related mortality. Radiology. 2013;266:226–235. doi: 10.1148/radiol.12120224. [DOI] [PubMed] [Google Scholar]
- 10.Amaral JG, Schwartz J, Chait P, et al. Sonographically guided percutaneous liver biopsy in infants: a retrospective review. AJR Am J Roentgenol. 2006;187:W644–649. doi: 10.2214/AJR.05.1536. [DOI] [PubMed] [Google Scholar]
- 11.Atwell TD, Smith RL, Hesley GK, et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am J Roentgenol. 2010;194:784–789. doi: 10.2214/AJR.08.2122. [DOI] [PubMed] [Google Scholar]
- 12.Azzam RK, Alonso EM, Emerick KM, Whitington PF. Safety of percutaneous liver biopsy in infants less than three months old. J Pediatr Gastroenterol Nutr. 2005;41:639–643. doi: 10.1097/01.mpg.0000184608.22928.f9. [DOI] [PubMed] [Google Scholar]
- 13.Beddy P, Lyburn IL, Geoghegan T, Buckley O, Buckley AR, Torreggiani WC. Outpatient liver biopsy: a prospective evaluation of 500 cases. Gut. 2007;56:307. doi: 10.1136/gut.2006.110460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevallier P, Ruitort F, Denys A, et al. Influence of operator experience on performance of ultrasound-guided percutaneous liver biopsy. Eur Radiol. 2004;14:2086–2091. doi: 10.1007/s00330-004-2407-0. [DOI] [PubMed] [Google Scholar]
- 15.Gilmore IT, Burroughs A, Murray-Lyon IM, Williams R, Jenkins D, Hopkins A. Indications, methods, and outcomes of percutaneous liver biopsy in England and Wales: an audit by the British Society of Gastroenterology and the Royal College of Physicians of London. Gut. 1995;36:437–441. doi: 10.1136/gut.36.3.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govender P, Jonas MM, Alomari AI, et al. Sonography-guided percutaneous liver biopsies in children. AJR Am J Roentgenol. 2013;201:645–650. doi: 10.2214/AJR.12.9802. [DOI] [PubMed] [Google Scholar]
- 17.Gunneson TJ, Menon KV, Wiesner RH, et al. Ultrasound-assisted percutaneous liver biopsy performed by a physician assistant. Am J Gastroenterol. 2002;97:1472–1475. doi: 10.1111/j.1572-0241.2002.05789.x. [DOI] [PubMed] [Google Scholar]
- 18.Hatfield MK, Beres RA, Sane SS, Zaleski GX. Percutaneous imaging-guided solid organ core needle biopsy: coaxial versus noncoaxial method. AJR Am J Roentgenol. 2008;190:413–417. doi: 10.2214/AJR.07.2676. [DOI] [PubMed] [Google Scholar]
- 19.Matos H, Noruegas MJ, Goncalves I, Sanches C. Effectiveness and safety of ultrasound-guided percutaneous liver biopsy in children. Pediatr Radiol. 2012;42:1322–1325. doi: 10.1007/s00247-012-2433-z. [DOI] [PubMed] [Google Scholar]
- 20.Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28:705–712. doi: 10.1111/j.1478-3231.2008.01691.x. [DOI] [PubMed] [Google Scholar]
- 21.Nobili V, Comparcola D, Sartorelli MR, et al. Blind and ultrasound-guided percutaneous liver biopsy in children. Pediatr Radiol. 2003;33:772–775. doi: 10.1007/s00247-003-1044-0. [DOI] [PubMed] [Google Scholar]
- 22.Padia SA, Baker ME, Schaeffer CJ, et al. Safety and efficacy of sonographic-guided random real-time core needle biopsy of the liver. J Clin Ultrasound. 2009;37:138–143. doi: 10.1002/jcu.20553. [DOI] [PubMed] [Google Scholar]
- 23.Potter C, Hogan MJ, Henry-Kendjorsky K, Balint J, Barnard JA. Safety of pediatric percutaneous liver biopsy performed by interventional radiologists. J Pediatr Gastroenterol Nutr. 2011;53:202–206. doi: 10.1097/MPG.0b013e3182183012. [DOI] [PubMed] [Google Scholar]
- 24.Riemann B, Menzel J, Schiemann U, Domschke W, Konturek JW. Ultrasound-guided biopsies of abdominal organs with an automatic biopsy system. A retrospective analysis of the quality of biopsies and of hemorrhagic complications. Scand J Gastroenterol. 2000;35:102–107. doi: 10.1080/003655200750024614. [DOI] [PubMed] [Google Scholar]
- 25.Scheimann AO, Barrios JM, Al-Tawil YS, Gray KM, Gilger MA. Percutaneous liver biopsy in children: impact of ultrasonography and spring-loaded biopsy needles. J Pediatr Gastroenterol Nutr. 2000;31:536–539. doi: 10.1097/00005176-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Seeff LB, Everson GT, Morgan TR, et al. Complication rate of percutaneous liver biopsies among persons with advanced chronic liver disease in the HALT-C trial. Clin Gastroenterol Hepatol. 2010;8:877–883. doi: 10.1016/j.cgh.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short SS, Papillon, Hunter CJ, et al. Percutaneous liver biopsy: pathologic diagnosis and complications in children. J Pediatr Gastroenterol Nutr. 2013;57:644–648. doi: 10.1097/MPG.0b013e3182a0e0d8. [DOI] [PubMed] [Google Scholar]
- 28.Sornsakrin M, Helmke K, Briem-Richter A, Ganschow R. Value of ultrasound-guided percutaneous liver biopsy in children following liver transplantation. J Pediatr Gastroenterol Nutr. 2010;51:635–637. doi: 10.1097/MPG.0b013e3181e7e832. [DOI] [PubMed] [Google Scholar]
- 29.Terjung B, Lemnitzer I, Dumoulin FL, et al. Bleeding complications after percutaneous liver biopsy. An analysis of risk factors. Digestion. 2003;67:138–145. doi: 10.1159/000071293. [DOI] [PubMed] [Google Scholar]
- 30.Thampanitchawong P, Piratvisuth T. Liver biopsy:complications and risk factors. World J Gastroenterol. 1999;5:301–304. doi: 10.3748/wjg.v5.i4.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Poorten D, Kwok A, Lam T, et al. Twenty-year audit of percutaneous liver biopsy in a major Australian teaching hospital. Intern Med J. 2006;36:692–699. doi: 10.1111/j.1445-5994.2006.01216.x. [DOI] [PubMed] [Google Scholar]
- 32.Vijayaraghavan G, Sheehan D, Zheng L, Hussain S, Ferrucci J. Unusual complication after left-lobe liver biopsy for diffuse liver disease: severe bleeding from the superior epigastric artery. AJR Am J Roentgenol. 2011;197:W1135–1139. doi: 10.2214/AJR.11.6545. [DOI] [PubMed] [Google Scholar]
- 33.West J, Card TR. Reduced mortality rates following elective percutaneous liver biopsies. Gastroenterology. 2010;139:1230–1237. doi: 10.1053/j.gastro.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Westheim BH, Aagenaes I, Ostensen AB, Sanengen T, Almaas R. Effect of operator experience and frequency of procedure performance on complication rate after ultrasound-guided percutaneous liver biopsies. J Pediatr Gastroenterol Nutr. 2013;57:638–643. doi: 10.1097/MPG.0b013e3182a0c7a5. [DOI] [PubMed] [Google Scholar]
- 35.Westheim BH, Ostensen AB, Aagenaes I, Sanengen T, Almaas R. Evaluation of risk factors for bleeding after liver biopsy in children. J Pediatr Gastroenterol Nutr. 2012;55:82–87. doi: 10.1097/MPG.0b013e318249c12a. [DOI] [PubMed] [Google Scholar]
- 36.Firpi RJ, Soldevila-Pico C, Abdelmalek MF, Morelli G, Judah J, Nelson DR. Short recovery time after percutaneous liver biopsy: should we change our current practices? Clin Gastroenterol Hepatol. 2005;3:926–929. doi: 10.1016/S1542-3565(05)00294-6. [DOI] [PubMed] [Google Scholar]
- 37.Thanos L, Zormpala A, Papaioannou G, Malagari K, Brountzos E, Kelekis D. Safety and efficacy of percutaneous CT-guided liver biopsy using an 18-gauge automated needle. Eur J Intern Med. 2005;16:571–574. doi: 10.1016/j.ejim.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Rossi P, Sileri P, Gentileschi P, et al. Percutaneous liver biopsy using an ultrasound-guided subcostal route. Dig Dis Sci. 2001;46:128–132. doi: 10.1023/A:1005571904713. [DOI] [PubMed] [Google Scholar]
- 39.Kim KW, Kim MJ, Kim HC, et al. Value of “patent track” sign on Doppler sonography after percutaneous liver biopsy in detection of postbiopsy bleeding: a prospective study in 352 patients. AJR Am J Roentgenol. 2007;189:109–116. doi: 10.2214/AJR.07.2071. [DOI] [PubMed] [Google Scholar]
- 40.Lindor KD, Bru C, Jorgensen RA, et al. The role of ultrasonography and automatic-needle biopsy in outpatient percutaneous liver biopsy. Hepatology. 1996;23:1079–1083. doi: 10.1002/hep.510230522. [DOI] [PubMed] [Google Scholar]
- 41.Gupta S, Wallace MJ, Cardella JF, Kundu S, Miller DL, Rose SC. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol. 2010;21:969–975. doi: 10.1016/j.jvir.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Compean D, Cortes C. Transjugular liver biopsy. An update. Ann Hepatol. 2004;3:100–103. [PubMed] [Google Scholar]
- 43.Shin JL, Teitel J, Swain MG, et al. A Canadian multicenter retrospective study evaluating transjugular liver biopsy in patients with congenital bleeding disorders and hepatitis C: is it safe and useful? Am J Hematol. 2005;78:85–93. doi: 10.1002/ajh.20263. [DOI] [PubMed] [Google Scholar]
- 44.Dohan A, Guerrache Y, Dautry R, et al. Major complications due to transjugular liver biopsy: Incidence, management and outcome. Diagn Interv Imaging. 2015 doi: 10.1016/j.diii.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Patel IJ, Davidson JC, Nikolic B, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012;23:727–736. doi: 10.1016/j.jvir.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 46.Veltri A, Bargellini I, Giorgi L, Almeida P, Akhan O. CIRSE guidelines on percutaneous needle biopsy (PNB) Cardiovasc Intervent Radiol. 2017;40:1501–1513. doi: 10.1007/s00270-017-1747-5. [DOI] [PubMed] [Google Scholar]
- 47.Filippiadis DK, Binkert C, Pellerin O, Hoffmann RT, Krajina A, Pereira PL. CIRSE quality assurance document and standards for classification of complications: the CIRSE classification system. Cardiovasc Intervent Radiol. 2017;40:1141–1146. doi: 10.1007/s00270-017-1703-4. [DOI] [PubMed] [Google Scholar]