Abstract
The effects of exercise on cognitive abilities have been studied. However, evidence regarding the neural substrates of sad emotion regulation is limited. Women have higher rates for affective disorders than men, but insufficient outcomes assess how aerobic exercises modulate central frontal activation in sad emotion inhibition and resilience among healthy women. This study investigated the effects of aerobic exercise-related brain activity on sad emotion inhibition processing in young women. Sad facial Go/No-Go and neutral Go/No-Go trials were conducted among 30 healthy young women to examine the changes in the N2 component, which reflects frontal inhibition responses, between pre-exercise and post-exercise periods. The first test was performed before aerobic exercise (baseline; 1st) and the second test was performed during an absolute rest period of 90 min after exercise. The sad No-Go stimuli that evoked N200 (N2) event-related potential were recorded and analyzed. The results showed that in the sad No-Go trials, N2 activation at the central-prefrontal cortex was significantly attenuated after exercise compared to the baseline N2 activation. Exercise-modulated N2 activation was not observed in the neutral No-Go trials. The behavioral error rates of sad No-Go trials did not differ between the two experiments. A reduced engagement of central-frontal activation to sad No-Go stimuli was shown after exercise. However, behavioral performance was consistent between the two measurements. The findings scope the benefits of the aerobic exercise on the neural efficiency in responding to sad emotion-eliciting cues as well as adaptive transitions reinstatement for regulatory capabilities in healthy young women.
Keywords: Exercise, Go/No-Go, Sad emotion regulation, Brain, EEG
Introduction
Background
The ability to effectively regulate emotions plays an important role in facilitating social interaction and psychological adaptation. The prefrontal cortex (PFC) is noteworthy as the controller of executive functions, controlling various cognitive processes that are also involved in self-regulation (Thayer et al. 2009; Williams et al. 2009). Over the past decade, several correlational studies have demonstrated a relationship between physical activity/aerobic fitness and brain function by using event-related brain potentials (ERPs) and functional magnetic resonance imaging techniques (Chaddock et al. 2012; Hillman et al. 2011; Kramer and Erickson 2007; Pontifex et al. 2011). Most evidences support the beneficial effects of chronic or acute participation in physical activity on brain health and cognition (Hillman et al. 2011), however, some of the reported findings of exercise-induced changes in cognitive performance are inconsistent (Lambourne and Tomporowski 2010). Systemic reviewer explained those moderators of facilitating effects of exercise on cognitive flexibility or variations were different from time-dependent properties (Ji et al. 2017) and that there were further differences between particular exercise interventions, study populations (Bayazit and Ungur 2018) and the types of cognitive tasks employed (Zschucke et al. 2015; Lambourne and Tomporowski 2010).
Subsequently, acute exercise was considered of the most effective behavioral techniques for exploring brain function, particularly mood self-regulation. Studies indicated that women have higher rates of affective disorders than men (Hyde et al. 2008; Pluchino et al. 2013a, b) and are more responsive to affective manipulations (Bradley et al. 2001; Guntekin et al. 2017; Morita et al. 2001; Yamamoto et al. 2001). The ability to effectively regulate emotions such as sadness plays an important role in facilitating social interaction and mental well-being. The effects of acute exercise could modulate emotional arousal through cortical–subcortical control via dopaminergic neurotransmission (Zheng and Hasegawa 2016). Increased physical exercise is associated with benefits for cognitive and affective adaptation as well as behavioral functioning (Budde et al. 2016). Physical activity-related response inhibition control has been investigated across different ages and populations in patients with psychological disorders (Hillman et al. 2009, 2011; Voss et al. 2010, 2013), however, the effects of exercise on the inhibition control neural processing of negative emotions like sadness (hereafter sad emotion processing) in women remain unclear.
Research has indicated that exercise has beneficial effects on human cognitive abilities and influences brain function, particularly frontal lobe-mediated cognitive processes (Dietrich and Sparling 2004; Hillman et al. 2003, 2011). Acute exercise is associated with significant increase in corticosterone levels and decreased negative emotions or stress responses through dopaminergic mechanisms, specifically by attenuating the acute exercise-induced increase in plasma corticosterone (Mika et al. 2015). Mitigation of stress is an important target for improving quality of life (Subhani et al. 2018). One study supports the idea that exercise engages arousal mechanisms, in the reticular-activating system, and the authors therefore propose a neurocognitive model of transient hypofrontality as a mechanism for the psychological effects of exercise to account for the psychological consequences of acute exercise (Dietrich 2006; Dietrich and Audiffren 2011). Research has shown that exercise enhances neurogenesis and neuroplasticity (Budde et al. 2016), duplicate from short-term physical activity (Austin et al. 2014; Ostadan et al. 2016; Puiu et al. 2016; Salame et al. 2016). The brain is inherently flexible and can tremendously change with experience. The current study, via electrophysiology, explores whether exercise modulates central-frontal activation performance in healthy women during a sad emotion inhibition challenging.
Electroencephalographic investigation of cognitive processing
Electroencephalography (EEG) is a safe and convenient method for measuring brain activity. ERPs are EEG changes that are time-locked to a stimulus event; in particular, the high temporal resolution of ERPs enables the measurement of neural activity. Thus, ERPs are ideal for studying a subset of processes that occur between a stimulus-encoding event and response production. Compared with task performance measures, EEG and ERP measures enable researchers to precisely analyze the effects of acute exercise on cognition. ERPs provide insights into the fine-grained mechanisms underlying the beneficial effects of physical fitness and acute exercise bouts on executive control processes.
Facial Go/No-Go task
Emotion regulation is mainly attributed to thePFC, and the cognitive Go/No-Go task is the most frequently used test to assess the effects of acute exercise on the functions of the PFC. Sad facial expressions constitute one type of fundamental negative emotional stimulus that conveys important information in social communications (Schneider et al. 1994a, b). The emotional Go/No-Go task is a modified variant of the probability Go/No-Go task, in which the control is measured in the context of emotional information. The emotional Go/No-Go task can serve as an effective probe for central-frontal lobe functions (Swainson et al. 2003). Response inhibition is a cognitive control process that coordinates the selection and execution of willed actions (Friedman et al. 2009). The sad facial No-Go task has been used to elicit negative emotions compared with passively viewing simple, unpleasant pictures for the No-Go condition (Boecker et al. 2007; Phan et al. 2004). Emotion regulation was measured after mood induction. The task efficiently commands attention toward evocative stimuli and potentially invokes sadness (negative emotion) during No-Go processing. The ERP component N200 (N2) is often elicited in No-Go trials in Go/No-Go tasks. The N2 component often reflects executive cognitive control functions and is a negative-going wave that peaks at 200–300 ms after a stimulus.
As mentioned above, the objective of the current study was to investigate the possible changes in aerobic exercise-related brain activity in a sad emotion Go/No-Go task and to compare the same in the neutral control task by analyzing N2 components (amplitude and latency) through EEG. We hypothesized that the neural indices of sad response inhibition would be altered in accordance with exercise for healthy women. Furthermore, deficits in PFC function are the most common finding in psychological illness or psychiatric disorders (Davidson et al. 2000), and deficient PFC function is known to interfere or impair cognitive function in responding to emotional challenges, such as distractibility, working memory, weak emotional regulation, poor error monitoring, or concentration (Lupien et al. 2009; Kessing 1998). The EEG signals was also observed to change in the local antero-frontal and distant regions in the brain hemisphere for mild cognitive impaired patients (John et al. 2018). To obtain objective information, the study would avoid those impacts by carefully recruiting participants and studying how aerobic exercise modulates central frontal activation or resilience in healthy women during a sad emotion inhibition challenge.
Methods
Ethical considerations and participants
This study was reviewed and approved by the Institutional Review Board of Chang Gung Memorial Hospital (CGMH), Taiwan. All experiments were performed in accordance with the guidelines and regulations of the Institutional Review Board of CGMH. Written informed consent was obtained from each participant before the experiment was conducted. Through advertisements, we recruited 30 paid, right-handed, female volunteers, aged approximately between 18 and 22 years (mean age: 20.4 years). Exclusion criteria were a history of neurological, psychiatric disorders (including premenstrual dysphoric disorder, as evaluated using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition); chronic cardiorespiratory illnesses; limb movement disorder; use of cortisol medications; and personality disorders. Furthermore, all subjects were not athletes who believed to have additive effects on spatio-temporal processing (Rao 2018; Isoglu-Alkac et al. 2018; Bayazit and Ungur 2018) or brain connectivity (Tharawadeepimuk and Wongsawat 2017). Before the experiment, each participant was asked to refrain from alcohol for at least 48 h and from caffeine/tobacco for 12 h.
Acute exercise session
We conducted 2 runs of the emotional paradigm (one Sad and one Neutral; the order was random). Each subject underwent two emotional Go/No-Go paradigms with EEG recordings. The 1st EEG was recorded before aerobic exercise as a baseline; the 2nd EEG was documented during a 90-min break after aerobic exercise.
In this study, moderate-intensity aerobic cycling exercise was conducted. The exercise intensity was primarily determined by calculating the maximal heart rate (HRmax) using the following formula: 220 − age. The exercise guidelines of the American College of Sports Medicine recommend that the exercise intensity should be 60–90% of the HRmax. The total caloric expenditure during a training session is determined by the intensity and duration of exercise, and these 2 factors are integrally related. To ensure participants’ safety during the exercise intervention, a pilot study was conducted to establish the physical activity protocols, particularly the exercise intensity, before the formal experiment was conducted. We measured heart rate variability 3 times across fitness training.
The exercise session consisted of 20 min of walking on a treadmill that ran at a consistent speed, approximately 25.6–28.8 mph (16–18 km/h). The graded practice was preceded by a warm-up (5 min, 1% grade). The participants spent 5 min at each of the 3% and 4% grades, and the last 5 min was a cool-down phase from 2% grade to 1% grade. The within-participant comparison design ameliorates inter-participant variations and thus yields a higher statistical power than that of a between-participant comparison experiment.
Task and stimuli
Participants were required to complete a sad facial Go/No-Go task with a 500-ms presentation of a fixed cross picture as a warning signal, followed 1000 ms later by a 500-ms presentation of a facial expression picture. Stimuli were displayed centrally on a white background at a viewing distance of approximately 60 cm. The stimulation set comprised digitized black-and-white pictures of faces taken from the Ekman collection of faces (Ekman 1992; Ekman et al. 1983). The task required participants to press a bar in response to an emotional facial expression (neutral, fearful, or happy; Go trials) or withhold the response to a sad expression (No-Go trials). In addition, an emotionally neutral Go/No-Go task was performed in a different experimental setting. Participants were required to respond to a symbol set (square, star, or triangle; Go trials) but withhold the response to a circle symbol (No-Go trials); this task was based on our previous work (Hwang et al. 2009). We used the symbol Go/No-Go task as the neutral control instead of using a neutral face as the No-Go event because a neutral face could be recognized by participants as a negative valence (Chiu et al. 2007). Participants performed identical experimental paradigms (sad and neutral Go/No-Go trials) during the pre-exercise (baseline) and post-exercise sessions. The Go/No-Go trial ratio was 80% over 20%. For successful No-Go trials, the ERP N2 component elicited by sad and neutral emotions was analyzed; it was calculated from 19 channels for each participant and for each condition.
ERP recording
EEG recordings were conducted in a quiet room by using elastic caps affixed to 19 electrodes that were designed to conform to the international 10-20 system (Version 5.4-16-2.0, Medicom MTD). The EEG signal was recorded from 19 sites (Fp1, Fp2, F3, F4, F7, F8, Fz, C3, C4, Cz, T3, T4, T5, T6, P3, P4, Pz, O1, and O2 for the standard 10-20 system) and was performed on symmetrical (anterior-frontal, frontal, anterior-temporal, temporal, posterior temporal, central, parietal, and occipital regions) brain areas. The epoching and initial processing of the continuous EEG recording was extracted from the 200 ms pre-stimulus to 1000 ms post-stimulus window. The filters were set from 0.5 to 70 Hz and the sampling frequency was 256 Hz. The impedance was maintained below 10 kΩ for all electrodes. Amplitudes were measured as the change from the pre-stimulus (baseline), and N2 latency was defined as the time point of the maximum amplitude. To prevent a memory effect, a few alienation errands were reliably given to participants during the approximately 1.5-h interval between the 2 EEG recording phases (baseline and post-exercise).
Analysis
Behavioral data
Descriptive statistics, including the averages and standard deviations (SDs; mean ± SD) of the behavioral performance and error rates (ERs) of No-Go trials, were evaluated. We calculated differences in the behavioral performance ERs of the sad and neutral No-Go trials between pre-exercise and post-exercise periods by using 2-tailed paired t tests.
ERP No-Go N2 component
ERPs were analyzed for successful No-Go trials as measured by correct responses to the No-Go trial (N2 latency and amplitude), and thus the error trials were excluded from processing. The NoGo-N2 voltage included all the negative points that were preceded or succeeded by negative values between 200 and 300 ms after the stimulus onset. The mean N2 amplitudes and peak latencies were calculated within windows that were determined on the basis of inspection of the grand average waveforms. The N2 effect associated with response inhibition was maximal at the central-frontal electrodes. We focused on the analysis over 10 frontal-central electrode sites. To assess the effects of exercise and emotion on sad No-Go N2 amplitudes and latency, we conducted 2 × 2 two-tailed repeated-measures analyses of variance (ANOVAs) to determine the main effects of exercise (pre-exercise vs. post-exercise) and emotion (sad vs. neutral) on central-frontal sites (Fp1, Fp2, F3, F4, F7, F8, Fz, C3, C4, and Cz). We performed post hoc pairwise comparisons when ANOVA yielded significant main effects. A successive comparison of the emotional N2 amplitude and latency was conducted using 2-tailed paired t tests.
Correlation between N2 component and ER
Pearson correlation coefficient analysis was used to evaluate the relationship between the N2 component (amplitude and latency) and ERs in the sad and neutral No-Go trials in the 2 sessions (pre-exercise vs. post-exercise). The correlation analysis was specifically conducted on the centering electrode, Fz, because this region plays a key role in response inhibition (Buodo et al. 2017). In the emotional Go/No-Go task, the main effect of exercise on N2 activation was primarily identified at the Fz site. The analysis was conducted using SPSS; significance was set at p = .05.
Results
ERP: emotional NoGo-evoked N2
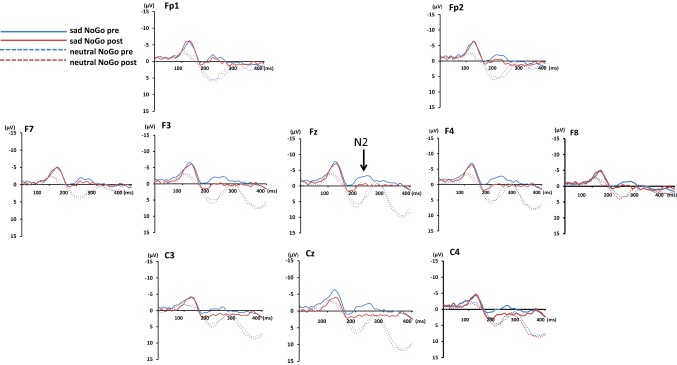
The data revealed a main effect of exercise for emotion NoGo-N2 amplitude among almost central PFC regions. Aerobic exercise did not affect the N2 latency in the sad and neutral trials (p > .05; Table 1); in contrast, N2 amplitude differed significantly (exercise × emotion) at the Fp1, Fp2, F7, F3, Fz, F4, C3, Cz, and C4 channels [F(1, 29) = 59.97, 35.29, 37.56, 35.99, 19.55, 18.95, 8.95, 21.05, and 8.18, respectively; p < .01; Geiser–Greenhouse corrected]. There were significant interactions between emotion (sad vs. neutral) and exercise (before vs. after) regarding the N2 amplitude at the frontal electrodes (F1, F3, Fz, and F4) [F (1, 29) = 4.83, 8.47, 12.3, 7.06; p < .05, < 0.01, = 0.001, < 0.05, respectively] (Geiser–Greenhouse corrected). A successive comparison with paired t-tests revealed that the mean N2 amplitude was significantly reduced after aerobic exercise at the F3, C3, Cz, and C4 channels: p < .05; F4; p < .01; Fz:p < .005 in the sad condition (Table 2); however, this effect was not observed in the neutral condition (Table 2). The data disclosed a relatively lesser PFC activation after exercise and a greater PFC activation at baseline in sad facial processing.
Table 1.
Repeated-measures ANOVA of the main effects of aerobic exercise and emotion in the No-Go trials in 30 healthy women
| Amplitude | Latency | |||
|---|---|---|---|---|
| F | p value | F | p value | |
| Repeated ANOVA within effects | ||||
| FP1 | ||||
| Sad | 59.968 | 0.000*** | 1.19 | 0.284 |
| Neutral | 0.011 | 0.919 | 1.016 | 0.322 |
| FP2 | ||||
| Sad | 35.292 | 0.000*** | 0.256 | 0.617 |
| Neutral | 1.918 | 0.177 | 0.002 | 0.965 |
| F7 | ||||
| Sad | 37.559 | 0.000*** | 0.153 | 0.698 |
| Neutral | 0.509 | 0.481 | 0.317 | 0.578 |
| F3 | ||||
| Sad | 35.989 | 0.000*** | 1.19 | 0.284 |
| Neutral | 3.042 | 0.092 | 2.159 | 0.153 |
| Fz | ||||
| Sad | 19.551 | 0.000*** | 1.366 | 0.252 |
| Neutral | 3.157 | 0.086 | 0.093 | 0.762 |
| F4 | ||||
| Sad | 18.948 | 0.000** | 0.949 | 0.338 |
| Neutral | 5.151 | 0.031* | 0.009 | 0.923 |
| F8 | ||||
| Sad | 35.227 | 1.903 | 0.52 | 0.476 |
| Neutral | 1.514 | 0.228 | 0.117 | 0.734 |
| C3 | ||||
| Sad | 8.951 | 0.006** | 0.026 | 0.873 |
| Neutral | 4.315 | 0.047* | 2.087 | 0.159 |
| Cz | ||||
| Sad | 21.054 | 0.000*** | 0.004 | 0.948 |
| Neutral | 8.349 | 0.007** | 2.207 | 0.148 |
| C4 | ||||
| Sad | 8.18 | 0.008** | 1.22 | 0.278 |
| Neutral | 8.985 | 0.006** | 1.006 | 0.324 |
A significant difference in the mean value of N2 amplitude was observed in Fp1, Fp2, F7, F3, Fz, F4, C3, Cz and C4 channels during the sad versus neutral No-Go task (*p < .05; **p < .01; ***p < .001)
Table 2.
Paired t test of the effects of aerobic exercise on ERP N2 activation in sad No-Go trials versus neutral No-Go trials (n = 30) (*p < 0.05; **p < 0.01); before = before exercise; after = after exercise
| Before Mean ± SE |
After Mean ± SE |
t(29) | p | |
|---|---|---|---|---|
| Fp1 | ||||
| Sad | − 5.850 ± 0.670 | − 5.158 ± 0.600 | − 1.012 | 0.32 |
| Neutral | − 1.208 ± 0.529 | − 2.000 ± 0.576 | 1.627 | 0.115 |
| Fp2 | ||||
| Sad | − 5.896 ± 0.824 | − 4.613 ± 0.675 | − 1.571 | 0.127 |
| Neutral | − 1.963 ± 0.536 | − 1.996 ± 0.567 | 0.07 | 0.945 |
| F7 | ||||
| Sad | − 5.113 ± 0.535 | − 4.304 ± 0.454 | − 1.389 | 0.175 |
| Neutral | − 1.558 ± 0.443 | − 1.871 ± 0.477 | 0.931 | 0.36 |
| F3 | ||||
| Sad | − 6.400 ± 0.792 | − 4.404 ± 0.562 | − 2.569 | 0.016* |
| Neutral | − 2.117 ± 0.493 | − 2.525 ± 0.512 | 1.048 | 0.303 |
| Fz | ||||
| Sad | − 7.704 ± 0.935 | − 5.008 ± 0.605 | − 3.09 | 0.004** |
| Neutral | − 3.229 ± 0.594 | − 3.975 ± 0.657 | 1.313 | 0.199 |
| F4 | ||||
| Sad | − 6.942 ± 0.940 | − 4.488 ± 0.637 | − 2.894 | 0.007** |
| Neutral | − 2.900 ± 0.522 | − 3.092 ± 0.552 | 0.366 | 0.717 |
| F8 | ||||
| Sad | − 5.163 ± 0.507 | − 4.083 ± 0.640 | − 1.595 | 0.122 |
| Neutral | − 2.379 ± 0.453 | − 2.513 ± 0.373 | 0.308 | 0.76 |
| C3 | ||||
| Sad | − 4.446 ± 0.676 | − 2.963 ± 0.445 | − 2.129 | 0.042* |
| Neutral | − 2.163 ± 0.414 | − 2.021 ± 0.429 | − 0.396 | 0.695 |
| Cz | ||||
| Sad | − 6.321 ± 0.888 | − 3.921 ± 0.495 | − 2.77 | 0.01** |
| Neutral | − 2.483 ± 0.516 | − 1.979 ± 0.580 | − 1.017 | 0.318 |
| C4 | ||||
| Sad | − 4.796 ± 0.815 | − 2.850 ± 0.441 | − 2.586 | 0.015* |
| Neutral | − 2.404 ± 0.406 | − 1.846 ± 0.429 | − 1.297 | 0.205 |
The mean N2 amplitude significantly reduced after aerobic exercise in the F3, F4, Fz, C3, C4, and Cz channels under the sad condition (p < .05)
Bold value indicates the greatest exercise-dependent decreases in the Sad NoGo-N2 amplitude were observed at the Fz electrodes (p < .005)
Behavioral data
The ER did not differ between pre-exercise and post-exercise periods in the Sad No-Go trials (20.5% vs. 18.9%) and Neutral symbol No-Go trials (9% vs. 10.4%) across the experiment [t (29) = 1.465 and − 1.03 in the sad and neutral trials, respectively; p > .05]. Behavioral results indicated that the acute exercise did not significantly affect the performance of emotional No-Go task for the participants.
Exercise-related heart rate measures
The mean (± SD) heart rate values were 81.03 ± 13.00, 140.20 ± 18.27, and 84.20 ± 8.31 before exercise, during exercise, and during the 90-min rest period after exercise, respectively. HRmax was estimated using the formula 220 − age. The average exercise intensity was 70–75% for 30 healthy participants.
Correlation between ERs and N2 component
No significant correlation was observed between behavioral ERs and the neural indices of Fz channel (amplitude and latency) in the sad No-Go trials or the Neutral No-Go trials in the 2 sessions (pre-exercise and post-exercise; p > .05).
Discussion
The study investigated exercise-related central-frontal activity in young women during a sad emotion inhibition task. Central PFC activation to Sad No-Go was significantly attenuated after exercise, but the behavioral ERs remained unchanged before and after exercise. The central PFC is part of the neural circuitry of emotional regulation. Neural manifestations of central PFC flexibility required less effort or processing resources after exercise while achieving equal outcomes in the sad inhibition task. The current EEG study provides the first evidence for the claim that exercise plays a critical role in regulating the neural basis of sad emotion inhibition processing in young women. Several possible mechanisms of the impact of exercise on the autonomic regulation of psychological states are discussed in the following sections.
Exercise attenuated N2 amplitudes in the sad No-Go trials
Aerobic exercise modulated the sad No-Go N2 amplitude (p < 0.05) (Table 1), with significant reductions of the N2 amplitude that emerged from the F4, Fz, C3, C4, and Cz channels (p < 0.05) after exercise in the sad condition (p < 0.01). However, the N2 amplitude was unaltered in the Neutral No-Go trials (p > .05). The tomography of the different brain areas are related to their different functions. Frontocentral NoGo-N2 activation was sensitive to engaging cognitive control or inhibiting response tendencies (Eimer 1993); specifically, the greatest exercise-dependent decreases in the sad NoGo-N2 amplitude were observed at the Fz and Cz electrodes (p < 0.005) (Fz, and Cz, source at the ACC), with smaller decreases at the F4, C3, and C4 channels (p < 0.05).
The brain network, including the anterior cingulate cortex (ACC) and PFC, has exhibited an important mechanism for the self-regulation of cognition and emotion (Balconi and Bortolotti 2013; Bush et al. 2000). In the current study, we implemented an emotionally neutral No-Go task to serve as a control in order to discern common cognitive components, such as executive function, attention, working memory, motor activity, and inhibition control. We did not detect any effects of exercise on the N2 component in the neutral tasks. It was observed that the N2 amplitude was significantly attenuated in the sad No-Go trials after exercise. The N2 amplitude is related to how much effort is put into a specific cognitive process with a given ERP, with more cognitive effort corresponding to higher ERP amplitudes. The ACC (Fz) is strongly linked to autonomic control centers. The neurodynamic resilience of sad response inhibition was altered in accordance with aerobic exercises, indicating the impact of aerobic exercise on the neural efficiency of young women’s sad emotion regulatory capabilities.
Exercise-induced neuroplasticity relates sadness emotional regulation
The data revealed that central PFC activation for sad facial processing significantly decreased after exercise (Table 2), which pinpoints a possible emotional regulation of sadness that is beneficial for healthy women who exercise, which can be explained by several possible mechanisms. The effects of acute exercise could modulate emotional arousal through cortical–subcortical control via dopaminergic neurotransmission (Zheng and Hasegawa 2016). Acute exercise can reduce stress reactivity by attenuating acute exercise-induced corticosterone increases in emotion neurocircuits. The effects of acute exercise on emotional function, as several studies have illustrated, are that exercise can attenuate negative emotions (Penedo and Dahn 2005; Mata et al. 2013) and decrease fear responses (Mika et al. 2015). ERP studies also suggest that exercise-related central-frontal N2 activation during mood inductions is associated with fluctuations or decreases in negative emotion properties, compared to the baseline (Penedo and Dahn 2005; Heijnen et al. 2015).
Neuroimaging studies have shown associations between sadness and significant loci of activation in the anterior temporal pole, midbrain, amygdala, insula, and PFC (Eugene et al. 2003; Levesque et al. 2003a, b). Emotional processing is influenced by top-down processes, such as the reappraisal of emotion-inducing events (Gan et al. 2015). Cognitive control processes rely on the PFC, and the target of control is located in the posterior subcortical limbic system (Basso et al. 2015). Affective neuroscientists suggest that greater PFC activity evoked by negative emotions represents higher vigilance or awareness and is associated with anxiety or mental illness (Davidson 2002). The exercise-attenuated central PFC N2 amplitude may function as an emotional reset, implicitly reducing the effort required and underlining a possible emotional regulation to a sad cue. With underlying emotional matters, the effects of physical exercise-induced arousal or attention varied (Lambourne and Tomporowski 2010). Affective facial displays have the potential to interact with an individual’s current emotional state (Maxwell et al. 2005). As previously mentioned, the exercise-attenuated central PFC N2 amplitude may function as an emotional reset, and this results in the reduction of the effort required to process possible emotional regulation to a sad cue. Therefore, the PFC activity findings reveal a dynamic exercise-induced plasticity that may mediate the automatic modulation of sad emotional regulation. Adequate measures of objective emotional intensity or exercise-induced arousal level alterations could be designed for the next manipulation.
Aerobic exercise modulated the sad No-Go N1 amplitude
Furthermore, based on the inspection of ERPs (Fig. 1), there seems to be a significant difference between the ERPs associated with sad No-Go stimuli before and after exercise during the N1 time range over Cz. Then, subsequent analysis was conducted by pair t test. We found that aerobic exercise modulated the sad No-Go N1 amplitude, i.e., we observed significant amplitude reductions in the Cz channel (p < 0.05) after exercise in the sad condition (p < 0.01) but not in the neutral trials (p > .05). The N1 often provides a means of examining the significance of an emotion in capturing attentional resources (Ho et al. 2015; Ma et al. 2016; Yang et al. 2012). Studies support the idea that emotional stimuli are more effective in capturing attentional resources than non-emotional stimuli. The N1 was greater for stimuli that were responded to, as opposed to those that were ignored. Studies reported that arousal modulates valence effects on both the early and late stages of affective picture processing (Feng et al. 2014; Yao et al. 2016). Aerobic exercises influenced the early stage of emotional face processing, implying that participants expended fewer attention resources into a sad cue by exercising.
Fig. 1.
The grand mean ERP waveforms in the sad No-Go trials versus neutral No-Go trials at the electrode site of central-frontal cortex. All the No-Go trials were averaged to produce the grand-mean waveforms. In each waveform, positive polarity was down and negative polarity was up. Each waveform was presented with 0–400 (ms) based on the onset of stimulus
Behavioral data across exercise
Acute exercise has been linked to the facilitation of executive function (Ji et al. 2017), but our preliminary behavioral analysis showed that the ERs were similar in both the sad and neutral conditions after exercise (p > .05). Despite growing evidence suggesting that various cognitive functions are improved by physical exercise, some meta-analyses indicate that single bouts of exercise had, overall, small positive effects on cognitive performance (Lambourne et al. 2010; Lambourne and Tomporowski 2010; Chang et al. 2012) or even the opposite result, i.e., decreasing performance during exercise (Del Giorno et al. 2010; Wang et al. 2013). Unlike the generally positive findings of long-term benefits of physical exercise on cognitive functions, it was reported that the immediate effects of exercise on cognitive performance had mixed results (Ji et al. 2017). Those effect sizes largely depended on the moderators that acted on the exercise-cognition relation, such as the physical activity protocols, cognitive task characteristics, the time between the physical and cognitive tasks, and fatigue-producing aspects (Ji et al. 2017). Moreover, the reticular-activating hypofrontality (RAH) (Dietrich and Audiffren 2011) model may be a counterpart of our ER findings; the baseline behavioral ERs of the sad No-Go trials were unchanged after exercise.
Data revealed that aerobic exercises significantly decrease N2 amplitude by sad emotional stimuli in central PFC regions (Table 1). Studies on cerebral blood flow and metabolism noted that exercise reduces neural activity in the PFC (Vissing et al. 1996; Vissing and Hjortso 1996). These studies supported and prompted the frontal hypofunction hypothesis of the effects of exercise on emotion and cognition (Dietrich 2006; Dietrich and Audiffren 2011). During exercise, the percentage of total cardiac output to the brain is drastically reduced because blood is shunted from numerous areas, including the brain and the muscles sustaining the workload (Dietrich 2006; Dietrich and Sparling 2004). Cognitive processing in the brain is competitive and mental effort is a limited resource. Acute exercise initially engages arousal mechanisms for emotional stimuli that are settled in the reticular-activating system, which is related to the facilitation of implicit information processing. Subsequently, PFC deactivation occurs partly due to the brain’s finite resources, thus affecting an inefficient explicit system or the occurrence of non-helpful emotional processes (Dietrich and Audiffren 2011). Such interrelated properties supported our findings that acute exercise has no positive impact on the emotion-related No-Go executive functions.
Behavioral data relates N2 amplitude
As abovementioned, those deliberations are applicable to our correlation findings, at least partially. There was no significant correlation observed between the behavioral ERs and the neural indices of the Fz site (amplitude and latency) in the sad No-Go trials or the neutral No-Go trials during the two sessions (p > .05). A previous study reported that the No-Go N2 was larger and earlier in good, compared to poor, inhibitors (as measured by the number of commission errors; Falkenstein et al. 1999). These inconsistent results concerning the relationship between behavioral inhibitions and corresponding ERPs in a Go/No-Go task could be affected by various issues or quite a number of procedures. The current study aimed to address how aerobic exercises modulate central-frontal PFC activation in sad emotion inhibition and resilience among healthy women. The exercise led to a reallocation of cognitive resources to the neural sub-processes that respond to the sad inhibitory control task.
In addition, this study emphasized the possible changes with aerobic exercise-related brain activity in a sad No-Go N2 (amplitude and latency) and the comparison of the same in the neutral control task. In visual Go/NoGo tasks, the ERP is usually characterized by frontal negativity after Nogo stimuli that (“Nogo-N2”) and reflects an inhibition process (Falkenstein et al. 1999). The ERPs elicited by Go stimuli had no report. One consideration was that, all subjects were asked to click the provided button as soon as they see the non-target stimuli. The Go-response was associated with motor preparation processes. Acute exercise could influence response preparation, primarily with maintenance of a stable motor preparation. Studies indicated that acute exercise can influence excessive sub-threshold motor preparation for non-target stimuli (Renaud et al. 2010), the skeletal muscle contractile properties (such as: muscular endurance, muscular power, et al.) or shows increase in the peripheral muscle fatigability. The yield in fatigue can also moderate the individual’s mental state and EEG signals in cognitive performance (Zeng et al. 2018). For this conceivable confounding factor from exercise, thus, did not present Go-response in this current study. It is possible to use EMG to solve the problem concerning these variables in the next study.
Limitations and future implications
Some limitations should be considered when interpreting the results of the present study. We analyzed the central-frontal N2 component alone. Future studies with a larger sample sizes should be conducted to analyze brain regions with sustained functional activity or late components in some series. The effects of exercise had not yet been tested on a sample of women performing sad emotion inhibition tasks at the time of testing in the neurobiology division. Future studies must conduct a detailed evaluation of the effects of exercise on sadness intensity or valence.
Conclusion
Our study revealed the effects of exercise on the neuroelectric indices of sad emotion inhibition. Our preliminary findings are that in the sad No-Go trials, exercise attenuated the N2 amplitude, whereas the behavioral ERs remained unchanged. These findings suggest that exercise can modulate the neural efficiency of executive control processing in the context of sad emotion regulation in healthy young women.
Acknowledgements
We thank the 30 participants as well as CGUST for providing administrative support. The authors would like to thank L- F Ni, Y-J Yan for statistical counseling; Yu-Ling Shih for her assistance in make aerobic exercise session is adequate and En-Zi Lin, RA for her administration support. The study was funded by the Ministry of Science and Technology in Taiwan (NSC 100-2410-H-255-005 -MY2; NSC 101-2629-B-255 -001 -MY2) and received financial assistance from Chang Gung Memorial Hospital (BMRPC52).
Compliance with ethical standards
Conflict of interest
The authors declare there is no conflict of interest.
Contributor Information
Ren-Jen Hwang, Phone: +886-3-2118999, Email: rjhuang@mail.cgust.edu.tw, Email: hr1202@ms37.hinet.net.
Hsin-Ju Chen, Email: hjchen01@hotmail.com.
Zhan-Xian Guo, Email: critisec@gmail.com.
Yu-Sheun Lee, Email: pikaojuice@gmail.com, Email: qoo60824@yahoo.com.tw.
Tai-Ying Liu, Phone: +886-2-2737-7690, Email: tyliu@stpi.narl.org.tw.
References
- Austin MW, Ploughman M, Glynn L, Corbett D. Aerobic exercise effects on neuroprotection and brain repair following stroke: a systematic review and perspective. Neurosci Res. 2014;87:8–15. doi: 10.1016/j.neures.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Balconi M, Bortolotti A. Emotional face recognition, empathic trait (BEES), and cortical contribution in response to positive and negative cues. The effect of rTMS on dorsal medial prefrontal cortex. Cogn Neurodyn. 2013;7:13–21. doi: 10.1007/s11571-012-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso JC, Shang A, Elman M, Karmouta R, Suzuki WA. Acute exercise improves prefrontal cortex but not hippocampal function in healthy adults. J Int Neuropsychol Soc JINS. 2015;21:791–801. doi: 10.1017/S135561771500106X. [DOI] [PubMed] [Google Scholar]
- Bayazit O, Ungur G. Neuroelectric responses of sportsmen and sedentaries under cognitive stress. Cogn Neurodyn. 2018;12:295–301. doi: 10.1007/s11571-018-9478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boecker M, Buecheler MM, Schroeter ML, Gauggel S. Prefrontal brain activation during stop-signal response inhibition: an event-related functional near-infrared spectroscopy study. Behav Brain Res. 2007;176:259–266. doi: 10.1016/j.bbr.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: sex differences in picture processing. Emotion. 2001;1:300–319. doi: 10.1037/1528-3542.1.3.300. [DOI] [PubMed] [Google Scholar]
- Budde H, Wegner M, Soya H, Voelcker-Rehage C, McMorris T. Neuroscience of exercise: neuroplasticity and its behavioral consequences. Neural Plast. 2016;2016:3643879. doi: 10.1155/2016/3643879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buodo G, Sarlo M, Mento G, Messerotti Benvenuti S, Palomba D. Unpleasant stimuli differentially modulate inhibitory processes in an emotional Go/NoGo task: an event-related potential study. Cogn Emot. 2017;31:127–138. doi: 10.1080/02699931.2015.1089842. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chaddock L, Erickson KI, Prakash RS, Voss MW, VanPatter M, et al. A functional MRI investigation of the association between childhood aerobic fitness and neurocognitive control. Biol Psychol. 2012;89:260–268. doi: 10.1016/j.biopsycho.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Chang YK, Labban JD, Gapin JI, Etnier JL. The effects of acute exercise on cognitive performance: a meta-analysis. Brain Res. 2012;1453:87–101. doi: 10.1016/j.brainres.2012.02.068. [DOI] [PubMed] [Google Scholar]
- Chiu Y-C, Lin C-H, Lin J-H (2007) The neutral face is not really expressionless and emotionless. In: Taiwanese Psychology Association 46th annual conference, p 35
- Davidson RJ. Anxiety and affective style: role of prefrontal cortex and amygdala. Biol Psychiatry. 2002;51:68–80. doi: 10.1016/S0006-3223(01)01328-2. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Jackson DC, Kalin NH. Emotion, plasticity, context, and regulation: perspectives from affective neuroscience. Psychol Bull. 2000;126:890–909. doi: 10.1037/0033-2909.126.6.890. [DOI] [PubMed] [Google Scholar]
- Del Giorno JM, Hall EE, O’Leary KC, Bixby WR, Miller PC. Cognitive function during acute exercise: a test of the transient hypofrontality theory. J Sport Exerc Psychol. 2010;32:312–323. doi: 10.1123/jsep.32.3.312. [DOI] [PubMed] [Google Scholar]
- Dietrich A. Transient hypofrontality as a mechanism for the psychological effects of exercise. Psychiatry Res. 2006;145:79–83. doi: 10.1016/j.psychres.2005.07.033. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Audiffren M. The reticular-activating hypofrontality (RAH) model of acute exercise. Neurosci Biobehav Rev. 2011;35:1305–1325. doi: 10.1016/j.neubiorev.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Dietrich A, Sparling PB. Endurance exercise selectively impairs prefrontal-dependent cognition. Brain Cogn. 2004;55:516–524. doi: 10.1016/j.bandc.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-W. [DOI] [PubMed] [Google Scholar]
- Ekman P. Are there basic emotions? Psychol Rev. 1992;99:550–553. doi: 10.1037/0033-295X.99.3.550. [DOI] [PubMed] [Google Scholar]
- Ekman P, Levenson RW, Friesen WV. Autonomic nervous system activity distinguishes among emotions. Science. 1983;221:1208–1210. doi: 10.1126/science.6612338. [DOI] [PubMed] [Google Scholar]
- Eugene F, Levesque J, Mensour B, Leroux JM, Beaudoin G, et al. The impact of individual differences on the neural circuitry underlying sadness. Neuroimage. 2003;19:354–364. doi: 10.1016/S1053-8119(03)00121-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 1999;101:267–291. doi: 10.1016/S0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Feng C, Li W, Tian T, Luo Y, Gu R, et al. Arousal modulates valence effects on both early and late stages of affective picture processing in a passive viewing task. Soc Neurosci. 2014;9:364–377. doi: 10.1080/17470919.2014.896827. [DOI] [PubMed] [Google Scholar]
- Friedman D, Nessler D, Cycowicz YM, Horton C. Development of and change in cognitive control: a comparison of children, young adults, and older adults. Cogn Affect Behav Neurosci. 2009;9:91–102. doi: 10.3758/CABN.9.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S, Yang J, Chen X, Yang Y. The electrocortical modulation effects of different emotion regulation strategies. Cogn Neurodyn. 2015;9:399–410. doi: 10.1007/s11571-015-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntekin B, Femir B, Golbasi BT, Tulay E, Basar E. Affective pictures processing is reflected by an increased long-distance EEG connectivity. Cogn Neurodyn. 2017;11:355–367. doi: 10.1007/s11571-017-9439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijnen S, Hommel B, Kibele A, Colzato LS. Neuromodulation of aerobic exercise: a review. Front Psychol. 2015;6:1890. doi: 10.3389/fpsyg.2015.01890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Snook EM, Jerome GJ. Acute cardiovascular exercise and executive control function. Int J Psychophysiol. 2003;48:307–314. doi: 10.1016/S0167-8760(03)00080-1. [DOI] [PubMed] [Google Scholar]
- Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159:1044–1054. doi: 10.1016/j.neuroscience.2009.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Kamijo K, Scudder M. A review of chronic and acute physical activity participation on neuroelectric measures of brain health and cognition during childhood. Prev Med. 2011;52(Suppl 1):S21–S28. doi: 10.1016/j.ypmed.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho HT, Schroger E, Kotz SA. Selective attention modulates early human evoked potentials during emotional face-voice processing. J Cogn Neurosci. 2015;27:798–818. doi: 10.1162/jocn_a_00734. [DOI] [PubMed] [Google Scholar]
- Hwang RJ, Wu CH, Chen LF, Yeh TC, Hsieh JC. Female menstrual phases modulate human prefrontal asymmetry: a magnetoencephalographic study. Horm Behav. 2009;55:203–209. doi: 10.1016/j.yhbeh.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev. 2008;115:291–313. doi: 10.1037/0033-295X.115.2.291. [DOI] [PubMed] [Google Scholar]
- Isoglu-Alkac U, Ermutlu MN, Eskikurt G, Yucesir I, Demirel Temel S, Temel T. Dancers and fastball sports athletes have different spatial visual attention styles. Cogn Neurodyn. 2018;12:201–209. doi: 10.1007/s11571-017-9469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji LY, Li XL, Liu Y, Sun XW, Wang HF, et al. Time-dependent effects of acute exercise on university students’ cognitive performance in temperate and cold environments. Front Psychol. 2017;8:1192. doi: 10.3389/fpsyg.2017.01192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John NT, Puthankattil DS, Menon R. Analysis of long range dependence in the EEG signals of Alzheimer patients. Cogn Neurodyn. 2018;12:183–199. doi: 10.1007/s11571-017-9467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessing LV. Cognitive impairment in the euthymic phase of affective disorder. Psychol Med. 1998;28:1027–1038. doi: 10.1017/S0033291798006862. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11:342–348. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Lambourne K, Tomporowski P. The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Res. 2010;1341:12–24. doi: 10.1016/j.brainres.2010.03.091. [DOI] [PubMed] [Google Scholar]
- Lambourne K, Audiffren M, Tomporowski PD. Effects of acute exercise on sensory and executive processing tasks. Med Sci Sports Exerc. 2010;42:1396–1402. doi: 10.1249/MSS.0b013e3181cbee11. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, Paquette V, Mensour B, et al. Neural circuitry underlying voluntary suppression of sadness. Biol Psychiatry. 2003;53:502–510. doi: 10.1016/S0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, Beaudoin G, Leroux JM, et al. Neural correlates of sad feelings in healthy girls. Neuroscience. 2003;121:545–551. doi: 10.1016/S0306-4522(03)00528-1. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Ma J, Liu C, Chen X. Emotional modulation of conflict processing in the affective domain: evidence from event-related potentials and event-related spectral perturbation analysis. Sci Rep. 2016;6:31278. doi: 10.1038/srep31278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata J, Hogan CL, Joormann J, Waugh CE, Gotlib IH. Acute exercise attenuates negative affect following repeated sad mood inductions in persons who have recovered from depression. J Abnorm Psychol. 2013;122:45–50. doi: 10.1037/a0029881. [DOI] [PubMed] [Google Scholar]
- Maxwell JS, Shackman AJ, Davidson RJ. Unattended facial expressions asymmetrically bias the concurrent processing of nonemotional information. J Cogn Neurosci. 2005;17:1386–1395. doi: 10.1162/0898929054985437. [DOI] [PubMed] [Google Scholar]
- Mika A, Bouchet CA, Bunker P, Hellwinkel JE, Spence KG, et al. Voluntary exercise during extinction of auditory fear conditioning reduces the relapse of fear associated with potentiated activity of striatal direct pathway neurons. Neurobiol Learn Mem. 2015;125:224–235. doi: 10.1016/j.nlm.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Morita K, Yamamoto M, Waseda Y, Maeda H. Effects of facial affect recognition on the auditory P300 in healthy subjects. Neurosci Res. 2001;41:89–95. doi: 10.1016/S0168-0102(01)00248-6. [DOI] [PubMed] [Google Scholar]
- Ostadan F, Centeno C, Daloze JF, Frenn M, Lundbye-Jensen J, Roig M. Changes in corticospinal excitability during consolidation predict acute exercise-induced off-line gains in procedural memory. Neurobiol Learn Mem. 2016;136:196–203. doi: 10.1016/j.nlm.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Dahn JR. Exercise and well-being: a review of mental and physical health benefits associated with physical activity. Curr Opin Psychiatry. 2005;18:189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager TD, Taylor SF, Liberzon I. Functional neuroimaging studies of human emotions. CNS Spectr. 2004;9:258–266. doi: 10.1017/S1092852900009196. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Russo M, Santoro AN, Litta P, Cela V, Genazzani AR. Steroid hormones and BDNF. Neuroscience. 2013;239:271–279. doi: 10.1016/j.neuroscience.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Pluchino N, Santoro A, Casarosa E, Wenger JM, Genazzani AD, et al. Advances in neurosteroids: role in clinical practice. Climacteric. 2013;16(Suppl 1):8–17. doi: 10.3109/13697137.2013.809647. [DOI] [PubMed] [Google Scholar]
- Pontifex MB, Raine LB, Johnson CR, Chaddock L, Voss MW, et al. Cardiorespiratory fitness and the flexible modulation of cognitive control in preadolescent children. J Cogn Neurosci. 2011;23:1332–1345. doi: 10.1162/jocn.2010.21528. [DOI] [PubMed] [Google Scholar]
- Puiu T, Kairys AE, Pauer L, Schmidt-Wilcke T, Ichesco E, et al. Association of alterations in gray matter volume with reduced evoked-pain connectivity following short-term administration of pregabalin in patients with fibromyalgia. Arthritis Rheumatol. 2016;68:1511–1521. doi: 10.1002/art.39600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao AR. An oscillatory neural network model that demonstrates the benefits of multisensory learning. Cogn Neurodyn. 2018;12:481–499. doi: 10.1007/s11571-018-9489-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud M, Bherer L, Maquestiaux F. A high level of physical fitness is associated with more efficient response preparation in older adults. J Gerontol B Psychol Sci Soc Sci. 2010;65B:317–322. doi: 10.1093/geronb/gbq004. [DOI] [PubMed] [Google Scholar]
- Salame S, Garcia PC, Real CC, Borborema J, Mota-Ortiz SR, et al. Distinct neuroplasticity processes are induced by different periods of acrobatic exercise training. Behav Brain Res. 2016;308:64–74. doi: 10.1016/j.bbr.2016.04.029. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Gur RE, Muenz LR. Standardized mood induction with happy and sad facial expressions. Psychiatry Res. 1994;51:19–31. doi: 10.1016/0165-1781(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Schneider F, Gur RC, Jaggi JL, Gur RE. Differential effects of mood on cortical cerebral blood flow: a 133xenon clearance study. Psychiatry Res. 1994;52:215–236. doi: 10.1016/0165-1781(94)90089-2. [DOI] [PubMed] [Google Scholar]
- Subhani AR, Kamel N, Mohamad Saad MN, Nandagopal N, Kang K, Malik AS. Mitigation of stress: new treatment alternatives. Cogn Neurodyn. 2018;12:1–20. doi: 10.1007/s11571-017-9460-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swainson R, Cunnington R, Jackson GM, Rorden C, Peters AM, et al. Cognitive control mechanisms revealed by ERP and fMRI: evidence from repeated task-switching. J Cogn Neurosci. 2003;15:785–799. doi: 10.1162/089892903322370717. [DOI] [PubMed] [Google Scholar]
- Tharawadeepimuk K, Wongsawat Y. Quantitative EEG evaluation for performance level analysis of professional female soccer players. Cogn Neurodyn. 2017;11:233–244. doi: 10.1007/s11571-017-9427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- Vissing SF, Hjortso EM. Central motor command activates sympathetic outflow to the cutaneous circulation in humans. J Physiol. 1996;492(Pt 3):931–939. doi: 10.1113/jphysiol.1996.sp021359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing J, Andersen M, Diemer NH. Exercise-induced changes in local cerebral glucose utilization in the rat. J Cereb Blood Flow Metab. 1996;16:729–736. doi: 10.1097/00004647-199607000-00025. [DOI] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Basak C, Chaddock L, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front Aging Neurosci. 2010;2:32. doi: 10.3389/fnagi.2010.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Hum Brain Mapp. 2013;34:2972–2985. doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Chu CH, Chu IH, Chan KH, Chang YK. Executive function during acute exercise: the role of exercise intensity. J Sport Exerc Psychol. 2013;35:358–367. doi: 10.1123/jsep.35.4.358. [DOI] [PubMed] [Google Scholar]
- Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: implications for stress regulation. Ann Behav Med. 2009;37:126–140. doi: 10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Morita K, Waseda Y, Ueno T, Maeda H. Changes in auditory P300 with clinical remission in schizophrenia: effects of facial-affect stimuli. Psychiatry Clin Neurosci. 2001;55:347–352. doi: 10.1046/j.1440-1819.2001.00874.x. [DOI] [PubMed] [Google Scholar]
- Yang H, Dong M, Chen S, Zheng X. The effect of early attention allocation on location-based attention toward a later threat: an ERP study. Neurosci Lett. 2012;523:62–66. doi: 10.1016/j.neulet.2012.06.042. [DOI] [PubMed] [Google Scholar]
- Yao Z, Yu D, Wang L, Zhu X, Guo J, Wang Z. Effects of valence and arousal on emotional word processing are modulated by concreteness: behavioral and ERP evidence from a lexical decision task. Int J Psychophysiol. 2016;110:231–242. doi: 10.1016/j.ijpsycho.2016.07.499. [DOI] [PubMed] [Google Scholar]
- Zeng H, Yang C, Dai G, Qin F, Zhang J, Kong W. EEG classification of driver mental states by deep learning. Cogn Neurodyn. 2018 doi: 10.1007/s11571-018-9496-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Hasegawa H. Central dopaminergic neurotransmission plays an important role in thermoregulation and performance during endurance exercise. Eur J Sport Sci. 2016;16:818–828. doi: 10.1080/17461391.2015.1111938. [DOI] [PubMed] [Google Scholar]
- Zschucke E, Renneberg B, Dimeo F, Wustenberg T, Strohle A. The stress-buffering effect of acute exercise: evidence for HPA axis negative feedback. Psychoneuroendocrinology. 2015;51:414–425. doi: 10.1016/j.psyneuen.2014.10.019. [DOI] [PubMed] [Google Scholar]