Abstract
Methadone is a cornerstone therapy for opioid addiction and a public health strategy for HIV/AIDS and hepatitis C reduction. Methadone is also used for acute and chronic pain. As use for chronic pain has grown, so too have adverse events. Constitutive and acquired (drug–interactions) inter- and intra-individual variability in methadone pharmacokinetics and pharmacodynamics confounds reliable clinical use. Identification of enzymes and transporters responsible for methadone disposition has been a long-sought ideal. Initial in vitro studies identified CYP3A4 as metabolizing methadone. Subsequently, by extrapolation, CYP3A4 was long assumed to be responsible for clinical methadone disposition. However, CYP2B6 is also a major catalyst of methadone metabolism in vitro. It has now been unequivocally established that CYP2B6, not CYP3A4, is the principle determinant of methadone metabolism, clearance, elimination, and plasma concentrations in humans. Methadone disposition is susceptible to inductive and inhibitory drug interactions. CYP2B6 genetics also influences methadone metabolism and clearance, which were diminished in CYP2B6*6 carriers and increased in CYP2B6*4 carriers. CYP2B6 genetics can explain, in part, interindividual variability in methadone metabolism and clearance. Thus, both constitutive variability due to CYP2B6 genetics, and CYP2B6-mediated drug interactions, can alter methadone disposition, clinical effect, and drug safety. Methadone is not a substrate for major influx or efflux transporters.
Keywords: methadone, EDDP, CYP2B6, CYP3A4, P-glycoprotein
Methadone is a cornerstone therapy for opioid addiction, and a public health strategy for HIV/AIDS and hepatitis C reduction. More than 300,000 patients in US opioid treatment programs receive methadone annually. Methadone is also used for acute and chronic pain, and analgesic use has grown markedly, particularly among primary care practitioners, with prescriptions increasing 5-fold from 2000 to 2009. Nevertheless, methadone fatalities have also increased more than 5-fold with that expanded use, and methadone was involved in about one-third of fatal prescription opioid-related overdoses from 1999 to 2009.1 In 2009, methadone accounted for only 2% of prescriptions, but 30% of prescription painkiller deaths. Methadone-related deaths peaked in 2007, but still remain frequent,2 with about 5,000 fatalities annually.1
Methadone toxicity is considered a serious public health problem by NIH/NIDA, FDA, CDC, DEA, GAO, SAMHSA), Department of Justice, and the White House Office of National Drug Control Policy. The National Methadone Assessment3 attributed methadone mortality to a) pharmacokinetic or pharmacodynamic drug interactions,4 and b) erroneous induction dosing, resulting in toxic accumulation, with the first 1–2 weeks of treatment constituting the highest risk period.5
Methadone pharmacology is complex. There is considerable constitutive and acquired (drug interactions) inter- and intra-individual variability in methadone pharmacokinetics that confounds reliable clinical use, with the consequent risk of drug accumulation causing untoward side effects or overdose deaths, or conversely, subtherapeutic concentrations causing withdrawal.6,7 Pharmacodynamic variability may also occur, as one-third of well-maintained methadone patients may experience withdrawal symptoms, and adverse events can occur despite normal therapeutic plasma concentrations.8
Methadone disposition has been intensively studied, yet persistently remains poorly understood, in the clinical pharmacology, practitioner, and regulatory communities.9 The identification of enzymes and transporters responsible for methadone disposition has been a long-sought ideal. So too has been a mechanistic understanding for constitutive and acquired (drug interactions) variability in methadone pharmacokinetics, particularly since co-therapy for opioid addiction and HIV/AIDS/HCV and/or tuberculosis is common, and some pharmacotherapeutics for these infections diseases are notorious perpetrators of drug interactions.7
Methadone has a long elimination half-life (1–2 days). It is cleared predominantly by hepatic metabolism, primarily via N-demethylation to 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP), which is pharmacologically inactive, and thence secondarily to 2-ethyl-5-methyl-3,3-diphenylpyrroline (EMDP). The disposition of methadone, which is dosed as a racemate, is stereoselective.
Methadone and cytochrome P4503A (CYP3A)
Methadone metabolism by human liver microsomes (HLM) and expressed cytochrome P450 enzyme (CYP) was extensively studied in the 1990’s, and every report before 2004 concluded that human liver and intestinal microsomal methadone N-demethylation to EDDP was catalyzed predominantly by CYP3A4.10–15 Based on these in vitro studies, and well-intended extrapolation, methadone metabolism and clearance in humans in vivo were ubiquitously and universally attributed to CYP3A4, and numerous practitioner dosing guidelines warned about the potential for CYP3A4-mediated methadone drug interactions and the need to adjust dosing accordingly. The 2007 methadone label, and even labels since revised, state that methadone metabolism is mediated primarily by CYP3A4, that CYP3A4 inducers may increase methadone clearance, that CYP3A4 inhibitors may decrease methadone clearance causing increased or prolonged effects, and generally warn about CYP3A4 drug interactions. Nevertheless, neither the role of CYP3A4 in clinical methadone clearance, nor effects of CYP3A4 induction or inhibition, were ever clinically tested.
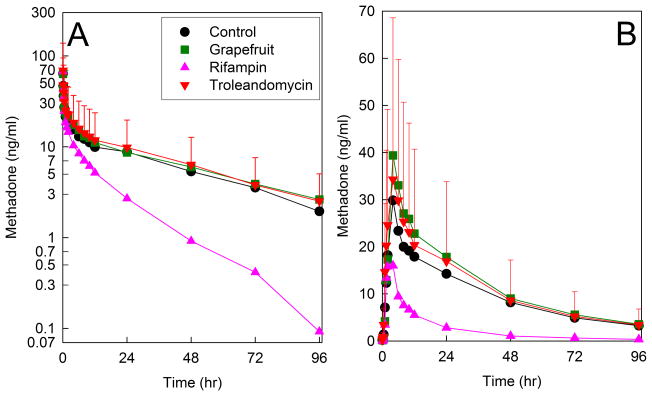
When the first ever clinical assessment of CYP3A activity and methadone clearance was performed, it was revealed that neither hepatic, intestinal, or first-pass CYP3A inhibition had any effect on methadone plasma concentrations, or systemic or apparent oral clearances or other kinetic parameters, and there was no correlation between systemic methadone clearance and hepatic CYP3A4 activity (Figure 1).16 Although rifampin, used to induce CYP3A, enhance methadone metabolism and clearance, this was later attributed to induction of CYP2B6 rather than CYP3A4 (vide infra). These results challenged the notion that CY3A4 was responsible for clinical methadone disposition.16
Figure 1.
Effect of CYP3A induction and inhibition on oral methadone plasma concentrations of (A) intravenous and (B) oral RS-methadone. Subjects were pretreated to achieve hepatic and intestinal CYP3A induction (rifampin, triangles), hepatic and intestinal CYP3A inhibition (troleandomycin, inverted triangles), selective intestinal CYP3A inhibition (grapefruit juice, squares), or control (no pretreatment, circles). Each data point is the mean ± SD (n=12). Some SDs are omitted for clarity. Redrawn from Kharasch et al16 with permission.
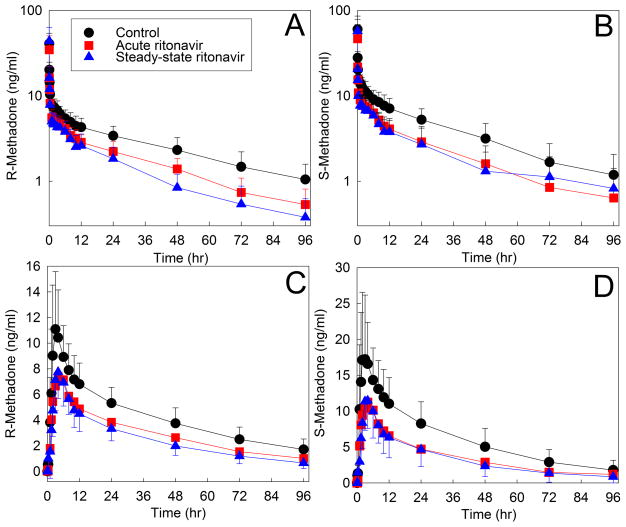
Due to a high incidence of HIV/AIDS and opioid addiction co-therapy, reports of drug interactions, and known inhibitory effects of HIV antiretrovirals on CYP3A4, a series of clinically applicable and mechanistic drug interaction studies between methadone (intravenous and oral) and HIV antiretrovirals (ritonavir,17,18 indinavir,19 ritonavir-indinavir,20 ritonavir-lopinavir,21 nelfinavir,22 efavirenz,23) was performed, along with assessment of hepatic, intestinal, and first-pass CYP3A4/5 activity. The first, between methadone and the strong CYP3A inhibitor ritonavir, found that methadone concentrations were actually and unexpectedly decreased, rather than increased (Figure 2), and methadone clearance was induced rather than inhibited, despite profound (up to 96%) inhibition of CYP3A activity (Figure 3). Figure 3 summarizes the results of these drug interaction studies. Despite substantial strong CYP3A4 inhibition by all the protease inhibitors (and troleandomycin and grapefruit juice), there was no inhibition of methadone metabolism or clearance; rather there was either induction or no effect. Strong hepatic and first-pass CYP3A inhibition by ritonavir/indinavir or indinavir alone had no significant effects on methadone plasma concentrations, systemic or apparent oral clearance, or bioavailability.19,20 Ritonavir alone, ritonavir-lopinavir, and nelfinavir, all induced methadone N-demethylation and clearance, despite strong hepatic and first-pass CYP3A inhibition.17,18,21,22 No investigation showed a significant correlation between CYP3A activity and methadone clearance or N-demethylation. Only efavirenz induced CYP3A4 and methadone clearance (effects later attributed to induction of CYP2B6 rather than CYP3A4; vide infra). These studies recapitulated, and mechanistically illuminated, earlier observational studies on methadone-HAART interactions.24
Figure 2.
Effect of acute and steady-state ritonavir on (A, B) intravenous and (C, D) oral methadone disposition. Shown are plasma R- and S-methadone concentrations after racemic methadone administration to healthy volunteers. Subjects received nothing (controls) or ritonavir, 200mg three times daily for 1d, 300mg twice daily for 6d, then 400mg twice daily for 2 weeks. Methadone disposition was determined on the third day of ritonavir and at steady-state (2 weeks). Each data point is the mean ± SD (n=12). Redrawn from Kharasch et al18 with permission). Hepatic and first-pass CYP3A activities (measured as the clearance of the CYP3A-selective probe alfentanil52) were also determined commensurate with methadone disposition. Hepatic CYP3A activity was inhibited 72% by steady-state ritonavir. First-pass CYP3A activity was inhibited 96% and 90%, respectively, by acute low-dose and steady-state higher dose ritonavir.17 Methadone metabolism and clearance were induced despite profound inhibition of CYP3A.
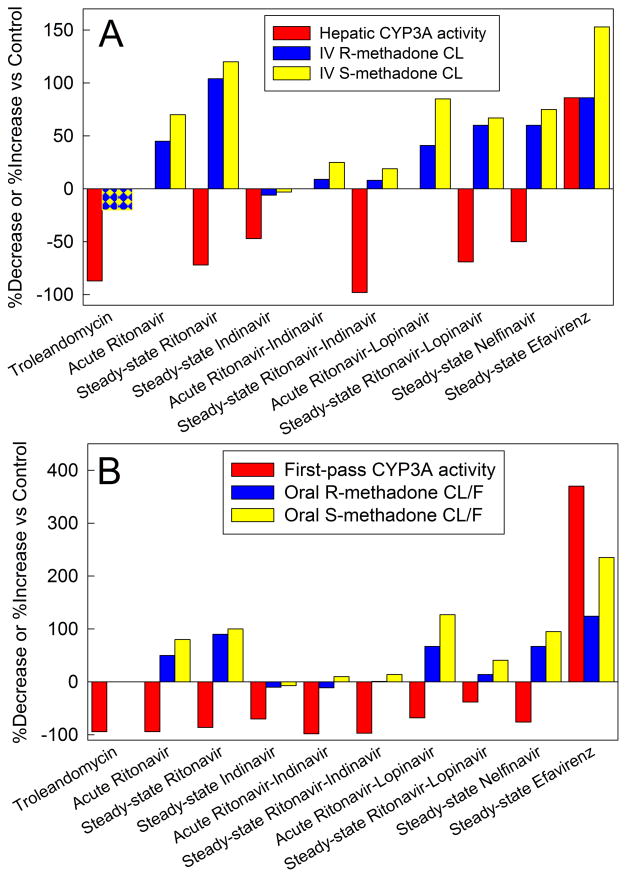
Figure 3.
Methadone disposition and cytochrome P4503A (CYP3A) activity. Results are from a series of drug interaction studies in healthy volunteers. Methadone was administered as the clinically used racemate. With the exception of the methadone-troleandomycin study, which determined total methadone concentrations (using an achiral assay), individual plasma R- and S-methadone enantiomers were determined using a stereoselective assay. Hepatic CYP3A activity and first-pass CYP3A activity were assessed by the clearance of intravenous alfentanil, and oral alfentanil respectively. Alfentanil is a CYP3A4/5-specific probe.52 For all of the methadone-antiretroviral interaction studies (ritonavir,17,18 indinavir,19 ritonavir-indinavir,20 ritonavir-lopinavir,21 nelfinavir,22 and efavirenz,23) alfentanil and methadone disposition were determined in the same subjects (on different days). For the effects of the CYP3A-selective inhibitor troleandomycin, effects on CYP3A activity (alfentanil clearance52) and on methadone disposition16 were determined in separate studies. Acute antiretroviral effects were determined after 2–3 days, and steady-state effects were determined after two weeks. Results are shown as the average per cent increase (ascending bars) or decrease (descending bars) in CYP3A activity (alfentanil clearance) and systemic (CL) or apparent oral (CL/F) methadone clearance.
(A) Intravenous methadone disposition and hepatic CYP3A activity. Racemic methadone was administered as a single intravenous (IV) bolus.
(B) Oral methadone disposition and first-pass CYP3A activity. Racemic methadone was administered as a single oral dose.
Together these investigations established that a) CYP3A has no influence on single-dose intravenous or oral methadone plasma concentrations, b) CYP3A plays a minimal (if any) role clinically in single-dose methadone N-demethylation and clearance, c) methadone is not a clinical CYP3A substrate, and d) clinical guidelines stating that methadone is a CYP3A4 substrate and warning about CYP3A4 drug interactions needed revision. In addition, CYPs 2C9, 2C19, and 2D6 do not appear to contribute materially to clinical methadone N-demethylation and clearance.25,26
Methadone and cytochrome P4502B6 (CYP2B6)
Disputation regarding methadone and CYP3A prompted a renewed search for CYP(s) catalyzing methadone metabolism. This identified CYP2B6, which was not initially considered or available as a homologously expressed enzyme, as a second predominant catalyst of HLM methadone metabolism.9,16,27 These and subsequent investigations identified that a) expressed CYP2B6 was as effective as CYP3A4 in N-demethylating methadone; b) HLM CYP2B6 content strongly influenced methadone metabolism; c) HLM methadone N-demethylation is stereoselective; d) CYP2B6 but not CYP3A4 is responsible for the stereoselective metabolism by recombinant CYPs and HLM; e) greater stereoselective metabolism (S- > R-methadone) occurred in HLM containing higher CYP2B6 than CYP3A4 content; f) HLM methadone metabolism was diminished more by CYP2B6 than CYP3A inhibitors, g) HLM CYP2B6 content predicted clinical plasma R/S methadone concentration ratios; and h) changes in plasma R/S methadone concentration ratios observed clinically after rifampin or troleandomycin pretreatment were successfully predicted by CYP2B6- but not CYP3A4-catalyzed methadone N-demethylation. 28–30 Together this suggested that CYP2B6 may be a major determinant of clinical methadone metabolism and disposition and drug interactions.
This supposition was verified in a pivotal clinical study using the CYP2B6-selective inhibitor ticlopidine, which does not inhibit CYP3A.31 Ticlopidine increased the plasma methadone area under the curve, did so stereoselectively (S>R), and diminished methadone N-demethylation and clearance, after both intravenous and oral dosing. This established the predominant role for CYP2B6 in clinical methadone disposition.
Such identification prompts the reevaluation of earlier methadone drug interaction studies, now comparing oral methadone clearance and hepatic CYP2B6 activity (Figure 4). In addition to inhibiting CYP3A, ritonavir induces CYP2B632 (as does ritonavir/lopinavir33). CYP2B6 induction, either alone (by ritonavir) or co-induction with CYP3A (by rifampin or efavirenz) increased methadone metabolism and clearance.16,18,23 Furthermore, time-dependent increases in the plasma R/S methadone ratio were enhanced with CYP2B6 induction by rifampin, ritonavir, or efavirenz,16,18,23,29,32 but unaffected from CYP3A inhibition by troleandomycin, ritonavir, nelfinavir, indinavir, or ritonavir/indinavir.16,18–20,22,23 The clear concordance between methadone clearance and CYP2B6 activity (Figure 4) contrasts with the clear discordance between methadone clearance and CYP3A activity (Figure 3). These drug interaction studies reinforce the predominant role for CYP2B6 in single-dose methadone N-demethylation, clearance, and plasma concentrations. Methadone interactions with HAART and anti-tuberculars (rifampin) are mediated by CYP2B6 induction.
Figure 4.
Oral methadone disposition and first-pass cytochrome P4502B6 (CYP2B6) activity. Results are from a series of drug interaction studies in healthy volunteers. Racemic methadone was administered as a single oral dose. With the exception of the methadone-rifampin study, which determined total methadone concentrations (using an achiral assay), individual plasma R- and S-methadone enantiomers were determined using a stereoselective assay. Effects of rifampin on methadone disposition16 and on CYP2B6 activity (oral bupropion hydroxylation)53 were determined in separate volunteer studies. Effects of ritonavir on methadone disposition,17 and on CYP2B6 activity (oral bupropion hydroxylation),32 were determined in separate studies. Efavirenz effects on methadone disposition and on CYP2B6 activity (determined as efavirenz hydroxylation) were assessed in the same subjects.23 Influence of the CYP2B6-selective inhibitor ticlopidine31,54 on methadone clearance31 and on CYP2B6 activity (oral bupropion hydroxylation)54 were determined separately. Acute antiretroviral effects were determined after 2–3 days, and steady-state effects were determined after two weeks. Results are shown as the average per cent increase (ascending bars) or decrease (descending bars) in first-pass CYP2B6 activity and apparent oral (CL/F) methadone clearance.
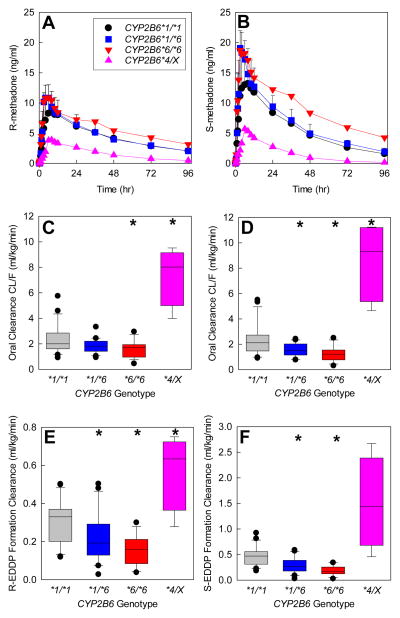
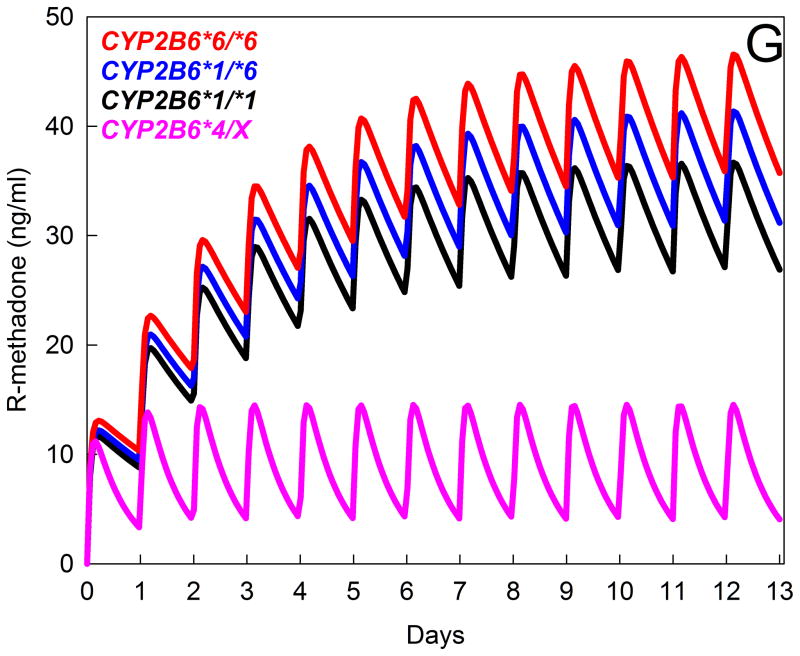
Pharmacogenetic studies reinforce the seminal role for CYP2B6 in methadone disposition.34 The CYP2B6 gene is highly polymorphic, with several variant alleles.35 The most common and clinically important variant is CYP2B6*6 (encoding CYP2B6.6 protein), with reduced hepatic expression and activity, and a less common variant (CYP2B6*4) causes increased expression and variably increased or decreased activity.35 CYP2B6 genetics influences methadone metabolism. N-demethylation at therapeutic concentrations by expressed human CYP in vitro was CYP2B6.4 ≥ CYP2B6.1 (wild-type) ≥ CYP2B6.5 > CYP2B6.9 ≥ CYP2B6.6, and undetectable from CYP2B6.18.36,37 HLM from CYP2B6*6 carriers had lower CYP2B6 content and methadone N-demethylation.36 Clinically, in CYP2B6*6 homozygotes vs heterozygotes and non-carriers, single steady-state dose-adjusted plasma R- and S-methadone concentrations were greater, and methadone dose requirements were lower.34,38 The mechanism for these associations was unknown (altered hepatic metabolism and clearance, renal clearance, other) until recently, when a fuller evaluation of clinical methadone disposition and CYP2B6 genetics was performed, with full sampling.39 Compared with wild-type individuals (CYP2B6*1/*1), methadone plasma concentrations were higher in CYP2B6*6 carriers and lower in CYP2B6*4 carriers (Figure 5, A and B). These effects were attributable to decreased and increased clearance in CYP2B6*6 and CYP2B6*4 carriers, respectively (Figure 5, C and D), in turn due to differences in N-demethylation (Figure 5E and F). Metabolism and clearance were lower in African Americans, compared with Caucasians, due to a larger proportion of CYP2B6*6 carriers and no CYP2B6*4 carriers. While these observations were from single-dose studies, simulations suggest that the effects of CYP2B6 genetic variants may be even greater at steady-state (Figure 5G). Thus CYP2B6 genetics can explain, in part, interindividual variability in methadone metabolism and clearance.
Figure 5.
Influence of CYP2B6 genotype on the disposition and metabolism of oral RS-methadone. Genotype cohorts were CYP2B6*1/*1 (n=21), CYP2B6*1/*6 (n=20), CYP2B6*6/*6 (n=17), and CYP2B6*4/X (n=4, with results for one CYP2B6*1/*4 and three CYP2B6*4/*6 subjects combined). *Significantly different from wild-type (CYP2B6*1/*1), P < 0.05. EDDP = 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidine, CL/F = apparent oral clearance.
(A and B) Plasma concentrations of R- and S-methadone after a single oral dose (mean ± SD, some SD values omitted for clarity). Reproduced from Kharasch et al39 with permission.
(C and D) Apparent oral clearance of R-methadone (C) and S-methadone (D). Results are shown as box plots (solid line within the box represents the median, dashed line within the box represents the mean, box boundaries are the 25th and 75th percentiles, error bars are the 10th and 90th percentiles, and individual points are outliers). Redrawn from Kharasch et al39 with permission.
(E and F) R- and S-EDDP formation clearance. Results are shown as box plots. Redrawn from Kharasch et al39 with permission.
(G) Influence of CYP2B6 genetic polymorphisms on plasma R-methadone concentrations during daily dosing, predicted from single-dose data. Multi-dose methadone enantiomer plasma concentrations were predicted for the various CYP2B6 genotypes using public-access models (http://copnt13.cop.ufl.edu/safezone/pat/pha5127/simulatn.htm) and the pharmacokinetic parameters obtained previously (apparent oral clearance, volume of distribution, bioavailability),39 and a reported oral absorption constant.42 Result are shown (predicted plasma R-methadone concentrations) for once-daily dosing of 20 mg oral racemic methadone (10 mg R-methadone). Simulations do not incorporate any influence of autoinduction of methadone clearance over the first 2 weeks of dosing, which is known to occur,42 and would decrease plasma concentrations during the second week and thereafter, because the relative susceptibility of various CYP2B6 genotypes to autoinduction is not known.
A particularly vexing problem is the high incidence of adverse events including overdose during the initiation of methadone therapy in relatively opioid-naïve patients, most commonly in the first weeks of treatment.5,40 It has long been appreciated methadone elimination undergoes time-dependent autoinduction with repeated dosing.41,42 After a week, plasma concentrations decreased 25%–40%, and elimination half-life was reduced in half, and with considerable interindividual variability.41 Autoinduction has been ascribed to increased clearance and metabolism,43 and speculatively to hepatic CYP3A4 induction.42 In human hepatocytes, methadone caused concentration-dependent autoinduction of N-demethylation, related to increased CYP2B6 and CYP3A4 mRNA and protein expression and catalytic activity. These in vitro findings, coupled to the above conclusions, suggest that clinical autoinduction of methadone metabolism and clearance result from CYP2B6 upregulation.
In summary, it is now obvious that CYP2B6 a) is a predominant catalyst of methadone metabolism in vitro; b) mediates clinical methadone metabolism, clearance, stereoselective disposition, and drug-drug interactions; and c) genetic polymorphisms influence methadone disposition. Thus, both constitutive variability due to CYP2B6 genetics, and CYP2B6-mediated drug interactions, can alter methadone disposition, clinical effect, and drug safety. Rewritten clinical guidelines stating that methadone is a CYP2B6 substrate and warning about CYP2B6 drug interactions may improve methadone use, treatment of pain and substance abuse, and patient safety. Nevertheless, despite clear and compelling evidence to the contrary, there is still persistent “CYP3A inertia” with regard to methadone disposition, which tenaciously endures in published literature and the methadone label.9 Additional effort is needed to overcome this inertia, redirect the field, and motivate updating the methadone label and practitioner guidelines.
Methadone and Drug Transporters
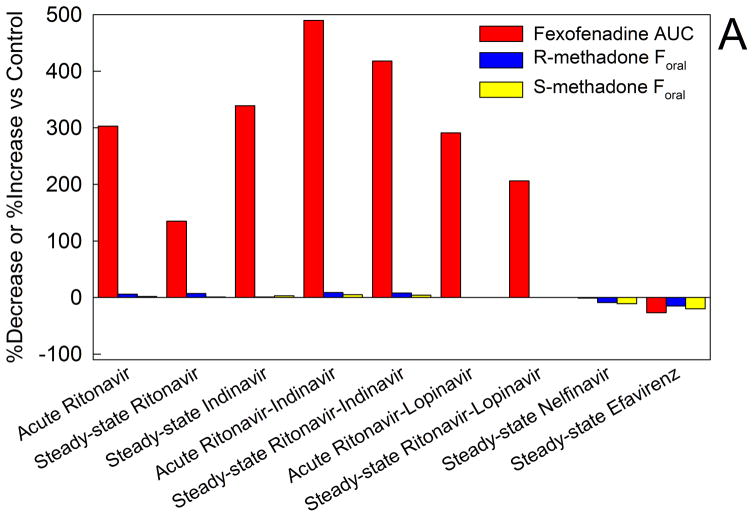
Influx and efflux transporters regulate drug absorption and excretion at most environment-organ interfaces (e.g. intestinal drug absorption, renal drug excretion), and vasculature-organ interfaces (e.g. hepatic drug concentrations, blood-brain barrier). Earliest studied among transporters was the efflux transporter P-glycoprotein (P-gp), and methadone was identified as a substrate for rodent intestinal P-gp.44 To assess this in humans, a series of clinically applicable and mechanistic studies was conducted to evaluate HIV antiretroviral effects on intestinal P-gp activity, and to compare changes in intestinal P-gp activity and methadone bioavailability (Figure 6A).17–23 All of the protease inhibitors studied, except nelfinavir, significantly increased fexofenadine plasma concentrations, which was not due to diminished elimination, suggesting impaired intestinal efflux. This suggested inhibition of intestinal P-gp efflux activity (although fexofenadine is also a substrate for organic anion uptake transporters), demonstrating a clinically meaningful effect of HIV antiretrovirals on intestinal drug transport. Nevertheless, despite this effect, there was no influence on methadone bioavailability, showing that the HIV antiretrovirals did not alter methadone intestinal absorption, and, mechanistically, that intestinal P-gp does not mediate methadone intestinal absorption. Similarly, the multi-transporter inhibitor cyclosporine delayed but only slightly (10%) decreased oral methadone maximum plasma concentrations.45
Figure 6.
Methadone disposition and clinical effects and drug transporters. (A) Effect of HIV antiretrovirals on intestinal transporter activity and methadone bioavailability. Transporter (P-glycoprotein) activity was assessed using oral fexofenadine as a substrate probe. Results are from a series of drug interaction studies in healthy volunteers. For all of the methadone-antiretroviral interaction studies fexofenadine and methadone disposition were determined in the same subjects on different days.17–23 Acute antiretroviral effects were determined after 2–3 days, and steady-state effects were determined after two weeks. Results are shown as the average per cent increase (ascending bars) or decrease (descending bars) in fexofenadine plasma area under the curve (AUC) and the bioavailability of oral methadone (Foral). Increased fexofenadine plasma AUC is interpreted as inhibition of intestinal P-glycoprotein efflux activity and increased drug absorption.
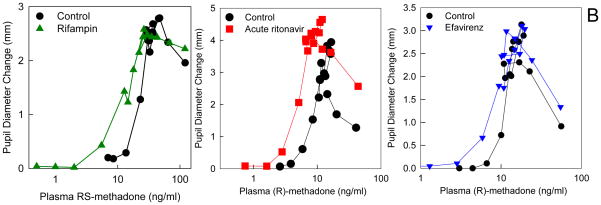
(B) Effect of HIV antiretrovirals and an antituberculosis drug on methadone pharmacodynamics (plasma concentration-effect relationships). Subjects simultaneously received intravenous and also oral methadone (accounting for the second concentration peak, 3–4 h after the initial intravenous peak, due to slow absorption of oral methadone). Results (representing the sum of intravenously and orally administered methadone) are shown for RS-methadone or for the more pharmacologically active R-methadone enantiomer. Methadone effect was determined from the decrease in pupil diameter (miosis). Leftward and/or upward shifts in the concentration-effect relationship suggest increased apparent potency or efficacy and increased brain methadone concentrations. Each data point is the mean of 12 subjects, with SDs omitted for clarity. Data are redrawn from previous publications16,18,23 with permission.
Variability in methadone pharmacodynamics (plasma concentration-effect relationships) has been observed, but is poorly understood.8 In the above-described methadone-HIV antiretroviral interaction studies, anti-tuberculosis (rifampin) and antiretroviral (ritonavir and efavirenz) drugs did influence methadone plasma concentration-effect relationships (determined from opioid decreases in pupil diameter), suggesting a pharmacodynamic interaction occurring at the blood-brain barrier (Figure 6B). Concentration-effect curves were shifted upward and leftward. It is known that influx and efflux transporters are an essential element of the blood-brain barrier, and these drug interactions suggested both that brain access of methadone is transporter-mediated, rather than by just passive permeability, and that transporter-mediated methadone drug interactions at the blood-brain barrier occur. An initially suspected transporter target was P-gp, because methadone was identified as a weak P-gp substrate in vitro and in animals in vivo, where it also influenced methadone analgesia.46 However in clinical studies, neither quinidine nor cyclosporine, used as an inhibitory in vivo probes for blood-brain barrier P-glycoprotein, had an influence on methadone miosis, or on R-methadone plasma concentration-miosis relationships.45,47 That cyclosporine did however markedly increase human brain uptake of the known P-gp substrate loperamide,48 and cyclosporine also increased apparent brain uptake of morphine,49 suggests that P-gp is not the principle determinant of methadone brain access in humans.50 Subsequent studies using cells which over-express specific transporters found that methadone was not a substrate for uptake (OATP1A2, OATP1B1, OATP1B3, OATP2B1, OCT1, OCT2, OCT3) or efflux (P-gp, BCRP) transporters.51 EDDP however was a good substrate for BCRP, P-gp, OATP1A2, OATP1B1, OCT1, and OCT3, and OATP1A2- and OCT3-mediated EDDP uptake, and BCRP-mediated EDDP efflux transport, were inhibited by several HIV antiretrovirals.51 Thus the search for responsible human methadone transporter(s) continues.
Acknowledgments
Supported by National Institutes of Health (Bethesda, MD) grants R01-DA14211, R01- GM63674, R01-DA25931, and K24-DA00417
Footnotes
http://www.cdc.gov/vitalsigns/MethadoneOverdoses/index.html. Last accessed October 30, 2016.
No author has any conflict of interest
References
- 1.Paulozzi LJ, Mack KA, Jones CM. Vital signs: risk for overdose from methadone used for pain relief - United States, 1999–2010. MMWR Morb Mortal Wkly Rep. 2012;61:493–7. [PubMed] [Google Scholar]
- 2.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths - United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64:1378–82. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 3.Summary report of the meeting. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration, DHHS; 2007. Methadone mortality - A reassessment. http://www.dpt.samhsa.gov/pdf/Methadone_Report_10%2018%07_Brief%20w%20attch.pdf. [Google Scholar]
- 4.Shah N, Lathrop SL, Landen MG. Unintentional methadone-related overdose death in New Mexico (USA) and implications for surveillance, 1998–2002. Addiction. 2005;100:176–88. doi: 10.1111/j.1360-0443.2004.00956.x. [DOI] [PubMed] [Google Scholar]
- 5.Baxter LE, Sr, Campbell A, Deshields M, Levounis P, Martin JA, McNicholas L, Payte JT, Salsitz EA, Taylor T, Wilford BB. Safe methadone induction and stabilization: report of an expert panel. J Addict Med. 2013;7:377–86. doi: 10.1097/01.ADM.0000435321.39251.d7. [DOI] [PubMed] [Google Scholar]
- 6.Eap CB, Buclin T, Baumann P. Interindividual variability of the clinical pharmacokinetics of methadone: implications for the treatment of opioid dependence. Clin Pharmacokinet. 2002;41:1153–93. doi: 10.2165/00003088-200241140-00003. [DOI] [PubMed] [Google Scholar]
- 7.McCance-Katz EF, Sullivan LE, Nallani S. Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am J Addict. 2010;19:4–16. doi: 10.1111/j.1521-0391.2009.00005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chugh SS, Socoteanu C, Reinier K, Waltz J, Jui J, Gunson K. A community-based evaluation of sudden death associated with therapeutic levels of methadone. Am J Med. 2008;121:66–71. doi: 10.1016/j.amjmed.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenblatt DJ. Drug interactions with methadone: Time to revise the product label. Clinical Pharm in Drug Dev. 2014;3:249–51. doi: 10.1002/cpdd.137. [DOI] [PubMed] [Google Scholar]
- 10.Iribarne C, Berthou F, Baird S, Dréano Y, Picart D, Bail JP, Beaune P, Ménez JF. Involvement of cytochrome P450 3A4 enzyme in the N-demethylation of methadone in human liver microsomes. Chem. Res. Toxicol. 1996;9:365–73. doi: 10.1021/tx950116m. [DOI] [PubMed] [Google Scholar]
- 11.Moody DE, Alburges ME, Parker RJ, Collins JM, Strong JM. The involvement of cytochrome P450 3A4 in the N-demethylation of L-α-acetylmethadol (LAAM), norLAAM, and methadone. Drug Metab. Dispos. 1997;25:1347–53. [PubMed] [Google Scholar]
- 12.Iribarne C, Dréano Y, Bardou LG, Ménez JF, Berthou F. Interaction of methadone with substrates of human hepatic cytochrome P450 3A4. Toxicology. 1997;117:13–23. doi: 10.1016/s0300-483x(96)03549-4. [DOI] [PubMed] [Google Scholar]
- 13.Iribarne C, Berthou F, Carlhant D, Dreano Y, Picart D, Lohezic F, Riche C. Inhibition of methadone and buprenorphine N-dealkylations by three HIV-1 protease inhibitors. Drug Metab Dispos. 1998;26:257–60. [PubMed] [Google Scholar]
- 14.Foster DJ, Somogyi AA, Bochner F. Methadone N-demethylation in human liver microsomes: Lack of stereoselectivity and involvement of CYP3A4. Br J Clin Pharmacol. 1999;47:403–12. doi: 10.1046/j.1365-2125.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J-S, DeVane CL. Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of (R)- and (S)-methadone in vitro. Drug Metab Dispos. 2003;31:742–7. doi: 10.1124/dmd.31.6.742. [DOI] [PubMed] [Google Scholar]
- 16.Kharasch ED, Hoffer C, Whittington D, Sheffels P. Role of hepatic and intestinal cytochrome P450 3A and 2B6 in the metabolism, disposition and miotic effects of methadone. Clin Pharmacol Ther. 2004;76:250–69. doi: 10.1016/j.clpt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Kharasch ED, Bedynek PS, Walker A, Whittington D, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. II. Ritonavir effects on CYP3A and P-glycoprotein activities. Clin Pharmacol Ther. 2008;84:506–12. doi: 10.1038/clpt.2008.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kharasch ED, Bedynek PS, Park S, Whittington D, Walker A, Hoffer C. Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics. I. Evidence against CYP3A mediation of methadone clearance. Clin Pharmacol Ther. 2008;84:497–505. doi: 10.1038/clpt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kharasch ED, Bedynek PS, Hoffer C, Walker A, Whittington D. Lack of indinavir effects on methadone disposition despite inhibition of hepatic and intestinal cytochrome P4503A (CYP3A) Anesthesiology. 2012;116:432–47. doi: 10.1097/ALN.0b013e3182423478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharasch ED, Hoffer C, Whittington D, Walker A, Bedynek PS. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport: Insights from methadone interactions with ritonavir/indinavir. Anesthesiology. 2009;110:660–72. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharasch ED, Stubbert K. Cytochrome P4503A does not mediate the interaction between methadone and ritonavir/lopinavir. Drug Metab Dispos. 2013;41:2166–74. doi: 10.1124/dmd.113.053991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kharasch ED, Walker A, Whittington D, Hoffer C, Bedynek PS. Methadone metabolism and clearance are induced by nelfinavir despite inhibition of cytochrome P4503A (CYP3A) activity. Drug Alcohol Depend. 2009;101:158–68. doi: 10.1016/j.drugalcdep.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kharasch ED, Whittington D, Ensign D, Hoffer C, Bedynek PS, Campbell S, Stubbert K, Crafford A, London A, Kim T. Mechanism of efavirenz influence on methadone pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2012;91:673–84. doi: 10.1038/clpt.2011.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bruce RD, Moody DE, Altice FL, Gourevitch MN, Friedland GH. A review of pharmacological interactions between HIV or hepatitis C virus medications and opioid agonist therapy: implications and management for clinical practice. Expert Rev Clin Pharmacol. 2013;6:249–69. doi: 10.1586/ecp.13.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crettol S, Deglon JJ, Besson J, Croquette-Krokar M, Hammig R, Gothuey I, Monnat M, Eap CB. ABCB1 and cytochrome P450 genotypes and phenotypes: Influence on methadone plasma levels and response to treatment. Clin Pharmacol Ther. 2006;80:668–81. doi: 10.1016/j.clpt.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Shiran MR, Lennard MS, Iqbal MZ, Lagundoye O, Seivewright N, Tucker GT, Rostami-Hodjegan A. Contribution of the activities of CYP3A, CYP2D6, CYP1A2 and other potential covariates to the disposition of methadone in patients undergoing methadone maintenance treatment. Br J Clin Pharmacol. 2009;67:29–37. doi: 10.1111/j.1365-2125.2008.03312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerber JG, Rhodes RJ, Gal J. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16:36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- 28.Totah RA, Allen KE, Sheffels P, Whittington D, Kharasch ED. Enantiomeric metabolic interactions and stereoselective human methadone metabolism. J Pharmacol Exp Ther. 2007;321:389–99. doi: 10.1124/jpet.106.117580. [DOI] [PubMed] [Google Scholar]
- 29.Totah RA, Sheffels P, Roberts T, Whittington D, Thummel K, Kharasch ED. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108:363–74. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- 30.Chang Y, Fang WB, Lin SN, Moody DE. Stereo-selective metabolism of methadone by human liver microsomes and cDNA-expressed cytochrome P450s: a reconciliation. Basic Clin Pharmacol Toxicol. 2011;108:55–62. doi: 10.1111/j.1742-7843.2010.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kharasch ED, Stubbert K. Role of cytochrome P4502B6 in methadone metabolism and clearance. J Clin Pharmacol. 2013;53:305–13. doi: 10.1002/jcph.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kharasch ED, Mitchell D, Coles R, Blanco R. Rapid clinical induction of hepatic cytochrome P4502B6 activity by ritonavir. Antimicrob Agents Chemother. 2008;52:1663–9. doi: 10.1128/AAC.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hogeland GW, Swindells S, McNabb JC, Kashuba AD, Yee GC, Lindley CM. Lopinavir/ritonavir reduces bupropion plasma concentrations in healthy subjects. Clin Pharmacol Ther. 2007;81:69–75. doi: 10.1038/sj.clpt.6100027. [DOI] [PubMed] [Google Scholar]
- 34.Somogyi AA, Barratt DT, Ali RL, Coller JK. Pharmacogenomics of methadone maintenance treatment. Pharmacogenomics. 2014;15:1007–27. doi: 10.2217/pgs.14.56. [DOI] [PubMed] [Google Scholar]
- 35.Zanger UM, Klein K. Pharmacogenetics of cytochrome P450 2B6 (CYP2B6): advances on polymorphisms, mechanisms, and clinical relevance. Front Genet. 2013;4:24. doi: 10.3389/fgene.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gadel S, Crafford A, Regina K, Kharasch ED. Methadone N-demethylation by the common CYP2B6 allelic variant CYP2B6. 6. Drug Metab Dispos. 2013;41:709–13. doi: 10.1124/dmd.112.050625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gadel S, Friedel C, Kharasch ED. Differences in methadone metabolism by CYP2B6 variants. Drug Metab Dispos. 2015;43:994–1001. doi: 10.1124/dmd.115.064352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dennis BB, Bawor M, Thabane L, Sohani Z, Samaan Z. Impact of ABCB1 and CYP2B6 genetic polymorphisms on methadone metabolism, dose and treatment response in patients with opioid addiction: a systematic review and meta-analysis. PLoS One. 2014;9:e86114. doi: 10.1371/journal.pone.0086114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kharasch ED, Regina KJ, Blood J, Friedel C. Methadone pharmacogenetics: CYP2B6 polymorphisms determine plasma concentrations, clearance, and metabolism. Anesthesiology. 2015;123:1142–53. doi: 10.1097/ALN.0000000000000867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson AE, Degenhardt LJ. Mortality related to pharmacotherapies for opioid dependence: a comparative analysis of coronial records. Drug Alcohol Rev. 2007;26:405–10. doi: 10.1080/09595230701373834. [DOI] [PubMed] [Google Scholar]
- 41.Änggärd E, Nilsson MI, Holmstrand J, Gunne LM. Pharmacokinetics of methadone during maintenance therapy: pulse labeling with deuterated methadone in the steady state. Eur J Clin Pharmacol. 1979;16:53–7. doi: 10.1007/BF00644967. [DOI] [PubMed] [Google Scholar]
- 42.Wolff K, Rostami-Hodjegan A, Hay AW, Raistrick D, Tucker G. Population-based pharmacokinetic approach for methadone monitoring of opiate addicts: potential clinical utility. Addiction. 2000;95:1771–83. doi: 10.1046/j.1360-0443.2000.951217717.x. [DOI] [PubMed] [Google Scholar]
- 43.Verebely K, Volavka J, Mulé S, Resnick R. Methadone in man: Pharmacokinetic and excretion studies in acute and chronic treatment. Clin Pharm Ther. 1975;18:180–90. doi: 10.1002/cpt1975182180. [DOI] [PubMed] [Google Scholar]
- 44.Bouër R, Barthe L, Philibert C, Tournaire C, Woodley J, Houin G. The roles of P-glycoprotein and intracellular metabolism in the intestinal absorption of methadone: In vitro studies using the rat everted intestinal sac. Fundam Clin Pharmacol. 1999;13:494–500. doi: 10.1111/j.1472-8206.1999.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 45.Meissner K, Blood J, Francis AM, Yermolenka V, Kharasch ED. Cyclosporine-inhibitable cerebral drug transport does not influence clinical methadone pharmacodynamics. Anesthesiology. 2014;121:1281–91. doi: 10.1097/ALN.0000000000000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson SJ, Koszdin KK, Bernards CM. Opiate-induced analgesia as increased and prolonged in mice lacking P-glycoprotein. Anesthesiology. 2000;92:1392–99. doi: 10.1097/00000542-200005000-00030. [DOI] [PubMed] [Google Scholar]
- 47.Kharasch ED, Hoffer C, Whittington D. The effect of quinidine, used as a probe for the involvement of P-glycoprotein, on the intestinal absorption and pharmacodynamics of methadone. Br J Clin Pharmacol. 2004;57:600–10. doi: 10.1111/j.1365-2125.2003.02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passchier J, Comley R, Salinas C, Rabiner E, Gunn R, Cunningham V, Wilson A, Houle S, Gee A, Laruelle M. Blood brain barrier permeability of [11C]loperamide in humans under normal and impaired P-glycoprotein function. J Nucl Med. 2008;49(Suppl 1):211P. [Google Scholar]
- 49.Meissner K, Avram MJ, Yermolenka V, Francis AM, Blood J, Kharasch ED. Cyclosporine-inhibitable blood-brain barrier drug transport influences clinical morphine pharmacodynamics. Anesthesiology. 2013;119:941–53. doi: 10.1097/ALN.0b013e3182a05bd3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crettol S, Deglon JJ, Besson J, Croquette-Krokar M, Hammig R, Gothuey I, Monnat M, Eap CB. No Influence of ABCB1 haplotypes on methadone dosage requirement. Clin Pharmacol Ther. 2008;83:668–9. doi: 10.1038/sj.clpt.6100305. [DOI] [PubMed] [Google Scholar]
- 51.Campbell SD, Gadel S, Friedel C, Crafford A, Regina KJ, Kharasch ED. Influence of HIV antiretrovirals on methadone N-demethylation and transport. Biochem Pharmacol. 2015;95:115–25. doi: 10.1016/j.bcp.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Kharasch ED, Walker A, Hoffer C, Sheffels P. Intravenous and oral alfentanil as in vivo probes for hepatic and first-pass cytochrome P450 3A activity: Noninvasive assessment using pupillary miosis. Clin Pharmacol Ther. 2004;76:452–66. doi: 10.1016/j.clpt.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 53.Kharasch ED, Mitchell D, Coles R. Stereoselective bupropion hydroxylation as an in vivo phenotypic probe for cytochrome P4502B6 (CYP2B6) activity. J Clin Pharmacol. 2008;48:464–74. doi: 10.1177/0091270008314254. [DOI] [PubMed] [Google Scholar]
- 54.Turpeinen M, Tolonen A, Uusitalo J, Jalonen J, Pelkonen O, Laine K. Effect of clopidogrel and ticlopidine on cytochrome P450 2B6 activity as measured by bupropion hydroxylation. Clin Pharmacol Ther. 2005;77:553–59. doi: 10.1016/j.clpt.2005.02.010. [DOI] [PubMed] [Google Scholar]