Abstract
Improvements in vehicle safety require understanding of the neural systems that support the complex, dynamic task of real‐world driving. We used functional near infrared spectroscopy (fNIRS) and pupilometry to quantify cortical and physiological responses during a realistic, simulated driving task in which vehicle dynamics were manipulated. Our results elucidate compensatory changes in driver behavior in response to changes in vehicle handling. We also describe associated neural and physiological responses under different levels of mental workload. The increased cortical activation we observed during the late phase of the experiment may indicate motor learning in prefrontal–parietal networks. Finally, relationships among cortical activation, steering control, and individual personality traits suggest that individual brain states and traits may be useful in predicting a driver's response to changes in vehicle dynamics. Results such as these will be useful for informing the design of automated safety systems that facilitate safe and supportive driver–car communication.
Keywords: drviving, functional near infrared spectroscopy, personality, pupilometry, steering control
1. INTRODUCTION
The goal of improving vehicle safety is a major focus of the automobile industry and has led to the design and implementation of increasingly automated vehicle safety systems. A comprehensive understanding of driving behavior under different circumstances, for example, how drivers respond to changes in vehicle handling, could significantly inform the design of these safety systems and improve overall driving safety. Functional neuroimaging during realistic driving scenarios offers a unique opportunity to examine the cognitive processes that support safe and skilled driving behavior.
Driving involves coordinated sensory, motor, and cognitive processes. Characterizing and quantifying the neural circuitry underlying these processes has been a goal among cognitive neuroscientists using various neuroimaging techniques with varying complexity of task design. Functional MRI (fMRI), which requires participants to lie supine in a tube, has been used to examine neural activation in simulated driving and navigation paradigms (Calhoun et al., 2002; Unni, Ihme, Jipp, & Rieger, 2017; Walter et al., 2001), and to investigate fundamental components of driving behavior, such as visuospatial and visuomotor processing (Ng et al., 2000; Pollmann, & von Cramon, 2000) and attention (Beauchamp et al., 2001). Functional near infrared spectroscopy (fNIRS), a portable, noninvasive optical imaging technique, has been used to measure cortical activation during on‐the‐road driving (Yoshino et al., 2013) and in realistic, simulated driving tasks (Tsunashima and Yanagisawa, 2009; Xu et al., 2017).
Driving requires widespread recruitment of neural resources with specific and unique neural circuitry subserving individual aspects of driving behavior. The parietal, occipital, and frontal lobes are most consistently engaged in driving tasks (Li et al., 2012); subcortical, cerebellar and occipital regions are also involved (Calhoun et al., 2002; Calhoun, & Adali, 2012). In particular, driving involves extensive recruitment of cognitive systems responsible for visuospatial integration and visuomotor mapping, including the fronto‐parietal and cerebellar networks (Walter et al., 2001). Executive control, planning and spatial working memory processes are required for driving, and these processes are associated with activation in prefrontal and parietal cortical regions (Spiers and Maguire, 2006; Spiers, & Maguire, 2007). The fronto‐parietal vigilance network is also active during driving, with increased activation at higher speeds (Calhoun et al., 2002). In contrast, error monitoring regions such as the anterior cingulate demonstrate decreased activation with increasing speed (Calhoun et al., 2002).
To better understand the neural basis of drivers' responses to changes in vehicle handling, we used an ultra‐portable fNIRS system to examine cortical activation under different vehicle steering control conditions, using an immersive, fixed‐base, full–cab driving simulator. fNIRS during simulated driving provides a unique opportunity to examine behaviors that occur in more “real world,” ecologically valid settings yielding results that are more interpretable than those collected during an MRI scan. We have greater experimental control over a simulated driving environment relative to natural driving conditions, and thus are able to examine particular aspects of driving behavior while prioritizing safety of the participant.
In this study, we manipulated the visuomotor mapping between the driver's steering commands and the behavior of the simulated vehicle. Participants completed a steering task based on the ISO standard double‐lane change maneuver (ISO 3888‐1 1999). During this task, half of the trials presented the participant with congruent visuomotor mapping in which turning the handwheel (steering wheel) to the right resulted in movement of the vehicle to the right, and vice versa. The other half of the trials presented the participant with incongruent visuomotor mapping in which the movement of the handwheel to the right corresponded with movement of the vehicle to the left, and vice versa. We used fNIRS to measure activation in prefrontal and parietal brain circuits in response to the cognitive demands of these two steering conditions. We included concurrent measurement of pupil diameter. As originally proposed by Kahneman (1973), pupil response as measured by change in pupil diameter is strongly associated with attention (Kang et al., 2014; Wierda, van Rijn, Taatgen, & Martens, 2012) and mental workload during visuospatial tasks such as object tracking (Alnæs et al., 2014) and driving (Recarte & Nunes, 2003). Additionally, our driving simulator (Figure 1) captured precise metrics of driver steering behavior, allowing us to link condition‐based changes in cortical activation with discrete changes in driving performance. Finally, we assessed personality characteristics that may be related to driving performance (Ulleberg & Rundmo, 2003) using the NEO Five‐Factor inventory (NEO‐FFI) (Mccrae & Costa, 2007). Personality characteristics were assessed as potential covariates of interest to predict driving performance, brain activity, and pupillary response. We hypothesized that the incongruent steering condition would be associated with increased cognitive load as evidenced by increased cortical activation, enhanced pupillary response and altered patterns of steering behavior.
Figure 1.

Driving simulator task. (a) Two views of a participant performing the simulated driving task. Eye tracking goggles and fNIRS optodes can be seen positioned on the participant's face and head, respectively. (b) Bird's‐eye view of the task, in which the participant is instructed to drive straight down the center of the lane through the first 3 pairs of cones, and then make a lane change left or right, indicated by the green direction arrow that appeared in their line of sight [Color figure can be viewed at http://wileyonlinelibrary.com]
2. MATERIALS AND METHODS
Participants (N = 21, 10 females, mean age = 23.48, range: 18–37) were right handed as measured by the Edinburg Handedness Inventory (Oldfield, 1971) (mean = 80.2, SD = 15.51), had a valid driver's license or permit, and no prior professional driving experience. Participants had 1–21 years of driving experience (mean = 7.5, SD =0.87) and, drove up to 10 hours per week (mean = 4.89, SD = 5.02) at the time of participation. All participants screened negative for significant psychiatric history including anxiety or depression, and other chronic or significant medical conditions. All participants had normal or corrected to normal vision. The participants were recruited locally via mailing lists, advertisements and fliers. The study was approved by the Stanford University Institutional Review Board (IRB protocol 31247). Written informed consent was obtained from all participants prior to their participation.
The driving simulator (Realtime Technologies Inc., United States, https://cars.stanford.edu/news/new-driving-simulator-human-vehicle-interface-research-now-operational) consisted of a full vehicle cab (Toyota Avalon), with projected dash, a high‐resolution, 270° field‐of‐view cylindrical projection screen, rear projector for the rear‐view mirror, and LCD‐screen side mirrors (Figure 1a). The vehicle was modified to communicate with the simulation software and provide the participant with a realistic, immersive experience. The handwheel provided force feedback to emulate the forces one would normally experience while driving a real automobile. Images from 5 projectors were blended on the cylindrical screen to form the forward and peripheral visual environment. Road and engine noises were provided by a customized surround sound system. The simulated environment was developed in internet scene assembler and saved using virtual reality modeling language (VRML).
During each trial, the participant was instructed via onscreen prompts to steer the car straight down the center lane, steer the car to either the right or left lane, and then steer the car back into the center lane (Figure 1b). Each trial consisted of three sections. (a) The trial began with straight driving in which the participant steered the vehicle down the center lane of the road (marked by traffic cones). (b) The turning portion of the trial began at the second to last cone in the straight portion of the course. At this point an arrow appeared in the participant's line of sight prompting the participant to turn left or right. Participants were also instructed to center the handwheel at the end of the double lane change concluding the active driving portion of the trial. (c) An “autonomous” driving condition began in which the vehicle was driven along a straight portion of the road by an experimenter who controlled the car from a separate console. Participants were unaware of the experimenter's control of the vehicle and experienced this as a fully autonomous condition. The arrow prompts indicating the direction for each trial were triggered by the longitudinal position of the vehicle. These prompts were programmed in Internet Scene Assembler, and were subsequently used as event markers for fNIRS, steering behavior, and pupil diameter data. The steering control of the vehicle was reversed for half of the experimental trials (incongruent). There were 32 trials total (8 left congruent, 8 right congruent, 8 left incongruent, and 8 right incongruent) presented in a pseudorandom order. Participants were instructed that both congruent and incongruent trials would be presented and were given two practice trials of each type prior to the experimental trials. The simulation software controlled the vehicle's longitudinal velocity in order to standardize the timing of experimental events and to isolate responses related to steering control. The velocity was 8 m/s (17.9 miles/h) and the timing for each portion of the course varied slightly for each participant depending upon the amount of lateral motion. Mean durations per portion were 23.43 s for the straight portion, 6.65 s for the turning portion, and 14.51 s for the autonomous portion.
Steering performance, fNIRS, and pupil diameter measures were only included in the group analysis if the trial was performed correctly. Correct performance was defined as the vehicle passing through either the left or right set of double cones (as indicated by the direction signal) and through the center lane. If a participant did not correctly complete a given trial, it was repeated at the end of the pseudorandom sequence. Eight participants performed all 32 trials correctly. Eleven participants repeated between 1 and 7 trials to achieve 32 correct trials. Due to a technical difficulty, one participant repeated 3 trials to achieve only 31 correct trials (for the learning analysis, this individual contributed only 15 trials to the late phase). One participant ended the experiment early after completing only 27 correct trials.
A tandem NIRSport (NIRx, Germany) system was used to record hemodynamic responses using two wavelengths (760 and 850 nm) with 16 LED illumination time‐multiplexed sources and 16 silicon photodiode sensors, sampling at a frequency of 7.8125 Hz. Illumination at two distinct wavelengths facilitated quantification of oxygenated hemoglobin (HbO) and deoxygenated hemoglobin (Hb). Thirty‐two optodes (16 sources, 16 detectors) were positioned over bilateral PFC and bilateral parietal cortex (10 channels per region, 40 channels total, Figure 2). Optodes were positioned over standard 10–20 system locations using individually sized caps (Brain Products, Germany) to maintain consistency across variation in head size (Okamoto et al., 2004; Tsuzuki et al., 2012). Plastic supports were placed between each source/detector pair that constituted a recording channel to maintain a 3 cm channel length. This consistency allowed us to subset the fNIRS channels of interest down to those directly measuring each region of interest (see functional localization section below). fNIRS data were not collected for a subset of participants (N = 4) due to technical errors.
Figure 2.
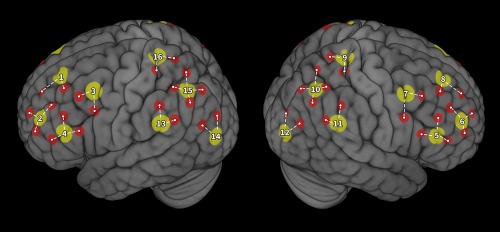
Locations of fNIRS channels. Source (yellow) and channel (red) locations are displayed on the cortical surface. Numbers indicate source number and dashed lines indicate source neighbor clusters that comprised each region of interest [Color figure can be viewed at http://wileyonlinelibrary.com]
SMI ETG 2.0 binocular eye tracking goggles (SensoMotoric Instruments, Germany) were used to measure pupil diameter with two infrared cameras (one for each eye) integrated in the inner eyeglass frame. The goggles recorded pupil diameter at a sampling rate of 30 Hz. Pupillary response was measured for a subset of participants (N = 15).
Participants also completed the NEO‐FFI personality self‐report (Mccrae and Costa, 2007) (N = 20) to examine potential relationships between personality factors and driving performance, cortical activation, and pupil response.
Primary analyses, determined a priori, compared incongruent and congruent trials during the double lane change maneuver (turning portion of the trial). The steering manipulation was active from the beginning of the trial. We hypothesized participants may have altered steering behavior, pupil diameter, and cortical activation during the straight portion of the trial as they probed the behavior of the vehicle in preparation for the double lane change. Accordingly, we also compared congruent and incongruent trials during the straight portion of the trial. Secondary analyses for fNIRS and pupil response datasets compared turning versus straight portions of the trial within the congruent condition, within the incongruent condition, and for both conditions combined. Behavioral, fNIRS, and pupil response data were excluded from any trials in which the participant did not perform the lane change correctly.
The following variables (described previously (Russell et al., 2016); see also Supporting Information, Figure 1) were recorded at 60 Hz, quantified for each trial and averaged during the time indicated for each turning and straight portions of the course.
Time to handwheel peak is a measurement of the elapsed time between the prompt indicating which direction the participant should turn and the first local peak of the handwheel angle. This represents the feed forward component of the driver's steering control, used to plan steering input necessary for a successful lane change.
Handwheel speed (degrees per second) is equal to the time derivative of handwheel angle, and measures how fast a driver is moderating his or her steering input. This metric is a measure of the feedback a driver uses to compensate for steering errors during the lane change.
Yaw rate (degrees per second) is the angular velocity of the vehicle, measured perpendicular to its vertical axis, and is therefore a measure of how rapidly the vehicle is turning.
Lateral acceleration (m/s2) measures the rate of change of the vehicle's velocity in the lateral direction (perpendicular to the centerline running from front to rear in the car). High levels of lateral acceleration and yaw rate are associated with uncomfortable driving maneuvers.
Lateral position deviation is equal to the difference between the lateral position of the vehicle's center of gravity and the mean lateral position of each participant's congruent trials at a given longitudinal position. A one‐sample t test was performed to quantify how different incongruent trials were from zero (i.e., how different they were from congruent trials).
An alpha of 0.05 was used and statistics for feedback metrics of driver behavior were corrected for multiple testing using the false discovery rate (FDR) procedure (Hochberg & Benjamini, 1990). We also examined the peak values for each of the feedback metrics (handwheel speed, yaw rate, lateral acceleration) by extracting the highest value from each trial and each portion of the course (turn and straight), then averaging across trials.
The pattern of cortical activation during each condition of interest was assessed using a general linear model (GLM) approach. The use of GLM for analysis of event‐related fNIRS designs has been well established. The data were analyzed using the HOMER2 package in MATLAB and the preprocessing pipeline outlined by Brigadoi et al. (2014). First, all optical density data were corrected for motion artifacts by the use of a wavelet motion correction procedure. Next, the optical density data were bandpass‐filtered between 0.01 and 0.5 Hz prior to being converted to HbO and Hb values using the modified Beer–Lambert law (Wyatt et al., 1986).
Separate GLM analyses were used to quantify activation from HbO and Hb signals. The time course of fNIRS signal (HbO or Hb) and the task parameters were included in each analysis with no additional covariates. The onset and duration of each condition of interest were submitted to the GLM procedure as predictor variables used to estimate standardized beta coefficients. The sign and magnitude of each beta coefficient provides an indicator of the direction (positive/negative) and intensity of blood‐oxygen level‐dependent change (i.e., cortical activity) that occurred during each condition. Beta coefficients for the congruent and incongruent trials, respectively, were estimated for the straight (preparatory) portion and the lane change (turning) maneuver of each trial. To make a priori comparisons between specific elements of our task, contrasts generated by calculating the difference between condition‐specific beta coefficients were submitted to statistical analysis.
We employed a functional localization approach (Hosseini et al., 2017) to allow for variation in cortical activation in response to our task. Thus, the only channels submitted for group statistical analysis were those that demonstrated a hemodynamic response to our task. This approach differs from the standard channel‐wise analysis approach, which groups all channels across participants on a one‐to‐one basis according to channel number (Tak & Chul, 2014).
First, all channels that shared a common fNIRS source were labeled together and constituted a “source neighbor” cluster. As our optode arrangement contained 16 source optodes, this procedure resulted in 16 source neighbor clusters. Moreover, due to the a priori design of our optode arrangement, the total number of channels within each source neighbor cluster included 2 (n = 10), 3 (n = 4), or 4 (n = 2) channels (Figure 2). Despite differences in the number of channels included in a cluster, grouping channels together based on their source optode constrained each region of interest to a 3 cm radius surrounding each source. Next, within each source neighbor cluster, the single channel with the largest contrast value for each contrast of interest was selected for submission to group analysis. Thus, each participant contributed 16 individual channels (i.e., one channel per cluster, S1–S16) to the group analyses. Additionally, we repeated group‐level statistical analyses including years of driving experience as a covariate.
Pupil diameter data for each eye were deblinked (Siegle et al., 2003) before the left and right eye pupil diameter was averaged to account for differences in pupil size between eyes. Linear detrending was applied to the time course of pupil diameter and the mean pupil diameter for each portion of the trail (straight and turn) and each condition (congruent and incongruent) was calculated.
We examined the relationship between significant cortical responses to the task and metrics of steering behavior, pupil diameter and personality factors (NEO‐FFI scores) using linear regression. A separate, stepwise regression was conducted predicting activity in each channel of interest from a combined set of behavior, pupil diameter, and personality factors.
3. RESULTS
3.1. Steering behavior
Vehicle position across all trials is summarized in Figure 3a. Primary comparisons demonstrated significantly smaller values for time to handwheel peak, for incongruent relative to congruent trials (t(20) = −10.12, p = 2.58e‐9, Table 1), and larger values during the turning portion of the trial for incongruent compared to congruent trials for handwheel speed (t(20) = 18.62, p(FDR) = 4.23e‐14), lateral acceleration (t(20) = 12.28, p(FDR) = 8.99e‐11), and yaw rate (t(20) = 12.55, p(FDR) = 6.11e‐11, Table 1). Vehicle lateral position deviation (representing position relative to the mean of each participant's congruent trials) during the turning maneuver was significantly different from zero for incongruent trials (t(20) = 13.48, p < 1.68e‐11, Table 1).
Figure 3.
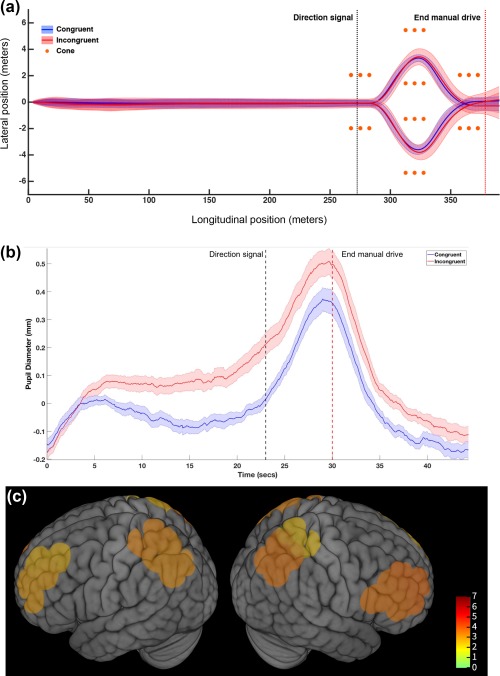
(a) Driving performance summarized by mean vehicle position across all trials and all participants. Straight portion begins at the start of the trial and ends with presentation of the direction signal. Turn potion of the trial starts at the direction signal and ends at the end of the manual drive. Left and right turns are shown separately. Bold lines represent means and shaded regions indicate standard error. (b) Pupil response across the trial measured by SMI eye tracking goggles indicate a significant main effect of driving. Bold lines represent means and shaded regions indicate standard error. (c) Cortical activation for incongruent versus congruent conditions during the double lane change maneuver. Colored regions indicate areas demonstrating significant activation differences between incongruent and congruent conditions using oxygenated hemoglobin (Oxy Hb, p(FDR) < .05) as the primary measure of cortical activity. Channel locations projected onto cortical surface with T values indicated in color bar. Channel locations are enlarged (diameter = 3 cm) to estimate spatial extent of measured underlying cortical region [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 1.
Descriptive statistics for steering metrics
| Congruent | Incongruent | ||||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | T | p | ||
| RMS handwheel speed (°/s) | Straight | 0.508a | 0.085 | 0.753 | 0.095 | 27.69 | 2.01E‐17 |
| Turn | 4.805a | 1.165 | 5.759 | 1.230 | 18.62 | 4.23E‐14 | |
| RMS yaw rate (°/s) | Straight | 0.530a | 0.123 | 0.826 | 0.124 | 24.30 | 2.55E‐16 |
| Turn | 6.238a | 1.011 | 7.112 | 1.143 | 12.55 | 6.11E‐11 | |
| RMS lateral acceleration (m/s2) | Straight | 0.087a | 0.019 | 0.144 | 0.020 | 21.49 | 2.73E‐15 |
| Turn | 1.623a | 0.242 | 1.838 | 0.277 | 12.28 | 8.99E‐11 | |
| Lateral position deviation (meters) | Straight | 0.164 | 0.010 | 0.309b | 0.036 | 22.27 | 1.38E‐15 |
| Turn | 0.198 | 0.010 | 0.535b | 0.117 | 13.48 | 1.68E‐11 | |
| Time to handwheel peak (s) | 2.467a | 0.326 | 1.613 | 0.368 | −10.12 | 2.58E‐09 | |
| Peak handwheel speed (°/s) | Straight | 0.312a | 0.197 | 0.419 | 0.253 | 5.98 | 7.58E‐06 |
| Turn | 2.989a | 1.011 | 3.372 | 1.094 | 6.18 | 4.84E‐06 | |
| Peak yaw rate (°/s) | Straight | 0.312a | 0.197 | 0.419 | 0.253 | 5.16 | 4.76E‐05 |
| Turn | 2.989a | 1.011 | 3.372 | 1.094 | 3.82 | 1.07E‐03 | |
| Peak lateral acceleration (m/s2) | Straight | 0.312a | 0.197 | 0.419 | 0.253 | 5.44 | 2.50E‐05 |
| Turn | 2.989a | 1.011 | 3.372 | 1.094 | 3.94 | 8.11E‐04 | |
Note. Abbreviation: RMS = root mean squared.
T and p values refer to incongruent versus congruent contrasts.
Indicates significant difference between incongruent and congruent conditions.
Lateral position deviation was significantly different from zero for the incongruent condition; T and p values refer to one‐sample T tests. The descriptive statistics for lateral position deviation for the congruent condition are presented for reference.
A similar pattern was observed during the straight portion of the trials; handwheel speed (t(20) = 27.69, p(FDR) = 2.01e‐17), lateral acceleration (t(20) = 21.49, p(FDR) = 2.73e‐15), and yaw rate (t(20) = 24.30, p(FDR) = 2.54e‐16) were significantly higher under the incongruent condition. Vehicle lateral position deviation was significantly different from zero for incongruent trials (t(20) = 22.27, p < 1.38e‐15). Results comparing peak values are presented in Table 1.
3.2. Pupil diameter
Pupil diameter was significantly greater for incongruent trials during the turning portion (t(16) = 4.524, p < .001) and the straight portion (t(16) = 4.4687, p < .001) of the trial (Figure 3b). Pupil diameter was also greater for turning relative to straight portions of the trial for incongruent trials (t(16) = 10.316, p < .001) congruent trials (t(16) = 10.316, p < .001) and overall (combined incongruent and congruent trials, t(16) =9.102, p < .001).
3.3. Cortical activation
Temporal decoupling of HbO and Hb signals indicates that either metric represents only a portion of the hemodynamic response associated with neural processing (Tam and Zouridakis, 2014). Thus, we report primary results for changes in HbO and include results for Hb in the Supplement for completeness (see Supporting Information, Text and Figure 2). A source neighbor localization procedure was used (see Methods and Figure 2, sources are referred to hereafter as S1–S16). During the turning portion of the trial, cortical activation was significantly greater for incongruent relative to congruent trials within the following regions: left prefrontal cortex (PFC, S1: t(16) = 2.634, p(FDR) = .018; S2: t(16) = 3.004, p(FDR) = .008), right PFC (S5: t(16) = 3.591, p(FDR) = .002; S6: t(16) = 3.802, p(FDR) = .001), right parietal cortex (S9: t(16) = 2.499, p(FDR) = .023; S10: t(16) = 3.610, p(FDR) = .002; S12: t(16) = 3.267, p(FDR) = .004), and left parietal cortex (S15: t(16) = 3.217, p(FDR) = .005; S16: t(16) = 3.365, p(FDR) = .003, Figures 3c and 4). After including years of driving experience as a covariate, activation for all source clusters listed in the previous sentence remained significant (see Supporting Information for further details). During the straight portion of the trial, there were no activation differences between incongruent and congruent trials (p(FDR) > .10). When both types of steering were combined, there were no significant differences between turning and straight driving portions, nor were there differences when turn and straight portions were compared within steering conditions (congruent trials only and incongruent trials only, p(FDR) > .10).
Figure 4.
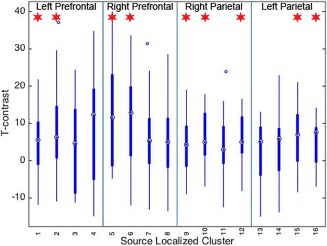
T contrast values for each region for incongruent versus congruent conditions during the double lane change maneuver. T values indicating activation differences between incongruent and congruent conditions based on oxygenated hemoglobin (Oxy Hb). Source clusters are listed along the x‐axis. * indicates p(FDR) < .05 [Color figure can be viewed at http://wileyonlinelibrary.com]
Although this experiment was not designed to assess learning, we performed post hoc analyses to compare early (trials 1–16) and late (trials 17–32) phases of the experiment. When comparing turning versus straight driving within incongruent trials, the late phase of the experiment was associated with significantly greater activation within left PFC (S1: t(16) = −2.834, p(FDR) = .012), right PFC (S8: t(16) = −3.064, p(FDR) = .007), right parietal cortex (S10: t(16) = −3.284, p(FDR) = .004; S11: t(16) = −3.569, p(FDR) = .002) and left parietal cortex (S13: t(16) = −4.925, p(FDR) < .001; S15: t(16) = −3.244, p(FDR) = .005). There was no difference between early and late phases when comparing turning versus straight driving within congruent trials (p(FDR) > .10). There also was no difference between early and late phases for incongruent versus congruent trials during turning or straight portions or when we compared turning to straight potions of the trial (all steering conditions combined or within congruent or incongruent trials only, p(FDR) > .10).
For each source cluster demonstrating a significant difference between incongruent and congruent trials during the turning portion, we conducted linear regression with step‐wise entry to identify the steering control metric contrasts that were significantly related to cortical activation. These analyses indicated that driver's peak yaw rate during the straight portion of the turn predicted cortical activation in right PFC (S5, F = 11.809, b = −18.77, p = .003, p(FDR) = .022, Figure 5). Analogous step‐wise regressions with personality factors indicated that extraversion significantly and negatively predicted cortical activation in the right parietal cortex (S10: F = 5.036, b = −0.262, p = .040, p(FDR) = .215) (Figure 6). Another set of step‐wise regressions indicated that a linear combination of openness and extraversion significantly predicted lateral position deviation (F = 13.652, b 1 = −0.099, b 2 = −0.008, p = .007, p(FDR) = .038) during the turning portion of the trial (Figure 7). Furthermore, openness significantly predicted time to handwheel peak (F = 4.372, b = 0.017, p = .046, p(FDR) = .127; Figure 7). Among source clusters that showed a significant learning effect within the incongruent condition (early vs late phase, turning vs straight driving), learning patterns in driver's handwheel rate (early vs late phase, incongruent vs congruent turn events) significantly predicted activation changes in the right parietal cortex (S11: F = 7.103, b = −28.905, p = .017, p(FDR) = .123; Figure 8).
Figure 5.
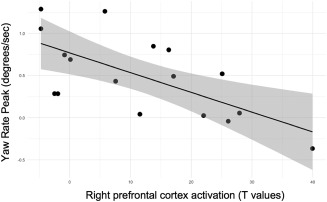
Relationship between steering behavior and cortical activation. The y‐axis corresponds to yaw rate peak for the incongruent—the congruent condition during the straight portion of the trial. The x‐axis corresponds to T contrast values reflecting incongruent—congruent conditions during the turn events for the right prefrontal cortex, source cluster 5
Figure 6.

Relationship between cortical activation and extraversion. The y‐axis corresponds to T contrast values reflecting the incongruent–congruent condition during the turn events for the right parietal region, source cluster 10
Figure 7.

Relationship between driving performance and NEO. The Y‐axis corresponds to T contrast values reflecting incongruent–congruent conditions during the turn events for the steering metric listed. The X‐axis corresponds to T scores for the personality factor listed
Figure 8.
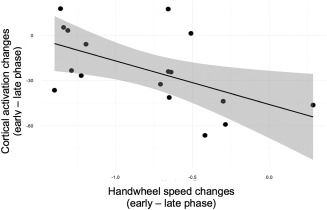
Relationship between learning signatures of neural activation and handwheel speed. The y‐axis corresponds to T contrast values reflecting activation in the right parietal region (S11) for turning–straight driving, for the early–late phase of the experiment, within incongruent trials. The x‐axis corresponds to turning–straight driving, for the early–late phase of the experiment, within incongruent trials
We also explored the association between driving experience and our primary outcomes. Specifically, we examined associations between driving experience and activation for each source cluster demonstrating a significant difference between incongruent and congruent trials during the turning portion of the trial. Stepwise regression results indicated that the number of years of driving experience predicted cortical activation in left parietal cortex (S15, F = 6.09, b = −1.39, p = .026, p(FDR) = .391, Figure 9). Next, we examined relationships between driving experience and steering control metric contrasts (incongruent vs. congruent trials) during the turning portion of the trial. The results of this analysis indicated that years of driving experience predicated yaw rate (F = 5.595, b = −0.059, p = .032, p(FDR) = .287, Figure 9). The regression analyses examining relationships between driving experience and the remaining variables did not reach statistical significance (p's > .10).
Figure 9.
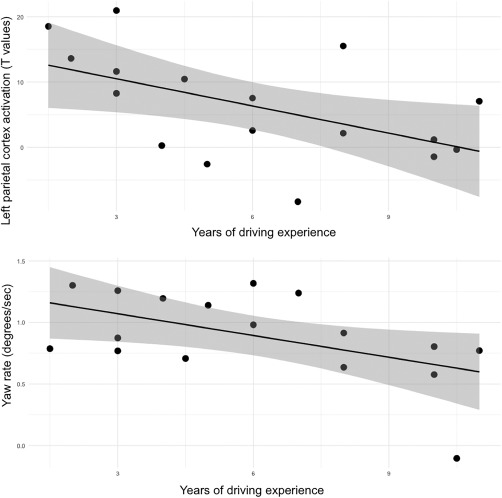
Relationship between driving experience and cortical activation/steering behavior. Top panel: the y‐axis corresponds to T contrast values reflecting incongruent–congruent condition during the turning portion of the trial, for the left parietal region, source cluster 15. Bottom panel: y axis corresponds to yaw rate for the incongruent–the congruent condition during the turning portion of the trial
4. DISCUSSION
This study employed a basic cognitive neuroscience paradigm – reverse motor mapping (Grafton et al., 2001)—within an immersive, naturalistic driving task, demonstrating a pattern of cortical activation response that lends ecological validity to previous, more basic research. The primary results demonstrate that when the steering dynamics of the vehicle were altered, the driver recruited more cognitive resources—signified by increased prefrontal and parietal cortical activation and enhanced pupillary response—and made corresponding adjustments in steering behavior to compensate for the altered condition. Individual differences in cortical activation and personality were related to steering performance suggesting that cortical activation patterns and personality traits may be useful in predicting a driver's response to changes in vehicle handling. Studies such as this, which quantify driver responses on several levels (behavioral, physiological, neurobiological) along with descriptions of individual personality traits and cortical activation patterns will be useful for informing the design of vehicle safety systems that support, rather than distract or confuse, a driver.
4.1. Cortical response to driving task
Specific cortical regions associated with increased activation in response to the steering manipulation (incongruent relative to congruent trials) included bilateral dorsolateral PFC and bilateral superior parietal cortices. The PFC and parietal cortex are critical for visuospatial working memory, attention, executive control and vigilance while driving (Calhoun et al., 2002; Spiers, & Maguire, 2006; Spiers et al., 2007; Walter et al., 2001). The right dorsolateral PFC and bilateral inferior parietal cortex responded to novel visuomotor mapping in previous research using a more basic cognitive task (Anguera et al., 2010). The parietal cortex also plays a role in updating motor plans based on visual feedback (Desmurget et al., 1999). Our results are consistent with previous fNIRS evidence that increased workload and attention demands during simulated driving are associated with increased activity in the PFC (Shimizu et al., 2013; Unni et al., 2017). Future studies will be required to tease apart the potentially overlapping cortical responses specific to visuomotor mapping and vehicle navigation (i.e., the baseline driving task) and the results of such studies could have important implications for the design of vehicle safety systems. The parietal cortex, which has previously been implicated in motor planning (Hanakawa et al., 2008) did not show any differences in activation measured by change in HbO during the straight portion (preparatory to making the lane‐change maneuver) under incongruent vs. congruent steering control, although we did note a significant difference in Hb signal in the right superior parietal cortex when comparing incongruent to congruent steering (Supporting Information). Lack of significant results for HbO during the preparatory period may be due to the timing of our experimental design and/or temporal decoupling of HbO and Hb signals during motor planning and motor execution (Tam and Zouridakis, 2014). As mentioned previously, considering both HbO and Hb signals is important in the evolving field of fNIRS research (Tachtsidis and Scholkmann, 2016).
4.2. Pupil response
Enlarged pupil diameter under the incongruent steering condition (relative to congruent steering) was observed during both the straight (preparatory) and turn portions of the driving task. Pupil response in association with cognitive processing is attributed to release of norepinephrine from the locus coeruleus (LC), which exerts inhibitory control on the parasympathetic oculomotor system (Wilhelm, 1999). Pupillary response corresponds with demands on attention (Kang et al., 2014) and mental workload during visuospatial tasks such as object tracking (Alnæs et al., 2014) and driving (Recarte and Nunes, 2003). The LC neuradrenergic system has also been linked to the fronto‐parietal attention network (Corbetta et al., 2008). Thus, elevated pupil diameter in this study can be considered evidence of increased mental workload and attention during the preparatory period as well as during the actual turning maneuver.
Measurement of pupil diameter represents a potentially important complement to fNIRS as pupillary response is related to activity in the LC (Alnæs et al., 2014; Murphy, O'Connell, O'Sullivan, Robertson, & Balsters, 2014), a deep brain structure that cannot be directly measured with fNIRS. It will be important to study pupillary response during preparation for maneuvers under a more realistic change in handling, to determine how useful it is as a measure of driver mental workload in the real world. However, the results presented here suggest that it is important to consider a driver's cognitive load in the period leading up to a challenging maneuver, and during the maneuver itself.
4.3. Steering behavior
Performance errors (i.e., unsuccessful attempts to complete the double lane change maneuver) were minimal: 8 participants completed the trials with no errors and 12 participants made between 1 and 8 errors across all trials. Although the significantly different steering behavior we observed (discussed in detail below) enabled drivers to successfully complete the maneuver under the incongruent steering condition, the paths driven during incongruent trials deviated significantly from the mean path taken during congruent trials (lateral position deviation). Participants were instructed to perform the lane‐change maneuver without hitting any cones but were not instructed to follow a specific path. Thus, there are two possible interpretations for deviation from the mean congruent path. First, the driver intended to follow a similar path under both conditions, but was less able to track this path under the incongruent steering condition. Second, the driver altered his or her path in order to gain an additional degree of freedom to complete the task. Evidence for the latter interpretation comes from our observation that there is no significant correlation between lateral deviation and handwheel speed during the turn portion of the task (p > .10). As discussed below, handwheel speed is a metric that captures feedback components of the driver's steering control, so the lack of correlation with lateral position deviation suggestions that drivers are allowing themselves a greater variation in path as a strategy to compensate for the increased difficulty of making the lane‐change under the incongruent condition.
We found that drivers exhibited a significantly smaller time to handwheel peak under the incongruent steering condition, relative to congruent steering, indicating that participants turned the handwheel earlier during incongruent trials to compensate for the change in vehicle steering control. The metric of time to handwheel peak includes feedback components, but, importantly, also represents the feed forward component of driver steering control (Russell et al., 2016). Our results suggest that the driver plans to start their lane‐change maneuver earlier under the incongruent steering condition, as a strategy to gain more time for any necessary steering corrections. Adjustments in feed forward control may be related to activity in the dorsolateral PFC, which is known to be involved in executive control (Hoshi, 2006; Miller, & Cohen, 2001), attention, and action inhibition (Cieslik et al., 2013). Thus, initiating the turn sooner may represent a top–down compensatory strategy that is linked to inhibition of the prepotent motor response to turn the wheel in the congruent direction.
Handwheel speed, lateral acceleration, and yaw rate capture feedback components of the driver's steering control. These metrics were significantly higher during incongruent trials, for the preparatory period leading up to the turn, and the turn itself. Increased handwheel speed indicates that participants made more rapid steering corrections to compensate for the incongruent condition. Similarly, the increase in lateral acceleration and yaw rate under incongruent steering indicates that the resulting motion of the vehicle displayed more abrupt changes. The PFC and parietal cortex facilitate remapping of visuomotor associations (Anguera et al., 2010; Murray, Bussey, & Wise, 2000; Staines, Padilla, & Knight, 2002). Thus, observed activation increases for these regions suggest they may be supporting the increase in feedback‐based steering behavior we observed during incongruent trials.
The steering manipulation we present here is an extreme change in vehicle handling. While the average driver would not experience such an extreme change in real‐world driving, our results are in line with a previous study from our group that involved control of a vehicle under more realistic changes in steering control (Russell et al., 2016). In particular, Russell et al. investigated driver responses to a change in steering ratio to simulate a driver regaining control of an automated vehicle travelling at a higher speed than the last time he or she was actively engaged in the driving task. Russell et al. report similar compensation in feed forward (initiating the turn sooner) and feedback (increased hand wheel speed) components of driver steering control. The results of the present study provide valuable new information regarding the cortical processes involved in driving and responding to changes in vehicle handling. These results can also help inform future studies investigating more realistic changes in vehicle handling.
4.4. Relationships among variables of interest
Interestingly, we did observe a relationship between steering behavior in the preparatory period and cortical activation during the turn maneuver. Specifically, this result indicated that peak yaw rate during the straight portion of the turn (incongruent vs. congruent) predicted activation in the right PFC during the turn. Peak yaw rate represents a single, sharp change in the rotation of the vehicle and thus captures a dynamic, rapid steering maneuver better than a mean value. Over 74% of the peaks in yaw rate occurred in the first 5 s of the straight portion across all conditions and all participants. The timing of the yaw rate peak suggests that the drivers probed the system to determine the congruency of each condition at the beginning of the straight portion of each trial (turning the handwheel and investigating which way the vehicle turns). Probing the system in this way may allow drivers to set up the feedback model used for the turning maneuver. The less aggressively a driver probed the vehicle steering control, the more he or she increased PFC resources to complete the turn in the incongruent condition. Those who use greater PFC resources may be approaching this steering challenge with a more overt cognitive strategy while those who use less PFC resources may be relying more heavily on feedback models. These results underscore the importance of examining the preparatory period to elucidate compensatory strategies drivers use to complete these maneuvers.
We also present regression results quantifying relationships between personality and cortical activation/driving performance. Individuals scoring higher on the extraversion scale demonstrated less increase in parietal resources and navigated the car with a more consistent path (i.e. less deviation in lateral position) during the incongruent condition. Previous studies have shown that extraversion is a significant predictor of distracted driving (Braitman and Braitman, 2017), and (when considering driving history) is positively correlated with number of traffic fatalities (Lajunen, 2001). To our knowledge, the results here are the first direct link between personality traits and different approaches to a particular driving maneuver.
Higher scores on the openness scale were associated with more consistent steering behavior across incongruent/congruent conditions. Individuals scoring higher on the openness scale drove a more consistent path (less deviation in lateral position) and were more consistent in the timing of the initiation of the lane change (time to handwheel peak) for incongruent relative to congruent conditions. Openness is associated with cognitive flexibility and working memory (DeYoung et al., 2005) and is positively related to one's ability to adapt to changing contexts in a cognitive task (LePine et al., 2000). Previous research has indicated a moderated relationship between openness and driving behavior (Clarke and Robertson, 2005) and specific associations between openness and risky driving behavior (Dahlen and White, 2006). Personality self‐report measures may offer an advantage over direct self‐reports of driving behavior because personality metrics measure internal traits (Dahlen et al., 2012). The results presented here are an important link between basic cognitive tasks and on‐the‐road driving history. Taken together, our results suggest that individuals with different personality traits use different strategies to compensate for changes in the vehicle's steering control. For example, allowing oneself an additional degree of freedom in vehicle path vs. planning to start the turn sooner. Further investigation into the interplay between personality and driving performance in the real world is warranted. The results we present here suggest the intriguing possibility that personality traits may be a predictor of individual brain activation patterns and driving performance.
We also observed evidence that the amount of previous driving experience was related to cortical activation and to steering behavior. Specifically, individuals with more years of driving experience demonstrated less increase in left parietal cortex and less increase in yaw rate when completing the turn in the incongruent condition. Previous driving experience is an important variable to consider (Shen et al., 2016). Our results suggest that with more driving experience a driver is better equipped to handle changes in a vehicle's behavior. However, we also confirmed that our activation results remained significant after accounting for years of driving experience.
4.5. Learning effects
We observed significantly greater activation in the bilateral PFC and bilateral parietal cortex during the late compared to the early phase of the experiment (turn relative to straight). These regions are involved in a range of motor learning tasks (Hikosaka et al., 2002), and increased activation in parietal cortex has been associated specifically with late phases of learning (Cavaco et al., 2015). In our study, individuals who demonstrated greater learning‐related activation changes in the right parietal cortex demonstrated more consistent handwheel speed, a measure of feedback, over the course of the experiment. Thus, greater learning in parietal cortex was associated with a more consistent feedback control strategy during the incongruent condition. These data provide further evidence that a driver's brain state might be predictive of how he or she will adapt to changes in vehicle handling. We interpret these results with caution, as the experiment was not designed to assess learning. Furthermore, fNIRS is not capable of imaging activation changes in deep brain structures, or the cerebellum, thus we were only able to partially describe motor learning pathways.
5. SUMMARY
In summary, we have demonstrated that when a driver is presented with a change in vehicle dynamics (reversed visuomotor mapping), his or her patterns of cortical activation, pupillary response and steering behavior are significantly altered relative to the nominal steering condition. The pattern of increased prefrontal and parietal resources provides an important link between laboratory‐ and real‐world studies, advancing the knowledge base regarding neural processes that support the complex, dynamic task of driving. Increased pupil diameter and steering corrections signify the importance of the period leading up to a challenging maneuver. To understand demands on driver's cognitive load and compensatory strategies, it is important to investigate the preparatory period and the maneuver itself when designing vehicle safety enhancements. Increased cortical activation during the late phase of the experiment may indicate motor learning in prefrontal–parietal networks. Finally, relationships among cortical activation, steering behavior, and individual personality traits suggest that individual brain states and traits may be useful in predicting a driver's response to changes in vehicle dynamics. Results such as these, that elucidate the cortical underpinnings of driver behavior, will be useful for informing the design of automated safety systems that facilitate safe and optimal driver–car communication.
6. DATA AVAILABILITY
The data that support the findings of this study are available from the authors upon reasonable request.
AUTHOR CONTRIBUTIONS
JLB, JMB, AG, LKH, SMHH, JCG, and ALR designed the study. JLB, JMB, AG, AMP, and ZS performed the research and analyzed the data. JLB, JMB, AG, LKH, SMHH, JCG, and ALR wrote and revised the manuscript. All authors discussed the results and made significant contribution to the manuscript.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
Support for this research was provided by the Revs Program at Stanford and The Center for Automotive Research at Stanford. This work was funded in part by the Toyota Class Action Settlement Safety Research and Education Program. The conclusions being expressed are the authors' only, and have not been sponsored, approved, or endorsed by Toyota or Plaintiffs' Class Counsel. The authors report no competing interests. SMHH's effort was supported in part by Brain & Behavior Foundation, NARSAD Young Investigator Award and NIH Career Development Award (K25‐AG050759).
Bruno JL, Baker JM, Gundran A, et al. Mind over motor mapping: Driver response to changing vehicle dynamics. Hum Brain Mapp. 2018;39:3915–3927. 10.1002/hbm.24220
Funding information Revs Program at Stanford; The Center for Automotive Research at Stanford; Toyota Class Action Settlement Safety Research and Education Program
REFERENCES
- Alnæs, D. , Sneve, M. H. , Espeseth, T. , Endestad, T. , van de Pavert, S. H. P. , & Laeng, B. (2014). Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. Journal of Vision, 14, 1–20. [DOI] [PubMed] [Google Scholar]
- Anguera, J. A. , Reuter‐Lorenz, P. A. , Willingham, D. T. , & Seidler, R. D. (2010). Contributions of spatial working memory to visuomotor learning. Journal of Cognitive Neuroscience, 22(9), 1917–1930. [DOI] [PubMed] [Google Scholar]
- Beauchamp, M. S. , Petit, L. , Ellmore, T. M. , Ingeholm, J. , & Haxby, J. V. (2001). A parametric fMRI study of overt and covert shifts of visuospatial attention. NeuroImage, 14(2), 310–321. [DOI] [PubMed] [Google Scholar]
- Braitman, K. A. , & Braitman, A. L. (2017). Patterns of distracted driving behaviors among young adult drivers: Exploring relationships with personality variables. Transportation Research Part F: Traffic Psychology and Behaviour, 46, 169–176. [Google Scholar]
- Brigadoi, S ., Ceccherini, L ., Cutini, S ., Scarpa, F ., Scatturin, P ., Selb, J ., … Cooper, R. J . (2014). NeuroImage Motion artifacts in functional near‐infrared spectroscopy: A comparison of motion correction techniques applied to real cognitive data. Neuroimage 85, 181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun, V. D. , & Adali, T. (2012). Multi‐subject independent component analysis of fMRI: A decade of intrinsic networks, default mode, and neurodiagnostic discovery. IEEE Reviews in Biomedical Engineering, 5, 60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun, V. D. , Pekar, J. J. , McGinty, V. B. , Adali, T. , Watson, T. D. , & Pearlson, G. D. (2002). Different activation dynamics in multiple neural systems during simulated driving. Human Brain Mapping, 16(3), 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaco, S. , Anderson, S. W. , Chen, K.‐H. , Teixeira‐Pinto, A. , & Damasio, H. (2015). Parietal damage impairs learning of a visuomotor tracking skill. Neuropsychologia, 79(Pt A), 106–112. [DOI] [PubMed] [Google Scholar]
- Cieslik, E. C. , Zilles, K. , Caspers, S. , Roski, C. , Kellermann, T. S. , Jakobs, O. , … Eickhoff, S. B. (2013). Is there one DLPFC in cognitive action control? Evidence for heterogeneity from Co‐activation‐based parcellation. Cerebral Cortex (New York, N.Y. : 1991), 23(11), 2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke, S. , & Robertson, I. T. (2005). A meta‐analytic review of the Big Five personality factors and accident involvement in occupational and non‐occupational settings. Journal of Occupational and Organizational Psychology, 78(3), 355–376. [Google Scholar]
- Corbetta, M. , Patel, G. , & Shulman, G. L. (2008). The reorienting system of the human brain: From environment to theory of mind. Neuron, 58(3), 306–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlen, E. R. , Edwards, B. D. , Tubré, T. , Zyphur, M. J. , & Warren, C. R. (2012). Taking a look behind the wheel: An investigation into the personality predictors of aggressive driving. Accident Analysis & Prevention, 45, 1–9. [DOI] [PubMed] [Google Scholar]
- Dahlen, E. R. , & White, R. P. (2006). The Big Five factors, sensation seeking, and driving anger in the prediction of unsafe driving. Personality and Individual Differences, 41(5), 903–915. [Google Scholar]
- Desmurget, M. , Epstein, C. M. , Turner, R. S. , Prablanc, C. , Alexander, G. E. , & Grafton, S. T. (1999). Role of the posterior parietal cortex in updating reaching movements to a visual target. Nature Neuroscience, 2(6), 563–567. [DOI] [PubMed] [Google Scholar]
- DeYoung, C. G. , Peterson, J. B. , & Higgins, D. M. (2005). Sources of openness/intellect: Cognitive and neuropsychological correlates of the fifth factor of personality. Journal of Personality, 73(4), 825–858. [DOI] [PubMed] [Google Scholar]
- Grafton, S. T. , Salidis, J. , & Willingham, D. B. (2001). Motor learning of compatible and incompatible visuomotor maps. Journal of Cognitive Neuroscience, 13(2), 217–231. [DOI] [PubMed] [Google Scholar]
- Hanakawa, T. , Dimyan, M. A. , & Hallett, M. (2008). Motor planning, imagery, and execution in the distributed motor network: A time‐course study with functional MRI. Cerebral Cortex (New York, N.Y. : 1991), 18(12), 2775–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka, O. , Nakamura, K. , Sakai, K. , & Nakahara, H. (2002). Central mechanisms of motor skill learning. Current Opinion in Neurobiology, 12(2), 217–222. [DOI] [PubMed] [Google Scholar]
- Hochberg, Y. , & Benjamini, Y. (1990). More powerful procedures for multiple significance testing. Statistics in Medicine, 9(7), 811–818. [DOI] [PubMed] [Google Scholar]
- Hoshi, E. (2006). Functional specialization within the dorsolateral prefrontal cortex: A review of anatomical and physiological studies of non‐human primates. Neuroscience Research, 54(2), 73–84. [DOI] [PubMed] [Google Scholar]
- Hosseini, H. , Bruno, J. L. , Baker, J. , Gundran, A. , Harbott, L. , Gerdes, J. C. , & Reiss, A. (2017). Neural, physiological, and behavioral correlates of visuomotor cognitive load. Scientific Reports, 7(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman, D. (1973). Attention and effort. Engelwood Cliffs, NJ: Prentice Hall. [Google Scholar]
- Kang, O. E. , Huffer, K. E. , & Wheatley, T. P. (2014). Pupil dilation dynamics track attention to high‐level information. PLoS One, 9(8), e102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajunen, T. (2001). Personality and accident liability: Are extraversion, neuroticism and psychoticism related to traffic and occupational fatalities? Personality and Individual Differences, 31(8), 1365–1373. [Google Scholar]
- LePine, J. A. , Colquitt, J. A. , & Erez, A. (2000). Adaptability to changing task contexts: Effects of general cognitive ability, conscientiousness, and openness to experience. Personnel Psychology, 53(3), 563–593. [Google Scholar]
- Li, Y.‐O. , Calhoun, V. D. , & Adali, T. (2012). Group study of simulated driving fMRI data by multiset canonical correlation analysis. Journal of Signal Processing Systems, 68(1), 31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccrae, R. R. , & Costa, P. T. (2007). Brief versions of the NEO‐PI‐3. Journal of Individual Differences, 28(3), 116–128. [Google Scholar]
- Miller, E. K. , & Cohen, J. D. (2001). An integrative theory of prefrontal cortex function. Annual Review of Neuroscience, 24, 167–202. [DOI] [PubMed] [Google Scholar]
- Murphy, P. R. , O'Connell, R. G. , O'Sullivan, M. , Robertson, I. H. , & Balsters, J. H. (2014). Pupil diameter covaries with BOLD activity in human locus coeruleus. Human Brain Mapping, 35(8), 4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray, E. A. , Bussey, T. J. , & Wise, S. P. (2000). Role of prefrontal cortex in a network for arbitrary visuomotor mapping. Experimental Brain Research, 133(1), 114–129. [DOI] [PubMed] [Google Scholar]
- Ng, V. W. K. , Eslinger, P. J. , Williams, S. C. R. , Brammer, M. J. , Bullmore, E. T. , Andrew, C. M. , … Benton, A. L. (2000). Hemispheric preference in visuospatial processing: A complementary approach with fMRI and lesion studies. Human Brain Mapping, 10(2), 80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, M ., Dan, H ., Sakamoto, K ., Takeo, K ., Shimizu, K ., Kohno, S ., … Dan, I . (2004). Three‐dimensional probabilistic anatomical cranio‐cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 21:99–111. [DOI] [PubMed] [Google Scholar]
- Oldfield, R. C. (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Plichta, M. M ., Heinzel, S ., Ehlis, A.‐C. , Pauli, P ., Fallgatter, A. J . (2007). Model‐based analysis of rapid event‐related functional near‐infrared spectroscopy (NIRS) data: A parametric validation study. Neuroimage 35:625–634. [DOI] [PubMed] [Google Scholar]
- Pollmann, S. , & von Cramon, D. Y. (2000). Object working memory and visuospatial processing: Functional neuroanatomy analyzed by event‐related fMRI. Experimental Brain Research, 133(1), 12–22. [DOI] [PubMed] [Google Scholar]
- Recarte, M. A. , & Nunes, L. M. (2003). Mental workload while driving: Effects on visual search, discrimination, and decision making. Journal of Experimental Psychology. Applied, 9(2), 119–137. [DOI] [PubMed] [Google Scholar]
- Russell, H. E. B. , Harbott, L. K. , Nisky, I. , Pan, S. , Okamura, A. M. , & Gerdes, J. C. (2016). Motor learning affects car‐to‐driver handover in automated vehicles. Science Robotics, 1(1), eaah5682. [DOI] [PubMed] [Google Scholar]
- Shen, H. , Li, Z. , Qin, J. , Liu, Q. , Wang, L. , Zeng, L. L. , … Hu, D. (2016). Changes in functional connectivity dynamics associated with vigilance network in taxi drivers. NeuroImage, 124(Pt A), 367–378. [DOI] [PubMed] [Google Scholar]
- Shimizu, T. , Hirose, S. , Obara, H. (2013). Measurement of frontal cortex brain activity attributable to the driving workload and increased attention. Kazuki Yanagisawa, Hitoshi Tsunashima and Yoshitaka Marumo Tomoki Haji and Masato Taira. SAE International Journal of Passenger Cars ‐ Mechanical Systems, 2, 736–744. [Google Scholar]
- Siegle, G. J. , Steinhauer, S. R. , Stenger, V. A. , Konecky, R. , & Carter, C. S. (2003). Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. NeuroImage, 20(1), 114–124. [DOI] [PubMed] [Google Scholar]
- Spiers, H. J. , & Maguire, E. A. (2006). Thoughts, behaviour, and brain dynamics during navigation in the real world. NeuroImage, 31(4), 1826–1840. [DOI] [PubMed] [Google Scholar]
- Spiers, H. J. , & Maguire, E. A. (2007). Neural substrates of driving behaviour. NeuroImage, 36(1), 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines, W. R. , Padilla, M. , & Knight, R. T. (2002). Frontal–parietal event‐related potential changes associated with practising a novel visuomotor task. Brain Research. Cognitive Brain Research, 13(2), 195–202. [DOI] [PubMed] [Google Scholar]
- Tachtsidis, I. , & Scholkmann, F. (2016). False positives and false negatives in functional near‐infrared spectroscopy: Issues, challenges, and the way forward. Neurophotonics, 3(3), 039801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak, S. , & Chul, J. (2014). NeuroImage statistical analysis of fNIRS data : A comprehensive review. NeuroImage, 85, 72–91. [DOI] [PubMed] [Google Scholar]
- Tam, N. D. , & Zouridakis, G. (2014). Temporal decoupling of oxy‐and deoxy‐hemoglobin hemodynamic responses detected by functional near‐infrared spectroscopy (fNIRS). Journal of Biomedical Engineering and Medical Imaging, 1(2), 18–28. [Google Scholar]
- Tsunashima, H. , & Yanagisawa, K. (2009). Measurement of brain function of car driver using functional near‐infrared spectroscopy (fNIRS). Computational Intelligence and Neuroscience, 2009, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuzuki, D. , Cai, D. , Dan, H. , Kyutoku, Y. , Fujita, A. , Watanabe, E. , & Dan, I. (2012). Stable and convenient spatial registration of stand‐alone NIRS data through anchor‐based probabilistic registration. Neuroscience Research, 72, 163–171. [DOI] [PubMed] [Google Scholar]
- Ulleberg, P. , & Rundmo, T. (2003). Personality, attitudes and risk perception as predictors of risky driving behaviour among young drivers. Safety Science, 41(5), 427–443. [Google Scholar]
- Unni, A. , Ihme, K. , Jipp, M. , & Rieger, J. W. (2017). Assessing the driver's current level of working memory load with high density functional near‐infrared spectroscopy: A realistic driving simulator study. Frontiers in Human Neuroscience, 11, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, H. , Vetter, S. , Grothe, J. , Wunderlich, A. , Hahn, S. , & Spitzer, M. (2001). The neural correlates of driving. NeuroReport, 12(8), 1763–1767. [DOI] [PubMed] [Google Scholar]
- Wierda, S. M. , van Rijn, H. , Taatgen, N. A. , & Martens, S. (2012). Pupil dilation deconvolution reveals the dynamics of attention at high temporal resolution. Proceedings of the National Academy of Sciences of the United States of America, 109(22), 8456–8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt, J. S. , Delpy, D. T. , Cope, M. , Wray, S. , Reynolds EOR . (1986). Quantification of cerebral oxygenation and haemodynamics in sick newborn infants by near infrared spectrophotometry. Lancet, 328, 1063–1066. [DOI] [PubMed] [Google Scholar]
- Xu, L. , Wang, B. , Xu, G. , Wang, W. , Liu, Z. , & Li, Z. (2017). Functional connectivity analysis using fNIRS in healthy subjects during prolonged simulated driving. Neuroscience Letters, 640, 21–28. [DOI] [PubMed] [Google Scholar]
- Yoshino, K. , Oka, N. , Yamamoto, K. , Takahashi, H. , & Kato, T. (2013). Functional brain imaging using near‐infrared spectroscopy during actual driving on an expressway. Frontiers in Human Neuroscience, 7, 882. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.


